4 Meninges
Cranial Meninges
Dura mater
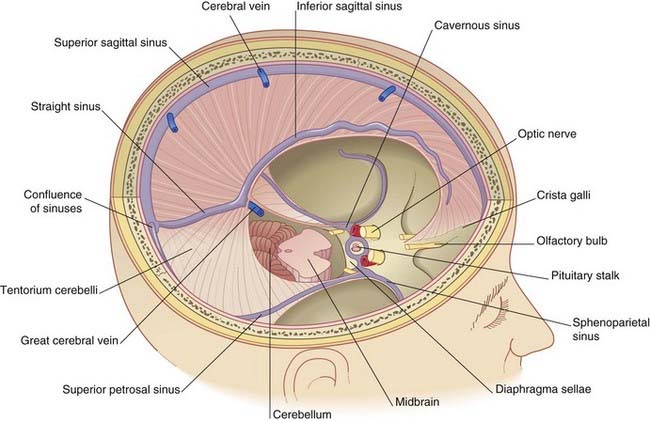
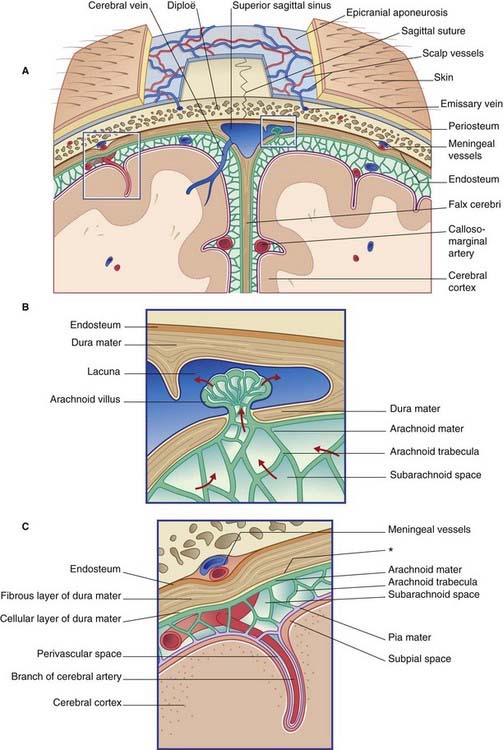
The terminology used to describe the cranial dura mater varies among different authors. It seems best to regard it as a single, tough layer of fibrous tissue that is fused with the endosteum (inner periosteum) of the skull, except where it is reflected into the interior of the vault or is stretched across the skull base. Wherever it separates from the periosteum, the intervening space contains venous sinuses (Figure 4.1).
The crescentic tentorium cerebelli arches like a tent above the posterior cranial fossa, being lifted up by the falx cerebri in the midline. The attached margin of the tentorium encloses the transverse sinuses on the inner surface of the occipital bone and the superior petrosal sinuses along the upper border of the petrous temporal bone. The attached margin reaches to the posterior clinoid processes of the sphenoid bone. Most of the blood from the superior sagittal sinus usually empties into the right transverse sinus (Figure 4.2).
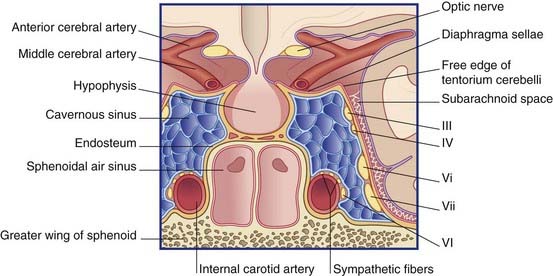
The free margin of the tentorium is U-shaped. The tips of the U are attached to the anterior clinoid processes. Just behind this, the two limbs of the U are linked by a sheet of dura, the diaphragma sellae, which is pierced by the pituitary stalk. Laterally, the dura falls away into the middle cranial fossae from the limbs of the U, creating the cavernous sinus on each side (Figure 4.3). Behind the sphenoid bone, the concavity of the U encloses the midbrain.
Innervation of the cranial dura mater
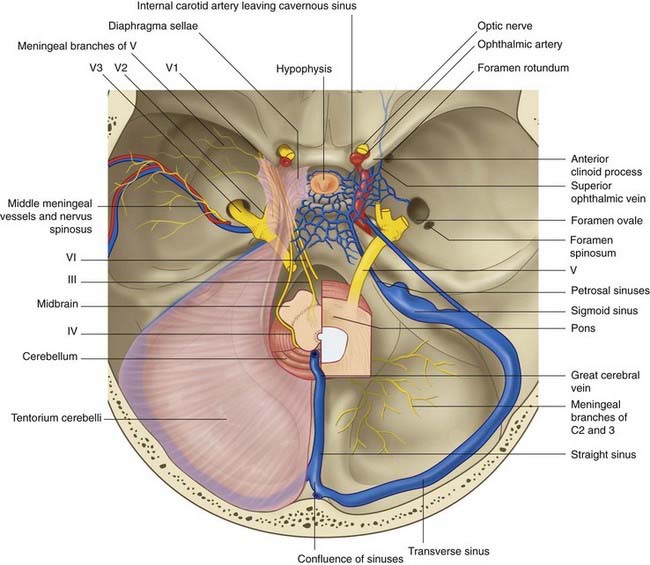
The dura mater lining the supratentorial compartment of the cranial cavity receives sensory innervation from the trigeminal nerve. That lining the anterior cranial fossa and anterior part of the skull vault is supplied by its ophthalmic branch; that lining the middle cranial fossa and midregion of the vault is mainly supplied by the nervus spinosus (Figure 4.2). This nerve leaves the mandibular nerve outside the foramen ovale, to return via the foramen spinosum and accompany the middle meningeal artery and its branches. Stretching or inflammation of the supratentorial dura gives rise to frontal or parietal headache.
The dura mater lining the infratentorial compartment is supplied by branches of upper cervical spinal nerves entering the foramen magnum (Figure 4.2). Occipital and posterior neck pains accompany disturbance of the infratentorial dura. Acute meningitis involving posterior cranial fossa meninges is associated with neck rigidity and often with head retraction brought about by reflex contraction of the posterior nuchal muscles, which are supplied by cervical nerves. Violent occipital headache follows subarachnoid hemorrhage (Ch. 35), where free blood swirls around the hindbrain.
Meningeal arteries
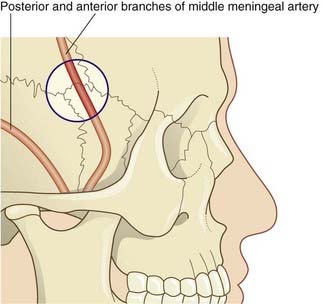
Embedded in the endosteum of the skull are several meningeal arteries whose main function is to supply the diploë (bone marrow). Much the largest is the middle meningeal artery, which ramifies over the inner surface of the temporal and parietal bones. Tearing of this artery, with its accompanying vein, is the usual source of an extradural hematoma (Clinical Panel 4.1).
Clinical Panel 4.1 Extradural and subdural hematomas
An extradural (epidural) hematoma is typically caused by a blow to the side of the head severe enough to cause a fracture with associated tearing of the anterior or posterior branch of the middle meningeal artery. Most cases remain unconscious unless treated. Occasionally, following the initial concussion of the brain, with loss of consciousness, there may be a lucid interval of several hours. Onset of increasing headache and drowsiness signals cerebral compression produced by expansion of the hematoma. Coma and death will supervene unless the hematoma is drained though a burr hole. The favored site of access is the H-shaped suture complex known as the pterion, which overlies the anterior branch of the middle meningeal artery (Figure CP 4.1.1).
Subdural hematomas are caused by rupture of superficial cerebral veins in transit from the brain to an intracranial venous sinus. An acute subdural hematoma most often follows severe head injury in children. It must always be suspected where a child remains unconscious after a head injury. Child-battering is a possible explanation if this situation arises in the home. A subacute subdural hematoma may follow head injury at any age. Symptoms and signs of raised intracranial pressure (described in Ch. 6) develop up to 3 weeks after the injury.
Arachnoid mater
The arachnoid (Gr. ‘spidery’) is a thin, fibrocellular layer in direct contact with the dura mater (Figure 4.4). The outermost cells of the arachnoid are bonded to one another by tight junctions that seal the subarachnoid space. Innumerable arachnoid trabeculae cross the space to reach the pia mater.
Pia mater
The pia mater invests the brain closely, following its contours and lining the various sulci (Figure 4.4). Like the arachnoid, it is fibrocellular. The cellular component of the pia is external and is permeable to CSF. The fibrous component occupies a narrow subpial space that is continuous with perivascular spaces around cerebral blood vessels penetrating the brain surface.
Note: Although the subarachnoid and subpial spaces are proven, there is no sign of any ‘subdural space’ in properly fixed material. Such a space can be created, however, by leakage of blood into the cellular layer of the dura mater following a tear of a cerebral vein at its point of anchorage to the fibrous layer. (See Subdural hematoma in Clinical Panel 4.1.)
Subarachnoid cisterns
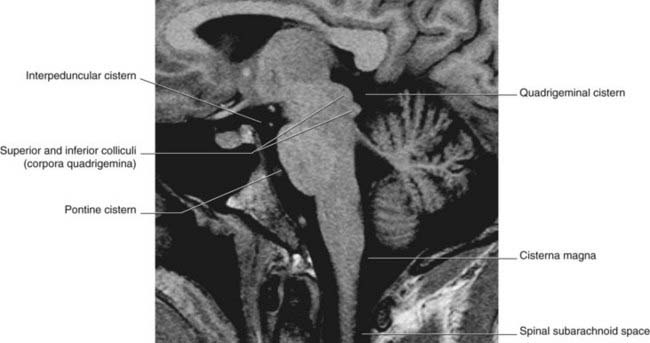
Along the base of the brain and the sides of the brainstem, pools of CSF occupy subarachnoid cisterns (Figures 4.5 and 4.6). The largest of these is the cisterna magna, in the interval between cerebellum and medulla oblongata. More rostrally are the cisterna pontis ventral to the pons, the interpeduncular cistern between the cerebral peduncles, and the cisterna ambiens at the side of the midbrain. The complete list of cisterns is in Table 4.1.
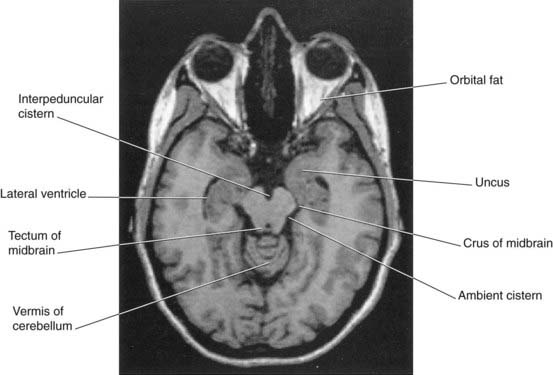
Figure 4.6 Horizontal MRI. Note the proximity of the uncus to the crus of the midbrain (cf. uncal herniation, Clinical Panel 6.2).
(From a series kindly provided by Professor J. Paul Finn, Director, Magnetic Resonance Research, Department of Radiology, David Geffen School of Medicine at UCLA, California, USA.)
| Cistern | Location |
|---|---|
| Posterior cerebellomedullary (cisterna magna) | Between cerebellum and dorsal surface of medulla oblongata |
| Lateral cerebellomedullary | Along each side of the medulla |
| Chiasmatic | Behind and above the optic chiasm |
| Cistern of lateral cerebral fossa | Along the lateral sulcus (Sylvian fissure) |
| Interpeduncular | Interpeduncular fossa |
| Ambient (cisterna ambiens) | On each side of the midbrain |
| Quadrigeminal | Surrounding the great cerebral vein dorsal to the midbrain colliculi (quadrigeminal bodies) |
Sheath of the optic nerve
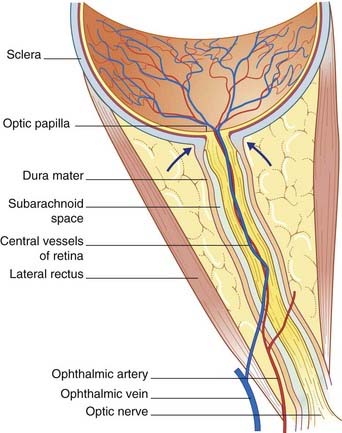
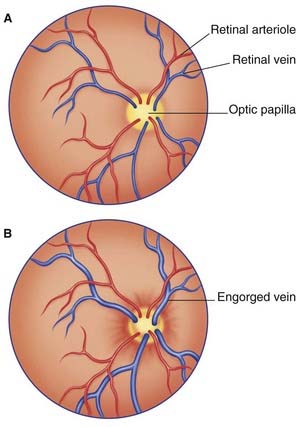
The optic nerve is composed of CNS white matter, and it has a complete meningeal investment. The dura mater fuses with the scleral shell of the eyeball; the subarachnoid space is a tubular cul de sac (dead end). The central vessels of the retina pierce the meninges to enter it (Figure 4.7). Any sustained elevation of intracranial pressure will be transmitted to the subarachnoid sleeve surrounding the nerve. The central vein will be compressed, resulting in swelling of the retinal tributaries of the vein and edema of the optic papilla, where the optic nerve begins. The condition is known as papilledema (Figure 4.8). It can be recognized on inspection of the retina with an ophthalmoscope.
Spinal Meninges (Figure 4.9)
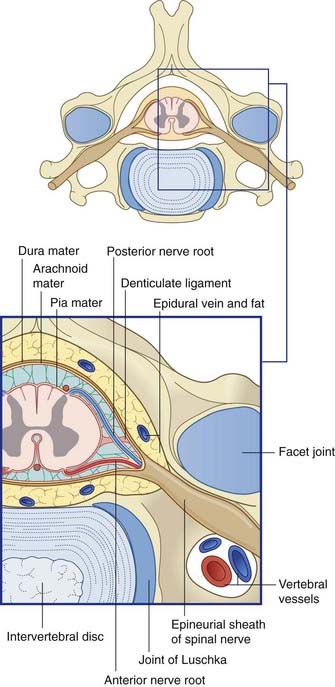
The spinal dural sac is like a test tube, attached to the rim of the foramen magnum and reaching down to the level of the second sacral vertebra. The outer surface of the tube is adherent to the posterior longitudinal ligament of the vertebrae in the midline; elsewhere, it is surrounded by fat containing the epidural, internal vertebral venous plexus (Ch. 14).
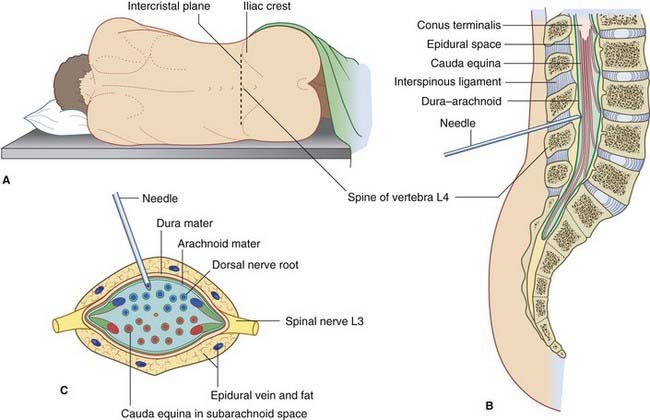
Because the spinal cord reaches only to first or second lumbar vertebral level, a large lumbar cistern is created, containing the free-floating motor and sensory roots of the sacral and lower lumbar spinal nerves (Ch. 14). The lumbar cistern may be tapped to procure samples of CSF for analysis (Clinical Panel 4.2) or to deliver a spinal anesthetic (Ch. 14).
Circulation of Cerebrospinal Fluid (Figure 4.10)
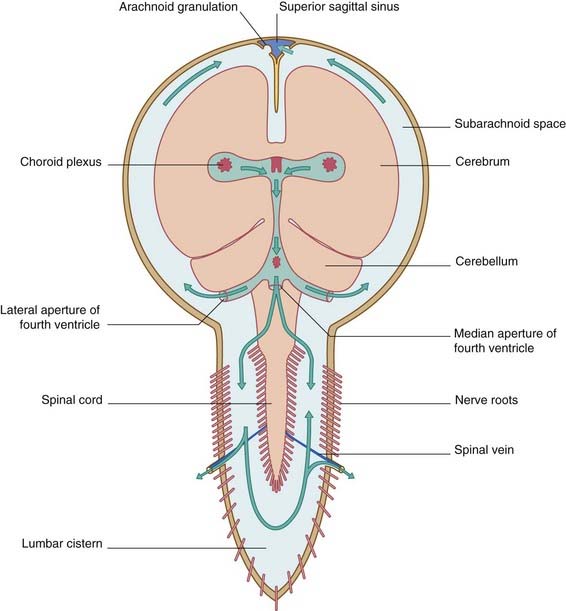
Within the subarachnoid space, some of the CSF descends through the foramen magnum, reaching the lumbar cistern in about 12 h. From the subarachnoid space at the base of the brain, the CSF ascends through the tentorial notch and bathes the surface of the cerebral hemispheres before being returned to the blood through the arachnoid granulations (Figure 4.4). The arachnoid granulations are pinhead pouches of arachnoid mater projecting through the dural wall of the major venous sinuses – especially the superior sagittal sinus and the small venous lacunae that open into it. CSF is transported across the arachnoid epithelium in giant vacuoles.
About 300 mL of CSF is secreted by the choroid plexuses every 24 h. Another 200 mL is produced from other sources, as described in Chapter 5. Blockage of flow through the ventricular system or cranial subarachnoid space will cause back-up within the ventricular system: a state of hydrocephalus (Clinical Panel 4.3).
Clinical Panel 4.3 Hydrocephalus
In the great majority of cases, hydrocephalus is caused by obstruction of the foramina opening the fourth ventricle to the subarachnoid space. A major cause of outlet obstruction in infancy is the Arnold–Chiari malformation, where the cerebellum is partly extruded into the vertebral canal during fetal life because the posterior cranial fossa is underdeveloped. In untreated cases, the child’s head may become as large as a football and the cerebral hemispheres paper-thin. The condition is nearly always associated with spina bifida (Ch. 14). Early treatment is essential to prevent severe brain damage. The obstruction can be bypassed by means of a catheter having one end inserted into a lateral ventricle and the other inserted into the internal jugular vein.
A major cause of outlet obstruction is displacement of the cerebellum into the foramen magnum by a space-occupying lesion (mass) such as a tumor or hematoma (see Ch. 6).