Chapter 21 Menopause
OVERVIEW
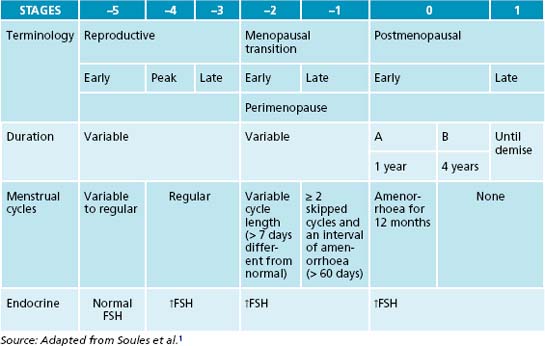
Menopause represents the natural transition from fertility to age-related non-fertility. The term ‘menopause’ specifically means the cessation of menstruation, and most often occurs between the ages of 45 and 55 years, with the average age for the last period being 51 years.
All healthy women will transition from a reproductive (premenopausal) period—marked by regular ovulation and cyclic menstrual bleeding—to a postmenopausal period marked by amenorrhoea. The onset of the menopausal transition is marked by changes in the menstrual cycle and in the duration or amount of menstrual flow (see Table 21.1). Subsequently, cycles are missed, but the pattern is often erratic early in the menopausal transition. Therefore the menopause is defined retrospectively after 12 months of continued amenorrhoea.
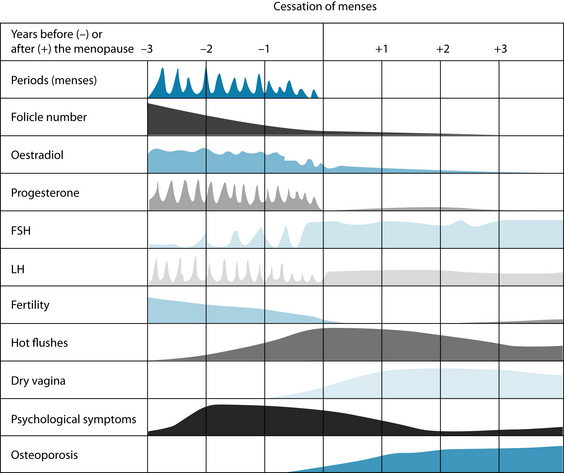
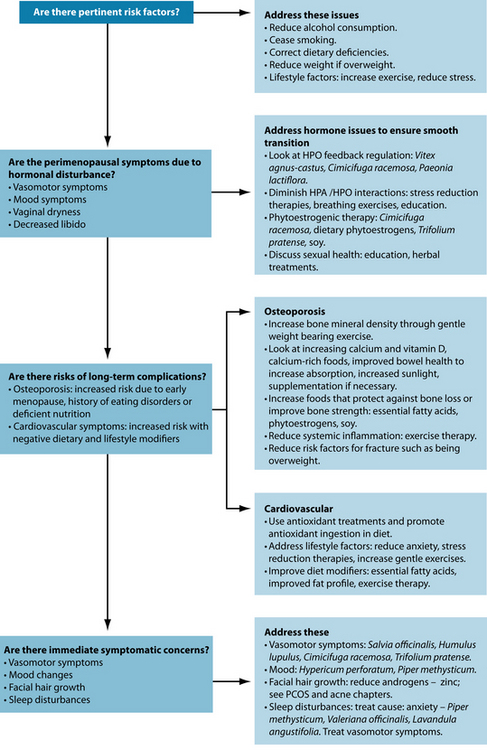
The term ‘perimenopause’ is most often used to describe the time leading up to and directly following menopause.2 During perimenopause, hormone levels fluctuate and women may experience a variety of symptoms including vasomotor symptoms (hot flushes and night sweats), vulvovaginal atrophy, emotional fluctuations and cognitive decline (memory problems). Some women experience very little in the way of these symptoms; for others this time is particularly debilitating. Low oestrogen levels postmenopause also increase the risk of a number of other physical conditions including osteoporosis and cardiovascular disease. The various symptoms of the menopausal transition are associated with a variety of physiological changes and the responses to these changes. Figure 21.1 shows the symptoms associated with changes in hormone levels.
In the postmenopausal phase FSH rise to levels 10–15 times the level that can be expected during the follicular phase of a reproductive cycle, while LH levels increase to around three times that experienced during menstruation. The ovaries do continue to excrete minimal amounts of oestrogens, and continue to excrete significant amounts of androgens.2
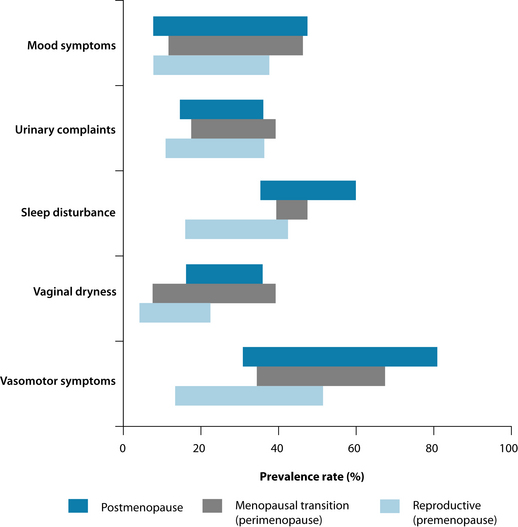
It should also be noted that individual women will experience the menopausal transition differently. The prevalence rates of the main symptoms can vary greatly across the different stages of menopause (see Figure 21.2).4
CONVENTIONAL TREATMENT
Conventional practitioners tend to underestimate the effects of perimenopausal symptoms on their patients.5 However, fewer than 25% of women experience a (relatively) symptom-free menopause and over 25% of women experience debilitating symptoms.6 Many women turn to conventional practitioners for explanation and reassurance rather than treatment.3
There is much conjecture and controversy surrounding the use of HT in menopause. There are definite benefits to using HT. HT is effective for vasomotor symptoms, vulvovaginal atrophy symptoms, osteoporosis-related fracture prevention, and benefits cardiovascular and central nervous systems.7 However, continued use of HT can increase the risk for venous thromboembolism and increases the risk of developing breast cancer.7
HT may be recommended in certain circumstances—specifically, distressing symptoms or significant osteoporosis.7 Longer-term use of HT is generally considered a matter for discussion between the physician and the patient, with risks taken into account in addition to potential benefit to bone health and quality of life. Generally, long-term use is discouraged and physicians are encouraged to use lower therapeutic doses.
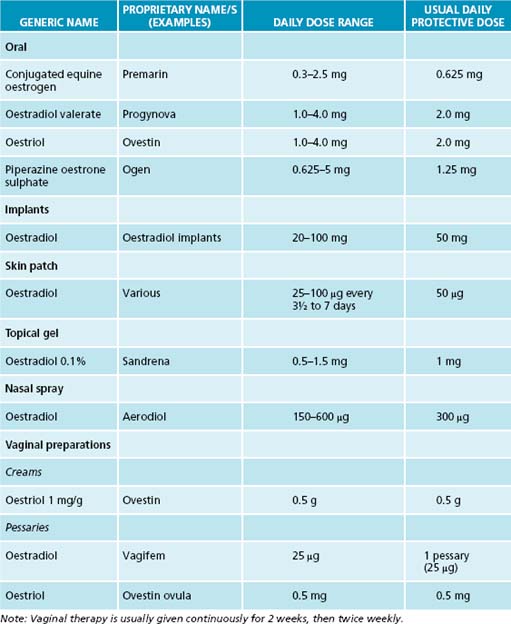
Various forms of oestrogen have been used for many years as a hormonal supplement to treat menopausal symptoms and are generally thought to be the most effective treatment for vasomotor symptoms. Oestrogen is therefore no longer recommended for prevention of chronic conditions, although it is effective and approved for osteoporosis prevention.4 Women with an intact uterus are usually prescribed the ‘opposed’ regimen (when oestrogen is combined with a progestin to avoid the development of endometrial hyperplasia and endometrial cancer). Testosterone may also be given to women. Examples of commonly used HT in Australia are listed in Tables 21.2 and 21.3.
| GENERIC NAME | DAILY DOSE RANGE | USUAL DAILY PROTECTIVE DOSE |
|---|---|---|
| Dydrogesterone | 10–20 mg | 10 mg |
| Medroxyprogesterone acetate | 2.5–20 mg | 10 mg |
| Norethisterone | 1.25–5 mg | 2.5 mg |
RISK FACTORS
Although much focus tends to be drawn towards the effect that HT can have on increasing breast cancer risk, other modifiable lifestyle factors such as little exercise, smoking, postmenopausal weight gain and high alcohol consumption may increase the risk of not only breast cancer but other menopausal symptoms as well.8
Risk factors for severity of symptoms during perimenopause include genetics (symptoms experienced by the mother may give a guide), body mass index (BMI)—women with a higher BMI may experience a lower level of perimenopause symptoms due to the production of oestrogen by aromatase in adipose tissue (see Chapter 19 on endometriosis).
The duration of symptoms appears independent of HT but is less in those groups with higher physical activity.9 Other lifestyle factors, such as increased alcohol and caffeine use and smoking, are also associated with worsened menopausal symptoms.10
KEY TREATMENT PROTOCOLS
Women undergoing the menopause transition turn to complementary medicines not only to seek effective treatment but also to gain greater control over their symptoms.17 It is therefore essential that a strong participatory relationship is encouraged during treatment and that the naturopath does not fall into the habit of product-prescribing only.
When looking at the literature there seems to be little relation to gross product sales (the most commonly sold complementary therapies for menopause) and specific clinical effectiveness. However, these therapies may be working on broader improvements or act on a psychosomatic level by offering the patients the opportunity to take control of their condition. For example, while acupuncture does not seem to have specific effects on menopausal symptoms such as hot flushes18 it does seem to offer improvements to quality of life scores in perimenopausal women overall.19
For example, treatment with a combination Hypericum perforatum and Cimicifuga racemosa product for 16 weeks resulted in a 50% reduction in Menopause Rating Scale (MRS) scores, with a 41.8% reduction in the Hamilton Depression Rating Scale.20
OESTROGEN-LIKE COMPOUNDS AND OESTROGEN RECEPTOR ACTIVITY
Many compounds—both natural and synthetic—may mimic endogenous sex hormones.11–13 Phytoestrogens may bind to and activate these oestrogen receptor sites. Given the use of hormone replacement therapy in conventional treatment, these compounds are gaining popularity amongst both the public and practitioners for relieving menopausal symptoms.
For example, the phytoestrogenic isoflavones found in Trifolium pratense and soy are thought to have beneficial effects on cognitive abilities, bone mineral density and plasma lipid concentrations in menopausal women.14 Phytoestrogenic activity may also be utilised in traditional Chinese medicine. Astragalus membranaceus and Scutellaria baicalensis are traditionally used in the treatment of menopausal symptoms in traditional Chinese medicine and exert oestrogenic activity in vivo.15
Dietary intake of phytoestrogens and an increased consumption of whole-grain foods may also be associated with a reduction in vasomotor symptoms.16
Many compounds exerting oestrogenic activity will be discussed in relation to symptoms. Many have not actually been studied extensively for menopausal symptoms, but phytoestrogens are discussed in more detail in Chapter 19 on endometriosis.
Although C. racemosa was effective in treating neurovegetative symptoms of depression in menopausal women, combination treatment was more effective than C. racemosa treatment alone.21 These changes seem more significant than the effects on vasomotor symptoms alone. And while H. perforatum does not seem to exert a significant effect on hot flush symptoms, it does proffer significant improvement on quality of life scales due to its effect on other symptoms associated with menopause and should therefore also be considered.22 C. racemosa also has positive effects on overall quality of life scales and other broader menopause rating scales when used alone in addition to its effects on individual symptoms.23 Several relaxation therapies have also proven to be effective in alleviating or reducing a number of perimenopausal symptoms, rather than having specific outcomes.
Major symptoms
Hot flushes
Exercise
Because exercise has demonstrated effects on sex steroids, it may moderate at least the severity, if not the frequency, of hot flushes.9,24–26 One epidemiological study found that women who belonged to a gymnasium club reported lower incidence of hot flushes than those who were not, although their individual exercise regimens were unreported.27 However, studies on exercise, while generally demonstrating reduced severity and incidence of hot flushes, suggest it has had the greatest effect on quality of life scores.
Herbal medicine
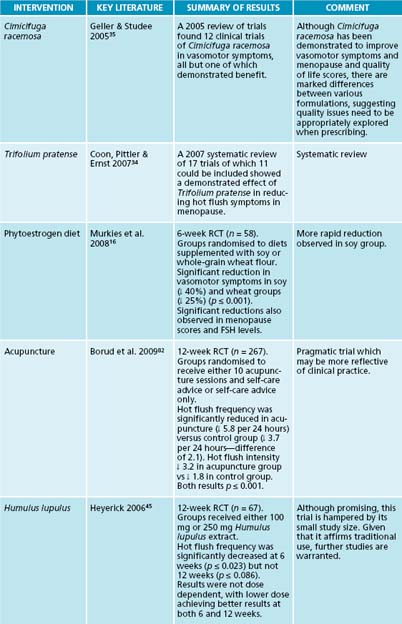
A 2007 systematic review of 17 trials of which 11 could be included showed a demonstrated effect of Trifolium pratense in reducing hot flush symptoms in menopause.34 A 2005 review of trials found 12 clinical trials of C. racemosa in vasomotor symptoms,
BLACK COHOSH SAFETY
Although concerns relating to the hepatotoxicity of Cimicifuga racemosa have been raised, systematic reviews have suggested it can be generally thought to be a safe medicine, with the main side effects being mild and reversible, and no direct causal link to Cimicifuga was found in cases of severe hepatotoxicity.28–31 In the most recent trial, long-term supplementation of Cimicifuga for a period of 1 year was found to have no effects on hepatic blood flow or liver function.32 However, an association does exist as hepatotoxicity has occurred in several people taking supplements containing Cimicifuga. This is thought to be related to issues of product quality, concomitant medications or individual genetic susceptibility. Although the specific mechanism relating to hepatotoxicity associated with Cimicifuga use is unknown, one theory gaining credence is an immunologic reaction to triterpene glycosides in the plant in susceptible individuals, rather than the direct result of inherent toxicity.33 Therefore these issues should be considered when advising or treating patients using these therapies, as they should for any other pharmacological intervention.
all but one of which demonstrated benefit,35 and a later non-specific review found significant benefit also.36
C. racemosa may act through a number of pathways independent of its activity as a phytoestrogen. Recent evidence suggests that it may have an effect on opiate receptors.37 Cimicifuga extract has also been demonstrated to exert dopaminergic activity in vitro.38 This may in part also explain the effects of Cimicifuga in menopause as dopaminergic drugs are often used to treat menopausal symptoms such as hot flushes. Some studies have suggested that Cimicifuga has an equipotent effect to oestrogen39 although this does not seem reflective of the entirety of the data.40,41 It should be noted, however, that significant heterogeneity exists in the results for differing formulations, suggesting that quality issues need to be considered before prescribing.
Angelica sinensis and Matricaria recutita in combination has been demonstrated to reduce hot flushes and improve sleep in menopausal women,42 but other trials have failed to demonstrate these results when Angelica sinensis was used alone.43,44
Humulus lupulus supplementation over 12 weeks was found to reduce discomforting symptoms of menopause, particularly hot flushes, even at low doses.45 This is thought to be due to the oestrogenic nature of H. lupulus.46 Salvia officinalis was also demonstrated to improve symptoms of hot flushes and nights sweats in perimenopausal women.47
Psychological interventions
Panax ginseng has been demonstrated to improve psychological symptoms, particularly fatigue, insomnia and depression, in menopausal women.54
In menopausal women with psychological and psychosomatic symptoms, sole therapy using Hypericum perforatum demonstrated significant improvement (76.4% self-evaluation and 79.2% physician evaluation), including improved sexual wellbeing.55
Piper methysticum as an adjuvant treatment has been demonstrated to reduce anxiety in perimenopausal women.56 It may also be useful in treating sleep disorders not associated with vasomotor symptoms that are often experienced by perimenopausal women (see Chapter 14 on insomnia).
Sexual function and libido
Women undergoing the perimenopausal transition often experience changes in libido or dyspareunia which can be influenced by a number of physiological or psychosocial ways. Hormone changes may influence sexual function in a number of ways. Low levels of oestrogens and androgens seem to be directly related to libido, as do low levels of testosterone.57 Physical changes enacted by alterations in hormone levels—for example, vaginal dryness associated with lower levels of oestrogen—will have a significant negative effect on sexual response. Lack of lubrication can result in soreness and tenderness, and ultimately less enjoyment during sexual encounters for these reasons. Anticipation of painful sex due to lack of lubrication after a negative experience may further decrease desire. Patients need to be counselled adequately on the practical implications of the menopausal transition as many women may not have previously needed to apply measures such as endogenous lubrication. Oestrogens also have a vasodilatory effect that can result in increased blood flow in vaginal, clitoral and urethral areas; this may reduce during menopause, and loss of oestrogen is also associated with relaxation of vaginal tissue and muscle tone in genital areas. Encouraging circulation and tone in these areas—for example, through pelvic floor exercises or more general regimens such as exercise, yoga or tai chi—may also be useful.
Physical changes alone are not solely responsible for the negative effects of the perimenopausal transition on sexual function and libido. Psychological changes such as depression, anxiety and low self-esteem can all affect sexual desire and enjoyment, as can many of the medicines prescribed for these conditions. Conversely, the effects of menopausal changes on a woman’s sex life can also precipitate psychological changes. The social construct of menopause in modern society can also have a deleterious effect in this regard. Menopause in Western societies is often viewed negatively, particularly in relation to the loss of femininity. This is in stark contrast to many traditional cultures, who often view the transition as a positive one that affords them respect as elders and relief from child-bearing. Patients need to be counselled appropriately throughout this period, encouraged to explore their femininity and sexual selves and reminded that these aspects of themselves need not diminish once menstruation ceases. One study has shown that while yoga therapy did not increase general self-esteem, it was significant in improving esteem relating to perceived sexual attractiveness.58
Many herbal medicines have been used to increase libido. Asparagus racemosus is colloquially referred to as ‘she with one thousand husbands’ in the Ayurvedic tradition in reference to its effects on sexual function and has demonstrated positive results in animal studies, but is yet to be tested through human trials.59 This situation is also true of other traditionally used aphrodisiac herbs such as Turnera diffusa and Tribulus terrestris. A product containing Panax ginseng, Turnera diffusa, Ginkgo biloba and l-arginine has been found to significantly increase libido in women at all stages of the menopausal transition compared to placebo in two small trials.60,61
Treating the psychological symptoms associated with menopause may also improve libido. Hypericum perforatum—a herb commonly used to treat the psychosocial problems associated with menopause—was found to improve libido when used to treat seasonal affective disorder62 and improved sexual wellbeing in 80% of perimenopausal women when prescribed for psychosocial symptoms.55 Trigonella foenum-graecum has been used for postmenopausal vaginal dryness in the North American naturopathic tradition.63
Vaginal atrophy and incontinence
After menopause the vaginal lining may thin due to lower oestrogen levels. This may result in painful intercourse and a greater susceptibility to vaginal infections. Regular intercourse may be beneficial as it increases blood flow to the genitalia, but adequate lubrication is required—natural or supplemental. Naturopathic treatment options for recurrent bladder infections are covered in Chapter 27.
Vitamin D may have a role in reducing vaginal atrophy. Supplementation with vitamin D has reduced vaginal atrophy in one small trial.64
Incontinence incidence may increase in postmenopausal women. Many women may resign themselves to this being a normal part of the ageing process, but there are several therapeutic options available. Pelvic floor exercises may reduce the incidence of incontinence in postmenopausal women and improve quality of life.65 Increased physical exercise more generally may also be beneficial in reducing the incidence or severity of urinary incontinence.66
Urinary tract infections
Approximately 15% of menopausal women will experience frequent bladder infections.2 General recommendations for urinary tract infections are discussed in Chapter 27 on urinary tract infection, but there are several factors to take into consideration when treating urinary tract infections in perimenopausal women, such as decreased acidity of the urine, loss of bladder elasticity, due to lower oestrogen levels and the fact that urinary tract infections are often symptomless during the menopause.
Long-term considerations
Although much focus tends to be drawn towards the association between hormone therapy and increasing breast cancer, other modifiable lifestyle factors such as little exercise, smoking, postmenopausal weight gain and high alcohol consumption have similar rates of increased risk of breast cancer.8 And although fat intake alone is not associated with
CAM AND OSTEOPOROSIS
increased risk of breast cancer, attention to this lifestyle factor can help to improve the cardiovascular outcomes usually associated with the menopausal transition.67 Attention needs to be paid to the long-term consequences of the menopausal transition as well as short-term symptomatic treatment.
Osteoporosis
In a clinical setting osteoporosis is often addressed in the context of menopausal treatment because it is found in postmenopausal women and can be largely prevented by correcting oestrogen deficiency. Approximately 30% of postmenopausal women are estimated to have osteoporosis.68 The hypo-oestrogenic state leads to loss of bone density in postmenopausal women by activation of the bone remodelling units with an excess of bone resorption compared to bone formation.69
Adequate dietary intakes are necessary for prevention and treatment of osteoporosis.
Another dietary intervention that may be beneficial for osteoporosis in postmenopausal women is omega-3 fatty acids. Several human and animal studies have shown significant decreases in bone resorption and a protective effect on bone with omega-3 fatty acids.70–72
Adequate calcium and vitamin D consumption are essential in the treatment of osteoporosis. It is generally recommended that 1500 mg of calcium and 800 IU per day of vitamin D are recommended for postmenopausal women.7 Dietary sources of calcium are listed in Table 21.4. However, dietary intake of calcium alone may not be sufficient, particularly in women who have undergone early menopause.73 Sunlight exposure of 5–10 minutes per day may be required, particularly considering that older people may synthesise vitamin D less well over time. Supplementation of both calcium and vitamin D may be necessary. However, large studies such as the Women’s Health Initiative have shown that reliance on supplementation can be fraught, as compliance can be low—only 59% of women were taking more than 80% of their recommended supplementation towards the study’s end.74 Dietary modification should therefore form the basis of treatment. More dietary recommendations for bone health can be found in Chapter 22 on osteoarthritis.
| FOOD | AMOUNT | CALCIUM CONTENT (MG) |
|---|---|---|
| Yoghurt | 200 g (1 small tub) | 520 |
| Calcium-enriched milk | 250 mL (1 cup) | 475 |
| Skim milk | 250 mL (1 cup) | 400 |
| Tahini | 20 g | 310 |
| Full fat milk | 250 mL (1 cup) | 300 |
| Calcium-enriched soy milk | 250 mL (1 cup) | 290 |
| Sardines | 5 whole | 286 |
| Cheddar cheese | 30 g (1 slice) | 232 |
| Parmesan cheese | 20 g | 230 |
| Salmon (tinned) | ½ cup with bones | 220 |
| Prawns | 1 cup | 132 |
| Mussels | 6 whole | 120 |
| Dried figs | 3 | 108 |
| Tofu | 80 g | 96 |
| Ice-cream | 60 g (2 scoops) | 90 |
| Soy beans, chickpeas or kidney beans | ½ cup | 70 |
| Dried apricots | 10 halves | 42 |
| Silverbeet or spinach | ½ cup | 38 |
| Oranges | 1 whole | 35 |
| Broccoli | 1 cup | 25 |
Phytoestrogenic therapy may offer a limited protective role.75 Considering that HT and SORM therapy are both used to prevent the effects of osteoporosis, this is worth exploring. Increased soy consumption may inhibit bone resorption and stimulate bone formation76,77 and population studies indicate that the incidence of osteoporosis is lower in countries with higher soy consumption.14
Regular exercise can increase bone density in postmenopausal women.68 However, high impact exercise may be counterproductive as more severe forms of exercise may result in micro-damage, increasing the risk of fracture.78 Regular tai chi exercise may improve bone density scores and offer protection against fracture in postmenopausal women.79 Further benefits of specific exercises are discussed in Chapter 22 on osteoarthritis.
Cardiovascular health
Women are offered a higher degree of protection against cardiovascular disease before the menopause due to higher oestrogen levels. However, reductions in oestrogen levels postmenopause result in women having the same incidence of cardiovascular disease as men by the age of 70 years.80 Despite this protective mechanism it is still estimated that 94% of adverse cardiovascular risk in women is associated with modifiable risk factors such as type II diabetes, hypertension, diet, stress, smoking and obesity.81 Therefore women who have previously not had to consider cardiovascular health implications may need to investigate this issue in more depth (see the section on the cardiovascular system).
Exercise
As well as the benefits of regular exercise in treating specific menopausal symptoms such as hot flushes previously outlined, exercise confers a number of other benefits in the perimenopausal patient. These benefits include decreased bone loss, improved circulation,
As menopause is not a defined medical condition but rather a collection of symptoms associated with a transitional stage through life, many of the treatments are covered in other relevant chapters. Table 21.5 focuses on vasomotor symptoms.
INTEGRATIVE MEDICAL CONSIDERATIONS
Acupuncture
Acupuncture does offer specific effects on menopausal symptoms such as hot flushes,24,82 but demonstrates significantly greater improvements to quality of life scores in perimenopausal women more broadly.19 Traditional Chinese medicine has a long history of treating menopausal symptoms and, typically, acupuncture would not be used alone.83 However, to be effective these treatments need to be prescribed according to traditional Chinese medicine theory.
Homoeopathy
Homoeopathic treatment has shown encouraging results, but requires further research. Homoeopathy has been successful in treating oestrogen withdrawal symptoms in women with breast cancer.84 As the symptoms treated in menopausal patients can vary substantially, individualised treatment is advised.
Example treatment
Prescription
She was prescribed the herbal tablet in the box on the right to be taken one tablet, once daily.
She was told to take three kava tablets half an hour before bed, and two tablets during periods of extreme irritability. She was counselled on sleep hygiene issues. She was also prescribed 1 tablespoon Ulmus fulva with 1 teaspoon of raw honey in warm water at night to encourage healthy intestinal bacterial populations, settle digestive dysfunction and reduce abdominal discomfort.
Expected outcomes and follow-up protocols
Perimenopause is a natural part of the lifecycle and therefore symptoms are not necessarily indicative of pathology. The changes will result in symptoms and these can be expected to continue for some time (1 year or more). Naturopathic treatments will not be instantaneous; for example, C. racemosa treatment will require 2 weeks to observe its effect and 3 months before optimal benefit can be observed.59 Patients should be moved towards a general health program as soon as possible as this will ultimately improve their symptoms and lessen their duration.
KEY POINTS
Coon J., et al. Trifolium pratense isoflavones in the treatment of menopausal hot flushes: a systematic review and meta-analysis. Phytomedicine. 2007;14(2–3):153-159.
Geller S., Studee L. Botanical and dietary supplements for menopausal symptoms: what works, what does not. J Womens Health (Larchmt). 2005;14(7):634-649.
Hudson T. Women’s encyclopedia of natural medicine. New york. McGraw-Hill. 2008.
Society of Obstetricians and Gynaecologists of Canada. Canada, menopause and osteoporosis update. J Obstet Gynaecol Can. 2009;31(1):S27-S30.
1. Soules M., et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76:874-878.
2. Edmonds K., editor. Obstetrics and gynaecology. London: Blackwell, 2007.
3. Murtagh J. General practice. Sydney: McGraw-Hill; 2007.
4. Nelson H. Menopause. Lancet. 2008;371(9614):760-770.
5. Ghali W., et al. Menopausal hormone therapy: physician awareness of patient attitudes. Am J Med. 1997;103:3-10.
6. Porter M., et al. A population based survey of women’s experience of the menopause. Br J Obstet Gynaecol. 2002;103:1025-1028.
7. Society of Obstetricians and Gynaecologists of Canada. Menopause and osteoporosis update. J Obstet Gynaecol Can. 2009;31(1 Suppl 1):S27-S30.
8. Reeves G., et al. Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134.
9. Col N., et al. Duration of vasomotor symptoms in middle-aged women: a longitudinal study. Menopause. 2009;16(3):453-457.
10. Greendale G., Gold E. Lifestyle factors: are they related to vasomotor symptoms and do they modify the effectiveness or side effects of hormone therapy? Am J Med. 2005;118(12):148-154.
11. Rier S. The potential role of exposure to environmental toxicants in the pathophysiology of endometriosis. Ann N Y Acad Sci. 2002;955:201-212.
12. Birnbaum L., Cummings A. Dioxins and endometriosis: a plausible hypothesis. Environ Health Persp. 2002;110(1):15-21.
13. Foster W., Agarwhal S. Environmental contaminants and dietary factors in endometriosis. Ann N Y Acad Sci. 2002;955:213-229.
14. Geller S., Studee L. Soy and red clover for mid-life and aging. Climacteric. 2006;9(4):245-263.
15. Zhang C., et al. In vitro estrogenic activities of Chinese medicinal plants traditionally used for the management of menopausal symptoms. J Ethnopharmacol. 2005;98(3):295-300.
16. Murkies A., et al. Dietary flour supplementation decreases post-menopausal hot flushes: effect of soy and wheat. Maturitas. 2008;61(1–2):27-33.
17. Gollschewski S., et al. Women’s perceptions and beliefs about the use of complementary and alternative medicines during menopause. Complement Ther Med. 2008;16(3):163-168.
18. Lee M., et al. Acupuncture for treating menopausal hot flushes: a systematic review. Climacteric. 2009;12(1):16-25.
19. Ailfhaily F., Ewies A. Acupuncture in managing menopausal symptoms: hope or mirage? Climacteric. 2007;10(5):371-380.
20. Uebelhack R., et al. Black cohosh and St John’s wort for climacteric complaints: a randomized trial. Obstet Gynecol. 2006;107(2.1):247-255.
21. Briese V., et al. Black cohosh with or without St John’s wort for symptom-specific climacteric treatment—results of a large-scale, controlled, observational study. Maturitas. 2007;57(4):405-414.
22. Al-Akoum M., et al. Effects of Hypericum perforatum (St John’s wort) on hot flashes and quality of life in perimenopausal women: a randomized pilot trial. Menopause. 2009;16(2):307-314.
23. Mollá M., et al. Cimicifuga racemosa treatment and health related quality of life in post-menopausal Spanish women. Gynecol Endocrinol. 2009;25(1):21-26.
24. Lindh-Astrand L., et al. Vasomotor symptoms and quality of life in previously sedentary postmenopausal women randomised to physical activity or estrogen therapy. Maturitas. 2004;48(2):97-105.
25. Ivarsson T., et al. Physical exercise and vasomotor symptoms in postmenopausal women. Maturitas. 1998;29(2):139-146.
26. Thurston R., et al. Physical activity and risk of vasomotor symptoms in women with and without a history of depression: results from the Harvard Study of Moods and Cycles. Menopause. 2006;13:553-560.
27. Hammar M., et al. Does physical exercise influence the frequency of postmenopausal hot flushes? Acta Obstet Gynecol Scand. 1990;69(5):409-412.
28. Huntley A., Ernst E. A systematic review of the safety of black cohosh. Menopause. 2003;10(1):58-64.
29. Teschke R., Schwarzenboeck A. Suspected hepatotoxicity by Cimicifugae racemosae rhizoma (black cohosh, root): critical analysis and structured causality assessment. Phytomedicine. 2009;16(1):72-84.
30. Firenzuoli F., et al. Black cohosh hepatic safety: follow-up of 107 patients consuming a special Cimicifuga racemosa rhizome herbal extract and review of literature. Evidence Based Complement Altern Med. 2008. doi:10.1093/ecam/nen009
31. Borrelli F., Ernst E. Black cohosh (Cimicifuga racemosa): a systematic review of adverse events. Am J Obstet Gynecol. 2008;199(5):455-466.
32. Nasr A., Nafeh H. Influence of black cohosh (Cimicifuga racemosa) use by postmenopausal women on total hepatic perfusion and liver functions. Fertil Steril. 2009;92(5):1780-1782.
33. Chitturi S., Farrell G. Hepatotoxic slimming aids and other herbal hepatotoxins. J Gastrolenterol Hepatol. 2008;23:366-373.
34. Coon J., et al. Trifolium pratense isoflavones in the treatment of menopausal hot flushes: a systematic review and meta-analysis. Phytomedicine. 2007;14(2–3):153-159.
35. Geller S., Studee L. Botanical and dietary supplements for menopausal symptoms: what works, what does not. J Womens Health (Larchmt). 2005;14(7):634-649.
36. Cheema D., et al. Non-hormonal therapy of post-menopausal vasomotor symptoms: a structured evidence-based review. Arch Gynecol Obstet. 2007;276(5):463-469.
37. Reame N., et al. Black cohosh has central opioid activity in postmenopausal women: evidence from naloxone blockade and positron emission tomography neuroimaging. Menopause. 2008;15(5):832-840.
38. Jarry H., et al. In vitro effects of the Cimicifuga racemosa extract BNO 1055. Maturitas. 2003;44(Supp 1):31-38.
39. Wuttke W., et al. The Cimicifuga preparation BNO 1055 vs. conjugated estrogens in a double-blind placebo-controlled study: effects on menopause symptoms and bone markers. Maturitas. 2003;44(Suppl):S67-S77.
40. Borrelli F., Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacy. Pharmacol Res. 2008;58(1):8-14.
41. Low Dog T. Menopause: a review of botanical dietary supplements. Am J Med. 2005;118(Suppl 12B):98-108.
42. Kupfersztain C., et al. The immediate effect of natural plant extract, Angelica sinensis and Matricaria chamomilla (Climex) for the treatment of hot flushes during menopause. A preliminary report. Clin Exp Obstet Gynecol. 2003;30(4):203-206.
43. Hirata J., et al. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril. 1997;68(6):981-986.
44. Haines C., et al. A randomized, double-blind, placebo-controlled study of the effect of a Chinese herbal medicine preparation (dang gui buxue tang) on menopausal symptoms in Hong Kong Chinese women. Climacteric. 2008;11(5):439-440.
45. Heyerick A., et al. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas. 2006;54(2):164-175.
46. Chadwick L., et al. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine. 2006;13(1–2):119-131.
47. De Leo V., et al. Treatment of neurovegetative menopausal symptoms with a phytotherapeutic agent. Minrva Ginecol. 1998;50(5):207-211.
48. Freedman R., Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol. 1992;167(2):436-439.
49. Irvin J., et al. The effects of relaxation response training on menopausal symptoms. J Psychosom Obstet Gynaecol. 1996;17(4):202-207.
50. Nedstrand E., et al. Applied relaxation and oral estradiol treatment of vasomotor symptoms in postmenopausal women. Maturitas. 2005;51(2):154-162.
51. Carmody J., et al. A pilot study of mindfulness-based stress reduction for hot flashes. Menopause. 2006;13(5):760-769.
52. Booth-LaForce C., et al. A pilot study of a hatha yoga treatment for menopausal symptoms. Maturitas. 2007;57(3):286-295.
53. Chattha R., et al. Treating the climacteric symptoms in Indian women with an integrated approach to yoga therapy: a randomized control study. Menopause. 2008;15(5):862-870.
54. Tode T., et al. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int J Gynaecol Obstet. 1999;67(3):169-174.
55. Grube B., et al. St John’s Wort extract: efficacy for menopausal symptoms of psychological origin. Adv Ther. 1999;16(4):177-186.
56. De Leo V., et al. Evaluation of combining kava extract with hormone replacement therapy in the treatment of postmenopausal anxiety. Maturitas. 2001;39(2):185-188.
57. Gracia C., et al. Predictors of decreased libido in women during the late reproductive years. Menopause. 2004;11(2):144-150.
58. Elavsky S., McAuley E. Exercise and self-esteem in menopausal women: a randomized controlled trial involving walking and yoga. Am J Health Promot. 2007;22(2):83-92.
59. Mills S., Bone K. Principles and practice of phytotherapy. Edinburgh: Churchill Livingstone; 2000.
60. Ito T., et al. The enhancement of female sexual function with ArginMax, a nutritional supplement, among women differing in menopausal status. J Sex Marital Ther. 2006;32(5):369-378.
61. Ito T., et al. A double-blind placebo-controlled study of ArginMax, a nutritional supplement for enhancement of female sexual function. J Sex Marital Ther. 2001;27(5):541-549.
62. Wheatley D. Hypericum in seasonal affective disorder (SAD). Curr Med Res Opin. 1999;15(1):33-37.
63. Braun L., Cohen M. Herbs and natural supplements: an evidence-based guide. Sydney: Elsevier; 2007.
64. Yildirim B., et al. The effects of postmenopausal vitamin D treatment on vaginal atrophy. Maturitas. 2004;49(4):334-337.
65. Borello-France D., et al. Continence and quality-of-life outcomes 6 months following an intensive pelvic-floor muscle exercise program for female stress urinary incontinence: a randomized trial comparing low- and high-frequency maintenance exercise. Phys Ther. 2008;88(12):1545-1553.
66. Peterson J. Minimize urinary incontinence: maximize physical activity in women. Urol Nurs. 2008;28(5):351-356.
67. Prentice R., et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629-642.
68. Bonaiuti D., et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. (3):2002. CD000333
69. Bjarnason N. Postmenopausal bone remodelling and hormone replacement. Climacteric. 1998;1(1):72-79.
70. Griel A., et al. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr J. 2007;6:2.
71. Sun D., et al. Dietary n-3 fatty acids decrease osteoclastogenesis and loss of bone mass in ovariectomized mice. J Bone Miner Res. 2003;18(7):1206-1216.
72. Fernandes G., et al. Protective role of n-3 lipids and soy protein in osteoporosis. Prostaglandins Leukot Essent Fatty Acids. 2003;68(6):361-372.
73. Bischoff-Ferrari H., et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257-2264.
74. Jackson R., et al. Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
75. Potter S., et al. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1998;83(Supp):1375S-1379S.
76. Ma D., et al. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr. 2008;27(1):57-64.
77. Ma D., et al. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2008;62(2):155-161.
78. Rittweger J. Can exercise prevent osteoporosis? J Musculoskelet Neuronal Interact. 2006;6(2):162-166.
79. Wayne P., et al. The effects of tai chi on bone mineral density in postmenopausal women: a systematic review. Arch Phys Med Rehabil. 2007;88(5):673-680.
80. Tunstall-Pedoe H. Myth and paradox of coronary risk and menopause. Lancet. 1998;351(9113):1425-1427.
81. Yusuf S., et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937-952.
82. Borud E., et al. The Acupuncture on Hot Flushes Among Menopausal Women (ACUFLASH) study, a randomized controlled trial. Menopause. 2009;16(3):484-493.
83. Maciocia G. Obstetrics and gynecology in Chinese medicine. Edinburgh: Churchill Livingstone; 1997.
84. Thompson E., Reilly D. The homoeopathic approach to the treatment of symptoms of oestrogen withdrawal in breast cancer patients. A prospective observational study. Homoeopathic. 2003;92(3):131-134.
85. Cleary C., Fox J. Menopausal symptoms: an osteopathic investigation. Complement Ther Med. 1994;2:181-186.