Chapter 32 Mechanistic and Methodological Considerations for the Imaging of Mental Stress Ischemia
INTRODUCTION
The role of emotion or stress in the provocation of angina pectoris has been described as far back in ancient history as Celsus, followed by Hunter in the 1700s, and by Osler at the turn of the 20th century.1 We have an intuitive sense of the importance of these factors, yet gathering empirical data to support or refute this intuition have been slow to unfold. The effort to do so has been hindered by the impact of the philosophical proposition of the duality of the mind and body entrenched in Western culture since Descartes, and by the compartmentalized structure of biomedical research.2 Modern epistemological/ontological constructs and emerging studies in neuroscience have questioned this duality. The latter enjoined with the expansion of integrative biomedical research will shed further light on the interaction of emotional processing/triggers and biological consequences in the next decade.
In the few short years since the previous edition of this volume, new information regarding the nature of mental stress–provoked ischemia, with refinement of key aspects of the pathophysiologic construct, has emerged. In this edition, we discuss new advances in diagnostic tools available to improve the accuracy of mental stress ischemia (MSI) diagnosis, such as peripheral arterial tonometry (PAT). We review MSI as a vulnerability factor for lethal arrhythmias and arrhythmia-induced implantable cardioverter defibrillator (ICD) shocks. We also describe the phenomenon of myocardial stunning due to sudden emotional stress (variably known as takotsubo cardiomyopathy or “broken heart syndrome”) as part of the continuum described by stress-provoked cardiac phenomena. In the previous edition, we outlined the importance of parasympathetic withdrawal and endothelial dysfunction in response to stress or emotionally provoked ischemia. We will discuss the possible links between these two consistent observations of MSI (parasympathetic withdrawal and endothelial dysfunction), based upon studies describing a new pathway that may mediate the vascular effects of MSI, termed the cholinergic antiinflammatory reflex.3
MENTAL STRESS ISCHEMIA
Myocardial ischemia that occurs in response to mental and emotional stress—MSI—is a phenomenon of clinical importance associated with a threefold increased risk of poor outcome in patients with coronary artery disease (CAD).4–9 The pathophysiology of MSI demonstrates a degree of overlap with that of demand-induced ischemia; however, MSI has distinct physiologic correlates, as suggested by differences in hemodynamic, vascular, and neuroendocrine responses to these distinct forms of stress.10,11 In addition, certain psychological factors related to the experience and expression of anger and hostility identify patients who are more vulnerable to MSI.12–15 The history of nuclear cardiology is replete with examples of our creativity in addressing difficult diagnostic challenges in order to demonstrate the manifestation of occult myocardial ischemia. Myocardial ischemia provoked by mental and emotional stress is pervasive and an important presentation within the spectrum of CAD. The overarching goal of this chapter is to provide a working conceptual construct grounded in empirical data on which to base future noninvasive testing for the identification of those at risk for MSI.
ECG Changes During Mental Stress
The ability to detect myocardial ischemia under real-life situations was made possible by the development of the Holter monitor in the 1960s, which records the ECG in real time on a medium that provides for later examination of a depression in the ST-segment—the marker of ischemia. By the latter part of that decade, cardiologists were using this technology to monitor coronary patients while they drove their car, while they engaged in routine daily activities, and while they performed stressful tasks in the laboratory. Studies using this approach demonstrated that among patients with CAD, ischemia is common during moderate to extreme mental and emotional activity independent of the degree of concurrent physical exertion,15–18 is most usually without symptoms of angina, and accounts for up to 75% of total ischemic burden (Fig. 32-1).14,16,19 Most ischemic episodes during mental stress were found to occur at lower heart rate and blood pressure than episodes during physical exertion, supporting the concept of dynamic coronary obstruction (i.e., ischemia caused by changes in arterial tone) as a pathophysiologic mechanism. This does not imply, however, that myocardial demand is not a contributor to MSI as well, since increases in diastolic blood pressure in particular are often seen.20
Left Ventricular Dysfunction During Mental Stress
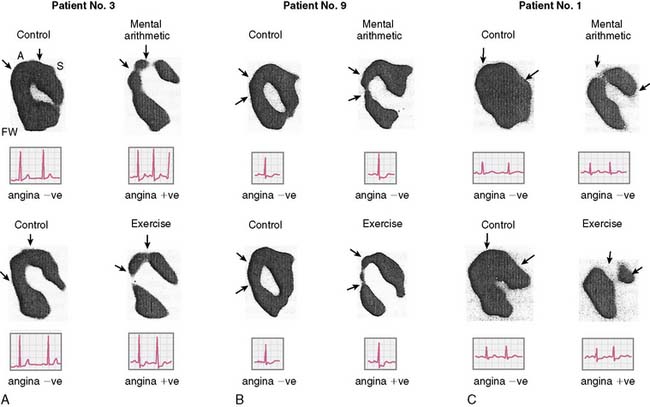
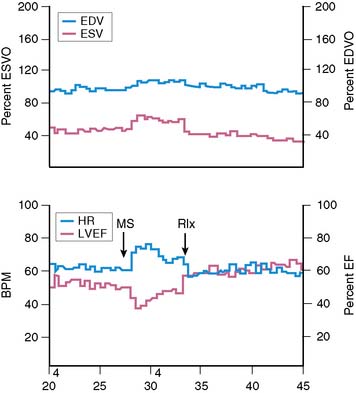
Assessment of left ventricular ejection fraction (LVEF) provides a sensitive (but potentially less specific) method to evaluate the acute cardiovascular effects of various interventions under controlled conditions. The development of radionuclide- and ultrasound-based methods to assess LVEF provided the opportunity to more directly examine the effects of stress on myocardial function as an index of ischemia, and marked the next step in the emergence of this research. The approaches used for this purpose have included equilibrium radionuclide angiography (ERNA), echocardiography, and volumetric measurement with a nonimaging nuclear probe. ERNA has been used in conjunction with mental stress for determination of LVEF and wall-motion abnormalities (WMAS). This approach is similar to that used for exercise ERNA studies, which are no longer routinely performed. In addition to this more standard approach, left ventricular (LV) function during mental stress has been assessed with a nonimaging nuclear probe after injection of radiolabeled red blood cells. This method has the advantage of providing beat-to-beat global LV volume curves, allowing assessment of rapid changes in LV function over an extended period (Fig. 32-2). The disadvantages are that regional wall motion cannot be assessed, and the technology is not widely available. The criteria used to define MSI by these techniques are similar to those used for other forms of stress: a reduction in LVEF of 5% or more and/or the development of a new regional WMA.
Overall, studies conducted in laboratory settings using such methods clearly established that mental stress—induced by having the patient perform any one of several mentally/emotionally demanding tasks—induced transient global and/or regional LV dysfunction in upwards of 50% of patients with CAD. Among patients who demonstrated this dysfunction, it occurred rapidly after the initiation of the stressful task, usually within 1 minute, and recovery was temporally related to the discontinuation of the task and likewise tended to occur rapidly.21 Consistent with the ambulatory ECG data, these abnormalities developed at a lower heart rate than with exercise, were usually asymptomatic, and occurred predominantly, although not exclusively, in patients with exercise tests that were positive for ischemia.12
Studies Using Radionuclide Angiography
Rozanski and colleagues12 were among the first to describe mental stress–induced LV dysfunction by ERNA in a study of CAD patients and normal controls during mental stress and exercise. Largely asymptomatic WMAs were observed during mental stress in 59% of the CAD patients, and 36% had a decrease in ejection fraction of more than 5%. This occurred at a lower heart rate than ischemia induced during exercise, and ECG changes indicative of ischemia were rarely seen. These researchers using the same criteria subsequently found MSI in up to 75% of CAD patients with exercise-induced ischemia. In other studies using only changes in global LV function as evidence of MSI (decrease in LVEF), 50% to 60% of CAD patients demonstrated global LV dysfunction during mental stress.22,23 These changes were usually without ECG changes and—again—asymptomatic.
Studies Using Echocardiography
Data from studies using echocardiography to assess changes in LV function during mental stress have been largely consistent with data from radionuclide-based studies. For example, Modena and colleagues24 reported mental stress–induced LV dysfunction in 38% of patients with chest pain who were referred for angiography. Gottdiener and colleagues25 similarly reported over 50% of patients with known CAD had new regional WMA during mental stress echocardiography. Patients with MSI defined in this manner also showed more frequent ischemia by ambulatory ECG monitoring during sedentary activities.
Despite evidence of LV dysfunction induced during mental stress, a number of unresolved issues remained. For example, the specificity of the LV response as an index of ischemia during mental versus physical stress was unclear. Mental stress causes a substantial sympathetic response that leads to an increase in systemic vascular resistance,11 which may affect LV function independent of ischemia. Indeed the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study10 found that 41% of older normal subjects (43 to 73 years old) exhibited a decrement in LVEF of 5% during mental stress. The reproducibility of LV functional changes during mental stress was and remains a second unresolved issue. The PIMI study data suggest that approximately two-thirds of patients have consistent responses when studied on different days, with the observed reproducibility a function of the type of stressor utilized.26 Others have corroborated these findings.27 A third issue concerned the association between LV functional changes and other potentially more specific indicators of ischemia, such as myocardial perfusion defects. These issues have implications for the development of standardized approaches for risk stratification in the clinical setting.
Myocardial Perfusion During Mental Stress
In an early clinical application of positron emission tomography (PET), Deanfield and colleagues14 found that 75% of patients with positive exercise tests had regional perfusion abnormalities during mental stress. Although the rate-pressure product was lower with mental stress than with exercise, the degree and location of perfusion defect were largely similar between the two forms of stress. A subsequent study by others using planar technetium-99m 99mTc sestamibi perfusion imaging found that 85% of CAD patients had myocardial perfusion abnormalities in the same vascular territory during both mental and physical stress, though the extent and degree of abnormalities were smaller during mental stress.28 These studies, using qualitative methods for assessment of myocardial perfusion, demonstrated that regional heterogeneity of blood flow develops in many patients during mental stress.
Quantitative assessment of myocardial blood flow by PET provides particular insights regarding the differential pathophysiology of MSI. This approach allows investigation of effects that may be more subtle than can be observed by more conventional approaches to assessment of LV function or myocardial perfusion. Several groups of investigators have used this approach to assess the effect of mental stress on absolute myocardial blood. For example, studying CAD patients and normal subjects, Schoder and colleagues found that despite similar increases in rate-pressure product in both cohorts, the magnitude of flow increase during mental stress was less in the patients.29 Subsequently, our group investigated regional differences in blood flow response to mental versus pharmacologic stress in CAD patients and found that with mental stress, a blunted flow response was observed, primarily in regions without significant epicardial coronary stenosis.30 These findings suggest that mental stress may more predominantly affect coronary microvascular tone, implicating this as an operative factor in the pathogenesis of myocardial ischemia at relatively low levels of work.
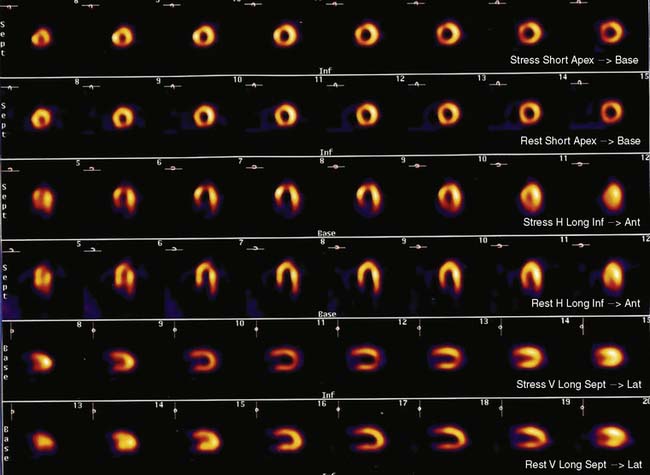
Flow heterogeneity during mental stress may be mild, variable, and perhaps insufficient to generate regional wall motion. Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI), less costly than approaches that rely upon PET, may therefore represent a best and most cost-effective approach to the diagnosis of MSI. Indeed, there is a growing body of research using this modality to study the effects of mental stress. It remains to be determined, however, whether this approach will result in greater sensitivity/specificity in the detection of MSI or incremental prognostic value for patients with CAD. In addition, the relationship of perfusion defects to LV dysfunction during mental stress is unclear. For example, our group has simultaneously assessed perfusion (using SPECT sestamibi) and function (using echocardiography), and has found a significant discordance between perfusion and functional changes during mental stress.31 Further study is needed to assess the significance of these findings. Nonetheless, MPI defects are a reliable indicator of ischemia (Fig. 32-3).
Comparison Between Mental Stress Imaging and Standard Exercise and Pharmacologic MPI
Although both mental stress and exercise induce hemodynamic changes, differences exist in the magnitude of change between these stressors. For example, the increase in heart rate during mental stress is much less than that during exercise.11,12 Systolic blood pressure response can be less than or similar to that during exercise, whereas diastolic blood pressure increases more during mental stress.11,12 Additionally, the rate of increase in the rate-pressure product is accelerated with mental stress and can peak within 1 minute of the onset of stress.21 These differences in hemodynamic responses likely relate to differential neurohormonal activation and peripheral vascular effects. For example, during mentally stressful tasks, epinephrine levels increase more than norepinephrine levels. The opposite is observed during exercise.32 Peripheral vascular resistance decreases during exercise but increases or remains constant during mental stress.11,33 The observation that MSI occurs at relatively low rate-pressure product suggests that other mechanisms such as coronary vasoconstriction and the neurohormonal effects on endothelium-dependent vasomotor tone may be operative. The importance of these findings to any differential pathophysiology between mental stress and exercise-induced ischemia, however, remains to be determined.
Recent studies suggest that vulnerability to either exercise or mental stress–provoked ischemia in the same individual does not reliably predict ischemia to both stimuli. For example, Ramachandruni et al.34 found that 6 of 21 CAD patients (29%) who had previously shown no flow defect during exercise or pharmacologic provocation had a reversible flow defect with mental stress. Conversely, as described earlier, only 40% to 60% of CAD patients with exercise-induced or pharmacologically induced ischemia have MSI. More recently, Hassan et al.35 demonstrated significant intraindividual variability in the severity and location of myocardial ischemia provoked by mental versus exercise or pharmacologic stress. This study of 187 patients with CAD found only 71% concordance for the provocation of ischemia between mental versus exercise/pharmacologic stress. In addition, 11% of the cohort demonstrated ischemia to mental but not exercise/pharmacologic stress, while 22% demonstrated ischemia to exercise/pharmacologic but not mental stress. Furthermore, the location of flow defect(s) provoked by mental versus exercise/pharmacologic stress was often different, as was the severity of the flow defect(s) observed. A prior study by Arrighi et al.30 using regional PET MPI may shed some light on the pathophysiology underlying the Hassan group’s results. In their study, Arrighi and colleagues found that pharmacologic stress caused an expected absolute reduction of myocardial blood flow in regions with significant epicardial disease (compared to regions without significant epicardial disease), and that this decreased flow was associated with a compensatory reduction in distal coronary microvascular resistance. Conversely, during mental stress, absolute myocardial blood flow was lower in regions without significant epicardial disease than in regions with significant epicardial disease, and was associated with an increase in distal coronary microvascular resistance. The study by Arrighi et al. therefore provides insights into the Hassan et al. findings by suggesting a prominent role for coronary microvascular dysfunction in mental versus exercise/pharmacologic stress–provoked ischemia. Overall, these studies describe contrasts in the coronary vascular response to mental stress and traditional clinical provocations to myocardial ischemia. Differences in CNS activity for these discordant groups are emerging and will be discussed under the heading “Functional PET Brain Imaging.”
Prognostic Significance of Myocardial Stress Ischemia
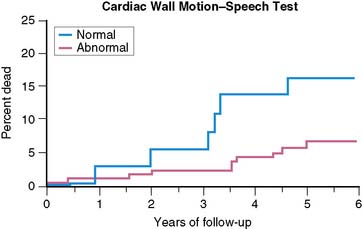
The prognostic significance of MSI has been explored by a number of investigators. In the first published report,33 there were a significantly greater (P < 0.025) number of events (MI, unstable angina) at 2 years in a group of patients who had previously demonstrated LV dysfunction—assessed by radionuclide methods—during mental stress than in the group who did not show this response. In a later study,36 patients with LV dysfunction—by ERNA—during mental stress were at increased 2-year risk of events (MI, unstable angina, revascularization; risk ratio = 2.40), even after adjusting for age, history of MI, and baseline EF. Similarly, in patients followed over a 4.4-year follow-up period (median = 3.5 years),37 almost 45% of patients with MSI in the lab—defined by WMA on ECHO or ERNA—experienced an event (death, MI, unstable angina), while less than 25% of patients without MSI experienced an event. Most recently, the PIMI investigators have reported on the prognostic significance of MSI in their multicenter study,38 with 17 patients dying during an average follow-up of 5.2 years (Fig. 32-4). MSI—defined by WMA on ERNA—had been demonstrated among 40% of those who died but only 17% of those who survived (rate ratio = 3.0; P < 0.04). Other indicators of ischemia during MS testing, including LVEF and/or ECG changes, did not predict death.
Psychological Factors and Prognosis in Mental Stress Ischemia
The contribution of psychological makeup to prognosis in patients with CAD deserves special mention inasmuch as factors including depression, anxiety, anger, and hostility have broadly been reported. Determination of the specific psychological factors that contribute to risk for MSI may help to determine when mental stress testing is likely to provide important prognostic information. Studies have found, for example, that patients with easily provoked anger and hostility have twice the incidence of CAD and a fivefold increased risk of recurrent MI over an extended follow-up period.37 High levels of hostility have also been associated with a higher incidence of restenosis after angioplasty38 and rapid progression of CAD.39 Similarly, chronic anger is associated with a 2.7 relative risk of cardiovascular death, myocardial infarction, and angina,40 whereas the experience of moderate to extreme anger increases risk of MI 2.5-fold for up to 2 hours.41
Data from our group and others specifically related mental stress–induced LV dysfunction to psychological makeup. In comparing patients with MSI to those without MSI, we found no difference on standard clinical indicators, on measures of cardiovascular performance during mental stress testing and exercise thallium testing, or on measures of CAD severity. Patients with MSI, however, scored higher on measures of emotional arousability, hostility, anger, and aggressive response to perceived provocation, and lower on a measure of anger control. In addition, the duration of ischemia evidenced during a clinical interview used as a measure of emotional arousability was highly correlated with the degree of emotional arousability measured by the interview (0.70, P < 0.0001). Hence, a hostile, angry psychological profile was associated with emotional arousability, which increased the risk of MSI.42
PATHOPHYSIOLOGY OF MENTAL STRESS ISCHEMIA
Mental Stress and Vascular Function
Coronary angiography studies show direct evidence of epicardial coronary artery segmental vasoconstriction during mental stress43–45 in concert with a paradoxical vasoconstriction of these segments to intracoronary administration of acetylcholine.44 This finding suggests that endothelial dysfunction is a substrate upon which mental stress acts. Further evidence from our lab, described earlier, indicates that rather than epicardial vasoconstriction, the greater impact of mental stress is on coronary blood flow in the microvascular bed.30,45 These findings overall suggest that endothelial dysfunction is a substrate upon which mental stress acts.
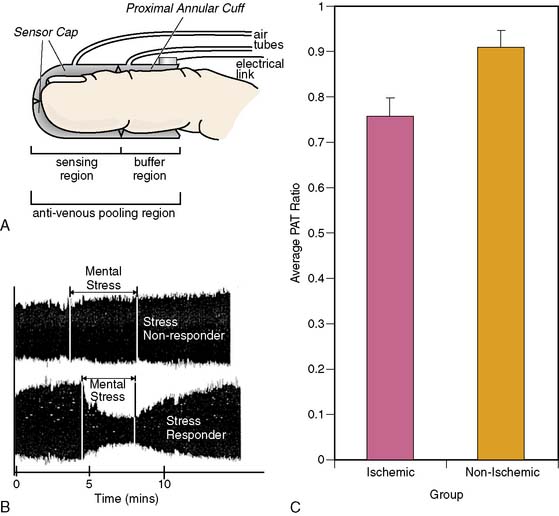
Vascular beds are richly innervated by adrenergic fibers46 through which regulation by sympathetic nervous system activity is accomplished. The performance of vascular beds in the periphery has been found to correlate with the performance of the coronary microvascular bed under given conditions. Therefore, measurement of pulse wave amplitude (PWA) in cutaneous vascular beds by peripheral arterial tonometry (PAT) is being evaluated for its added value as a noninvasive diagnostic tool to identify vulnerability to mental stress.
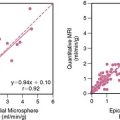
The EndoPAT-2000 (Itamar Inc.) is a self-contained, computer-operated device that has been validated for use in the assessment of sympathetic activity in the setting of exercise stress testing.47,48 It has also recently demonstrated moderate correlation between PWA in cutaneous beds and peripheral endothelial function assessed by brachial artery ultrasound.48 Goor et al. used PAT for the assessment of mental stress effects in patients with stable CAD. During simultaneous assessment of ejection fraction and WMA by ERNA, and PWA assessment by PAT, the PWA began to change as soon as 4 seconds after the transition from rest to stress. A positive PAT tracing—defined as PWA reductions of at least 20% from rest to mental stress—was 88% concordant with ERNA results.49 Our group has tested the utility of PAT in predicting MSI in patients with CAD. Patients with MSI had significantly lower ratios of PWA during stress to rest than those patients without MSI (0.76 versus 0.91) (Fig. 32-5). This PAT ratio had a sensitivity of 62% and a specificity of 63% in detecting MSI. In patients who were on angiotensin-converting enzyme (ACE) inhibitors, the sensitivity improved to 73% and specificity to 86%.50 This new diagnostic modality, properly utilized, might serve as a screening tool for primary and secondary prevention of MSI-vulnerable patients.
Several studies have also demonstrated that mental stress can provoke sustained impairment in endothelial function, assessed by forearm hyperemic flow, with one study in particular demonstrating that this effect is mediated by endothelin-A receptors.51 Using this approach, Cardillo and colleagues52 found that inhibition of nitric oxide synthesis attenuated a previously observed vasodilator response to mental stress. Each of these findings is consistent with the concept that vasomotor response to mental stress is endothelially mediated. Thus, mental stress–induced transient endothelial dysfunction may be an important factor in the pathogenesis of MSI in patients with known CAD.
Mental Stress and Inflammation
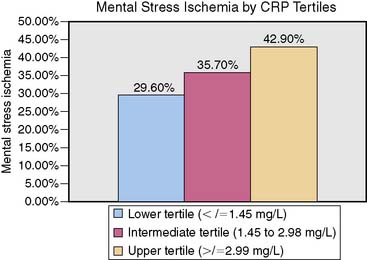
An indicator of chronic inflammation, C-reactive protein (CRP) has been identified as a risk marker for acute coronary syndromes (ACS) and a direct participant in the progression of atherosclerosis.53,54 Our group55 has found a positive correlation between serum CRP and MSI (r = 0.23; P = 0.04) and a dose-response relationship, with 42.7% of patients showing clinically meaningful CRP levels also demonstrating MSI. Each unit (1 mg/L) increase in CRP level was associated with 20% higher risk of MSI (OR 1.2; 95% CI 1.01–1.29; P = 0.04) (Fig. 32-6). These findings indicate that inflammatory processes may play a role in the provocation of MSI.
Cholinergic Antiinflammatory Reflex
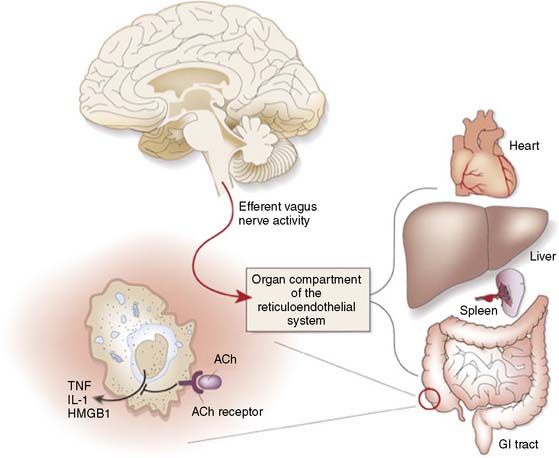
As described, mental stress provokes an increase in the sympathetic response, often paired with a decrease in parasympathetic tone.1 The complex interplay between the CNS and the periphery that maintains the balance of homeostasis and mounts responses to both internal and external threats is rich with multiple interacting systems and complex negative-feedback loops. A growing body of evidence points to a role for the autonomic nervous system in the regulation of inflammatory processes germane to CAD. The relationship between autonomic balance and the release of inflammatory factors is complex and dependent upon variables that are presently not fully understood in a human in vivo model. Increased sympathetic activation can play an important proinflammatory role, working through A2 receptors to increase production of TNF-α and comparable markers. In addition, there is emerging evidence linking decrements in parasympathetic tone to the generation of inflammatory cytokines such as TNF-α.3 The latter has been described as the cholinergic antiinflammatory reflex (Fig. 32-7). The involvement of specific brain regions (including the medial prefrontal cortex) in the vagal component of heart rate regulation during self-generated emotions has been demonstrated,56 and we have also found these regions activated in concert with parasympathetic withdrawal during mental stress (see later discussion).
TNF-α is a proinflammatory molecule involved in atherosclerotic plaque rupture, coronary artery vasospasm, and ischemic injury.57 In CAD patients, brief exposure to TNF-α depresses endothelium-dependent relaxation58–62 and causes coronary vasoconstriction63 and decreased coronary flow rate.64 Furthermore, it can provoke release of endothelin-1 (ET-1) from macrophages65,66 and has been observed in combination with ET-1 to promote constriction in the microvascular bed.67 The effect is specific for endothelium-dependent vasodilators.58 In addition to promoting release of ET-1, TNF-α alters endothelial vasomotor responses by blocking the activation of endothelial nitric oxide synthase (eNOS), which is essential for flow-dependent relaxation of blood vessels.68 TNF-α directly degrades eNOS mRNA69 and contributes to the posttranscriptional inactivation of eNOS.
With these findings in mind, one could describe a model whereby during mental stress, the observed parasympathetic withdrawal would leave macrophages without the cholinergic inhibitory input from the vagus nerve. The resulting increase in local release of cytokines and related vasoactive proteins (e.g., ET-1) would favor vasoconstriction in vascular beds. Furthermore, the increase of sympathetic output, also evident during metal stress, would lead to an unopposed vasoconstrictive effect of catecholamines. The speed with which this response operates and the wide distribution of macrophages in diseased coronary vessels may implicate this reflex as a mechanism by which the effects of mental stress on coronary microvascular beds are modulated. Preliminary evidence is emerging that CAD patients who demonstrate parasympathetic withdrawal during anger recall in the laboratory are more likely to demonstrate increases in levels of ET-1 during stress.70
Neurocardiac Central Nervous System Correlates
Functional PET Brain Imaging
We and others have developed techniques to study the effects of mental stress on both brain activity (using 15O-water PET) and cardiac function (using simultaneous echocardiography or SPECT MPI) to investigate the role of the central nervous system (CNS) in MSI. These studies are yielding a number of interesting observations. First, during mental arithmetic stress, cortical frontal-limbic circuits implicated in affect and cognition are activated in patients with CAD but not in healthy subjects.71 The greater activation in cortical areas may indicate that the task of mental calculation requires more effort in CAD patients than in healthy subjects and may indicate more intense activation of areas associated with emotion and memory as well. Second, comparison of CAD patients with and without MSI showed distinct activation during MSI in the hippocampus and anterior cingulate region, suggesting important influences of the brain regions implicated in mediation of stress, emotion, and memory (Fig. 32-8).72,73 The cortical regions implicated in these studies may be integral to stress effector systems that transduce cardiovascular reactivity and vasomotor tone. These effector systems are mediated by neurohormonal constituents in vascular and tissue compartments. Thus, brain PET imaging is a useful research tool to investigate the effect of the CNS in the pathophysiology of MSI.
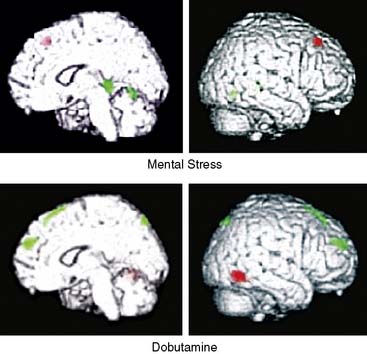
Our group evaluated 58 subjects with CAD with simultaneous measurement of cerebral blood flow with 15O PET and cardiac wall-motion analysis with echocardiography during arithmetic mental stress and dobutamine stress.74 Thirteen patients (22%) were ischemic during mental stress but not during dobutamine stress. When brain PET images were analyzed, cerebral hyperactivation was observed in the frontolimbic circuits and neocortical regions of the brain during mental stress relative to dobutamine stress. These areas are associated with emotion, memory, fear, anxiety, and autonomic regulation. These findings support the notion that mental stress may produce ischemia in some subjects with CAD by different operative mechanisms than those described in exercise or pharmacologic-induced ischemia.56
MENTAL STRESS AND DISEASE—EMERGING AREAS
Takotsubo Cardiomyopathy
Reversible myocardial stunning in response to extreme emotional stress—takotsubo cardiomyopathy—is a phenomenon that is attracting growing attention among clinicians and those who study the effects of stress on the heart. This clinical presentation is associated with markedly increased serum catecholamine levels accompanying a transient and profound decrease in LV systolic function, with no angiographic evidence of significant CAD.75 Takotsubo cardiomyopathy was initially described by Dote in Japan,76 with subsequent case reports and series around the world demonstrating a similar presentation. Characteristically, there is apical ballooning and compensatory hyperkinesis of the basal segments of the heart.
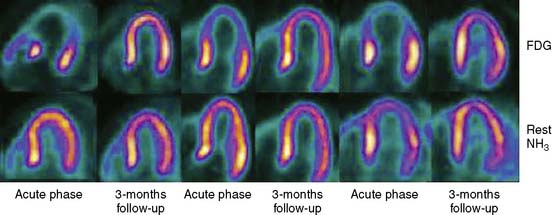
A number of pathways have been proposed to account for this phenomenon, including multivessel coronary vasospasm, abnormalities in coronary microvascular function, and catecholamine-mediated cardiotoxicity. The definite pathophysiology of this syndrome, however, remains unknown. SPECT MPI with these patients77–79 consistently shows impairment immediately after hospital admission, with considerable improvement at 3 to 5 days. The presentation seems to be more frequent in Caucasian or Asian females (age > 50). Most of the reports and case series typically describe the absence of significant coronary disease. Nevertheless, evaluations of TIMI frame counts in patients being admitted with transient LV apical ballooning syndrome have shown significant abnormalities in one or more epicardial coronary vessels, compared to matched controls.78,80 PET studies using nitrogen-13-ammonia and 18-fluorodeoxyglucose have documented a regional transient decrease in myocardial blood flow and coronary flow reserve during the acute phase of takotsubo cardiomyopathy that completely resolves after 3 months (Fig. 32-9).81–84 The use of iodine-123-metaiodobenzylguanidine (I-123 MIBG) has recently shown regional cardiac denervation in the apex and in the inferior wall of the left ventricle during the acute phase.85 Takotsubo is a multifaceted and apparently stress-provoked phenomenon that is defined by elements of flow, function, and metabolism. A multimodal imaging approach that captures each of these elements by including echocardiography, coronary angiography with left ventriculography, cardiac magnetic resonance imaging, and PET/SPECT metabolic imaging may allow the precise depiction of the various aspects of the diagnosis. Takotsubo represents a potentially important window into the processes by which mental/psychological stress can profoundly and acutely affect myocardial performance and myocardial perfusion.
Electrophysiologic Abnormalities and Arrhythmic Heart Disease
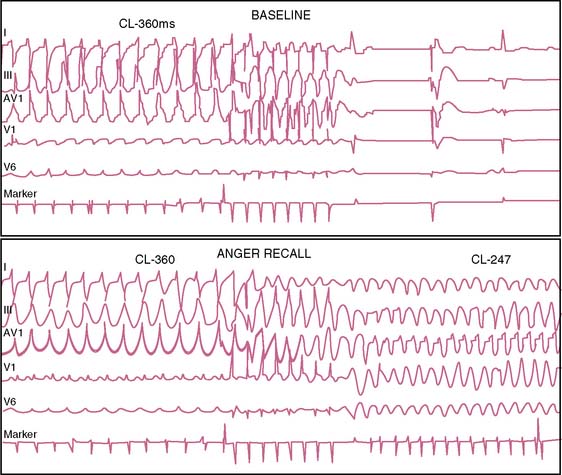
Case series have described individuals experiencing cardiac arrest or sudden death in the setting of acute grief, fear, or anger,86,87 while anger and anxiety are described as potent triggers of life-threatening arrhythmias88 and capable of provoking ICD shock in both the laboratory and natural setting (Fig. 32-10).89,90 Comparable to findings for the provocation of ischemia, anger appears to be a particularly important emotional component, both as a stressor89 and as an underlying part of the person’s psychological makeup.90
T-wave alternans (TWA) is a marker of repolarization instability91,92 that is a recognized factor in the development of ventricular fibrillation,91,93,94 and that immediately precedes development of ventricular fibrillation.95 TWA increases with heart rate,91,96,97 and sympathetic activation/catecholamine release play a role.89 TWA induced by exercise or atrial pacing under controlled conditions predicts subsequent ventricular arrhythmias,97,98 cardiac arrest,97,99 and mortality.100–102 TWA increases during anger in daily life, and experimental studies demonstrate that as the level of TWA increases, the likelihood of developing an arrhythmia increases as well.103,104 TWA increases in ICD patients during the recall of a previously anger-provoking incident,89,105 suggesting that mental stress induced in the laboratory predicts increases in TWA with anger in daily life and might predict subsequent arrhythmia.106
SUGGESTED PROTOCOL FOR MENTAL STRESS SPECT
Imaging
Mental stress SPECT MPI is similar to other stress SPECT imaging (see Chapters 14 and 15). SPECT is performed during two conditions: rest and mental stress. We recommend the use of a 99mTc-based perfusion agent with low-dose rest and high-dose stress studies. Resting SPECT MPI is performed after intravenous injection of 7 to 10 mCi 99mTc sestamibi or 99mTc tetrofosmin. One hour after injection, SPECT MPI is acquired. On completion of resting MPI, the patient is removed from the camera, and mental stress testing may begin. After 90 seconds of mental stress, the patient is injected with 25 to 30 mCi 99mTc sestamibi or 99mTc tetrofosmin. SPECT MPI is acquired 30 minutes after radiotracer injection.
Mental Stress Protocol
Various mental stress protocols are described in the literature, but we recommend mental arithmetic and anger recall. For the anger recall technique, the subject is asked to vividly recall a recent incident that caused moderate to extreme anger. The subject is given 1 minute to fully recall the incident and is then told to describe it fully to the experimenter. The subject is asked specifically to “describe it as if you were describing it to a friend.” During the description, the subject is prompted for details about actual words exchanged during the event and affective experiences. The task takes approximately 6 minutes to complete. One advantage of this technique is its reproducibility. Further, in patients with CAD, anger appears to be a particularly potent psychological stressor. In a study by Ironson and colleagues,107 anger recall reduced LVEF more than exercise and other psychological stressors, and more patients with CAD had a significant reduction in LVEF (7%) during anger (7 of 18) than during exercise (4 of 18). The protocol necessitates that a clinical psychologist or someone with focused training in this technique be present.
CONCLUSION
It is not often that our specialty can take the lead in the identification of distinct cardiovascular presentations. We outlined the incremental impact of MSI on prognosis and its distinct central and peripheral pathophysiologic features. The emergence of simplified finger probes and stress protocols since the last edition serves as evidence of the growing interest in this phenomenon. Epidemiologic evidence suggests that acute coronary events occur in individuals who do not necessarily have the traditional high-risk profile for CAD.8 This observation, in concert with the high frequency of myocardial infarction during catastrophic events, suggests that the identification of patients who are vulnerable to MSI is highly relevant. MSI predisposes vulnerable individuals to adverse clinical outcomes, angina, and fatal arrhythmias. In this chapter, we outlined a simplified algorithm for clinicians interested in conducting mental stress examinations. As work in this field progresses, more laboratories will likely incorporate mental stress imaging into the management of patients with CAD. The main economic limitation is a specific Current Procedural Terminology code for testing for ischemia in response to mental stress. Policy guidelines from professional societies are planned, because they are the customary antecedents toward reimbursement. Nonetheless, clinical demand to aid in the identification of such patients has not diminished. The rationale and logistics outlined in this chapter can provide the framework for this testing.
1. Soufer R. Neurocardiac interaction during stress-induced myocardial ischemia: how does the brain cope? Circulation. 2004;110:1710-1713.
2. Damasio A.R. Remembering when. Sci Am. 2002;287:66-73.
3. Tracey K.J. The inflammatory reflex. Nature. 2002;420:853-859.
4. Powell L.H., Thoresen C.E. Behavioral and physiologic determinants of long term prognosis after myocardial infarction. J Chron Dis. 1985;38:253-263.
5. Jain D., Burg M., Soufer R., et al. Prognostic implications of mental stress–induced silent left ventricular dysfunction in patients with stable angina pectoris. Am J Cardiol. 1995;76:31-35.
6. Sheps D.S., McMahon R.P., Becker L., et al. Mental stress–induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780-1784.
7. Jiang W., Babyak M., Krantz D.S., et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA. 1996;275:1651-1656.
8. Ruberman W., Weinblatt E., Goldberg J.D., et al. Psychosocial influences on mortality after myocardial infarction. N Engl J Med. 1984;311:552-559.
9. Rosengren A., Tibblin G., Wilhelmsen L. Self-perceived psychological stress and incidence of coronary artery disease in middle-aged men. Am J Cardiol. 1991;68:1171-1175.
10. Becker L.C., Pepine C.J., Bonsall R., et al. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) Study. Circulation. 1996;94:2768-2777.
11. Jain D., Shaker S.M., Burg M., et al. Effects of mental stress on left ventricular and peripheral vascular performance in patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1314-1322.
12. Rozanski A., Bairey C.N., Krantz D.S., et al. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N Engl J Med. 1988;318:1005-1012.
13. Specchia G., De Servi S., Falcone C., et al. Mental arithmetic stress testing in patients with coronary artery disease. Am Heart J. 1984;108:56-63.
14. Deanfield J.E., Shea M., Kensett M., et al. Silent myocardial ischaemia due to mental stress. Lancet. 1984;2:1001-1005.
15. Barry J., Selwyn A.P., Nabel E.G., et al. Frequency of ST-segment depression produced by mental stress in stable angina pectoris from coronary artery disease. Am J Cardiol. 1988;61:989-993.
16. Specchia G., Falcone C., Traversi E., et al. Mental stress as a provocative test in patients with various clinical syndromes of coronary heart disease. Circulation. 1991;83:II-108-II-114.
17. Schang S.J.Jr, Pepine C.J. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol. 1977;39:396-402.
18. Stern S., Tzivoni D. Early detection of silent ischaemic heart disease by 24-hour electrocardiographic monitoring of active subjects. Br Heart J. 1974;36:481-486.
19. Bosimini E., Galli M., Guagliumi G., et al. Electrocardiographic markers of ischemia during mental stress testing in postinfarction patients—Role of body surface mapping. Circulation. 1991;83:II-115-II-127.
20. Sherwood A., Johnson K., Blumenthal J.A., et al. Endothelial function and hemodynamic responses during mental stress. Psychosom Med. 1999;61:365-370.
21. LaVeau P.J., Rozanski A., Krantz D.S., et al. Transient left ventricular dysfunction during provocative mental stress in patients with coronary artery disease. Am Heart J. 1989;118:1.
22. Stone P.H., Krantz D.S., McMahon R.P., et al. Relationship among mental stress-induced ischemia and ischemia during daily life and during exercise: the Psychophysiologic Investigations of Myocardial Ischemia (PIMI) study. J Am Coll Cardiol. 1999;33:1476-1484.
23. Vassiliadis I.V., Fountos A.I., Papadimitriou A.G., et al. Mental stress induced silent myocardial ischemia detected during ambulatory ventricular function monitoring. Int J Card Imaging. 1998;14:171-177.
24. Modena M.G., Corghi F., Fantini G., et al. Echocardiographic monitoring of mental stress test in ischemic heart disease. Clin Cardiol. 1989;12:21-24.
25. Gottdiener J.S.K.D., Howell R.H., Hecht G.M., et al. Induction of silent myocardial ischemia with mental stress testing: Relation to the triggers of ischemia during daily life activities and to ischemic functional severity. J Am Coll Cardiol. 1994;24:1645-1651.
26. Carney R.M., McMahon R.P., Freedland K.E., et al. Reproducibility of mental stress-induced myocardial ischemia in the Psychophysiological Investigations of Myocardial Ischemia (PIMI) study. Psychosom Med. 1998;60:64-70.
27. Jain D., Joska T., Lee F.A., et al. Day-to-day reproducibility of mental stress-induced abnormal left ventricular function response in patients with coronary artery disease and its relationship to autonomic activation. J Nucl Cardiol. 2001;8:347-355.
28. Giubbini R., Galli M., Campini R., et al. Effects of mental stress on myocardial perfusion in patients with ischemic heart disease. Circulation. 1991;83:II-100-II-107.
29. Schoder H., Silverman D.H., Campisi R., et al. Effect of mental stress on myocardial blood flow and vasomotion in patients with coronary artery disease. J Nucl Med. 2000;41:11-16.
30. Arrighi J.A., Burg M., Cohen I.S., et al. Myocardial blood-flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310-311.
31. Arrighi J.A., Burg M., Cohen I.S., et al. Simultaneous assessment of myocardial perfusion and function during mental stress in patients with chronic coronary artery disease. J Nucl Cardiol. 2003;10:267-274.
32. Dimsdale J.E., Moss J. Plasma catecholamines in stress and exercise. JAMA. 1980;243:340-342.
33. Goldberg A.D., Becker L.C., Bonsall R., et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation. 1996;94:2402-2409.
34. Ramachandruni S., Fillingim R.B., McGorray S.P., et al. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J Am Coll Cardiol. 2006;47:987-991.
35. Hassan M., York K.M., Li Q., et al. Variability of myocardial ischemic responses to mental versus exercise or adenosine stress in patients with coronary artery disease. J Nucl Cardiol. 2008;15:518-525.
36. Krantz D.S., Santiago H.T., Kop W.J., et al. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol. 1999;84:1292-1297.
37. Rosenman R.H., Brand R.J., Jenkins D., et al. Coronary heart disease in Western Collaborative Group Study. Final follow-up experience of 8 1/2 years. JAMA. 1975;233:872-877.
38. Goodman M., Quigley J., Moran G., et al. Hostility predicts restenosis after percutaneous transluminal coronary angioplasty. Mayo Clin Proc. 1996;71:729-734.
39. Julkunen J., Salonen R., Kaplan G.A., et al. Hostility and the progression of carotid atherosclerosis. Psychosom Med. 1994;56:519-525.
40. Kawachi I., Sparrow D., Spiro A.III, et al. A prospective study of anger and coronary artery disease. Circulation. 1996;94:2090-2095.
41. Mittleman M.A., Maclure M., Sherwood J.B., et al. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720-1725.
42. Burg M.M., Jain D., Soufer R., et al. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J Am Coll Cardiol. 1993;22:440-448.
43. Rebecca G., Wagner R., Zebede T. Pathogenic mechanism causing transient myocardial ischemia with mental arousal in patients with coronary artery disease (abstract). Clin Res. 1989;34:338A.
44. Yeung A.C., Vekshtein V.I., Krantz D.S., et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551-1556.
45. Kop W.J., Krantz D.S., Howell R.H., et al. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J Am Coll Cardiol. 2001;37:1359-1366.
46. Burton A. The range and variability of the blood flow in the human fingers and the vasomotor regulation of body temperature. Am J Physiol. 1939;127:437-453.
47. Rozanski A., Qureshi E., Bauman M., et al. Peripheral arterial responses to treadmill exercise among healthy subjects and atherosclerotic patients. Circulation. 103, 2001.
48. Kuvin J.T., Patel A.R., Sliney K.A., et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168-174.
49. Goor D.A., Sheffy J., Schnall R.P., et al. Peripheral arterial tonometry: A diagnostic method for detection of myocardial ischemia induced during mental stress tests: A pilot study. Clin Cardiol. 2004;27:137-141.
50. Burg M.M., Graeber B., Vashist A., et al. Noninvasive detection of risk for emotion provoked myocardial ischemia. Psychosom Med. 2009;71:14-20.
51. Spieker L.E., Hurlimann D., Ruschitzka F., et al. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation. 2002;105:2817-2820.
52. Cardillo C., Kilcoyne C.M., Quyyumi A.A., et al. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol. 1997;80:1070-1074.
53. Sabatine M.S., Morrow D.A., Jablonski K.A., et al. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528-1536.
54. Albert M.A., Glynn R.J., Ridker P.M. Plasma concentration of C-reactive protein and the calculated Framingham Coronary Heart Disease Risk Score. Circulation. 2003;108:161-165.
55. Shah R., Burg M.M., Vashist A., et al. C-reactive protein and vulnerability to mental stress-induced myocardial ischemia. Mol Med. 2006;12:269-274.
56. Soufer R., Burg M.M. The heart-brain interaction during emotionally provoked myocardial ischemia: implications of cortical hyperactivation in CAD and gender interactions. Cleve Clin J Med. 2007;74(Suppl 1):S59-S62.
57. Mizia-Stec K., Gasior Z., Zahorska-Markiewicz B., et al. Serum tumour necrosis factor-alpha, interleukin-2 and interleukin-10 activation in stable angina and acute coronary syndromes. Coron Artery Dis. 2003;14:431-438.
58. Bhagat K., Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96:3042-3047.
59. Chia S., Qadan M., Newton R., et al. Intra-arterial tumor necrosis factor-alpha impairs endothelium-dependent vasodilatation and stimulates local tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol. 2003;23:695-701.
60. Nakamura M., Yoshida H., Arakawa N., et al. Effects of tumor necrosis factor-alpha on basal and stimulated endothelium-dependent vasomotion in human resistance vessel. J Cardiovasc Pharmacol. 2000;36:487-492.
61. Patel J.N., Jager A., Schalkwijk C., et al. Effects of tumour necrosis factor-alpha in the human forearm: blood flow and endothelin-1 release. Clin Sci (Lond). 2002;103:409-415.
62. Rask-Madsen C., Dominguez H., Ihlemann N., et al. Tumor necrosis factor-alpha inhibits insulin’s stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation. 2003;108:1815-1821.
63. Edmunds N.J., Woodward B. Effects of tumour necrosis factor-alpha on the coronary circulation of the rat isolated perfused heart: a potential role for thromboxane A2 and sphingosine. Br J Pharmacol. 1998;124:493-498.
64. Edmunds N.J., Lal H., Woodward B. Effects of tumour necrosis factor-alpha on left ventricular function in the rat isolated perfused heart: possible mechanisms for a decline in cardiac function. Br J Pharmacol. 1999;126:189-196.
65. Woods M., Mitchell J.A., Wood E.G., et al. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: evidence for intracellular endothelin-converting enzyme. Mol Pharmacol. 1999;55:902-909.
66. Kahaleh M.B., Fan P.S. Effect of cytokines on the production of endothelin by endothelial cells. Clin Exp Rheumatol. 1997;15:163-167.
67. Hohlfeld T., Klemm P., Thiemermann C., et al. The contribution of tumour necrosis factor-alpha and endothelin-1 to the increase of coronary resistance in hearts from rats treated with endotoxin. Br J Pharmacol. 1995;116:3309-3315.
68. Dimmeler S., Fleming I., Fisslthaler B., et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601-605.
69. Yoshizumi M., Perrella M.A., Burnett J.C.Jr, et al. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res. 1993;73:205-209.
70. Soufer A., Ranjbaran H., Graeber B., et al. Parasympathetic withdrawal and sympathetic arousal during anger correlate with elevated endothelin-1 in patients with coronary artery disease. Psychosom Med. 70, 2008.
71. Soufer R., Bremner J.D., Arrighi J.A., et al. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci U S A. 1998;95:6454-6459.
72. Bremner J.D., Krystal J.H., Southwick S.M., et al. Functional neuroanatomical correlates of the effects of stress on memory. J Trauma Stress. 1995;8:527-553.
73. LeDoux J. The Emotional Brain. New York: Touchstone Books, 1998.
74. Vashist A., Burg M.M., Arrighi J.A., et al. Central nervous system correlates of myocardial ischemia: Neurocardiac distinctions between mental stress and dobutamine provocation. Psychosom Med. 2005;67:A8-A9.
75. Gianni M., Dentali F., Grandi A.M., et al. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27:1523-1529.
76. Dote K., Sato H., Tateishi H., et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol. 1991;21:203-214.
77. Akashi Y.J., Nakazawa K., Sakakibara M., et al. 123I-MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J Nucl Med. 2004;45:1121-1127.
78. Kurisu S., Inoue I., Kawagoe T., et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J. 2004;68:77-81.
79. Ito K., Sugihara H., Katoh S., et al. Assessment of takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT—comparison with acute coronary syndrome. Ann Nucl Med. 2003;17:115-122.
80. Bybee K.A., Prasad A., Barsness G.W., et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343-346.
81. Feola M., Rosso G.L., Casasso F., et al. Reversible inverse mismatch in transient left ventricular apical ballooning: perfusion/metabolism positron emission tomography imaging. J Nucl Cardiol. 2006;13:587-590.
82. Feola M., Chauvie S., Rosso G.L., et al. Reversible impairment of coronary flow reserve in takotsubo cardiomyopathy: a myocardial PET study. J Nucl Cardiol. 2008;15:811-817.
83. Malafronte C., Farina A., Tempesta A., et al. Tako-tsubo: a transitory impairment of microcirculation? A case report. Ital Heart J. 2005;6:933-938.
84. Alexanderson E., Cruz P., Talayero J.A., et al. Transient perfusion and motion abnormalities in takotsubo cardiomyopathy. J Nucl Cardiol. 2007;14:129-133.
85. Scholte A.J., Bax J.J., Stokkel M.P., et al. Multimodality imaging to diagnose takotsubo cardiomyopathy. J Nucl Cardiol. 2006;13:123-126.
86. Reich P., DeSilva R.A., Lown B., et al. Acute psychological disturbances preceding life-threatening ventricular arrhythmias. JAMA. 1981;246:233-235.
87. Engel G.L. Sudden and rapid death during psychological stress. Folklore or folk wisdom. Ann Intern Med. 1971;74:771-782.
88. Albert C.M., Lampert R., Conti J.B., et al. Episodes of anger trigger ventricular arrhythmias in patients with implantable cardioverter defibrillators. Circulation. 2006;114:II-831.
89. Lampert R., Shusterman V., Burg M.M., et al. Effects of psychologic stress on repolarization and relationship to autonomic and hemodynamic factors. J Cardiovasc Electrophysiol. 2005;16:372-377.
90. Burg M.M., Lampert R., Joska T., et al. Psychological traits and emotion-triggering of ICD shock-terminated arrhythmias. Psychosom Med. 2004;66:898-902.
91. Pastore J.M., Girouard S.D., Laurita K.R., et al. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385-1394.
92. Shimizu W., Antzelevitch C. Cellular and ionic basis for t-wave alternans under long-QT conditions. Circulation. 1999;99:1499-1507.
93. Han J., Moe G.K. Nonuniform recovery of excitability in ventricular muscle. Circ Res. 1964;14:44-60.
94. Nearing B.D., Verrier R.L. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol. 2002;92:541-549.
95. Shusterman V., Goldberg A., London B. Upsurge in T-wave alternans and non-alternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation. 2006;113:2880-2887.
96. Hohnloser S.H., Klingenheben T., Zabel M., et al. T wave alternans during exercise and atrial pacing in humans. J Cardiovasc Electrophysiol. 1997;8:987-993.
97. Rosenbaum D.S., Jackson L.E., Smith J.M., et al. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235-241.
98. Cantillon D.J., Stein K.M., Markowitz S.M., et al. Predictive value of microvolt t-wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol. 2007;50:166-173.
99. Gold M.R., Bloomfield D.M., Anderson K.P., et al. A comparison of T-wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol. 2000;36:2247-2253.
100. Bloomfield D.M., Steinman R.C., Namerow P.B., et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004.
101. Klingenheben T., Zabel M., D’Agostino R.B., et al. Predictive value of T-wave alternans for arrhythmic events in patients with congestive heart failure (letter). Lancet. 2000;356:651-652.
102. Nieminen T, Lehtimaki T, Viik J, et al: T-wave alternans predicts mortality in a population undergoing a clinically indicated exercise test, Eur Heart J 28:2332–2337
103. Nearing B.D., Huang A.H., Verrier R.L. Dynamic tracking of cardiac vulnerability by complex demodulation of the T wave. Science. 1991;252:437-440.
104. Nearing B., Oesterle S., Verrier R. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dogs and humans. Cardiovasc Res. 1994;28:1440-1449.
105. Kop W.J., Krantz D.S., Nearing B.D., et al. Effects of acute mental stress and exercise on T-wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation. 2004;109:1864-1869.
106. Lampert R., Shusterman V., Burg M., et al. Arrhythmias in patients with Implantable cardioverter defibrillators. J Am Coll Cardiol. 2009;53:774-778.
107. Ironson G., Taylor C.B., Boltwood M., et al. Effects of anger on left ventricular ejection fraction in coronary artery disease. Am J Cardiol. 1992;70:281-285.