Mediastinal Tumors
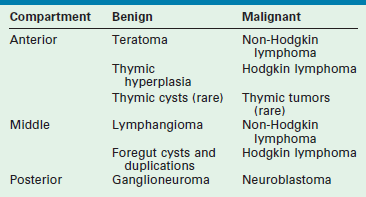
The mediastinum can be divided into three compartments (Fig. 25-1). The anterior one contains the thymus, vessels, lymphoid structures, and nerves. The middle one contains the trachea, main-stem bronchi, the heart and great vessels, and the hilar lymph nodes. The posterior mediastinum accommodates the aorta, the thoracic esophagus, and the sympathetic nerve chains.
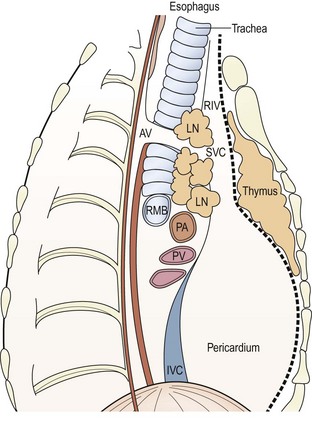
FIGURE 25-1 Anatomic division of the mediastinum: anterior compartment extends from the sternum to the dotted line anterior to the pericardium. Middle mediastinum extends posteriorly to the anterior border of the vertebrae (solid line). AV, azygous vein; IVC, inferior vena cava; LN, lymph node; PA, pulmonary artery; PV, pulmonary vein; RMB, right main-stem bronchus; RIV, right internal jugular vein; SVC, superior vena cava.
The tracheobronchial tree derives from the embryonic foregut that is innervated by neural crest cells that form the sympathetic chains. Embryonal germ cells migrate toward their final settlement in the gonads through the anterior mediastinum. The thymus primordia from the third pharyngeal pouches migrate into the anterior mediastinum and fuse to form a single organ. Finally, huge vascular structures develop in the mediastinum. All these embryonal events explain the nature of the tumors located in each compartment. Their most frequent location is summarized in Table 25-1.
Clinical Features
Mediastinal tumors and cysts are occasionally diagnosed before birth and may even benefit from prenatal instrumentation.1 However, most of them remain silent during infancy and are discovered serendipitously. Respiratory embarrassment, orthopnea, stridor, wheezing, severe distress, and superior vena cava syndrome are occasionally seen. Large anterior tumors may cause sternal bulging. Recurrent laryngeal or phrenic nerve palsies or Horner syndrome may also lead to diagnosis. Sudden paraplegia can occur in dumbbell tumors involving the spinal cord. Finally, secretion of catecholamines, α-fetoprotein, or gonadotropins may also uncover the tumor.
Diagnostic Methods
Plain radiographs of the thorax or ultrasonography may show widening of the superior mediastinal shadow or masses in either hemithorax.2,3 Computed tomography (CT) provides information about the location of the tumor, its cystic or solid nature, depicting calcifications, necrotic areas, and widening of the spinal foramina in cases with intraspinal extension. Calcifications are suggestive of neuroblastoma or teratoma, but other tumors, such as lymphangiomas or histiocytoses, can also have calcified areas.4,5 CT is crucial for assessing the patency of the airway and the risks of anesthesia.6 Magnetic resonance imaging (MRI) is used to better define the nature of the masses of vascular origin, and also for evaluating intraspinal invasion.7,8
For many tumors, histology, cell markers, and molecular biology features can be identified before undertaking operative therapy. Cells can be obtained by biopsy of cervical or suprasternal lymph nodes, by fine needle aspiration (FNA), or by pleural or pericardial fluid aspiration.9–11 When this is not feasible, operative biopsy or excision is required. Thoracoscopy12,13 or anterior mini-thoracotomy through the bed of the second or third rib (the Chamberlain approach)14,15 may provide material for biopsy or for complete removal. Mediastinoscopy is rarely used in children.16
Principles of Management
Children with anterior mediastinal masses can undergo respiratory collapse after induction of anesthesia. Ventilation can be critically difficult, particularly in children with lymphoma. Cross-sectional surfaces of the tracheal lumen decreased by 50% or more on CT scan carry a high anesthetic risk.14 These patients also have marked reductions in the maximum expiratory flow rates, and special modalities of anesthesia (e.g., spontaneous ventilation, laryngeal mask, rigid tubes) may be necessary.6
Except for lymphomas that respond to chemotherapy and/or radiotherapy and in which surgery is usually adjuvant, mediastinal tumors should be removed. Median sternotomy17 or standard thoracotomy are the preferred approaches. Sternotomy extended to the neck allows safe approach for large cervicothoracic masses (Fig. 25-2).18 Thoracoscopy is an alternative approach, and should respect the principles of oncologic surgery, avoiding spillage of tumor cells or fluids (particularly true for germ cell tumors).17,19,20 Robotic surgery has also been described.21
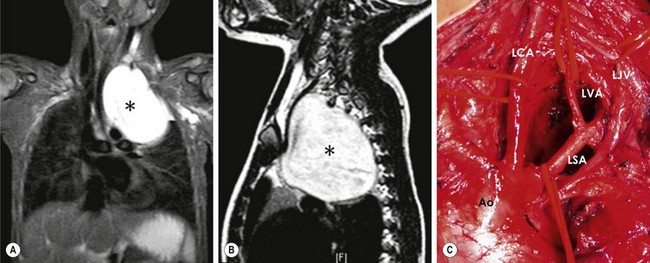
FIGURE 25-2 (A,B) Coronal and sagittal views from an MRI of a 5-year-old girl with a high mediastinal ganglioneuroma (asterisk) extending from the hilum of the lung to the neck and involving the vessels and nerves of this area. (C) This was safely excised through a cervico-transternal approach seen after removal of the mass. Ao, aorta; LCA, left carotid artery; LSA, left subclavian artery; LVA, left vertebral artery; LJV, left jugular vein.
Lymphoma
Non-Hodgkin Lymphoma (NHL)
This systemic disease is one of the more frequent mediastinal tumors characterized by massive proliferation of lymphoblasts in the lymph nodes. These cells can ontogenically differentiate into B-cells (related to humoral immunity and produced in the bone marrow) and T-cells (involved in cellular immunity and processed in the thymus). In children, B-cells cause undifferentiated Burkitt or non-Burkitt lymphomas, which together constitute about 50% of all lymphomas. T-cells cause lymphoblastic lymphomas (about 40%) that are generally found in the thymus and/or anterior mediastinal lymph nodes. The remaining 10% correspond to large cell lymphomas. These are similar to undifferentiated tumors, but may be of T- or B-cell origin and appear on either side of the diaphragm.
Undifferentiated lymphomas express surface IgM and CD19 and CD20 antigens, whereas lymphoblastic lymphomas contain the enzyme TdT (terminal deoxynucleotidyl transferase) and express T-cell markers such as CD5 and CD7.22 Burkitt-type lymphomas often have 8 : 14, and less often 8 : 22 or 2 : 8 translocations, involving the MYCC proto-oncogene, whereas T-cell lymphomas occasionally have 14q11 translocations.15,23
Rapid growth of mediastinal lymphomas may cause respiratory distress, wheezing, orthopnea, cervicofacial edema, and jugular ingurgitation. Systemic symptoms are possible, and bone marrow invasion causes hematologic disturbances. Enlarged lymph nodes may become palpable in the neck, axillae, and supraclavicular or suprasternal regions.
Imaging depicts an enlarged mediastinum and airway involvement. Pleural or pericardial fluid can often be obtained for cytologic analysis.11 If neither peripheral lymph nodes nor fluids are available for biopsy or cytology, FNA or biopsy by thoracoscopy or the anterior Chamberlain approach may be necessary. In all cases, the anesthetic risks and precautions should be emphasized.24
Mediastinal NHL cases are stage III except when bone marrow and/or central nervous system are involved (stage IV).25 Gallium-67 isotopic scanning may be helpful for staging.26,27 The role of combined positron emission tomography (PET) and CT in children is still undefined.
Mediastinal NHL respond well to chemotherapy and corticosteroids, and the contribution of surgery is limited to retrieval of biopsy material for cytologic assessment. Chemotherapy leads to long-term survival in more than 80% of these children.25,28
Hodgkin Lymphoma
Mediastinal Hodgkin lymphoma is less frequent and occurs more often in adolescents. The main feature is the Reed–Sternberg cell, the malignant counterpart of the dendritic interdigitating cell that has a role in antigen presentation. These cells are embedded in lymph nodes in which the proportions of fibrous stroma, lymphocytes, and plasma cells are variable, allowing classification into lymphocyte predominant, lymphocyte depleted, mixed cellularity, and nodular sclerosis types. Most children with Hodgkin lymphoma have the nodular sclerosis type, but the youngest ones may have lymphocyte predominant or mixed cellularity varieties.29 Hodgkin lymphoma originates in one group of lymph nodes and spreads to contiguous or distant nodes. Accurate staging is imperative before selection of the treatment protocol. The Ann Arbor classification of four stages with subgroups, according to the presence or absence of systemic symptoms, remains the most widely used system, but mapping of the involved organs or lymph node groups requires refined imaging tools.
Hodgkin lymphoma may be localized primarily in the mediastinum and may cause the same compressive effects as NHL (Fig. 25-3). Biopsies of extrathoracic nodes are sometimes possible. If not, the Chamberlain operation or thoracoscopy should be used. The preference for chemotherapy over radiotherapy has limited the use of staging laparotomy in children. This should be reserved for cases with thoracic stage II cases in which localized radiotherapy as the sole treatment requires excluding transdiaphragmatic involvement.30 PET/CT seems promising as a noninvasive staging modality.29,31
FIGURE 25-3 Imaging studies in an 11-year-old boy with Hodgkin lymphoma. (A) Widening of the mediastinum is accompanied by compression of the upper trachea that is seen on the chest radiograph. (B) Transverse section on a CT scan of the upper mediastinum shows the extent of this tracheal compression by adenopathy that displaces the vessels laterally.
Surgery is not usually the primary treatment of Hodgkin lymphoma, except in very localized cases. However, it may occasionally be needed in children.32
Germ Cell Tumors
Teratomas
These comprise only 8–16% of the tumors in this region and are uncommon in children.33,34 They consist of solid or organoid masses containing tissues derived from all three blastodermic layers. Their histologic features are heterogeneous and may include cystic or solid areas as well as mature and immature components. Their incidence is higher in individuals with Klinefelter syndrome.35,36
Mediastinal teratomas originate most often from the thymus or pericardium and are therefore anterior. They are as frequent in girls as in boys, in contrast to the situation in adults in whom there is a clear male predominance.37,38 They develop relatively early in fetal life, and may cause hydrops and fetal demise.1,39 These masses can be diagnosed prenatally (Fig. 25-4), but they are more often detected after birth because of respiratory distress or later in life because of vague symptoms (thoracic or cervical pain, dyspnea). The diagnosis is often incidental. Mediastinal teratomas should be suspected whenever a mass with or without calcifications is detected in the anterior mediastinal compartment. However, this diagnosis often is not made until the operation. Most mediastinal teratomas are benign in children, but the prognosis is definitely worse if they contain elements of yolk sac, embryonal carcinoma, seminoma, germinoma, or choriocarcinoma.35,40,41 Some of these lesions may induce precocious puberty or detectable pancreatic secretions.35,42
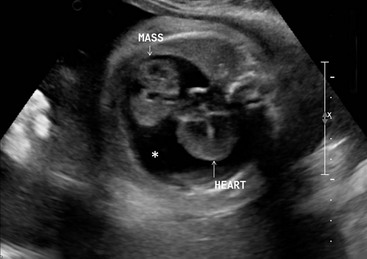
FIGURE 25-4 This fetal echocardiogram is on a 30 week gestational age baby shows a 4 cm × 4 cm intrapericardial teratoma (MASS) which is closely associated with the aortic root. The bulk of the mass is in the right chest and is in direct contact with the right atrium, but does not cause any right atrial compression or systemic venous inflow or outflow obstruction. There is a large pericardial effusion (asterisk) around both the heart and the tumor. Following delivery, the baby was taken to the operating room for excision of the teratoma and has recovered uneventfully.
Operative excision is the treatment of choice. Median sternotomy allows excellent exposure, but lateral thoracotomies may be preferred when the tumor extends into either hemithorax. Thoracoscopy has also been used.43 Chemotherapy with carboplatin, bleomycin, and etoposide may allow for complete removal after tumor shrinkage in cases that cannot be initially excised.35 Although wide adherence to adjacent tissues usually makes a complete resection difficult, it is essential to avoid recurrence. In fact, these tumors have an excellent prognosis when resection is complete. α-Fetoprotein is a good tumor marker because it is usually elevated in malignant and immature tumors and very seldom in mature ones.44
Nonteratomatous Germ Cell Tumors
Very rarely, pure extraembryonal germ cell tumors (seminoma/dysgerminoma, embryonal carcinoma, yolk sac tumor, or choriocarcinoma) may develop in the anterior mediastinum. These are malignant and require complete removal and chemotherapy, as explained earlier for the malignant components of teratomas.35,44
Tumors and Cysts of the Thymus
Thymic Cysts
Cysts derived from the thymopharyngeal ducts may appear in the anterior mediastinum and/or in the neck. They are lined by pharyngeal or ciliated epithelium. They have secretory and thymic elements (Hassall’s corpuscles) and may be inflamed or infected. When they reach a certain size, they become palpable (in the neck) or detected on imaging of the mediastinum.45–47 They may also cause respiratory symptoms by compression.48 Thymic cysts can also be found in rare instances of malignant lymphoma and in patients with human immunodeficiency virus (HIV) infection in whom they can be multilocular.32,49
Vascular Tumors and Anomalies
Modern classifications of vascular anomalies and tumors has allowed an understanding of their unpredictable clinical course. Vascular tumors like noninvoluting (NICH) and rapidly involuting (RICH) congenital hemangioma, kaposiform hemangioendothelioma, and the capillary, venous, arteriovenous, or lymphatic vascular malformations have different clinical behaviors and require an individualized approach.53 These conditions may occasionally locate in the mediastinum where they may pose difficult therapeutic problems.54 (See Chapter 72 for more information about vascular tumors and anomalies.)
Vascular Tumors
Most hemangiomas located in the anterior mediastinum are in continuity with cervicofacial components that sometimes cause respiratory compromise when they extend into the airway. If they are asymptomatic, they should not be treated because they tend to regress over time. However, if airway compression is present, active anti-angiogenic treatment with corticosteroids and/or interferon-2α or vincristine have been used. Interferon-2α has been found to cause severe neurologic complications.53 Propranolol, which induces rapid regression of hemangiomas, has also been used with success in mediastinal locations.55
Kaposiform hemangioendothelioma may be accompanied by a Kasabach–Merritt syndrome with massive platelet trapping and risks of hemorrhage. These tumors involve the thoracic wall and often the mediastinum. Full anti-angiogenic therapy together with close hematologic monitoring and eventually surgery are required. This tumor has a mortality rate close to 20%.53
Vascular Malformations
Venous and arteriovenous malformations, and particularly lymphangiomas, are occasionally located near the confluences of large venous and lymphatic collectors in the mediastinum.56,57 The tumor may extend into either hemithorax and eventually into the neck and the base of the mouth. Lymphangiomas are usually multicystic and infiltrate the anatomic structures. Reaction to local infections may make the mass swell or even suppurate. Respiratory symptoms may arise in cases with airway compromise.58 MRI in the best imaging method for diagnosis.
Mediastinal lymphangiomas do not tend to involute. Treatment strategies must take into account that they are benign, that total removal is often impossible, and that a too radical operation may endanger nerve trunks or other structures (Fig. 25-5).59,60 Sclerosis with OK-432 or bleomycin is an alternative or a complement in cases in which incomplete removal has already reduced the volume of the tumor.53 Only in cases of single, wide cysts is the result of sclerosing procedures generally satisfactory.61 For masses with multiple infiltrating cysts of small size, partial debulking may be needed to reduce symptoms.60
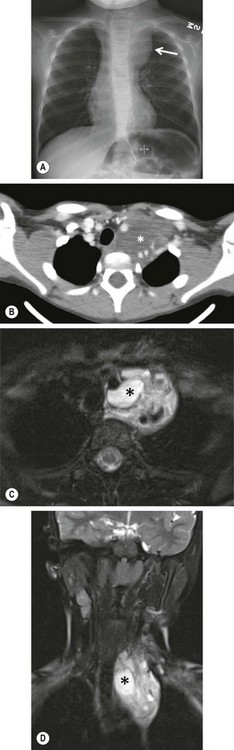
FIGURE 25-5 This 7-year-old was found to have a cervico-thoracic lymphangioma following imaging evaluation for a left cervical soft tissue mass. (A) On the chest radiograph, a mass effect is noted in the left superior mediastinum with displacement of the trachea to the right. (B) The CT scan shows a low density soft tissue mass (asterisk) with splaying of the vasculature. Again, the tracheal deviation is noted. (C) Axial and (D) coronal T2 fat saturated MRI images are seen. Both reveal a multicystic soft tissue mass which is classic for a lymphatic malformation. An asterisk is noted in a macrocyst.
Foregut Cysts and Duplications
Abnormal branching of the tracheobronchial anlage may create closed spaces lined by either esophageal or bronchial mucosa. These cysts are in close contact with either the trachea and main-stem bronchi or the esophagus. The secreting mucosa progressively enlarges the cysts, which can then become symptomatic. In rare instances, the foregut malformation is more extensive and involves both the respiratory and digestive tracts, and combines esophageal duplications with airway malformations.62,63 When the foregut cyst is due to a persistence of the embryonic neuroenteric communication, it involves both the foregut and the neural tube. It splits the notochord and, as a consequence, is accompanied by split vertebral bodies. This rare variety is termed a neuroenteric cyst.64–66
Prenatal detection of mediastinal cysts is possible.67 However, most cases are diagnosed at birth or later in life because of symptoms of respiratory or gastrointestinal compression (Fig. 25-6). Very seldom, mediastinal cysts may bleed due to mucosal ulceration.
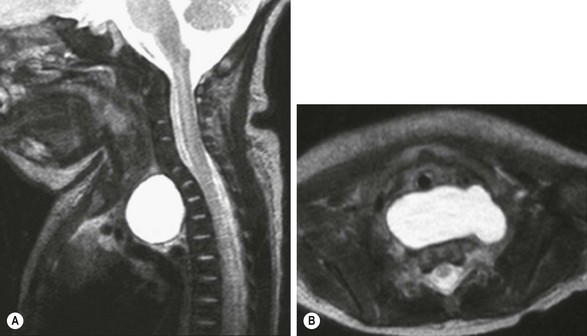
FIGURE 25-6 This newborn with the prenatal diagnosis of an upper mediastinal cyst had respiratory distress at birth. These T2-weighted MR images show a large prevertebral mucus cyst that (A) displaces the trachea forward and (B) extends to both sides. In A, deformation of the anterior vertebral bodies due to prolonged prenatal compression can be seen. This bronchogenic cyst was successfully removed thoracoscopically.
The cysts located close to the trachea and main-stem bronchi are lined by ciliated epithelium and are termed ‘bronchogenic cysts’ (Fig. 25-7).68,69 Those located in contact or within the wall of the esophagus are lined by esophageal or mixed epithelium and are known as ‘esophageal duplication cysts.’
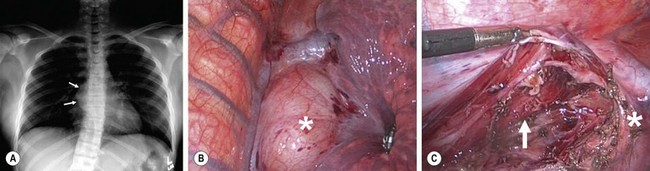
FIGURE 25-7 (A) This 12-year-old developed some respiratory distress that prompted the chest radiograph that shows a right bronchogenic cyst (arrows). (B) The lesion (asterisk) is seen at thoracoscopy. (C) After removal of the cyst, the esophagus (arrow) is seen to lie in the posterior bed of the resection. The cyst was intimately adherent to the right main-stem bronchus (asterisk).
Foregut cysts and duplications should be excised because of their secretory nature. Prenatal diagnosis may allow intrauterine treatment.70 Thoracoscopy has become the preferred approach.71–74 Most esophageal duplications and bronchogenic cysts can be removed by this approach. Dissection from the esophageal wall may be delicate, but because these cysts generally do not communicate with the esophageal lumen, they can be mobilized without damage to the mucosa. As in other duplications, it is essential to completely remove the secreting mucosa to prevent recurrence or cancer later in adulthood.68 (See Chapter 39 for more information about duplications.)
Neural Tumors
These tumors originate from the sympathetic chains located on both sides of the spine and may exhibit different degrees of differentiation, ranging from malignant neuroblastomas to mature ganglioneuromas that may appear intermixed in the same tumor (Fig. 25-8). For unknown reasons, thoracic neural tumors are less malignant than abdominal ones and tend to behave less aggressively.75 The proportion of stages 1 and 2 and the favorable histologic patterns are definitely higher than in other locations. Moreover, these tumors often have less MYCN amplification.75–78 The tumors involve the sympathetic trunks, and can extend into the spinal canal through one or several foramina (dumbbell- or hourglass-shaped tumors).79,80 The upper or apical tumors may extend to the neck and often involve the stellate ganglion. The lower ones can extend into the abdomen through the posterior diaphragmatic insertions and/or the aortic hiatus. The aorta and the esophagus on the left side and the azygos vein on the right may be in close contact with the tumor, which sometimes passes across the midline. Intercostal arteries and veins are often intratumoral.
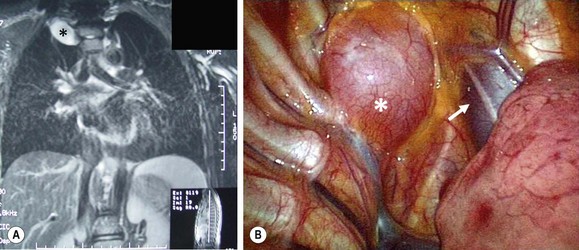
FIGURE 25-8 A 16-year-old presented with Horner syndrome and was found to have a right superior mediastinal mass. (A) MR image of the mass showing the ganglioneuroma (asterisk). (B) At thoracoscopy, the mass (asterisk) can be visualized cephalad to the azygous vein and just lateral to the superior vena cava (arrow). This ganglioneuroma was able to be removed thoracoscopically.
Horner syndrome and/or heterochromia of the iris may lead to the diagnosis.81,82 Paraplegia due to spinal cord compression can occur suddenly.83 However, most cases are silent and are discovered by imaging procedures for concurrent conditions or vague symptoms. Secreting tumors and paraneoplastic symptoms such as hypertension and diarrhea are rare in this location. A limited number of malignant tumors spread from the primary site and metastasize to distant regions such as the bone marrow or the bones.78
Imaging reveals a round or fusiform mass with sometimes hemorrhagic, necrotic, or calcified areas. The ribs and the spinal pedicles may be distorted and the foramina enlarged. In dumbbell tumors, the intraspinal component is better depicted by MRI (Fig. 25-9).79
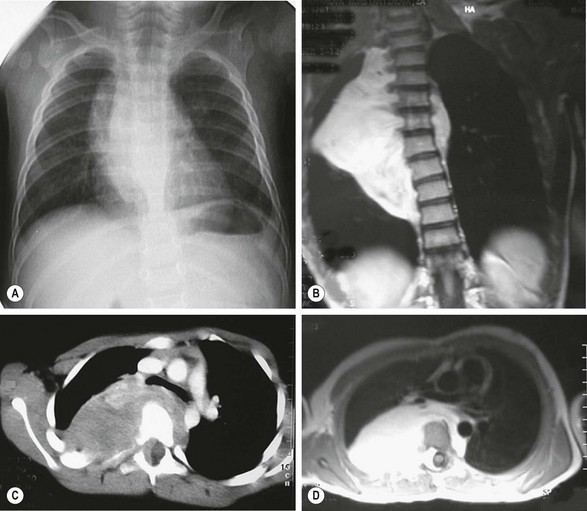
FIGURE 25-9 This 7-year-old patient had a long-standing history of neuroblastoma that was treated with chemotherapy but the lesion was not resected. The tumor is seen as a fusiform mass extending to both sides of the thorax on the (A) plain radiograph and (B) and MR image. (C) CT scan shows rib and vertebral body deformations with widening of the foramina and displacement of the great vessels. (D) MR image depicts the dumbbell-shaped nature of the tumor, which invaded the spinal canal, although without paraplegia. Combined spinal and right thoracotomy approaches allowed complete resection of the mass.
In cases with paraplegia, emergency laminectomy or laminotomy with removal of the intraspinal extension is generally preferred, although some groups advise chemotherapy initially.83–85 Except in very extensive tumors and in those with bone metastases in which chemotherapy should precede surgical removal, mediastinal neural tumors are primarily excised.86 This operation can be difficult, particularly when the tumor extends beyond the midline or into the neck, the spinal canal, or below the diaphragm. Mobilization of the aorta with division of several intercostal vessels may be required. Clearance of the tumor should be as complete as possible, although in many cases minimal macroscopic residual disease is unavoidable. In cases of thoracoabdominal tumors, splitting the diaphragm and dissecting the retroperitoneal mass from above at the same operative setting is advantageous. Thoracoscopic excision is being increasingly utilized.19,20,87
Irrespective of their histology, the survival of patients with thoracic neuroblastoma is encouraging and always better than that of abdominal primaries. Some sequelae are unavoidable. Paraplegia may persist when the cord has been permanently damaged (this happens in tumors that cause prenatal compression).88,89
Permanent paraplegia may rarely occur due to intraoperative spinal cord ischemia.90 Division of the segmental vessels may cause localized paralysis of upper abdominal or thoracic muscles. Removal of the stellate ganglion usually leads to permanent Horner syndrome. Finally, scoliosis can develop after laminectomies.
Other Rare Mediastinal Tumors
Rarely, other mediastinal tumors have been reported in children. These include pseudoinflammatory tumor, Langerhans’ cell histiocytosis with calcification, thymolipoma, sarcoma, and liposarcoma.91–95
References
1. Sbragia, L, Paek, BW, Feldstein, VA, et al. Outcome of prenatally diagnosed solid fetal tumors. J Pediatr Surg. 2001; 36:1244–1247.
2. Lemaitre, L, Leclerc, F, Marconi, V, et al. Ultrasonographic findings in thymic lymphoma in children. Eur J Radiol. 1987; 7:125–129.
3. Borecky, N, Gudinchet, F, Laurini, R, et al. Imaging of cervico-thoracic lymphangiomas in children. Pediatr Radiol. 1995; 25:127–130.
4. Ichikawa, T, Ohtomo, K, Araki, T, et al. Ganglioneuroma: Computed tomography and magnetic resonance features. Br J Radiol. 1996; 69:114–121.
5. Wyttenbach, R, Vock, P, Tschappeler, H. Cross-sectional imaging with CT and/or MRI of pediatric chest tumors. Eur Radiol. 1998; 8:1040–1046.
6. Shamberger, RC, Holzman, RS, Griscom, NT, et al. CT quantitation of tracheal cross-sectional area as a guide to the surgical and anesthetic management of children with anterior mediastinal masses. J Pediatr Surg. 1991; 26:138–142.
7. Castellote, A, Vazquez, E, Vera, J, et al. Cervicothoracic lesions in infants and children. Radiographics. 1999; 19:583–600.
8. Zhang, Y, Nishimura, H, Kato, S, et al. MRI of ganglioneuroma: Histologic correlation study. J Comput Assist Tomogr. 2001; 25:617–623.
9. Motoyama, T, Yamamoto, O, Iwamoto, H, et al. Fine needle aspiration cytology of primary mediastinal germ cell tumors. Acta Cytol. 1995; 39:725–732.
10. Shabb, NS, Fahl, M, Shabb, B, et al. Fine-needle aspiration of the mediastinum: A clinical, radiologic, cytologic, and histologic study of 42 cases. Diagn Cytopathol. 1998; 19:428–436.
11. Chaignaud, BE, Bonsack, TA, Kozakewich, HP, et al. Pleural effusions in lymphoblastic lymphoma: A diagnostic alternative. J Pediatr Surg. 1998; 33:1355–1357.
12. Rothenberg, SS. Thoracoscopy in infants and children. Semin Pediatr Surg. 1998; 7:194–201.
13. Tsao, K, St Peter, SD, Sharp, SW, et al. Current application of thoracoscopy in children. J Laparoendosc Adv Surg Tech A. 2008; 18:131–135.
14. Shamberger, RC. Preanesthetic evaluation of children with anterior mediastinal masses. Semin Pediatr Surg. 1999; 8:61–68.
15. Glick, RD, Pearse, IA, Trippett, T, et al. Diagnosis of mediastinal masses in pediatric patients using mediastinoscopy and the Chamberlain procedure. J Pediatr Surg. 1999; 34:559–564.
16. Gun, F, Toker, A, Kaya, S, et al. Cervical mediastinoscopy for paratracheal masses in pediatric patients. Pediatr Hematol Oncol. 2008; 25:393–397.
17. Koga, H, Yamataka, A, Kobayashi, H, et al. Median sternotomy provides excellent exposure for excising anterior mediastinal tumors in children. Pediatr Surg Int. 2005; 21:864–867.
18. Grosfeld, JL, Weber, TR, Vane, DW. One-stage resection for massive cervicomediastinal hygroma. Surgery. 1982; 92:693–699.
19. Partrick, DA, Rothenberg, SS. Thoracoscopic resection of mediastinal masses in infants and children: An evaluation of technique and results. J Pediatr Surg. 2001; 36:1165–1167.
20. Petty, JK, Bensard, DD, Partrick, DA, et al. Resection of neurogenic tumors in children: Is thoracoscopy superior to thoracotomy? J Am Coll Surg. 2006; 203:699–703.
21. Meehan, JJ, Sandler, AD. Robotic resection of mediastinal masses in children. J Laparoendosc Adv Surg Tech A. 2008; 18:114–119.
22. Long, JC, McCaffrey, RP, Aisenberg, AC, et al. Terminal deoxynucleotidyl transferase positive lymphoblastic lymphoma: A study of 15 cases. Cancer. 1979; 44:2127–2139.
23. Kaneko, Y, Variakojis, D, Kluskens, L, et al. Lymphoblastic lymphoma: Cytogenetic, pathologic, and immunologic studies. Int J Cancer. 1982; 30:273–279.
24. Ricketts, RR. Clinical management of anterior mediastinal tumors in children. Semin Pediatr Surg. 2001; 10:161–168.
25. Marky, I, Bjork, O, Forestier, E, et al. Intensive chemotherapy without radiotherapy gives more than 85% event-free survival for non-Hodgkin lymphoma without central nervous involvement: A 6-year population-based study from the Nordic Society of Pediatric Hematology and Oncology. J Pediatr Hematol Oncol. 2004; 26:555–560.
26. Drossman, SR, Schiff, RG, Kronfeld, GD, et al. Lymphoma of the mediastinum and neck: Evaluation with Ga-67 imaging and CT correlation. Radiology. 1990; 174:171–175.
27. Hamrick-Turner, JE, Saif, MF, Powers, CI, et al. Imaging of childhood non-Hodgkin lymphoma: Assessment by histologic subtype. Radiographics. 1994; 14:11–28.
28. Goubin, A, Auclerc, MF, Auvrignon, A, et al. Survival in France after childhood acute leukaemia and non-Hodgkin’s lymphoma (1990–2000). Eur J Cancer. 2006; 42:534–541.
29. Donaldson, SS. Pediatric Hodgkin’s disease–up, up, and beyond. Int J Radiat Oncol Biol Phys. 2002; 54:1–8.
30. Tebbi, CK, Mendenhall, N, London, WB, et al. Treatment of stage I, IIA, IIIA1 pediatric Hodgkin disease with doxorubicin, bleomycin, vincristine and etoposide (DBVE) and radiation: A Pediatric Oncology Group (POG) study. Pediatr Blood Cancer. 2006; 46:198–202.
31. Metwally, H, Courbon, F, David, I, et al. Coregistration of prechemotherapy PET-CT for planning pediatric Hodgkin’s disease radiotherapy significantly diminishes interobserver variability of clinical target volume definition. Int J Radiat Oncol Biol Phys. 2011; 80:793–799.
32. Nogues, A, Tovar, JA, Sunol, M, et al. Hodgkin’s disease of the thymus: A rare mediastinal cystic mass. J Pediatr Surg. 1987; 22:996–997.
33. Weidner, N. Germ-cell tumors of the mediastinum. Semin Diagn Pathol. 1999; 16:42–50.
34. Takeda, S, Miyoshi, S, Ohta, M, et al. Primary germ cell tumors in the mediastinum: A 50-year experience at a single Japanese institution. Cancer. 2003; 97:367–376.
35. Billmire, D, Vinocur, C, Rescorla, F, et al. Malignant mediastinal germ cell tumors: An intergroup study. J Pediatr Surg. 2001; 36:18–24.
36. Beresford, L, Fernandez, CV, Cummings, E, et al. Mediastinal polyembryoma associated with Klinefelter syndrome. J Pediatr Hematol Oncol. 2003; 25:321–323.
37. Grosfeld, JL, Billmire, DF. Teratomas in infancy and childhood. Curr Probl Cancer. 1985; 9:1–53.
38. Moran, CA, Suster, S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer. 1997; 80:681–690.
39. Froberg, MK, Brown, RE, Maylock, J, et al. In utero development of a mediastinal teratoma: A second-trimester event. Prenat Diagn. 1994; 14:884–887.
40. Lakhoo, K, Boyle, M, Drake, DP. Mediastinal teratomas: Review of 15 pediatric cases. J Pediatr Surg. 1993; 28:1161–1164.
41. De Backer, A, Madern, GC, Hakvoort-Cammel, FG, et al. Mediastinal germ cell tumors: Clinical aspects and outcomes in 7 children. Eur J Pediatr Surg. 2006; 16:318–322.
42. Kallis, P, Treasure, T, Holmes, SJ, et al. Exocrine pancreatic function in mediastinal teratomata: An aid to preoperative diagnosis? Ann Thorac Surg. 1992; 54:741–743.
43. Feo, CF, Chironi, G, Porcu, A, et al. Videothoracoscopic removal of a mediastinal teratoma. Am Surg. 1997; 63:459–461.
44. Dehner, LP. Germ cell tumors of the mediastinum. Semin Diagn Pathol. 1990; 7:266–284.
45. Samuel, M, Spitz, L, Deleval, M, et al. Mediastinal thymic cysts in children. Pediatr Surg Int. 1995; 10:146–147.
46. Sturm-O’Brien, AK, Salazar, JD, Byrd, RH, et al. Cervical thymic anomalies—the Texas Children’s Hospital experience. Laryngoscope. 2009; 119:1988–1993.
47. De Caluwe, D, Ahmed, M, Puri, P. Cervical thymic cysts. Pediatr Surg Int. 2002; 18:477–479.
48. Wagner, CW, Vinocur, CD, Weintraub, WH, et al. Respiratory complications in cervical thymic cysts. J Pediatr Surg. 1988; 23:657–660.
49. Kontny, HU, Sleasman, JW, Kingma, DW, et al. Multilocular thymic cysts in children with human immunodeficiency virus infection: Clinical and pathologic aspects. J Pediatr. 1997; 131:264–270.
50. Takeda, S, Miyoshi, S, Akashi, A, et al. Clinical spectrum of primary mediastinal tumors: A comparison of adult and pediatric populations at a single Japanese institution. J Surg Oncol. 2003; 83:24–30.
51. Yaris, N, Nas, Y, Cobanoglu, U, et al. Thymic carcinoma in children. Pediatr Blood Cancer. 2006; 47:224–227.
52. Stachowicz-Stencel, T, Bien, E, Balcerska, A, et al. Thymic carcinoma in children: A report from the Polish Pediatric Rare Tumors Study. Pediatr Blood Cancer. 2010; 54:916–920.
53. Fishman, SJ. Vascular anomalies of the mediastinum. Semin Pediatr Surg. 1999; 8:92–98.
54. Moran, CA, Suster, S. Mediastinal hemangiomas: A study of 18 cases with emphasis on the spectrum of morphological features. Hum Pathol. 1995; 26:416–421.
55. Fulkerson, DH, Agim, NG, Al-Shamy, G, et al. Emergent medical and surgical management of mediastinal infantile hemangioma with symptomatic spinal cord compression: Case report and literature review. Childs Nerv Syst. 2010; 26:1799–1805.
56. Ratan, J, Bhatnagar, V, Mitra, DK. Mediastinal cystic hygroma in infancy and childhood. Pediatr Surg Int. 1992; 7:380–381.
57. Wright, CC, Cohen, DM, Vegunta, RK, et al. Intrathoracic cystic hygroma: A report of three cases. J Pediatr Surg. 1996; 31:1430–1432.
58. Sumner, TE, Volberg, FM, Kiser, PE, et al. Mediastinal cystic hygroma in children. Pediatr Radiol. 1981; 11:160–162.
59. Glasson, MJ, Taylor, SF. Cervical, cervicomediastinal and intrathoracic lymphangioma. Prog Pediatr Surg. 1991; 27:62–83.
60. Alqahtani, A, Nguyen, LT, Flageole, H, et al. 25 years’ experience with lymphangiomas in children. J Pediatr Surg. 1999; 34:1164–1168.
61. Okazaki, T, Iwatani, S, Yanai, T, et al. Treatment of lymphangioma in children: Our experience of 128 cases. J Pediatr Surg. 2007; 42:386–389.
62. Kitano, Y, Iwanaka, T, Tsuchida, Y, et al. Esophageal duplication cyst associated with pulmonary cystic malformations. J Pediatr Surg. 1995; 30:1724–1727.
63. Horwitz, JR, Lally, KP. Bronchogenic and esophageal duplication cyst in a single mediastinal mass in a child. Pediatr Pathol Lab Med. 1996; 16:113–118.
64. Almog, B, Leibovitch, L, Achiron, R. Split notochord syndrome—prenatal ultrasonographic diagnosis. Prenat Diagn. 2001; 21:1159–1162.
65. Schurink, M, van Herwaarden-Lindeboom, MY, Coppes, MH, et al. Neurenteric cyst—a case report of this rare disorder. J Pediatr Surg. 2007; 42:E5–E7.
66. Kumakura, A, Takahara, T, Asada, J, et al. Split notochord syndrome with congenital unilateral Horner’s sign. Pediatr Neurol. 2008; 38:47–49.
67. Kawahara, H, Kamata, S, Nose, K, et al. Congenital mediastinal cystic abnormalities detected in utero: Report of two cases. J Pediatr Gastroenterol Nutr. 2001; 33:202–205.
68. Suen, HC, Mathisen, DJ, Grillo, HC, et al. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg. 1993; 55:476–481.
69. Ribet, ME, Copin, MC, Gosselin, B. Bronchogenic cysts of the mediastinum. J Thorac Cardiovasc Surg. 1995; 109:1003–1010.
70. Martinez Ferro, M, Milner, R, Voto, L, et al. Intrathoracic alimentary tract duplication cysts treated in utero by thoracoamniotic shunting. Fetal Diagn Ther. 1998; 13:343–347.
71. Schier, F, Waldschmidt, J. Thoracoscopy in children. J Pediatr Surg. 1996; 31:1640–1643.
72. Michel, JL, Revillon, Y, Montupet, P, et al. Thoracoscopic treatment of mediastinal cysts in children. J Pediatr Surg. 1998; 33:1745–1748.
73. Merry, C, Spurbeck, W, Lobe, TE. Resection of foregut-derived duplications by minimal-access surgery. Pediatr Surg Int. 1999; 15:224–226.
74. Engum, SA. Minimal access thoracic surgery in the pediatric population. Semin Pediatr Surg. 2007; 16:14–26.
75. Suita, S, Tajiri, T, Sera, Y, et al. The characteristics of mediastinal neuroblastoma. Eur J Pediatr Surg. 2000; 10:353–359.
76. Morris, JA, Shcochat, SJ, Smith, EI, et al. Biological variables in thoracic neuroblastoma: A Pediatric Oncology Group study. J Pediatr Surg. 1995; 30:296–302.
77. La Quaglia, MP, Kushner, BH, Su, W, et al. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004; 39:412–417.
78. Escobar, MA, Grosfeld, JL, Powell, RL, et al. Long-term outcomes in patients with stage IV neuroblastoma. J Pediatr Surg. 2006; 41:377–381.
79. Shadmehr, MB, Gaissert, HA, Wain, JC, et al. The surgical approach to ‘dumbbell tumors’ of the mediastinum. Ann Thorac Surg. 2003; 76:1650–1654.
80. Takeda, S, Miyoshi, S, Minami, M, et al. Intrathoracic neurogenic tumors—50 years’ experience in a Japanese institution. Eur J Cardiothorac Surg. 2004; 26:807–812.
81. Jaffe, N, Cassady, R, Petersen, R, et al. Heterochromia and Horner syndrome associated with cervical and mediastinal neuroblastoma. J Pediatr. 1975; 87:75–77.
82. McRae, D, Jr., Shaw, A. Ganglioneuroma, heterochromia iridis, and Horner’s syndrome. J Pediatr Surg. 1979; 14:612–614.
83. Mam, MK, Mathew, S, Prabhakar, BR, et al. Mediastinal enterogenic cyst presenting as paraplegia—a case report. Indian J Med Sci. 1996; 50:337–339.
84. Akwari, OE, Payne, WS, Onofrio, BM, et al. Dumbbell neurogenic tumors of the mediastinum. Diagnosis and management. Mayo Clin Proc. 1978; 53:353–358.
85. Plantaz, D, Rubie, H, Michon, J, et al. The treatment of neuroblastoma with intraspinal extension with chemotherapy followed by surgical removal of residual disease. A prospective study of 42 patients—results of the NBL 90 Study of the French Society of Pediatric Oncology. Cancer. 1996; 78:311–319.
86. Kang, CH, Kim, YT, Jeon, SH, et al. Surgical treatment of malignant mediastinal neurogenic tumors in children. Eur J Cardiothorac Surg. 2007; 31:725–730.
87. Sue, K, Yamanaka, K, Nakamura, M. Thoracoscopic resection of a ganglioneuroma in the posterior mediastinum of 3-year-old boy. Pediatr Surg Int. 1998; 14:151.
88. Rothner, AD. Congenital ‘dumbbell’ neuroblastoma with paraplegia. Clin Pediatr (Phila). 1971; 10:235–236.
89. Shimada, Y, Sato, K, Abe, E, et al. Congenital dumbbell neuroblastoma. Spine. 1995; 20:1295–1300.
90. Boglino, C, Martins, AG, Ciprandi, G, et al. Spinal cord vascular injuries following surgery of advanced thoracic neuroblastoma: An unusual catastrophic complication. Med Pediatr Oncol. 1999; 32:349–352.
91. Gorospe, L, Fernandez-Gil, MA, Torres, I, et al. Misleading lead: Inflammatory pseudotumor of the mediastinum with digital clubbing. Med Pediatr Oncol. 2000; 35:484–487.
92. Lee, BH, George, S, Kutok, JL. Langerhans cell histiocytosis involving the thymus. A case report and review of the literature. Arch Pathol Lab Med. 2003; 127:e294–e297.
93. Moran, CA, Rosado-de-Christenson, M, Suster, S. Thymolipoma: Clinicopathologic review of 33 cases. Mod Pathol. 1995; 8:741–744.
94. Burt, M, Ihde, JK, Hajdu, SI, et al. Primary sarcomas of the mediastinum: Results of therapy. J Thorac Cardiovasc Surg. 1998; 115:671–680.
95. Plukker, JT, Joosten, HJ, Rensing, JB, et al. Primary liposarcoma of the mediastinum in a child. J Surg Oncol. 1988; 37:257–263.