Meconium Aspiration Syndrome
After reading this chapter, you will be able to:
• List the anatomic alterations of the lungs associated with meconium aspiration.
• Describe the causes of meconium aspiration.
• List the cardiopulmonary clinical manifestations associated with meconium aspiration syndrome (MAS).
• Describe the general management of meconium aspiration.
• Describe the clinical strategies and rationales of the SOAPs presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs
First, the physical presence of the meconium results in upper airway obstruction at birth because of the high viscosity of the meconium. Shortly after birth (within 1 hour), and especially if gasping inspirations are present, clumps of meconium rapidly migrate past the glottis and penetrate the smaller airways (see Figure 32-1). In cases of severe intrauterine hypoxemia, meconium may already be present in the distal airways at birth.
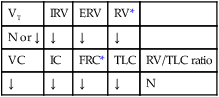
When thick particulate meconium is aspirated into the small airways, the meconium can partially or totally obstruct the airways. Airways that are partially obstructed are affected by a “ball-valve” effect, in which air can enter but cannot readily leave the distal airways and alveoli. This condition in turn leads to air trapping and alveolar hyperinflation. Excessive hyperinflation commonly leads to alveolar rupture and air leak syndromes (see Chapter 35) such as pneumomediastinum or pneumothorax. Totally obstructed airways lead to alveolar shrinkage and atelectasis. This combination of areas of overexpanded alveoli adjacent to areas of atelectasis creates both an increased functional residual capacity (FRC) and a decrease in air flow during exhalation.
The major pathologic or structural changes associated with MAS are as follows:
• Physical presence of the meconium leading to:
• Partially obstructed airways, air trapping, and alveolar hyperinflation
• Pulmonary air leak syndromes (pneumomediastinum or pneumothorax)
• Edema of the bronchial mucosa and alveolar epithelium
• Excessive bronchial secretions
CASE STUDY
Meconium Aspiration Syndrome (MAS)
Admitting History and Physical Examination
Respiratory Assessment and Plan
O Apneic at birth, hypoactive, cyanotic, covered with meconium. 1 minute Apgar 1, 5 minute Apgar 6. Bilateral crackles and rhonchi. Meconium suctioned from oral and pharyngeal areas.
• Possible MAS (meconium in airway)
• Airway secretions (meconium?) (crackles and rhonchi)
• Probable asphyxic episode; likely combined respiratory and metabolic acidosis (history, cyanosis)
P Mechanical Ventilation Protocol in combination with Oxygen Therapy and Hyperinflation Therapy Protocol (RR 40, Fio2 100%, PIP +25, flow 8 L/min, and PEEP 5). Bronchopulmonary Hygiene Protocol (suction prn and CPT qid). Assist physician with surfactant administration. Monitor closely (oximetry, vital signs, watch for signs of acute air leak, pulmonary hemorrhage).
Respiratory Assessment and Plan
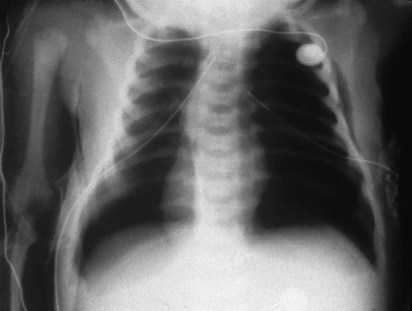
O Pink skin. Bilateral crackles and rhonchi. Atelectasis in the right upper and middle lobes. Air trapping right and left lower lobes. ABGs: pH 7.19, Paco2 37,  14, Pao2 87, and Spo2 94% (on Fio2 1.0).
14, Pao2 87, and Spo2 94% (on Fio2 1.0).
P Continue Mechanical Ventilation Protocol in combination with Oxygen Therapy and Hyperinflation Therapy Protocols (RR 40, Fio2 100%, PIP +30 cm H2O, and PEEP +5 cm H2O). Continue Bronchopulmonary Hygiene Protocol (suction prn and CPT qid). Monitor closely (vital signs, watch for signs of acute air leak, pulmonary hemorrhage).
Discussion
As with adult subjects, several of the clinical manifestations in this case can be traced back through the “clinical scenarios” associated with Atelectasis (see Figure 9-8) and Excessive Bronchial Secretions (see Figure 9-12). For example, the increased lung density caused by the atelectasis was revealed on the chest x-ray film, and the crackles and rhonchi were produced by the excessive airway secretions recorded in the second SOAP.

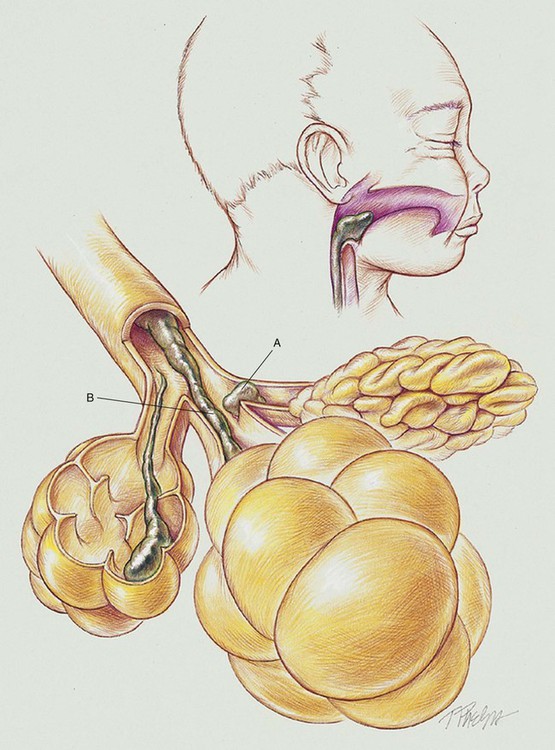


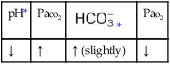
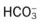 values will be lower than expected for a particular Pa
values will be lower than expected for a particular Pa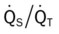
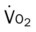
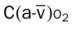
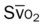

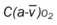 , Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;  , pulmonary shunt fraction;
, pulmonary shunt fraction; 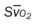 , mixed venous oxygen saturation;
, mixed venous oxygen saturation;  , oxygen consumption.
, oxygen consumption.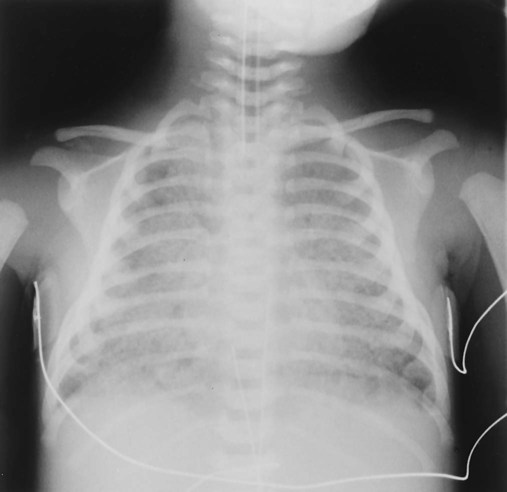
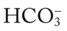 14, Pa
14, Pa