Chapter 3 Medical Considerations in Spinal Cord Stimulation
 When considering SCS in a patient on anticoagulation, the implanting physician should have a thorough understanding of the most recent American Society of Regional Anesthesia and Pain Medicine (ASRA) consensus guidelines for patients receiving anticoagulation, while also recognizing that there are no SCS specific guidelines.
When considering SCS in a patient on anticoagulation, the implanting physician should have a thorough understanding of the most recent American Society of Regional Anesthesia and Pain Medicine (ASRA) consensus guidelines for patients receiving anticoagulation, while also recognizing that there are no SCS specific guidelines. The concern for interaction between SCS and a pacemaker is inability to pace, whereas in defibrillators it is inappropriate shock. To minimize this interaction, the SCS should be set to a bipolar configuration, whereas the cardiac device should be set to bipolar sensing. Coordination should be undertaken with the patient’s cardiologist.
The concern for interaction between SCS and a pacemaker is inability to pace, whereas in defibrillators it is inappropriate shock. To minimize this interaction, the SCS should be set to a bipolar configuration, whereas the cardiac device should be set to bipolar sensing. Coordination should be undertaken with the patient’s cardiologist.Background
Electrical stimulation for the treatment of pain has been used for over 4500 years.1 In 1967 neurosurgeon Dr. C. Norman Shealy and colleagues from Case Western Reserve University were the first to implement spinal cord stimulation (SCS) in the treatment of chronic pain at University Hospitals of Cleveland.2 Shealy proved the clinical feasibility of SCS, and subsequently there has been tremendous growth in its application. Currently SCS is approved by the Food and Drug Administration (FDA) for chronic pain of the trunk and limbs, pain from failed back surgery syndrome (FBSS), and intractable low back pain. “Off label,” SCS has been used for neuropathic painful conditions and vascular and visceral pain, with diverse applications ranging from vulvodynia to cervicalgia. The full range of considerations for SCS is beyond the scope of this chapter.
Specific Considerations
Surgical Site Infection
Surgical site infection (SSI), in general, has an overall prevalence of 2% to 7%3; and, consistent with this, a rate of 3% to 8% has been found with SCS implantation.4–6 In expert panel recommendations, Kumar and colleagues state that the “use of antibiotics is recommended by the panel and others and should be started intravenously, 1.5 hours prior to surgery.”4
By comparison, infection rates for implanted cardiac devices (ICDs—pacemakers and defibrillators) were reported as 0.5% to 6% in early studies7,8 but have more recently been found to be as low as 1%.9 Although there have been no studies in SCS comparing infection rates in those with and without preoperative antibiotics, a prospective, randomized, double-blind, placebo-controlled trial evaluated infection risk for ICDs in those receiving either prophylactic cefazolin or a placebo.10 This trial was interrupted early by the safety committee because of the dramatically higher rate of infection in those who did not receive antibiotics vs. those who did (3.28% vs. 0.63%). The authors also found that the presence of postoperative hematoma and procedure duration were positively correlated with infection risk. A recent American Heart Association (AHA) scientific statement also identified ICD infection risk factors to include diabetes mellitus (DM), congestive heart failure (CHF), renal dysfunction, oral anticoagulation, revision surgery, hematoma formation, corticosteroid use, and surgeon inexperience.11 This statement also notes that “there is currently no scientific basis for the use of prophylactic antibiotics before routine invasive dental, gastrointestinal, or genitourinary procedures.” Although these findings are in the setting of ICDs, the similarity between minimally invasive surgeries such as these and SCS may provide guidance. There are no similar studies in the SCS population and until such a time this literature may be used as a prudent reference.
Gaynes and colleagues12 have also found that American Society of Anesthesiologists’ (ASA) classification, the National Nosocomial Infection Surveillance (NNIS) wound classification, and prolonged operative time—defined as ≥75th percentile compared to average duration of the operation—are associated with SSI. In a retrospective review of >10,000 patients over 6 years, Haridas and Malangoni13 identified several other significant risk factors for SSI: hypoalbuminemia (≤3.4 mg/dL), anemia (Hgb ≤10 g/dL), excessive alcohol use (not defined), history of chronic obstructive pulmonary disease, history of CHF, infection at remote site, and current operation through a previous incision (Box 3-1). Most of these risk factors can be identified through an appropriate history and physical examination and preoperative laboratory work and can be addressed in conjunction with the patient’s primary care physician or appropriate specialist. However, operation through a previous incision site may be of greater concern. One of the most common indications for SCS use is FBSS. Many times old scars are used as an entry points for the new procedure, either because they provide adequate anatomic access or to prevent further cosmetic disfiguration. Haridas and Malangoni13 suggest that using a previous incision may predispose to SSI because of the decreased vascularity of scar tissue.
The pathogen most commonly involved in SSI is Staphylococcus aureus, which is responsible for more than 50% of infections,14–16 with most cases occurring in patients who are themselves carriers of the organism. The carriage site is most often the anterior nares,17 and multiple studies have shown that nasal carriage is one of the most important risk factors for the development of surgical site infection.14,18,19 Given this, there is a new body of research specifically focused on identifying and treating nasal carriers of Staphylococcus aureus, with resultant dramatic decreases in SSI. Studies in cardiothoracic,14 orthopedic,20–22 and dialysis23,24 populations have shown that treatment is feasible and cost-effective, decreases infection rates by 57% to 93%, and reduces morbidity and mortality. A recent, randomized, double-blind, placebo-controlled, multicenter trial showed that treatment with mupirocin nasal ointment and chlorhexidine soap reduced the infection rate to 3.4%, compared to 7.7% in the placebo control group.25
Although different treatment protocols have been used, there is accumulating evidence for a combination of intranasal mupirocin and chlorhexidine showers preoperatively, and vancomycin intra-operatively. When patients are seen in presurgical screening (or during a routine office visit for potential SCS patients), a polyester (Dacron) nasal swab of the nasal passage may be taken. Polymerase chain reaction (PCR)-based rapid testing is used to identify methicillin-resistant Staphylococcus aureus, and standard cultures are used to identify methicillin-sensitive Staphylococcus aureus. If patients test positive for either strain, they are treated with 2% intranasal mupirocin (Bactroban) twice daily for a five-day treatment course prior to implant date and continued for two days post-implant. Additionally, a shower wash of 2% chlorhexidine (Hibiclens) is taken the evening prior to surgery.20 A combination of vancomycin and cefazolin dosed for weight can be used intra-operatively, as β-lactam antibiotics may provide better coverage for methicillin-sensitive Staphylococcus aureus strains.26,27 SSI can be particularly devastating and difficult to treat in patients with implanted hardware, and, although the ideal regimen has yet to be determined, these developments allow another opportunity to minimize patient morbidity.
Tobacco Use/Smoking Cessation
It is now clear that smoking is an important and significant factor in perioperative complications.28–31 Although there are no SCS studies that have looked at the increased risk of SSI in smokers, there is an abundance of evidence in the general surgery literature from which to draw conclusions. Smokers have up to eight times the risk of wound infection (≈8% vs. 1%) after surgery.32 Although the exact etiologic mechanism is unclear, carbon monoxide and the hypoxemic state it creates are likely important factors. The role of nicotine itself is unclear. It is a known vasoconstrictor that impairs tissue revascularization.33 However, nicotine replacement therapy (NRT) does not increase infection rates in experimental or clinical studies and there is no evidence that it adversely affects wound healing.34–36
The risks of smoking have been unequivocally shown, and evidence continues to accumulate that smoking cessation can drastically reduce perioperative morbidity. In one study preoperative smoking cessation before joint replacement surgery reduced wound infection rates from 27% in smokers to 0% in those who quit.37 At this time there is no consensus on the duration of smoking cessation for maximum benefit before surgery. Increased length of abstinence is certainly beneficial for a patient’s overall health, and the ideal situation would be for this to continue permanently after surgery. However, given the difficulty most patients experience with quitting smoking, the search continues for the shortest amount of time that will still yield clinical benefit operatively. Initial studies showed clear benefit from smoking cessation for 6 to 8 weeks before surgery, consistent with physiological improvements in pulmonary and cardiac function.32,38,39 Moller and associates37 found a 65% decrease in postoperative complications with 6 to 8 weeks of preoperative smoking cessation before orthopedic surgeries. Even 4 weeks of smoking cessation reduced wound infection rates to that of nonsmokers in those having skin biopsies.34 The 3-week mark may be the cutoff point to see benefit from smoking cessation. One study found that the complication rate for colorectal surgery was unchanged with smoking cessation ≤3 weeks,40 whereas two separate studies found a reduction in complications in head and neck and breast reduction surgery with cessation ≥3 weeks.41,42
With the clear and proven increased risks from continued smoking, discussing smoking cessation with patients considering SCS may be an important part of preoperative education and teaching. Perioperative intervention can directly and dramatically decrease complication rates and can lead to sustained smoking cessation for up to 1 year after surgery.43,44 Peters and colleagues45 gave important perspective to the need for smoking cessation: “the adverse effect of failing to quit smoking is similar to that of omitting antibiotic prophylaxis.” Unfortunately, despite this overwhelming increase in risks, many patients still continue to smoke.
Human Immunodeficiency Virus+
Currently there is the misperception that HIV-positive status alone increases the risk of postoperative complications. With the exception of certain transoral procedures,46,47 review of the literature does not support this belief.48–51 The most important risk factor for postoperative complications in the HIV+ patient is the one routinely assessed in all patients: ASA classification.49 However, there are markers used to monitor disease status that are predictive of increased risk (Box 3-2). Increased morbidity and mortality rates are associated with CD4 count ≤200 cell/mm3 and viral load >10,000 copies/mL.50,52–55 In addition, a postoperative CD4 percent of ≤18 ± 3 and a decrease in percent CD4 of ≥3 are associated with increased morbidity.54 All these values can easily be tested for, and any physician operating on an HIV+ patient should strongly consider ordering these laboratory values routinely. If there are abnormalities, both SCS trial and implant should be delayed, and the patient referred to an infectious disease specialist. To date there are no SCS studies that have specifically looked at the increased risk of infection in HIV+ patients.
Thrombocytopenia (platelets <50,000/µL) is a frequent finding in HIV+ patients, with prevalence rates from 9% to 37% in various study populations.56 Therefore thorough preoperative evaluation of platelet count and correction of a possible coagulation disorder is mandatory before proceeding with surgical intervention. Most implanters believe that an implant should be delayed until platelets are above 50,000 by either disease correction or platelet infusion.
Obesity
There is considerable stigma associated with obesity (body mass index [BMI] >30 kg/m2), and outcomes are impacted in many areas of medicine. Most physicians are aware of the deterioration of cardiac, pulmonary, and immunological function associated with obesity.57–59 Obesity is also associated with decreased quality of life and life expectancy.60,61 The co-morbidities of obesity are well known, and the list of associated disease states continues to grow annually. Given this, there is the commonly held deduction that obesity is a significant risk factor for perioperative complications. Although there is an increased risk of wound infections,62,63 Dindo and colleagues64 have shown that obesity alone is not a risk factor for postoperative complications. Further, their prospective study of >6000 patients over 10 years showed no significant difference in median operation time or need for blood transfusions. The latter results are especially encouraging given the high prevalence of obesity among chronic pain patients. However, the increased risk of wound infection is particularly worrisome. With implantable technologies, simple wound infections can lead to significant morbidity, often requiring explanation of an otherwise well-functioning device. To date there have been no SCS studies specifically assessing the increased risk of wound infection in obese patients and whether obesity leads to increased rates of explantation or further morbidity in SCS. At this time it is appropriate to counsel the obese patient of his or her increased risk of infection. At worst this allows the patient to make a better-informed decision; at best it may provide further motivation toward weight loss. Anecdotal data suggest that, with improved pain control, patients may be able to engage in the behavioral modifications necessary for weight loss.
Rheumatoid Arthritis
Compared to the general population, patients with RA have an increased incidence of SSI, as high as 15%. Concomitant steroid use has been associated with increased risk, whereas continued methotrexate use has been linked to decreased risk.65–67 den Broeder and associates68 examined the risk of SSI in those using anti–tumor necrosis factor (TNF) therapy and found no effect on SSI. However, the patients on anti-TNF therapy did have higher rates of wound dehiscence and bleeding. Interestingly, they found sulfasalazine to have a strong protective effect against SSI and hypothesized that this may be because of the bactericidal effect of the sulfapyridine component. Currently it would seem prudent to withhold anti-TNF medications before surgery. This would require stopping anti-TNF treatment for at least four drug half-lives before surgery (12 days for etanercept [Enbrel], 39 days for infliximab [Remicade], 56 days for adalimumab [Humira]).69 Changes in the patient’s disease-modifying agents are best coordinated with their rheumatologist.
Diabetes Mellitus
As the rate of DM increases,70 the proportion of patients who are candidates for SCS with diabetes will likewise grow. Currently pain from peripheral diabetic neuropathy shows excellent response to SCS.71,72 The stress from surgery induces the release of counterregulatory hormones, which leads to insulin resistance, increased glucose production, decreased insulin secretion, and ultimately hyperglycemia.73 Subsequently this hyperglycemic state inhibits leukocyte function74 and collagen formation, decreasing wound tensile strength.75,76 Perioperative hyperglycemia is known to be an independent risk factor for the development of SSI.77 Interestingly, in a retrospective review of over 38,000 surgeries by Acott, Theus, and Kim,78 there was no correlation between hemoglobin A1c levels and risk of complication, type of complication, or death.
Although the terms “strict” and “optimal” glycemic control are used in the management of DM, there is no consensus definition of these terms in the surgical patient. There is evidence that maintaining postoperative blood glucose <200 mg/dL reduces the incidence of SSI,79 but there is no clear guide as to what an ideal preoperative blood glucose range is. Bergman80 has developed guidelines for the management of persons with type I diabetes (Boxes 3-3 and 3-4) and those with type II diabetes undergoing minor surgery (Box 3-5). He further recommends that persons with type II diabetes should take their oral medication as soon as they resume eating/drinking. There are no recommendations for the discontinuation of rosiglitazone (Avandia) or pioglitazone (Actos) before surgery. With their long duration of action, it is unclear if there is a reason to stop them at all.81 After surgery persons with type I diabetes should monitor their blood glucose every 2 hours; persons with type II diabetes should monitor every 4 hours. To date no SCS studies have specifically addressed the increased risk of infection in patients with diabetes.
Box 3-3
Recommendations for Management of Persons with Type I Diabetes Undergoing Spinal Cord Stimulation
 Begin an infusion of 10% dextrose in
Begin an infusion of 10% dextrose in 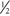 NS. Flow rate should be consistent with fluid maintenance for the patient (≈100 mL/hr in an average adult). Add 20 mEq of KCl to each liter if no renal failure.
NS. Flow rate should be consistent with fluid maintenance for the patient (≈100 mL/hr in an average adult). Add 20 mEq of KCl to each liter if no renal failure. If blood glucose is >200 mg/dL, rapid-acting insulin is administered subcutaneously using the Rule of 1500 to determine dose (see Box 3-4).
If blood glucose is >200 mg/dL, rapid-acting insulin is administered subcutaneously using the Rule of 1500 to determine dose (see Box 3-4). Check blood glucose every hour. Rapid-acting insulin is administered if >200 mg/dL as stated previously.
Check blood glucose every hour. Rapid-acting insulin is administered if >200 mg/dL as stated previously.Adapted from Bergman S: Perioperative management of the diabetic patient, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:731-737, 2007.
Box 3-4
Rule of 1500
Adapted from Bergman SA: Perioperative management of the diabetic patient, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:731-737, 2007.
Box 3-5
Recommendations for Management of Patients With Type II Diabetes Undergoing Spinal Cord Stimulation
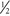 normal saline. Flow rate should be consistent with fluid maintenance for the patient (≈100 mL/hr in an average adult). Add 20 mEq of KCl to each liter if no renal failure. Rapid-acting insulin lispro (Humalog) or aspart (NovoLog) 0.1 U/kg is given subcutaneously.
normal saline. Flow rate should be consistent with fluid maintenance for the patient (≈100 mL/hr in an average adult). Add 20 mEq of KCl to each liter if no renal failure. Rapid-acting insulin lispro (Humalog) or aspart (NovoLog) 0.1 U/kg is given subcutaneously.Adapted from Bergman SA: Perioperative management of the diabetic patient, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:731-731, 2007.
Anticoagulation
One of the most devastating complications involving SCS procedures in the patient with a compromised coagulation status is epidural hematoma. There are no figures to cite regarding epidural hematoma following SCS trial/implant; however, the anesthesia literature shows the rate to be <1 in 150,000 following epidural anesthesia.82 As dimensions of a percutaneous lead are similar to an epidural catheter, familiarity with guidelines in regional anesthesia are necessary until such time that there are studies specifically applicable to SCS. A synthesis of recommendations from the 2010 American Society of Regional Anesthesia and Pain Medicine (ASRA) consensus guidelines for patients receiving anticoagulation is presented in Table 3-1.83 A patient on warfarin (Coumadin) should stop it for at least 5 days before the procedure and have their prothrombin time (PT)/international normalized ratio (INR) checked before surgery. The INR should be <1.5 before proceeding with regional anesthesia per ASRA guidelines. For those patients in whom stopping warfarin poses too high a risk without continued anticoagulation, low–molecular–weight heparin (LMWH) should be used as a bridge (Box 3-6).84 Clopidogrel (Plavix), an antiplatelet agent that binds to the cysteine residue of platelet receptor P2Y12, is commonly used in conjunction with aspirin to attenuate platelet aggregation. However, unlike warfarin, there is no drug with a shorter half-life that can be adequately substituted before surgery.85,86 Although heparin “bridge” therapy has been advocated for those on clopidogrel and considered high risk for thrombotic events, there is no literature to support this.87 In this case, when the risk of thrombosis is so high that any interruption of antiplatelet therapy could result in significant morbidity, elective surgery should be reconsidered. Arguably, with both SCS trial and implant, the greater concern is bleeding rather than thrombotic risk: the procedure is short and performed on an outpatient basis, and many patients have improved mobility after it is completed. In this situation stopping clopidogrel 7 days before the procedure and resuming 12 to 24 hours after surgery are logical.88,89 When weighing the decision to stop clopidogrel, it is recommended that there be no interruption in therapy for the first year after it is initiated.88,90 Physicians should strongly consider coordinating changes in the patient’s anticoagulant medications with the prescribing physician and/or a hematologist.
Table 3-1 2010 ASRA Precautions and Recommendations in Anticoagulated Patients
| Medication | Recommendation |
|---|---|
| Aspirin |
Abciximab (ReoPro), eptifibatide (Integrilin), tirofiban (Aggrastat)
Enoxaparin (Lovenox), dalteparin (Fragmin)
Argatroban (Acova), bivalirudin (Angiomax), desirudin (Iprivask/Revasc), lepirudin (Refludan)
ASRA, American Society of Regional Anesthesia and Pain Medicine; INR, international normalized ratio; LMWH, low–molecular–weight heparin; NSAID, nonsteroidal antiinflammatory drug; PT, prothrombin time; UFH, unfractionated heparin.
Adapted from Horlocker TT et al: Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy, Reg Anes Pain Med 35(1):64-101, 2010.
Box 3-6
Bridge Use of Low–Molecular–Weight Heparin in Patients Requiring Warfarin
Postoperative Protocol
CBC, Complete blood count; INR, international normalized ratio; LMWH, low–molecular–weight heparin.
Adapted from Jaffer AK, Brotman DJ, Chukwumerije N: When patients on warfarin need surgery, Cleve Clin J Med 70(11):973-984, 2003.
Other Considerations
At this time the only SCS that are approved for use with magnetic resonance imaging (MRI) are those produced by Medtronic. Only an MRI of the head with a magnet strength ≤1.5 Tesla is considered safe. MRI can only be conducted in completely implanted systems and cannot be conducted during a trial. Even following these precautions, there is still a risk of permanent damage to the SCS requiring revision or explant.91 Given the considerable risk to the patient and necessary precautionary steps, including reprogramming the SCS to manufacturer recommended specifications, these changes are best made in conjunction with a representative from the company.
Previously the combination of SCS and ICDs (pacemaker, defibrillator) was contraindicated. However, review of the manufacturers’ prescriptive information shows that their stance is softening, no doubt based on the successful use of these devices together in case reports.92–94 Nonetheless, the official statements for the three manufacturers are as follows:
Is Spinal Cord Stimulation Disease-Modifying?
Despite our current neuroanatomical knowledge, conditions such as complex regional pain syndrome prove that there is still much more to be learned and deciphered. Most chronic pain states almost certainly have a neuropathic component and some degree of central sensitization, and it is these features that can be exploited via SCS. As SCS use expands and it is used for a greater variety of patients with multiple medical conditions, there is evidence that it modulates more than just pain. Krames and Mousad98 describe a case in which reductions in diarrheal episodes were found in a patient initially implanted for the pain of irritable bowel syndrome. In those with critical limb ischemia, a long-term outcome study in Italy showed that those implanted with SCS had statistically significant improved limb survival at 1 year.99 Kapural and associates100 describe a patient with complex regional pain syndrome (CRPS), type 1 of the left lower extremity and type 2 DM who had ≈50% decrease in insulin requirement after successful SCS implantation. It has also been found that, even in FBSS cases in which SCS provides only minimal pain relief, there can still be improvements in leg muscle strength and gait.101
Conclusion
As the safety and efficacy of SCS expand, a greater number of patients may benefit from its use. Although many patient factors are now considerations rather than contraindications,102,103 there are still many questions left unanswered. As the use of SCS grows, there may be evidence to direct clinicians as to how specific disease processes affect and in turn are affected by SCS. Until that time, knowledgeable physicians will continue to rely on and synthesize information from a variety of fields to best treat their patients—a familiar situation for any of us who treat those in pain.
1 Kane K, Taub A. A history of local electrical analgesia. Pain. 1975;1:25-138.
2 Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46(4):489-491.
3 Culver DH, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. Am J Med. 1991;91(S):152-157.
4 Kumar K, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomized controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179-188.
5 Stojanovic MP, Abdi S. Spinal cord stimulation. Pain Phys. 2002;5:156-166.
6 Rosenow JM, et al. Failure modes of SCS hardware. J Neurosurg. 2004;100(3):254-267.
7 Hill PE. Complications of permanent transvenous cardiac pacing: a 14-year review of all transvenous pacemakers inserted at one community hospital. Pacing Clin Electrophysiol. 1987;10:564-570.
8 Kearney R, Eisen HJ, Wolf JE. Nonvalvular infections of the cardiovascular system. Ann Intern Med. 1994;121:219-230.
9 Nery PB, Fernandes R, Nair G. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol. 2010;21(7):786-790.
10 Cesar de Oliveira J, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythmia Electrophysiol. 2009;2:29-34.
11 Baddour LM, et al. Update on cardiovascular implantable electronic device infections and their management. Circulation. 2010;121:458-477.
12 Gaynes RP, et al. Surgical site infection (SSI) rates in the United States, 1992-1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin Infect Dis. 2001;33(S2):S69-S77.
13 Haridas M, Malangoni MA. Predictive factors for surgical site infection in general surgery. Surgery. 2008;144:496-503.
14 Walsh EE, Greene L, Kirshner L. Sustained reduction in methicillin-resistant Staphylococcus aureus wound infections after cardiothoracic surgery. Arch Intern Med. 2011;171(1):68-73.
15 Sharma M, Berriel-Cass D, Baran JJr. Sternal surgical-site infections following coronary artery bypass graft: prevalence, microbiology, and complications during a 42-month period. Infect Control Hosp Epidemiol. 2004;25(6):468-471.
16 Filsoufi F, Castillo JG, Rahmanian PB, et al. Epidemiology of deep sternal wound infection in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23(4):488-494.
17 Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S344-S349.
18 Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S. aureus nasal carriage. Ann Pharmacother. 1998;32:S7-16.
19 Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J Hosp Infect. 1995;31:13-24.
20 Kim DH, Spencer M, Davidson SM, et al. Institutional prescreening for detection and eradication of methicillin-resistant Staphylococcus aureus in patients undergoing elective orthopedic surgery. J Bone Joint Surg Am. 2010;92:1820-1826.
21 Rao N, Cannella B, Crossett LS, et al. A preoperative decolonization protocol for Staphylococcus aurues prevents orthopaedic infections. Clin Orthop Relat Res. 2008;466:1343-1348.
22 Kalmeijer MD, Coertjens H, van Nieuwland-Rollen PM, et al. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35:353-358.
23 Herwaldt LA. Reduction of Staphylococcus aureus nasal carriage and infection in dialysis patients. J Hosp Infect. 1998;40(Suppl B):S13-S23.
24 Kluytmans JA, Manders MJ, van Bommel E, et al. Elimination of nasal carriage of Staphylococcus aureus in hemodialysis patients. Infect Control Hosp Epidemiol. 1996;17:780-785.
25 Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9-17.
26 Engelman R, Shahian D, Shemin R, et al. Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, II: antibiotic choice. Ann Thorac Surg. 2007;83(4):1569-1576.
27 Stevens DL. The role of vancomycin in the treatment paradigm. Clin Infect Dis. 2006;42(suppl 1):S51-S57.
28 Schwilk B, et al. Perioperative respiratory events in smokers and nonsmokers undergoing general anaesthesia. Acta Anaesthesiol Scand. 1997;41:348-355.
29 Morton HJV. Tobacco smoking and pulmonary complications after operation. Lancet. 1944;1:368-370.
30 Bluman LG, et al. Preoperative smoking habits and postoperative pulmonary complications. Chest. 1998;113:883-889.
31 Wetterslev J, et al. PaO2 during anaesthesia and years of smoking predict late postoperative hypoxaemia and complications after upper abdominal surgery in patients without preoperative cardiopulmonary dysfunction. Acta Anaesthesiol Scand. 2000;44:9-16.
32 Padubidri AN, et al. Complications of postmastectomy breast reconstructions in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg. 2001;107:342-349.
33 Krueger JK, Rohrick RJ. Clearing the smoke: the scientific rationale for tobacco abstention with plastic surgery. Plast Reconstr Surg. 2001;108:1063-1073.
34 Sorensen LT, Karlsmakr T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg. 2003;238:1-5.
35 Glassman SD, et al. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25:2608-2615.
36 Bennett-Guerrero E, et al. The use of a postoperative morbidity survey to evaluate patients with prolonged hospitalization after routine, moderate-risk, elective surgery. Anesth Analg. 1999;89:514-519.
37 Moller AM, et al. Effect of preoperative smoking intervention on postoperative complications: a randomized clinical trial. Lancet. 2002;359:114-117.
38 Buist AS, et al. The effect of smoking cessation and modification of lung function. Am Rev Respir Dis. 1976;114:115-122.
39 Kinsella JB, et al. Smoking increases facial skin flap complications. Ann Otol Rhinol Laryngol. 1999;108:139-142.
40 Sorensen JT, Jorsengen T. Short-term pre-operative smoking cessation intervention does not affect postoperative complications in colorectal surgery: a randomized clinical trial. Colorectal Dis. 2003;5:347-352.
41 Kuri M, et al. Determination of the duration of preoperative smoking cessation to improve wound healing after head and neck surgery. Anesthesiology. 2005;102:892-896.
42 Chan L, Withey S, Butler P. Smoking and wound healing problems in reduction mammoplasty: is the introduction of urine nicotine testing justified? Ann Plast Surg. 2006;56:111-115.
43 Azodi OS, et al. The efficacy of a smoking cessation programme in patients undergoing planned surgery—a randomized clinical trial. Anaesthesia. 2009;64:259-265.
44 Villebro NM, et al. Long-term effects of a preoperative smoking cessation programme. Clin Resp J. 2008;2:175-182.
45 Peters MJ, Morgan LC, Gluch L. Smoking cessation and elective surgery: the cleanest cut. Med J Aust. 2004;180:317-318.
46 Reilly MJ, Burke KM, Davison SP. Wound infection rates in elective plastic surgery for HIV-positive patients. Plast Reconstr Surg. 2009;123:106-112.
47 Rose DN, Collins M, Kleban R. Complications of surgery in HIV-infected patients. AIDS. 1998;12:2243-2251.
48 Ayers J, Howton M, Layon J. Postoperative complications in patients with human immunodeficiency virus disease. Chest. 1993;103:1800-1807.
49 Jones S, et al. Is HIV infection a risk factor for complications of surgery? Mt Sinai J Med. 2002;29:329-333.
50 Horberg M et al: Surgical outcomes of HIV+ patients in the era of HARRT (Abstract 82). Presented at the 11th Conference of Retroviruses and Opportunistic Infections, San Francisco, California. February 8-11, 2004.
51 Dodson TB. HIV status and the risk of post-extraction complications. J Dent Res. 1997;76:1644-1652.
52 Emparan C, et al. Infective complications after abdominal surgery in patients infected with human immunodeficiency virus: role of CD4 lymphocytes in prognosis. World J Surg. 1998;22(8):778-782.
53 Saltzman DJ, et al. The surgeon and AIDS: twenty years later. Arch Surg. 2005;140:961-967.
54 Tran HS, et al. Predictors of operative outcome in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. Am J Surg. 2000;180(3):228-233.
55 Consten EC, et al. Severe complications of perianal sepsis in patients with human immunodeficiency virus. Br J Surg. 1996;83(6):778-780.
56 Sullivan P, et al. Surveillance for thrombocytopenia in persons infected with HIV. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14(4):374-379.
57 Berkalp B, et al. Obesity and left ventricular diastolic dysfunction. Int J Cardiol. 1995;52:23-26.
58 Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655-660.
59 Tanaka S, et al. Impaired immunity in obesity: suppressed but reversible lymphocyte responsiveness. Int J Obes Relat Metab Disord. 1993;17:631-636.
60 Manson JE, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677-685.
61 Allison DB, et al. Meta-analysis of the effect of excluding early deaths on the estimated relationship between body mass index and mortality. Obes Res. 1999;7:342-354.
62 Moulton MJ, et al. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation. 1996;94(S):II87-92.
63 Shapiro M, et al. Risk factors for infection at the operative site after abdominal or vaginal hysterectomy. N Engl J Med. 1982;307:1661-1666.
64 Dindo D, et al. Obesity in general elective surgery. Lancet. 2003;361:2032-2035.
65 Grennan DM, et al. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopedic surgery. Ann Rheum Dis. 2001;60:214-217.
66 Hamalainen M, Raunio P, Von Essen R. Postoperative wound infection in rheumatoid arthritis surgery. Clin Rheumatol. 1984;3:329-335.
67 Bongartz T, et al. Incidence and risk factors for prosthetic joint infections in patients with rheumatoid arthritis following total knee and total hip replacement. Arthritis Rheum. 2005;52(S):1449.
68 den Broeder AA, et al. Risk factors for surgical site infections and other complications in elective surgery in patients with rheumatoid arthritis with special attention for anti-tumor necrosis factor: a large retrospective study. J Rheumatol. 2007;34:689-695.
69 Nederlandse Vereniging voor Reumatologie. Medicijnen: het toepassen van TNF blokkade in de behandeling van reumatoïde artritis. Utrecht: Dutch Society for Rheumatology; November 2003.
70 Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clinic Pract. 2010;87(1):4-14.
71 Daousi C, Benbow SJ, MacFarlane IA. Electrical spinal cord stimulation in the long-term treatment of chronic painful diabetic neuropathy. Diabet Med. 2005;22(4):393-398.
72 Tesfaye S, et al. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348(9043):1698-1701.
73 Shamoon H, Hendler R, Sherwin RS. Synergistic actions among antiinsulin hormones in pathogenesis of stress hyperglycemia in humans. J Clin Endocrinol Metab. 1981;52:1235-1241.
74 Marhoffer W, et al. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15:256-260.
75 Gottrup F, Andreassen TT. Healing of incisional wounds in stomach and duodenum: the influence of experimental diabetes. J Surg Res. 1981;31:61-68.
76 McMurray JFJ. Wound healing with diabetes mellitus: better glucose control for better wound healing in diabetes. Surg Clin North Am. 1984;64:769-778.
77 Golden SH, et al. Perioperative glycemic control and risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408-1414.
78 Acott AA, Theus SA, Kim LT. Long-term glucose control and risk of perioperative complications. Am J Surg. 2009;198(5):596-599.
79 Zerr KJ, et al. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63:356-361.
80 Bergman SA. Perioperative management of the diabetic patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:731-737.
81 Marks JB. Perioperative management of diabetes. Am Fam Phys. 2003;67:93-100.
82 Tryba M. Ru ckmarksnahe regionalana sthesie und niedermolekulare heparine. Pro Ana sth Intensivmed Notfallmed Schmerzther. 1993;28:179-181.
83 Horlocker TT, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Reg Anes Pain Med. 2010;35(1):64-101.
84 Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med. 2003;70(11):973-984.
85 Gonzalez-Correa JA, et al. Effects of dexibuprofen on platelet function in humans: comparison with low-dose aspirin. Anesthesia. 2007;106(2):218-225.
86 Samama CM. Preoperative nonsteroidal anti-inflammatory agents as substitutes for aspirin: already too late? Anesthesia. 2007;106(2):205-206.
87 Collet JP, Montalescot G. Premature withdrawal and alternative therapies to dual oral antiplatelet therapy. Eur Heart J Suppl. 2006;8(suppl G):G46-G52.
88 Thacil J, Gatt A, Martlew V. Management of surgical patients receiving anticoagulation and antiplatelet agents. Br J Surg. 2008;95:1437-1448.
89 Di Minno MND, et al. Perioperative handling of patients on antiplatelet therapy with need for surgery. Intern Emerg Med. 2009;4:279-288.
90 O’Riordan JM, et al. Antiplatelet agents in the perioperative period. Arch Surg. 2009;144(1):69-76.
91 Medtronic. MRI guidelines, accessed February 13, 2010, from. http://professional.medtronic.com/interventions/spinal-cord-stimulation/mri-guidelines/index.htm#anchor1.
92 Kosharskyy B, Rozen D. Feasibility of spinal cord stimulation in a patient with a cardiac pacemaker. Pain Phys. 2006;9:249-252.
93 Hoelzer BC, Burgher AH, Huntoon MA. Thoracic spinal cord stimulation for post-ablation cardiac pain in a patient with permanent pacemaker. Pain Prac. 2008;8(2):110-113.
94 Andersen C, Pedersen HS, Scherer C. Management of spinal cord stimulators in patients with implantable cardioverter-defibrillators. Neuromodulation. 2002;5(3):133-136.
95 Boston Scientific. Precision plus SCS system, accessed February 26, 2010, from. http://www.bostonscientific.com/Device.bsci?page=ResourceDetail&navRelId=1000.1003&method=DevDetailHCP&id=10068931&resource_type_category_id=1&resource_type_id=91&pageDisclaimer=Disclaimer.ProductPage.
96 Medtronic. Spinal cord stimulation—indications, safety and warnings, accessed February 26/2010 from. http://professional.medtronic.com/interventions/spinal-cord-stimulation/indications-safety-and-warnings/index.htm.
97 St. Jude Medical. Eon mini rechargeable IPG system, accessed February 26, 2010, from. http://www.sjmneuropro.com/Products/US/Eon-Mini-Rechargeable-IPG-System.aspx.
98 Krames E, Mousad D. Spinal cord stimulation reverses pain and diarrheal episodes of irritable bowel syndrome: a case report. Neuromodulation. 2004;7(2):82-88.
99 Tedesco A, D’Addato M. Spinal cord stimulation for patients with critical limb ischemia: immediate and long-term clinical outcome from the prospective Italian register. Neuromodulation. 2004;7(2):97-102.
100 Kapural L, et al. Decreased insulin requirements with spinal cord stimulation in a patient with diabetes. Anesth Analg. 2004;98:745-746.
101 Buonocore M, Demartini L, Bonezzi C. Lumbar spinal cord stimulation can improve muscle strength and gait independently of analgesic effect: a case report. Neuromodulation. 2006;9(4):309-313.
102 Segal R. Spinal cord stimulation, conception, pregnancy, and labor: case study in a complex regional pain syndrome patient. Neuromodulation. 1999;2(1):41-45.
103 Saxena A, Eljamel S. Spinal cord stimulation in the first two trimesters of pregnancy: case report and review of the literature. Neuromodulation. 2009;12(4):281-283.



















