Medical Management of Ocular Surface Disease
Topical Treatment
Method of Action
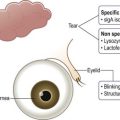
The tear layer represents a complex mixture of mucins for increased viscosity, antimicrobial proteins, growth factors, inflammatory suppressors, and electrolytes for proper osmolarity (Fig. 34.1). Artificial tears cannot completely substitute this complex composition of human tears. Their mechanism of action includes adding volume to the tear film while in contact with the ocular surface. In order to remain in contact with the ocular surface, hydrogels are an essential ingredient of artificial tears. Hydrogels are polymers that swell in water and retain moisture to increase viscosity. The mucous adhesive properties of hydrogels prolong the contact time of artificial tears on the eye. The following hydrogels have been used in artificial tears: hydroxypropyl methylcellulose (HPMC), carboxymethylcellulose (CMC), polyvinyl alcohol (PVA), carbopol, polyvinyl pyrrolidone, polyethylene glycol (PEG), polyvinyl alcohol (PVA), dextran, hyaluronic acid, glycerin, and carbomer 940 (polyacrylic acid). There have been no large scale, masked, comparative clinical trials to evaluate the wide variety of hydrogels available. However, using wavefront sensing and ocular coherence tomography, PEG drops showed significant worsening of visual quality, compared with CMC, PVA, and glycerin-containing artificial tears.1
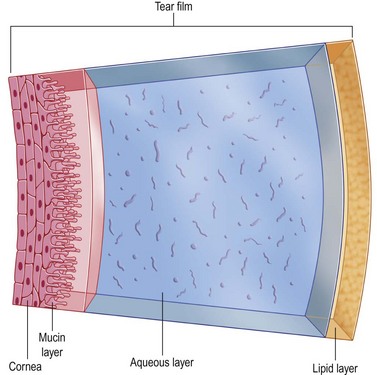
Figure 34.1 Composition of tears. (Reproduced from Allergan. ‘Tear film.’ Online image. Mydryeyes.com. 2012. 15 August 2012. http://www.mydryeyes.com/what_is_a_healthy_tear_film.cfm.)
There are many different types of preservatives in artificial tears. Benzalkonium chloride (BAK) and chlorobutanol, which can be toxic when used more than four times a day, are older preservatives. Preservatives, such as GenAqua (Sodium Perborate), Purite (sodium chlorite) and Polyquad (Polyquaternium-1) are less damaging to the ocular surface than BAK.2,3 Purite degrades to chloride ions and water after instillation. GenAqua is converted to water and oxygen on contact with the tear film.
The presence of ‘inactive ingredients’ provides unique surface protective properties to artificial tears. For example, compatible solutes may help surface healing by osmoprotection. HP-Guar forms a cross-linked viscoelastic gel when exposed to eye surface (pH 7.5). This gel increases viscosity and bioadhesive properties while promoting the retention of its two demulcents (polyethylene glycol 400 and polypropylene glycol). It has been suggested that HP-guar preferentially binds to the more dry or damaged areas of the surface epithelial cells, providing protection for these cells.4–6
Viscous tears have a longer retention time, as they are not easily drained out of the eye through the lacrimal outflow system. Oil-containing eye drops may be added if meibomian gland dysfunction is present. These eye drops will replenish the lipid layer of the tear film and prevent tear evaporation. Moderately hypotonic artificial tears containing PVA, as well as bicarbonate-containing tears have been shown to promote OSD healing in severe dry eyes.7–10
Topical CorticoSteroids
Delivery System
Corticosteroids are available in a diverse range of preparations, including solutions, suspensions, emulsions and ointments (Table 34.1). Topical steroids in a suspension, or viscous formulation have increased ocular contact time and can thereby double the corneal and aqueous steroid concentrations, compared with the same drug used as a solution.11 There is no prospective clinical trial comparing the relative efficacy of generic with branded topical steroids, though studies point to the possible advantage of smaller particle size in branded drops conferring greater efficacy and bioavailability.12 The relative potency of corticosteroids depends on the molecular structure, concentration, and release from the vehicle. For example, dexamethasone is a very potent steroid, but does not penetrate ocular tissues well. In contrast, prednisolone is less potent and has better ocular penetration. Steroids, such as loteprednol etabonate and fluorometholone are less potent, but have safer side effect profiles. Compared to dexamethasone and prednisolone, loteprednol etabonate and fluorometholone are reported to have lower rates of intraocular pressure (IOP) spikes. A retrospective review of 30 patients with IOP elevation to a mean of 31.1 millimeters of mercury after prednisolone acetate use for keratoplasty reported a 41% reduction in IOP after switching to loteprednol etabonate. Yet in one study, IOP elevations with loteprednol occurred more commonly in females after an average of 2 months of treatment.13
Table 34.1
Active Ingredients and Available Formulations for Topical Ophthalmic Steroid Preparations
| Topical Ocular Steroid | Concentration/Formulation |
| Prednisolone acetate | 0.125, 1.0% suspension |
| Difluprednate | 0.05% emulsion |
| Prednisolone sodium phosphate | 0.125, 0.5 1.0% solution |
| Dexamethasone alcohol | 0.05–0.1% susp, ointment |
| Fluorometholone acetate | 0.1% suspension |
| Fluorometholone alcohol | 0.1% suspension, ointment 0.25% suspension |
| Rimexolone | 0.5–1% suspension |
| Medrysone alcohol | 1.0% suspension |
| Lotoprednol etabonate | 0.2, 0.5% suspension |
Dosage
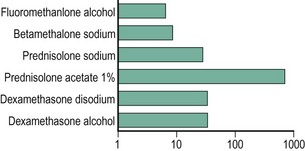
To our knowledge, no studies have conclusively outlined the optimal steroid concentrations for treating various OSD conditions. Studies have evaluated topical corticosteroid penetration into human aqueous humor (Fig. 34.2).14 For conditions primarily affecting the ocular surface where intraocular antiinflammatory therapy is not needed, fluorometholone alcohol 0.1%, loteprednol etabonate 0.5% and prednisolone sodium phosphate 0.5% are viable options. When intraocular penetration is indicated, the more potent prednisolone acetate, dexamethasone or difluprednate emulsion should be considered. In randomized, controlled studies, loteprednol etabonate has demonstrated excellent results in treating external inflammation, such as seasonal allergic conjunctivitis and giant papillary conjunctivitis with a favorable side effect profile.15,16 Topical corticosteroids with lower aqueous penetration are not as effective as those with higher intraocular penetration in the management of intraocular inflammation.14 Compared to prednisolone acetate 1%, loteprednol etabonate 0.5% has been found to be less effective in patients with acute anterior uveitis.17
Side Effects
Considered a corticosteroid primarily targeting ocular surface inflammation, fluorometholone alcohol 0.1% may adequately control signs and symptom of dry eyes in patients with keratoconjunctivitis sicca (KCS). In a randomized, controlled trial, fluorometholone alcohol 0.1% used q.i.d. for 30 days demonstrated a beneficial effect on both subjective and objective clinical parameters of moderate-to-severe KCS patients, reportedly without complications.18 A mild corticosteroid with low intraocular penetration, fluorometholone alcohol 0.1% may have a lower likelihood of increasing intraocular pressure, compared to prednisolone or dexamethasone.14 A short-term treatment option for patients with moderate keratoconjunctivitis sicca, topical loteprednol etabonate 0.5% used four times a day for 4 weeks may be beneficial in improving objective clinical parameters with an acceptable benefit–risk ratio. A randomized, masked clinical trial comparing loteprednol to vehicle reported greater improvement in objective variables, including corneal fluorescein staining and conjunctival injection, particularly in a subset of patients with more severe clinical findings of inflammation.19
Topical Cyclosporine
Cyclosporine A (CsA) is a fungal-derived peptide that prevents activation and nuclear translocation of transcription factors that are required for inflammatory cytokine production and T-cell activation. Its major clinical effect is disruption of expression of interleukin-2 by helper T cells, thereby preventing T-cell proliferation. Studies have demonstrated that cyclosporine 0.05% can be an effective treatment for management of herpetic stromal keratitis, graft-versus-host disease (GVHD), ocular rosacea, vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC), and post-LASIK dry eye.20
Dosage
In severe cases, higher concentration cyclosporine A 2% can provide symptomatic relief. A double-masked, placebo-controlled trial to evaluate the short-term efficacy and safety of topical 2% cyclosporine A in a preservative-free vehicle for patients with severe VKC demonstrated a statistically significant decrease in both signs and symptoms with no observed side effects over the course of the study.21 For the treatment of patients with steroid-dependent atopic keratoconjunctivitis (AKC), cyclosporine A 2% dissolved in maize oil and dosed four times daily to both eyes appears to be an effective means to improve signs and symptoms, allowing patients to be weaned off topical corticosteroids.22
Cyclosporine 0.05% may lead to improved tear film regularity and ocular surface health. In a randomized clinical study of moderate to severe dry eye patients, baseline tear production, as determined by Schirmer testing (with anesthesia), increased to a significantly greater degree in the cyclosporine 0.05% group than in the vehicle group. Also, compared to vehicle, patients treated with cyclosporine 0.05% demonstrated greater improvements in blurred vision that could be explained by the improvement in corneal fluorescein staining.23 Combination therapy with topical cyclosporine A and topical steroids has been successfully used in treating moderate to severe DES. While cyclosporine does not contribute to the rapid anti-inflammatory effect of corticosteroids, it has a better side effect profile and is safer for long-term use. It is possible to begin treatment of the ocular surface by prescribing both topical steroids and cyclosporine at the same time. The concurrent short-term use of a topical steroid tapered over 1–2 months provides the benefit of faster symptom relief and improvement of ocular signs without accompanying serious complications.24
Azithromycin
Azithromycin is a broad-spectrum macrolide antibiotic that contains nitrogen on its macrolide ring. It inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit of susceptible microorganisms. It has high tissue penetration and a prolonged half-life. Macrolide antibiotics, such as azithromycin exhibit antiinflammatory properties. Studies have demonstrated that they can inhibit the production of proinflammatory cytokines and the production of matrix metalloproteinases (MMPs).25
Although the specific antiinflammatory mechanism of action remains unknown, the suppression of the nuclear transcription factor nuclear factor kappa B (NF-κB) has been shown to play a role. Furthermore, the concentration of macrolide antibiotics, such as azithromycin within polymophonuclear leukocytes (PMNs) may also modulate their role in infection-mediated inflammation.26,27 With respect to ocular inflammatory diseases, an in vitro study found azithromycin in DuraSite as effective in suppressing MMPs in the corneal epithelium and endothelium as doxycycline (a tetracycline analog with known antiinflammatory properties, see below) in both human and bovine cells.28
Delivery System
Azithromycin is extremely lipophillic, making a stable aqueous preparation difficult. When coupled with a vehicle containing polycarbophil it can be formulated into a stable aqueous preparation. It binds to the mucin-coated surfaces of the eye (including the palpebral conjunctiva), resulting in the formation of a sustained-release gel that prolongs the release and availability of the drug on the ocular surface and enhances the penetration of the drug into the eyelids, conjunctiva, and cornea.29,30
Dosage
The recommended dose of azithromycin is one drop twice a day for the first 2 days and then one drop a day for the next 5 days. A single topical dose in healthy individuals demonstrated azithromycin achieved significant tissue concentrations and maintained those levels for up to 24 hours.31 Peak concentrations of azithromycin of more than 200 µg/g of tissue were achieved in human eyelid tissue as the drug accumulated over the 7 days of therapy. Furthermore, 5 days after discontinuing the medication, tissue concentrations of azithromycin of more than 50 µg/g were still present in the eyelids. Similar results were found for corneal and conjunctival samples.30
Topical Vitamin A
Vitamin A is essential for maintaining the health of epithelial cells throughout the body. Vitamin A deficiency adversely affects conjunctival and corneal epithelial cells, causing loss of goblet cells and leading to increased epidermal keratinization and squamous metaplasia of the mucous membranes.32 Vitamin A can exist in three forms: retinol, retinal, and retinoic acid. Many tissues requiring vitamin A store the vitamin as an ester of retinal. Vitamin A is stored as fatty acyl esters of retinol in the lacrimal gland. It is also present as retinol in the tears of rabbits and humans. Its presence in tears provides the rationale for treating OSD with vitamin A.32
Dosage
Topical vitamin A used four times daily has demonstrated improvement in blurred vision, Schirmer scores without anesthesia, and impression cytologic analysis in patients with dry eye.32 Topical therapy with retinoic acid ointment 0.05% can be an effective treatment of keratinization seen in cicatrizing diseases involving the cornea and conjunctiva. Vitamin A eye drops can be used as adjunct therapy with artificial lubricants for treatment of dry eyes.
Autologous Serum
Natural human tears contain essential components for maintaining the ocular surface, such as vitamin A, epidermal growth factor, fibronectin, and other cytokines. Because these components are also present in serum, the application of autologous serum is thought to offer advantages over artificial tear substitutes that lack these essential components. Compared to pharmaceutical lubricants, autologous serum eye drops more closely mirror the biochemical components of natural tears, containing vitamins, immunoglobulins, and growth factors, some in higher concentrations than natural tears. The pH of natural tears and serum is 7.4 and the osmolarity of tears and serum is approximately equivalent. Certain components are present in higher concentration in serum, compared with natural tears: vitamin A, TGF-β, lysozyme, and fibronectin (Table 34.2).34 It is possible that serum concentration of certain components may be harmful to the ocular surface, which explains the rationale for using dilute serum.
Table 34.2
Biochemical Comparison of Serum and Normal, Unstimulated Human Tears
| Serum | Unstimulated Tears | |
| pH | 7.4 | 7.4 |
| Osmolality (SD) | 296 | 298 (10) |
| EGF (ng/mL) | 0.5 | 0.2–3.0 |
| Vitamin A (mg/mL) | 46 | 0.02 |
| TGF-β | 6–33 | 2–10 |
| Surface IgA (µg/mL) (SD) | 2 | 1190 (904) |
| Lysozyme (mg/mL) (SD) | 6 | 1.4 (0.2) |
| Fibronectin (µg/mL) | 205 | 21 |
Delivery System
In previously reported studies, the serum concentration has ranged from 20% to 100%.35 In order to formulate autologous serum eye drops, blood is drawn from the patient and allowed to coagulate. Autologous serum is separated from other blood components through centrifugation. The separated serum is then re-suspended in balanced salt solution or artificial tears in either a 20% or 50% concentration and frozen in small aliquots. Prior to freezing, the containers should be covered to prevent exposure of the contents to light, as some of the serum components may degrade. The lack of a preservative in the autologous serum requires the necessary aliquot or small amount be defrosted and refrigerated between applications.
Dosage
Protocols reported for preparation and use of autologous serum eye drops exhibit considerable variation. Currently, no data have definitively defined the optimal concentration of autologous serum most appropriate for the treatment OSD. Studies have described the use of serum eye drops for treatment of persistent epithelial defects, severe dry eyes, KCS associated with Sjögren’s syndrome and graft-versus-host disease, superior limbal keratoconjunctivitis, and recurrent erosion syndrome.35–38 In patients with neurotrophic keratopathy, topical autologous serum drops used six to eight times per day, results in improved visual acuity, decreased corneal fluorescein staining, and increased corneal sensitivity that correlated with improvement in corneal nerve morphology as measured with confocal microscopy.39 Autologous serum has been shown to be superior to conventional treatment with artificial tears in a randomized, crossover study, including patients with severe KCS. After 3 months of 50% autologous serum, patients showed significant improvement in subjective symptoms and impression cytology analysis, corneal staining with rose bengal staining, Schirmer’s test without anesthesia, and fluorescein tear clearance test in comparison with those who instilled artificial tears.40 The use of 50% autologous serum eye drops appears to be an efficacious medical treatment modality for persistent corneal epithelial defects that are recalcitrant to conventional medical therapy.41
Side Effects
A potential disadvantage of serum eye drops is the limited stability. The bottles of autologous serum must be kept in the freezer or the refrigerator. Currently, no studies delineate the minimum freezer temperature required for adequate storage. After approximately 30 days, the bottle kept in the refrigerator must be discarded and replaced with a new bottle. Another disadvantage is the risk of blood-borne infection arising for patients and others handling serum. A donor could potentially transmit hepatitis to a recipient or to staff handling the serum. With prolonged use, an initially sterile dropper could become contaminated. Bacterial contamination poses a potential risk either during the production of the serum tears or during prolonged use of the eye drops. The drops are also not typically covered by insurance and must be made by a compounding pharmacy, so continued use can become expensive. Caution should be exercised in prescribing autologous serum eye drops as scleral vasculitis and melting has been reported in a patient with rheumatoid arthritis. Immune complex deposition in the peripheral cornea and subsequent inflammation could be encountered given that antibodies are found in serum. Other possible side effects include discomfort, worsening corneal epitheliopathy, microbial keratitis and conjunctivitis, and eyelid eczema.34
Tacrolimus
Tacrolimus is a macrolide immunosuppressive agent produced by the fermentation of Streptomyces tsukubaensis. The method of action of tacrolimus involves the blockage of transcription of cellular communications molecules, including interleukin 2, inhibiting the antigen presenting role of Langerhans cells, and reducing the activation of T cells.42,43)
Dosage
Tacrolimus can be compounded into a 0.02% ophthalmic suspension or ointment by a compounding pharmacy. Its use in the eye is considered off-label. There have been no prospective comparative trials of the efficacy of the different strengths. Tacrolimus dermatologic ointment has been reported to control ocular surface inflammation in all three strengths when applied 1–2 times a day in the inferior fornix, without any detectable blood levels. Tacrolimus has been demonstrated to treat intractable allergic conjunctivitis, atopic blepharoconjunctivitis, atopic keratitis, recalcitrant vernal keratoconjunctivitis, and giant papillary conjunctivitis.44–47 In a retrospective interventional case series, tacrolimus 0.02% placed twice daily in the inferior fornix was effective in severe ocular surface inflammatory diseases.48 Application of tacrolimus dermatological ointment may present an alternative treatment in advanced ocular surface inflammation without the potential side effects of long-term steroid use.
Systemic Therapies
Mechanism of Action
Derived from a South American shrub Pilocarpus jaborandi, pilocarpine is a muscarinic cholinergic parasympathomimetic agonist that binds to muscarinic M3 receptors. In humans, it can cause pharmacologic smooth muscle contraction and exocrine gland stimulation.49 Cevimeline hydrochloride is an orally administered derivative of acetylcholine that also binds to muscarinic M3 receptors in exocrine glands and stimulates exocrine gland secretion.
Delivery System
Pilocarpine (Salagen tablets, Novartis Pharmaceuticals, Basel, Switzerland) is currently available in 5 and 7.5 mg tablets for oral use. Cevimeline (Evoxac, Daiichi Pharmaceutical Corp, Montvale, NJ) 30 mg tablets are typically taken three times daily; maximum dosage is 90 mg/day.50
Dosage
Use of oral secretogogues is beneficial for addressing both the ocular and oral mucosal manifestations of Sjögren’s syndrome. Most patients require 20 mg/d of pilocarpine in divided doses for a therapeutic response. According to recommendations in a review article published in JAMA, in cases with residual salivary gland function, oral pilocarpine and cevimeline are the treatment of choice. Recommended doses balancing efficacy and adverse effects are the following: pilocarpine 5 mg q.i.d. and cevimeline 30 mg t.i.d.51 In a placebo-controlled clinical trial of Sjögren’s syndrome patients suffering from xerosis of the eyes and mouth, significant relief in reported ocular symptoms, including decreased use of artificial tears was noted at a dose of 30 mg/day after 12 weeks of therapy.49 Response to pilocarpine may require 6–12 weeks of therapy.
Oral Cyclines
Delivery Systems
The tetracyclines are available in numerous oral compounds, including tetracycline, the semisynthetic doxycycline hyclate, doxycycline monohydrate and minocycline.52 Tetracycline must be taken on an empty stomach half an hour before, or 2 hours after meals. This is because food prevents absorption of tetracycline into the bloodstream. Minocycline and doxycycline absorption are not affected by food and can be taken at mealtime. All of these tetracyclines must be taken with a full glass of water to prevent esophageal ulceration.
Side Effects
In addition to gastrointestinal (GI) concerns, all of the tetracyclines may stain developing teeth in children, induce vaginal or oral candidiasis, and cause dose-related dizziness and photosensitivity. Tetracycline may make the skin more sensitive to sunlight (photosensitivity); this effect depends on the variety of tetracycline and the amount taken. Photosensitization is most likely with doxycycline and least likely with minocycline. Additionally, minocycline may be associated with the development of autoantibodies, including antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA) and antiphospholipid antibodies with or without associated clinical symptoms. Minocycline may result in dose-related dizziness. It has been reported to cause pseudotumor cerebri and to precipitate arthritis and systemic lupus erythematosus, especially in young women.53,54
Oral Vitamin A
Vitamin A is essential for the differentiation of mucus-secreting epithelium of the eyes, skin, gastrointestinal tract, and genitourinary tract. Loss of mucin and goblet cells in the conjunctiva leads to squamous metaplasia with keratinization of the conjunctiva and cornea. Severe forms of hypovitaminosis A, such as xerosis, corneal ulceration, and keratomalacia, can be seen in countries with a high prevalence of malnutrition.55 In the developed world, hypovitaminosis A can present in the wake of malnutrition from a variety of causes, such as psychiatric conditions, chronic gastrointestinal and liver disease and bariatric surgery through the disruption of fat-soluble vitamin absorption.56 It is important to have a high clinical suspicion of hypovitaminosis A, as it can initially be misdiagnosed or overlooked, in part, due to clinical signs being easily represented as simple epithelial erosions seen in keratoconjunctivitis sicca or exposure keratopathy. Without appropriate vitamin A supplementation, ocular surface conditions can be refractory to medical or surgical intervention and lead to progressive blinding disease. With the increasing popularity of bariatric surgery, the risk of vitamin A deficiency is increasing in the developed world. As a result, the ophthalmologist must be mindful of this possible etiology in the differential diagnosis of ocular surface disease.
Dosage
Recommended daily intake and various dosing regimens are highlighted in Table 34.3. The dose of vitamin A is variable, depending on the level of deficiency. Vitamin A levels can be determined by blood test.
Table 34.3
Adult Dosing of Oral Vitamin A Supplementation
| Dietary Reference Intake | Dosing |
| Male | 3 000 units p.o. daily; max: 10 000 units/day |
| Female | 2 330 units p.o. daily; max: 10 000 units/day |
| Vitamin A deficiency | 100 000 units p.o. daily × 3 days, then 50 000 units p.o. daily × 14 days |
| Severe vitamin A deficiency with xerophthalmia | 500 000 units p.o. daily × 3 days, then 50 000 units p.o. daily × 14 days, then 10 000- 20 000 units p.o. daily × 2 mon |
| Prophylaxis, malabsorption syndromes | 10 000–50 000 units p.o. daily |
| Retinitis pigmentosa | 15 0000 units p.o.daily |
| Ichthyosis vulgaris | 50 000–500 000 units p.o. daily |
| Keratosis follicularis | 50 000–500 000 units p.o. daily |
Nutritional Supplements
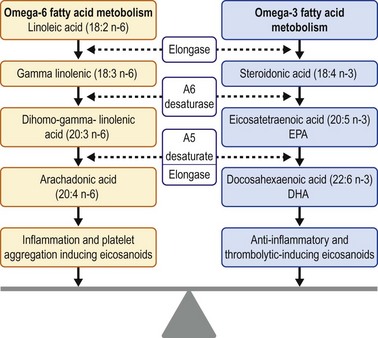
Dietary modifications and nutritional supplements can promote ocular surface health. An essential part of normal growth and development, essential fatty acids (EFA) cannot be synthesized and must therefore be acquired through dietary intake. Precursors of eicosanoids, omega-3 and omega-6 EFAs are hormones involved in the inflammatory cascade. Four groups of eicosanoids have been identified: leukotrienes, thromboxanes, prostacyclins, and prostaglandins. Omega-3 molecules act as antiinflammatory and anticoagulation mediators. On the converse, omega-6 molecules are proinflammatory and proplatelet aggregator mediators. The overall inflammatory state in the body is influenced by the ratio of omega-6 to omega-3 fatty acids. Omega-3 and omega-6 fatty acids compete for the same enzyme in two distinct pathways that result in antiinflammatory and proinflammatory derivatives, respectively (Fig. 34.3).57
Delivery System
Essential fatty acids can be obtained via dietary supplementation. Omega-3 essential fatty acids include docosahexaenoic acid (DHA), alpha linoleic acid (ALA), and eicosapentaenoic acid (EPA). Omega-3 fatty acids can be acquired by consuming the following oily, cold-water fish: salmon, sardines, tuna, mackerel, and herring.58 One serving of salmon or mackerel could provide 1.5–3.5 g of omega-3 fatty acids. Omega-3 fatty acids are also available in vegetarian dietary choices. A number of different seeds and their oils and some nuts contain varying amounts of ALA. Flaxseeds (linseeds) and their oil typically contain ≈45–55% of fatty acids as ALA.58 Omega-6 EFAs include linoleic acid (LA), gamma linoleic acid (GLA), dihomogamma-linoleic acid (DGLA), and arachidonic acid. Sources of omega-6 fatty acids include soybean oil, palm oil, grapeseed oil, sunflower oil, poultry, nuts, and cereals.59 The omega-6 fatty acid linoleic acid (distinct from alpha linoleic acid) is the main polyunsaturated fatty acid in most Western diets and is typically consumed in 5- to 20-fold greater amounts than the omega-3 fatty acid, alpha linoleic acid.60 Omega-3 derived from fish oil has a distinct fishy odor. Removal of the ester will remove the fish odor from the oil. Re-esterification of the omega-3 molecule may increase both the efficacy, as well as the serum concentration of omega-3.
Dosage
In a prospective randomized controlled trial, daily doses of 6 g of flaxseed oil (equivalent to 3.3 g/day of omega-3 fatty acids) resulted in a decrease in the RBC and plasma ratios of omega-6 to omega-3 fatty acids, compared to controls. Additionally, improvements in overall ocular surface disease index (OSDI) score, tear break-up time (TBUT), and meibum score were noted. This prospective clinical study found that dietary supplementation with omega-3 fatty acids induced a change in the fatty acid saturation content in meibum.57,59
In a small study, patients receiving lower doses of omega-3 fatty acids (450 mg of eicosapentaenoic acid, 300 mg of docosahexaenoic acid and 1000 mg of flaxseed oil) had an increase in aqueous tear secretion. No significant effect in meibum lipid composition or aqueous tear evaporation rate was reported, perhaps, due to the choice of active ingredients in the omega-3 dose, the trial length, and the dosing schedule in a population with chronic dry eye syndrome.60 Review of dietary diaries of > 32 000 patients enrolled in the Women’s Health Study revealed that a higher dietary intake of omega-3 fatty acids is associated with a decreased incidence of dry eye syndrome in women. Also, low dietary intake of omega-3, a high omega-6 to omega-3 ratio, or both increase the risk of clinically diagnosed dry eye.61 In clinical studies, supplementation with the combination of LA and GLA or with evening primrose oil (EPO), which contains both of these fatty acids, resulted in improvement of keratoconjunctivitis sicca syndrome.62
Medical Management of Ocular Surface Disease
At the same time, it is imperative to assess the eyelid margins for blepharitis and meibomitis, both of which can result in exacerbation of ocular surface irritation, as well as the evaporative dry eye. Meibomitis can be difficult to diagnose without compressing the meibomian glands to evaluate their contents. Continual hygiene of the eyelash base and the meibomian gland orifice is the mainstay of treatment of these lid margin conditions. Eyelash shampoo in the shower, as opposed to warm compresses and lid scrubs, is recommended. In describing this technique to patients it is preferable to use the term eyelash shampoo, as it is not the lids that we want them to clean, but rather the eyelashes. Patients are instructed to shampoo the base of their lashes with a non-irritating dilute detergent (baby shampoo) on a washcloth at the end of their daily shower. The warm moist environment of the shower will provide the same effect as a warm compress, while the ease of the process increases patient compliance with this chronic therapy. In some patients a topical ointment (loteprednol) can be applied to the lashes at bedtime to soften the collarettes and decrease inflammation of the meibomian gland orifices. The oily effect of the ointment on the eyelid increases compliance with eyelash shampooing in the morning. In severe cases of blepharomeibomitis or recurrent chalazia, oral doxycycline 100 mg twice a day may be of use with a taper to 20 mg per day for long-term use. However, even in these low antiinflammatory doses the potential for systemic side effects remains, including photosensitivity. Topical azithromycin has been shown to have a role in the treatment of blepharitis, as well as the associated dry eye symptoms.63–65
While an increase in tear volume may temporarily decrease irritation symptoms, it does not decrease the inflammation of the ocular surface. A topical steroid will have the quickest effect in reducing the ocular surface inflammation. However, due to the potential for side effects with long-term use of topical steroids, cyclosporine or other nonsteroidal antiinflammatory therapies should be considered. Coupling topical steroids with topical cyclosporine provides the beneficial effect of decreasing burning and stinging that may be seen in up to 18% of patients using cyclosporine alone.66 Long-term use of topical cyclosporine will both reduce ocular inflammation and increase tear production. In blepharitis patients, chronic topical cyclosporine has been shown to improve patient symptoms.20 In patients with keratinization of the eyelid margin, topical vitamin A ointment may be substituted for a topical steroid ointment and applied to the eyelid margin at bedtime.
In the patient with moderate to severe OSD and decreased tear production, topical corticosteroids along with doxycycline suppress metalloproteinases and cytokine activation.67 The use of a topical steroid suspension (prednisolone acetate) may be contraindicated and a topical solution (prednisolone phosphate) preferred. The steroid suspension may result in further irritation of the ocular surface if the particulates in the suspension precipitate out and abrade the ocular surface. In addition, the particulates in the suspension may combine with the mucus in the tear film of the aqueous deficient patient and result in filament formation. In these cases, the use of a topical steroid solution, such as prednisolone sodium phosphate is recommended. In recalcitrant OSD or patients who are steroid intolerant, topical tacrolimus ointment may be indicated for control of surface inflammation. In severe cases, autologous serum tears may also be useful, though long-term use is somewhat impractical.68 However, in non-healing epithelial defects, autologous serum may be required to aid in healing of the epithelial defect and reduce the ocular surface inflammation. Future development of epithelial growth factors for clinical use may also play a role in refining the management of OSD.
References
1. Tung, CI, Kottaiyan, R, Koh, S, et al. Noninvasive, objective, multimodal tear dynamics evaluation of 5 over-the-counter tear drops in a randomized controlled trial. Cornea. 2012;31:108–114.
2. Debbasch, C, De La Salle, SB, Brignole, F, et al. Cytoprotective effects of hyaluronic acid and Carbomer 934P in ocular surface epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3409–3415.
3. Doughty, MJ. Acute effects of chlorobutanol- or benzalkonium chloride- containing artificial tears on the surface of rabbit corneal epithelial cells. Optom Vis Sci. 1994;71:562–572.
4. Ubels, JL, Clousing, DP, Van Haitsma, TA, et al. Pre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agent. Curr Eye Res. 2004;28:437–444.
5. Hartstein, I, Khwarg, S, Przydryga, J. An open-label evaluations of HP-Guar gellable lubricant eye drops for the improvement of dry eye signs and symptoms in a moderate dry eye adult population. Curr Med Res Opin. 2005;21:255–260.
6. Foulks, GN. Clinical evaluation of the efficacy of PEG/PG lubricant eye drops with gelling agent (HP-Guar) for the relief of the signs and symptoms of dry eye disease: a review. Drugs Today. 2007;43:887–896.
7. Benitez-del-Castillo, JM, Aranguez, C, Garcia-Sanchez, J. Corneal epithelial permeability and dry eye treatment. Adv Exp Med Biol. 2002;506:703–706.
8. Dogru, M, Tsubota, K. Pharmacotherapy of dry eye. Expert Opin Pharmacother. 2011;12:325–334.
9. Albietz, JM, Lenton, LM, McLennan, SG, et al. A comparison of the effect of refresh plus and bion tears on dry eye symptoms and ocular surface health in myopic LASIK patients. CLAO J. 2002;28:96–110.
10. Ridder, WH, 3rd., Lamotte, JO, Ngo, L, et al. Short-term effects of artificial tears on visual performance in normal subjects. Optom Vis Sci. 2005;82:370–377.
11. McGhee, CN. Pharmacokinetics of ophthalmic corticosteroids. Br J Ophthalmol. 1992;76:681–684.
12. Roberts, CW, Nelson, PL. Comparative analysis of prednisolone acetate suspensions. J Ocul Pharmacol Ther. 2007;23:182–187.
13. Rajpal, RK, Digby, D, D’Aversa, G, et al. Intraocular pressure elevations with lotoprednol etaborate; a retrospective chart review. J Ocul Pharmacol Ther. 2011;27:305–308.
14. Awan, MA, Agarwal, PK, Watson, DG, et al. Penetration of topical and subconjunctival corticosteroids into human aqueous humour and its therapeutic significance. Br J Ophthalmol. 2009;93:708–713.
15. Dell, SJ, Shulman, DG, Lowry, GM, et al. A controlled evaluation of the efficacy and safety of loteprednol etabonate in the prophylactic treatment of seasonal allergic conjunctivitis. Loteprednol Allergic Conjunctivitis Study Group. Am J Ophthalmol. 1997;123:791–797.
16. Friedlaender, MH, Howes, J. A double-masked, placebo-controlled evaluation of the efficacy and safety of loteprednol etabonate in the treatment of giant papillary conjunctivitis. The Loteprednol Etabonate Giant Papillary Conjunctivitis Study Group I. Am J Ophthalmol. 1997;123:455–464.
17. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Loteprednol Etabonate US Uveitis Study Group. Am J Ophthalmol. 1999;127:537–544.
18. Avunduk, AM, Avunduk, MC, Varnell, ED, et al. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003;136:593–602.
19. Pflugfelder, SC, Maskin, SL, Anderson, B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444–457.
20. Donnenfeld, E, Pflugfelder, SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009;54:321–338.
21. Kilic, A, Gurler, B. Topical 2% cyclosporine A in preservative-free artificial tears for the treatment of vernal keratoconjunctivitis. Can J Ophthalmol. 2006;41:693–698.
22. Hingorani, M, Moodaley, L, Calder, VL, et al. A randomized, placebo-controlled trial of topical cyclosporine A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology. 1998;105:1715–1720.
23. Sall, K, Stevenson, OD, Mundorf, TK, et al. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639.
24. Byun, YJ, Kim, TI, Kwon, SM, et al. Efficacy of combined 0.05% cyclosporine and 1% methylprednisolone treatment for chronic dry eye. Cornea. 2012;31:509–513.
25. Ianaro, A, Ialenti, A, Maffia, P, et al. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exper Ther. 2000;292:156–163.
26. Amsden, GW. Anti-inflammatory effects of macrolides – an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? Rev J Antimicrob Chemother. 2005;55:10–21.
27. Shinkai, M, Rubin, BK. Macrolides and airway inflammation in children. Paediatr Respir Re. 2005;6:227–235.
28. Luchs, J. Azithromycin in Durasite for the treatment of blepharitis. Clin Ophthalmol. 2010;30:681–688.
29. Akpek, EK, Vittitow, J, Verhoeven, RS, et al. Ocular surface distribution and pharmacokinetics of a novel ophthalmic 1% azithromycin formulation. J Ocul Pharmacol Ther. 2009;25:433–439.
30. Friedlander, MH, Protzko, E. Clinical development of 1% azithromycin in DuraSite, a topical azalide anti-infective for ocular surface therapy. Clin Ophthalmol. 2007;1:3–10.
31. Torkildsen, G, O’Brien, TP. Conjunctival tissue pharmacokinetic properties of topical azithromycin 1% and moxifloxacin 0.5% ophthalmic solutions: a single-dose, randomized, open-label, active-controlled trial in healthy adult volunteers. Clin Ther. 2008;30:2005–2014.
32. Kim, EC, Choi, JS, Joo, CK. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. 2009;147:206–213. [e3].
33. Herbort, CP, Zografos, L, Zwingli, M, et al. Topical retinoic acid in dysplastic and metaplastic keratinization of corneoconjunctival epithelium. Graefes Arch Clin Exp Ophthalmol. 1988;226:22–26.
34. Geerling, G, Maclennan, S, Hartwig, D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004;88:1467–1474.
35. Tsubota, K, Goto, E, Fujita, H, et al. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br J Ophthalmol. 1999;83:390–395.
36. Goto, E, Shimmura, S, Shimazaki, J, et al. Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea. 2001;20:807–810.
37. Ogawa, Y, Okamoto, S, Mori, T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2003;31:579–583.
38. Matsumoto, Y, Dogru, M, Goto, E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111:1115–1120.
39. Rao, K, Leveque, C, Pflugfelder, SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94:584–591.
40. Noble, BA, Loh, RS, MacLennan, S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. Br J Ophthalmol. 2004;88:647–652.
41. Jeng, BH, Dupps, WJ, Jr. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28:1104–1108.
42. Koo, JY, Fleisher, AB, Abramovits, W, et al. Tacrolimus ointment is safe and effective in the treatment of atopic dermatitis: results in 8000 patients. J Am Acad Dermatol. 2005;53:S195–S205.
43. Hooks, M. Tacrolimus, a new immunosuppressant: a review of the literature. Ann Pharmacother. 1994;28:501–511.
44. Attax-Fox, L, Barkana, Y, Iskhakov, V, et al. Topical tacrolimus 0.03%ointment for intractable allergic conjunctivitis: an open label pilot study. Curr Eye Res. 2008;33:545–549.
45. Joseph, MS, Kaufman, HE, Insler, M. Topical tacrolimus ointment for treatment of refractory anterior segment inflammatory disorders. Cornea. 2005;24:417–420.
46. Kymionis, GD, Goldman, D, Ide, T, et al. Tacrolimus 0.03% ointment in the eye for the treatment of giant papillary conjunctivitis. Cornea. 2008;27:228–229.
47. Garcia, DP, Alperte, JI, Cristobal, JA, et al. Topical tacrolimus ointment for treatment of intractable atopic keratoconjunctivitis: A case report and review of the literature. Cornea. 2011;30:462–464.
48. Miyazaki, D, Tominaga, T, Kakimaru-Hasegawa, A, et al. Therapeutic effects of tacrolimus ointment for refractory ocular surface inflammatory diseases. Ophthalmology. 2008;115:988–992.
49. Papas, AS, Sherrer, YS, Charney, M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjogren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169–177.
50. Akpek, EK, Lindsley, KB, Adyanthaya, RS, et al. Treatment of Sjogren’s syndrome-associated dry eye an evidence-based review. Ophthalmology. 2011;118:1242–1252.
51. Ramos-Casals, M, Tzioufas, AG, Stone, JH, et al. Treatment of primary Sjogren syndrome: a systematic review. JAMA. 2010;304:452–460. [28].
52. Maibach, H. Second generation tetracyclines, a dermatologic overview: clinical uses and pharmacology. Cutis. 1991;48:411–417.
53. Sloan, B, Scheinfeld, N. The use and safety of doxycycline hyclate and other second generation tetracyclines. Expert Opin Drug Saf. 2008;7:571–577.
54. Kircik, LH. Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol. 2010;11:1407–1411.
55. Sommer, A. Vitamin a deficiency and clinical disease: an historical overview. J Nutr. 2008;138:1835–1839.
56. Lin, P, Fintelmann, RE, Khalifa, YM, et al. Ocular surface disease secondary to vitamin A deficiency in the developed world: it still exists. Arch Ophthalmol. 2011;129:798–799.
57. Macsai, MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:336–356.
58. Calder, PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S.
59. Rand, AL, Asbell, PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22:279–282.
60. Wojtowicz, JC, Butovich, I, Uchiyama, E, et al. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30:308–314.
61. Miljanovic, B, Trivedi, KA, Dana, MR, et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893.
62. Barabino, S, Rolando, M, Camicione, P, et al. Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003;22:97–101.
63. Veldman, P, Colby, K. Current evidence for topical azithromycin 1% ophthalmic solution in the treatment of blepharitis and blepharitis-associated ocular dyness. Int Ophthalmol Clin. 2011;51:43–52.
64. Optiz, DL, Tyler, KF. Efficacy of azithromycin 1% ophthalmic solution for the treatments of ocular surface disease from posterior blepharitis. Clin Exp Optom. 2011;94:200–206.
65. Haque, RM, Torkildsen, GL, Brubaker, K, et al. Multicenter open-label study evaluating the efficacy of azithromycin ophthalmic solution 1% on the signs and symptoms of subjects with blepharitis. Cornea. 2010;29:871–877.
66. Sheppard, JD, Scoper, SV, Samudre, S. Topical lotoprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. J Ocul Pharmacol Ther. 2011;27:23–27.
67. De Paiva, CS, Corrales, RM, Villarreal, AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activationin the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535.
68. Alio, JL, Abad, M, Artola, A, et al. Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology. 2007;114:1286–1293.