Megaloblastic anaemia
Why does deficiency of vitamin B12 or folate lead to megaloblastic anaemia?
Key characteristics of these essential vitamins are summarised in Table 13.1.
Table 13.1
| Characteristic | Vitamin B12 | Folate |
| Average dietary intake/day (µg) | 20 | 2501 |
| Minimum adequate intake/day (µg) | 1–2 | 1501 |
| Major food sources | Animal produce only | Liver, vegetables |
| Normal body stores | Sufficient for several years | Sufficient for a few months |
| Mode of absorption | Combined with transport protein (IF) secreted by gastric parietal cells – then absorbed through ileum via special receptors | Dietary folate converted to methyl THF and absorbed in duodenum and jejunum |
Both folate and vitamin B12 are necessary for the synthesis of DNA (Fig 13.1). Folate is needed in its tetrahydrofolate form (FH4) as a cofactor in DNA synthesis. Deficiency of B12 leads to impaired conversion of homocysteine to methionine causing folate to be ‘trapped’ in the methyl form. The resultant deficiency in methylene FH4 deprives the cell of the coenzyme necessary for DNA formation.
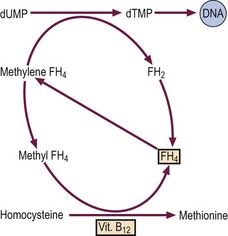
Fig 13.1 The cause of megaloblastic anaemia.
Both vitamin B12 and folate (FH4) are necessary for normal synthesis of DNA (see text).
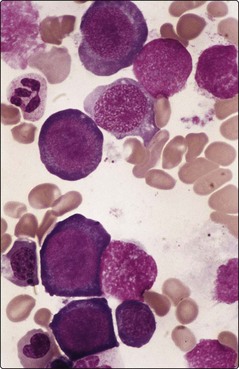
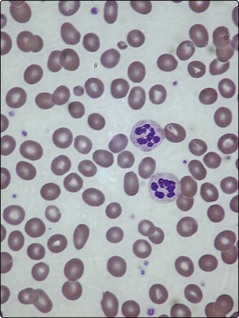
All dividing cells in the body suffer from the impaired DNA synthesis of B12 and folate deficiency. However, the actively proliferating cells of the bone marrow are particularly affected. As RNA synthesis progresses unhindered in the cytoplasm, the erythroid cells develop nuclear–cytoplasmic imbalance with abundant basophilic cytoplasm and enlarged nuclei. The chromatin pattern in the nucleus is characteristically abnormal; one author has described it as resembling ‘fine scroll work’, another as ‘sliced salami’ (Fig 13.2). The slowed synthesis of DNA leads to prolonged cell cycling and the cells being discharged into the blood without the normal quota of divisions. Red cells are enlarged and egg-shaped and the neutrophils hypersegmented due to retention of surplus nuclear material (Fig 13.3).
Clinical syndromes
Pernicious anaemia
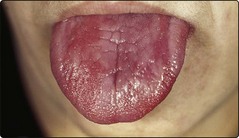
Patients usually have symptoms of anaemia and the generalised epithelial abnormality can manifest as glossitis (Fig 13.4) and angular stomatitis. The archetypal neurological complication – ‘subacute combined degeneration’ – arises from demyelination of the dorsal and lateral columns of the spinal cord. Patients most commonly complain of an unsteady gait, and if B12 deficiency is not corrected there can be progression to irreversible damage of the central nervous system with paralysis and dementia. There is a possible increased incidence of carcinoma of the stomach and colorectal cancer in pernicious anaemia.
1. Blood count and film. There is a macrocytic anaemia with the typical film appearance of megaloblastic anaemia. There may be leucopenia and thrombocytopenia.
2. Bone marrow aspirate. This is not always necessary. It will confirm megaloblastic anaemia but will not illuminate the underlying cause.
3. Estimation of vitamin B12 and folate levels. In pernicious anaemia the serum vitamin B12 level is normally very low but the assay is not entirely reliable and a trial of therapy may be justified where clinical and blood features strongly suggest deficiency. Serum methylmalonate and homocysteine levels are raised in B12 deficiency but their role in diagnosis is limited by their being often increased in normal people and a range of other disorders. Serum folate may be elevated and the red cell folate reduced (folate is trapped in its extracellular methyl FH4 form – see Fig 13.1).
4. Autoantibodies. Parietal cell antibodies are found more commonly in the serum than IF antibodies (90% vs 50%) but whereas IF antibodies are almost diagnostic of pernicious anaemia, parietal cell antibodies occur in about 15% of healthy elderly people.
5. Tests for vitamin B12 absorption. The urinary secretion (Schilling) test was formerly much used but radioactive cyanocobalamin is not available now and the test is obsolete.
Treatment: Vitamin B12 levels are usually replenished by intramuscular injection of the vitamin. Several injections of 1 mg hydroxycobalamin are given over the first few weeks and then either one injection every 3 months or daily oral vitamin B12 1–2 mg daily for life. The increase in reticulocytes in the blood peaks 6–7 days after the start of treatment.
Folate deficiency
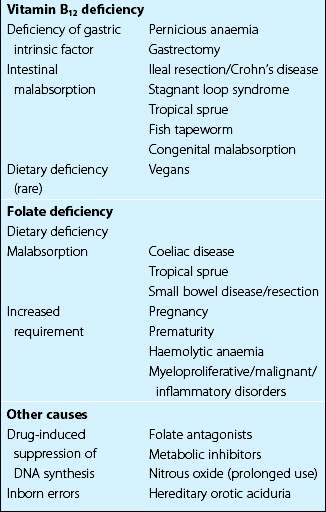
Folate deficiency is caused by dietary insufficiency, malabsorption, excessive utilisation or a combination of these (Table 13.2). Patients may complain of symptoms of anaemia or of an underlying disease. The increased risk of thrombosis is because of associated hyperhomocysteinaemia (see p. 79). There is a macrocytic anaemia and a megaloblastic bone marrow. In significant deficiency both serum and red cell folate are usually low but the latter is the better measure of tissue stores. In addition to a thorough dietary history patients may need investigations for malabsorption (e.g. jejunal biopsy).
Folate deficiency is treated with oral folic acid 5 mg once daily. This is given for several months at least, the precise duration of therapy depending on the underlying cause. Folate is prescribed prophylactically in pregnancy (400 µg daily) with a reduction in neural tube defects in the fetus and also in groups of patients at high risk of deficiency (Table 13.2). Before folate is prescribed, vitamin B12 deficiency must be excluded (or corrected) as subacute combined degeneration of the cord can be precipitated.