Mechanisms of Atrioventricular Nodal Excitability and Propagation
Anatomy and Molecular Characteristics of the Mammalian Atrioventricular Node
Molecular Characteristics of Transitional Cells and Inferior Nodal Extension Cells
Anatomy of the Compact Atrioventricular Node and the Bundle of His
Molecular Characteristics of the Compact Atrioventricular Node and Bundle of His
Functional Heterogeneity of the Atrioventricular Junction: Atrionodal Cells, Nodal Cells, and Nodo-His Cells
Dual-Pathway Electrophysiology of the Atrioventricular Node: Slow and Fast Pathways
His Bundle Activation: Longitudinal Dissociation of Atrioventricular Junctional Conduction
Antegrade and Retrograde Conduction Properties of the Atrioventricular Junction
Decremental Conduction and Wenckebach Periodicity
Atrioventricular Junctional Pacemaker
Autonomic Innervation of the Atrioventricular Junction
The atrioventricular node (AVN) is a critical component of the cardiac conduction system, primarily responsible for the conduction of electrical impulses from the atria to the ventricles, and has a secondary function as an escape pacemaker. Since its discovery by Tawara a little more than a century ago, many important advances have been made in terms of understanding the complex structure and function of the AV node, as well as its role in cardiac physiology and pathophysiology. In 1906, Tawara first discovered the spindle-shaped compact network of small cells, which he called a knoten (node).1 Subsequently, Mines provided the first clear and elegant description of reentry involving the AVN in 1916.2 More than half a century later, Moe et al. described the existence of dual-pathway AV nodal physiology and demonstrated the slow and fast pathways with AV nodal reentrant tachycardia (AVNRT) in the dog,3 and Janse et al.4 subsequently confirmed the same in the rabbit.
Anatomy and Molecular Characteristics of the Mammalian Atrioventricular Node
Anatomy of the Triangle of Koch and Atrioventricular Junction Structures
The cardiac pacemaking and conduction system comprises specialized cells composed of pacemaker cells that generate electrical impulses as well as a His-Purkinje system that rapidly conducts the electrical impulses to enable properly timed synchronous myocardial contraction. The electrical impulse generated from the sinoatrial node (SAN), embedded at the junction of superior vena cava and the right atrium, exits the SAN via sinoatrial exit pathways, and travels through the right atrium via the crista terminalis and atrial septum to reach the compact AVN located at the apex of the triangle of Koch. As shown in Figure 28-1, A, the triangle of Koch is defined as the area enclosed by the septal leaflet of the tricuspid valve, the ostium of coronary sinus, and the tendon of Todaro. The apex point of the triangle of Koch is formed by the membranous portion of the ventricular septum, which is another anatomic landmark for the AVN.5 Cells of the compact AVN at the apex of the triangle of Koch are small and spindle shaped, with no clear cellular orientation. In contrast, atrial myocardial cells are large, densely packed, and oriented parallel with one another. Two distinct cell populations exist between cells of the atrial myocardium and those of the compact AVN: (1) transitional cells with intermediate features between the atrial myocytes and spindle-shaped compact AVN, and (2) cells of one or two inferior nodal extension(s), which are regarded as extension(s) of the compact AVN. These two cell types have traditionally been regarded as being responsible for the two atrial electrical inputs into the compact AVN; transitional cells have been thought to compose the “fast pathway,”6,7 whereas cells of inferior nodal extensions constitute the “slow pathway.”8,9
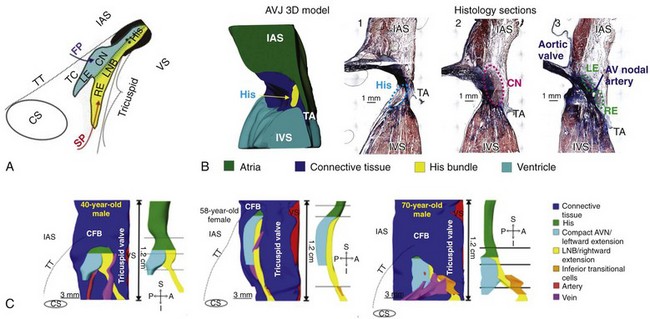
Figure 28-1 Triangle of Koch and AVJ three-dimensional model of the human heart. A, Schematic of the triangle of Koch and AVJ structures. The AV nodal portion is composed of the compact AV node (CN) and the lower nodal bundle (LNB). The right inferior nodal extension (RE) extends proximally from the lower nodal bundle and the left inferior nodal extension (LE) extends from the compact AV node. B, Three-dimensional anatomic reconstruction of the AVJ and histologic sections through the bundle of His, the compact AV node, and extensions (RE and LE). C, Three-dimensional reconstruction of the AVJ, conduction system. AVJ, Atrioventricular junction; TA, tricuspid annulus; IAS, interatrial septum; VS, ventricular septum; FP, fast pathway; SP, slow pathway; CN, compact AV node; CS, coronary sinus; CFB, central fibrous body; TT, tendon of Todaro; A-P, anterior–posterior; S-I, superior–inferior. (A, Reproduced with permission from Kurian T, Ambrosi C, Hucker W, et al: Anatomy and electrophysiology of the human AV node. Pacing Clin Electrophysiol 33:754–762, 2010. B, Reproduced with permission from Fedorov VV, Ambrosi CM, Kostecki G, et al: Anatomic localization and autonomic modulation of atrioventricular junctional rhythm in failing human hearts. Circ Arrhythm Electrophysiol 4:515–525, 2011. C, Reproduced with permission from Hucker WJ, McCain ML, Laughner JI, et al: Connexin 43 expression delineates two discrete pathways in the human atrioventricular junction. Anat Rec [Hoboken] 291:204–215, 2008.)
Transitional Cells
The transitional cells extend anteriorly from the anterior limbus of fossa ovalis, located just above the noncoronary cusp of the aortic valve, and cover the middle and anterior part of the triangle of Koch after crossing the tendon of Todaro, before connecting to the proximal portion of the compact AVN. Such a distribution of transitional cells enables the electrical impulse to spread inside the triangle of Koch without conduction block by the tendon of Todaro.10–12 The area of transitional cells distributed at the right anterior interatrial septum just outside the tendon of Todaro and behind the bundle of His corresponds approximately to the fast pathway site that was targeted during early attempts to cure AVNRT using radiofrequency ablation.6,13 Transitional cells have also been observed in the left atrial septum,14 supporting clinical observations of earliest atrial activation at that site during retrograde fast pathway conduction. Although transitional cells have traditionally been considered to constitute the “fast pathway,” experimental observations do not confirm fast conduction velocity in this pathway.15–17 A shorter conduction delay in this pathway is primarily caused by anatomically shorter distance compared with the “slow pathway.”
Inferior Nodal Extension
The inferior nodal extension (INE) lies parallel to the superior portion of the tricuspid annulus within the triangle of Koch, extends towards the coronary sinus, and then merges with the compact AVN (see Figure 28-1, B). The distal portion of the INE contacts atrial myocytes directly. In the middle of the triangle of Koch, loosely packed transitional cells and the INE converge, and transitional cells overlie the INE at the anterior part of the triangle of the Koch.14,18 The anatomic relationship between the transitional cells and the INE within the triangle of Koch may explain the two different types of double potentials observed on intracardiac electrograms while targeting the slow pathway potential in the ablation of AVNRT. The site where the double potential consists of a low-frequency deflection (nodal component) followed by high-frequency deflection (atrial component), as described by Jackman and colleagues,19 corresponds approximately to the distal part of the INE beneath the orifice of the coronary sinus.20 The double potential of high-frequency followed low-frequency deflections described by Haissaguerre et al.21 may be generated by the two populations of cells in the middle and anterior parts of the triangle of Koch.20
There are important species differences in the anatomy of the AVJ. In contrast to rabbits, which have only one INE, humans typically have two INEs,4,8 which are referred to as the rightward and leftward extensions. As shown in a study of the anatomy of the human AVJ, there is significant anatomic variability in human.8 The majority of humans have two INEs (13 of 21 hearts), some have only the rightward INE (7 of 21 hearts), and, rarely, others have only the leftward INE.8 The leftward INE in humans extends from the compact AVN and lies more superiorly to and is usually shorter than the rightward extension (see Figure 28-1, B).8 Interestingly, three-dimensional reconstruction of the human AVJ shows age-related changes of the INEs and the compact AVN (see Figure 28-1, C). Compared with the left extension, the length of the right extension increases with age, accompanied by a widening of transitional cell zone.22 Evidence of age-related change of INEs may explain the high incidence of AVNRT within an older aged population compared with children (i.e., >10 years of age compared with <5 years of age). The rightward INE in humans has been regarded to constitute the slow pathway, yet the existence of the leftward extension should also be noted, because it is associated with the atypical left variant of AVNRT.23
Molecular Characteristics of Transitional Cells and Inferior Nodal Extension Cells
Histologically, the transitional cells and cells of the INE are relatively small and dispersed among connective tissues, and although these two populations of cells share some similar features, there are important molecular and electrophysiologic differences between these cells. It has recently been demonstrated in human tissue that the transitional cells and the INE cells both express the intermediate levels of Tbx3, a transcription factor that regulates the development of the cardiac conduction system compared with the compact AVN.14,24 However, in rabbit, transitional cells stain negative for neurofilament, and INE cells are neurofilament positive, suggesting that these two cell populations have different embryologic origins.14,16 An important electrophysiologic difference is that, compared with atrial cells, transitional cells express connexin43 (Cx43), an important cardiac gap junction protein, at the intermediate level, whereas the cells of the compact AVN and the leftward INE scantly express Cx43. However, detailed histologic and molecular discrimination between the rightward and the leftward INE in the human AVJ is currently lacking.
Another important difference is that pacemaker activity is observed in INE cells but not in transitional cells in rabbit studies.25 This is supported by gene expression data from humans showing that messenger RNA (mRNA) levels for HCN4, responsible for the hyperpolarization-activated “funny” (If) current, and Cav1.3, an alternative L-type calcium-channel isoform, are highly abundant in the INE, whereas expression of SCN5A, the gene encoding for the cardiac sodium channel protein Nav 1.5, is low.24 In contrast, the expression of HCN4 and Cav1.3 is lower in transitional cells, and the expression of SCN5A is high.24 The INE cells have slower Ca2+-dependent action potential upstrokes, like the nodal cells, whereas transitional cells have fast sodium current–dependent upstrokes. This may account for relatively slow conduction across the INE. These differences in gene expression may explain why sodium-channel blocking Class IA and IC antiarrhythmic drugs are effective in blocking the fast pathway, whereas L-type calcium-channel blockers such as verapamil are effective in blocking the slow pathway.26 The presence of nodal-like cells in the INE could also explain the occurrence of junctional rhythms during radiofrequency application at the inferior triangle of Koch.27
Anatomy of the Compact Atrioventricular Node and the Bundle of His
The AVN and the His bundle are demarcated by the central fibrous body, as initially described by Tawara.1 Morphologically18 and based on Cx43 expression,28,29 the AVN can be subdivided into the lower nodal bundle and the compact AVN. The compact node is the superior portion of the AVN connected to transitional tissue and the INEs, which was previously referred to as the “open node” by earlier investigators.18 The lower nodal bundle indicates the inferior portion that is in some cases enveloped by connective tissue, which was previously referred to as the “closed node.” Cells of the lower nodal bundle are longer and arranged more parallel to each other than in the compact AVN and possess an intermediate functional phenotype between that of the compact AVN and the His bundle. Some investigators have recently described the lower nodal bundle as the penetrating bundle of His because it is enclosed by connective tissue.5 The lower nodal bundle has been shown to connect to the rightward INE, whereas the leftward INE and the compact AVN are a continuous structure (see Figure 28-1, C).28 Regarding age-related change, the compact AVN is known to change from an oval shape to a spindle shape as age increases (see Figure 28-1, C).22
Molecular Characteristics of the Compact Atrioventricular Node and Bundle of His
Both the compact AVN and His bundle express high quantities of connexin40 mRNA and protein, which are gap junction proteins that form large conductance channels, whereas Cx43 is scantly expressed in the compact AVN and expressed at an intermediate level in the lower nodal bundle/His bundle (Figure 28-2).24,28 mRNA levels for Nav1.5, the cardiac sodium channel protein, and a determinant of conduction velocity are scant in the compact AVN (Figure 28-3, A) compared with their high expression levels in the His bundle.24 The abundant expression of connexin proteins and Nav1.5 mRNA in His bundle enables the electrical wave to pass rapidly through the His bundle.
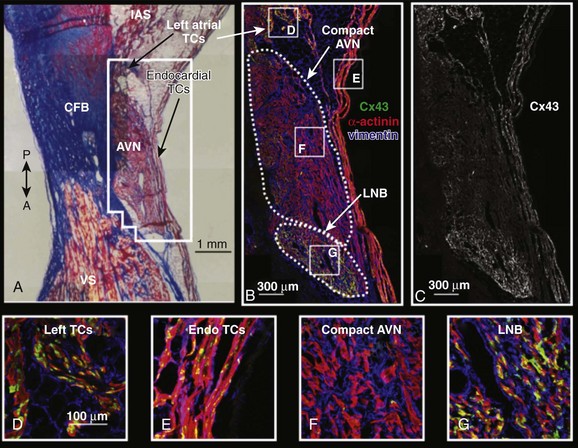
Figure 28-2 Connexin43 (Cx43) expression of the AVJ. A, Masson’s trichrome stain of the AV node. The outlined area surrounding the AV node corresponds to immunohistochemistry shown in B and C. B, Immunohistochemistry of the AV node showing α-actinin in red, vimentin in blue, and Cx43 in green. C, Cx43 expression in the AV node. D-G, Higher magnification of Cx43, vimentin, and α-actinin expression in various areas of the AV node region. (Reproduced with permission from Hucker WJ, McCain ML, Laughner JI, et al: Connexin 43 expression delineates two discrete pathways in the human atrioventricular junction. Anat Rec [Hoboken] 291:204–215, 2008.)
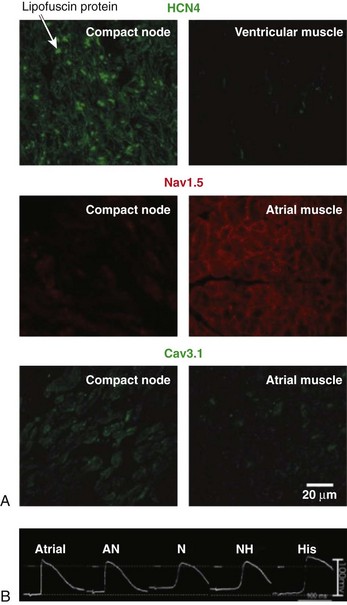
Figure 28-3 Action potentials and immunofluorescence labeling of ion channels. A, Immunofluorescence labeling of HCN4, Nav1.5, and Cav3.1 of human atrioventricular (AV) node and working myocardium. HCN4 (top; green signal) is expressed at the compact AV node (primarily within the cell membrane) but is less expressed in the ventricular muscle. Nav1.5 (middle; red signal) is expressed in the atrial muscle (primarily within the cell membrane) but is less expressed at the compact AV node. Cav3.1 (bottom; green signal) is expressed at the compact AV node but is less expressed in the atrial muscle. Scale bars in each panel are shown in the bottom right corner. B, Action potential recordings from atrial, atrionodal, nodal, nodo-His, and His cells at the rabbit AV junction. (A, Reproduced with permission from Greener ID, Monfredi O, Inada S, et al: Molecular architecture of the human specialised atrioventricular conduction axis. J Mol Cell Cardiol 50:642–651, 2011. B, Reproduced with permission from De Carvalho AP, De Almeida DF: Spread of activity through the atrioventricular node. Circ Res 8:801–809, 1960.)
Functional Heterogeneity of the Atrioventricular Junction: Atrionodal Cells, Nodal Cells, and Nodo-His Cells
As described in the previous section, the AVJ is composed of numerous components, with different cell types that possess different cell morphologies and varying expression of sarcolemmal ion channels and cardiac connexins. The effect of this variability of cell types is the marked functional heterogeneity within the AVJ. Characteristics of the action potential for the different cells in the AVJ have previously been demonstrated in the rabbit heart through the combined study of microelectrodes and histology.30 Significant heterogeneity of action potential morphology in the rabbit AVJ was found,31–33 and three main types of cells have been described: atrionodal (AN), nodal (N), and nodo-His (NH) cells, according to the morphology of the action potential (see Figure 28-3, B). Compared with characteristic atrial myocardial cells, N cells in the compact AVN have less negative resting potentials, smaller amplitudes, and slower upstrokes, and they possess pacemaker activity. AN cells have intermediate features between the atrial cells and the N cells—that is, more negative resting membrane, steeper action potential upstroke, and large amplitude compared with the N cells. NH cells also show intermediate electrophysiologic characteristics between N cells and the His bundle cells. These cells also have more negative resting potentials and steeper action potential upstrokes compared with N cells. Attempts have been made to correlate the functional characteristics to the various cell populations within the AVJ with specific molecular and morphological characteristics, and it appears that AN cells are the transitional cells, N cells are in the compact AVN, and NH cells are in the lower nodal bundle. To date, these observations of variability of action potential morphology in animal models remain unconfirmed in the human AVJ.
Dual-Pathway Electrophysiology of the Atrioventricular Node: Slow and Fast Pathways
It has been demonstrated both in experimental studies3 and in the clinical setting that there are often two conduction pathways through the AVJ, known as the “fast pathway” and the “slow pathway,” which reference the conduction delay with which these pathways conduct an electrical impulse. It has been thought that conduction longitudinally along the anterior interatrial septum represents the fast pathway (anterior input) because earliest retrograde atrial activation is observed at the anterior interatrial septum or near the bundle of His during right ventricular pacing and during slow-fast AVNRT.13 Transverse conduction along the triangle of Koch has been thought to represent the slow pathway (posterior input) because the inferior triangle of Koch is often the site of successful slow pathway ablation to eliminate typical AVNRT.19
However, attempting to correlate these functional pathways to specific cell types and to clearly identify them on histology has proved to be challenging. Optical coherence tomography of intact rabbit AVJ has demonstrated a great degree of variability of cell orientation at different depths.29 Because the transitional cells were found at the anterior interatrial septum, it has been thought that the transitional cells are responsible for the fast pathway. Similarly, the INE cells found within the triangle of Koch have been thought to represent the slow pathway. In reality, transitional cells are widely distributed across the triangle of Koch and coexist with the INEs within the triangle of Koch and are not located exclusively at the anterior interatrial septum. Similarly, correlation of the functional slow pathway to specific cells or histology may be difficult because the posterior inputs to the INE exhibit profound functional complexity, and the triangle of Koch includes not only INE but also transitional cells covering INE and the lower nodal bundle beneath INE.14,28 Such complex distributions of different cell types surrounding the AVJ may give rise to the conceptual confusion of what constitutes the slow and the fast pathways.
Histologically, two inputs (atrial cells and transitional cells), the INEs and the compact AVN, and two His compartments (the lower nodal bundle and His bundle, respectively) exist in the human heart. As described before, the transitional cells in the anterior septum and INE are thought to be the fast and slow pathways, respectively. Clinically, there are two distinct sites of earliest retrograde atrial activation (the anterior septum near His and near the ostium of coronary sinus) during AVNRT or ventricular pacing, which has contributed to the concept that the input through the anterior atrial septum represents the fast pathway and the input through the inferior triangle of Koch represents the slow pathway. Functionally, the traditional terminology of the slow pathway (posterior input) and the fast pathway (anterior input) may not necessarily reflect differences in conduction velocity. Visualization of electrical propagation by optical mapping has revealed that the wavefront during sinus rhythm or electrical stimulation at the right atrium propagates broadly toward the AVN rather than proceeding via two electrically distinguishable, isolated pathways.12,15,17 This finding suggests that, at least during AVNRT, the atrial myocardium/transitional cells enveloping the areas of slow conduction, in which the entry or exit sites are the anterior interatrial septum or the inferior triangle of Koch, may play the role of true fast pathway.16 Furthermore, it should be noted that because techniques of visualizing electrical propagation have developed and improved, they may be better considered as different distances of electrical activation pathways.
His Bundle Activation: Longitudinal Dissociation of Atrioventricular Junctional Conduction
Previous rabbit and human AVN studies have demonstrated that the fast pathway and the slow pathway differentially activate the His bundle.29,34 Fast pathway activation has been shown to engage the superior portion of the His bundle, whereas slow pathway activation engages the inferior portion of the His bundle, suggesting the possible existence of functional longitudinal dissociation of conduction through the AVJ. Furthermore, it has been shown that the time interval between pacing and His activation by direct pacing of the slow pathway is shorter than by fast pathway activation involving transitional cells in animal studies, and it was in reverse relationship to the distance from His.29 Some investigators postulate that, although conduction through the slow pathway is slower than that of the fast pathway, as supported by the gene expression profiles described before,35 direct pacing of the slow pathway may activate the His bundle earlier than fast pathway activation because the rightward INE connects directly to the lower nodal bundle that lies beneath the compact AVN. Therefore, there is the possibility that activation of the slow pathway can bypass the compact AVN and proceed directly to the lower nodal bundle and His bundle. Further evidence comes from optical mapping studies in human hearts, where two different amplitudes of the bipolar His electrogram are seen depending on whether the slow pathway or the fast pathway is activated, indicating that the His is differentially activated by these two pathways.17 Figure 28-4, B-D, shows reentrant echo beats using two dissociated pathways for antegrade conduction and retrograde conduction. These experimental findings suggest that there may be two functionally dissociated axes of the AV conduction system: (1) the fast pathway: transitional cells–(leftward INE)—the compact AVN-His bundle; (2) the slow pathway: the atrial cells/transitional cells–rightward INE—the lower nodal bundle–His bundle.24,29,36–39 Intra-His Wenckebach block may be a clinical manifestation of longitudinal dissociation of AV nodal conduction (see Figure 28-4, E). It should be noted that interactions between two functionally dissociated axes may exist and these two axes may not be completely electrically isolated because of their anatomic proximity. The presence of two longitudinal axes of AV conduction gives rise to potential clinical strategies to modulate ventricular rate in atrial fibrillation.40
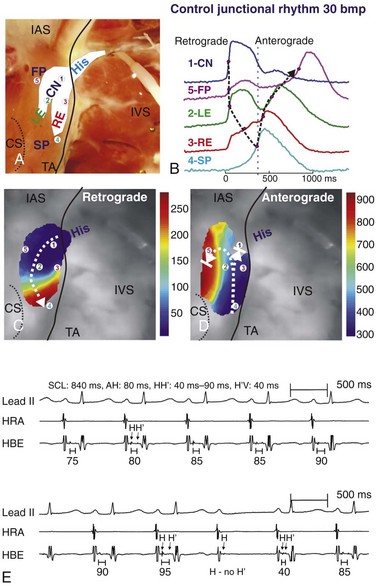
Figure 28-4 Atrioventricular (AV) junctional beat with a retrograde reentrant beat and intra-His Wenckebach block. A, Photograph of the coronary-perfused human AVJ preparation with an optical field of view that included compact node (CN), leftward (LE), and rightward extensions (RE), and the bundle of His (His). B, Optical action potentials, from the five locations indicated in A, showing retrograde and anterograde activation separated by the vertical dashed line. C and D, Activation maps of retrograde and anterograde activations. Dashed arrows show the main conduction directions. E, Intracardiac electrograms recorded at the high right atrium (HRA) and His bundle (HBE), showing intra-hisian Wenckebach block with progressive increase of the H-H′ interval, followed by block of the H′ potential. The lead II surface electrocardiogram is also shown. (A-D, Reproduced with permission from Fedorov VV, Ambrosi CM, Kostecki G, et al: Anatomic localization and autonomic modulation of atrioventricular junctional rhythm in failing human hearts. Circ Arrhythm Electrophysiol 4:515–525, 2011. E, Provided by S.S. Kim, Yonsei University College of Medicine, Seoul, Korea.)
Antegrade and Retrograde Conduction Properties of the Atrioventricular Junction
Antegrade Atrioventricular Conduction
Antegrade AV conduction describes the propagation of electrical impulse from the atria to the ventricles through the AVJ. Experimental optical imaging studies, which can generate thousands of simultaneous recordings, have helped to improve our understanding of AV conduction. Recently, the successful optical mapping of human AVJs has allowed, for the first time, the visualization of the electrical activation patterns through the “slow” and “fast” pathways during atrial pacing (Figure 28-5).39 Compared with the rapid electrical conduction over atrial tissue (up to 117 cm/s), conduction was significantly slower across both the slow and fast pathways, and the action potential upstrokes in both pathways were slower than that of atrial tissue (see Figure 28-5, B). Conduction is slower in transitional cells, thought to constitute the “fast pathway,” than in atrial myocardium because they express lower levels of Cx43 and Nav1.5, which are responsible for the action potential upstroke.24,35 This observation may explain why a phenomenon known as the “AH jump,” defined as the abrupt prolongation of the atrial-His (AH) interval as a result of the large difference in conduction delay between the two pathways, is not common (<10% of the population).
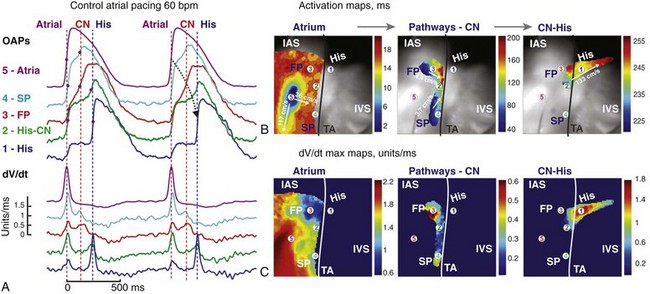
Figure 28-5 Optical mapping of atrioventricular junction during atrial pacing. A, Optical action potentials (OAPs) and their derivatives recorded from sites 1 to 5, as shown in B and C, during atrial pacing at 60 beats/min (cycle length = 1000 ms). Red dots on OAP upstrokes correspond to dV/dt peaks. B, Atrial, atrioventricular node, and His bundle activation maps superimposed on the optical field of view (30 × 30 mm2). C, dV/dtmax maps corresponding to the atrial, atrionodal, and His activation from B. The black line demarcates the tricuspid valve annulus (TA). FP, fast pathway; SP, slow pathway; IAS, interatrial septum; IVS, intraventricular septum. (Reproduced with permission from Fedorov VV, Ambrosi CM, Kostecki G, et al: Anatomic localization and autonomic modulation of atrioventricular junctional rhythm in failing human hearts. Circ Arrhythm Electrophysiol 4:515–525, 2011.)
Rate-dependent decreases in excitability have been observed in transitional cells located at the anterior interatrial septum.6,41 Microelectrode experimental studies in animal models demonstrated that as cycle length was decreased, the transitional cells in the anterior interatrial septum proximal to the AVN had less negative resting membrane potentials and reduced action potential amplitude and upstroke compared with those of transitional cells distal to the AVN.6,41 Thus, the anterior interatrial septum (anterior input) has significantly steeper conduction velocity restitution compared with the posterior input.41 These differences in rate-dependent excitability and conduction velocity restitution between the anterior and posterior inputs cause conduction to delay or block in the “fast pathway” at short cycle lengths, enabling conduction across the slow pathway to reach the AVN and thereby allowing AVNRT to initiate. These experimental observations may explain how AVNRT can be initiated without an abrupt conduction delay during continuous rapid pacing.
Retrograde VA Conduction
Retrograde VA conduction refers to conduction from the ventricles to the atria through the AVJ. Retrograde VA conduction is not common during ventricular pacing in the electrophysiology laboratory and is also infrequently seen during ventricular arrhythmias such as premature ventricular beats, ventricular tachycardia, and accelerated idioventricular rhythm. One significant difference between retrograde AV conduction and antegrade AV conduction is that the retrograde electrical impulse potentially encounters significant source-sink mismatch42 when it exits the insulated His bundle and compact AVN and encounters the large mass atrial tissue, which increases the likelihood of conduction block. Block of retrograde conduction through the AVJ has been demonstrated in human optical mapping studies,39 where electrical impulses originating from the His bundle were seen to be delayed at distal sites of the fast pathway and the slow pathway, ultimately resulting in exit block (Figure 28-6, A). Interestingly, as is shown in Figure 28-6, retrograde conduction of AV junctional ectopic beats originating from the NH region can follow different patterns of electrical wave propagation.39 One junctional beat originating from the NH region can be seen to be conducted preferentially through the fast pathway (see Figure 28-6, A), whereas another beat is conducted through the slow pathway, prominent with a shift in the electrical wave propagation pattern (see Figure 28-6, B). This finding potentially supports the possibility of two longitudinally dissociated retrograde conduction pathways. Similarly, Figure 28-4 shows a junctional beat originating in the NH region, conducting retrogradely via the fast pathway, then turning and reentering anterograde via the slow pathway.
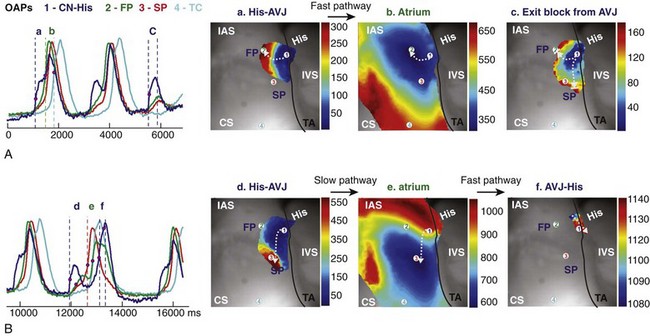
Figure 28-6 Optical mapping of atrioventricular junction during intrinsic rhythm under control conditions. A, Optical action potentials (OAPs) from the nodal-His region (CN-His), fast and slow conduction pathways (FP and SP), and the transitional cell (TC) region near the coronary sinus (CS) recorded from sites 1 to 4 in (B) during intrinsic AVJ rhythm. B, Activation maps of the different tissue layers during FP conduction between atrioventricular node (AVN) (a) and atria (b); exit block from the AVN (c); and during SP conduction between AVN (d) and atria (e), and then AVJ again (f). White arrows indicate the wave propagation directions from the different tissue layers. (Reproduced with permission from Fedorov VV, Ambrosi CM, Kostecki G, et al: Anatomic localization and autonomic modulation of atrioventricular junctional rhythm in failing human hearts. Circ Arrhythm Electrophysiol 4:515–525, 2011.)
Decremental Conduction and Wenckebach Periodicity
Hoffman and Cranefield43 explained the mechanism of slow AV nodal conduction using a decremental conduction hypothesis or the “decremental driving force hypothesis.” In this hypothesis, decremental conduction is a result of increased rates causing reduced excitability and a reduction in action potential upstroke velocity and amplitude. However, this hypothesis does not adequately explain the Wenckebach phenomenon. A different hypothesis regarding the AVN properties was put forward by Rosenblueth, who postulated that a step-delay in conduction at a particular location in the AVN could explain the appearance of Wenckebach cycles at fast atrial rates, also referred to as the “constant driving force hypothesis.”44 It was postulated that during rapid constant continuous stimuli, incomplete recovery from excitation by the previous beat creates a conduction delay for the next beat, and thus progressively accumulating step-delays ultimately result in block, providing time for full recovery of excitation. A theoretical study demonstrated that a reduction in intercellular conductance (such as that seen in the AV node) could produce a step delay in conduction, reduce conduction velocity, and paradoxically increase the safety factor of conduction.45 In a microelectrode experiment of single AV nodal cell of rabbit,46 Wenckebach periodicity was found to be associated with a gradual loss of diastolic potential, resulting in progressive impairment of cellular recovery from beat to beat, and thereby progressive conduction slowing because of postrepolarization refractoriness.
Although most theoretical and experimental studies have implicated the compact AVN as playing a key role in the decremental properties of the AV conduction and the Wenckebach phenomenon, some investigators have demonstrated a role for the fast and slow pathways. Transitional cells have also been shown to exhibit less negative resting membrane potentials, and a reduction in AP amplitude and upstroke as cycle lengths were decreased, contributing to decremental conduction.41 Some studies have also demonstrated the occurrence of 2 : 1 AV block in the fast pathway and 3 : 2 Wenckebach block in the slow pathway conduction. Recent studies have shown that the decremental conduction and Wenckebach block in the fast and slow pathways can also affect the Wenckebach phenomenon of the compact AVN.36,37 These experiments showed that electrical input from the slow pathway affects the compact AVN, thereby slowing the electrical conduction emanating from the fast pathway, ultimately creating complex Wenckebach block. This suggests a dynamic electrotonic interaction of the longitudinally dissociated functional pathways.
Atrioventricular Junctional Pacemaker
In addition to playing a critical role in conducting electrical impulses from the atria to the ventricles, cells in the AVJ can also play a pacemaking role. Clinically, AV junctional rhythm is commonly observed during periods of sinus node pauses, when it fulfills the role of an escape pacemaker. Accelerated junctional rhythm can also occur during acute illnesses, postoperative cardiac surgery, and sympathetic overdrive. The notion that the AVJ has a pacemaking function is not new. In fact, when Tawara first published his discovery of the AVN, his mentor Ludwig Aschoff suggested that the AVN may be the pacemaker of the heart.1
The pacemaking function of the AVJ has been clearly demonstrated in optical mapping studies in animals and in humans. In the rabbit, the dominant AV junctional pacemaker was identified in the INE, where HCN4 is abundantly expressed, with activation spreading toward the bundle of His without a significant delay.25 Recently, we have demonstrated that the AV junctional pacemaker rhythm originates from the NH region or His bundle in optical mapping studies on the failing human heart.39 As shown in the example of a human AVJ in Figures 28-6 and 28-7, pacemaking activity originates from the NH/His bundle, which is electrically isolated from the ventricular myocardium, and then spreads retrogradely into the atrium. Diastolic depolarization preceded each action potential upstroke in the NH/His region, demonstrating the pacemaker function of the proximal NH/His bundle. In response to isoproterenol, the AV junctional rate increased from 41 bpm to 80 bpm. Interestingly, β-adrenergic stimulation also shifted the location of pacemaking activity from the proximal His to the AVN, accompanied by the movement of the predominant site of diastolic depolarization (see Figure 28-7, A and B). Moreover, isoproterenol also altered the preferential retrograde conduction pattern of the junctional impulses (see Figure 28-7, C). Retrograde atrial activation, which occurs predominantly via the fast pathway in intact heart, occurred simultaneously through both the slow and fast pathways during β-adrenergic stimulation. Junctional pacemaker activity was suppressed by acetylcholine. Acetylcholine in a second human heart resulted in a shift of preferential conduction toward the slow pathway, with no shift of pacemaker site of the NH/His bundle (see Figure 28-7, C). These data suggest that modulation of adrenergic and cholinergic tone can affect the preferential conduction pathway as well as the location of the dominant pacemaker within the AVJ.
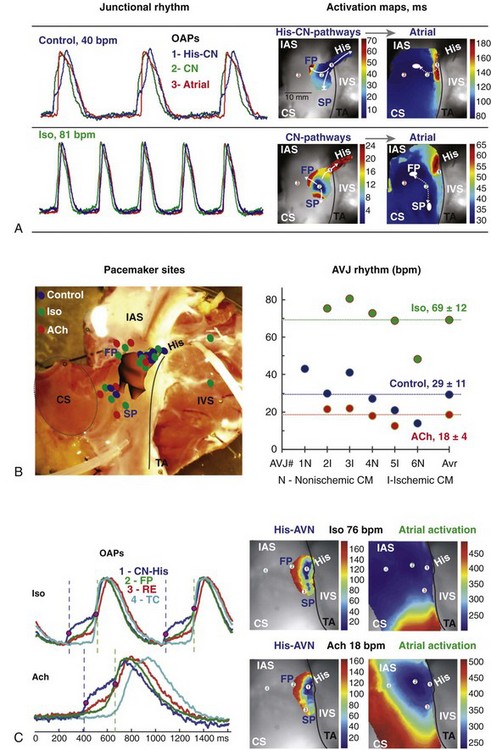
Figure 28-7 Optical mapping of atrioventricular junction (AVJ) during autonomic stimulation. A, Optical action potentials (OAPs) and activation maps of the different tissue layers during spontaneous AVJ rhythm in control (upper panel) and after a 10-minute perfusion of isoproterenol (Iso) (lower panel). OAPs were recorded from the nodo-His (NH) region (1, His-CN, blue), compact node (2, CN, green), and interatrial septum (IAS) (3, atria, red). White ovals show atrial breakthrough sites as recorded by optical mapping. B, Summary of anatomic locations of leading pacemaker sites in the human AVJ (left panel). Individual for each heart and average values for AVJ rhythm in control and during perfusion of Iso and acetylcholine (Ach; right panel). C, OAPs from sites 1 to 4 during Iso and ACh perfusion (left panel). His-AVN and atrial activation maps showing the leading pacemaker remaining in the same location (NH region) but different wave propagation during both Iso and ACh perfusion (right panel). CM, cardiomyopathy. (Reproduced with permission from Fedorov VV, Ambrosi CM, Kostecki G, et al: Anatomic localization and autonomic modulation of atrioventricular junctional rhythm in failing human hearts. Circ Arrhythm Electrophysiol 4:515–525, 2011.)
It is currently thought that the voltage-dependent “funny current” (If current) and the “calcium clock” are two important molecular mechanisms involved in the spontaneous diastolic depolarization of pacemaking cells.47–49 HCN4, which encodes for the If channel, is expressed abundantly in the His bundle in the human heart,24 as well as the compact AVN and INE as described before, and contributes to the pacemaking activity of the His bundle and the NH region. Another molecular mechanism of pacemaking activity of the SAN relates to the “calcium clock” hypothesis, whereby sarcoplasmic reticulum calcium release and the related activation of sodium-calcium exchanger (NCX) current play roles in diastolic depolarization, especially during β-adrenergic stimulation.50,51 This mechanism may also play a substantial role in the human AVN. In humans, the mRNA levels of NCX1 were shown to be elevated in the compact AVN compared with the INE.24 This observation may support the superior shift of the AVJ pacemaker from the His bundle to compact node during β-adrenergic stimulation.
Autonomic Innervation of the Atrioventricular Junction
In addition to modifying pacemaker activity, autonomic innervation of the AVJ can also modulate AVN function and affect the effective refractory period.52,53 In the clinical setting, alterations of autonomic tone in the AVJ may also be related to initiation of AVNRT54 or transient AV block, and may be important in determining the AVN conduction properties and thus the ventricular rate during atrial tachyarrhythmias such as atrial fibrillation. The epicardial ganglionated plexus embedded in the atrioventricular fat pad located at the junction of both atria and inferior vena cava innervates the AVJ, and high-frequency nerve stimulation has been shown to slow AVN conduction without altering sinus rate, leading to a slowing of ventricular rate, or transient complete heart block.55–57 Autonomic nerve stimulation at the ganglionated plexus innervating the AVJ was also shown to shorten the local effective refractory period of the atrial tissue of the AVJ.55 Attempts have also recently been made to understand the functional complexity and the distribution of the autonomic innervation of the AVJ. Studies in rabbits have demonstrated that the inferior nodal extension expresses higher levels of parasympathetic innervation, and the His bundle was innervated with both sympathetic and parasympathetic neurons, in contrast to the sparsely innervated compact AVN region.58 Despite a lack in understanding of autonomic innervation, modulation of AVN conduction may be an emerging therapeutic target, because atrial fibrillation with rapid rate caused by high AVN conduction may lead to fatal hemodynamic instability, especially in heart failure.
Optical Mapping of the Human Atrioventricular Junction
For many years, it has been difficult to study the electrophysiology of the AVJ in detail because it is a complex and deep structure, with multiple histologically and electrophysiologically distinct cell types and components. Although His bundle–potential recordings using bipolar electrodes in clinical settings had advanced our understanding of the functional properties of the AVJ, integrated and simultaneous recording of electrical activity of the compact AVN, slow pathway, and fast pathway in the intact human heart had remained elusive. However, recent advances in optical imaging capabilities, such as the design of high-resolution complementary metal-oxide-semiconductor cameras and the advent of novel near-infrared potentiometric dyes, such as di-4-ANBDQBS, have made possible the simultaneous imaging of transmembrane voltage transients in the different components of the intact human AVJ.39 Optical mapping using di-4-ANBDQBS allows the imaging of electrical activity from deeper structures such as the INE, in contrast to traditional potentiometric dyes such as di-4-ANEPPS, which only allows for imaging of superficial, subendocardial human AVJ structures such as the His bundle and parts of the fast and slow pathways, but not the complete AV conduction pathway.17
Using combined optical mapping, microelectrode recordings, and surface electrogram recordings, it has been clearly demonstrated that optical action potentials from the superficial and deeper structure can be distinguished in the rabbit heart.59 With optical mapping, we were able to map electrical activation in human AVJs. As shown in Figure 28-5, action potentials from different tissues (i.e., atria, transitional cells, INEs, compact AVN, His) with different morphologies could be recorded, allowing detailed activation patterns to be discerned. Interestingly, the first derivative traces of optical AP (dV/dt) displayed very similar morphologies to that of bipolar electrogram recordings at those sites (see Figure 28-5, A). For example, the first derivative of the optical signal recorded at the site of the slow pathway has a high-amplitude deflection followed by a low-amplitude deflection as a result of atrial and rightward INE activation, respectively, which is similar to that of slow pathway potential in bipolar electrode recording.
Using such optical mapping techniques, we have been able to demonstrate many of the physiologic properties of the intact human AVJ. This includes demonstrating antegrade conduction through the slow pathway and the fast pathway (see Figure 28-5), two retrograde conduction patterns through two distinct pathways, suggesting the presence of longitudinal dissociation (see Figure 28-6), shift of AV junctional pacemaker activity by modulation by autonomic tone (see Figure 28-7), and reentry within the AVJ structures (see Figure 28-4, A).
References
1. Tawara, S, Das reizleitungssystem des saugertierherzens (the conduction system of the mammalian heart) (Translated into English by Kozo Suma and Munehiro Shimada). Imperial College Press, London, 2000.
2. Mines, GR. On dynamic equilibrium in the heart. J Physiol. 1913; 46:349–383.
3. Moe, GK, Preston, JB, Burlington, H. Physiologic evidence for a dual a-v transmission system. Circ Res. 1956; 4:357–375.
4. Janse, MJ, van Capelle, FJ, Freud, GE, et al. Circus movement within the av node as a basis for supraventricular tachycardia as shown by multiple microelectrode recording in the isolated rabbit heart. Circ Res. 1971; 28:403–414.
5. Anderson, RH, Yanni, J, Boyett, MR, et al. The anatomy of the cardiac conduction system. Clin Anat. 2009; 22:99–113.
6. Antz, M, Scherlag, BJ, Patterson, E, et al. Electrophysiology of the right anterior approach to the atrioventricular node: Studies in vivo and in the isolated perfused dog heart. J Cardiovasc Electrophysiol. 1997; 8:47–61.
7. Patterson, E, Scherlag, BJ. Fast pathway-his bundle connections in the rabbit heart. J Interv Card Electrophysiol. 2004; 10:121–129.
8. Inoue, S, Becker, AE. Posterior extensions of the human compact atrioventricular node: A neglected anatomic feature of potential clinical significance. Circulation. 1998; 97:188–193.
9. Moe, GK, Preston, JB, Burlington, H. Physiologic evidence for a dual a-v transmission system. Circ Res. 1956; 4:357–375.
10. Chang, BC, Schuessler, RB, Stone, CM, et al. Computerized activation sequence mapping of the human atrial septum. Ann Thorac Surg. 1990; 49:231–241.
11. Pandozi, C, Ficili, S, Galeazzi, M, et al. Propagation of the sinus impulse into the koch triangle and localization, timing, and origin of the multicomponent potentials recorded in this area. Circ Arrhythm Electrophysiol. 2011; 4:225–234.
12. McGuire, MA, Bourke, JP, Robotin, MC, et al. High resolution mapping of Koch’s triangle using sixty electrodes in humans with atrioventricular junctional (av nodal) reentrant tachycardia. Circulation. 1993; 88:2315–2328.
13. Jazayeri, MR, Hempe, SL, Sra, JS, et al. Selective transcatheter ablation of the fast and slow pathways using radiofrequency energy in patients with atrioventricular nodal reentrant tachycardia. Circulation. 1992; 85:1318–1328.
14. Li, J, Greener, ID, Inada, S, et al. Computer three-dimensional reconstruction of the atrioventricular node. Circ Res. 2008; 102:975–985.
15. Efimov, IR, Fahy, GJ, Cheng, Y, et al. High-resolution fluorescent imaging does not reveal a distinct atrioventricular nodal anterior input channel (fast pathway) in the rabbit heart during sinus rhythm. J Cardiovasc Electrophysiol. 1997; 8:295–306.
16. Efimov, IR, Nikolski, VP, Rothenberg, F, et al. Structure-function relationship in the av junction. Anat Rec. 2004; 280A:952–965.
17. Hucker, WJ, Fedorov, VV, Foyil, KV, et al. Optical mapping of the human atrioventricular junction. Circulation. 2008; 117:1474–1477.
18. Meijler, FL, Janse, MJ. Morphology and electrophysiology of the mammalian atrioventricular node. Physiol Rev. 1988; 68:608–647.
19. Jackman, WM, Beckman, KJ, McClelland, JH, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry, by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992; 327:313–318.
20. McGuire, MA, de Bakker, JM, Vermeulen, JT, et al. Origin and significance of double potentials near the atrioventricular node. Correlation of extracellular potentials, intracellular potentials, and histology. Circulation. 1994; 89:2351–2360.
21. Haissaguerre, M, Gaita, F, Fischer, B, et al. Elimination of atrioventricular nodal reentrant tachycardia using discrete slow potentials to guide application of radiofrequency energy. Circulation. 1992; 85:2162–2175.
22. Waki, K, Kim, JS, Becker, AE. Morphology of the human atrioventricular node is age dependent: A feature of potential clinical significance. J Cardiovasc Electrophysiol. 2000; 11:1144–1151.
23. Otomo, K, Okamura, H, Noda, T, et al. “Left-variant” atypical atrioventricular nodal reentrant tachycardia: Electrophysiological characteristics and effect of slow pathway ablation within coronary sinus. J Cardiovasc Electrophysiol. 2006; 17:1177–1183.
24. Greener, ID, Monfredi, O, Inada, S, et al. Molecular architecture of the human specialised atrioventricular conduction axis. J Mol Cell Cardiol. 2011; 50:642–651.
25. Dobrzynski, H. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circ Res. 2003; 93:1102–1110.
26. Goy, JJ, Fromer, M. Antiarrhythmic treatment of atrioventricular tachycardias. J Cardiovasc Pharmacol. 1991; 17(Suppl 6):S36–40.
27. Nikoo, MH, Emkanjoo, Z, Jorat, MV, et al. Can successful radiofrequency ablation of atrioventricular nodal reentrant tachycardia be predicted by pattern of junctional ectopy? J Electrocardiol. 2008; 41:39–43.
28. Hucker, WJ, McCain, ML, Laughner, JI, et al. Connexin 43 expression delineates two discrete pathways in the human atrioventricular junction. Anat Rec. 2008; 291:204–215.
29. Hucker, WJ, Sharma, V, Nikolski, VP, et al. Atrioventricular conduction with and without av nodal delay: Two pathways to the bundle of his in the rabbit heart. Am J Physiol: Heart Circ Physiol. 2007; 293:H1122–H1130.
30. Anderson, RH, Janse, MJ, van Capelle, FJ, et al. A combined morphological and electrophysiological study of the atrioventricular node of the rabbit heart. Circ Res. 1974; 35:909–922.
31. Billette, J. Atrioventricular nodal activation during periodic premature stimulation of the atrium. Am J Physiol. 1987; 252:H163–177.
32. De Carvalho, AP, De Almeida, DF. Spread of activity through the atrioventricular node. Circ Res. 1960; 8:801–809.
33. Hoffman, BF, De Carvalho, AP, Mello, WC, et al. Electrical activity of single fibers of the atrioventricular node. Circ Res. 1959; 7:11–18.
34. Zhang, Y, Bharati, S, Mowrey, KA, et al. His electrogram alternans reveal dual-wavefront inputs into and longitudinal dissociation within the bundle of his. Circulation. 2001; 104:832–838.
35. Greener, ID, Tellez, JO, Dobrzynski, H, et al. Ion channel transcript expression at the rabbit atrioventricular conduction axis. Circ Arrhythm Electrophysiol. 2009; 2:305–315.
36. Zhang, Y, Mazgalev, TN. Av nodal dual pathway electrophysiology and Wenckebach periodicity. J Cardiovasc Electrophysiol. 2011; 22:1256–1262.
37. Patterson, E, Scherlag, BJ, Lazzara, R. Stable patterns of ah block arising from longitudinal dissociation and reentry within the superfused rabbit av junction. J Interv Card Electrophysiol. 2010; 28:5–18.
38. Patterson, E, Scherlag, BJ. Longitudinal dissociation within the posterior av nodal input of the rabbit : A substrate for av nodal reentry. Circulation. 1999; 99:143–155.
39. Fedorov, VV, Ambrosi, CM, Kostecki, G, et al. Anatomic localization and autonomic modulation of atrioventricular junctional rhythm in failing human hearts. Circ Arrhythm Electrophysiol. 2011; 4:515–525.
40. Zhang, Y, Bharati, S, Mowrey, KA, et al. His electrogram alternans reveal dual atrioventricular nodal pathway conduction during atrial fibrillation: The role of slow-pathway modification. Circulation. 2003; 107:1059–1065.
41. Patterson, E, Scherlag, BJ. Decremental conduction in the posterior and anterior av nodal inputs. J Interv Card Electrophysiol. 2002; 7:137–148.
42. Rohr, S, Kucera, JP, Fast, VG, et al. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997; 275:841–844.
43. Hoffman, BF, Cranefield, PF. Electrophysiology of the heart. New York: McGraw-Hill, Blakiston Division; 1960.
44. Rosenblueth, A. Mechanism of the Wenckebach-Luciani cycles. Am J Physiol. 1958; 194:491–494.
45. Shaw, RM, Rudy, Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and l-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997; 81:727–741.
46. Hoshino, K, Anumonwo, J, Delmar, M, et al. Wenckebach periodicity in single atrioventricular nodal cells from the rabbit heart. Circulation. 1990; 82:2201–2216.
47. Bogdanov, KY, Vinogradova, TM, Lakatta, EG. Sinoatrial nodal cell ryanodine receptor and na(+)-ca(2+) exchanger: Molecular partners in pacemaker regulation. Circ Res. 2001; 88:1254–1258.
48. Baruscotti, M, Bucchi, A, Difrancesco, D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol Ther. 2005; 107:59–79.
49. Hata, T, Noda, T, Nishimura, M, et al. The role of ca2+ release from sarcoplasmic reticulum in the regulation of sinoatrial node automaticity. Heart Vessel. 1996; 11:234–241.
50. Vinogradova, TM, Bogdanov, KY, Lakatta, EG. Beta-adrenergic stimulation modulates ryanodine receptor ca(2+) release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002; 90:73–79.
51. Joung, B, Tang, L, Maruyama, M, et al. Intracellular calcium dynamics and acceleration of sinus rhythm by beta-adrenergic stimulation. Circulation. 2009; 119:788–796.
52. Lazzara, R, Scherlag, BJ, Robinson, MJ, et al. Selective in situ parasympathetic control of the canine sinoatrial and atrioventricular nodes. Circ Res. 1973; 32:393–401.
53. O’Toole, MF, Ardell, JL, Randall, WC. Functional interdependence of discrete vagal projections to sa and av nodes. Am J Physiol. 1986; 251:H398–404.
54. Stellbrink, C, Diem, B, Schauerte, P, et al. Differential effects of atropine and isoproterenol on inducibility of atrioventricular nodal reentrant tachycardia. J Interv Card Electrophysiol. 2001; 5:463–469.
55. Quan, KJ, Lee, JH, Van Hare, GF, et al. Identification and characterization of atrioventricular parasympathetic innervation in humans. J Cardiovasc Electrophysiol. 2002; 13:735–739.
56. Hou, Y, Scherlag, BJ, Lin, J, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: Effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007; 50:61–68.
57. Rossi, P, Bianchi, S, Monari, G, et al. Vagal tone augmentation to the atrioventricular node in humans: Efficacy and safety of burst endocardial stimulation. Heart Rhythm. 2010; 7:683–689.
58. Hucker, WJ, Nikolski, VP, Efimov, IR. Autonomic control and innervation of the atrioventricular junctional pacemaker. Heart Rhythm. 2007; 4:1326–1335.
59. Efimov, IR, Mazgalev, TN. High-resolution, three-dimensional fluorescent imaging reveals multilayer conduction pattern in the atrioventricular node. Circulation. 1998; 98:54–57.