CHAPTER 55 Mediastinum
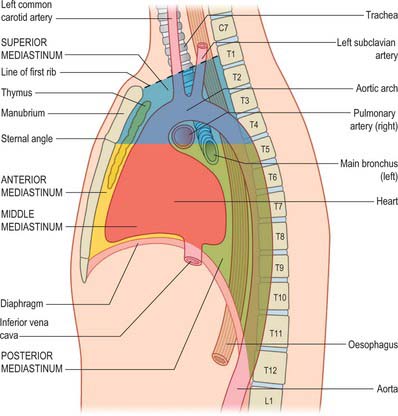
Strictly speaking, the mediastinum is the partition between the lungs and includes the mediastinal pleura. However, the term is commonly applied to the region between the two pleural sacs bounded anteriorly by the sternum and posteriorly by the thoracic vertebral column and extending vertically from the thoracic inlet to the diaphragm. For descriptive purposes, this region is arbitrarily divided into superior and inferior mediastina, and the latter is subdivided into anterior, middle and posterior parts. The plane of division into superior and inferior mediastina crosses the manubriosternal joint and the lower surface of the fourth thoracic vertebra (Fig. 55.1). Detailed accounts of some mediastinal contents are also included with descriptions of the respiratory organs (see Ch. 57) and the heart (see Ch. 56).
SUBDIVISIONS OF THE MEDIASTINUM
SUPERIOR MEDIASTINUM
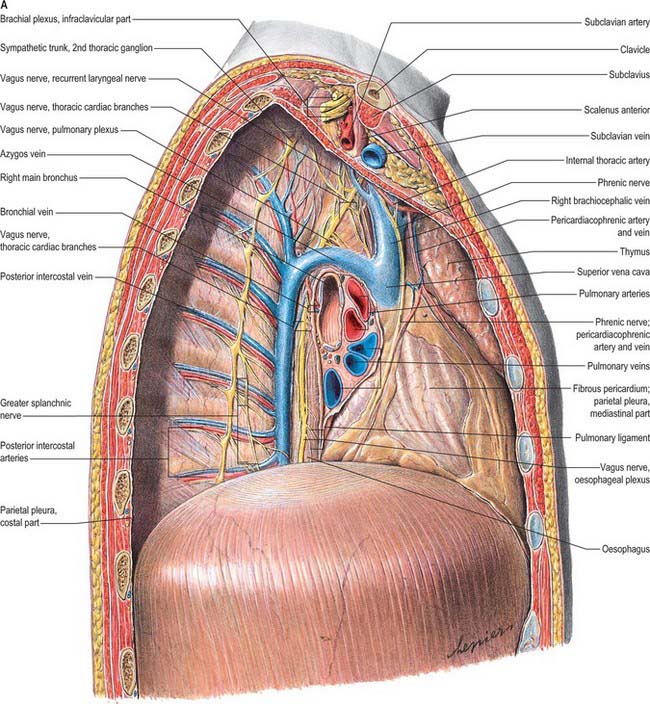
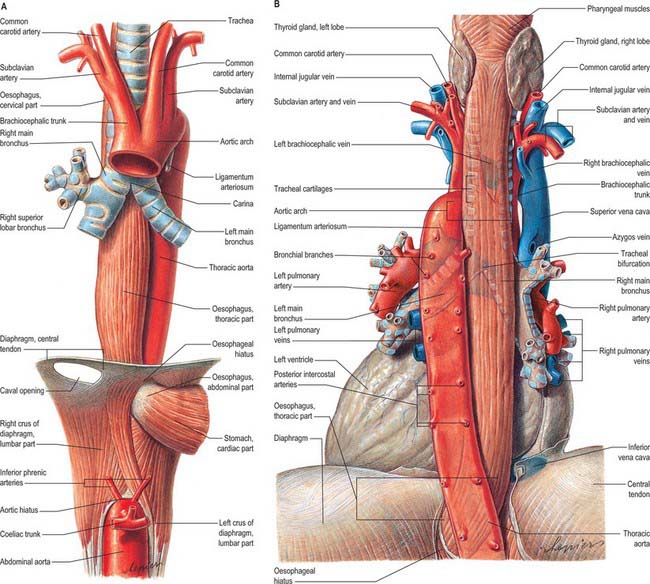
The superior mediastinum lies between the manubrium sterni and the upper four thoracic vertebrae (see Fig. 55.19A–C). It is bounded below by the sternal plane, above by the plane of the thoracic inlet, and laterally by the mediastinal pleurae. It contains the lower ends of sternohyoid, sternothyroid and longus colli; thymic remnants; the internal thoracic arteries and veins, the brachiocephalic veins and the upper half of the superior vena cava, the aortic arch, the brachiocephalic, left common carotid and subclavian arteries and the left superior intercostal vein; the right and left vagi and phrenic nerves, the left recurrent laryngeal nerve, the cardiac nerves and the superficial part of the cardiac plexus; the trachea, oesophagus and thoracic duct. It also contains the paratracheal, brachiocephalic and tracheobronchial lymph nodes associated with their named structures.
INFERIOR MEDIASTINUM
Anterior mediastinum
The anterior mediastinum lies between the sternal body and pericardium (see Fig. 55.19D,E). It narrows above the fourth costal cartilages, where the pleural sacs come close to each other, and contains loose connective tissue, the sternopericardial ligaments, a few lymph nodes and the mediastinal branches of the internal thoracic artery. The anterior mediastinum may sometimes contain part of the thymus gland or its degenerated remains.
Middle mediastinum
The middle mediastinum is the broadest part of the inferior mediastinum (see Fig. 55.19D,E). It contains the pericardium, the heart and the ascending aorta; the lower half of the superior vena cava receiving the azygos venous arch posteriorly; the tracheal bifurcation and both main bronchi; the pulmonary trunk and right and left pulmonary arteries and veins; the right and left phrenic nerves; the deep part of the cardiac plexus; the tracheobronchial lymph nodes.
Posterior mediastinum
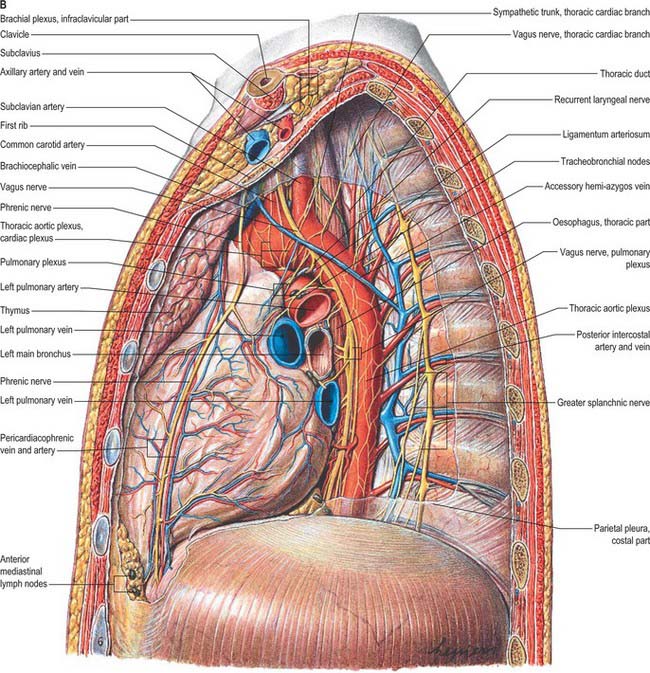
The posterior mediastinum is bounded anteriorly by the bifurcation of the trachea, pulmonary vessels, pericardium and the posterior part of the upper surface of the diaphragm (see Fig. 55.19D,E). Posteriorly, it is bounded by the vertebral column, from the lower border of the fourth to the twelfth thoracic vertebrae, and on each side by the mediastinal pleura. It contains the descending thoracic aorta and the azygos, hemiazygos and accessory azygos veins; the right and left sympathetic chains, the splanchnic nerves and the right and left vagi; the oesophagus; the thoracic duct and posterior mediastinal lymph nodes.
MEDIASTINAL COMMUNICATIONS WITH THE NECK
Anatomical pathways exist between the oral cavity and the thorax via the parapharyngeal space and other fascial planes of the neck. The parapharyngeal space is more likely to be infected than any of the other potential tissue spaces in the head and neck: infection can pass from this space to the retropharyngeal and pretracheal spaces and so reach the superior mediastinum, from where it can track down into the anterior part of the inferior mediastinum (see Ch. 28).
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Great vessels of the superior mediastinum
The aortic arch, descending thoracic aorta, pulmonary trunk and superior vena cava are described in Chapter 56.
Azygos venous system
Azygos vein
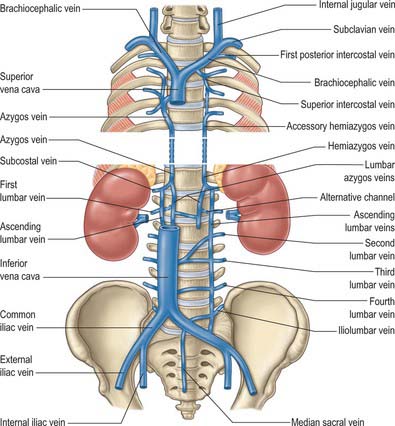
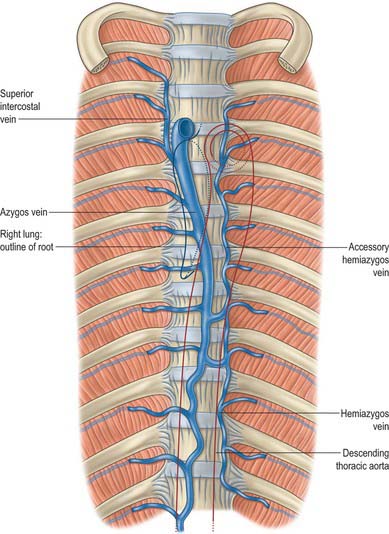
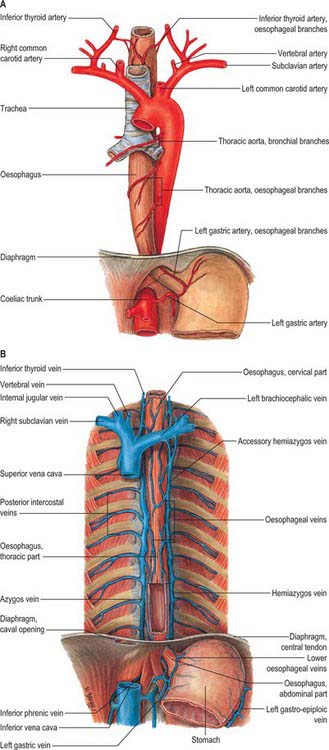
The azygos vein (Gr. azygos = ‘unpaired’) typically starts from the posterior aspect of the inferior vena cava, at or below the level of the renal veins, although the origin is not constant (Figs 55.2, 55.3; see Fig. 55.14B). When present, the lumbar azygos ascends anterior to the upper lumbar vertebrae. It may pass behind the right crus of the diaphragm or pierce it, or it may traverse the aortic hiatus to the right of the cisterna chyli. Anterior to the twelfth thoracic vertebral body, the azygos is joined by a large vessel formed by the right ascending lumbar and subcostal veins that passes forward and to the right of the twelfth thoracic vertebra behind the right crus: in the absence of a lumbar azygos, this common trunk may form the azygos vein itself. Whatever its origin, the azygos vein ascends in the posterior mediastinum to the level of the fourth thoracic vertebra, where it arches forward above the right pulmonary hilum. It ends in the superior vena cava, before the latter pierces the pericardium. The azygos lies anterior to the bodies of the lower eight thoracic vertebrae, anterior longitudinal ligament and right posterior intercostal arteries. The right greater splanchnic nerve, lung and pleura are right lateral relations. The thoracic duct and aorta and, where the vein arches forward, the oesophagus, trachea and right vagus, are left lateral relations. In the lower thorax the azygos is covered anteriorly by a recess of the right pleural sac and by the oesophagus; it emerges from behind the oesophagus to ascend behind the right hilum. The azygos vein lies close to the right posterolateral aspect of the descending thoracic aorta: aortic pulsations may assist venous return in the azygos and hemiazygos veins.
Hemiazygos vein
The hemiazygos vein is formed on the left side from the lower three posterior intercostal veins, a common trunk formed by the left ascending lumbar and subcostal veins, and by oesophageal and mediastinal tributaries (Fig. 55.2). It ascends anterior to the level of the vertebral column to the eighth thoracic level then crosses the vertebral column posterior to the aorta, oesophagus and thoracic duct, and ends in the azygos vein (Fig. 55.3). Its lower end is often connected to the left renal vein.
Accessory hemiazygos vein
The accessory hemiazygos vein descends to the left of the vertebral column, and receives veins from the fourth or fifth to eighth left intercostal spaces; it crosses the seventh thoracic vertebra to join the azygos vein (see Figs 55.5 and 55.6). The accessory hemiazygos vein sometimes receives the left bronchial veins, and it may join the hemiazygos vein, in which case their common trunk opens into the azygos vein.
Variations of the azygos veins
The azygos veins vary greatly in their mode of origin, course, tributaries, anastomoses and termination. The accessory hemiazygos is the most variable, and may drain into the left brachiocephalic, azygos or hemiazygos vein. The arrangement shown in Fig. 55.3 represents a common pattern. Commonly, there is a main ‘right-sided’ azygos and at least some representative of the hemiazygos veins. The latter vary, and one or other may be absent or poorly developed. Very occasionally, independent left and right azygos veins (the early embryonic form) persist, or a single azygos vein may occur in a midline position without hemiazygos tributaries. Retro-aortic transvertebral connections from hemiazygos and accessory hemiazygos veins to the azygos are also extremely variable: there may be up to five connections. When either of the hemiazygos veins is absent, the relevant intercostal veins cross the vertebral bodies and end in the azygos. These transvertebral routes are often very short, because the azygos vein is more commonly anterior to the vertebral column and often passes to the left of the midline for part of its course. When there is congenital interruption of the inferior vena cava (IVC), the azygos vein can become as large as the IVC that it has replaced. Rarely, the azygos arch in the right tracheobronchial angle can be placed more superolaterally in a diagonal accessory azygos fissure in the right upper lobe of the lung as a consequence of failure of embryonic descent.
MEDIASTINAL LYMPH NODES
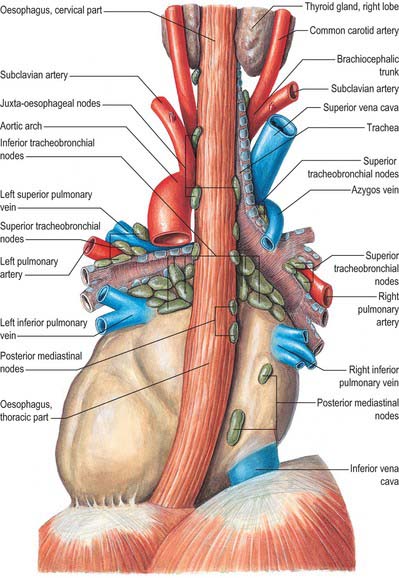
The mediastinal lymph nodes (Fig. 55.4; see Fig. 56.3) are classified into regional lymph node stations by thoracic surgeons for the purposes of staging lung cancer. The stations are defined as follows: Station 1: highest mediastinal nodes lie above a horizontal line of the level at which the left brachiocephalic vein crosses the trachea. Station 2: upper paratracheal nodes lie below the line of the highest mediastinal nodes and above a line drawn horizontally at the level of the upper border of the aortic arch. Station 3: prevascular and retrotracheal nodes lie behind the trachea but in front of the great vessels. Station 4: lower paratracheal nodes lie below the upper margin of the aortic arch and down to the upper margin of the corresponding upper lobe bronchus. On the right side, this is the upper margin of the right upper lobe bronchus; the majority of nodes in this area tend to be positioned anterolateral to the trachea. On the left side, the nodes are located below the upper margin of the aortic arch and above the margin of the left upper lobe bronchus. They lie medial to the ligamentum arteriosum and are usually lateral to the trachea. Station 5: subaortic nodes lie in the aortopulmonary window and are situated lateral to the ligamentum arteriosum or aorta or left pulmonary artery, but proximal to the first division of the left pulmonary artery. Station 6: para-aortic nodes lie between the upper margin of the aortic arch and lateral to the ascending aorta and aortic arch. Station 7: subcarinal nodes lie below the carina of the trachea, but are not associated with the lower lobe bronchi. Station 8: para-oesophageal nodes lie at either side of the oesophagus, well below the level of the subcarinal nodes. Station 9: pulmonary ligament nodes lie within the pulmonary ligament.
Involvement of these lymph nodes by cancer cells has important prognostic implications and influences the choice of treatment (Mountain & Dresler 1997). The staging system for lung cancer classifies involvement of ipsilateral hilar lymph nodes as N1, ipsilateral mediastinal lymph nodes as N2, contralateral mediastinal/hilar nodes as N3 and supraclavicular/scalene nodes as M1 (distant metastases).
These groups are not sharply demarcated. Pulmonary nodes become continuous with the bronchopulmonary nodes, and these in turn merge with the inferior and superior tracheobronchial nodes, which are continuous with the paratracheal group. Afferents of tracheobronchial nodes drain the lungs and bronchi, the thoracic part of the trachea, the heart and some efferents of the posterior mediastinal nodes. Their efferent vessels ascend on the trachea to unite with efferents of the parasternal and brachiocephalic nodes as the right and left bronchomediastinal trunks. The right trunk may occasionally join a right lymphatic duct or another right-sided lymph trunk and the left trunk may join the thoracic duct, but usually they open independently in or near the ipsilateral jugulosubclavian junction (see Ch. 28).
THORACIC DUCT
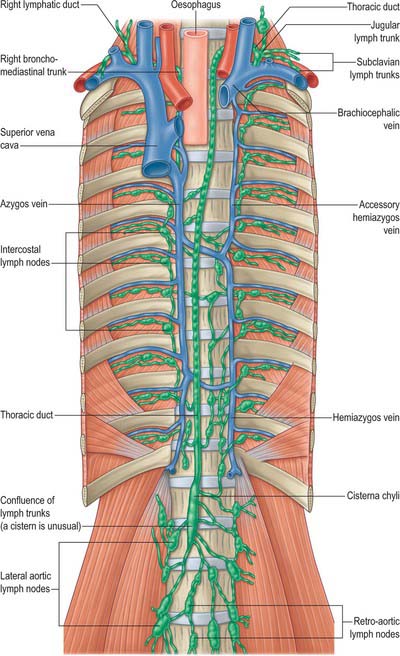
In adults, the thoracic duct, including the confluence of lymph trunks (or the cisterna chyli in the small proportion of individuals in whom the latter is saccular), is 38–45 cm in length and extends from the second lumbar vertebra to the base of the neck (Fig. 55.5). Starting from the superior pole of the confluence near the lower border of the twelfth thoracic vertebra (see Ch. 62), the thoracic duct traverses the retrocrural space of the diaphragm with the aorta, azygos and hemiazygos veins, then ascends in the posterior mediastinum, on the right of the midline, between the descending thoracic aorta (on its left) and the azygos vein (on its right). The vertebral column, the right aortic intercostal arteries and terminal segments of the hemiazygos and accessory hemiazygos veins are posterior relations. The diaphragm and oesophagus are anterior; a recess of the right pleural cavity may separate the duct and oesophagus. At the level of the body of the fifth thoracic vertebra, the duct gradually inclines to the left, enters the superior mediastinum and then ascends to the thoracic inlet along the left border of the oesophagus. In this part of its course the duct is first crossed anteriorly by the aortic arch and then runs posterior to the initial segment of the left subclavian artery, in close contact with the left mediastinal pleura. Passing into the neck, it arches laterally at the level of the transverse process of the seventh cervical vertebra. Its arch rises 3 or 4 cm above the clavicle and curves anterior to the vertebral artery and vein, the left sympathetic trunk, the thyrocervical trunk or its branches, the left phrenic nerve and the medial border of scalenus anterior. It passes posterior to the left common carotid artery, vagus nerve and internal jugular vein. Finally, the duct descends anterior to the arched cervical first part of the left subclavian artery and ends by opening into the junction of the left subclavian and internal jugular veins (see Fig. 28.16A). It may also open into either of the great veins, near the junction, or it may divide into a number of smaller vessels before terminating. In rare individuals there is no apparent thoracic duct on the left. Several terminal openings sometimes occur. Patterns vary greatly in different studies, but the commonly reported sites of termination are the internal jugular vein, jugulosubclavian junction and subclavian vein. Termination in the left brachiocephalic vein occurs occasionally.
Anomalies of the thoracic duct may be delineated by dissection into the inguinal lymphatics followed by cannulation and subsequent injection of an oily contrast medium (lipiodol) followed by plain films or CT.
INJURY DURING OESOPHAGEAL SURGERY
The thoracic duct is vulnerable to damage after thoracic, particularly oesophageal, surgery (Wemyss-Holden et al 2001). The incidence is between 0.2% and 3% and increases with transhiatal and thoracoscopic procedures. Thoracic duct laceration is a potentially life-threatening complication: mortality rates are more than 50% with conservative management and as high as 10–16% even after early surgical duct ligation. Rupture of the thoracic duct leads to leakage of chyle, which is rich in lipid, protein and lymphocytes, and hence a progressive nutritional and immune deficit occurs. Fortunately, the incidence of true thoracic duct transection is rare, and cases in which there are some postoperative chylous effusions are usually the result of damage to some of the tributaries of the thoracic duct, rather than to the duct itself. These are usually self-limiting and respond to conservative treatment.
RIGHT LYMPHATIC TRUNK
The right lymphatic trunk (Fig. 55.5) has a variable anatomy, which includes doubling of the duct, left-sided, right or bilateral termination. The plexiform nature of the trunks from which the thoracic duct develops leads to a number of possible abnormalities. The most common is duplication of the duct for a variable part of its course and subsequent merging to form a single duct which drains into the left internal jugular trunk. Occasionally the duct has dual terminations in the right and left internal jugular veins, and rarely it terminates only into the right internal jugular vein.
AUTONOMIC NERVOUS SYSTEM
The autonomic nervous system in the thorax consists of right and left ganglionated sympathetic chains and vagi and the cardiac, pulmonary and oesophageal plexuses. The cardiac plexus is described in Chapter 56, the pulmonary plexus is described in Chapter 57, and the oesophageal plexus is described below.
THORACIC SYMPATHETIC TRUNK
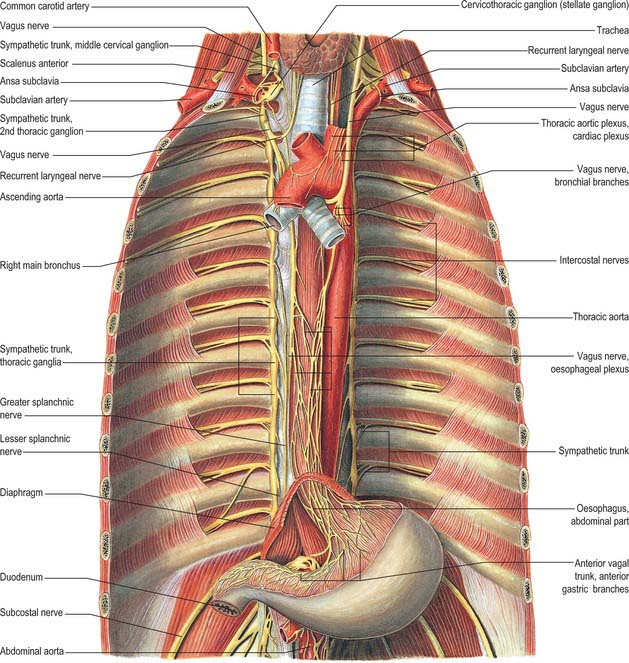
The thoracic sympathetic trunk (Fig. 55.7; see Fig. 15.13) contains ganglia almost equal in number to those of the thoracic spinal nerves (usually 11, occasionally 12, rarely 10 or 13). Almost always, the first thoracic ganglion is fused with the inferior cervical ganglion, forming the cervicothoracic ganglion. Occasionally, the second thoracic ganglion may be included in this fusion, in which case the succeeding ganglion is counted as the second in order to make the other ganglia correspond numerically with other segmental structures. Except for the second and lowest two or three, the thoracic ganglia lie against the costal heads, posterior to the costal pleura. The second thoracic sympathetic ganglion is commonly located in the second intercostal space, and the lowest two or three ganglia lie lateral to the bodies of the corresponding vertebrae. Caudally, the thoracic sympathetic trunk passes dorsal to the medial arcuate ligament (or through the crus of the diaphragm) to become the lumbar sympathetic trunk.
The ganglia are small and interconnected by intervening segments of the trunk. The classic description is that two or more rami communicantes, named white and grey, connect each ganglion with its corresponding spinal nerve, the white rami joining the nerve distal to the grey. Variation in this pattern is not uncommon, especially in the upper thoracic levels: these variations are rarely bilaterally symmetrical. Additional ascending and descending rami to higher and lower levels respectively issue from the second sympathetic ganglion (54% and 46% respectively), from the third ganglion (6% and 25%), and from the fourth ganglion (5% and 8%) (Cho et al 2005). The ascending ramus from the second ganglion is sometimes called the nerve of Kuntz. The anatomical variations displayed by the communicating rami and in the location of the second sympathetic ganglion might explain some surgical failures and symptom recurrences). Occasionally, a grey and a white ramus fuse to form a ‘mixed’ ramus.
The medial branches from the upper five ganglia are very small, and supply filaments to the thoracic aorta and its branches; they form a fine thoracic aortic plexus on the aorta with filaments from the greater splanchnic nerve. Rami of the second to fifth or sixth ganglia enter the posterior pulmonary plexus. Others, from the second to fifth ganglia, pass to the deep (dorsal) part of the cardiac plexus. Small branches of these pulmonary and cardiac nerves pass to the oesophagus and trachea. The medial branches from the lower seven ganglia are large; they supply the aorta and unite to form the greater, lesser and lowest splanchnic nerves (the last two are not always identifiable).
Thoracic sympathectomy
The effect is immediately evident: the patient awakes from anaesthesia with dry and warm hands. In many cases, even hyperhidrosis of the feet improves, but the underlying anatomical/physiological mechanisms are not yet properly understood (Gofeld & Faclier 2006). Surgical complications are very rare: Horner’s syndrome is the most feared, and is caused by damage to the stellate ganglion and interruption of the sympathetic fibres from T1 which ascend around the arteries supplying the head and neck (see Ch. 28).
VAGUS NERVE IN THE MEDIASTINUM
Cardiac branches join the cardiac plexuses and relay in ganglia that are distributed freely over both atria in the subepicardial tissue. The terminal fibres are distributed to the atria and the atrioventricular bundle; they are concentrated around the sinuatrial and, to a lesser extent, the atrioventricular nodes (see Ch. 56). Cardiac branches slow the cardiac cycle and diminish the force of contraction. It has been claimed that the vagi can only influence ventricular muscle via their effect on the atrioventricular node, even though postganglionic parasympathetic innervation of the ventricles is sparse. The smaller branches of the coronary arteries are innervated mainly via the vagus, whereas larger arteries, with a dual innervation, are chiefly supplied by sympathetic fibres. Pulmonary branches are motor to the circular smooth muscle fibres of the bronchi and bronchioles and are therefore bronchoconstrictor; synaptic relays occur in the ganglia of the pulmonary plexuses.
Right vagus
The right vagus nerve descends posterior to the internal jugular vein and crosses the first part of the subclavian artery to enter the thorax. It descends through the superior mediastinum, at first behind the right brachiocephalic vein, and then to the right of the trachea and posteromedial to the right brachiocephalic vein and superior vena cava. The right pleura and lung are lateral to it above and are separated from it below by the azygos vein, which arches forwards above the right pulmonary hilum (Fig. 55.6). It passes behind the right main bronchus and lies on the posterior aspect of the right hilum, where it divides into the posterior pulmonary (or bronchial) branches. The latter unite with rami from the second to fifth or sixth thoracic sympathetic ganglia to form the right posterior pulmonary plexus. Two or three branches descend from the caudal part of this plexus on the posterior surface of the oesophagus and join a left vagal branch to form the posterior oesophageal plexus. A vagal trunk containing fibres from both right and left vagi leaves the plexus and runs down on the posterior surface of the oesophagus. It enters the abdomen by passing through the diaphragmatic oesophageal opening.
Left vagus
The left vagus enters the thorax between the left common carotid and subclavian arteries and behind the left brachiocephalic vein. It descends through the superior mediastinum and crosses the left side of the aortic arch to pass behind the left pulmonary hilum (Fig. 55.6). Above the aortic arch, it is crossed anterolaterally by the left phrenic nerve, and on the arch by the left superior intercostal vein. Behind the hilum it divides into the posterior pulmonary (or bronchial) branches, which unite with rami of the second to fourth thoracic sympathetic ganglia to form the left posterior pulmonary plexus. Two or three branches descend anteriorly on the oesophagus and join with a ramus from the right posterior pulmonary plexus to form the anterior oesophageal plexus. A trunk containing fibres from both vagi descends anterior to the oesophagus and enters the abdomen through the oesophageal diaphragmatic opening.
THYMUS
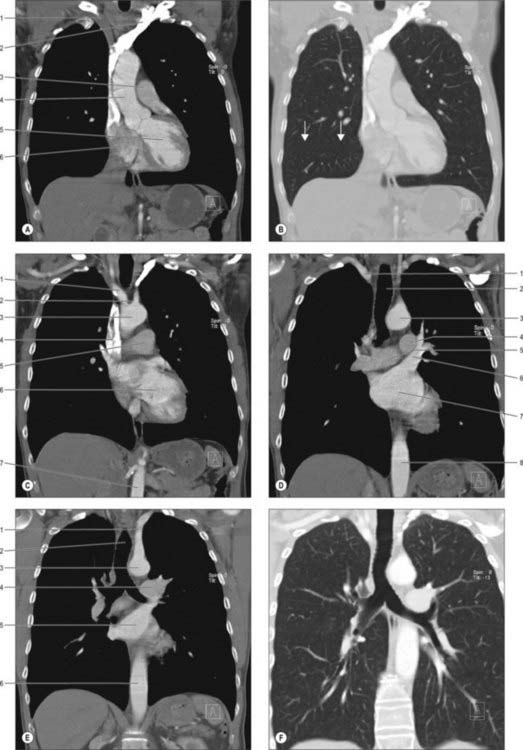
The thymus (Figs 55.8, 55.9) is one of the two primary lymphoid organs, the other being the bone marrow. It is an encapsulated soft, bilobed organ, the two parts being joined in the midline by connective tissue that merges with the capsule of each lobe. The latter may normally have adhesions to the fibrous pericardium that necessitate limited pericardiectomy during thymectomy. The thymus is visible on CT and MRI axial sections just anterior to the ascending aorta and inferior to the left brachiocephalic vein; the CT attenuation in younger individuals is homogeneous and similar to or greater than that of muscle, and the signal intensity with MRI on T2-weighted images is similar to or greater than that of fat.
RELATIONS
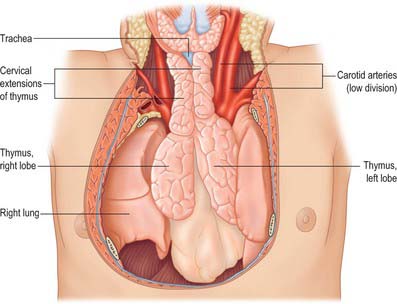
The thymus is largest in the early part of life, particularly around puberty, and persists actively into old age despite considerable fibrofatty degeneration which sometimes hides the existence of thymic tissue. The greater part of the thymus lies in the superior mediastinum and anterior part of the inferior mediastinum, and the lower border of the thymus reaches the level of the fourth costal cartilages. Superiorly, extensions into the neck are common, reflecting the (bilateral) embryonic origins of the thymus from the third pharyngeal pouch. Its upper poles join at, and extend above, the level of the suprasternal notch; the left usually extends higher and is seen first behind the strap muscles during the initial stages of transcervical thymectomy (see p. 949). It sometimes reaches the inferior poles of the thyroid gland or even higher, and is connected to the thyroid gland by the thyrothymic ligament. Its shape is largely moulded by adjacent structures. Inferiorly, the lower end of the right lobe commonly lies between the right side of the ascending aorta and the right lung, anterior to the superior cava. Anteriorly lie sternohyoid, sternothyroid and fascia (in the neck), and the manubrium sterni, internal thoracic vessels and upper three costal cartilages (in the thorax). The pleurae lie laterally and the phrenic nerves are anterolateral and inferior. Posteriorly, the thymus is in contact with the vessels of the superior mediastinum (the left brachiocephalic vein may be partly embedded in the gland), the upper part of the thoracic trachea and the anterior surface of the heart. Thoracic surgeons performing thymectomies must be aware of the anatomic variation where the upper poles may lie posterior to the left brachiocephalic vein.
MICROSTRUCTURE
General architecture
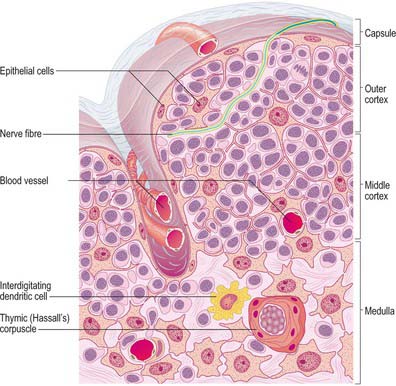
It is useful to consider the embryological origins of the thymus in order to understand its cellular organization. The thymus is derived from a number of sources, including epithelial derivatives of the pharyngeal pouches, mesenchyme, haemolymphoid cells and vascular tissue. In section, the thymus can be seen to consist of an outer cortex of densely packed cells mainly of the T-lymphocyte lineage (thymocytes) and an inner medulla with fewer lymphoid cells (Fig. 55.10).
Both thymic lobes have a loose fibrous connective tissue capsule, from which septa penetrate to the junction of the cortex and medulla, and partially separate the irregular lobules (see Fig. 55.12), which are each 0.5–2.0 mm in diameter. The connective tissue septa form a route of entry and exit for blood vessels and nerves and carry efferent lymphatics. Most migrant cells enter or leave the thymus by this route.
Epithelial framework
Unlike other lymphoid structures, in which the supportive framework is chiefly collagenous reticular tissue, the thymus contains a network of interconnected epithelial cells (Fig. 55.10) which create an appropriate microenvironment, by cell–cell contact and the release of paracrine factors, in which thymic lymphocytes (T cells) develop and mature. Although differing in morphology, all the epithelial cells of the thymus share a common origin from pharyngeal endoderm. They vary in size and shape according to their positions within the thymus. Typically they have pale, oval nuclei, a rather eosinophilic cytoplasm and intercellular desmosomal attachments. Intermediate filament bundles of cytokeratin lie within their cytoplasm. The subcapsular cells form a continuous external lining to the thymus beneath its fibrous capsule, and follow its lobulated profile, ensheathing the vessels that pass into it, and contributing to the functional blood–thymus barrier. Other cortical epithelial cells form a loose mesh of long cytoplasmic processes, whereas medullary epithelial cells tend to form more solid cords as well as thymic or Hassall’s corpuscles: lymphocytes lie within the interstices of the mesh or between the cords. Large epithelial cells may be associated with around 50 or more thymocytes and are sometimes called thymic nurse cells.
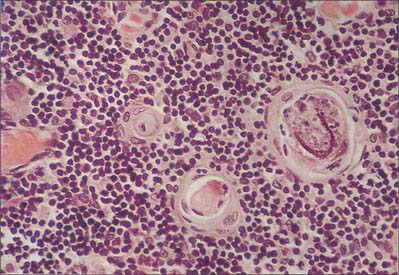
Hassall’s corpuscles are whorls of flattened, concentrically layered medullary epithelial cells, from 30 to 100 μm in diameter, and are characteristic features of the thymic medulla (Figs 55.10, 55.11). They start to form before birth and their numbers increase throughout life. Their function is not clear, although they may represent a site for removal of dying, apoptotic thymocytes, because their centres are eosinophilic, partly keratinized and often contain cellular debris. Corpuscles with a similar appearance have been described in the palatine tonsil.
Thymocytes
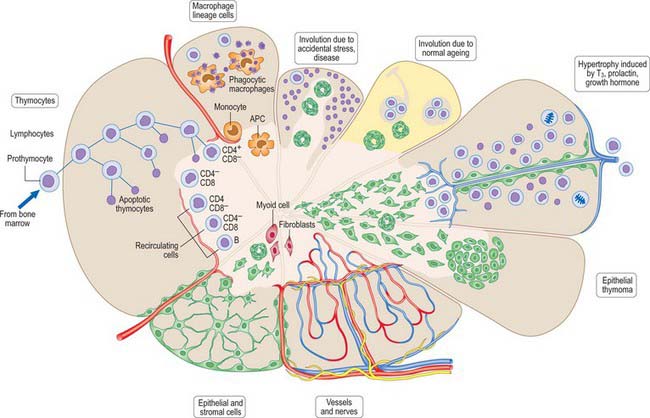
Thymocytes undergo mitosis in all cortical zones as the differentiating T cells mature, gradually moving deeper into the cortex (Fig. 55.12). The processes of thymocyte development and maturation to generate T cells depend on the microenvironment provided by epithelial cells, dendritic cells, macrophages and fibroblasts. T cells which either fail to recognize self-MHC (histocompatibility) molecules or which recognize self-antigens die by apoptosis, in order to achieve functional immune reactivity and maintain self-tolerance, respectively. Over 95% of cortical thymocytes die within the thymus; the surviving T cells migrate through the walls of venules and efferent lymphatics to enter the circulation and populate secondary lymphoid tissues.
Microcirculation
Cortex
The pattern of blood flow differs in the cortex and medulla. Major blood vessels enter the gland at the corticomedullary junction and pass within each lobe, giving off small capillaries to the cortex and larger vessels to the medulla. Most cortical capillaries loop around at different depths in the cortex and join venules at the corticomedullary junction; some continue through the cortex and join larger venules running in the capsule which leave the thymus. These smaller cortical capillaries usually have a narrow perivascular space, which sometimes contains pericytes and other cells, but rarely nerves. Sheaths of thymic epithelial cells of the blood–thymus barrier lie between the perivascular space and cortical thymocytes.
DEVELOPMENT
The embryology and prenatal development of the thymus are described in Chapter 35.
Thymic changes during postnatal life
At birth the thymus is most often bilobar. It is 4–6 cm long, 2.5–5 cm wide and 1 cm thick. The thickest part of the gland at birth is not at the thoracic inlet, but immediately above the base of the heart. During childhood the thymus narrows and lengthens and the cervical portion becomes less noticeable. CT and imaging studies of the thorax reveal that the right lobe of the thymus typically measures 9 mm in thickness, and the left 11 mm, in normal children. After the age of 20 years, it decreases to 5–6 mm in thickness (Fig. 55.12).
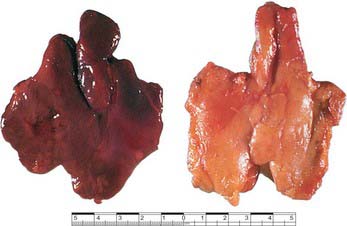
In children the gland is more pyramidal in shape and firmer than in later life, when the amount of lymphoid tissue is greatly reduced. In the fresh state it is deep red, reflecting its rich blood supply (Fig. 55.9). With age it becomes thinner and greyer, and infiltrated by yellow adipose tissue. Each of the two lobes is partially divided by the ingrowth of shallow septa, so that, superficially, the gland appears lobulated. As fatty atrophy proceeds, this lobulation becomes more distinct. The older thymus can be distinguished from the surrounding mediastinal fat only by the presence of its capsule. However, even within greatly atrophied glands there are usually greyer areas around blood vessels formed by persistent lymphoid tissue. Thymocyte production and differentiation persist throughout life: T cells from the thymus continue to populate the peripheral lymphoid tissue, blood and lymph.
Mediastinal parathyroid adenoma
When primary hyperparathyroidism is diagnosed, surgical excision of the causative parathyroid adenoma (occasionally multiple) is usually performed whether or not symptoms are present. Approximately 3% of parathyroid tumours are found in glands in the mediastinum; 80% of these ectopic adenomas are found in the superior or anterior part of the inferior mediastinum, derived from inferior glands that descend with the thymus in the embryo, and the remainder are found in the posterior mediastinum. Preoperative localization may help decrease surgical exploration time and morbidity: the sensitivity of technetium-99m sestamibi imaging for identification of parathyroid adenomas exceeds 90%. MRI has a sensitivity of up to 75%, while CT and suprasternal sonography are less sensitive (Iyer et al 1999). Venous sampling may also be used in cases of diagnostic difficulty.
OESOPHAGUS
The oesophagus (Figs 55.13, 55.14; see also Figs 55.4, 55.7) is a muscular tube, typically 25 cm long, which connects the pharynx to the stomach. It begins in the neck, level with the lower border of the cricoid cartilage and the sixth cervical vertebra, and descends largely anterior to the vertebral column through the superior and posterior mediastina, passes through the diaphragm, level with the tenth thoracic vertebra, and ends at the gastric cardiac orifice level with the eleventh thoracic vertebra. Generally vertical in its course, the oesophagus has two shallow curves. It starts in the median plane, but inclines to the left as far as the root of the neck, gradually returns to the median plane near the fifth thoracic vertebra, and at the seventh thoracic vertebra deviates left again, before it pierces the diaphragm. The oesophagus also bends in an anteroposterior plane to follow the cervicothoracic curvatures of the vertebral column; it can also bend slightly to the right as it is pushed by the aorta before bending to the left to reach the oesophageal hiatus. It is the narrowest part of the alimentary tract (except for the vermiform appendix) and is constricted at the beginning (15 cm from the incisor teeth), where it is crossed by the aortic arch (22.5 cm from the incisor teeth), where it is crossed by the left principal bronchus (27.5 cm from the incisors) and as it passes through the diaphragm (40 cm from the incisors). These measurements are important clinically with regard to the passage of instruments along the oesophagus.
CERVICAL OESOPHAGUS
The cervical oesophagus (see Fig. 55.4) is posterior to the trachea and attached to it by loose connective tissue. The recurrent laryngeal nerves ascend on each side in or near the tracheo-oesophageal groove. Posteriorly are the vertebral column, longus colli and prevertebral layer of deep cervical fascia. Laterally on each side are the common carotid arteries and posterior part of the thyroid gland. In the lower neck, where the oesophagus deviates to the left, it is closer to the left carotid sheath and thyroid gland than it is on the right. The thoracic duct ascends for a short distance along its left side (see Fig. 55.5).
THORACIC OESOPHAGUS
The thoracic oesophagus (Figs 55.13, 55.14) is situated a little to the left in the superior mediastinum between the trachea and the vertebral column. It passes behind and to the right of the aortic arch to descend in the posterior mediastinum along the right side of the descending thoracic aorta. Below, as it inclines left, it crosses anterior to the aorta and enters the abdomen through the diaphragm at the level of the tenth thoracic vertebra. From above downwards, the trachea, right pulmonary artery, left main bronchus, pericardium (separating it from the left atrium) and the diaphragm are anterior. The vertebral column, longus colli, right posterior intercostal arteries, thoracic duct, azygos vein and the terminal parts of the hemiazygos and accessory hemiazygos veins, and, near the diaphragm, the aorta are posterior. A long recess of the right pleural sac lies between the oesophagus (in front) and the azygos vein and vertebral column (behind) in the posterior mediastinum.
In the superior mediastinum, the terminal part of the aortic arch, the left subclavian artery, thoracic duct, the left pleura and the recurrent laryngeal nerve are left lateral relations. In the posterior mediastinum, the oesophagus is related to the descending thoracic aorta and left pleura. The right pleura, and the azygos vein as it arches forwards above the right main bronchus to join the superior vena cava, are right lateral relations. Below the pulmonary roots, the vagus nerves descend in contact with the oesophagus, the right mainly behind and the left in front; the vagi subsequently unite to form a plexus around the oesophagus. Low in the posterior mediastinum, the thoracic duct is behind and to the right of the oesophagus; at higher levels the duct is posterior, crossing to the left of the oesophagus at about the level of the fifth thoracic vertebra and then ascending on the left. On the right of the oesophagus, just above the diaphragm, a small serous infracardiac bursa may occur; it represents the detached apex of the right pneumatoenteric recess.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
Arteries
The cervical oesophagus is supplied by the inferior thyroid artery (see Ch. 28). The thoracic oesophagus is supplied by bronchial and oesophageal branches of the thoracic aorta (Fig. 55.14A). Four or five oesophageal branches arise from the anterior surface of the aorta and descend obliquely to the oesophagus, where they form a vascular chain that anastomoses above with the oesophageal branches of the inferior thyroid arteries, and below with ascending branches from the left phrenic and left gastric arteries.
Veins
Blood from the oesophagus drains into a submucous plexus and thence into a peri-oesophageal venous plexus from which oesophageal veins arise. Those from the thoracic oesophagus drain predominantly into the azygos veins and, to a lesser extent, the hemiazygos, intercostal and bronchial veins (Fig. 55.14B). Those from the cervical oesophagus drain into the inferior thyroid vein. The left gastric vein meets the lower oesophageal veins at the oesophageal opening in the lesser curvature and then drains into the portal vein.
Oesophageal varices
Varices in the distal oesophagus are easily visible at endoscopy, because they are situated superficially in the lamina propria. The blood from the superficial veins drains into a superficial venous plexus, then into a deeper intrinsic venous plexus and finally into the perioesophageal veins via perforating veins. Bidirectional flow is normally possible in this region, a phenomenon that permits pressure changes during breathing and Valsalva manoeuvres. However, in portal hypertension, the valves within the perforating vessels become incompetent and blood flow is retrograde, causing dilatation of the deep intrinsic veins. The greater pressure within this region predisposes the varices to brisk and life-threatening bleeding. Treatment is directed towards controlling the formation of a collateral circulation and the obliteration of varices that are susceptible to bleeding, and is achieved by paravariceal endoscopic injection of a sclerosant, which causes obliteration of the varices as a result of thrombus formation. Fibrosis is also induced within the mucosa, which reduces the formation of new collateral vessels. Alternatively, rubber bands may be applied in an attempt to ligate the varices. Bleeding from varices is associated with a mortality rate of 25%, reflecting the problems of rebleeding and underlying comorbidity.
INNERVATION
The upper oesophagus is supplied by the branches of the recurrent laryngeal nerve and by postganglionic sympathetic fibres that reach it by travelling along the inferior thyroid arteries (Fig. 55.7) (see Ch. 28). The lower oesophagus is supplied by the oesophageal plexus, a wide-meshed autonomic network that surrounds the oesophagus below the level of the lung roots, and which contains a mixture of parasympathetic and sympathetic fibres.
MICROSTRUCTURE
The tissues forming the thoracic oesophageal wall, from lumen outwards, are the mucosa (consisting of epithelium, lamina propria and muscularis mucosae), submucosa, muscularis externa and adventitia (Fig. 55.15; see Fig. 60.9).
Mucosa
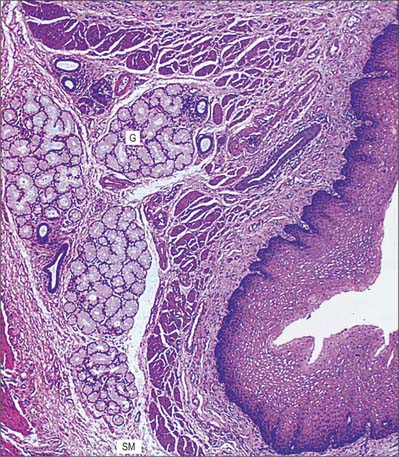
Epithelium
The epithelium is a non-keratinized, stratified squamous epithelium, continuous with that of the oropharynx. In humans this protective layer is quite thick (300–500 μm) (Fig. 55.15), and is not affected by oesophageal distension. The boundary between the oesophageal epithelium and its lamina propria is distinct but markedly uneven, because tall connective tissue papillae invaginate the epithelial base, assisting in the anchorage of the epithelium to underlying tissues (Fig. 55.15). These papillae are permanent structures, also unaffected by oesophageal distension, and they are rich in blood vessels and nerve fibres. At the base of the epithelium there is a basal lamina, to which epithelial cells are attached by hemidesmosomes, as occurs in the oral mucosa.
OESOPHAGOSCOPY AND TRANSOESOPHAGEAL ULTRASOUND
Endoscopic transoesophageal ultrasound permits assessment of the oesophageal wall and para-oesophageal structures such as lymph nodes. This technique is more sensitive than CT scanning in detecting metastases in mediastinal lymph nodes, because it allows lymph nodes less than 10 mm in size to be assessed for possible malignant involvement. Normal lymph nodes cannot be easily identified by endoscopic ultrasound, because they have the same echogenicity characteristics as surrounding tissue; a hypoechoic or inhomogeneous pattern on ultrasound is considered more suspicious of malignancy. Endoscopic ultrasound-guided fine needle aspiration allows accurate diagnosis of lymph nodes, but only mediastinal nodes adjacent to the oesophagus can be fully evaluated. Transbronchial fine needle aspiration is an alternative approach for sampling mediastinal nodes, with lymph node choice based on CT findings. PET CT scanning is rapidly becoming the modality of choice for mediastinal nodal staging.
BARIUM STUDIES
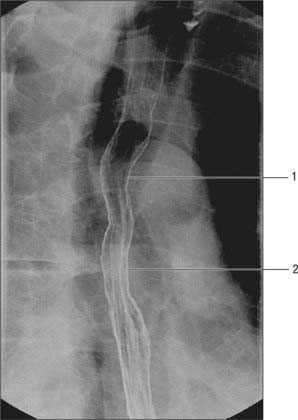
A double-contrast barium swallow with fluoroscopy enables anatomical and functional assessment of the oesophagus (see Fig. 55.18). A standard examination involves the patient swallowing a barium solution and gas granules, and images be taken in the erect, supine and prone recumbent positions. The upright posture fully dilates the oesophagus and is useful in evaluating any constrictions. The narrow tubular lumen of the oesophagus is slightly indented by the aortic arch and the left main bronchus. The supine view allows better evaluation of any motor dysfunction and varices, and moving into the prone position provokes underlying gastro-oesophageal reflux. Both repeating single digital images and cine loops of fluoroscopy can be obtained. The fluoroscopic images demonstrate defective opening of the pharyngeal oesophageal sphincter and the nature of the contractions in the body of the oesophagus. The cardio-oesophageal sphincter can be assessed in achalasia. Other abnormalities such as hiatus hernia and areas of narrowing or dilatation of the oesophagus may be demonstrated. Mucosal relief images are obtained once the bulk of the contrast media has passed into the stomach, and this provides fine mucosal detail. Cross-sectional imaging is usually used for the preoperative planning and staging of oesophageal tumours, and is of value in radiotherapy planning.
OESOPHAGEAL SPHINCTERIC MECHANISMS
Radiological studies show that swallowed food stops momentarily in the gastric end of the oesophagus, before entering the stomach (see Fig. 55.18), suggesting the presence of a sphincter at this point. In the past there was much controversy as to the reason for this behaviour, because only slight thickening of the muscle coat has been found in humans. There is now ample physiological and clinical evidence that closure depends on two major mechanisms. The more important of these is the lower oesophageal sphincter, a specialized zone of circular smooth muscle surrounding the oesophagus at its transit through the diaphragm and for much of its short abdominal course. This region of the oesophagus is maintained under tonic contraction, except during swallowing, when it relaxes briefly to admit ingesta to the stomach, and during vomiting (see p. 954). It is controlled by the intramural plexuses of the enteric nervous system; in addition, the neural release of nitric oxide contributes to its relaxation. The second mechanism is a functional external sphincter provided by the crural diaphragm (usually the right crus), which encircles the oesophagus as it passes into the abdomen and is attached to it by the phreno-oesophageal ligament. Radiological, electromyographic and manometric analyses have shown that its muscular fibres contract around the oesophagus during inspiration and when intra-abdominal pressure is increased, thus helping to prevent gastro-oesophageal reflux, even when the lower oesophageal sphincter is inhibited experimentally with atropine. The relative importance of these two agents in the prevention of oesophageal reflux is still a matter for debate. Clinically, there is a good correlation between this condition and lower oesophageal sphincter dysfunction in some cases, whereas in others, failure of the diaphragmatic component, as seen in hiatus hernia, appears to be a major factor. The anatomical configuration of the gastro-oesophageal orifice may also play some part in these processes (see p. 954).
OESOPHAGEAL DYSMOTILITY
Achalasia of the cardia is a primary motor disorder of the oesophagus in which there is failure of relaxation of the cardio-oesophageal sphincter and loss of peristalsis in the oesophageal body with degeneration of neuronal cell bodies in the myenteric nerve plexus. The clinical presentation is dysphagia, regurgitation of undigested food, retrosternal chest pain and occasional weight loss. A simple chest radiograph may show dilatation of the oesophagus and retention of food products as a mottled double-contoured mediastinal widening. Barium swallow shows a classical bird-beak appearance as a result of failure of relaxation of the lower oesophageal sphincter and an absence of peristalsis. Oesophageal manometry with pressure measurements confirms the diagnosis and demonstrates absent or impaired relaxation of the lower oesophageal sphincter. Treatment consists of pneumatic balloon dilatation during endoscopy or minimal-access surgical myotomy of the cardia. An alternative in frail patients is intrasphincteric injection of botulinum toxin. The advantages are lower morbidity and mortality, with a much lower risk of oesophageal perforation.
MEDIASTINAL IMAGING
Mediastinal structures may be viewed using X-rays, CT and MRI.
NORMAL MEDIASTINAL CONTOURS ON FRONTAL CHEST RADIOGRAPH
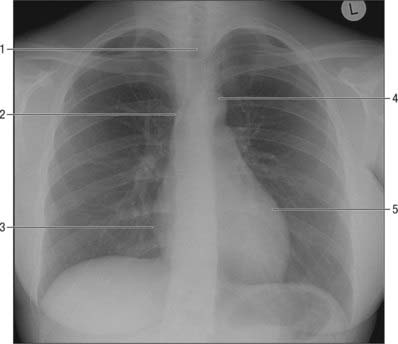
In a standard posteroanterior chest radiograph the X-ray beam passes from back to the front of the chest. The standing patient breath-holds in full inspiration, and elevates and abducts the arms over the radiographic plate, movements which protract the scapulae and depress the medial ends of the clavicles to clear the peripheral lung fields and apices respectively. In this view the heart and large blood vessels appear as the ‘mediastinal silhouette’ (Fig. 55.16). Forming its left border, from above down, are the aortic arch (‘aortic knuckle’), sometimes with the left superior intercostal vein (‘aortic nipple’) visible, the pulmonary trunk and left ventricle. The descending aorta is projected as a continuous left paravertebral border all the way down to the diaphragm. The right border is delineated by the right brachiocephalic vein, superior vena cava, right atrium and thoracic inferior vena cava. A right paratracheal stripe (normally under 3 mm) is usually seen with the oval silhouette of the azygos arch at its lower margin. An accessory lung fissure, the azygos fissure, is seen rarely and extends from the apex of the lung to the right tracheobronchial angle. The azygos arch width should be no more than 5 mm, but may measure up to 10 mm in the third trimester of pregnancy as a result of a physiological blood volume increase. Enlargements or displacements of any of these structures accentuate the normal bulges on the borders of the mediastinal silhouette, thus an enlarged left auricle is seen between the pulmonary trunk and left ventricle. On both sides, opacities caused by pulmonary vessels associated with the roots of the lungs form hilar contours. In the upper thorax, the less dense projection of the trachea is visible in the median plane. The anterior junctional line, where the two lungs approximate above the heart, is sometimes visible. The posterior junctional line, which represents pleural apposition behind the oesophagus, is also sometimes seen: it projects up into the neck because of the obliquity of the thoracic inlet. The azygo-oesophageal recess is an area where a portion of the right lung is in contact with the azygos vein and the lateral wall line. The paraspinal lines are also visible parallel to the right and left margins of the thoracic spine.
NORMAL MEDIASTINAL CONTOURS ON A LATERAL CHEST RADIOGRAPH
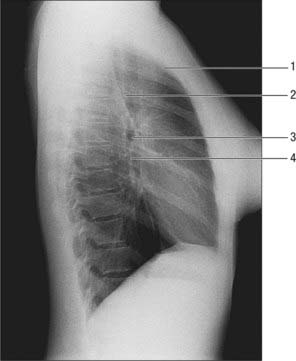
On a lateral view, the cardiac silhouette is seen above the anterior part of the diaphragm (Fig. 55.17). The anterior and posterior cardiac silhouettes are formed by the right ventricle and left atrium respectively. Behind is the retrocardiac space (posterior mediastinum) containing the descending thoracic aorta and the oesophagus: the oesophagus can be visualized with swallowed air or barium on a chest radiograph or fluoroscopically in the barium swallow examination (Fig. 55.18). Above, the less dense trachea and main bronchi are recognizable.
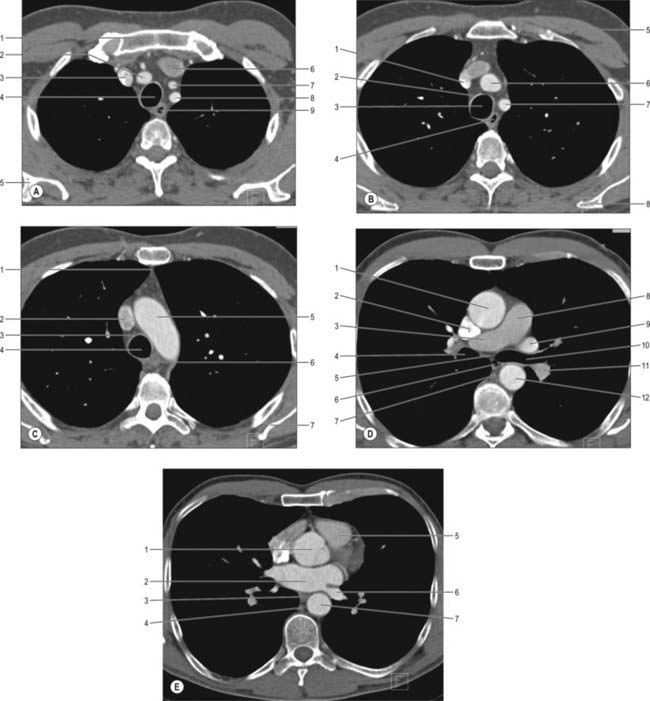
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING OF THE MEDIASTINAL SPACES
CT scans provide excellent cross-sectional views of the anatomy of the mediastinum and are complemented by MRI (Figs 55.19, 55.20). Intravenous contrast enhancement of the vessels improves the detail of the scan and improves distinction of blood vessels from lymph nodes and soft tissue opacities. Cross-sectional CT scanning is very useful in evaluating the mediastinal spaces, which include the pretracheal, subcarinal, right paratracheal and posterior tracheal spaces and aortopulmonary window: these areas are where mediastinal lymph node masses are located and hence of importance in the staging of lung cancer.
Armstrong P. The normal chest. In: Armstrong P, Wilson AG, Dee P, Hansell DM. Imaging of Diseases of the Chest. 3rd edn. London: Mosby; 2000:21-62.
Cho HM, Lee DY, Sung SW. Anatomical variations of rami communicantes in the upper sympathetic trunk. Eur J Cardiothoracic Surg. 2005;27:320-324.
Gofeld M, Faclier G. Bilateral pain relief after unilateral thoracic percutaneous sympathectomy. Can J Anaesth. 2006;53:258-262.
Iyer RB, Whitman GJ, Sahin AA. Parathyroid adenoma of the mediastinum. Am J Radiol. 1999;173:94.
Jaretzki A, Wolff M. ‘Maximal’ thymectomy for myasthenia gravis: surgical anatomy and operative technique. J Thorac Cardiovasc Surg. 1988;96:711-716.
Masaoka A, Yamakawa Y, Niwa H, et al. Extended thymectomy for myasthenia gravis patients: a 20-year review. Ann Thorac Surg. 1996;62:853-859.
Meyers BF, Cooper JD. Transcervical thymectomy for myasthenia gravis. Chest Surg Clin N Am. 2002;11:363-368.
Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718-1723.
Paquet K-J. Causes and pathomechanisms of oesophageal varices development. Med Sci Monit. 2000;6:915-928.
Singh TP, Bala Sanju, Kalsey G, Singla Rajan K. Applied anatomy of fascial spaces in the head and neck. J Anat Soc India. 2000;49:78-88.
Spitz L. Esophageal atresia: past, present and future. J Paediatr Surg. 1996;31:19-25.
Wemyss-Holden SA, Launois B, Maddern GJ. Management of thoracic duct injuries after oesophagectomy. Br J Surg. 2001;88:1442-1448.