8 Medytoxin / Neuronox®
Summary and Key Features
• Neuronox® is a botulinum toxin type A (BoNT-A) product approved in 23 countries
• Neuronox® shows similar microbiological, physicochemical, and biochemical features to Botox®
• Neuronox® has proven its non-inferiority to Botox® in terms of efficacy and safety in various clinical studies in 1 : 1 dose ratio
• The recommended dosage of botulinum toxin type A for wrinkle treatment in Asians is usually lower than that in Caucasians
• The combination technique of multiple intradermal injection of botulinum toxin type A with conventional intramuscular injection is widely utilized in Asia for the purpose of facial rejuvenation
• The use of botulinum toxin type A to reduce the volume of muscles such as masseter, temporalis, calf, and deltoid is becoming more popular and promise to have increasingly broader applications
Introduction
Neuronox® (Medytox Inc., Ochang, South Korea) is a botulinum toxin type A (BoNT-A) product first approved in 2006 by the Korean Food and Drug Administration. Since then, it has been approved under different brand names as Botulift®, Siax®, Cunox®, and Meditoxin® in 23 countries world wide (Fig. 8.1). Among currently available BoNT-A products, Neuronox® seems to be one of the viable alternatives to Botox® in terms of microbiological, physicochemical, and biochemical features, and clinical interchangeability in 1 : 1 dose ratio.
The use of Neuronox® in Asians
Another concern is the optimal dose of BoNT-A for the treatment of wrinkles. Although more recently published recommendations by Carruthers et al in 2004 and 2007 modified the original techniques and decreased dosage of BoNT-A, the optimal initial doses for wrinkle reduction in Asians seems to be still lower than that of Caucasians (Table 8.1). From the above-mentioned data of clinical studies on the treatment of glabellar wrinkles using BoNT-A, we found differences in the dosages of BoNT-A between Asians and Caucasians. Intrinsic ethnic differences in the muscle mass as well as cultural factors, such as Asians being less facially expressive, could be the reason why lower doses are more appropriate.
Table 8.1 Recommended dose of BoNT-A for the reduction of dynamic wrinkles in Asians
| Wrinkles | Muscles | Initial dose (in units) |
|---|---|---|
| Forehead wrinkles | Frontalis | 3–10 |
| Glabellar frown lines | Corrugator and procerus | 10–14 |
| Crow’s feet | Orbicularis oculi | 6–8 |
| Nasal bunny lines | Nasalis | 6 |
| Perioral wrinkles | Orbicularis oris | 2–4 |
| Cobbled chin | Mentalis | 6–8 |
| Neck platysmal bands | Platysma | 20–30 |
Combining multiple intradermal injections with the conventional intramuscular injection seems more promising than using either method in isolation. Conventional intramuscular injections can be used for supplementation in areas where more targeted paralysis is needed (Fig. 8.2). This could tailor a more comprehensive approach to using BoNT for facial rejuvenation.
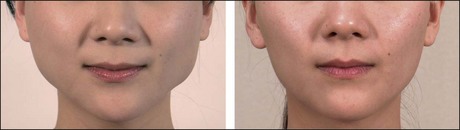
The third striking feature of BoNT-A treatment in Asians is its expanding role in facial and body contouring. Since Dr Smyth et al first reported the use of BoNT-A to treat masseteric hypertrophy with favorable results, BoNT-A has proven reduce muscle volume through disuse atrophy. This novel method has become a great success among Asians, whose faces tend to be wider or flatter compared with Caucasians and who aspire to having a more oval and slimmer face. Before BoNT-A became widely used for masseter reduction, mandibular angle resection surgery was the mainstay of treatment. Considering the downtime, risks, and costs, BoNT-A treatment for masseteric hypertrophy is far more attractive than surgery. In patients where muscles contribute more to their lower facial contours than bone or fat, BoNT-A treatment can change their appearance dramatically (Fig. 8.3). Indeed, it is highly suitable for Asians, considering that even normal Asians have masseteric muscles 0.8 cm thick per side.
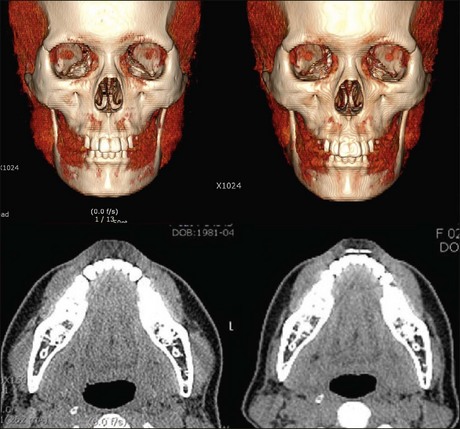
Usually 10–40 units per side are required, depending on masseteric volume (Table 8.2). In our clinical study using three-dimensional computed tomography, the overall masseter muscle volume had decreased by 27.5% on average at 12 weeks’ post-treatment with 20–40 units of Neuronox®. Volume reduction of the lower part of masseter, lying below an imaginary line connecting the anterior tragus and the corner of the mouth, attributed more to the contouring of the lower face, by up to 35.1% (Fig. 8.4) (publication in progress). Other published studies yielded comparable results. The duration of effect varied from 3 to more than 12 months depending on the individual’s dietary habits, bruxism, and so forth. Although this procedure is generally considered simple and safe, the possibility of adverse effects should be anticipated before proceeding with treatment and our suggestions considered.
Table 8.2 Recommended dosage of BoNT-A for the reduction of muscle volume.
| Muscle | Dose of botulinum toxin type A per side (in units) |
|---|---|
| Masseter | 10–40 |
| Temporalis | 20–40 |
| Gastrocnemius | 70–150 |
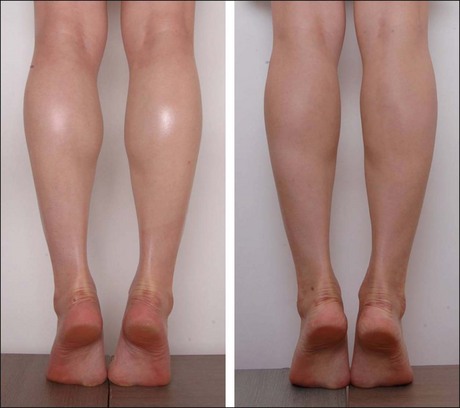
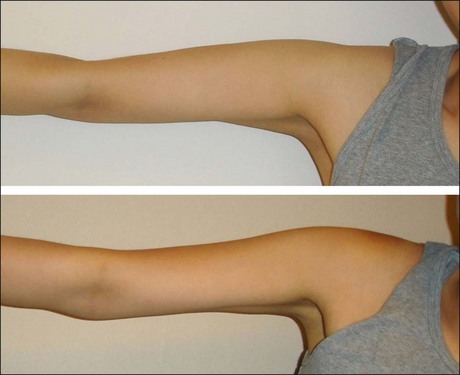
Disuse muscular atrophy induced by BoNT-A could be applied to other areas such as the temple, calves and upper arms (see Table 8.2). Hypertrophied temporalis muscle may result in a less attractive bulging appearance, and BoNT-A can create smooth and neat contours by volume reduction of temporalis muscle. Calf muscle reduction has rapidly become popular in Asia, as a close second to masseter reduction in terms of muscle volume reduction by BoNT-A. Patients are more keen on reducing the muscular bulge during walking or in tip-toe position than in the diameter itself. Reducing muscle bulge makes the lower legs look slimmer (Fig. 8.5). Shaping the upper arms by reducing deltoid muscle is another novel indication of BoNT-A utilizing disuse atrophy. With 50 Unit per side, it can deliver impressive results and patient satisfaction (Fig. 8.6).
Carruthers A, Carruthers J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatologic Surgery. 1998;24(11):1244–12447.
Carruthers A, Carruthers J. Botulinum toxin products overview. Skin Therapy Letter. 2008;13(6):1–4.
Carruthers J, Fagien S, Matarasso SL. Botox consensus group. Consensus recommendations on the use of botulinum toxin type A in facial aesthetics. Plastic and Reconstructive Surgery. 2004;114(6 suppl):1S–22S.
Carruthers J, Glogau RG, Blitzer A. Facial Aesthetic Consensus Group Faculty. Advances in facial rejuvenation: botulinum toxin type A, hyaluronic acid dermal fillers, and combination therapies – consensus recommendations. Plastic and Reconstructive Surgery. 2008;121(5 suppl):5S–30S.
Chang SP, Tsai HH, Chen WY, et al. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. International Journal of Dermatology. 2008;47:1287–1294.
Dessy LA, Mazzocchi M, Rubino C, et al. An objective assessment of botulinum toxin A effect on superficial skin texture. Annals of Plastic Surgery. 2007;58:469–473.
Kim K, Shin H-I, Kwon BS, et al. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: a randomized, double-blinded, controlled multicentre clinical trial. Developmental Medicine and Child Neurology. 2011;53(3):239–244.
Kim HJ, Yum KW, Lee SS, et al. Effects of botulinum toxin type A on bilateral masseteric hypertrophy evaluated with computed tomographic measurement. Dermatologic Surgery. 2003;29:484–489.
Lietzow MA, Gielow ET, Le D, et al. Subunit stoichiometry of the Clostridium botulinum type A neurotoxin complex determined using denaturing capillary electrophoresis. Protein Journal. 2008;27(7-8):420–425.
Rho NK, Kim HS, Kim YS, et al. Botulinum toxin type A for facial wrinkles and benign masseter hypertrophy in Korean patients. Korean Journal of Dermatology. 2010;48(10):823–831.
Seo KK. Clinical tips and recent advances in cosmetic uses of botulinum toxin including mesobotox. Journal of the Korean Medical Association. 2005;48:1225–1232.
Shim WH, Yoon SH, Park JH, et al. Effect of botulinum toxin type A injection on lower facial contouring evaluated using a three-dimensional laser scan. Dermatologic Surgery. 2010;36(suppl 4):2161–2166.
Smyth AG. Botulinum toxin treatment of bilateral masseteric hypertrophy. British Journal of Oral and Maxillofacial Surgery. 1994;32(1):29–33.
Stone AV, Ma J, Whitlock PW, et al. Effects of Botox and Neuronox on muscle force generation in mice. Journal of Orthopaedic Research. 2007;25(12):1658–1664.
Xu JA, Yuasa K, Yoshiura K, et al. Quantitative analysis of masticatory muscles using computed tomography. Dentomaxillofacial Radiology. 1994;23:154–158.
Yoon JS, Kim JC, Lee SY. Double-blind, randomized, comparative study of Meditoxin versus Botox in the treatment of essential blepharospasm. Korean Journal of Ophthalmology. 2009;23(3):137–141.
Zhang L, Lin W-J, Li S, et al. Complete DNA sequences of the botulinum neurotoxin complex of Clostridium botulinum type A-Hall (Allergan) strain. Gene. 2003;315:21–32.