CHAPTER 8 Meniscal Transplantation
Surgical treatment of meniscus lesions has changed significantly over time. Meniscal tears were traditionally treated with meniscal excision, but it became understood that loss of the meniscus alters the biologic and biomechanical environment of the knee.1,2 The resulting degenerative changes in the involved compartment led us away from meniscus removal and toward meniscus preservation. Partial meniscectomy and meniscus repair procedures have become the standard of care. For patients for whom meniscal preservation is not an option, meniscal allograft transplantation can be done for a select subset of patients who have become symptomatic from their meniscal deficiency. This offers restoration of anatomic and biomechanical function.
ANATOMY
The menisci are semilunar-shaped fibrocartilaginous structures that function in shock absorption,3 load transmission,4–6 secondary mechanical stability,7,8 joint lubrication,9 and nutrition.10 Circumferentially oriented collagen fibers provide resistance to hoop stresses whereas radially oriented fibers hold the circumferential fibers together and provide resistance to shear.11,12 The anterior and posterior horns attach to bone by interdigitating collagen fibers oriented to transmit load and shear optimally from the meniscus to the tibia.13
The menisci are composed of 74% water,14 allowing for optimization in force transmission. The lateral meniscus carries 70% of the lateral compartment load, compared with 50% by the medial meniscus.6,15 The menisci transmit 50% of the joint load when in knee extension and 90% when the knee is in flexion.6,15 Loss of the meniscus, therefore, increases the load on the articular cartilage surfaces and facilitates the development of early degenerative changes. Loss of just 16% to 35% of the meniscal tissue can lead to a 350% increase in contact forces.4 Clinical studies support meniscus preservation, because a greater size of meniscal resection is associated with a poor clinical outcome.16–19
PATIENT EVALUATION
Physical Examination
Examination often reveals full range of motion. Depending on recent activity, a joint effusion may be present. Joint line or femoral condyle tenderness is occasionally found. A thorough physical examination is essential to reveal malalignment, ligament deficiency, or articular cartilage lesions that would modify the treatment plan. These findings need to be addressed, either as a concurrent or staged procedure.
TREATMENT
Indications and Contraindications
Although not absolute contraindications, chondral defects, malalignment, or ligamentous instability all require consideration for concurrent or staged procedures to ensure that all joint pathology is addressed. In the past, full-thickness chondral defects were considered a contraindication; however, cartilage degeneration is not a significant risk factor for meniscal allograft failure.20 Outcomes of many concurrent procedures, including meniscal transplantation with concurrent autologous chondrocyte implantation (ACI)21,22 and osteochondral allograft32 have shown excellent results in the carefully selected patient.
Concurrent or staged corrective osteotomy is indicated for patients with deviation toward the involved compartment. Axial malalignment can exert abnormal pressure on the newly placed graft, which can lead to loosening, overload, degeneration, and failure.23–25
Anterior cruciate ligament (ACL)–deficient patients who have had a prior medial meniscectomy may benefit from concomitant ACL reconstruction (ACLR) and meniscal transplantation. Many studies have shown that meniscectomized ACL-deficient knees lead to worsening degenerative changes. The more aggressive approach of combination ACLR and meniscal transplantation has good long-term follow-up as opposed to untreated (left alone) knees. In addition, the posterior horn of the medial meniscus is an important secondary stabilizer to anterior translation and may be important in preventing secondary “stretch” of the ACL reconstructed knee.7,26–28
Conservative Management
Patients who have had a prior meniscectomy should have a trial of conservative treatment before consideration of operative measures. Activity modifications, anti-inflammatory medications, and occasionally injections can be recommended to help determine which patients can function without surgical intervention. More aggressive management of the relatively young patient following lateral meniscectomy might be considered, especially in female athletes with slight valgus who are at significant risk for the development of progressive lateral compartment arthritis.29
Arthroscopic Technique
Preoperative Planning
Allograft Sizing.
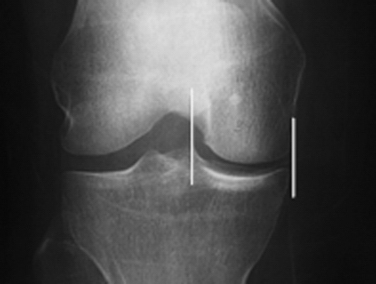
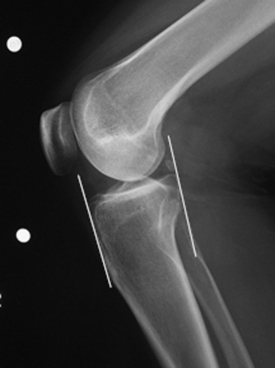
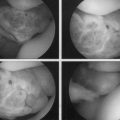
The success of meniscal transplantation is dependent on careful size matching of the meniscus allograft to the native meniscus. Meniscal allografts are compartment- and size-specific. Anteroposterior and lateral preoperative radiographs with sizing markers are important for meniscal sizing (Figs. 8-1 and 8-2). Allograft sizing is of significant importance, because oversized meniscal allografts lead to greater forces across the articular cartilage.30 On the other hand, undersized allografts result in greater forces seen by the meniscal tissue.30 The meniscus width is determined on the AP radiograph by measuring from the edge of the ipsilateral tibial spine to the edge of the tibial plateau. Meniscal length is determined on the lateral radiograph as determined by the AP dimension of the ipsilateral tibial plateau. These measurements, after correction for magnification, are multiplied by 0.8 for medial and 0.7 for lateral meniscus. Other methods using height and weight have been proposed, but are not routinely used.31,32
Meniscal Graft Processing and Preservation.
Meniscal allografts are harvested using sterile surgical technique ideally within 24 hours after death and frozen to −80° C. Although other graft preservation methods are used, including secondary sterilization methods, fresh-frozen grafts remain the most commonly used allograft preservation method.33–35
Stringent donor selection is based on comprehensive medical and social history. The risk of disease transmission is further reduced by screening for human immunodeficiency virus (HIV), human T-cell lymphotropic virus (HTLV), hepatitis B and C, and syphilis. Blood cultures for aerobic and anaerobic bacteria, as well as lymph node sampling, may be performed. Graft processing, including débridement, ultrasonic-pulsatile washing, and use of ethanol to denature proteins, further lowers the risk of disease transmission.36
Surgical Anatomy, Incisions, and Portals
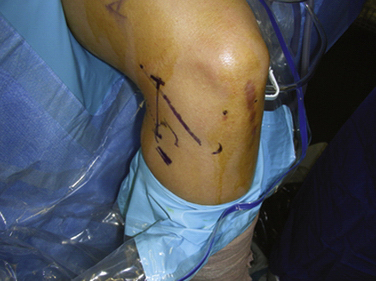
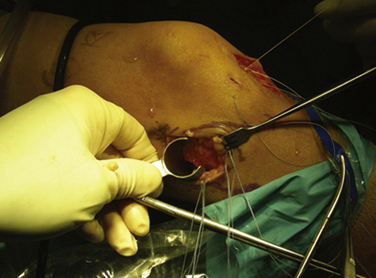
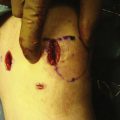
Important surgical landmarks to be identified are the patella, patellar tendon, tibial plateau, and fibular head. Portals and incisions include inferomedial and inferolateral arthroscopy portals, an accessory outflow portal, a posterolateral or posteromedial incision, and a miniarthrotomy adjacent to or splitting the patellar tendon on the transplant side (Fig. 8-3).
Caution should be taken while making incisions because many structures are at risk, depending on the approach. These include the peroneal nerve and lateral collateral ligament with the posterolateral approach, the saphenous nerve and medial collateral ligament with the posteromedial approach, and the patellar tendon with the anterior miniarthrotomy. In addition, the posterior neurovascular bundle can be damaged during needle passage when suturing the meniscus in place, especially on the lateral side.
Examination Under Anesthesia and Diagnostic Arthroscopy
PEARLS& PITFALLS
Arthroscopic Preparation.
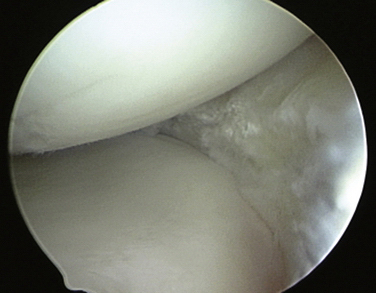
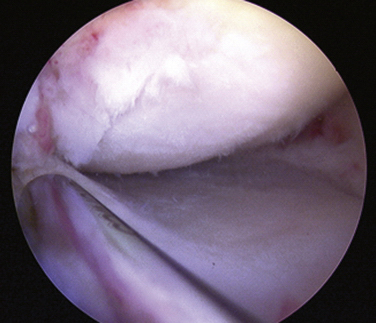
Initially, the steps for medical and lateral meniscal transplantation are similar. The remaining meniscus is arthroscopically débrided to a 1- to 2-mm peripheral rim until punctate bleeding occurs (Fig. 8-4). The most anterior part of the meniscus can be excised with an no. 11 scalpel through the respective anterior portal. The anterior and posterior horn insertion sites should be maintained, because they are helpful markers during preparation of the slot. In addition, limited notchplasty along the posterior and inferior femoral condyle allows for improved visualization of the posterior horn during passage of the graft.
Slot Preparaztion.
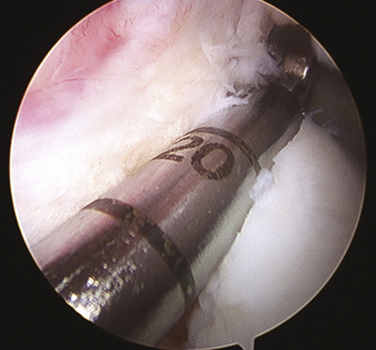
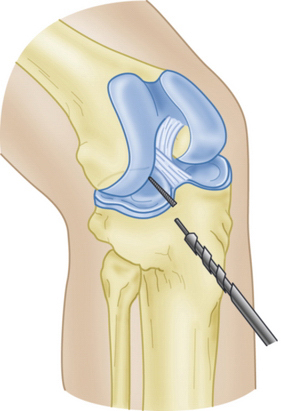
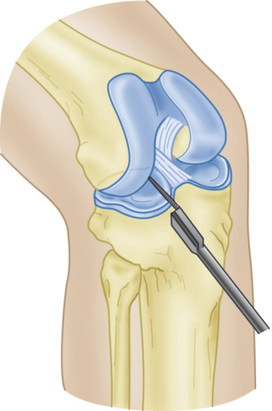
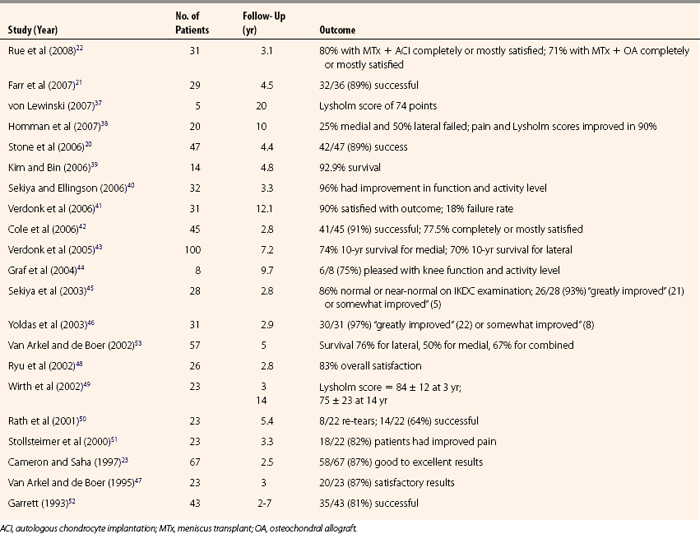
Slot orientation follows the normal anatomy of the meniscal attachment sites. Electrocautery is used to establish a line connecting the center of the anterior and posterior horn attachment sites. Using this line as a guide, a 4-mm burr is used to make a straight anterior to posterior reference slot in the tibial plateau (Fig. 8-5). Slot height and width will equal the dimensions of the burr, and its alignment in the sagittal plane should parallel the slope on the tibial plateau. Slot measurements, including the AP length of the tibial plateau, are confirmed by placement of a depth gauge in the reference slot (Fig. 8-6). A drill guide is used to place a guide pin just distal and parallel to the reference slot (Figs. 8-7 and 8-8). The drill guide is advanced to but not through the posterior cortex. The pin is subsequently overreamed with a 7- or 8-mm cannulated drill bit (Fig. 8-9). A box cutter osteotome is then used to widen the trough to 7 to 8 mm and deepen it to 10 mm (Fig. 8-10). This is then refined with a 7- to 8-mm rasp to allow insertion of the bone bridge of the allograft. Final slot preparation is shown in Figure 8-11.
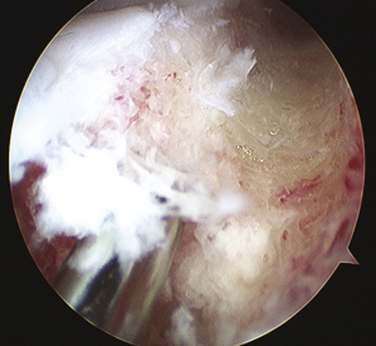
FIGURE 8-7 Intraoperative depiction of the burr slot with the guide pin inserted. Partial reaming has been performed to ensure that the trajectory and depth are appropriate prior to finishing reaming to the posterior cortex.
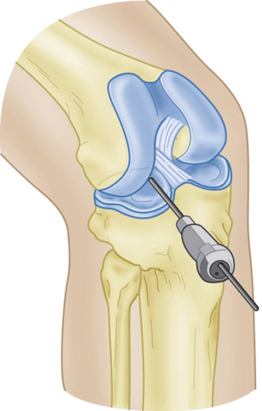
FIGURE 8-8 Schematic representation of a guide pin positioned in the superficial tibial plateau reference slot.
PEARLS& PITFALLS
Meniscal Allograft Preparation.
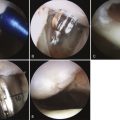
The meniscus allograft tissue arrives as a hemiplateau with an attached meniscus. All nonmeniscal soft tissue is removed. Attachment sites of the meniscus are identified on the bone block, and the accessory attachments are débrided, leaving only true attachment sites (approximately 5 to 6 mm wide). The meniscus is then prepared to achieve the desired width and length, as determined by the slot preparation. The width of the bone bridge is intentionally undersized by 1 mm to facilitate graft passage into the slot and reduce inadvertent bridge fracture during placement. The bone bridge is then cut to a width of 7 mm and a height of 10 mm. Bone extending beyond the posterior horn attachment is removed so that the posterior wall of the bone bridge will be flush with the most posterior edge of the prepared slot. Bone extending to the anterior horn, however, should be preserved to maintain graft integrity and ease in graft insertion. A vertical mattress traction suture (0 polydioxanone [PDS]) is placed at the junction of the posterior horn and middle thirds of the meniscus (Fig. 8-12).
If the anterior horn attachment is larger (up to 9 mm wide), the attachment should be left intact and the width of the bone bridge should be increased accordingly in the area of the anterior horn insertion only. The remainder of the bone bridge should be the intended 7 mm. To accommodate the increased width, the corresponding area of the recipient slot should be widened.
Meniscus Insertion and Fixation.
Once the meniscus is reduced, the knee is cycled to ensure proper placement and capturing by the tibiofemoral articulation. A guidewire is inserted between the bone bridge and the medial eminence side of the slot. A tap is inserted over the guidewire to create a path for the interference screw, with the bone bridge held in place manually (typically, a freer; Fig. 8-13). The bone bridge is then secured within the tibial slot with a 7 × 25-mm bioabsorbable cortical interference screw. This step is typically done in flexion under direct visualization.
Finally, the graft is attached to the capsule with eight to ten standard inside-out vertical mattress sutures (2-0 Ethibond) placed equally on the superior and inferior meniscal surfaces (Figs. 8-14 and 8-15). Sutures should be placed peripherally on the meniscus, because sutures placed in the middle or inner third of the meniscus can weaken the implant. For medial transplants, this fixation can be modified with the use of appropriate all-inside fixation devices placed most posteriorly and outside-in sutures placed most anteriorly.
PEARLS& PITFALLS
Concurrent Procedures
Anterior Cruciate Ligament and Medial Meniscal Transplantation.
PEARLS& PITFALLS
Postoperative Rehabilitation Protocol
Immediate partial weight-bearing is allowed in a hinged knee brace, with range of motion limited to 0 to 60 degrees of flexion for the first 2 weeks and to 90 degrees at 4 weeks. Non–weight-bearing flexion beyond 90 degrees is also allowed immediately. Progression to full weight bearing and range of motion as well as strengthening exercises are achieved at 4 weeks postoperatively. Squatting and pivoting are avoided until 16 weeks, at which in-line running is permitted. Return to full activity is permitted after 6 months as long as strength is 80% that of the contralateral leg.
CONCLUSIONS
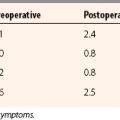
Meniscal allograft transplantation yields good to excellent results in almost 85% of patients (Table 8-1). Patients demonstrate significant decrease in pain, as well as an increase in activity. Long-term success is encouraging in well-selected patients but it is unknown whether meniscal transplantation is protective against the progression of degenerative changes.
1. Allen PR, Denham RA, Swan AV. Late degenerative changes after meniscectomy. Factors affecting the knee after operation. J Bone Joint Surg Br. 1984;66:666-671.
2. Baratz ME, Fu FH, Mengato R. Meniscal tears: the effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. Am J Sports Med. 1986;14:270-275.
3. Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5:157-161.
4. Seedhom BB, Hargreaves DJ. Transmission of load in the knee joint with special reference to the role of the menisci: part II. Experimental results, discussions, and conclusions. Eng Med Biol. 1979;8:220-228.
5. Seedhom BB, Dowson D, Wright V. Proceedings: functions of the menisci. A preliminary study. Ann Rheum Dis. 1974;33:111.
6. Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;(109):184-192.
7. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64:883-888.
8. Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee—the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58:583-594.
9. MacConaill MA. The movements of bones and joints; the synovial fluid and its assistants. J Bone Joint Surg Br. 1950;32:244-252.
10. Renstrom P, Johnson RJ. Anatomy and biomechanics of the menisci. Clin Sports Med. 1990;9:523-538.
11. Bullough PG, Munuera L, Murphy J, et al. The strength of the menisci of the knee as it relates to their fine structure. J Bone Joint Surg Br. 1970;52:564-567.
12. McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990;(252):8-18.
13. Gao J, Wei X, Messner K. Healing of the anterior attachment of the rabbit meniscus to bone. Clin Orthop Relat Res. 1998;(348):246-258.
14. Cole BJ, Carter TR, Rodeo SA. Allograft meniscal transplantation:: background, techniques, and results. Instr Course Lect. 2003;52:383-396.
15. Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints. Part I: tibial surface of the knee. J Biomech Eng. 1983;105:216-225.
16. Bonneux I, Vandekerckhove B. Arthroscopic partial lateral meniscectomy long-term results in athletes. Acta Orthop Belg. 2002;68:356-361.
17. Higuchi H, Kimura M, Shirakura K, et al. Factors affecting long-term results after arthroscopic partial meniscectomy. Clin Orthop Relat Res. 2000;(377):161-168.
18. Meredith DS, Losina E, Mahomed NN, et al. Factors predicting functional and radiographic outcomes after arthroscopic partial meniscectomy: a review of the literature. Arthroscopy. 2005;21:211-223.
19. Rockborn P, Gillquist J. Outcome of arthroscopic meniscectomy. A 13-year physical and radiographic follow-up of 43 patients under 23 years of age. Acta Orthop Scand. 1995;66:113-117.
20. Stone KR, Walgenbach AW, Turek TJ, et al. Meniscus allograft survival in patients with moderate to severe unicompartmental arthritis: a 2- to 7-year follow-up. Arthroscopy. 2006;22:469-478.
21. Farr J, Rawal A, Marberry KM. Concomitant meniscal allograft transplantation and autologous chondrocyte implantation: minimum 2-year follow-up. Am J Sports Med. 2007;35:1459-1466.
22. Rue JP, Yanke AB, Busam ML, et al. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008;36:1770-1778.
23. Cameron JC, Saha S. Meniscal allograft transplantation for unicompartmental arthritis of the knee. Clin Orthop Relat Res. 1997;(337):164-171.
24. de Boer HH, Koudstaal J. Failed meniscus transplantation. A report of three cases. Clin Orthop Relat Res. 1994;(306):155-162.
25. Noyes FR, Barber-Westin SD, Rankin M. Meniscal transplantation in symptomatic patients less than fifty years old. J Bone Joint Surg Am. 2005;87(suppl 1):149-165.
26. Alford W, Cole BJ. Failed ACL reconstruction and meniscus deficiency. Background, indications and techniques for revision ACL reconstruction with allograft meniscus transplantation. Sports Med Arthrosc Rev. 2005;13:93-102.
27. Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28:446-452.
28. Shoemaker SC, Markolf KL. The role of the meniscus in the anterior-posterior stability of the loaded anterior cruciate-deficient knee. Effects of partial versus total excision. J Bone Joint Surg Am. 1986;68:71-79.
29. Alford JW, Lewis P, Kang RW, et al. Rapid progression of chondral disease in the lateral compartment of the knee following meniscectomy. Arthroscopy. 2005;21:1505-1509.
30. Dienst M, Greis PE, Ellis BJ, et al. Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med. 2007;35:34-42.
31. Stone KR, Freyer A, Turek T, et al. Meniscal sizing based on gender, height, and weight. Arthroscopy. 2007;23:503-508.
32. Van Thiel G, Verma NN, McNickle AG, et al. Meniscal allograft size can be predicted by height, weight and gender. Arthroscopy. 2009;25:722-727.
33. Alford W, Cole BJ. The indications and technique for meniscal transplant. Orthop Clin North Am. 2005;36:469-484.
34. McNickle AG, Wang VM, Shewman EF, et al. Performance of a sterile meniscal allograft in an ovine model. Clin Orthop Relat Res. 2009;467:1868-1876.
35. Rodeo SA. Meniscal allografts—where do we stand. Am J Sports Med. 2001;29:246-261.
36. Vangsness CTJr, Garcia IA, Mills CR, et al. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474-481.
37. von Lewinski G, Milachowski KA, Weismeier K, et al. Twenty-year results of combined meniscal allograft transplantation, anterior cruciate ligament reconstruction and advancement of the medial collateral ligament. Knee Surg Sports Traumatol Arthrosc. 2007;15:1072-1082.
38. Hommen JP, Applegate GR, Del Pizzo W. Meniscus allograft transplantation: ten-year results of cryopreserved allografts. Arthroscopy. 2007;23:388-393.
39. Kim JM, Bin SI. Meniscal allograft transplantation after total meniscectomy of torn discoid lateral meniscus. Arthroscopy. 2006;22:1344-1350.
40. Sekiya JK, Ellingson CI. Meniscal allograft transplantation. J Am Acad Orthop Surg. 2006;14:164-174.
41. Verdonk PC, Verstraete KL, Almqvist KF, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14:694-706.
42. Cole BJ, Dennis MG, Lee SJ, et al. Prospective evaluation of allograft meniscus transplantation: a minimum 2-year follow-up. Am J Sports Med. 2006;34:919-927.
43. Verdonk PC, Demurie A, Almqvist KF, et al. Transplantation of viable meniscal allograft. Survivorship analysis and clinical outcome of one hundred cases. J Bone Joint Surg Am. 2005;87:715-724.
44. Graf KWJr, Sekiya JK, Wojtys EM, et al. Long-term results after combined medial meniscal allograft transplantation and anterior cruciate ligament reconstruction: minimum 8.5-year follow-up study. Arthroscopy. 2004;20:129-140.
45. Sekiya JK, Giffin JR, Irrgang JJ, et al. Clinical outcomes after combined meniscal allograft transplantation and anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:896-906.
46. Yoldas EA, Sekiya JK, Irrgang JJ, et al. Arthroscopically assisted meniscal allograft transplantation with and without combined anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2003;11:173-182.
47. Van Arkel ER, de Boer HH. Human meniscal transplantation. Preliminary results at 2- to 5-year follow-up. J Bone Joint Surg Br. 1995;77:589-595.
48. Ryu RK, Dunbar VWH, Morse GG. Meniscal allograft replacement: a 1-year to 6-year experience. Arthroscopy. 2002;18:989-994.
49. Wirth CJ, Peters G, Milachowski KA, et al. Long-term results of meniscal allograft transplantation. Am J Sports Med. 2002;30:174-181.
50. Rath E, Richmond JC, Yassir W, et al. Meniscal allograft transplantation. Two- to eight-year results. Am J Sports Med. 2001;29:410-414.
51. Stollsteimer GT, Shelton WR, Dukes A, et al. Meniscal allograft transplantation: a 1- to 5-year follow-up of 22 patients. Arthroscopy. 2000;16:343-347.
52. Garrett JC. Meniscal transplantation: a review of 43 cases with two- to seven-year follow-up. Sports Med Arthrosc Rev. 1993;2:163-167.
53. Van Arkel ER, de Boer HH. Survival analysis of human meniscal transplantations. J Bone Joint Surg Br. 2002;84:227-231.