Mechanisms of surgical disease and surgery in practice
Approaches to surgical problems
What do surgeons do?
What sort of patients come to surgeons?: Different types of surgeon practise in very different ways. In the UK, most patients are referred by another doctor, e.g. GP, accident and emergency (ER) officer or physician. The exceptions include trauma patients who self-refer or arrive by ambulance. In some countries, patients can self-refer to the specialist they consider most appropriate. Regardless of the route, surgical patients fall into the following categories:
• Emergency/acute, i.e. symptoms lasting minutes to hours or up to a day or two—often obviously surgical conditions such as traumatic wounds, fractures, abscesses, acute abdominal pain or gastrointestinal bleeding
• Intermediate urgency—usually referrals from other doctors based on suspicious symptoms and signs and sometimes investigations, e.g. suspected colonic cancer, gallstones, renal or ureteric stones
• Chronic conditions likely to need surgery, e.g. varicose veins, hernias, arthritic joints, cardiac ischaemia or rectal prolapse
The diagnostic process: To manage surgical patients optimally, a working diagnosis needs to be formulated to guide whether investigations are necessary and their type and urgency, and to determine what intervention is necessary. The process depends upon whether immediate life-saving intervention is required or, if not, the perceived urgency of the case. For example, a patient bleeding from a stab wound might need pressure applied to the wound immediately whilst resuscitation and detailed assessment are carried out. At the other end of the scale, if symptoms suggest rectal carcinoma, a systematic approach is needed to obtain visual and histological confirmation of the diagnosis by colonoscopy and radiological imaging. Tumour staging (see Ch. 13, p. 178) aims to determine the extent of cancer spread to direct how radical treatment needs to be. Treatment may be curative (surgery, chemotherapy, radiotherapy) or palliative if clearly beyond cure (stenting to prevent obstruction, local tumour destruction using laser, palliative radiotherapy).
Formulating a diagnosis: The traditional approach to surgical diagnosis is to attempt to correlate a patient’s symptoms and signs with recognised sets of clinical features known to characterise each disease. While most diagnoses match their ‘classical’ descriptions at certain stages, this may not be so when the patient presents. Patients often present before a recognisable pattern has evolved or at an advanced stage when the typical clinical picture has become obscured. Diagnosis can be confusing if all the clinical features for a particular diagnosis are not present, or if some seem inconsistent with the working diagnosis.
Principal mechanisms of surgical disease
Surgical patients present with disorders resulting from inherited abnormalities, environmental factors or combinations in varying proportions. These are summarised in Box 1.1, as a useful ‘first principles’ framework or aide-mémoire upon which to construct a differential diagnosis. This is useful when clinical features do not immediately point to a diagnosis. This approach is known as the ‘surgical sieve’; however, it is not a substitute for logical thought based on the clinical findings.
Acquired conditions
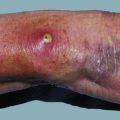
Trauma: Tissue trauma, literally injury, includes damage inflicted by any physical means, i.e. mechanical, thermal, chemical or electrical mechanisms or ionising radiation. Common usage tends to imply blunt or penetrating mechanical injury, caused by accidents in industry or in the home, road traffic collisions, fights, firearm and missile injuries or natural disasters such as floods and earthquakes. Damage varies with the causative agent, and the visible injuries may not indicate the extent of deep tissue damage.
Inflammation: Many surgical disorders result from inflammatory processes, most often stemming from infection. However, inflammation also results from physical irritation, particularly by chemical agents, e.g. gastric acid/pepsin in peptic ulcer disease or pancreatic enzymes in acute pancreatitis.
Infection: Primary infections presenting to surgeons include abscesses and cellulitis, primary joint infections and tonsillitis. Typhoid may cause caecal perforation, and abdominal tuberculosis may be discovered at laparotomy. Amoebiasis can cause ulcerative colitis-like effects. Preventing and treating infection is an important factor in surgical emergencies such as acute appendicitis or bowel perforation. Despite the rational use of prophylactic and therapeutic antibiotics, postoperative infection remains a common complication of surgery.
Neoplasia: Certain benign tumours such as lipomas are common and are excised mainly for cosmetic reasons. Less commonly, benign tumours cause mechanical problems such as obstruction of a hollow viscus or surface blood loss, e.g. gastrointestinal stromal tumours (GIST). Benign endocrine tumours may need removal because of excess hormone secretion (see Endocrine disorders later). Finally, benign tumours may be clinically indistinguishable from malignant tumours and are removed or biopsied to obtain a diagnosis.
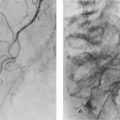
Vascular disorders: A tissue or organ becomes ischaemic when its arterial blood supply is impaired; infarction occurs when cell life cannot be sustained. Atherosclerosis progressively narrows arteries often resulting in chronic ischaemia, causing symptoms such as angina pectoris or intermittent claudication. It also predisposes to acute-on-chronic ischaemia when diseased vessels finally occlude. Other common causes of acute arterial insufficiency are thrombosis, embolism and trauma. Arterial embolism causes acute ischaemia of limbs, intestine or brain; emboli often originate in the heart. If blood supply is restored after a period of ischaemia, further damage can ensue as a result of reperfusion syndrome.
Degenerative disorders: This is an inhomogeneous group of conditions characterised by deterioration of body tissues as life progresses. In the musculoskeletal system, osteoporosis decreases bone density and impairs its structural integrity, making fragility fractures more likely. Spinal disc and facet joint degeneration is common, causing back pain and disability, and osteoarthritis is widely prevalent in later life: the almost universal musculoskeletal aches and pains are probably caused by degeneration of muscle, tendon, joint and bone.
Metabolic disorders: Metabolic disorders may be responsible for stones in the gall bladder (e.g. haemolytic diseases causing pigment stones) or in the urinary tract (e.g. hypercalciuria and hyperuricaemia causing calcium and uric acid stones, respectively). Hypercholesterolaemia is a major factor in atherosclerosis and hypertriglyceridaemia is a rare cause of acute pancreatitis.
Endocrine disorders and hormonal therapy: Hypersecretion of hormones, as in thyrotoxicosis and hyperparathyroidism, may require surgical removal or reduction of glandular tissue. Endocrine tumours, benign and malignant, may present with metabolic abnormalities such as hypercalcaemia caused by a parathyroid adenoma, Cushing’s syndrome resulting from an adrenal adenoma or episodic hypertension caused by a phaeochromocytoma.
Other abnormalities of tissue growth: Growth disturbances such as hyperplasia (increase in number of cells) and hypertrophy (increase in size of cells) may cause surgical problems, in particular benign prostatic hyperplasia, fibroadenosis of the breast and thyroid enlargement (goitre).
Iatrogenic disorders: Iatrogenic damage or injury results from the action of a doctor or other health care worker. It may be an unfortunate outcome of an adequately performed investigation or operation, e.g. perforated colon during colonoscopy or pneumothorax from attempted aspiration of a breast cyst. These are termed surgical misadventure. However, if the damage results from a patently wrong procedure, e.g. amputation of the wrong leg or removal of the wrong kidney, then negligence is likely to be proven. Such wrong site surgery is easily avoided by preoperative site marking. Other potentially negligent actions include retained surgical swabs after laparotomy, or vascular trauma during central venous line insertion. Complications of bowel surgery such as anastomotic leakage may result from poorly performed surgery but can occur in expert hands; only audited results can demonstrate whether the surgeon is proficient. Wrong drugs or doses are usually iatrogenic. It is unusual for iatrogenic problems to be due simply to one person’s failure. More often it is a system failure, with inadequate checks and balances in the system.
Drugs, toxins and diet: Problems with prescribed drugs include unavoidable toxic effects of certain chemotherapeutic agents, e.g. neutropenia, and the side-effects of drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) causing duodenal perforation, or codeine phosphate causing constipation. Drug allergy, idiosyncrasy or anaphylaxis may result from individual responses to almost any drug, and interactions between drugs cause adverse effects; in this respect warfarin is a prime culprit. Maladministration of drugs may also cause problems with, for example, the wrong drug given for intrathecal chemotherapy causing paralysis.
Psychogenic disorders: Psychogenic disorders are not often a source of surgical disease but Munchausen syndrome patients may present with abdominal pain and become subjects of repeated laparotomies, psychiatric patients living rough may suffer from exposure and frostbite, and others may repeatedly cause self-harm or swallow foreign bodies, even such items as razor blades or safety pins.
Disorders of function: A range of common disorders are defined by the functional abnormalities they cause, although their pathogenesis often remains ill understood. The gastrointestinal tract is particularly susceptible, with conditions such as idiopathic constipation, irritable bowel syndrome and diverticular disease.
Medical ethics and confidentiality
To some extent, the practice of surgery is influenced by the need for self-protection but in trying to avoid litigation, a surgeon may over-treat or over-investigate in ways that are unnecessary and may even be unethical. A degree of self-interest is inevitable but the guiding principle should be that the patient’s interests are paramount. Desirable attributes in a surgeon are listed in Box 1.2.
• Doctors must be instructed and then registered to protect the public from amateurs and charlatans
• Medicine is for the benefit of patients, and doctors must avoid doing anything known to cause harm
• Euthanasia and abortion are prohibited
• Operations and procedures must be performed only by practitioners with appropriate expertise
• Doctors must maintain proper professional relationships with their patients and treatment choices should not be governed by motives of profit or favour
• Doctors should not take advantage of their professional relationships with their patients
Confidentiality: Patients allow the NHS to gather sensitive information about their health and personal matters as part of seeking treatment. They do this in confidence and legitimately expect staff will respect this trust.
Do not resuscitate (DNR) orders: A DNR order on a patient’s file means that doctors are not required to resuscitate a patient if their heart stops. It is designed to prevent unnecessary suffering and potential side-effects such as pain, broken ribs, ruptured spleen or brain damage. The British Medical Association and the Royal College of Nursing say that DNR orders can be issued only after discussion with patients or family, difficult though this may be. Decisions should not be made by junior doctors alone but in consultation with seniors. The most difficult cases are those involving patients who know they are going to die and are suffering pain or other severe symptoms but who could live for months.
Guidelines for when a DNR may be issued::
• If a patient’s condition is such that resuscitation is unlikely to succeed
• If a mentally competent patient has consistently stated or recorded they do not want to be resuscitated
• If an advance notice or living will says the patient does not want to be resuscitated
• If successful resuscitation would not be in the patient’s best interest because it would lead to a very poor quality of life
In the UK, NHS Trust Hospitals must agree explicit resuscitation policies that respect patients’ rights and are readily available to patients, families and carers; policies must be regularly monitored.
Communication
Palliative care: Sometimes cure is not possible. Then quality of life may become the goal, with palliative treatment being offered. Patients generally want to know what will happen, including their mode of dying. Whilst this can be hard to predict, they need to know their symptoms, particularly pain, will be managed effectively and that they will be looked after. Experience teaches it is usually impossible to say with accuracy when a patient will die except a few days before it will happen, so it is unwise to predict life span except in general terms.
Breaking bad news: All doctors in clinical practice experience the need to break bad news, such as an unfavourable outcome, unsatisfactory care, a cancer diagnosis or a poor prognosis. It is an event doctors tend to remember and a moment in the patient or relative’s life they will never forget.
• Bad news is private. Find a quiet space, preferably an office with chairs (you don’t need a desk)
• Avoid hiding behind jargon: ‘the metastatic nature of the neoplasm makes it inoperable’ is useless. ‘I’m sorry to say that the cancer has spread and an operation won’t help’ is better
• Give time and space; turn off pagers and phones if possible
• Don’t be defensive and don’t be afraid to express regret
• Avoid filling the silence of grief with continuous chatter
• Allow time for questions. If you don’t know the answer, say so and try to find out
• Always offer another meeting, ideally with the head of the team
• Many patients/families will wish to discuss what has been imparted with their family doctor, so it is vital that you get all information to the GP before that visit
Communicating with colleagues
Communicating with colleagues involves speaking, both face to face and on the telephone (Box 1.3), and writing (handwriting, dictating, typing, emailing) patient notes, information letters to patient or family practitioners, e.g. after an outpatient consultation, referral letters, discharge summaries, reports and presentations for local or larger-scale medical meetings. All of these need to be honest, accurate and timely, particularly when communicating patient information. Remember, recipients are entitled to rely on what you have written in their later treatment of a patient. Also, any written information may be called in evidence in a court of law should something go wrong later. Patient notes must never be altered later, although rarely, amendments may be added provided they are signed and dated.
Communication via the clinical record: Reduced junior hospital doctors’ hours make it imperative to keep the written records for every patient up to date, including management plans and what to do if predictable changes occur. Date and legibly sign each entry giving your name in capitals and grade, record important test results and write instructions for antibiotic and DVT prophylaxis. In high operative risk patients, seniors should document discussions before surgery. After operation, write or type an operation note with clear postoperative instructions so these are immediately available to recovery and ward staff.
Evidence-based medicine and guidelines
History: Evidence-based medicine (EBM) as now understood really began when Professor Archie Cochrane, a Scottish epidemiologist, published his book Effectiveness and Efficiency: Random Reflections on Health Services in 1972 and continued with his later advocacy of its principles. EBM has gradually gained political support and acceptance within the medical profession. EBM calls into question the traditional belief that ‘we’ve always based our practice on science’. Cochrane’s work has been recognised by the proliferation of Cochrane Centres and the international Cochrane Collaboration, all devoted to meticulously evaluating evidence and promoting its use.
Austin Bradford Hill, the grandfather of modern medical research, who was fundamental in discovering the link between smoking and lung cancer, produced a set of guidelines, as given in Box 1.4, for assessing causality, i.e. the relationship between an exposure and an outcome, and these remain the foundation of evidence-based medicine today.
Cherry-picking the evidence versus systematic review: Cherry-picking is a dubious means of reinforcing what you already believe, the very opposite of systematic review. It involves relying only on published work that supports your view and finding reasons to ignore what goes against it. The solution is a process of systematic review as conducted by the Cochrane Collaboration. Their methodologies were largely established at McMaster University. The term EBM first appeared in 1992 and now journals devoted to the subject include the BMJ’s Clinical Evidence, the Journal of Evidence-Based Healthcare and Evidence Based Health Policy, all co-founded by Anna Donald, an Australian pioneer.
Longitudinal or cohort studies: For predicting prognosis, the highest level of evidence is a systemic review of inception cohort studies, i.e. groups of patients assembled near the onset of the disorder. These groups are followed over years to determine how variables such as smoking habits, exercise, occupation and geography may affect outcome. Prospective studies take years to perform but are valued more than retrospective studies which are more likely to generate bias.
Ranking the quality of evidence (Box 1.5): The strongest evidence for therapeutic interventions is by systematic review of randomised, double- or triple-blind, placebo-controlled trials with allocation concealment and complete follow-up, in a homogeneous patient population and medical condition. In contrast, patient testimonials, case reports, and even expert opinion have lesser value because of the placebo effect, biases inherent in observation and reporting, and personal and institutional biases.
• Systematic reviews of randomised controlled trials (RCTs)
• Controlled observational studies—cohort and case control studies
• Uncontrolled observational studies and case reports
• Established practice and expert opinion (not to be confused with personal experience, sometimes dubbed eminence-based medicine). Expert opinion may be the best guide in the absence of good research evidence
Quality and limitations of clinical trials: Trials must now be registered in advance: the Declaration of Helsinki 2008 requires that every clinical trial be registered in a publicly accessible database before recruitment of the first subject. The International Committee of Medical Journal Editors refuses to publish clinical trial results if the trial was not recorded in this way. This should eliminate the bias inherent in the failure to publish negative trials.
In 1993, 30 medical journal editors, clinical trialists, epidemiologists and methodologists met in Ottawa to develop a new scale to assess the quality of randomised controlled trial (RCT) reports. This eventually resulted in the Consolidated Standards of Reporting Trials (CONSORT) Statement, published in 1996 and now largely adhered to by respected medical journals (http://www.consort-statement.org/home/). Cochrane adheres to similar standards and employs software ‘RevMan’ to help reviewers evaluate published studies.
• Cochrane Library: http://www.thecochranelibrary.com/view/0/index.html
• UK National Institute for Health and Clinical Excellence (NICE): http://www.nice.org.uk/
• NHS search engine for Evidence in Health and Social Care (from NICE): https://www.evidence.nhs.uk/
Guidelines: Clinical guidelines, practice policies, protocols and codes of practice are locally or more widely published mechanisms aimed at harmonising processes of care using best practice. Some are produced by surgical societies such as the Association of Surgeons of GB &I (ASGBI). Guidelines should be just that—providing a structure rather than absolute ways to proceed in every case; they may be varied if clinical conditions dictate. Guidelines should have an evidence basis or be of proven clinical effectiveness and need regular review as evidence accumulates. Local guidelines are a natural outcome of clinical audit studies (see p. 13 below).
Consent to treatment
When is consent necessary?: Ideally, medical treatment should not proceed without first obtaining the patient’s consent. Consent may be expressed, or it may be implied, as when a patient presents for examination and acquiesces in the suggested procedure. Expressed permission can be based on an oral or a written agreement. Most invasive investigations (such as upper GI endoscopy or arteriography) and any surgical operation should be preceded by written consent, ideally well in advance to give the patient time to think it over. If oral consent alone has been obtained, then a note should be made in the patient’s record.
The unconscious patient: Under the necessity principle, a surgeon is justified in treating a patient without expressed consent if what he seeks to protect is more valuable than the wrongful act, i.e. treating without consent, provided there is no objection to treatment. Treatment must be no more extensive than is essential and procedures not needed for the patient’s survival must not be performed. For example, a diseased testis could be removed during a hernia repair but sterilising a patient during a Caesarean section without consent constitutes assault.
Practical aspects of consent for treatment: In British law, there is no such thing as informed consent. Surgeons like to feel they obtain informed consent after explaining to the patient in non-technical language the nature, purpose and risks of the proposed investigation or treatment, together with alternatives and the likely outcome of treatment. The patient must be capable of understanding the explanation and if this is not the case then informed consent has not been obtained. It follows that consent cannot be obtained from patients who are unconscious or of unsound mind.
Obtaining consent (Box 1.6): Consent should be obtained by a doctor sufficiently knowledgeable to explain the treatment, any alternatives, the likely outcome and any significant risks. Sometimes trained nurses obtain a first stage consent which is confirmed by a doctor later.
Consent in children: Consent can be obtained from children aged 16 and over and occasionally in those under 16. It is always sensible to liaise with parents wherever possible in young people aged 17 and 18. In the absence of parents, another relative or person ‘in loco parentis’ can give consent for children.
Jehovah’s Witnesses: Adult Jehovah’s Witnesses usually refuse blood or blood product transfusion even in an extreme emergency because of their interpretation of part of the Bible. If permission to transfuse is withheld then blood should not be given. Failure to respect the patient’s wish may result in an accusation of battery. The moral dilemma of allowing a patient to die when blood transfusion is likely to prevent death is uncomfortable but the law is clear. General advice is that a surgeon cannot refuse to treat simply because the patient imposes conditions on that treatment, although it may be possible to transfer the patient to a compliant surgeon’s care. In these circumstances, it is wise to interview the patient in the presence of a witness and explain the risks. The discussion should be noted and the witness should sign the hospital record.
Clinical governance and clinical audit
Clinical governance is a systematic approach to preserving and advancing the quality of patient care within a health system. Since the 1970s, there has been a growing realisation that looking critically at the way we run our clinical practice and then taking active steps to move ahead is much more effective than simply following time-honoured practices or even opening new avenues of research. In the UK, this movement is now universal but with varying degrees of success. Clinical governance starts with the mindset that the quality of care matters; it embodies a range of activities described here and elsewhere in this chapter. See also NHS Scotland clinical governance: http://www.clinicalgovernance.scot.nhs.uk/section1/introduction.asp.
Management attitude to quality of care: Health service managers have to keep quality of care high on their long list of priorities and facilitate clinicians’ initiatives.
Education and training of clinical staff: Thorough and well-rounded teaching in medical and nursing school, including anatomy and surgery, is the starting point. Training posts then need to offer a wide range of experience in an apprenticeship model, including step-by-step learning of procedures to back up continuing medical education as well as specific courses such as Advanced Trauma Life Support (ATLS). During training, good behaviours, attitudes and judgement can be acquired (see attributes of a good surgeon earlier). All clinicians need to remain open-minded to change and remember it is their professional duty to remain up to date.
Clinical audit: Clinical audit reviews clinical performance against agreed standards, refining clinical practice and then re-auditing—a cyclical process of improving quality.
Clinical effectiveness: Clinical effectiveness studies evaluate the extent to which an intervention works, its efficiency, safety, appropriateness and value for money. Studies of this type can be instructive and worthwhile for trainees to undertake.
Research and development: Professional practice can change in the light of good research evidence, provided it can be implemented effectively. Evidence-based medicine involves critical appraisal of the literature and development of evidence-based guidelines, protocols and implementation strategies from research.
Clinical performance: Poor performance and poor practice often thrive behind closed doors but can be revealed by a local climate of openness; this also demonstrates the organisation meets the needs of its population. In surgery, trouble may come to light through morbidity and mortality meetings, clinical audit, via patient complaints or by ‘whistle blowing’, and these should provide the motor for change. Critical incident meetings, for example, can thoroughly examine particular adverse events and recommend change.
Nationally in the UK, the National Patient Safety Agency (http://www.npsa.nhs.uk/) ‘informs, supports and influences healthcare organisations and individuals’ by handling patient safety incidents, by running national independent Confidential Enquiries (NCEPOD in surgery and anaesthesia), by encouraging ethical research, and by developing and implementing safety recommendations, advice and strategies. Through the National Clinical Assessment Service (http://www.ncas.npsa.nhs.uk/), it endeavours to solve concerns about the performance of health practitioners short of referral to the General Medical Council.
Risk management: This is a prospective process to identify hazards that could cause harm, decide who might be harmed and how, then evaluate the risks and decide on precautions. Risks in a health service include: risks to patients, risks to practitioners and risks to the organisation itself. Recognising in advance where particular risks lie is the first step to minimising those risks. Areas of potentially high risk include:
The elderly: surgeons deal with an increasingly elderly population. The likelihood of co-morbid disease is higher, although chronological age by itself is less important than biological age
Emergency surgery: this carries a higher risk of complications and death than elective surgery. Patients may be more physiologically disrupted or not fully resuscitated, intervention may be required out-of-hours when the ideal mix of staff is not available; investigations such as CT scanning may also not be available
Day surgery: preoperative assessment can preselect patients for day surgery and minimise risk
Critically ill patients: these patients need optimising before surgery, often with shared care with a senior anaesthetist, physician or other specialist. More preoperative investigations and resuscitation may be needed, perhaps in an ICU or HDU. The initial surgical approach may become a damage limitation exercise with more realistic expectations about outcome
Operative risk assessment: the American Association of Anaesthesiologists (ASA) grade scheme gives anaesthetist and surgeon a subjective idea of how sick the patient is and the likely outcome.
Information management: Information management is vital to facilitate good, effective and economical practice. For example, high quality and available patient notes, systems for ordering laboratory and imaging tests and receiving results, accurate and prompt discharge summaries, easy outpatient booking, good feedback to family practitioners and reliable A&E systems. Hand-written methods have been employed for many years, with recent attempts to employ computers to streamline processes. These have been successful in countries such as Denmark, but in the UK, system design has largely been driven by computer companies rather than clinicians, so systems are often unfriendly and ineffective. However, the use of individual smart cards for patients to hold their own records, and easily portable devices such as the iPad hold promise for the future, provided clinicians take sufficient interest in their development.
Medical research versus medical audit: Medical research is employed on a one-off basis to determine scientifically how interventions affect outcomes. Clinical audit measures how effectively aspects of good health care are put into practice. Every doctor can improve the way patients are cared for by critically examining local practices against current standards using audit methods.
Carrying out an audit (Box 1.7): Selecting topics for audit means taking into consideration how frequent the condition or treatment is, how high the risk to patients is, whether there is doubt about which treatment is the best, where care crosses specialty boundaries and finally, any topics of particular concern to clinicians or professions allied to medicine.
Peer group review of medical audit data: Using audit indicators has advantages over raw data analysis or informal morbidity meetings. As standards have of necessity been agreed, any numbers of cases can be screened to select out for further discussion only those that vary from the standard. In itself, the process of refining and employing audit indicators is an educational experience that encourages self-analysis by individuals, departments, units or regions.
Examples of how clinical audit can improve the quality of care::
• Reduction of risk of morbidity or mortality
• Improved effectiveness of care such as streamlined processes of treatment
• Improvement in diagnosis—availability, appropriateness or quality
• Improved timing of care—reduced delay, better planning, efficient use of facilities
• Better use of resources—equipment, beds, support services, money
• Consumer satisfaction—patients and referring doctors
• Access to care—availability of diagnostic services and treatment
• Documentation and records—improved recording of the process of care
• Identifying educational needs by audit activity—e.g. pain management
Confidential enquiry into perioperative deaths (CEPOD): The pilot study was designed in 1983 jointly by the ASGBI and the Association of Anaesthetists to examine perioperative deaths and the delivery of surgical and anaesthetic care in Britain. This was followed by a review of all deaths within 30 days of surgery (all specialties) in three English Regions for the whole of 1986: 500 000 operations were reviewed with 4000 deaths (0.8%); 79% of deaths occurred in patients over 65 years of age. More information is available from: http://www.ncepod.org.uk/, including all published reports from 1987 onwards.
Educational lessons from CEPOD: Many of the substandard practices identified could be put down to a lack of education or training in particular fields. These included:
• When to give prophylaxis against infection and thromboembolism
• When to delay operation in order to resuscitate
• Managing co-morbid disease and the elderly
• Safe use of local anaesthetics
• Local protocols for referral, handover and transfer
• Organising effective audit or morbidity and mortality meetings
Research in surgery
How are potentially improved methods evaluated?: When new surgical techniques appear they must be dispassionately evaluated and compared with existing practices, ideally by people with no vested interest. For a new technique to be introduced, it must be at least as good as existing methods or better in some way, for example, in achieving oncological clearance. New methods should be easily and quickly learnt—an operation that requires a learning curve of 500 patients is little use to those 500. Methods need to be reasonably economical in equipment and in operating time and high-level hazards should be no greater than existing operations. While this may seem utopian, ‘the greatest uncontrolled medical experiment of all’, namely the introduction of laparoscopic cholecystectomy, was undoubtedly at the expense of a massive increase in common bile duct injuries. The proper view should be that the safety of the many outweighs the foibles of the few.
Design of research and experiments: All British health authorities have to establish an Ethics Committee charged with examining and sanctioning each research project before it is launched. They help ensure that all projects are ethical and can be justified and that the methodology is sound. Among medical members, these committees generally include lawyers, ethicists, statisticians and lay members.
Drug trials: Once a potential drug has been identified, say from a likely plant molecule, a cell receptor that might be influenced or a modification of an old drug, it is tested for toxicity in animals and to see if it works. Then Phase I trials ‘first in man’ are performed on a few healthy young people. This is for toxicity, excretion rates and pathways, etc. If this works, Phase II trials in perhaps 200 people with the relevant illness are performed as ‘proof of concept’ to see if the drug is effective and to work out the dose. Many drugs fail at this point. Then Phase III trials are performed in hundreds or thousands of patients. These are randomised, blinded trials comparing the new drug against placebo or comparable treatments. More data on efficacy and safety is collected. Once successful trials are complete, the company applies for a licence to sell the drug. After it reaches market, the company and others usually conduct further trials and studies to look out for unnoticed side-effects. However, trials do not tell the whole story: in the 1960s thalidomide, a very effective drug for morning sickness, had not been tested in pregnancy, and this led to many avoidable birth deformities in countries where it had been licensed.
Patient safety
Dealing with an adverse event:
• Apologise to the patient for the failure as soon as the error is recognised
• Report to your consultant and other responsible people
• Take steps to correct the error and make sure you see the patient often
• If an official complaint is made, patient letters are usually sent to the hospital manager then to a complaints officer
• If asked to comment, provide full and honest detail
• If legal action is threatened, contact your medical insurance society
• Adverse outcomes should be discussed at local meetings to seek system problems
Introduction: ‘First do no harm’, an aphorism attributed to Thomas Sydenham, an English physician in the mid-1600s, is sound advice for surgeons too. All surgical treatments should be thought of in terms of their potential harm as well as benefit.
General hazards: The two most common sources of error leading to patient harm are communication failures and drug prescribing errors. Some 26% of 100 consecutive cases referred to the Medical Protection Society resulted from communication failure. There need to be explicit systems for dealing with risky situations, for example, informing seniors about sick patients, handing over properly to staff coming on duty, knowing who to call about patients that have ‘gone off’ during unsocial hours. This applies especially to anyone not familiar with the patient’s current state, particularly locums, who are unlikely to be familiar with how things work locally.
Theatre safety: The period between a patient entering the operating department and leaving the recovery unit is potentially hazardous for both the patient and the staff (Boxes 1.8 and 1.9). A fully conscious patient has automatic defence mechanisms to avoid injury but when anaesthetised or recovering, relies on the care of trained staff.
All operating theatres have safety protocols, with patients’ identities, nature and type of operation, allergies, etc. being repeatedly checked—but errors still occur. The World Health Organization (WHO) has developed a well-tested tool for minimising errors using a simple three-stage checklist for each case: before induction of anaesthesia (with at least nurse and anaesthetist), before the skin incision (with nurse, anaesthetist and surgeon) and before the patient leaves the operating room (with nurse, anaesthetist and surgeon). This is now used extensively around the world; see Box 1.8 and: http://www.who.int/patientsafety/safesurgery/en/
Surgical mishaps: Surgical mishaps in the operating theatre range from dramatic uncontrolled haemorrhage to the harder to define inadequate surgery leading to complications, slow recovery or avoidable recurrence of cancer. Surgeons have long had clear evidence of poor results of surgical treatment and at last, improvements are occurring with audit, specialisation, training and continuing medical education after specialist accreditation. Governments eager to save money sometimes mandate excessively short training and this is likely to impair outcomes and in the end do more damage and cost more.
Injuries and hazards of moving and positioning patients:
Damage to the cervical spine may occur if the unsupported head is allowed to fall backwards or sideways in unconscious patients, particularly those with rheumatoid arthritis of the cervical spine
Falls to the floor usually occur only if several things go wrong simultaneously
Damage to upper limbs can occur during transfer and positioning, and lower limb damage can occur when placing diseased hips into flexed abduction
Traction on infusion lines, tubes and catheters can cause tissue injury or interfere with monitoring or intravenous therapy, or both
Drains and catheters are at similar risk. Chest drains require special attention as detachment allows air to enter the pleural cavity causing pneumothorax
Peripheral nerve injuries: Peripheral nerve injuries after anaesthesia are probably caused by nerve ischaemia and can occur after as little as 30 minutes in an adverse position. Examples include:
Eye injuries: Irritant fluids such as antiseptics, sprays or gastric acid may be spilled on the cornea causing chemical injury. The eyelids are usually taped gently shut during operation to prevent direct trauma and drying which causes damage after 10 minutes.
Hypothermia: Unintentional hypothermia is a danger to children and to adults undergoing prolonged surgical procedures and is largely avoidable. Reduced core temperature causes changes in drug metabolism, impaired coagulation and an increase in tissue oxygen requirement during the postoperative period and consequent acidosis. This has been shown to predispose to serious postoperative complications. Maintaining normothermia is a mainstay of enhanced recovery protocols.