CHAPTER 38 Medical and Surgical Management of Chronic Subdural Hematomas
Chronic subdural hematoma (CSDH) is seen frequently in daily neurosurgical practice. Initially, it was called “chronic subdural haemorrhage of traumatic origin” by Trotter.1 This term anticipates two main aspects of the disease:
Previously, CSDH was seen simply as the chronic form of acute subdural hematoma. It was thought that its development was continuous from acute to subacute and then to chronic subdural hematoma.2 These entities, however, do not share much more than the anatomic location where the hematoma is found. Even though 1% to 6% of patients with untreated acute subdural hematoma experience transformation to CSDH,3,4 the latter develops its own dynamics. Until now, there was only limited understanding of its pathophysiology, which is discussed elsewhere in this volume (see Chapter 37). Major differences between these entities are risk factors, typical age groups, and outcome.
Dramatic improvement in the outcome of CSDH has been achieved as a result of the wide availability of modern imaging methods and refinement of operative techniques.5 Currently, CSDH is considered to be a rather benign disease of the elderly. Nevertheless, the relatively high mortality rates of up to 13% reported in the contemporary literature reflect the fact that as many as four deaths per year are still related to this ailment in a typical neurosurgical department.
History
As early as the Neolithic Period, humans started to open the skull for uncertain reasons. It is unclear whether CSDH was a common condition at a time when people died in their fourth decade. If so, CSDH might have been one of the ailments with a spectacular course of salvation after trephination. There is no doubt that head injury afflicted humanity from its earliest beginnings. Most likely, Stone Age humans had already realized that injury to the head could render an adversary unconscious.6 It has been a matter of debate, however, whether our ancestors from the initial stages of neurosurgery were able to establish a logical relationship between trauma and CSDH on the one hand and trephination on the other.7,8 Clearly, science and magic were interwoven at that time.9 Various diseases were ascribed to evil spirits, and cure was achieved by allowing these spirits to escape from the skull.10–12 Indian folktales, for example, tell us about extraction of worm-like structures from the skull.13 It is conceivable that CSDH, in contrast to other disorders, might have been a basis for such magic embodiment in folktales and myths.
The first pathologic description of CSDH was attributed to Wepfer, who described two cases of “serum accumulation” between the dura mater and pia mater in 1675.14,15 About a century later, Morgagni, the “father of modern pathology,”16 added more cases of “bleeding between the meninges” and speculated about the cause of the “delayed apoplexy.”17 Initially, it was assumed that CSDH was caused by spontaneous bleeding17,18 or trauma.19 Subsequently, the concept of an inflammatory process with lethal hemorrhage into a pseudomembranous sac was favored. The term pachymeningitis hemorrhagica interna was introduced by Virchow.20 Decompression of CSDH was sought via the auditory meatus. The failure of surgical treatment trials, in general, led to the assumption that “pachymeningitis” was an incurable disorder. In the following years, a series of publications reported on lifetime sufferers of pachymeningitis,21–23 and a large number of unfortunate patients spent their life in almshouses and asylums.24
Successful neurosurgical treatment was marked by the publication of Hulke in The Lancet in 1883.25 However, only in the early 20th century did neurosurgery become important in the treatment of CSDH.1 It was the work of Cushing that fueled the development of neurosurgical treatment strategies and advocated for early diagnosis.26 At that time the diagnosis of CSDH depended on the skills of the individual performing the neurological examination. This was limited by the overall low familiarity with the entity of CSDH and the diagnostic dilemma of CSDH not providing pathognomonic signs or symptoms. Osler reported on an autopsy study in which 197 of 1185 patients were found to suffer from CSDH.24 The cardinal symptom was dementia in more than a third of the patients. Underestimation of the incidence was obvious, and scientists as well as physicians tried to find certain characteristics providing diagnostic clues.21,23,27,28
Enabled by the introduction of pneumencephalography by Dandy29 and angiography by Moniz,30 radiologic diagnosis had an enormous impact on diagnostic decision making. As a consequence, CSDH was diagnosed much earlier and surgical outcome improved continuously.31
In 1937, Horrax and Poppen reported a cure rate of 83% after surgery. During the following decades, similar results were achieved by less invasive methods in larger series. Cure rates after simple bur-hole craniotomy (BHC) with saline rinsing2,32 approximated those of large craniotomies with marsupialization of the membranes,33 but the former technique reduced the risk for perioperative morbidity and mortality.34
The problem of reexpansion of the brain was identified as a major cause of recurrence. Treatment concepts were aimed at direct expansion of the brain by provoking cerebral edema,35 reducing venous outflow,31 or expanding cerebrospinal fluid (CSF) volume.36,37 Fitting the skull to its content was another rarely performed procedure in pediatric patients.38 The introduction of closed system drainage eased the problem,39–41 and the establishment of cross-sectional imaging techniques was the last milestone in the treatment of CSDH because it allowed further minimization of neurosurgical approaches.
Definition
CSDH is defined as a fluid collection within the layers of the dura mater.42–44 It must be distinguished from other entities that might have a similar appearance on cerebral computed tomography (CT), including subdural hygroma, formerly called subdural hydroma,45 and external hydrocephalus.46 Although the former has a watery or xanthochromic consistency and often lacks a surrounding membrane, the latter is a variant of hydrocephalus and may develop after tearing of the arachnoid layer in cases of internal hydrocephalus. It is often seen after vascular surgery.47 Subdural hygroma can transform into CSDH. A comprehensive literature review on the relationship of CSDH and subdural hygroma showed that up to 58% of cases of subdural hygroma evolved into CSDH, depending mainly on the length of observation.48
Epidemiology
The incidence of CSDH has been estimated to be 1 to 2 cases per 100,000 inhabitants per year in publications in the early CT era.49,50 According to newer studies, however, the incidence appears to be as high as 13.1 cases per 100,000 inhabitants,51 which might reflect primarily an increase in diagnosis. An increasing frequency is observed in all advanced societies throughout the world, which might also have primarily been driven by an increasing percentage of the population older than 65 years.52–54 Patients older than 40 years account for 80% of cases.32,55 The peak incidence currently occurs in the eighth decade. There is a clear gender difference. In older publications, at least 70% to 80% of patients with CSDH were male.56,57 Newer studies describe a male-to-female ratio of 3 : 2.55,58–61 The higher incidence of CSDH in men most likely reflects the overrepresentation of men with head injury.53
Trauma is probably the most important risk factor for the development of CSDH, with two thirds of CSDH patients remembering some type of minor trauma.53,55,62 Patients with seizures are also thought to be at higher risk.63 Traumatic or postsurgical communication of the subarachnoid space with the subdural space is thought to increase the risk for CSDH considerably.64 As a consequence, neurosurgical treatment of vascular disorders involving opening the subarachnoid space bears the highest risk for surgery-related CSDH.65 CSDH is rarely a complication in sports with a propensity for cranial trauma, such as boxing,66 capoeira,67 soccer,68 and snowboard racing.69
Craniocerebral disorders and therapeutic procedures that alter the volume ratios of intracranial compartments and thereby result in intracranial hypotension also increase the risk for CSDH. Thus, CSDH is seen in conjunction with dehydration70 and with brain atrophy71 or degenerative cerebral diseases such as Huntington’s disease.72,73 CSF shunting is another well-recognized risk factor.74 Up to 8% of patients undergoing shunting for normal-pressure hydrocephalus may suffer from this complication.75 Other procedures that reduce the volume of CSF acutely, such as lumbar puncture,76 spinal anesthesia,77 or transventricular approaches for tumor surgery,65 are seldom followed by the formation of CSDH.
Primary coagulopathies in children,78 as well as secondary coagulopathies or anticoagulant treatment in adults, are further risk factors for the development of CSDH.79 In acute subdural hematoma, the risk increases 7-fold in men and 26-fold in women.80 Similar mechanisms might be involved when CSDH complicates leukemia,81 thrombopathies,82 or hepatogenic coagulopathies.83
Chronic alcoholism is often cited as a major risk factor for CSDH. Kremiansky had already noted the importance of alcoholism in 1868 and provided an animal model in which it was demonstrated that feeding 45% brandy to dogs could cause CSDH.84 In an epidemiologic study from Helsinki, alcoholism was present in as many as 50% of CSDH patients.49 The pathoetiologic mechanisms are not known precisely, but the propensity of alcohol-dependent individuals to experience trauma is considered the single most important cause.53 Other possible factors are alcohol-induced hepatopathy, generalized vascular fragility as a result of avitaminosis, dysfunction of thrombocytes, brain atrophy with intracranial hypotension, and increased serum levels of estrogens.85
Older age predisposes to the development of CSDH in a multifaceted fashion. Older patients suffer falls more often,86 treatment with anticoagulative medications is frequent,87 the risk for concomitant bleeding rises with age88 and, finally, brain atrophy is a physiologic phenomenon in advanced age.89
Diagnosis
Patient History
The clinical picture of CSDH varies widely. There are no pathognomonic signs or symptoms that allow one to make the diagnosis solely on the basis of clinical data. It is not even clear which pathophysiologic aspect causes the clinical symptoms. The variety of mechanisms may include cortical dysfunction, which is responsible, for example, for Gerstmann’s syndrome associated with CSDH,90 adverse effects on the basal ganglia resulting in parkinsonian symptoms,91 conduction failure of fiber tracts leading to hemiparesis,92 or simply decreased regional cerebral blood flow.93–95
According to more recent data, CSDH is asymptomatic in a large number of patients, but it may also cause high intracranial pressure resulting in coma. Between these extreme states, nearly every constellation of speech, sensorimotor, neuropsychiatric, or mood disturbances may occur. It might not be surprising that CSDH was called the “great imitator” in the past.96
Common symptoms in the largest series with medical reports on 2300 patients were simple refractory headache and sensorimotor and neuropsychiatric changes such as amnestic deficits or lack of concentration.62 The development of symptoms is slow in the majority of patients, but stroke-like onset can occur.97,98 A relevant number of patients have seizures of different semiology.63,99–101 Diagnostic evaluation for CSDH has improved in some areas such that no individual in a group of 560 demented patients was referred to a neurological ward with dementia caused by CSDH.102
The use of clinical grading systems for CSDH is reserved for scientific purposes and has no impact on therapeutic decision making (Tables 38-1 and 38-2).103,104
TABLE 38-1 Original Clinical Score Introduced by Bender and Christoff103
| 1 Fully alert and conscious, normal mental function, few or no focal signs |
| 2 Drowsy or lethargic, organic mental syndrome, focal neurological signs |
| 3 Very drowsy or stuporous, conspicuous organic mental syndrome, pronounced focal signs |
| 4 Coma or signs of herniation |
TABLE 38-2 Original Clinical Score Introduced by Markwalder and Reulen104
| 0Patient neurologically normal |
| 1Patient alert and oriented, mild symptoms such as headache, absent or mild neurological deficits such as reflex asymmetry |
| 2Patient drowsy or disoriented with variable neurological deficits such as hemiparesis |
| 3Patient stuporous but responding appropriately to noxious stimuli, severe focal signs such as hemiplegia |
| 4Patient comatose with absent motor responses to painful stimuli, decerebrate or decorticate posturing |
Imaging
Preoperative CT
The introduction of cross-sectional imaging revolutionized the diagnosis of CSDH.105 Because of the widespread use of modern imaging techniques in developed countries, the clinical picture of CSDH has changed within the past 30 years,106 a process that also occurs in developing countries after the introduction of CT.107,108 Cranial CT and magnetic resonance imaging (MRI) made former techniques obsolete. Cerebral angiography and ventriculography, which look for deviation of a calcified pineal body on radiographs of the skull, no longer play a role in the diagnostic evaluation for suspected CSDH. Today, experience with the superior image quality of modern multislice computed tomographic scanners allows one to estimate that the rate of false-negative scans might approximate zero. As a consequence, CT of the cranium is the diagnostic tool of choice in many hospitals.109 It enables establishment of the diagnosis within a few minutes.
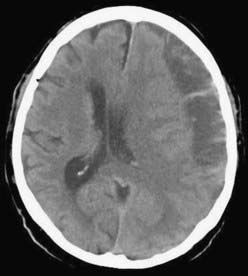
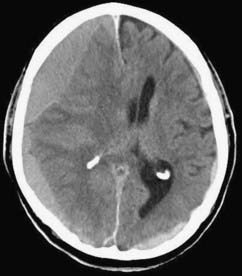
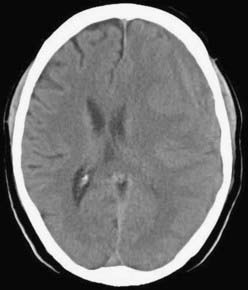
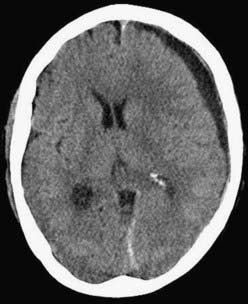
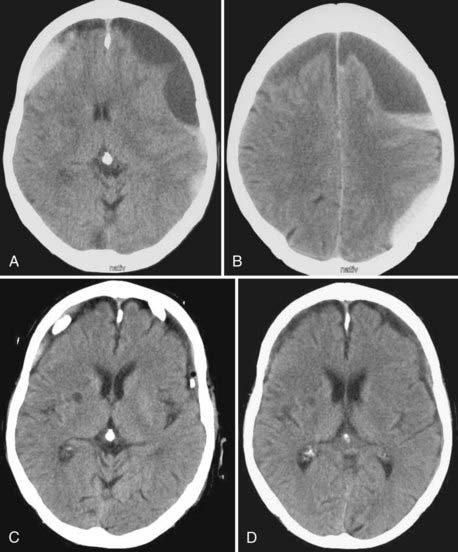
CSDH appears as a sickle-shaped lesion between the cortical surface and the calvaria. The gyri beneath the hematoma may still be visualized, a finding indicating that the arachnoid space is preserved. Compression of the ipsilateral gyri or the ventricle and midline shift are frequent observations. Although in acute subdural hematoma, a midline shift is regularly correlated with signs of elevated intracranial pressure, it is not uncommon in CSDH that shifts of greater than 1 cm are compensated for without neurological deficit. In one study, an average of 90 mL of hematoma fluid was compensated for without a detrimental rise in intracranial pressure.110 The computed tomographic appearance of CSDH varies widely (Figs. 38-1 to 38-5) and has been categorized by different classification systems (Tables 38-3 and 38-4).111,112 The scales were positively correlated with the age of the hematoma, rate of recurrence,113 amount of exudation,114 and the state of consciousness before surgery.115 It was emphasized that the highest risk for recurrence is seen with a mixed-density or layering type of hematoma on cranial CT because both types may be due to recent rebleeding into the cavity.111 An isodense appearance on CT can hamper establishment of the diagnosis, especially in patients with bilateral hematomas, which are characterized by no asymmetry of ventricles or midline shift, although intracranial pressure may be elevated because of the bilateral extracerebral space-occupying lesions. Bilateral hematomas are noted in up to 25% of cases.62
TABLE 38-3 CT Classification According to Nomura and Colleagues111
| 1Hyperdensity |
| 2Isodensity |
| 3Hypodensity |
| 4Mixed density |
| 5Layering type |
TABLE 38-4 CT Classification According to Naganuma and Colleagues112
| 1Homogeneous density |
| 2Laminar type |
| 3Layering or separated type |
| 4Trabecular density type |
Postoperative CT
It is useful to repeat CT after surgery before the drains are removed because there is a recurrence rate of up to 29% after BHC and up to 76% after twist drill craniostomy (TDC). Notably, recurrence after TDC is tacitly accepted as a complication because it is the least invasive technique. Caution must be exercised not to misdiagnose any remaining subdural fluid collection as recurrence after surgery. Residual fluid was present in 78% of cases on day 10 and in 15% in the sixth week after surgery.116 It is therefore recommended that one reoperate only in cases in which the hematoma increases and the patient deteriorates as a result of the recurrent hematoma. In the first days after surgery, intracranial air can be detected as black hypodense areas within the hematoma cavity. The amount of intracranial air after surgery was positively correlated with recurrence.117–119 Severe complications may arise from tension pneumocephalus after decompression of CSDH.120 In bilateral CSDH, a collapsed brain, especially in its frontal aspect, leads to the characteristic “Mount Fuji sign” on CT.121,122
MRI
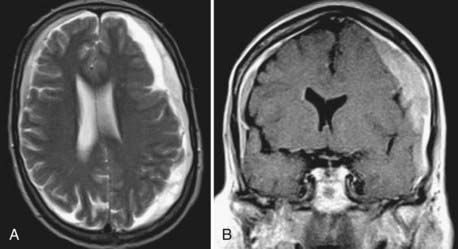
MRI has the advantage of higher resolution, which allows better differentiation of tissue and fluids.123,124 In children with CSDH, MRI is the preferred diagnostic tool. In the elderly, MRI is considered a second-line option for diagnostic evaluation,109 possibly because of historical, technical, and practical reasons. CT was the first widely available imaging modality that allowed visualization of CSDH, and neurosurgeons may be more familiar with interpretation of blood and its degradation products on CT than on MRI. Furthermore, MRI is a time-consuming and more expensive method, and it depends on a cooperative patient. In that regard, it should be considered that 18% of patients with CSDH have neuropsychiatric deficits when CSDH is diagnosed. Up to 15% of patients are comatose and need intensive care equipment,62 which often is not compatible with the high magnetic fields of MRI scanners.
In the majority of cases, CSDH appears hyperintense on T2-weighted (Fig. 38-6A) or proton-weighted images because blood degradation products, especially methemoglobin, give a hyperintense signal on such images. The variability in signal intensity is greater on T1-weighted images. Although nearly 50% of CSDH images appear hyperintense (Fig. 38-6B), hypointense, isointense, and mixed intensities are also seen in CSDH. Hypointense or isointense signals were interpreted as fresh rebleeding into the cavity.123 Therefore, it is not surprising that the recurrence rate is higher with the latter than with high-intensity hematomas on T1-weighted images. The inner architecture of CSDH noted on MRI suggests similarities with the findings on CT, but no correlation was found between the appearance in the two different techniques.109 Hematomas with low density, isodensity, and mixed density on CT may all appear as homogeneous hyperintense lesions on MRI.
CSF is characterized by a hypointense signal on proton-weighted images. Subdural fluid collections with low intensity on proton-weighted images may therefore be lesions other than CSDH.109 Because subdural hygroma is an important differential diagnosis, MRI can provide elementary information to differentiate both entities.
Contemporary Treatment
The approach to management of patients with CSDH ranges from a simple “watch and wait” strategy to large craniotomies with marsupialization of hematoma membranes.5 Pharmaceutical treatment was suggested in the past.103,127,128 Spontaneous resolution of CSDH occurs only rarely.112 Scientific reports on successful nonsurgical treatment of CSDH are also rare and date back to the 1960s and 1970s. The results are controversial and in reality cannot be construed as providing an alternative to surgical treatment. Conservative treatment has thus far included corticosteroids and bed rest. Glover and Labadie demonstrated a reduced rate of membrane formation in an animal model with corticosteroid treatment.129 The effect of dexamethasone was thought to be anti-inflammatory129 or antiangiogenic.130 Another approach included long-term application of mannitol and bed rest.131 The promising results of a pilot study, however, could not be reproduced in a randomized investigation because the side effects of long-term immobilization became more prominent in elderly patients.132 No parameters were defined in the past that might help decide in favor of a nonsurgical approach. It is common sense to carefully monitor asymptomatic patients in whom the presence of CSDH was proved by CT or MRI. Once clinical symptoms develop, however, surgical management is mandatory in the majority of cases.
New pathophysiologic aspects might have an impact on conservative treatment in the future. In particular, detection of the angiogenic cytokines responsible for development of the well-known leaky vessels within the outer membrane of a hematoma might offer new and promising targets to be blocked by pharmacologic agents. Recently, it was shown that the antiangiogenic properties of angiotensin-converting enzyme inhibitors could reduce the rate of recurrences in CSDH, as well as levels of vascular endothelial growth factor within the hematoma.133 Prospective trials with other antiangiogenic substances are under way.
An important issue in the perioperative and postoperative management of patients with CSDH is the question of whether anticonvulsive prophylaxis should be administered. The literature on this topic does not provide any guidelines. As a consequence, a Cochrane review came to the conclusion that because of the controversial findings in mainly retrospective studies, no formal recommendation could be given.100 Detailed data on the frequency of early or late seizures caused by CSDH are not available to date. However, posttraumatic or postoperative epilepsy is thought to have a low incidence in patients with CSDH.134 In a retrospective analysis of their own patient data and data obtained from a literature survey, Rubin and Rappaport found a 5.6% incidence of preoperative seizures versus 3% in the literature and a 4.3% incidence of postoperative seizures versus 1.8% in the literature.101 This is in accordance with a generally low standardized incidence ratio of between 1.5 and 2.8 for epilepsy in patients with mild head injury.135 From a pathophysiologic point of view, epileptic foci develop mainly from cortical injuries.136 Subdural hematoma in the chronic state, however, is an exclusively extracerebral lesion in the majority of cases. Pathoanatomically, a fibrous visceral membrane separates potentially epileptogenic blood degradation products within the hematoma from the cerebral cortex.101 Presumably, a higher risk for seizures might exist in cases in which the inner membrane was opened during surgery. Other risk factors are alcohol abuse and age older than 65 years.101,135
Patient posture in the early days after surgery was also thought to influence the rate of recurrence. In the first prospective study no difference was found with regard to recurrence and outcome.137 A recent randomized controlled trial, however, found an increased incidence of CSDH recurrence when patients did not maintain a flat position in the first 3 days after surgery. The number of adverse events caused by the flat position was equal in both groups. No effect on final outcome was demonstrated.138
Postoperative reexpansion of the brain was found early on to be a factor associated with recurrence and hence considered to influence outcome. Generous hydration of patients with intravenous fluids postoperatively was thought to increase the brain’s volume and decrease the risk for recurrence. However, attention must be paid to elderly patients because postoperative hyperemia in areas of cortex that were covered by hematoma was demonstrated in up to 41% of patients older than 75 years after rapid decompression of CSDH.139 Hyperemia might be one reason for postoperative subcortical hemorrhage140–145 or postoperative hyperperperfusion syndrome in agitated or delirious patients.126
Surgical Treatment
Surgical treatment of CSDH in symptomatic patients is still the “gold standard” of therapy because it allows immediate decompression of the space-occupying lesion and significantly improves outcome.106 However, a standardized approach to the treatment of CSDH does not exist,5 in part because of the fact that until recently, the advances in determining the pathophysiology of CSDH discussed earlier were of little practical relevance for treatment.
CSDH is still one of the most frequent problems encountered in neurosurgery. The progress in its surgical treatment is not comparable, however, to the sophisticated development of treatment concepts and surgical techniques in other subspecialities of neurosurgery, such as functional, spinal, or vascular neurosurgery. Unfortunately, even in light of the relatively frequent occurrence of unilateral and bilateral CSDH, there has been no definitive study or studies to define the best primary treatment. The number of randomized controlled trials on topics concerning CSDH has increased since the beginning of the 21th century, but there is still a surprisingly low level of scientific evidence for the different surgical approaches used for the treatment of CSDH.5 All these studies report successful resolution of hematomas, albeit in a varying number of cases, and there are two possible explanations. First, resolution of hematomas occurs independently of the treatment chosen. Second, CSDH is not a uniform entity, and different manifestations of the disease might demand different approaches. If the latter is true, it offers the possibility of tailoring treatment to an individual patient. One challenge, therefore, is to characterize individual cases according to relevant parameters.
Parameters identified thus far aim at predicting risk for recurrence and complications. Both risks were appreciated by the appearance of CSDH on cross-sectional images. However, to date, the specificity of parameters is not high enough to allow more precise prediction of outcome or to tailor treatment on the basis of these parameters. Furthermore, to the best of our knowledge, no study has compared different approaches for different appearances of CSDH on cross-sectional imaging. Moreover, the very definition of recurrence may vary substantially from site to site. For example, Torihashi and colleagues defined a recurrent CSDH as one in which the increased hematoma volume resulted in a neurological deficit.146 Many neurosurgeons would intervene earlier for recurrence, before the development of a neurological deficit, if the patient suffered severe and progressive headache, which correlates with reaccumulation of fluid and a mass effect in the subdural space. Finally, the issue of recurrence cannot be considered in an isolated fashion. One must also consider the complication rate and morbidity associated with the various treatments, and given the lack of large controlled studies, this is quite difficult. We have therefore tried to treat the problem according to the principles of evidence-based medicine while always keeping in mind that such medicine is not “cookbook medicine”147 and what appears to be the best treatment for a patient population is not necessarily the best option for an individual patient.
A systematic review of 48 publications from the MEDLINE database and from reference lists was conducted. The articles were written in English or German. Pediatric series and series with more than 10% of patients lost to follow-up were not included. The articles were classified as providing class I, class II, or class III evidence according to the criteria of the American Academy of Neurology.148 The various surgical treatment options are summarized in Table 38-5 and range from single-needle trephination without intraoperative irrigation or postoperative drainage to large craniotomies with marsupialization of the membranes.55,58–61,105,148–187 No study met the criteria for class I evidence, and six studies provided class II evidence. For analysis and comparison of results, the following uniform criteria were defined: morbidity—any complication during or after surgery other than recurrence; mortality—any death reported between surgery and discharge from the hospital; recurrence—clinical or radiologic deterioration requiring further surgery; and cure—complete patient autonomy after surgery (grade 0 or 1 in the classification of Markwalder and associates116 or Bender and Christoff103 and grade 5 in the Glasgow Outcome Scale).188 Morbidity and mortality can be considered as a measure of the safety of a procedure, whereas the cure rate reflects the efficiency of a particular surgical method.
TABLE 38-5 Overview of Contemporary Neurosurgical Treatment Options for Chronic Subdural Hematoma in the Elderly
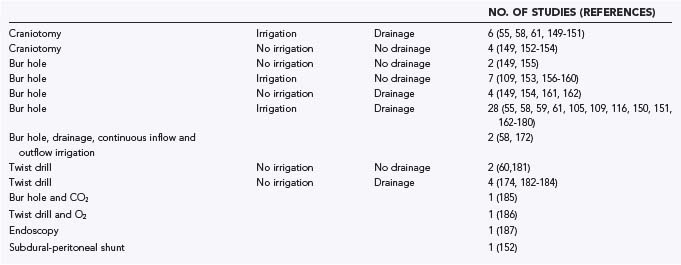
As with other analyses on topics such as unruptured aneurysms189 or Parkinson’s disease,148 there are several methodologic problems inherent in such types of systematic review. Publication bias is related to the arbitrary selection of language and by primarily selecting manuscripts from a database such as MEDLINE, which yields papers that are more likely to report on positive results. Furthermore, the lack of any studies providing class I evidence and the paucity of studies providing class II evidence preclude the application of statistical procedures for meta-analysis. In the following section we briefly summarize the results of our analysis and provide “the best available external clinical evidence from systematic research.”147
Surgical Approach
The principal techniques used for the treatment of CSDH at present are TDC (up to a diameter of 5 mm),60,174,181–184,186 BHC or enlarged BHC (between 5 and 30 mm in diameter),* and craniotomy (larger than 30 mm in diameter).55,58,61,147,150–154 The term craniostomy is an artificial descriptive term that was established in the past. It underlines the miniaturization of the surgical approach. Additional measures such as intraoperative irrigation and postoperative drainage increase the number of neurosurgical treatment options. During the search for reduction of recurrence rates it was also suggested that the hematoma cavity be filled with 100% oxygen186 or carbon dioxide.185 In single studies, subdural-peritoneal shunting was used.152 Recurrent hematomas were successfully treated via BHC under endoscopic guidance.187 The variety of strategies reflects the dilemma of the search for the optimal procedure (see Table 38-5).
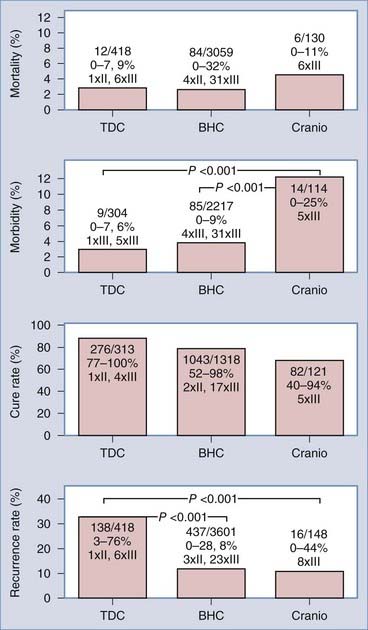
No significant difference in mortality rates was found with the three principal techniques (Fig. 38-7). Craniotomy was burdened with the highest morbidity rate (12.3%), but it is conceivable that selection bias may have resulted in a somewhat distorted picture of the morbidity of craniotomy inasmuch as series involving primary craniotomy in general included patients who were presumed to be at higher risk for recurrence or who had less satisfactory performance preoperatively.150–153
The three principal techniques also do not differ significantly in cure rates. BHC and craniotomy are the most efficacious techniques and provide the lowest recurrence rates. Thus, it can be concluded that BHC shares the advantages of TDC, a high cure rate and safety, and the advantage of primary craniotomy, high efficiency. These results were supported by a recently published series that directly compared BHC and TDC in 62 patients. The authors demonstrated the superiority of BHC in terms of postoperative computed tomographic findings and the number of recurrences (7% versus 64%).190 TDC and BHC can be performed under local anesthesia, but a cooperative patient is mandatory. Implementation of rigid fixation of the head during craniotomy for recurrent CSDH strongly favors performing the operation under general anesthesia. The number of bur holes needed to drain CSDH successfully is still a matter of debate. Although many CSDHs appear to be loculated on CT, it was demonstrated with fiberoptic endoscopy that communication exists in the majority of cases.187,191 A recent publication based on retrospective data, however, found a negative correlation between the number of bur holes and the rate of recurrence.192
Irrigation
The purpose of irrigation is to remove the hematoma completely or at least dilute its contents. Only a few articles allowed comparison of the results of patients treated with irrigation or without irrigation. Irrigation in conjunction with bur-hole techniques did not demonstrate a significant reduction in the recurrence rate.162,169,177 In TDC, however, irrigation resulted in a significant decrease in recurrences.181 Although often stated, the rate of infection was not increased with the use of irrigation.5
Two papers reported on the use of continuous inflow and outflow irrigation after surgical decompression of CSDH. Both described fewer recurrences with postoperative irrigation; however, a significant difference was seen in one publication only because of the small number of recurrences in the other paper.58,172
Drainage
The most convincing data on the treatment of CSDH refer to the use of a drain after BHC. Four of six studies with class II evidence were concerned with the question of whether drainage systems should be used; they highlighted different aspects of drainage systems. The use of a drain after BHC significantly reduced the number of recurrences.178 One series reported faster recovery in patients treated with a closed system drainage, but ultimate outcome per se was not affected.158 A frontal position of the drain was correlated with a reduced rate of recurrence.171 Postoperative drainage volume less than 200 mL was associated with a higher recurrence rate, thus supporting the concept of a beneficial effect of total or subtotal evacuation of the hematoma.168 One recently published paper on a consecutive series of 500 patients with subacute subdural hematoma or CSDH confirmed that recurrences were avoided when drains were used.193
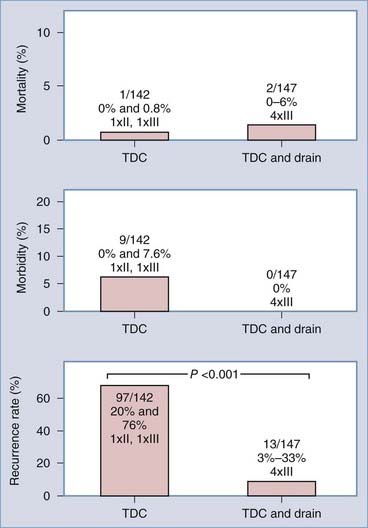
With TDC the situation is less clear. Because we found no data on direct comparison of TDC alone and TDC with drainage, we pooled the available data from six publications.60,174,182–184,194 There was a significant reduction in recurrences with closed system drainage (Fig. 38-8).
Treatment of Recurrences
A major concern is determining the best possible treatment of recurrence and how one can reduce the risk for recurrence, perhaps by altering the primary treatment used. Detailed information on recurrence after TDC was available from seven publications.60,174,182–184,186,194 Repeating TDC once or twice was successful in 106 of 151 recurrences (70%). In 35 patients, a bur-hole procedure was chosen for reoperation (23%), and in 10 patients (7%) a craniotomy was the most useful procedure. For BHC, data on recurrences from 20 publications were available.* A total of 190 of 229 patients (83%) were successfully treated by the same procedure as previously, and 32 patients (14%) underwent craniotomy. Three patients died (1.3%). It seems that BHC is more effective than TDC in treating recurrent hematoma. Craniotomy is considered the treatment of last choice. Occasionally, interventional techniques were used in which the blood supply of the outer membrane was reduced by embolization of the middle meningeal artery.83
Pragmatic Recommendations for Surgery
In our personal experience with more than 500 treated cases of CSDH (JKK; RW), one bur hole most frequently sufficed to treat CSDH in the majority of cases (see Fig. 38-5A to D). An alternative is to place two bur holes just a few centimeters apart. We use a ventricular catheter to irrigate the cavity with warm saline in all directions until clear fluid exits. Several strategies can be used to minimize the amount of intracranial air after surgery. Some recommend that the patient’s head be fixed so that the bur hole is located at the highest point and that the cavity be filled with saline before closing the wound. Others work with a drain located frontally and another located occipitally. The cavity is filled through the occipital drain after closing the wound tightly until no more air leaves the cavity through the frontal drain. After this maneuver the occipital drain can be removed, and the frontal drain is left in place for at least 3 days. While removing the drain, special care must be taken to avoid entrance of air into the cavity. In bilateral hematomas, it might be useful to connect both drains to only one reservoir to avoid a major pressure gradient between both sides, which can lead to a devastating midline shift. Continuous large drainage volumes do not argue against removing a drain. Theoretical considerations show that 7% of hematoma fluid is renewed every day196 from an average volume of 90 mL.110 Drainage volumes of greater than 50 mL/day are seen frequently, which argues for tearing of the inner membrane to allow CSF to escape via the drain.
Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31:73.
Benzel EC, Bridges RMJr, Hadden TA, et al. The single burr hole technique for the evacuation of non-acute subdural hematomas. J Trauma. 1994;36:190.
Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:399.
Friede RL, Schachenmayr W. The origin of subdural neomembranes II. Fine structure of neomembranes. Am J Pathol. 1978;92:69.
Glover D, Labadie EL. Physiopathogenesis of subdural hematomas. Part 2: Inhibition of growth of experimental hematomas with dexamethasone. J Neurosurg. 1976;45:393.
Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993;33:67.
Heckmann JG, Ganslandt O. Images in clinical medicine. The Mount Fuji sign. N Engl J Med. 2004;350:1881.
Hohenstein A, Erber R, Schilling L, et al. Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and -2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma. 2005;22:518.
Hosoda K, Tamaki N, Masumura M, et al. Magnetic resonance images of chronic subdural hematomas. J Neurosurg. 1987;67:677.
Ito H, Yamamoto S, Saito K, et al. Quantitative estimation of hemorrhage in chronic subdural hematoma using the 51Cr erythrocyte labeling method. J Neurosurg. 1987;66:862.
Lee KS, Bae WK, Doh JW, et al. Origin of chronic subdural haematoma and relation to traumatic subdural lesions. Brain Inj. 1998;12:901.
Loew F, Kivelitz R. Chronic subdural hematomas. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology, vol 24. Amsterdam: North Holland Publishing Company; 1976:297. part 2
Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637.
Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256.
Rubin G, Rappaport ZH. Epilepsy in chronic subdural haematoma. Acta Neurochir (Wien). 1993;123:39.
Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47:418.
Stroobandt G, Fransen P, Thauvoy C, et al. Pathogenetic factors in chronic subdural haematoma and causes of recurrence after drainage. Acta Neurochir (Wien). 1995;137:6.
Tabaddor K, Shulmon K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46:220.
Tokmak M, Iplikcioglu AC, Bek S, et al. The role of exudation in chronic subdural hematomas. J Neurosurg. 2007;107:290.
Virchow R. Das Hämatom der Dura mater. Verh Phys Med Ges Würzburg. 1857;7:134.
Wakai S, Hashimoto K, Watanabe N, et al. Efficacy of closed-system drainage in treating chronic subdural hematoma: a prospective comparative study. Neurosurgery. 1990;26:771.
Weigel R, Hohenstein A, Schlickum L, et al. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma probably by an angiogenic mechanism. Neurosurgery. 2007;61:788.
Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74:937.
1 Trotter W. Chronic subdural haemorrhage of traumatic origin, and its relation to pachymeningitis haemorrhagica interna. Br J Surg. 1914;2:271.
2 McKissock W, Richardson A, Bloom WH. Subdural hematoma. A review of 389 cases. Lancet. 1960;1:1365.
3 Adams JH. Brain damage in fatal non-missile head injury. In: Vinken PJ, Bruyn GW, Klawans HL, editors. Handbook of Clinical Neurology, Vol 57. Amsterdam: Elsevier Science; 1990:43.
4 McCormick WF. Pathology of closed head injury. In: Wilkins WH, Rengachary SS, editors. Neurosurgery. New York: McGraw Hill; 1985:1544.
5 Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74:937.
6 Bucy PC. Historical evolution of topographic diagnosis in neurosurgery. In: Carrea R, editor. Neurological Surgery with Emphasis on Non-invasive Methods of Diagnosis and Treatment. Amsterdam-Oxford: Excerpta Medica; 1978:3.
7 Broca P. Sur les trépanations préhistoriques. Bull Soc Antrop. 1876;2:236.
8 Horsley V. Trephining in the Neolithic period. J R Anthrop Inst. 1888;17:100.
9 Needham J. Chinese-Western cultural and scientific contacts as recorded by chinese historians, Vol 1. London, New York: Cambridge University Press. 1954.
10 Forgue E. Histoire de la chirurgie. In: Laignel-Lavastine M, editor. Histoire Génerale de la Médicine, de la Pharmacie, de l’Art Dentaire et de l’Art Vétérinaire, Vol 2. Paris: Albin Michel; 1938:350.
11 Guiard E. La trépanation cranienne chez les néolithiques et chez les primitifs modernes. In: Faculté de Médecine et de Pharmacie. Bordéaux: Université de Bordéaux; 1930:132.
12 Thompson CJS. The evolution and development of surgical instruments. Br J Surg. 1938;25:726.
13 Müller RFG. Schädeleröffnung nach indischen Sagen. Centaurus. 1959;6:68.
14 Hoessly GF. Intracranial hemorrhage in the seventeenth century. A reappraisal of Johann Jacob Wepfer’s contribution regarding subdural hematoma. J Neurosurg. 1965;24:493.
15 Wepfer JJ. Observationes anatomicae ex cadaveribus eorum, quos sustulit apoplexia cum exercitatione de eius loco affecto. Schaffhausen: Waldkirch Alexandri Riedingii; 1675.
16 Virchow R. Morgagni and anatomical thought. Br Med J. 1894;1:725.
17 Morgagni GB. De sedibus et causis morborum: E. Lovanii. 1767, Vol Liber 1.
18 Houssard M. Observation d’un kyste considerable, developé dans la cavité de l’arachnoide chez un subject qui succumbé avec les symptomes d’une apoplexie sanguine. Biblioth Med. 1817;55:67.
19 Abercrombie J. Pathological and practical researches on diseases of the brain and the spinal cord, 4th ed. London: Maclachlan, Stewart, and Company; 1845.
20 Virchow R. Das Hämatom der Dura mater. Verh Phys Med Ges Würzburg. 1857;7:134.
21 Fürstner C. Zur Genese und Symptomatologie der Pachymeningitis haemorrhagica. Arch Pathol Anat. 1878;8:1.
22 Probst M. Über Pachymeningitis cervicalis hypertrophica und über Pachymeningitis interna haemorrhagica bei chronisch fortschreitenden Verblödungsprocessen in der Jugend. Arch Psych. 1903;36:114.
23 Schuberg W. Das Haematoma durae matris bei Erwachsenen. Arch path Anat. 1859;16:464.
24 Osler W. The Principles and Practice of Medicine, 4th ed. New York: D. Appleton and Company; 1901.
25 Hulke JW. Severe blow on the right temple, followed by right hemiplegia and coma, and then by spastic rigidity of the left arm; trephining; evacuation of inflammatory fluid by incision through dura mater; quick disappearance of cerebral symptoms; complete recovery. Lancet. 1883;2:814.
26 Putnam TJ, Cushing H. Chronic subdural hematoma. Arch Surg. 1925;11:329.
27 Osler W. The Principles and Practice of Medicine, 1st ed. New York: D. Appleton and Company; 1892.
28 v. Saar H. Zur Symptomatologie und Therapie der Pachymeningitis haemorrhagica interna. Deutsch Z Chir. 1918;145:398.
29 Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg. 1918;68:5.
30 Moniz E. L’encephalographie artérielle, son importance dans la localisation des tumeurs cérébrales. Rev Neurol. 1927;34:72.
31 Loew F, Kivelitz R. Chronic subdural hematomas. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology, vol 24. Amsterdam: North Holland Publishing Company; 1976:297. part 2
32 Cameron MM. Chronic subdural haematoma: a review of 114 cases. J Neurol Neurosurg Psychiatry. 1978;41:834.
33 Robinson RG. The treatment of subacute and chronic subdural hematomas. Br Med J. 1955;1:21.
34 Svien HJ, Gelety JE. On the surgical management of encapsulated subdural hematoma. A comparison of the results of membranectomy and simple evacuation. J Neurosurg. 1964;21:172.
35 Holmes J. Intracranial hypotension associated with subdural hematoma. Br Med J. 1953;1:1363.
36 Krebs E, Puech P, Brunhes J. Collapsus des ventricules cérébreaux dans les traumatismes craniens. Rev Neurol. 1937;68:831.
37 Lalonde AA, Gardner WJ. Chronic subdural hematoma. Expansion of compressed cerebral hemispheres and relief of hypotension by spinal injection of physiologic saline solution. N Eng J Med. 1948;239:493.
38 Faulhauer K, Herrmann HD, Loew F. Operative treatment of extremely large bilateral subdural effusions in infancy. Acta Neurochir (Wien). 1973;28:179.
39 Pennybacker J, Gye R, Sato F. The unexpanded brain in chronic subdural hematoma. Excerpta Med I C S. 1963;60:112.
40 Tabaddor K, Shulmon K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46:220.
41 Yashon D, White RJ, Bryk JP, et al. Simplified supplementary treatment of chronic subdural fluid collections. Neurochirurgia (Stuttg). 1971;14:8.
42 Friede RL, Schachenmayr W. The origin of subdural neomembranes II. Fine structure of neomembranes. Am J Pathol. 1978;92:69.
43 Sato S, Suzuki J. Ultrastructural observations of the capsule of chronic subdural hematoma in various clinical stages. J Neurosurg. 1975;43:569.
44 Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. 1983;59:298.
45 Dandy WE. Chronic subdural hydroma and serous meningitis (pachymeningitis serosa; localizes external hydrocephalus). In: Lewis D, editor. Practise of Surgery. Hagerstown, MD: WF Prior Co; 1932:306.
46 Kuzma BB, Goodman JM. Differentiating external hydrocephalus from chronic subdural hematoma. Surg Neurol. 1998;50:86.
47 Mori K, Maeda M. Risk factors for the occurrence of chronic subdural haematomas after neurosurgical procedures. Acta Neurochir (Wien). 2003;145:533.
48 Lee KS, Bae WK, Doh JW, et al. Origin of chronic subdural haematoma and relation to traumatic subdural lesions. Brain Inj. 1998;12:901.
49 Fogelholm R, Waltimo O. Epidemiology of chronic subdural hematoma. Acta Neurochir. 1975;32:247.
50 Vanderfield GK, Berry G, Dan NG, et al. Experience with chronic subdural haematomas in New South Wales. Aust N Z J Surg. 1986;56:577.
51 Kudo H, Kuwamura K, Izawa I, et al. Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992;32:207.
52 Adhiyaman V, Asghar M, Ganeshram KN, et al. Chronic subdural haematoma in the elderly. Postgrad Med J. 2002;78:71.
53 Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:399.
54 Lee KS. Natural history of chronic subdural haematoma. Brain Inj. 2004;18:351.
55 Ernestus RI, Beldzinski P, Lanfermann H, et al. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48:220.
56 Bouzarth WF, Bhatti TH. Subdural hematomata in the elderly. J Am Med Womens Assoc. 1968;23:631.
57 Tomita T, Ischida Y, Kagawa M. Clinical study on chronic subdural hematoma in aged patients. Clin Neurol. 1968;8:204.
58 Hennig R, Kloster R. Burr hole evacuation of chronic subdural haematomas followed by continuous inflow and outflow irrigation. Acta Neurochir (Wien). 1999;141:171.
59 Krupp WF, Jans PJ. Treatment of chronic subdural haematoma with burr-hole craniostomy and closed drainage. Br J Neurosurg. 1995;9:619.
60 Reinges MH, Hasselberg I, Rohde V, et al. Prospective analysis of bedside percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. J Neurol Neurosurg Psychiatry. 2000;69:40.
61 van Havenbergh T, van Calenbergh F, Goffin J, et al. Outcome of chronic subdural haematoma: analysis of prognostic factors. Br J Neurosurg. 1996;10:35.
62 Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47:418.
63 Kotwica Z, Brzeinski J. Epilepsy in chronic subdural haematoma. Acta Neurochir (Wien). 1991;113:118.
64 Mori K, Mitsuoka H, Cho K, et al. Rate constant of gadolinium (Gd)-DTPA transfer into chronic subdural hematomas. Neurol Res. 1996;18:126.
65 Mori K, Maeda M. Risk factors for the occurrence of chronic subdural hematomas after neurosurgical procedures. Acta Neurochir (Wien). 2003;145:533.
66 Miele VJ, Bailes JE, Cantu RC, et al. Subdural hematomas in boxing: the spectrum of consequences. Neurosurg Focus. 2006;21:E10.
67 Turkoglu E, Serbes G, Sanli M, et al. Chronic subdural hematoma in capoeira sport. Turk Neurosurg. 2008;18:39.
68 Prabhu VC, Bailes JE. Chronic subdural hematoma complicating arachnoid cyst secondary to soccer-related head injury: case report. Neurosurgery. 2002;50:195.
69 Uzura M, Taguchi Y, Matsuzawa M, et al. Chronic subdural haematoma after snowboard head injury. Br J Sports Med. 2003;37:82.
70 Kopitnik TAJr, de Andrade RJr, Gold MA, et al. Pressure changes within a chronic subdural hematoma during hemodialysis. Surg Neurol. 1989;32:289.
71 Aronson SM, Okazaki H. A study of some factors modifying response of cerebral tissue to subdural hematoma. J Neurosurg. 1963;20:89.
72 Bae SH, Vates TSJr, Kenton EJ. Generalized chorea associated with chronic subdural hematomas. Ann Neurol. 1980;8:449.
73 Pechlivanis I, Andrich J, Scholz M, et al. Chronic subdural haematoma in patients with Huntington’s disease. Br J Neurosurg. 2006;20:327.
74 Illingworth RD. Subdural hematoma after the treatment of chronic hydrocephalus by ventriculocaval shunts. J Neurol Neurosurg Psychiatry. 1970;33:95.
75 Weiner HL, Constantini S, Cohen H. Current treatment of normal-pressure hydrocephalus: Comparison of flow-regulated and different-pressure shunt valves. Neurosurgery. 1995;37:877.
76 Lavie F, Herve D, Le Ber I, et al. [Bilateral intracranial subdural hematoma following lumbar puncture: report of a case.]. Rev Neurol (Paris). 1998;154:703.
77 Kunz U, Panning B, Stolke D. [Chronic subdural hematoma following spinal anesthesia.]. Reg Anaesth. 1989;12:34.
78 Mayser P. Die Rolle der Koagulopathien beim chronisch subduralen Hämatom des Säuglings. Mschr Kinderheilk. 1962;110:208.
79 Sato M, Endo Y, Takahagi S, et al. [Chronic subdural hematoma with bleeding tendency; clinical analysis of 11 surgical cases.]. No Shinkei Geka. 1995;23:49.
80 Wintzen AR. The clinical course of subdural haematoma. A retrospective study of aetiological, chronological and pathological features in 212 patients and a proposed classification. Brain. 1980;103:855.
81 Jourdan E, Dombret H, Glaisner S, et al. Unexpected high incidence of intracranial subdural haematoma during intensive chemotherapy for acute myeloid leukaemia with a monoblastic component. Br J Haematol. 1995;89:527.
82 Talmant JC, Collet M, Sartre R, et al. [Central nervous system hemorrhages associated with acute leukemia. (Apropos of a rare localization revealing the disease).]. Sem Hop. 1967;43:3022.
83 Mandai S, Sakurai M, Matsumoto Y. Middle meningeal artery embolization for refractory chronic subdural hematoma. Case report. J Neurosurg. 2000;93:686.
84 Kremiansky J. Über die Pachymeningitis interna haemorrhagica bei Menschen und Hunden. Arch Pathol Anat. 1868;42:129.
85 Giuffre R, Palma E, Liccardo G, et al. Sex steroid hormones in the pathogenesis of chronic subdural haematoma. Neurochirurgia (Stuttg). 1992;35:103.
86 Alexander BH, Rivara FP, Wolf ME. The cost and frequency of hospitalization for fall-related injuries in older adults. Am J Public Health. 1992;82:1020.
87 Kannel WB, Abbott RD, Savage DD, et al. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306:1018.
88 Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897.
89 Markwalder TM. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637.
90 Maeshima S, Okumura Y, Nakai K, et al. Gerstmann’s syndrome associated with chronic subdural haematoma: a case report. Brain Inj. 1998;12:697.
91 Wiest RG, Burgunder JM, Krauss JK. Chronic subdural haematomas and Parkinsonian syndromes. Acta Neurochir (Wien). 1999;141:753.
92 Yokoyama K, Matsuki M, Shimano H, et al. Diffusion tensor imaging in chronic subdural hematoma: correlation between clinical signs and fractional anisotropy in the pyramidal tract. AJNR Am J Neuroradiol. 2008;29:1159.
93 Kuwabara H. Regional cerebral blood flow and metabolism in chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:499.
94 Tanaka A, Nakayama Y, Yoshinaga S. Cerebral blood flow and intracranial pressure in chronic subdural hematomas. Surg Neurol. 1997;47:346.
95 Tanaka A, Yoshinaga S, Kimura M. Xenon-enhanced computed tomographic measurement of cerebral blood flow in patients with chronic subdural hematomas. Neurosurgery. 1990;27:554.
96 Potter JF, Fruin AH. Chronic subdural hematoma—the “great imitator.”. Geriatrics. 1977;32:61.
97 Kotwica Z, Brzezinski J. Chronic subdural hematomas presenting as cerebral stroke. Zentralbl Neurochir. 1988;49:54.
98 Welsh JE, Tyson GW, Winn HR, et al. Chronic subdural hematoma presenting as transient neurologic deficits. Stroke. 1979;10:564.
99 Hilt DC, Alexander GE. Jacksonian somatosensory seizures as the sole manifestation of chronic subdural hematoma. Arch Neurol. 1982;39:786.
100 Ratilal B, Costa J, Sampaio C. Anticonvulsants for preventing seizures in patients with chronic subdural haematoma. Cochrane Database Syst Rev. 2005;3:CD004893.
101 Rubin G, Rappaport ZH. Epilepsy in chronic subdural haematoma. Acta Neurochir (Wien). 1993;123:39.
102 Knopman DS, Petersen RC, Cha RH, et al. Incidence and causes of nondegenerative nonvascular dementia: a population-based study. Arch Neurol. 2006;63:218.
103 Bender MB, Christoff N. Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31:73.
104 Markwalder TM, Reulen HJ. Influence of neomembranous organisation, cortical expansion and subdural pressure on the post-operative course of chronic subdural haematoma—an analysis of 201 cases. Acta Neurochir (Wien). 1986;79:100.
105 Richter HP, Klein HJ, Schafer M. Chronic subdural haematomas treated by enlarged burr-hole craniotomy and closed system drainage. Retrospective study of 120 patients. Acta Neurochir (Wien). 1984;71:179.
106 Weigel R, Krauss JK, Schmiedek P. Concepts of neurosurgical management of chronic subdural haematoma: historical perspectives. Br J Neurosurg. 2004;18:8.
107 Agunloye AM, Adeyinka AO, Obajimi MO, et al. Computerised tomography of intracranial subdural haematoma in Ibadan. Afr J Med Med Sci. 2003;32:235.
108 Dakurah TK, Iddrissu M, Wepeba G, et al. Chronic subdural haematoma: review of 96 cases attending the Korle Bu Teaching Hospital, Accra. West Afr J Med. 2005;24:283.
109 Tsutsumi K, Maeda K, Iijima A, et al. The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. J Neurosurg. 1997;87:870.
110 Kawakami Y, Chikama M, Tamiya T, et al. Coagulation and fibrinolysis in chronic subdural hematoma. Neurosurgery. 1989;25:25.
111 Nomura S, Kashiwagi S, Fujisawa H, et al. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994;81:910.
112 Naganuma H, Fukamachi A, Kawakami M, et al. Spontaneous resolution of chronic subdural hematomas. Neurosurgery. 1986;19:794.
113 Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256.
114 Tokmak M, Iplikcioglu AC, Bek S, et al. The role of exudation in chronic subdural hematomas. J Neurosurg. 2007;107:290.
115 Amirjamshidi A, Eftekhar B, Abouzari M, et al. The relationship between Glasgow Coma/Outcome scores and abnormal CT scan findings in chronic subdural hematoma. Clin Neurol Neurosurg. 2007;109:152.
116 Markwalder TM, Steinsiepe KF, Rohner M, et al. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55:390.
117 Amirjamshidi A, Abouzari M, Eftekhar B, et al. Outcomes and recurrence rates in chronic subdural haematoma. Br J Neurosurg. 2007;21:272.
118 Hirsh LF. Intracranial air following subdural haematoma drainage with delayed recovery. Neurochirurgia (Stuttg). 1980;23:55.
119 Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791.
120 Sharma BS, Tewari MK, Khosla VK, et al. Tension pneumocephalus following evacuation of chronic subdural haematoma. Br J Neurosurg. 1989;3:381.
121 Heckmann JG, Ganslandt O. Images in clinical medicine. The Mount Fuji sign. N Engl J Med. 2004;350:1881.
122 Sadeghian H. Mount Fuji sign in tension pneumocephalus. Arch Neurol. 2000;57:1366.
123 Hosoda K, Tamaki N, Masumura M, et al. Magnetic resonance images of chronic subdural hematomas. J Neurosurg. 1987;67:677.
124 Sipponen JT, Sepponen RE, Sivula A. Chronic subdural hematoma: demonstration by magnetic resonance. Radiology. 1984;150:79.
125 Ishikawa T, Kawamura S, Hadeishi H, et al. Cerebral blood flow and oxygen metabolism in hemiparetic patients with chronic subdural hematoma. Quantitative evaluation using positron emission tomography. Surg Neurol. 1995;43:130.
126 Ogasawara K, Ogawa A, Okuguchi T, et al. Postoperative hyperperfusion syndrome in elderly patients with chronic subdural hematoma. Surg Neurol. 2000;54:155.
127 Ambrosetto C. Osservazioni sul trattamento dell’ ematoma subdurale cronico posttraumatico. Sist Nerv. 1958;10:435.
128 Bender MB. Recovery from subdural hematoma without surgery. J Mt Sinai Hosp. 1960;27:52.
129 Glover D, Labadie EL. Physiopathogenesis of subdural hematomas. Part 2: Inhibition of growth of experimental hematomas with dexamethasone. J Neurosurg. 1976;45:393.
130 Hohenstein A, Erber R, Schilling L, et al. Increased mRNA Expression of VEGF within the hematoma and imbalance of angiopoietin-1 and -2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma. 2005;22:518.
131 Suzuki J, Takaku A. Nonsurgical treatment of chronic subdural hematoma. J Neurosurg. 1970;33:548.
132 Gjerris F, Schmidt K. Chronic subdural hematoma. Surgery or mannitol treatment. J Neurosurg. 1974;40:639.
133 Weigel R, Hohenstein A, Schlickum L, et al. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma probably by an angiogenic mechanism. Neurosurgery. 2007;61:788.
134 Ohno K, Maehara T, Ichimura K, et al. Low incidence of seizures in patients with chronic subdural haematoma. J Neurol Neurosurg Psychiatry. 1993;56:1231.
135 Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11.
136 Asikainen I, Kaste M, Sarna S. Predicting late outcome for patients with traumatic brain injury referred to a rehabilitation programme: a study of 508 Finnish patients 5 years or more after injury. Brain Inj. 1998;12:95.
137 Nakajima H, Yasui T, Nishikawa M, et al. The role of postoperative patient posture in the recurrence of chronic subdural hematoma: a prospective randomized trial. Surg Neurol. 2002;58:385.
138 Abouzari M, Rashidi A, Rezaii J, et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61:794.
139 Ogasawara K, Koshu K, Yoshimoto T, et al. Transient hyperemia immediately after rapid decompression of chronic subdural hematoma. Neurosurgery. 1999;45:484.
140 d’Avella D, De Blasi F, Rotilio A, et al. Intracerebral hematoma following evacuation of chronic subdural hematomas. Report of two cases. J Neurosurg. 1986;65:710.
141 Modesti LM, Hodge CJ, Barnwell ML. Intracerebral hematoma after evacuation of chronic extracerebral fluid collections. Neurosurgery. 1982;10:689.
142 Nakahara N, Masuzawa T, Kuno K, et al. Intracerebral hemorrhage immediately following surgical treatment of chronic subdural hematoma. Report of two cases. Neurol Med Chir (Tokyo). 1984;24:27.
143 Ovul I, Oner K. Intracerebral hematoma after evacuation of chronic subdural hematoma. Neurochirurgia (Stuttg). 1988;31:160.
144 Ramamurthi B, Ganapathi K, Ramamurthi R. Intracerebral hematoma following evacuation of chronic subdural hematoma. Neurosurg Rev. 1989;12(Suppl 1):225.
145 Turtas S, Orunesu G. Intracerebral hematoma following removal of chronic subdural hematoma. Report of two cases. Zentralbl Neurochir. 1989;50:115.
146 Torihashi K, Sudamason N, Yoshito K, et al. Independent predictions for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63:1125.
147 Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71.
148 Hallett M, Litvan I. Evaluation of surgery for Parkinson’s disease: a report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. The Task Force on Surgery for Parkinson’s Disease. Neurology. 1999;53:1910.
149 Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993;33:67.
150 Piotrowski WP, Krombholz-Reindl MA. [Surgical outcome in chronic subdural hematoma.]. Unfallchirurgie. 1996;22:110.
151 Tanikawa M, Mase M, Yamada K, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien). 2001;143:613.
152 Probst C. Peritoneal drainage of chronic subdural hematomas in older patients. J Neurosurg. 1988;68:908.
153 Robinson RG. Chronic subdural hematoma: surgical management in 133 patients. J Neurosurg. 1984;61:263.
154 Schulz W, Saballus R, Flugel R, et al. [Chronic subdural hematoma. A comparison of bore hole trepanation and craniotomy.]. Zentralbl Neurochir. 1988;49:280.
155 Patrick D, Gates PC. Chronic subdural haematoma in the elderly. Age Ageing. 1984;13:367.
156 Benzel EC, Bridges RMJr, Hadden TA, et al. The single burr hole technique for the evacuation of non-acute subdural hematomas. J Trauma. 1994;36:190.
157 Iwabuchi T, Sekiya T, Suzuki S. Neurosurgical aspects of the parietal boss in patients with chronic subdural hematomas. Neurosurgery. 1981;9:531.
158 Markwalder TM, Seiler RW. Chronic subdural hematomas: to drain or not to drain? Neurosurgery. 1985;16:185.
159 Ohaegbulam SC. Surgically treated traumatic subacute and chronic subdural haematomas: a review of 132 cases. Injury. 1981;13:23.
160 Yoshimoto Y, Kwak S. Frontal small craniostomy and irrigation for treatment of chronic subdural haematoma. Br J Neurosurg. 1997;11:150.
161 Aydin IH, Aydin Y, Akdemir D, et al. Chronic subdural hematomas (clinical analysis). Zentralbl Neurochir. 1987;48:308.
162 Kuroki T, Katsume M, Harada N, et al. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien). 2001;143:1041.
163 Choudhury AR. Avoidable factors that contribute to complications in the surgical treatment of chronic subdural haematoma. Acta Neurochir (Wien). 1994;129:15.
164 Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991;5:467.
165 Grisoli F, Graziani N, Peragut JC, et al. Perioperative lumbar injection of Ringer’s lactate solution in chronic subdural hematomas: a series of 100 cases. Neurosurgery. 1988;23:616.
166 Harders A, Eggert HR, Weigel K. [Treatment of chronic subdural haematoma by closed external drainage.]. Neurochirurgia (Stuttg). 1982;25:147.
167 Kotwica Z, Brzezinski J. Chronic subdural haematoma treated by burr holes and closed system drainage: personal experience in 131 patients. Br J Neurosurg. 1991;5:461.
168 Kwon TH, Park YK, Lim DJ, et al. Chronic subdural hematoma: evaluation of the clinical significance of postoperative drainage volume. J Neurosurg. 2000;93:796.
169 Matsumoto K, Akagi K, Abekura M, et al. Recurrence factors for chronic subdural hematomas after burr-hole craniostomy and closed system drainage. Neurol Res. 1999;21:277.
170 Mellergard P, Wisten O. Operations and re-operations for chronic subdural haematomas during a 25-year period in a well-defined population. Acta Neurochir (Wien). 1996;138:708.
171 Nakaguchi H, Tanishima T, Yoshimasu N. Relationship between drainage catheter location and postoperative recurrence of chronic subdural hematoma after burr-hole irrigation and closed-system drainage. J Neurosurg. 2000;93:791.
172 Ram Z, Hadani M, Sahar A, et al. Continuous irrigation-drainage of the subdural space for the treatment of chronic subdural haematoma. A prospective clinical trial. Acta Neurochir (Wien). 1993;120:40.
173 Rohde V, Graf G, Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg Rev. 2001;69:40.
174 Smely C, Madlinger A, Scheremet R. Chronic subdural haematoma—a comparison of two different treatment modalities. Acta Neurochir (Wien). 1997;139:818.
175 Spallone A, Giuffre R, Gagliardi FM, et al. Chronic subdural hematoma in extremely aged patients. Eur Neurol. 1989;29:18.
176 Stroobandt G, Fransen P, Thauvoy C, et al. Pathogenetic factors in chronic subdural haematoma and causes of recurrence after drainage. Acta Neurochir (Wien). 1995;137:6.
177 Suzuki K, Sugita K, Akai T, et al. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998;50:231.
178 Wakai S, Hashimoto K, Watanabe N, et al. Efficacy of closed-system drainage in treating chronic subdural hematoma: a prospective comparative study. Neurosurgery. 1990;26:771.
179 Weir BK. Results of burr hole and open or closed suction drainage for chronic subdural hematomas in adults. Can J Neurol Sci. 1983;10:22.
180 Weisse A, Berney J. Chronic subdural haematomas. Results of a closed drainage method in adults. Acta Neurochir (Wien). 1994;127:37.
181 Aoki N. Subdural tapping and irrigation for the treatment of chronic subdural hematoma in adults. Neurosurgery. 1984;14:545.
182 Camel M, Grubb RLJr. Treatment of chronic subdural hematoma by twist-drill craniotomy with continuous catheter drainage. J Neurosurg. 1986;65:183.
183 Carlton CK, Saunders RL. Twist drill craniostomy and closed system drainage of chronic and subacute subdural hematomas. Neurosurgery. 1983;13:153.
184 Rychlicki F, Recchioni MA, Burchianti M, et al. Percutaneous twist-drill craniostomy for the treatment of chronic subdural haematoma. Acta Neurochir (Wien). 1991;113:38.
185 Kitakami A, Ogawa A, Hakozaki S, et al. Carbon dioxide gas replacement of chronic subdural hematoma using single burr-hole irrigation. Surg Neurol. 1995;43:574.
186 Aoki N. A new therapeutic method for chronic subdural hematoma in adults: replacement of the hematoma with oxygen via percutaneous subdural tapping. Surg Neurol. 1992;38:253.
187 Hellwig D, Kuhn TJ, Bauer BL, et al. Endoscopic treatment of septated chronic subdural hematoma. Surg Neurol. 1996;45:272.
188 Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480.
189 Brennan JW, Schwartz ML. Unruptured intracranial aneurysms: appraisal of the literature and suggested recommendations for surgery, using evidence-based medicine criteria. Neurosurgery. 2000;47:1359.
190 Williams GR, Baskaya MK, Menendez J, et al. Burr-hole versus twist-drill drainage for the evacuation of chronic subdural haematoma: a comparison of clinical results. J Clin Neurosci. 2001;8:551.
191 Hellwig D, Heinze S, Riegel T, et al. Neuroendoscopic treatment of loculated chronic subdural hematoma. Neurosurg Clin N Am. 2000;11:525.
192 Taussky P, Fandino J, Landolt H. Number of burr holes as independent predictor of postoperative recurrence in chronic subdural haematoma. Br J Neurosurg. 2008;22:279.
193 Lind CR, Lind CJ, Mee EW. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J Neurosurg. 2003;99:44.
194 Aoki N. Percutaneous subdural tapping for the treatment of chronic subdural haematoma in adults. Neurol Res. 1987;9:19.
195 Kuroki T, Matsumoto M, Kushida T, et al. [Nontraumatic subdural hematoma secondary to dural metastasis of lung cancer: case report and review of the literature.]. No Shinkei Geka. 1994;22:857.
196 Ito H, Yamamoto S, Saito K, et al. Quantitative estimation of hemorrhage in chronic subdural hematoma using the 51Cr erythrocyte labeling method. J Neurosurg. 1987;66:862.