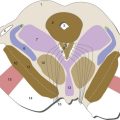
4 Meningeal Coverings of the Brain and Spinal Cord
There Are Three Meningeal Layers: The Dura Mater, Arachnoid, and Pia Mater
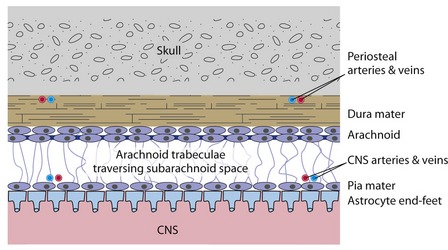
The dura mater, or dura, is a thick connective tissue membrane that also serves as the periosteum of the inside of the skull (Fig. 4-1). The arachnoid and the pia mater (or pia) are much thinner collagenous membranes. The arachnoid is attached to the inside of the dura and the pia is attached to the outer surface of the CNS. Hence the only space normally present between or around the cranial meninges is subarachnoid space (not counting the venous sinuses found within the dura). The arrangement of spinal meninges is slightly different, as described later in this chapter.
The Dura Mater Provides Mechanical Strength
The thickness and abundant collagen of the dura make it the mechanical link that connects the skull to the delicate strands of arachnoid (arachnoid trabeculae) that suspend the CNS in its bath of cerebrospinal fluid (CSF). This combination of partial flotation of the CNS in subarachnoid CSF, together with mechanical suspension by the skull-dura-arachnoid-arachnoid trabeculae-pia-CNS connections (see Fig. 4-3), stabilizes the fragile CNS during routine head movements.
The Dura Mater Has Its Own Blood Supply
In addition to the venous sinuses that drain the brain, the dura contains a mostly separate set of arteries and veins that help with its periosteal role for the skull (see Fig. 4-1). Meningeal arteries can be important clinically because tearing one can cause bleeding between the skull and dura (epidural bleeding, described later).
The Dura Mater Has an Arachnoid Lining
The arachnoid is attached to the skull by virtue of its attachment to the dura. The pia is attached to the CNS. Hence, the arachnoid trabeculae that interconnect the pia and arachnoid form a relatively weak mechanical suspension system for the CNS. The CNS is completely immersed in the CSF that fills subarachnoid space. The partial flotation effect of this CSF reduces the effective weight of the CNS to the point that the meningeal suspension system can support the brain and spinal cord (see Fig. 4-3).
CSF Enters the Venous Circulation through Arachnoid Villi
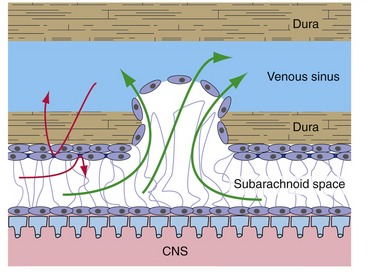
Arachnoid outpouchings poke through holes in the walls of venous sinuses as arachnoid villi (Fig. 4-2). Here only an arachnoid layer and an endothelial layer separate CSF and venous blood, the arachnoid barrier (see next section) is missing, and CSF can move directly from subarachnoid space into venous blood. Arachnoid villi act like mechanical flap valves, so when CSF pressure is higher than venous pressure (the usual situation), CSF moves into the venous system. If the reverse situation occurs, the villi snap shut and venous blood doesn’t enter subarachnoid space.
The Arachnoid Has a Barrier Function
The arachnoid includes a layer of cells that are connected to each other by bands of tight junctions, represented by dark bars in Figs. 4-1 and 4-2. This forms a barrier to the diffusion of extracellular substances from the dura into CSF, or in the reverse direction. The arachnoid barrier is part of a system, summarized in Chapter 6, that effectively separates the extracellular fluids of the nervous system from those of the rest of the body.
The Vertebral Canal Contains Spinal Epidural Space
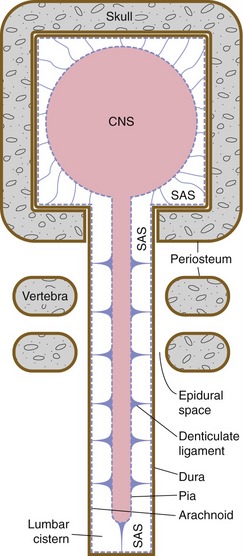
Spinal meninges are similar in principle to cranial meninges: thick dura lined by arachnoid, subarachnoid space filled with CSF, and suspensory interconnections between arachnoid and pia (Fig. 4-3). However, at the level of the foramen magnum the periosteal component of the cranial dura continues around onto the outer surface of the skull. The result is a real spinal epidural space between the spinal dura and the vertebral periosteum (as opposed to the potential cranial epidural space, which when present is located between the periosteum and the skull).
Arachnoid trabeculae are thickened around the spinal cord to form denticulate ligaments. The spinal cord is shorter than the vertebral canal (see Chapter 10), so there’s a large subarachnoid cistern, the lumbar cistern, extending from vertebral levels L1/L2 to S2.
Bleeding Can Open Up Potential Meningeal Spaces
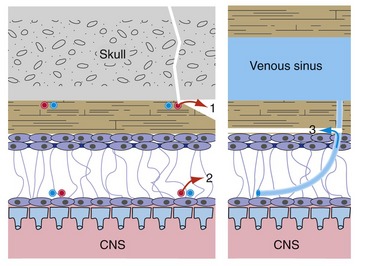
Spaces are not normally present between the dura and either the skull or the arachnoid, but these potential epidural and subdural* spaces can be opened up in certain pathological conditions (Fig. 4-4; see THB6 Figure 4-14, p. 92). Most often this is caused by tearing of a meningeal artery (leading to epidural bleeding) or of a cerebral vein as it enters a dural venous sinus (leading to subdural bleeding). Rupture of ordinary cerebral arteries and veins causes subarachnoid bleeding because these vessels reside in subarachnoid space. Each has a characteristic appearance in clinical images: Epidural hematomas are usually localized and lens-shaped (THB6 Figure 4-15, p. 93), subdural hematomas are usually larger and crescent-shaped (THB6 Figure 4-18, p. 95), and subarachnoid bleeding fills sulci and cisterns (THB6 Figure 6-25, p. 141).
Parts of the CNS Can Herniate from One Intracranial Compartment into Another
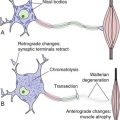
Because the dural septa are tough and taut, and brain is soft and squishy, expanding masses (e.g., hematomas, tumors) can cause parts of the brain to herniate from one side of a dural septum to another (THB6 Figure 4-19, p. 96). The most common such herniations are for one cingulate gyrus to herniate under the falx, or (more ominously) for the uncus of one temporal lobe to herniate through the tentorial notch and compress the midbrain. Similarly, an expanding mass in the posterior fossa can cause low-hanging parts of the cerebellum (the cerebellar tonsils; see Fig. 20-1) to herniate through the foramen magnum and compress the medulla.