CHAPTER 7 Mechanism of Action of Sclerotherapy
General Mechanism for Producing Endothelial Damage
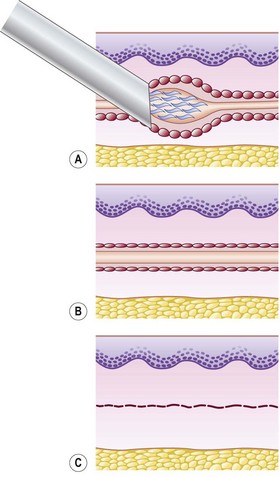
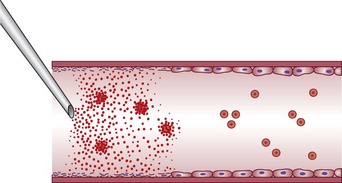
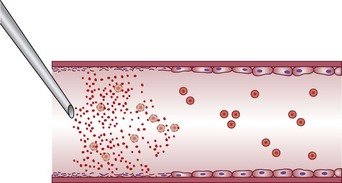
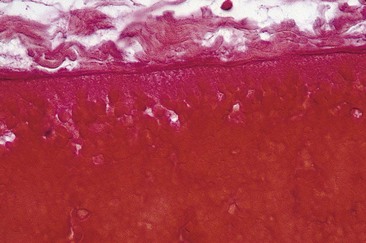
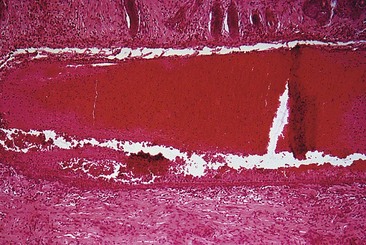
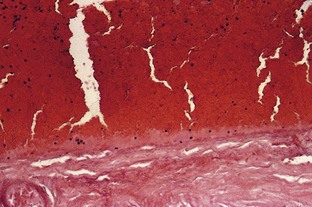
Sclerotherapy refers to the introduction of a foreign substance into the lumen of a vessel, aiming to create venous wall damage leading to occlusion of the vessel (Fig. 7.1). This procedure, when performed on telangiectasias, is referred to as microsclerotherapy.1
In addition, endothelial cells in one organ and in one location may act differently than endothelial cells elsewhere. They appear to be specialized to their area. Thus it is paradoxical that sclerotherapy treatment is not without significant adverse sequelae (see Chapter 8).
Endothelial destruction by sclerosing solutions is both dose and time dependent.6 In vitro studies of cultured endothelial cells demonstrated activation of calcium signaling and nitric oxide pathways followed by cell death after exposure to sclerosing solutions. Cell death occurred within 15 minutes with 0.3% polidocanol (POL) or 0.1% sodium tetradecyl sulfate (STS). At less than 0.003% POL or 0.005% STS cells remained alive after 60 minutes. Combined with protein-binding of detergent sclerosing solutions and a 10,000-fold dilution after release into the circulation, these studies demonstrate the safety of sclerotherapy, since sclerosing solutions are rapidly diluted to ‘safe’ concentrations distal to the point of injection.
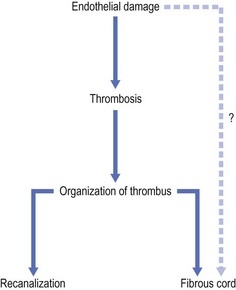
Total endothelial destruction results in the exposure of subendothelial collagen fibers, causing platelet aggregation, adherence, and release of platelet-related factors. This series of events initiates the intrinsic pathway of blood coagulation by activating factor XII. Ideally, sclerosing solutions otherwise should not cause activation or release of thromboplastic activity because this would initiate the extrinsic pathway of blood coagulation. Excessive thrombosis is detrimental to the production of endofibrosis because it may lead to recanalization of the vessel as well as excessive intravascular and perivascular inflammation and its resulting sequelae (see Chapter 8). This is thought to be prevented or at least minimized with postsclerotherapy compression (see Chapter 6). However, thrombosis usually occurs to some degree as a result of sclerotherapy.
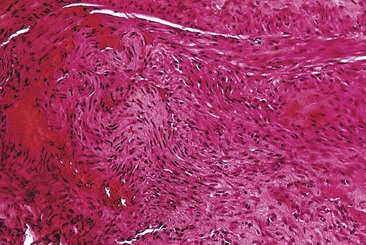
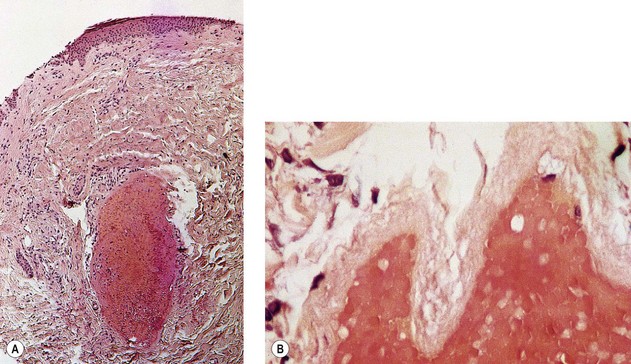
If a thrombus is formed, it should be well anchored to the venous wall to prevent embolization. Wolf7 in 1920 established that effective sclerosis causes thrombosis that penetrates the full thickness of the adventitia of the vessel wall. Schneider8 has shown in histologic examinations of sclerosed varices that the strongest fixation of a thrombus occurs in areas where the entire endothelium is destroyed. Therefore, endothelial damage must be complete and should result in minimal thrombus formation with subsequent organization and fibrosis (Fig. 7.2). In addition, after sclerotherapy, maximum full-thickness fibrosis of the treated segment occurs after 6 weeks of compression.9 Therefore, in addition to limiting the extent of thrombosis, compression may facilitate endofibrosis (see Chapter 6). Chleir and Vin10 have described the differences between a clot observed during a spontaneous thrombosis ‘thrombus’ and during the sclerosing process, for which they suggested the neologism ‘sclerus’ (Table 7.1).
Table 7.1 Thrombus versus sclerosis: comparison of vein content during a thrombosis and during a sclerosing process
| Thrombus | Sclerosis | |
|---|---|---|
| Pathophysiology | Hematological phenomenon | Tissue phenomenon |
| Activation of Virchow’s triad | Parietal mechanism | |
| Recanalization without fibrosis | Fibrosis | |
| Clinical | Painful | More discomfort than pain |
| Inflammatory | No inflammation | |
| Indurate plaques | No indurate plaque | |
| Biology | Positive d-dimers | Negative d-dimers |
| B-mode ultrasound | Convex toward junction | Concave toward junction |
| ‘Rosette’ picture | Parietal thickening | |
| No parietal adhesion | Parietal adhesion | |
| Dilatation | Retraction | |
| Pathology | No parietal lesion | Inflammatory infiltration of venous wall |
| Thrombus rich in RBCs and platelets | Sclerosis contains more WBCs and less RBCs than thrombus | |
| Rare WBCs |
RBCs, red blood cells; WBCs, white blood cells.
From Chleir F, Vin F: Actualités Vasculaires Internationales 35:18, 1995.
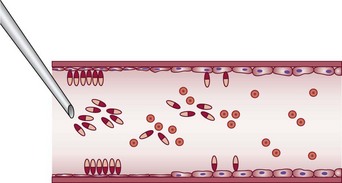
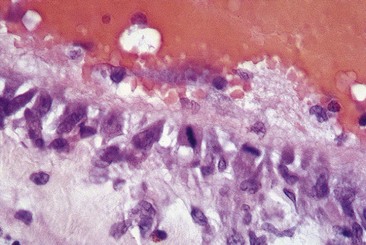
Endothelial damage can be provoked by a number of mechanisms, such as a change in the surface tension of the plasma membrane or modification of the physical–chemical milieu of the endothelial cell through a change of intravascular pH or osmolality. The endothelium can be destroyed directly by caustic chemicals or by other physical factors such as heat and cold. For sclerotherapy to be effective without recanalization of the thrombotic vessel, the endothelial damage and resulting vascular necrosis must be extensive enough to destroy the entire blood vessel wall.11
Destruction of the entire vessel wall and not just the endothelium is necessary, as demonstrated in animal studies described later in this chapter. The reason may relate to the multifunctional nature of vascular smooth muscle cells. These cells, which are found in significant concentration within superficial veins (see Chapter 2), have a large number of functions, including the synthesis of collagen, elastin, and proteoglycans.12 It is hypothesized that if they remain viable, they can regenerate a foundation that promotes migration of undamaged adjacent endothelial cells that allow recanalization of the treated vessel.13,14
In addition, for effective destruction of a varicosity or telangiectasia, the entire vessel must be sclerosed to prevent recanalization. Recanalization occurs easily in vessels where only a section of endothelium is damaged. This is due to rapid endothelial regeneration, which has been measured at a turnover rate of 0.1% to 10% per day, or higher.15 Endothelial migration has been estimated to proceed at a rate of 0.07 mm/day in the circumferential direction and six times faster in an axial direction in rat aortas.16 In fact, endothelial cell regeneration may be sufficiently rapid to replace dying endothelium after small areas are denuded.17 In the future, the practitioner may be able to estimate total endothelial destruction as a marker for effective sclerosis by counting circulating endothelial cells.18,19
Categories of Sclerosing Solutions
Detergent solutions
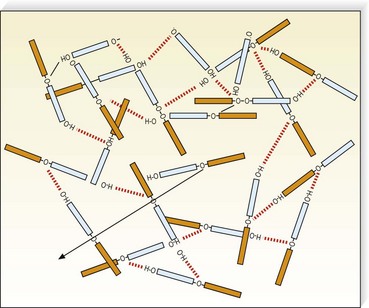
Detergent sclerosing solutions commonly used to treat varicose and telangiectatic veins include sodium morrhuate (SM), ethanolamine oleate (EO), STS, and POL (lauromacrogol 400, laureth-9). They produce endothelial damage through interference with cell surface lipids (Fig. 7.3). Strong detergents, such as STS and SM, produce maceration of the endothelium within 1 second of exposure.20 The intercellular ‘cement’ is disrupted, causing desquamation of endothelial cells in plaques. Because the hydrophilic and hydrophobic poles of the detergent molecule orient themselves so that the polar hydrophilic part is within the water and the hydrophobic part is away from the water, they appear as aggregates in solution (micelles) or fixed onto the endothelial surface (Fig. 7.4). Because the practitioner cannot ensure that the solution is entirely in contact with the endothelial surface (if the injected vein contains blood), the decrease in surface tension on the endothelial cells may not be in direct proportion to the concentration of the solution. Strong detergent sclerosants therefore have a low safety margin.20
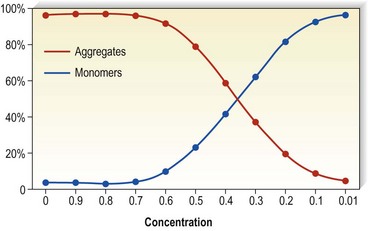
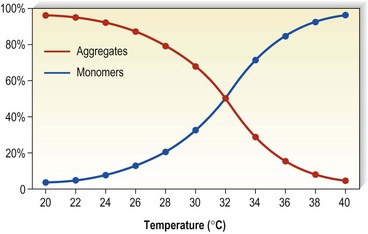
Detergents act as micelles when injected into a nondetergent environment (blood). Their destructive action on endothelial cells is enhanced when they act as aggregates rather than monomers. Thus, the concentration of the sclerosing solution in the vessel is an important factor regarding endothelial destruction and activity (Fig. 7.5). They have been found to aggregate to a significant extent at lower temperatures as opposed to room temperatures (Fig. 7.6).
Detergent sclerosing agents have been studied regarding their direct toxic effects on the formed elements of blood. One study found that the addition of SM, EO, STS, or POL to citrated plasma did not cause clotting nor shorten either the prothrombin time (PT) or the partial thromboplastin time (PTT).21 However, all of the sclerosing agents examined were directly toxic to both granulocytes and red blood cells (RBCs) at dilutions of up to 1 : 1000. When tested against cultured endothelial cells, all solutions were toxic to approximately 60% to 80% of cells at 1 : 100 dilutions, but only SM and EO were toxic at a further dilution of 1 : 1000. None of these tested solutions were toxic at 1 : 10,000 dilutions. Therefore, this study confirms that effective endosclerosis occurs through damage to endothelium and not through thrombosis induced by destruction or damage to red and/or white blood cells. Another in vitro study, however, found that the activated PTT was prolonged in proportion to the fall of factor XII and prekallikrein activity when POL was added to citrated serum.22 This indicates that in addition to its action on endothelial cells, POL is capable of acting on blood coagulation through activation of the early phase of the intrinsic pathway. The clinical relevance of this finding is unclear because additional studies have failed to demonstrate its significance, as explained later in this Chapter.23,24
Osmotic solutions
Hypertonic solutions, such as hypertonic saline (HS), probably cause dehydration of endothelial cells through osmosis, causing endothelial destruction (Fig. 7.7).22 It is speculated that fibrin deposition with thrombus formation on the damaged vessel wall occurs through modification of the electrostatic charge of the endothelial cells.20 For the vessel wall to be completely destroyed, the osmotic solution must be of sufficient concentration to diffuse throughout the entire vein wall.25 In contrast to the immediate action of detergent sclerosing solutions, experimental studies have shown that endothelial destruction with HS 22% or glucose 66% occurs only after 3 minutes.20 The destroyed endothelial cells do not appear to be desquamated as with detergent sclerosing solutions.20
Hypertonic solutions have a predictable destructive power that is proportional to their osmotic concentration. This was demonstrated in a comparative study of multiple hypertonic solutions used on the superficial and internal saphenous veins of 27 dogs.26 The degree of endothelial damage was assessed histologically at multiple times from 30 minutes to 8 weeks after sclerotherapy. The authors ranked the solutions from strongest to weakest as follows:
The authors concluded that maximal endothelial destruction occurred as early as 30 minutes to 4 days after injection, after which time the injected vessel went through either a reparative or a fibrotic process. Because dilution occurs with intravascular serum and blood, osmotic solutions have their greatest effect at or near the site of injection. In contrast, detergent sclerosing solutions can exert effective sclerosis for 5 to 10 cm along the course of the injected vessel. Sadick27 examined the sclerosing effect of HS 23.4% and POL 0.5%. He found equal sclerosing effect (length) for these two solutions injected in a similar type of vein using identical techniques. Unfortunately, HS 23.4% is more potent (about two to three times more) than POL 0.5%. Therefore, he inadvertently demonstrated that detergent solutions have about twice the therapeutic efficacy of osmotic solutions. A better comparison would have been with HS 11.7%.
Chemical solutions
Chemical irritants also act directly on endothelial cells to produce endosclerosis. Lindemayr and Santler28 studied the sclerosing effect of 4% polyiodinated iodine (PII; Variglobin) with standard and immunofluorescent microscopy and demonstrated fibrin deposition on the sclerosed veins only. Platelets fixed only to elastin, collagen, the basement membrane, and the amorphous material of the subendothelial layer, not to intact endothelial cells. It is also thought that the chemical destruction is in part related to the dissolution of intercellular cement, which has been demonstrated to occur after 30 seconds of exposure.20 Thus, this chemical irritant sclerosing solution produces its end result of vascular fibrosis through the irreversible destruction of endothelial cells with resultant thrombus formation on the subendothelial layer (Fig. 7.8).
The aforementioned mechanism of action has been demonstrated visually with scanning electron microscopy of sclerosed rabbit veins (agent not noted) by Merlen.29 He demonstrated intimal cracks and fissures that left intimal connective tissue fibers and elastic lamina exposed. Ultrastructural damage involving stasis of blood and platelet aggregation on intact endothelial intima occurred in 5 minutes in the dorsal rabbit ear vein.
Factors Predisposing to Thrombosis
As discussed previously, optimal clinical results occur when sclerotherapy-induced thrombosis is minimized. Factors predisposing to thrombus formation include decreased velocity of blood flow, hypercoagulability, and endothelial cell damage.30 The velocity of blood flow is unaffected by the sclerosing agent itself, but flow in general is usually slower in varicose veins and telangiectasia. This relative decrease in blood flow may predispose to thrombus formation in varicose veins and may be a significant contributing factor to the increased incidence of thrombophlebitis and deep vein thrombosis (DVT) in patients with varicose veins (see Chapter 2).
Hypercoagulability predisposes the patient to thrombus formation. Wuppermann31 studied fibrinolysis in subjects injected with POL and found a slight, statistically insignificant hyperfibrinolysis in blood drawn from the antecubital vein. He hypothesized that because coagulation factors II, VII, VIII, and IX and platelet function are damaged directly by the sclerosing solution, coagulation at the injection site is delayed. Endosclerosis, as measured by fibrinogen, gradually occurred over 5 days only at the injection site and did not result in systemic hypercoagulability. Therefore, sclerotherapy should not cause a sudden thrombosis. In fact, a hemolytic effect was detected with STS even at a 0.1% concentration, with POL at a 0.05% dilution, and with PII at a 2% concentration.23 The PTT, PT, and thrombin time (TT) were unchanged with injection of these agents into whole blood. Earlier work also demonstrated the lack of effect of POL on coagulation parameters in rabbits.32 MacGowen et al33 combined STS with whole normal blood, causing a homogeneous RBC lysate without the formation of thrombin. In vivo studies have also demonstrated a lack of hypercoagulability from sclerosing solutions. Cepelak24 found that platelet aggregation occurs only at the site of the sclerosing solution injection, with aggregation-inhibiting effects occurring in the efferent deep veins distal to the femoral vein. Thus, endothelial damage probably causes a release of various factors that produce anticoagulant effects, lowering the risk of thrombotic complications of sclerosing therapy. This effect was confirmed by Raymond-Martimbeau and Leclerc,34 who measured fibrinopeptide A and fibrin degradation of D-dimer fragments after injection of sodium iodine. They found no evidence for activation of blood coagulation. Therefore, experimental findings do not support the theory of intrinsic hypercoagulability of sclerosing solutions as the mechanism of action for thrombus formation during sclerotherapy (see Chapter 1).
The lack of hypercoagulability just mentioned correlates with clinical experience using POL. When POL is used in patients who are taking systemic anticoagulants, a decrease in its sclerosing action does not occur.35 The addition of heparin to STS also had no effect on the sclerotherapy results in a paired comparison of 100 patients.36 Thus it appears that the mechanism of action of POL and STS is to produce endothelial damage,37 but not thrombus formation associated with platelet aggregation. This mode of action is also seen with other (nondetergent) sclerosing agents. The low incidence of DVT after sclerotherapy (less than 1 per 10,000 sessions38) is the ultimate evidence that thrombosis is an epiphenomenon of the sclerosing process, not a goal.
Factors Predisposing to Endofibrosis
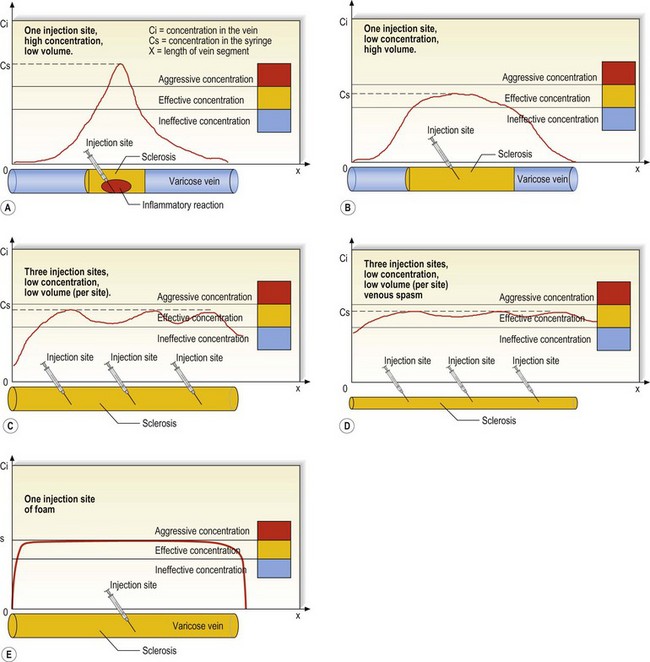
Whether vessels altered by changes of being varicose or stretched are more susceptible to the action of sclerosing agents than are normal vessels is unknown. At times, human varicose and telangiectatic vessels are noted to sclerose focally after the injection of various solutions. The focal nature of endothelial necrosis and thrombus formation may be related to toxic effects of the sclerosing solutions on the surrounding media. This effect is commonly observed when injecting varicose veins under duplex control. With this technique (described in Chapter 9), the sclerosing solution is injected and/or held in place until the varicosity is seen to spasm. This indicates effective sclerosis. Venograms of varicose veins injected with STS demonstrate segmental, intense, and diffuse spasm, both proximally and distally, at the time of injection and 6 minutes after injection.39 Effective endosclerosis occurs at points of vessel spasm where the entire endothelium is adherent. This agrees with the clinical impression that total compression of the sclerosed vessel is necessary for ideal, long-lasting, and complication-free sclerosis.40,41
At one time it was thought that ‘any solution which will not produce a slough when injected perivenously will generally not be strong enough to obliterate a vein.’42 However, some very effective sclerosing solutions are thought to act selectively on ‘damaged’ varicose endothelium. In fact, experimental studies have documented that effective sclerosing solutions do not have to produce tissue necrosis on intradermal injection (see Chapter 8). The manufacturers of POL state that this agent acts selectively on damaged vessels.a In addition, a number of histologic studies of the effect of sclerotherapy on varicose veins have also concluded that damaged varices are preferentially sclerosed.b,8,20,29 However, experimental injection of sclerosing agents into normal dorsal veins of rabbit ears yields effective vessel sclerosis in a concentration-dependent manner.43,44 Therefore, in addition to the type and concentration of sclerosing solution, other factors, including vessel diameter, rate of blood flow, and anatomic site of the vessel, may also be important.
Sclerosing solutions affect arteries in a different manner than they do veins. Although thrombosis occurs, intimal damage may not. MacGowen et al33 studied the local effects of intra-arterial injection of STS. They injected STS 3.0% into the central auricular artery at the base of the rabbit ear and visualized the resulting chain of events through a Perspex ear chamber (Lucite International, Southampton, UK) with high-power and oil-immersion lenses. Spasm was not noted in any vessels, but within minutes the erythrocytes appeared distorted and broken, with the formation of a central homogeneous thrombus that moved down the arteriole and lodged in a capillary. Intimal damage did not occur. Thus, the major effect of STS was on the blood cell mass that it destroyed and converted into an intravascular embolus. Subsequent biopsies of the ears at 1 hour and at 5 days demonstrated thrombus only, without evidence of intimal damage. These results are distinctly contrary to the effects of sclerosis on veins.
The reason for the different mechanism of action is unknown but may relate to the difference in velocity of blood flow in arteries and veins. Specifically, STS injected intra-arterially may not have enough time to react with endothelium, being both absorbed and inactivated by formed elements in the blood and serum factors and thereby being diluted to a ‘safe’ concentration by the more rapid arterial flow. Safe is a relative term, since inadvertent intra-arterial injections of STS have produced gangrene through thrombosis of vessels downstream of injection (see Chapter 8).
Experimental Evaluation of Sclerosing Solutions
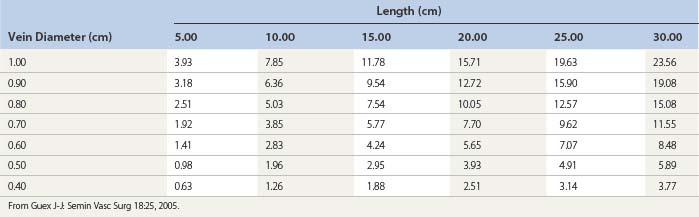
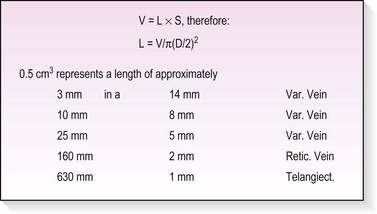
An important question regarding the studies in this section is whether the experimental animal model is an appropriate system in which to compare the efficacy of various sclerosing solutions. The dorsal marginal rabbit ear vein is similar in size (0.35 to 0.45 mm in diameter) to telangiectasias in humans. Reiner45 found that it was difficult to measure the rate of dilution of sclerosing solutions in the rabbit ear vein because of the greater number of collaterals and rapid blood flow caused by the thermoregulatory nature of the ear. Therefore, after injection of the solution, 20 seconds of occlusion on the proximal and distal aspects of the injected vein wall caused by firm pressure helped simulate the more sluggish blood flow of human telangiectasias.43,44 In vitro studies of the effect of sclerosing solution on saphenous veins harvested for coronary bypass surgery have confirmed the histologic effect of sclerosing solution type and concentration with the rabbit ear vein model.46 However, study of an animal model may not produce accurate data because the researcher is comparing the action of a sclerosing solution on a normal vessel. In addition, the injected vessels are not compressed in rabbit ear vein studies, thereby resulting in the formation of a larger thrombus, which may allow for a more rapid or increased incidence of recanalization. Finally, thrombogenesis and thrombolytic effects are different in the rabbit ear than in the human artery and vein.47 However, despite all these shortcomings, as a model the rabbit ear vein does allow the physician to compare the mechanism of action of various sclerosing solutions both clinically and histologically. On the basis of these studies, the physician can achieve a similar therapeutic effect in humans by varying the concentration and type of solution (Table 7.2).
| Vein Diameter | Sclerosing Solution |
|---|---|
| 0.4–1 mm | Glycerin 70% |
| Chromated glycerin 50% | |
| Polidocanol 0.25%–0.5% | |
| Sodium tetradecyl sulfate 0.1% | |
| Hypertonic saline 11.7% | |
| Sclerodex | |
| Ethanolamine oleate 2% | |
| Polyiodinated iodine 0.1% | |
| Sodium morrhuate 0.25%–0.5% | |
| 1–3 mm | Sodium tetradecyl sulfate 0.25% foam |
| Polidocanol 0.5% foam | |
| Polyiodinated iodine 0.5%–1.0% | |
| Ethanolamine oleate 5% | |
| Hypertonic saline 23.4% | |
| Sodium morrhuate 1.0%–2.5% | |
| 3–5 mm | Polidocanol 1% foam |
| Sodium tetradecyl sulfate 0.5%–1.0% foam | |
| Sodium morrhuate 5% | |
| Polyiodinated iodine 2% | |
| >5 mm | Polidocanol 3%–4% foam |
| Perforators | Sodium tetradecyl sulfate 2%–3% foam |
| Saphenofemoral/popliteal junctions | Polyiodinated iodine 3%–12% |
In the 1920s, the first studies to elucidate the mechanism of action of sclerosing agents were performed using the dorsal vein of the rabbit ear. Sclerosing agents tested included 1% bichloride of mercury,48 30% sodium chloride and 50% grape sugar,49,50 30% sodium salicylate,51 50% to 60% calorose,52,53 and SM.54,55 All of these solutions achieved venous obliteration through endothelial cell alteration, with inflammation resulting in thrombus formation and the eventual production of a fibrous cord.
An evaluation of foamed sclerosing solutions has also been performed in vivo on isolated saphenous veins before stripping.56 Pathologic damage from 3% STS foam prepared with 1 mL of STS and 4 mL of air was extremely rapid, with complete damage to the endothelium within 2 minutes. Edema of the intima with progressive separation from the tunica media and formation of thrombus occurred at 15 and 30 minutes.
Sodium tetradecyl sulfate
The mechanism of sclerosis for intravascular STS was elucidated by Schneider8 and by Schneider and Fischer55 in human varicose veins, by Dietrich and Sinapius57 in rabbit external jugular veins, and by Imhoff and Stemmer20 in the dorsal rabbit ear vein. With STS, endothelial damage is dependent on concentration and occurs immediately after injection, with resulting rapid thrombus formation leading to vascular sclerosis.
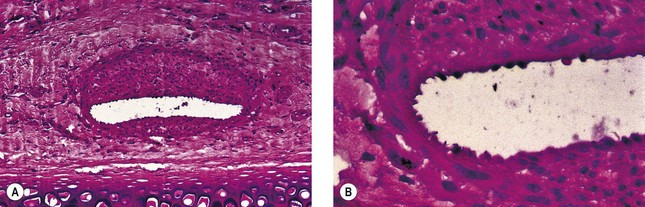
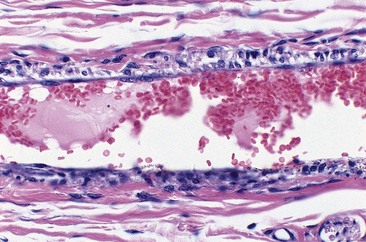
In two studies, sclerosis with STS produced similar results in a concentration-dependent manner.43,44 Endothelial damage occurred within 1 hour (Fig. 7.9), followed by the rapid onset of vascular thrombosis with subsequent organization (Fig. 7.10). Histologic recanalization occurred after 30 days with solution concentrations of 0.1% to 0.5%. The histologic findings explained the clinical appearance, which demonstrated initial thrombosis, followed by partial reappearance of the vessel injected with STS 0.1%. Therefore, there may be a concentration gradient in which an ideal concentration depends on many factors, including vessel diameter, rate of blood flow, animal model, and anatomic region within each animal model.
Sodium morrhuate
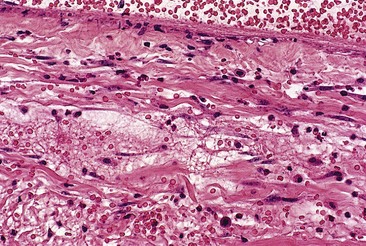
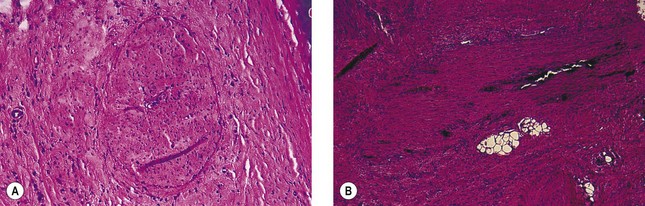
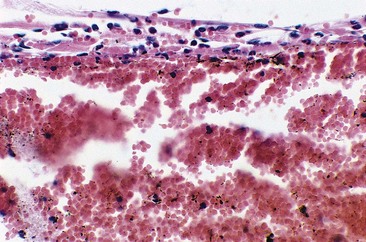
Sodium morrhuate, a mixture of sodium salts of the saturated and unsaturated fatty acids present in cod-liver oil, has been studied in the rabbit ear vein model.58 A 0.5% concentration of SM produced no clinical evidence of endothelial damage. Temporary histologic evidence of thrombosis was noted at 1 hour only, with a mild perivascular mixed cellular infiltrate (MCI). There was no evidence for extravasation of RBCs. Vessels injected with SM 1.0% were thrombosed between 2 and 10 days, after which the vessels normalized. Histologically, the SM 1.0%-injected vessel demonstrated a partially destroyed endothelium with extravasation of RBCs. The vessels injected with SM 2.5% demonstrated clinical fibrosis with histologic evidence of microangiopathic recanalization through a fibrotic cord at 45 days after injection. A unique finding noted with injection of SM, both 1.0% and 2.5%, was the presence of large numbers of perivascular mast cells (Fig. 7.11). This finding may correlate with the increased inflammatory nature and allergenicity of SM as compared with other sclerosing solutions.
Ethanolamine oleate
A synthetic mixture of ethanolamine and oleic acid, EO is another sclerosing solution that has been studied in the rabbit ear vein model.58 No histologic or clinical changes were noted with injection of EO 0.5%. Although an organizing thrombus was produced, complete recanalization occurred in the vessel injected with EO 1%, causing the returned clinical appearance of the injected vessel. Vessels injected with EO 2.5% had a partially destroyed endothelium followed by luminal recanalization. Evidence of phagocytosis of lipid-like material was noted in a vessel injected with EO 2.5% at 48 hours (Fig. 7.12). This may indicate extravasation of sclerosing solution either during injection or with endothelial destruction. Large numbers of perivascular mast cells were also noted 2 days after injection with EO 1% and 2.5%. Extravasated RBCs occurred in vessels injected with EO 1% and 2.5% at 1 hour and 2 days, but not in vessels injected with EO 0.5%.
A previous study comparing EO with STS was performed using the rat tail vein model.59 In this model, EO 5% was compared with STS 3% and 1%. Solution measuring 0.1 ml was injected and the veins were biopsied at 4 weeks. In this study, EO 5% was effective in sclerosing only 25% of the treated veins, as opposed to a 73% efficacy with STS 1% and a near 100% efficacy with STS 3%. Therefore, results in the rabbit ear vein compare well with those in the rat tail vein.
Polidocanol
A concentration gradient was also demonstrated in a study of POL in concentrations of 0.25%, 0.5%, and 1.0%.43 Only vessels injected with POL 0.5% and 1% were clinically sclerosed, and only the vessels injected with POL 1% maintained sclerosis without revascularization by 60 days.
An examination of the histologic effects of POL on the endothelium specifically, between 1 hour and 4 days after injection, illustrates the effect of varying the concentration of sclerosing solutions. Endothelial cells exposed to POL 0.25% were at first only partially damaged (Fig. 7.13). Mitotic figures indicating endothelial regeneration were noted 4 days after injection (Fig. 7.14). Likewise, with POL 0.5%, partial luminal recanalization occurred through an initial fibrotic cord in vessels (Fig. 7.15). Only vessels sclerosed with POL 1.0% developed complete endosclerosis (Fig. 7.16). Therefore, POL is probably a weaker detergent type of sclerosing solution than STS, and higher concentrations are necessary to produce complete vascular sclerosis.
Polidocanol: liquid versus foam
Hamel-Desnos et al and Wollmann have demonstrated both clinical60,61 and microscopic62 superior efficacy of foam versus liquid. In vitro studies have been conducted on single layers of endothelial cells in contact for 1.5 seconds with various concentrations of foam and liquid POL. Histologic examination demonstrated identical cell destruction with 0.5% foam and 3% liquid. As indicated by Hamel-Desnos et al, better efficacy had been supposed to be related to a longer time of contact; they have observed that the effect happened in a very short time, therefore there should be missing explanatory links. From a macroscopic point of view, foam seems to work because of the delayed dilution and closer contact between nondiluted sclerosing agent and endothelium; some microscopic phenomena will perhaps be found explaining more precisely what happens. (Foam sclerosants are described in detail at the end of this chapter and in Chapter 9.)
Hypertonic saline
The sclerosing effect of HS was examined histologically in the external jugular vein of the dog by Kern and Angle,63 and in human varicose veins by McPheeters and Anderson.53 These investigators noted endothelial damage with thrombus formation within 1 hour of injection, with ultimate conversion into a fibrous cord within 2 to 4 weeks. This was confirmed in the rabbit ear vein model43 both clinically and histologically. Examination of the marginal ear vein 1 hour after exposure to HS 23.4% demonstrated complete endothelial destruction (Fig. 7.17).
However, subsequent evaluation of HS 11.7% in the rabbit ear vein model58 demonstrated an immediate thrombosis that lasted only 48 hours before complete normalization. Endothelial destruction was patchy at 1 hour with perivascular and intraluminal margination of polymorphonuclear cells and eosinophils. There was no evidence of extravasation of RBCs in the veins injected with HS 11.7%, whereas extravasation was noted in 30% of vessels injected with HS 23.4%. Therefore, the degree of endothelial damage and resulting extravasation of RBCs is proportional to the concentration of HS used.
Hypertonic glucose/saline
Sclerodex (SX; Omega Laboratories, Montreal) is a mixture of dextrose, sodium chloride, propylene glycol, and phenethyl alcohol. When SX was studied in the rabbit ear vein model,58 it produced an immediate thrombosis that lasted for 2 days, after which the vessel recanalized. At 1 hour, perivascular and intraluminal margination of polymorphonuclear cells and eosinophils were present with patchy endothelial destruction. Endothelial mitoses were present at 2 days within a regenerative endothelium. Extravasation of RBCs was not noted. Therefore, SX has a potency similar to HS 11.7%.
Chromated glycerin and 72% glycerin
The effect of chemical irritant sclerosing solutions has been studied in the rabbit ear model.44 Chromated glycerin (CG; Sclérémo, Laboratoires Bailleul, Paris, France), 50% and 100%, was injected into the dorsal marginal rabbit ear vein, producing clinical and histologic thrombosis that lasted only 2 to 8 days, after which the vessel appeared clinically and histologically normal. As noted with POL 0.25% above, the endothelium 1 hour after biopsy was almost undamaged. Therefore, in this experimental model, CG is a weak solution with a sclerosing effect similar to POL 0.25%. This correlates well with its clinical profile. The sclerosing effect of CG is not dependent upon chromium. Excellent results can be achieved in treating leg veins less than 1 mm in diameter with the use of a 72% glycerin solution mixed 2 : 1 with 1% lidocaine with epinephrine, as described later in this chapter.
Polyiodinated iodine
Various iodine solutions, with or without hypertonic solutions, have been examined in the rabbit ear vein model.64 Sclerodine (Omega Laboratories, Montreal) is a mixture of iodine USP (60 mg/mL) and sodium iodide USP (90 mg/mL). This solution was compared with an American iodine formula consisting of iodine and sodium iodide, compounded by the Women’s Hospital of Texas in Houston. The two sclerosants were identical in composition. After being mixed with either normal saline, SX (Omega Laboratories), or a solution of dextrose 250 mg/mL and sodium chloride 100 mg/mL (compounded by the Women’s Hospital of Texas in Houston), each sclerosant was injected in concentrations of 0.1% and 0.5%. Thrombosis occurred with all solutions at 1 hour, with an attenuated endothelium and focal endothelial necrosis. A mild perivascular mixed cellular infiltrate consisting of eosinophils and polymorphonucleocytes was present with margination along the endothelial border. With the 0.1% solutions, the endothelium was hyperplastic, with regenerative changes noted by 8 days (Fig. 7.18) and complete normalization by 28 days. With the 0.5% solutions, the endothelium was necrotic with multiple brown spherules approximately 1 µm in diameter seen within the endothelial wall (Fig. 7.19). These may represent iodine crystals. By 28 days, a fibrous cord was present without inflammation. Interestingly, veins treated with iodine/normal saline mix showed a greater incidence and extent of extravasated erythrocytes compared to those treated with iodine/SX (hypertonic saline/dextrose) solutions. The PII 0.1% solution had an experimental efficacy equivalent to HS 11.7%, SM 2.5%, STS 0.25%, and POL 0.5%. The PII 0.5% solution had an experimental efficacy similar to HS 23.4%, STS 0.5%, and POL 1.0%.
Comparative efficacy in the animal model
The mechanism of action for all sclerosing solutions injected into veins in the aforementioned studies was basically similar; that is, endothelial damage and simultaneous thrombus formation occurred almost immediately after injection. Endothelial damage was less in the vessels injected with CG, POL 0.25%, SX, and EO 0.5%, which showed early recanalization and a continued normal clinical appearance. POL 0.5%, SM 0.5% and 1%, EO 1%, and HS 11.7% produced endothelial attenuation, not necrosis. Although an organizing thrombus was produced, recanalization occurred, causing the returned clinical appearance of the injected vessel. Vessels injected with PII 0.1%, STS 0.5%, SM 2.5%, and EO 2.5% also demonstrated recanalization, although endothelial necrosis was demonstrated. In contrast to the luminal recanalization that occurred with POL 0.5% and EO 2.5%, recanalization with STS 0.5% and SM 2.5% occurred with multiple minute vascular channels. Vessels sclerosed with STS 0.25% and 0.5% and with SM 2.5% never totally reappeared clinically in the 60-day span of this study. The only vessels to histologically demonstrate fibrous cord formation that did not recanalize were sclerosed with PII 0.5%, HS 23.4%, and POL 1.0%. Therefore there is a minimal sclerosant concentration (MSC) that is essential to produce endosclerosis. This term, coined by Neil Sadick,65 is useful in determining which solution and concentration is best at sclerosing a specific vessel.
Comparative efficacy in the human model
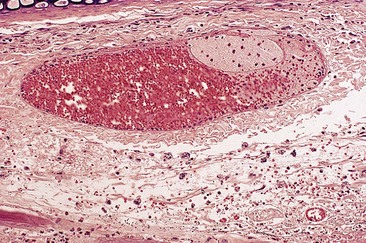
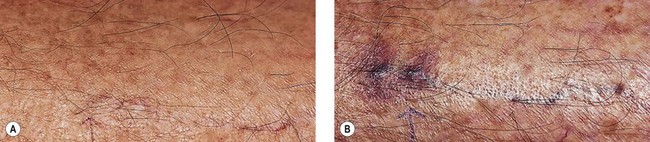
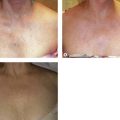
In an effort to assess the effect of sclerosing agents in human leg telangiectasias, the author injected 0.1 mL of either POL 0.5% or STS 0.5% into two nearly identical telangiectasias (0.4 mm in diameter) over the anterior tibia in a 65-year-old man. The vessels did not have any associated ‘feeding’ reticular veins, and there was no evidence of associated varicose veins or signs of venous insufficiency. Neither injected vessel was compressed, and a biopsy of each was taken 48 hours after treatment. The vessel injected with POL 0.5% demonstrated a blue thrombus (Fig. 7.20) that was histologically confirmed, and an endothelium that was relatively intact with extensive cellular vacuolization (Fig. 7.21). The vessel injected with STS 0.5% demonstrated a deep blue thrombus (Fig. 7.22). Histologically, the endothelium was totally destroyed, showing extensive intravascular thrombosis and early organization (Fig. 7.23). Therefore, this limited human study correlates with the aforementioned studies on the marginal rabbit ear vein, demonstrating that STS is a stronger sclerosing agent than POL.
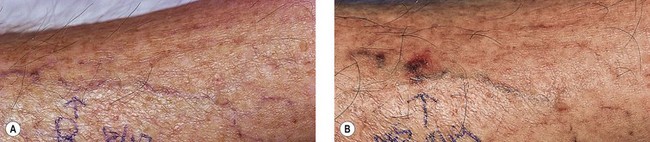
Figure 7.20 Anterior tibial telangiectasia. A, Before treatment and, B, 48 hours after injection of polidocanol 0.5%.
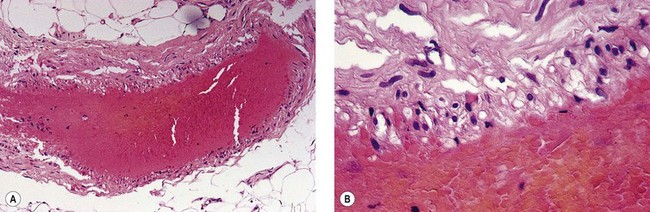
Figure 7.21 Histologic examination of vein in Figure 7.11. A, ×40. B, ×100; shows endothelial cell vacuolization with thrombosis (hematoxylin–eosin).
Clinical Use of Sclerosing Agents
The only sclerosing agents approved for use in the United States by the Food and Drug Administration (FDA) are SM, EO, and STS. All of these agents were approved for use before 1950 and thus have never been subjected to the rigorous toxicity and efficacy studies that would be required by the FDA today. Hypertonic saline in a 23.4% concentration is available and approved for use as an abortifacient. However, it is commonly used in various concentrations, with and without the addition of heparin, procaine, or lidocaine, for sclerosis of telangiectasias and superficial varicosities. Polidocanol, widely used in the United States today, has undergone investigative trials and was approved for use in June 2010. SX, a solution of dextrose 5% and sodium chloride 10%, is commonly used in Canada for sclerosis of superficial varicosities and telangiectasias. Chromated glycerin, and now glycerin, is perhaps the most widely used sclerosing agent worldwide for the treatment of leg telangiectasias. Ouvry66 and Goldman67 have popularized it in the English literature. PII is the most powerful sclerosing agent and is commonly used outside of the United States for sclerotherapy of the saphenofemoral junction (SFJ). It is not yet approved for use by the FDA in the United States but is discussed because of its importance in sclerotherapy. Another solution rarely used now, sodium salicylate, is briefly discussed.
Osmotic agents
Hypertonic saline
Hypertonic saline was first used to sclerose varicose veins by Linser20 in 1926 and Kern and Angle63 in 1929. With the advent of more effective, synthetic, detergent sclerosing solutions in the 1940s, its use declined. Renewed interest in its use occurred in the 1970s, spurred on by numerous publications in the dermatology literature and multiple lectures on its use presented at major medical meetings.
A double-blind, paired-comparison study of various concentrations of HS, with and without heparin, was performed in different-sized varicose and telangiectatic leg veins.65 It was found that an 11.7% HS solution was as effective as a 23.4% solution in treating vessels less than 8 mm in diameter, and more effective than a 5.8% solution, with less burning, pigmentation, and cramping. The addition of heparin only decreased thrombosis formation requiring puncture evacuation in vessels greater than 4 mm in diameter. This demonstration of MSC produced superior cosmetic results with an improved therapeutic-to-complication index.
Advantages
Part of the currently experienced popularity stems from the lack of allergenicity of unadulterated HS solution compared with the exaggerated claims of allergenicity associated with all other sclerosing agents. However, HS is not without significant adverse sequelae (see Chapter 8).
Disadvantages
Unlike detergent sclerosing solutions, all hypertonic solutions act nonspecifically to destroy all cells (including RBCs) within their osmotic gradients. Osmotic agents damage cellular tissues and readily produce ulceration if injected extravascularly or if diffused through the vessel extravascularly (see Chapter 8). Therefore, injection technique is critically important with use of this type of sclerosing agent.
Because HS diffuses to some extent through the blood vessel wall, nerves in the adventitia of the vein may be stimulated, causing pain (see Chapter 3). This diffusion may also lead to transient muscle cramping. Hemolysis of RBCs occurs through hyperosmosis, causing the release of hemosiderin, which may readily diffuse across the damaged endothelium. This may lead to post-treatment hyperpigmentation, especially in a punctate pattern. Finally, because osmotic agents are rapidly diluted in the bloodstream, they lose their potency within a short distance of injection. Thus, these agents are only rarely effective in treating veins larger than 3 to 4 mm in diameter.
Modification of the Solution and the Technique
Various modifications of the HS solutions have been made in an effort to increase the efficacy and decrease the pain of injection and other adverse sequelae. In 1975, Foley68 described the microinjection of ‘venous blemishes’ with a 30-gauge needle using 20% hypertonic saline, 100U/mL of heparin, and 1% procaine, which he patented as Heparsal. He reported no allergic or anaphylactic reactions and only rare pigmentary problems in more than 1000 treatments to more than 100 patients. Foley theorized that the addition of heparin helped to prevent thrombi in larger vessels, and the addition of procaine helped alleviate the pain on injection. Sadick,65,69 in randomized, double-blind, 800-patient and 600-patient, paired-comparison studies, found that the addition of heparin to HS provided no benefit in the treatment of varicose and telangiectatic veins less than 4 mm in diameter. Bodian,70,71 because of his personal clinical comparison experience, also did not believe that the addition of heparin was necessary for effective sclerosis. Finally, it has been demonstrated that the addition of heparin to the culture medium enhances proliferation and increases the lifespan of endothelial cells.72 Therefore its use may be counterproductive.
A number of modifications in injection technique have also been made to limit the pain of HS. Bodian71 found that muscle cramps occurring at the site of injection last 3 to 5 minutes and are relieved with gentle massage or ambulation. To limit the risk of extravasation, he recommended injecting a small air bolus before injecting 0.5 to 1 mL of HS; this ensures undiluted contact of the HS with the intima to produce maximum irritation of the vessel. He believed that hemolysis caused by the sclerosing solution may lead to or exacerbate hemosiderin staining and thus should be lessened by the prior injection of air, which washes out the RBCs from the vessel.73 Finally, regarding the possible exacerbation of hypertension with injection of a large sodium bolus, he stated that 1 g of sodium chloride injected during a ‘long treatment session’ (5 mL of a 20% HS solution) is well tolerated.
Alderman74 was the first to advocate dilution of the HS solution to better adjust the osmotic damage to the caliber of the vessel. From his experience with 150 patients with telangiectasias treated over 8 years with 18% to 30% HS, he recommended the following HS concentrations for sclerosis of varicose and telangiectatic veins: 18% to 25% HS for ‘venous telangiectasias’ (blue telangiectasias), 22% to 25% HS for ‘arterial lesions’ (red telangiectasias), and 30% HS for rare, large ‘arterial’ lesions. He diluted the saline with lidocaine to achieve a 0.4% concentration of lidocaine. The only adverse side effects reported were mild, temporary burning at injection and residual brownish pigmentation that occurred in up to one-third of patients. The pigmentation usually resolved, for the most part, over 1 year.
Lidocaine, when used as a diluent, can be used with or without epinephrine. Animal studies have demonstrated that solutions with less than a 1% concentration of lidocaine are vasoconstrictive.75,76 In addition, the author routinely uses it with epinephrine as a diluent to enhance vasospasm and partially stabilize perivascular mast cells.
Hypertonic glucose–saline
Sclerodex is a mixture of dextrose 250 mg/mL, sodium chloride 100 mg/mL, propylene glycol 100 mg/mL, and phenethyl alcohol 8 mg/mL (as a local anesthetic/preservative) at a pH of 5.9, mainly used in Canada for sclerosis of telangiectasias and small-diameter superficial varicosities.77 It is essentially a hypertonic solution with a mechanism of action similar to HS. The manufacturer states that the sodium chloride reinforces the sclerosing potency of dextrose.
The manufacturer of SX recommends that the maximum quantity to be injected during one visit is 10 mL in divided doses, with a 5-cm interval between each site of injection.a (The maximum recommended amount to be injected at any one site is 1 mL.) The average dose per treated vein varies between 1 mL in the upper thigh and 0.1 mL in the lower leg. The reason for these recommended doses by the manufacturer is unclear.
Advantages
Omega Laboratories, which produces SX, claims that the addition of dextrose allows for a reduction in the concentration of sodium chloride, thereby minimizing the pain and local discomfort that would occur with injection of sodium chloride alone.a However, it is probably the decrease in osmolarity relative to 23.4% HS that allows SX to produce less pain and muscle cramping.
Disadvantages
Despite the lower osmolarity of SX, like HS, it is slightly painful for the patient on injection.78 Superficial necrosis may occur rarely, with an incidence of less than that with HS.a,77,79 The author has noted postsclerotic pigmentation occurs with a frequency similar to that of other sclerosing agents, although Mantse79 noted a decreased incidence of complications with SX as compared with POL and STS (see Chapter 8).
Another disadvantage of SX use is that the solution becomes sticky in the syringe when blood is withdrawn to ensure an intravenous position. Unfortunately, unlike unadulterated HS, allergic reactions may occur to the phenethyl alcohol component of the solution.a Mantse77 noted one allergic reaction in 500 patients treated with SX, giving an incidence of 0.2%.
Chemical irritants
Chromated glycerin/glycerin
Chromated glycerin 72% (Sclérémo, Laboratories Bailleul, Paris, France; Chromex, Omega Laboratories, Montreal, Canada; Skleremo, Elvetium-Alet Laboratorios, Buenos Aires, Argentina) is a sclerosing solution that is popular in Europe, whereas clinical experience in the United States remains limited. The maximum recommended amount per injection session is 10 mL of pure solution. Concentrations of 25% to 100% have been used.80 Its clinical efficacy has been shown to be dose dependent. Although not approved by the FDA, CG is a widely used sclerosing agent for leg telangiectasias in the world; 500,000 vials were sold in 1986.a More recent information is not available from the manufacturers.
The glycerin component of CG is rapidly absorbed by the intestine and transformed into carbon dioxide or glycogen or is directly used for the synthesis of fatty acids.81 Therefore, this solution must be used with caution in diabetic patients. One case of reactive hypoglycemia to an infusion of glycerol occurred in a child, resulting in a comatose state within 4 minutes of infusion.82
The sclerosing quality of glycerin was first studied in 1925 by Jausion et al,83 who found that it induced a mild, rapid, and complete endosclerosis. Isosmotic glycerol (2.6%m/v) produces 100% hemolysis in 45 minutes.81,84 This usually occurs with rapid infusions of 60 g in 15 minutes, 70 g in 30 minutes, and 80 g in 60 minutes.85 However, a review of 500 patients who received glycerol intravenously (at 50 g/500 mL) 6 hours a day for 7 to 10 days demonstrated hemoglobinuria in less than 1% of patients.86
The chromium alum component of CG is a potent coagulating factor that increases the sclerosing power of glycerin. It also prevents the mild hematuria induced through the use of glycerin alone.81,87,88
We compared the effects of STS 0.25% with those of glycerin 72% mixed 2 : 1 with lidocaine 1% with epinephrine in 13 patients, to determine the relative safety and efficacy of the two sclerosant solutions.67 Each patient’s leg veins from 0.2 to 0.4 mm in diameter that did not have incompetence from the SFJ, and whose feeding reticular veins had been already treated in a prior sclerotherapy session, were randomly treated with either STS 0.25% or glycerin 72% solution. Patients were evaluated from 2 to 6 months postsclerotherapy for overall clinical improvement and incidence of adverse sequelae. We found that glycerin was comparable to STS in the discomfort of injection, but demonstrated a significant decrease in bruising, swelling, and postprocedural hyperpigmentation. Glycerin also demonstrated a better, more rapid clearance of treated telangiectasias. Thus, the chromate salt addition to glycerin was not necessary for effective sclerotherapy.
Advantages
The relatively weak sclerosing power of CG corresponds to its promotion as a mild sclerosing solution with more versatile use and a low incidence of side effects. Pigmentation and cutaneous necrosis are exceedingly rare at recommended dosages, and minimal extravascular injection causes only a small temporary ecchymosis without any cutaneous damage.89,90 Reportedly, the incidence of adverse sequelae is very low.a,41,80
Disadvantages
The disadvantages of CG are its high viscosity and local pain at injection.91,92 Both of these drawbacks can be overcome partially by dilution with lidocaine. Hypersensitivity is a rare complication.93,94 Hematuria associated with ureteral colic can occur transiently after injection of large doses. Ocular manifestations, including blurred vision and a partial visual field loss, have been reported by a single author, with resolution in less than 2 hours.95 These latter two complications may be a result of excessive, nonspecific destruction of RBCs.
A case of fatal anaphylaxis has recently been reported with chromated glycerin.96 The case is well documented and little doubt is left regarding responsibility of the sclerosing agent. This case is the only one published and no known cases of anaphylaxis with glycerin alone exist.
Ethanol
Ethanol is a sclerosing agent most commonly used for treating arteriovenous malformations. It kills cells by fixation, preserving cell morphology, and is thus listed as a ‘chemical’ sclerosing agent. The precipitant thrombus-forming effect makes it useful for high-flow lesions. Using an in vitro model, Mol et al found that almost all cells die within 5 seconds when exposed to a 30% concentration.97 A 3% concentration, to which cells were exposed for 12 hours, did not cause any damage. As a comparison, all cells exposed to 0.025% POL for 5 seconds also died. As discussed previously, POL kills cells by disrupting the cell membrane through protein-theft denaturization.
Detergent sclerosing solutions
Sodium morrhuate
Sodium morrhuate (Palisades Pharmaceuticals, Tenafly, NJ; American Regent Laboratories, Shirley, NY) is a mixture of sodium salts of the saturated and unsaturated fatty acids present in cod-liver oil (Table 7.3). It is prepared by the saponification of selected cod-liver oils. Each milliliter contains morrhuate sodium, 50mg; benzyl alcohol, 2% (as a local anesthetic); water for injection (as much as will suffice); and hydrochloric acid and/or sodium hydroxide to adjust the pH to approximately 9.5. It is available as a 5% concentration that can be diluted with normal saline (to the appropriate concentration) for the vessel to be treated.
| Component | Percentage |
|---|---|
| Linoleic acid | 28.2 |
| Unknown | 20.8 |
| Eicosadienoic acid | 15.5 |
| Palmitoleic acid | 12.1 |
| Arachidonic acid | 8.2 |
| Palmitic acid | 8.1 |
| Myristic acid | 4.2 |
| Oleic acid | 1.8 |
| Stearic acid | 1.1 |
From Monroe P et al: Gastroenterology 85:693, 1983.
This sclerosing agent was first prepared for injection by Ghosh98 or Cutting99 and was met with enthusiasm in the United States by Biegeleisen54 and others. However, extensive cutaneous necrosis occurs when SM is inadvertently injected perivascularly. Many cases of anaphylactic reactions within a few minutes after injection have been reported. More commonly, these reactions occur when therapy is reinstituted after a few weeks. Anaphylaxis has resulted in fatalities, albeit rarely (see Chapter 8). The FDA has approved the usage of SM for sclerosis of varicose veins. However, because of its extremely caustic nature, it is not recommended for use as a sclerosing agent for telangiectasias, although Gallagher89 advocates its use diluted to a 0.25% to 0.5% concentration.
Most patients have minimal discomfort after injection, with an occasional tenderness at the injected site for a few days.100 Gallagher’s 25-year experience with 20,000 patients treated with SM is notably free of significant adverse sequelae, with only one episode of ‘full anaphylactoid reaction’. He describes more than 20 patients who had immediate postinjection ‘early anaphylactoid reactions’ manifesting as chest pain, shortness of breath, tachycardia, and hypotension, who responded to intravenous dexamethasone and intramuscular Benadryl. Gallagher states that STS has a higher incidence of adverse reactions than SM, so he prefers the latter for telangiectasia.
Ethanolamine oleate
Marketed under the name of Ethamolin (QOL Medical, USA), ethanolamine oleate (EO) is a synthetic mixture of ethanolamine and oleic acid with an empiric formula of C20H41NO3. It is available as a 5% aqueous solution containing approximately 50 mg of EO per milliliter. Benzyl alcohol, 2% by volume, is used as a preservative. The pH ranges from 8.0 to 9.0. The minimum lethal intravenous dose in rabbits is 130 mg/kg.101
The oleic acid component is responsible for the inflammatory action. Oleic acid may also activate coagulation in vitro by release of tissue factors and Hageman factor XII. It is not known whether EO is secreted into breast milk, so its use during lactation cannot be recommended. A 5% concentration used in gross varicose veins (diameter not specified) completely destroyed the veins 8 weeks after injection.102
Advantages
Ethanolamine oleate was first reported to be an ideal sclerosing agent by Biegeleisen103 in the medical literature in 1937. No toxic effects were noted in 500 injections and it is thought to be less likely to cause allergic reactions than either SM or STS.104 However, pulmonary toxicity has been associated with this sclerosing agent (see Chapter 8).
The sclerosing action is thought to occur as a dose-dependent, extravascular inflammatory reaction caused by diffusion of EO through the venous wall.a Autopsy findings regarding its use in esophageal varix injection demonstrated that variceal obliteration occurred as a result of mural necrosis, followed by fibrosis, and that thrombosis was a transient phenomenon.105,106
Disadvantages
Ethanolamine oleate is a viscous solution that can be injected only through a 30-gauge needle with dilution. Some degree of nonspecific RBC hemolysis may occur with its use. A hemolytic reaction occurred in 5 of 900 patients with injection of over 12 mL of EO 0.5% per patient per treatment session.107 Acute renal failure with spontaneous recovery followed injection of 15 to 20 mL of Ethamolin in two women.b The patients were described as ‘feeling generally unwell and shivery, with aching in the loins and passage of red-brown urine. All rapidly recovered with bed-rest and were perfectly normal the next day.’ One hundred and four injections of less than 12 mL per treatment session did not result in this reaction.
In addition, even a 0.5% solution produces an unacceptable incidence of eschar, ulceration, or pigmentation when injecting telangiectasias of less than 1 mm in diameter.102
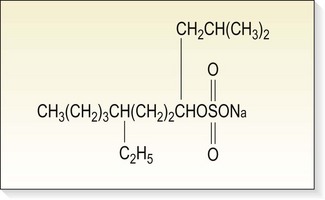
Sodium tetradecyl sulfate
Sodium tetradecyl sulfate (Sotradecol, Bioniche Pharma Group, Inverin, Co. Galway, Ireland; Thromboject, Omega Laboratories, Montreal, Canada; Trombovar, Laboratoires Innothéra, Arcueil, France) is a synthetic, surface-active substance first described by Reiner45 in 1946 (Fig. 7.24). It is composed of sodium 1-isobutyl-4-ethyloctyl sulfate plus benzoyl alcohol 2% (as an anesthetic agent) and phosphate buffered to a pH of 7.6. It is recommended that solutions be protected from light. It is a long-chain fatty acid salt of an alkali metal with the properties of soap. The solution is clear, non-viscous, has a low surface tension and is readily miscible with blood, leading to a uniform distribution after injection.108 It primarily acts on the endothelium of the vein, because, if diluted with blood, the molecules attach to the surface of RBCs, causing hemolysis. The recommended maximum dosage suggested by STD Pharmaceutical Products in a treatment session is 4 mL of a 3% solution or up to 10 mL of lower concentrations (e.g. 1%).a The recommended maximum dosage from Bioniche Pharma Group and Omega Laboratories is 10 mL of a 3% solution, with intervals between treatments of 5 to 7 days.b,c
The solution just mentioned should not be mixed with other anesthetic solutions because it will become turbid and form a new compound.109 In addition, heparin should not be included in the same syringe as STS because the two are incompatible (product insert, rev. October 1988). Yet, a paired comparison study of STS with and without heparin disclosed no difference in the therapeutic effect between the two solutions.36
In the United States, STS is available as a 1% or 3% solution that can be diluted with sterile water or normal saline to achieve an appropriate therapeutic concentration. It is also available – with the same pH and preservative – as Fibro-Vein (STD Pharmaceutical Products, Hereford, England), in 5-ml multiuse vials in concentrations of 0.2% and 3% and in 2-ml ampules in concentrations of 0.5%, 1%, and 3%. The Canadian manufacturer recommends dilution with phosphate-buffered saline to preserve the original pH level.c (This also prevents pain from injection.) It is limpid and does not stick to the syringe cylinder when blood is withdrawn to ensure accurate needle placement. Concentrations of 0.1% to 0.3% are commonly used for the treatment of telangiectatic veins 0.2 to 1.0 mm in diameter; 0.5% to 1% for treatment of uncomplicated varicose veins 2 to 4 mm in diameter; and 1.5% to 3% for the treatment of larger varicose veins, incompetent perforating veins, or an incompetent SFJ.
Advantages
Sodium tetradecyl sulfate became widely used in the 1950s after its introduction in 1946 by Reiner.45 Tretbar110 in 1978 first reported the injection of a 1% solution into spider angiomata. He noted excellent results in virtually all 144 patients treated. He also noted an unspecified number of episodes of epidermal necrosis without significant sequelae and a 30% incidence of postsclerosis pigmentation that resolved within a few months.
Shields and Jansen111 in 1982 were the first to describe microsclerosis of telangiectasias with STS in the dermatologic literature. They injected STS 1% into 105 patients and reported only one episode of necrosis in more than 600 treatments of vessels less than 5 mm in diameter. There were no systemic reactions, and the majority of postsclerosis pigmentary changes resolved in 3 to 4 months. However, as more experience with its use in the treatment of leg telangiectasias occurred, even further dilutions (0.1% to 0.3%) were recommended, both to achieve clinical efficacy and to limit adverse sequelae (see Chapter 12).
Disadvantages
Approved for use by the FDA for vein sclerosis, STS nevertheless has a number of disadvantages. Epidermal necrosis frequently occurs with extravasation of concentrations higher than 1%; however, telangiectasias and spider veins are usually treated with 0.1% to 0.2% and extravasation at this concentration rarely causes a problem. Allergic reactions occur rarely. Postsclerotherapy hyperpigmentation occurs in proportion to its concentration (see Chapter 8), therefore its dilution is critical. The previously mentioned percentages per diameter of treated vein provide only a preliminary guide for effective treatment. In addition, the Canadian and US manufacturers recommend that as a precaution against anaphylactic shock, 0.3 mL of a 1% solution should be injected into the varicosity, and then the patient should be observed for several hours before proceeding with further injections.a The reason for this recommendation is unclear, impractical, and potentially hazardous; it is discussed further in Chapter 8.
Historical Manufacturing of STS Injections
Carbitol has about the same toxicity as ethylene glycol when ingested, which has a mean lethal dose in humans of 3 to 4 oz (90–120 mL).112 The LD50 for intraperitoneal carbitol was 5.39 mL/kg in both rats and mice. The Ames test was very weakly mutagenic for Salmonella typhimurium and Saccharomyces cerevisiae. Carbitol is reported to be teratogenic in rats and mice.113 Cutaneous contact with carbitol also can produce a dermatitis with both immediate and delayed hypersensitivity.114,115
Many compounding pharmacies supply STS. Various loopholes in Federal and State regulations allow pharmacies to compound a variety of medications for use in humans. The FDA has no regulatory power over this ‘branch’ of the pharmaceutical industry. Although the compounding pharmacy industry has voluntary standards, no organization exists to test the quality and accuracy of medications provided by the compounding pharmacies. Our analysis found both a discrepancy between the stated concentration and the actual concentration of STS in bottles from all three compounding pharmacies. The concentration of the sclerosing solution should be matched to the size and type of vein treated to produce the minimal sclerosing effect.116
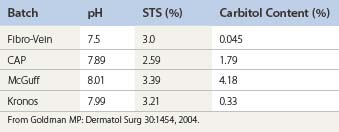
The reason for the difference in concentration may be related to the variability of the percentage of the bulk STS industrial solution. At temperatures below 15°C, the product fractionates so that the concentration of STS is greater than 27% at the bottom of the drum and below 27% at the top of the drum. An analysis of this 27% STS solution by the Professional Compounding Centers of America (PCCA; Houston, Tex) performed in July 2003 found that the 27% STS contained 27.94% of STS. No analysis of the carbitol component or any other component in the industrial solution was performed. The presence of impurities in any intravenous injection is worrisome (Table 7.4).
Compounding pharmacies manufacture STS from industrial source material. Carbitol is a known contaminant of industrial STS. If a company is going to use an industrial chemical to prepare a pharmaceutical injectable product, then it is duty bound to disclose other chemical compounds present too. However, by far the most important consideration in our opinion is that carbitol, like STS, is a high-molecular-weight organic molecule. The presence of both molecules in an injectable product will increase the risk of unwanted side effects relating to sensitivity and anaphylaxis. It is not a coincidence that Fibro-Vein has displayed a very low incidence of such side effects, but rather that it is due to the very low levels of carbitol that have been present in Fibro-Vein for the last 25 years. We find it very difficult to justify the deliberate administration by intravenous injection of two large-molecule organic compounds when physicians are being led to believe that they are only injecting the one compound, namely STS. In fact, Alemeida and Raines have compared the therapeutic effect of compounded STS with Sotradecol and found that compounded STS was less effective in sclerosing varicose veins.117
Current Manufacturing of STS Injections
The Bioniche active pharmaceutical ingredient (API) was tested for the presence of carbitol by gas chromatography analysis.a A carbitol reference standard was sourced from Sigma Aldrich Inc., St Louis, Mo. USA. The test results revealed that no carbitol was detected.
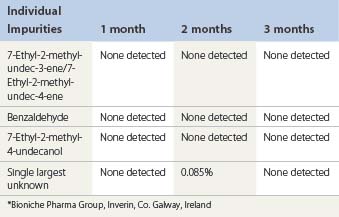
Accelerated stability studies of Sotradecol 3% and 1% manufactured by Bioniche were performed at 40°C and analyzed for impurities. The results for Sotradecol 3% are shown in Table 7.5.
| 7-Ethyl-2-methyl-undec-3-ene/7-Ethyl-2-methyl-undec-4-ene | NMT 0.15% |
| 7-Ethyl-2-methyl-4-undecanol | NMT 0.10% |
| Individual unknown | NMT 0.10% |
| Total impurities | NMT 1.0% |
| Assay | 96.0% to 100.0% |
Forced degradation of the API at 80°C for 2 hours increased the impurities:
| 7-Ethyl-2-methyl-4-undecanol | 5.0% |
| 7-Ethyl-2-methyl-undec-4-ene | 2.1% |
| 7-Ethyl-2-methyl-undec-3-ene | 0.4% |
If a temperature of 80°C was required to distill out carbitol (which is not the case with the Bioniche product), then there is an increased likelihood that the above-mentioned impurities would appear. A full discussion on the toxicity of carbitol is found in Chapter 9.
Polidocanol
Varying from one country to another, POL is available in 2-ml ampules in concentrations of 0.25%, 0.5%, 1%, 2%, 3%, and 4%, as well as multiuse 30-ml vials in 0.5% and 1% concentrations. In the US, it is available as 2 mL ampules of 0.5% and 1.0%. The other ingredients are disodium hydrogen orthophosphate dihydrate and potassium dihydrogen orthophosphate. Sclerovein (Globopharm, Switzerland) contains chlorobutanolum as a preservative, 0.5g/100 mL. Its pH varies from 4.8 to 6.1. The manufacturer has no stability data on POL when it is diluted with bacteriostatic water or normal saline, but Sadick and Farber118 have determined that it remains sterile for at least 3 months following dilution. The sterility is confirmed even when used daily through a multiuse vial. Manufactured by Craveri (Buenos Aires, Argentina), AET is diluted in absolute alcohol and is available in 2-ml ampules of 0.25%, 0.5%, 1%, 1.5%, 2%, 3%, and 4%, as well as in 2-ml syringes at concentrations of 0.25%, 0.5%, and 1%.
POL was synthesized by BASF and introduced in 1936 as a local and topical anesthetic under the tradename Sch 600. Unlike the two main groups of local anesthetics – esters (procaine, benzocaine, and tetracaine) and the amides (lidocaine, prilocaine, mepivacaine, procainamide, and dibucaine) – POL has a noncyclic chemical structure. The anesthetic effect is not a direct function of its concentration but is optimum at a concentration between 3% and 4%.a Animal trials on its use as a local anesthetic demonstrated the occurrence of obliteration of vessels as a side effect.
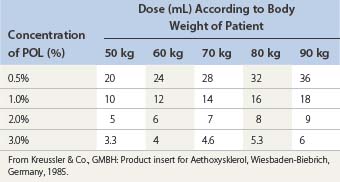
Henschel, the former Medical Director of Kreussler Pharma, first started clinical trials of several concentrations of POL to treat varicose veins (correspondence from B. Olesch, 1992).The maximum daily dose recommended by Kreussler Pharma is found in Table 7.6. Blenkinsopp119 recommended a higher maximum daily dosage of 10 mL of a POL 6% solution for an average person based on toxicity experiments extrapolated from rats. However, rats are much less sensitive to POL. Since the LD50 in rabbits is approximately 11.7 mg/kg, Blenkinsopp’s minimum dose may be toxic (Pfahler B, Kreussler: Personal communication, 29 March 1990).
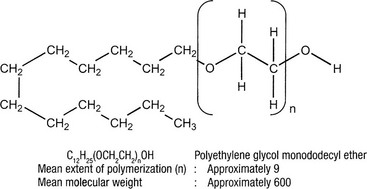
POL is unique among local anesthetics in its lack of an aromatic ring. As an aliphatic molecule, it is composed of a hydrophilic chain of polyethylene glycolic ether and a lipo-soluble radical of dodecylic alcohol. It is used as a topical anesthetic agent in ointments and lotions for mucous membranes, including hemorrhoidal treatment.120 It is also used as a local anesthetic for skin irritation, burns, and insect bites and as an epidural anesthetic.121–123 The subcutaneous anesthetic effect of a 0.4% solution is equal to a 2% solution of novocaine.121 The LD50 in rabbits at 2 hours is 0.2 g/kg, which is three to six times greater than the LD50 for novocaine.122 The LD50 in mice has been found to vary between 110 mg/kg (personal correspondence from Kreussler Pharma, 1992) and 1.2 g/kg.121 The systemic toxicity is similar to that of procaine and lidocaine.124 Thus, POL was considered an ideal local anesthetic. However, it soon became apparent that intravascular and intradermal instillation produced sclerosis of small-diameter blood vessels and ‘moderate, clinically unimportant reversible damage to healthy tissue’.a Therefore, compound Sch 600 was considered for use as a sclerosing agent. The polidocanol preparation Aethoxysklerol was registered at the German health authority, the Bundesgesundheitsamt (BGA), in 1967.123
Experimental evaluation of absorption, distribution, metabolism, and excretion of POL has been studied in dogs, rats, and humans.123 It is rapidly distributed throughout the body within minutes of injection.123 The compound is rapidly metabolized and eliminated, having a terminal elimination half-life of 1.4 to 1.7 hours in dogs. After 72 hours, 97% of the administered compound is excreted (61% in urine, 37% in feces).
In humans, the elimination half-life is 4 hours, with 89% of the dose eliminated from the blood within 12 hours. Amounts excreted in the urine and feces are equal, and almost 80% of the injected compound is excreted via respiration through a breakdown into low-molecular-weight products. Polidocanol is completely eliminated from body organs whether the patient receives one dose or repeated doses. Therefore, no accumulation takes place, nor does POL cross the blood–brain barrier.123 Sixty-four percent of POL is bound to protein, with a volume of distribution of 24.5 L/hour. The total clearance is 11.7 L/hour, with renal clearance of 2.01 L/hour and biliary clearance of 3.08 L/hour.b
The capacity of POL to cross the placental barrier was investigated in rats.123 Of radioactivity from labeled POL, 15% to 87% was recovered from fetal tissue after 13 days. The striking variations in an earlier differentiation phase may come from weight differences between fetuses. The fetus of day 19 accumulated less activity per gram of tissue than those of day 13 and showed only 18% to 19% of the maternal blood values. From the data obtained, the placenta is evidently only a partial barrier for POL, and its penetration capacity declines on increasing differentiation of the fetus.
Polidocanol belongs to the class of detergent sclerosing solutions that are nonionic compounds. It consists of an apolar hydrophobic part (dodecyl alcohol) and a polar hydrophilic part (polyethylene-oxide chain) that is esterified (Fig. 7.25). In solution, POL is associated as macromolecules through electrostatic hydrogen bonding between the H+atom of the OH– group in one molecule, and the free electron-pair of an oxygen atom of a second molecule. This bonding results in the formation of a network (see Fig. 7.4). The sclerotherapeutic activity results from this double hydrophobic and hydrophilic action, and thus POL is a ‘detergent’. The optimal efficacy of the compound coincides with the highest concentration that still permits the existence of nonaggregated molecules, 3%.a However, Kreussler Pharma states that POL 4% is also optimal and soluble (personal correspondence, 1992).
Telangiectasias are treated with concentrations of 0.25% to 0.75%. A randomized study determined that a 0.5% concentration may be ideal for sclerosis of leg telangiectasias.125 Varicose veins are treated with concentrations of 1% to 4%. Small vessels and telangiectasias respond well. Efficacy is decreased in the treatment of large or medium-sized varicose veins. Kreussler Pharmab recommends the use of POL 4% for treatment of varicosities greater than 8 mm in diameter. A 3% solution is recommended for varicose veins 4 to 8 mm in diameter, 2% is recommended for varicose veins 2 to 4 mm in diameter, and 1% for veins 1 to 2 mm in diameter. The practitioner should take care not to exceed the maximum dose of POL, which is 2 mg/kg per day.
Advantages
The safety and efficacy of this agent is such that in the 1950s the Vick Chemical Company developed a derivative of POL, polyoxyethylene dodecanol, as a mucolytic wetting agent for use in vaporizers.126 Toxicity studies on rats demonstrated a lack of sensitization to cutaneous application and no toxicity with oral ingestion or with exposure to steaming electric vaporizers. A clinical study carried out on 168 infants and children treated with this compound in vaporizers showed no harmful effects.127
Henschelc stated that the selective activity on damaged endothelium results from the steric structure of POL: ‘The macromolecules retard the individual molecules and thus shield the tissue from their uninhibited action.’ This damage is therefore said to be reversible in normal tissue. Henschel goes on to claim that since the surface-active-induced absorption on the varix wall is greatest at the point of injection and falls off rapidly with increasing distance, large quantities can be injected without danger of damage to the deep venous system. He recommends the injection of a maximum of 2 mL of POL 3% at each site, with a maximum of 6 mL of POL 3% injected in one sclerotherapy session. However, Goldman et al43 have demonstrated that POL scleroses normal vessels (rabbit ear vein) and that the concentration injected is critical to the final outcome of vein sclerosis. Polidocanol is a weaker detergent-type of sclerosing solution than STS. These experimental studies indicate that its sclerosing power is approximately 50% of the strength of STS.
Polidocanol is unique among sclerosing agents in that it is both painless to inject and does not produce cutaneous ulcerations, even with intradermal injection of concentrations less than 1.0% (see Chapter 8). Allergic reactions rarely have been reported. The degree of pigmentation produced may be less than that of other detergent sclerosing agents (see Chapter 8).
An open clinical trial comparing POL with STS and HS was conducted in Australia by 120 physicians.128 The results at 2 years showed that 55 of 65 physicians considered that POL had a better efficacy than STS, with two claiming decreased efficacy and eight seeing no difference. When compared with HS, 49 of 58 claimed better efficacy for POL, with one physician reporting decreased efficacy and eight physicians finding no difference. Pain on injection with POL was less than with STS in the experience of 54 of 65 physicians, and was less than with HS as reported by 56 of 58 physicians. Finally, 58 of 65 physicians considered that, overall, POL caused fewer complications (pigmentation, ulceration, phlebitis, telangiectatic matting) than STS, and 43 of 58 considered that it caused fewer complications overall when compared with HS.
A double-blind prospective comparative trial between POL and STS in 129 patients found no therapeutic difference between the two agents.129 Concentrations of POL were twice that of STS for comparable-sized veins. Adverse effects were also not statistically different. All patients had an average 70% improvement with one treatment. An Italian study which did not mention exact numbers of patients treated or concentrations of solutions also compared POL with STS.130 This study found an increase in closure from 66% to 89% in favor of POL. The adverse reaction of skin inflammation was also increased with STS compared to with POL. Thus, POL may have better efficacy than STS as well as fewer adverse effects. In Japan, a 6-year study of 261 patients treated in 21 centers found a 70% to 100% efficacy of POL in treating variably sized leg veins.131 No subject developed any treatment-related systemic adverse effect.
Sclerosing solution combinations
Another method for sclerosing varicose veins is to combine solutions either together or in a sequential manner. Certainly, diluting PII with HS or SX increases the potency and localizes the sclerosing effect to the point of injection. This technique may be useful when sclerosing junctions between the superficial and deep systems (saphenofemoral-saphenopopliteal junctions and/or perforating veins).132
Adding 66% glucose as a diluent can modify the viscosity of POL. This increases the sclerosant endothelium contact time, which in turn increases the surfactant action of the detergent. Making the solution thicker lowers the force of injection and minimizes the diffusion of the sclerosing solution. A mixture of one-third POL 0.5% with one-third glucose 66% and one-third sterile water has been found to decrease thrombosis and telangiectatic matting in the treatment of telangiectasias.133
Sequential injections of different sclerosing solutions
Sequential injections may be useful to enhance the efficacy of a milder sclerosing solution, either by increasing its potency or by the act of sequentially damaging endothelium. After mechanical trauma, endothelial cells are unable to generate various substances or to respond to circulating or locally produced substances.134 In this damaged state, further injury may produce irreparable damage. In addition, combining a solution with another may produce an additive effect on its potency.
Sodium tetradecyl sulfate 3% is currently the strongest sclerosant approved by the FDA. When treatment with this alone may prove ineffective, such as in patients with a large varicose vein or an area of high reflux, sequential use of HS produces a stronger, synergistic effect. This technique has been reported recently using ultrasound guidance to sclerose the SFJ.135 One-year follow-up of 66 patients treated with STS 3% alone under ultrasound guidance at the SFJ demonstrated a recanalization rate of 25%. When a second group of 70 patients with similar pathology were treated with STS 3% immediately followed by HS 23.4%, only 12% demonstrated recanalization at 1-year follow-up. Thus, the sclerosing effect was enhanced. A second report of the identical technique performed on 100 patients reported that a ‘small number’ of treatment failures have occurred.136 Unfortunately, when the author reported on the 1- to 2-year follow-up in a response to a ‘letter to the editor’,137 he noted that gradual recanalization was the rule on duplex evaluation, even though the clinical outcome was good.
Volumes, concentrations, and progressive dilution of sclerosing agents
The following is a method to compute theoretically how much sclerosing agent is necessary to fill up a vein. The inner volume V of a vein segment is: V = L × π × (D/2)2, where L is the length, and D the inner diameter. It is very simple to calculate that – for example – a length of 10 cm of a vein of 0.7 cm inner diameter has an inner volume of 3.85 cm3. Other examples are computed in Table 7.7 (Fig. 7.26). It is interesting to note that since volumes are proportional to the square of the radius, it is possible to inject a very long vein of small diameter with a small volume of liquid. Figure 7.27 emphasizes the fact that the injection of 0.5 cm3 has very different diffusions in veins of different diameters. This leads us to understand that when deciding the injected volume, the most important reference is the venous diameter (Fig. 7.28).
For all these reasons, we presented a theoretical model that is useful for predicting subsequent sclerosing reactions from a given dilution (Fig. 7.29).138 The concentration of sclerosing agent decreases progressively when drifting away from the point of injection (Fig. 7.30A). Practically, when the liquid sclerosing agent is injected at a single point, the injected concentration is usually too high (in order to obtain a sufficiently long sclerosed zone, despite some dilution), and can induce side effects. To some extent, the problem can be addressed by injecting a greater volume of a milder solution (Fig. 7.30B) or by injecting small volumes in multiple points close to each other (Fig. 7.30C). If a venous spasm occurs, or if some means allows a reduction in the vein diameter, the laminar flow will further improve the phenomenon (Fig. 7.30D). The ultimate evolution of this thinking is the use of foam, as described below.
Foam sclerosants (foamed sclerosing agents, sclerofoam)
The first foam sclerosants were described 60 years ago, and Wollmann139 has demonstrated well that it is hard to tell who really invented the technique. However, it remains obvious that two authors – Cabrera in Spain140 and Monfreux in France141 – have boosted its use in the past 15 years.
The first advantage of foam is that it does not mix much with blood, and, therefore, little dilution occurs in the body. Provided the diameter is not too large, the foam ‘pushes’ the blood like liquid does, ensuring an even effect on the endothelium (Fig. 7.30E). However, when diameters are too high, the foam floats and only the upper wall is in contact. In these cases, additional maneuvers should be undertaken in order to ensure a full contact (alternative massages, compression, elevation, creation of spasm).
Many different types of foam have been used and presented, using different sclerosing agents (only detergent solutions like STS, POL and morrhuate can foam), different gases (room air, sterile air, CO2, O2, CO2 + O2, N2O), different gas/liquid ratios, different preparation tools, etc. At present, two techniques are predominant: the Provensis, which has been manufactured and standardized, and the double-syringe technique. The double-syringe method mixes gas and liquid through either a three-way stopcock (Tessari) or a female–female Luer lock connector (DSS technique). The DSS has been mechanized by use of an automated device: Turbofoam (Kreussler France, Paris) (Fig. 7.31).
Foam sclerosants also offer the advantage of being an excellent contrast medium for B-mode echography since ultrasounds are scattered by the multiple air/liquid interfaces and foam is recognized by its white cloud aspect and dark shade cone. Thanks to this property, control of diffusion within the desired vein is simple and accurate.142 In veins smaller than 3 mm, theoretical advantages of foam sclerosants are less obvious, since experiments have demonstrated that a laminar flow ensures replacement of blood by injected liquid sclerosants. The increased sclerosing power must be taken into account with care, and adverse reactions caused by transparietal burn are common. Chapter 9 details the use and Chapter 8 describes side effects related to the use of foam for the treatment of varicose, spider, and reticular veins.
A complete discussion on the use of foam in sclerotherapy treatment of varicose veins is found in Chapter 9.
1 Green D. Compression sclerotherapy techniques. Dermatol Clin. 1989;7:137.
2 Hoeffner U, Vanhoutte PM. Endothelial factors and regulation of vascular tone. In: Vanhoutte PM, editor. Return circulation and norepinephrine: an update. Paris: John Libbey Eurotext, 1991.
3 Ralevic V, Lincoln J, Burnstock G. Release of vasoactive substances from endothelial cells. In: Ryan U, Rubanyi GM, editors. Endothelial regulation of vascular tone. New York: Marcel Dekker, 1992.
4 Van Hinsbergh VWM, Kooistra T, van den Berg EA, et al. Tumor necrosis factor increases the production of plasminogen activator inhibitor in human endothelial cells in vitro and in rats in vivo. Blood. 1988;72:1467.
5 Munro JM, Pober JS, Cotran RS. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989;135:121.
6 Kobayashi S, Crokks S, Eckmann DM. Dose-and time-dependent liquid sclerosant effects on endothelial cell death. Dermatol Surg. 2006;32:1444.
7 Wolf E. Die histologischen Veranderungen der Venen nach intravenosen sub limatein Spritzungen. Med Klin. 1920;16:806.
8 Schneider W. Contribution to the history of the sclerosing treatment of varices and to its anatomo-pathologic study. Soc Fran Phlebol. 1965;18:117.
9 Fegan WG. Varicose veins: compression sclerotherapy. London: Heinemann; 1967.
10 Chleir F, Vin F. Sclérus versus thrombus. Actua Vasc Inter. 1995;35:18.
11 Hanschell HM. Treatment of varicose veins. Br Med J. 1947;2:630.
12 Campbell GR, Campbell JH, Manderson JA, et al. Arterial smooth muscle: a multifactorial mesenchymal cell. Arch Pathol Lab Med. 1988;112:977.
13 Thulesius O. Physiological and pathophysiological variations of smooth muscle. Presented at the European Congress of the International Union of Phlebology, Budapest, September 6, 1993, Multiscience Publishing.
14 Westerband A, Crouse D, Richter LC, et al. Vein adaptation to arterialization in an experimental model. J Vasc Surg. 2001;33:561.
15 Schwartz SM, Gajdusek CM, Reidy MA, et al. Maintenance of integrity in aortic endothelium. Fed Proc. 1980;39:2618.
16 Haudenschild CC, Schwartz SM. Endothelial regeneration, II: restitution of endothelial continuity. Lab Invest. 1979;41:407.
17 Schwartz SM, Gajdusek CM, Selden SC. Vascular wall growth control: the role of endothelium. Arteriosclerosis. 1981;1:107.
18 Bouvier CA, Gaynor E, Cintron JR, et al. Circulating endothelium as an indication of vascular injury. In: Koller F, et al, editors. Vascular factors and thrombosis. Stuttgart: Schattauer Verlag, 1970.
19 Fronek A, Strejcek J, Korinkova Z, Buchtova L. Changes in endothelial activity caused by venoactive drugs as measured by photoplethysmography and circulating endothelial cells. Presented at the 2003 UIP World Congress Chapter Meeting. San Diego, CA, August 2003.
20 Imhoff E, Stemmer R. Classification and mechanism of action of sclerosing agents. Soc Fran Phlebol. 1969;22:143.
21 Stroncek DF, Hutton SW, Silvis SE, et al. Sodium morrhuate stimulates granulocytes and damages erythrocytes and endothelial cells: probable mechanism of an adverse reaction during sclerotherapy. J Lab Clin Med. 1985;106:498.
22 Cacciola E, Giustolisi R, Musso R, et al. Activation of contact phase of blood coagulation can be induced by the sclerosing agent polidocanol: possible additional mechanism of adverse reaction during sclerotherapy. J Lab Clin Med. 1987;109:225.
23 Brenn H, Imhoff E, Duckert F. Wirkung verschiedener sklero-sierungsmittel auf einige Gerinnungsparameter in vitro und bei Patienten wahrend Varizenverodung. Vasa. 1976;5:199.
24 Cepelak V. Effect of sclerosing agents on platelet aggregation. Folia Angiol. 1982;30:363.
25 Cooper WM. Clinical evaluation of sotradecol, a sodium alkyl sulfate solution, in the injection therapy of varicose veins. Surg Gynecol Obstet. 1946;83:647.
26 Oscher A, Garside E. Intravenous injection of sclerosing substances: experimental comparative studies of changes in vessels. Ann Surg. 1932;96:691.
27 Sadick N. Hyperosmolar vs detergent sclerosing agents in sclerotherapy: effect on distal vessel obliteration. Presented at the Seventh Annual Congress of the North American Society of Phlebology, Maui, February 1994.
28 Lindemayr H, Santler R. The fibrinolytic activity of the vein wall. Phlebologie. 1977;30:151.
29 Merlen JF, Curri SB, Saout J, et al. Histological changes in sclerosed vein. Phlebologie. 1978;31:17.
30 Virchow R. Gesammelte abhandlungen zur wissenschaftlichen Medicin. Frankfurt: Meidingersohn; 1856.
31 Wuppermann TH. Study of sclerosis of varicose veins: comparison between the natural fibrinolysis in the blood of the antecubital vein and the test with labelled fibrinogen in the sclerosed leg. Phlebologie. 1977;30:145.
32 Soehring K, Nasemann TH. Beitrage zur pharmakologie der Alkylpolyathylenoxyderivate, III: Gerinnungszeit bei wiederholtzer Applikation, hamolytische Wirkungen. Arch Int Pharmacodyn. 1952;91:96.
33 MacGowen WAL, Holland PD, Browne HI, et al. The local effects of intra-arterial injections of sodium tetradecyl sulfate (STD) 3%: an experimental study. Br J Surg. 1972;59:101.
34 Raymond-Martimbeau P, Leclerc JR. Effects of sclerotherapy on blood coagulation: a prospective study. In: Raymond-Martinbeau P, Prescott R, Zummo M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
35 Dastain JY. Sclerotherapy of varices when the patient is on anticoagulants, with reference to 2 patients on anticoagulants. Phlebologie. 1981;34:73.
36 Kanter AH. Complications of sotradecol sclerotherapy with and without heparin. In: Raymond-Martimbeau P, Prescott R, Zummo M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
37 Klein-Fein J. What happens during the injection of sclerosant? Phlebologie. 1977;30:165.
38 Guex J-J, Allaert F-A, Gillet J-L, Chleir F. Immediate and mid-term complications of sclerotherapy. Report of a prospective multi-center registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31:123.
39 Williams RA, Wilson E. Sclerosant treatment of varicose veins and deep vein thrombosis. Arch Surg. 1984;119:1283.
40 Wenner L. Sind endovarikose hamatische Ansammlungen eine normalerscheinung bei Sklerotherapie? Vasa. 1981;10:174.
41 Fegan WG. Continuous compression technique of injecting varicose veins. Lancet. 1963;282:109.
42 Schmier AA. Clinical comparison of sclerosing solutions in injection treatment of varicose veins. Am J Surg. 1937;36:389.
43 Goldman MP, Kaplan RP, Oki LN, et al. Sclerosing agents in the treatment of telangiectasia: comparison of the clinical and histologic effects of intravascular polidocanol, sodium tetradecyl sulfate, and hypertonic saline in the dorsal rabbit ear vein model. Arch Dermatol. 1987;123:1196.
44 Martin DE, Goldman MP. A comparison of sclerosing agents: clinical and histologic effects of intravascular sodium tetradecyl sulfate and chromated glycerine in the dorsal rabbit ear vein. J Dermatol Surg Oncol. 1990;16:18.
45 Reiner L. The activity of anionic surface active compounds in producing vascular obliteration. Proc Soc Exp Biol Med. 1946;62:49.
46 Rotter SM, Weiss RA. Human saphenous vein in vitro model for studying the action of sclerosing solutions. J Dermatol Surg Oncol. 1993;19:59.
47 Feied C, Kessler CM. Personal communication, 1993.
48 Regard GL. The treatment of varicosities by sclerosing injections. Rev Med Suisse Rom Geneve. 1925;65:102.
49 Doerffel J. Klinisches und Experimentelles uber Venenverodung mit Kochsalzlosung und Traubenzucker. Deutsche Med Wohnschr. 1927;53:901.
50 Binet L, Verne J. Evolution histo-physiologie de la veine a la suite de son obliteration experimentale. Presse Med. 1925;1:761.
51 Schwartz E, Ratschow M. Experimentelle und klinische Erfahrungen bei der kunstlichen verodung von Varicen. Arch Klin Chir. 1929;156:720.
52 Biegeleisen HI. Fatty acid solutions for the injection treatment of varicose veins: an evaluation of 4 new solutions. Ann Surg. 1937;105:610.
53 McPheeters HO, Anderson JK. Injection treatment of varicose veins and hemorrhoids. Philadelphia: FA Davis; 1938.
54 Biegeleisen HI. The evaluation of sodium morrhuate therapy in varicose veins: a critical study. Surg Gynecol Obstet. 1933;57:696.
55 Schneider W, Fischer H. Fixierung und bindegewebige organization artefizieller thromben bei der Varizenuerodung. Deutsche Med Wochenschr. 1964;89:2410.
56 Orsini C, Brotto M. Immediate pathologic effects on the vein wall of foam sclerotherapy. Dermatol Surg. 2007;33:1250.
57 Dietrich VHP, Sinapius D. Experimental endothelial damage by varicosclerosation drugs. Arzneimittelforschung. 1968;18:116.
58 Goldman MP. A comparison of sclerosing agents: clinical and histologic effects of intravascular sodium morrhuate, ethanolamine oleate, hypertonic saline (11.7%), and Sclerodex in the dorsal rabbit ear vein. J Dermatol Surg Oncol. 1991;17:354.
59 Blenkinsopp WK. Comparison of tetradecyl sulfate of sodium with other sclerosants in rats. Br J Exp Pathol. 1968;49:197.
60 Hamel-Desnos C, Desnos P, Wollmann JC, et al. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg. 2003;29:1170.
61 Hamel-Desnos C, Allaert FA, Benigni JP, et al. Etude 3/1. Mousse de Polidocanol 3% versus 1% dans la grande veine saphène. Premiers résultats. Phlebologie. 2005;58:165.
62 Wollmann JC. Schaum-zwischen Vergangenheit und Zukunft, 8. Bonner Venentage 15–16 Feb. Vasomed. 2002;16:34.
63 Kern HM, Angle LW. The chemical obliteration of varicose veins: a clinical and experimental study. JAMA. 1929;93:595.
64 Goldman MP, Raymond-Martimbeau P. Clinical and histologic evaluation of polyiodinated iodine with and without hypertonic saline/dextrose in the rabbit ear model. Dermatol Surg. 1997;23:701.
65 Sadick NS. Sclerotherapy of varicose and telangiectatic leg veins: minimal sclerosant concentration of hypertonic saline and its relationship to vessel diameter. J Dermatol Surg Oncol. 1991;17:65.
66 Ouvry PA. Telangiectasia and sclerotherapy. J Dermatol Surg Oncol. 1989;15:177.
67 Leach B, Goldman MP. Comparative trial between sodium tetradecyl sulfate and glycerin in the treatment of telangiectatic leg veins. Dermatol Surg. 2003;29:612.
68 Foley WT. The eradication of venous blemishes. Cutis. 1975;15:665.
69 Sadick N. Treatment of varicose and telangiectatic leg veins with hypertonic saline: a comparative study of heparin and saline. J Dermatol Surg Oncol. 1990;16:24.
70 Bodian EL. Techniques of sclerotherapy for sunburst venous blemishes. J Dermatol Surg Oncol. 1985;11:696.
71 Bodian EL. Sclerotherapy. Semin Dermatol. 1987;6:238.
72 Thornton SC, Mueller SN, Levine EM. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983;222:623.
73 Bodian E. Sclerotherapy. Dialogues Dermatol. 13(3), 1983. (tape recording)
74 Alderman DB. Therapy for essential cutaneous telangiectasias. Postgrad Med. 1977;61:91.
75 Haines PC, et al. Effects of lidocaine concentration on distal capillary blood flow in a rabbit ear model. Microsurgery. 1987;8:54.
76 Johns RA, DiFazio CA, Longnecker DE. Lidocaine constricts or dilates rat arterioles in a dose-dependent manner. Anesthesiology. 1985;62:141.
77 Mantse L. A mild sclerosing agent for telangiectasias. J Dermatol Surg Oncol. 1985;11:9.
78 Nguyen VB. Sklerotherapie des varices des membres inferieurs etude de 522 cas. Saguenay Medical. 1976;23:134.
79 Mantse L. More on spider veins. J Dermatol Surg Oncol. 1986;12:1022.
80 Ouvry PA. Telangiectasia and sclerotherapy. J Dermatol Surg Oncol. 1989;15:177.
81 Martindale W. The extra pharmacopoeia, 28th ed. London: Pharmaceutical Press; 1982.
82 Maclaren NK, Cowles C, Ozand PT, et al. Glycerol intolerance in a child with intermittent hypoglycemia. J Pediatr. 1975;86:43.
83 Jausion H, Carrot E, Ervais A. Une methode simple de phlebosclerose: la cure des varices par les injections de glycerine diluee. Bull Soc Fran Dermatol Syph. 1931;38:171.
84 Hammarlund ER, Pedersen-Bjergaard L. Hemolysis of erythrocytes in various iso-osmotic solutions. J Pharm Sci. 1961;50:24.
85 Hagnevik K, Gordon E, Lins LE, et al. Glycerol-induced haemolysis with haemoglobinuria and acute renal failure. Lancet. 1974;303:75.
86 Welch KMA, Meyer JS, Okamoto S, et al. Glycerol-induced hemolysis. Lancet. 1974;303:416. (letter)
87 Jausion H. Glycerine chromée et sclerose des ectasies veineuses. Presse Med. 1933;53:1061.
88 Jausion H, et al. La sclerose des varices et des hemorroides par le glycerine chromée. Bull Memoires Soc Med Hospitaux Paris. 1932:587.
89 Hutinel B. Esthetique dans les scleroses de varices et traitement des varicosites. Vie Med. 1978;20:1739.
90 Nebot F. Quelques points tecniques sur le traitement des varicosites et des telangiectasies. Phlebologie. 1968;21:133.
91 Ducros R, Gruffaz J. Indications, techniques et resultats du traitement sclerosant. Revue Praticien. 1970;20:2027.
92 Stemmer R, Kopp C, Voglet P. Etude physique del injection sclerosante. Phlebologie. 1969;22:149.
93 Ouvry P, Arlaud R. Le traitement sclerosant des telangiectasies des membres inferieurs. Phlebologie. 1979;32:365.
94 Ouvry P, Davy A. Le traitement sclerosant des telangiectasies des membres inferieurs. Phlebologie. 1982;35:349.
95 Wallois P. Incidents et accidents de la sclerose. In Tournay R, editor: La sclerose des varices, 4th ed, Paris: Expansion Scientifique Francaise, 1985.
96 Albino P, Monteiro Castro J. Réaction allergique grave après traitement de télangiectasies à la glycérine chrome. Phlebologie. 2005;58:151.
97 Mol W, Furukawa H, Sasaki S, et al. Evaluation of the sclerotherapeutic efficacy of ethanol, polidocanol, and OK-432 using an in vitro model. Dermatol Surg. 2007;33:1452.
98 Ghosh S. Chemical investigation in connection with leprosy inquiry. Indian J Med Res. 1920;8:211.
99 Cutting RA. The preparation of sodium morrhuate. J Lab Clin Med. 1926;11:842.
100 Gallagher PG. Varicose veins – primary treatment with sclerotherapy. J Dermatol Surg Oncol. 1992;18:39.
101 Meyer NE. Monoethanolamine oleate: a new chemical for obliteration of varicose veins. Am J Surg. 1938;40:628.
102 Mosley JG, Gupta I. The clinical and histological effects of ethanolamine in varicose veins. Phlebology. 1998;13:29.
103 Biegeleisen HI. Fatty acid solutions for the injection treatment of varicose veins: evaluation of four new solutions. Ann Surg. 1937;105:610.
104 Hedberg SE, Fowler DL, Ryan LR. Injection sclerotherapy of esophageal varices using ethanolamine oleate: a pilot study. Am J Surg. 1982;143:426.
105 Ayres SJ, Goff JS, Warren GH. Endoscopic sclerotherapy for bleeding esophageal varices: effects and complications. Ann Intern Med. 1983;98:900.
106 Evans DMD, Jones DB, Cleary BK, et al. Osophageal varices treated by sclerotherapy: a histopathological study. Gut. 1982;23:615.
107 Reid RG, Rothine NG. Treatment of varicose veins by compression sclerotherapy. Br J Surg. 1968;55:889.
108 Nabatoff RA. Recent trends in the diagnosis and treatment of varicose veins. Surg Gynecol Obstet. 1950;90:521.
109 Orbach EJ. Histopathological findings of telangiectasies treated with sodium tetradecyl sulfate-procaine precipitate. Angiopatias (Brasil). 1968;8:103.
110 Tretbar LL. Spider angiomata: treatment with sclerosant injections. J Kansas Med Soc. 1978;79:198.
111 Shields JL, Jansen GT. Therapy for superficial telangiectasias of the lower extremities. J Dermatol Surg Oncol. 1982;8:857.
112 Muir CK. The toxic effects of some industrial chemicals on rabbit ileum in vitro compared with eye irritancy in vivo. Toxicol Lett. 1983;19:309.
113 Berte F, Bianchi A, Gregotti C, et al. In vivo and in vitro toxicity of carbitol. Boll Chim Farm. 1986;125:401.
114 Pereira F, Pereira C, Lacerda MH. Contact dermatitis due to a cream containing chitin and a carbitol. Contact Dermatitis. 1998;38:290.
115 Dawson TA, Black RJ, Strang WC, et al. Delayed and immediate hypersensitivity to carbitols. Contact Dermatitis. 1989;21:52.
116 Goldman MP. Sodium tetradecyl sulfate for sclerotherapy treatment of veins: is compounding pharmacy solution safe? Dermatol Surg. 2004;30:1454.
117 Almeida JI, Raines JK. FDA-approved sodium tetradecyl sulfate (STS) versus compunded STS for venous sclerotherapy. Dermatol Surg. 2007;33:1037.
118 Sadick NS, Farber B. A microbiologic study of diluted sclerotherapy solutions. J Dermatol Surg Oncol. 1993;19:450.
119 Blenkinsopp WK. Choice of sclerosant: an experimental study. Angiologica. 1970;7:182.
120 Schulz KH. Uber die verwendung von alkyl-polathylenoxyd-derivaten als Oberflachenanaesthetica. Dermatol Wochen. 1952;126:657.
121 Soehring K, et al. Beitrage zur pharmakologie der Alkylpolyathylerioxyd derivate, I: Untersuchungen uber die acute und subchronische Toxizitat bein verschiedenen Tierarten. Arch Int Pharmacodyn. 1951;87:301.
122 Siems KJ, Soehring K. Die ausschaltug sensibler Nerven duren peridurale und paravertebrale Injektion von Alkylpolyathylenoxydathern bei Meerschweinchen. Arzneimittelforschung. 1952;2:109.
123 Olesch B. Neuere Erkenntisse zur Pharmakokinetik von polidocanol (Aethoxysklerol). Vasomed Aktuell. 1990;4:22.
124 Soehring K, Frahm M. Studies on the pharmacology of alkylpolyethyleneoxide derivatives. Arzneimittelforschung. 1955;5:655.
125 Carlin MC, Ratz JL. Treatment of telangiectasia: comparison of sclerosing agents. J Dermatol Surg Oncol. 1987;13:1181.
126 Grubb TC, Dick LC, Oser M. Studies on the toxicity of polyoxyethylene dodecanol. Toxicol Appl Pharmacol. 1960;2:133.
127 Larkin V De P. Polyethylene dodecanol vaporization in the treatment of respiratory infections of infants and children. NY State J Med. 1957;57:2667.
128 Conrad P, Malouf GM. The Australian polidocanol (Aethoxysklerol) open clinical trial results at two years. Presented at the Eighth Annual Meeting of the North American Society of Phlebology, Maui, 24 February, 1994.
129 Goldman MP. Treatment of varicose and telangiectatic leg veins: double blind prospective comparative trial between Aethoxysklerol and Sotradecol. Dermatol Surg. 2002;28:52.
130 Belcaro G, Cesarone MR, Dugal M, et al. Primary ‘classic’ sclerotherapy registry and trial. The sclero randomized 10-year follow-up study. Presented at the UIP World Congress Chapter Meeting, San Diego, CA, 2003.
131 Satokawa H, Hoshino S, Sakaguchi S, Yamada C. The Japanese polidocanol (Aethoxysclerol) clinical trial. Presented at the UIP World Congress Chapter Meeting, San Diego, CA, 2003.
132 Raymond-Martimbeau P. Intravascular ultrasound and evaluation of treatment results. Presented at the 50th Annual Meeting of the American Academy of Dermatology, Dallas, December 11, 1991.
133 Zuccarelli F. Combination of aetoxisclerol with glucose for the treatment of varicose veins and telangiectasias. In: Negus etal, editor. Phlebology ’95. London: Libby, 1995.
134 Vanhoutte PM. The endothelium-modulator of vascular smooth-muscle tone. N Engl J Med. 1988;319:512.
135 Mauriello J, Zygmunt J Jr: Synergistic effect of sclerosing agents. Presented at the Eighth Annual Meeting of the North American Society of Phlebology, Maui, February 22, 1994.
136 Miller D, Biegeleisen K. Sequential injection of 3% sodium tetradecyl sulfate and 20% sodium chloride in the treatment of refractory varicosity of the greater saphenous vein. Dermatol Surg. 1994;20:329.
137 Biegeleisen K. Response to sequential injection of 3% sodium tetradecyl sulfate and 20% sodium chloride in the treatment of refractory varicosity of the greater saphenous vein. Dermatol Surg. 1995;20:355. (letter)
138 Guex J-J. Indications for the sclerosing agent polidocanol. J Dermatol Surg Oncol. 1993;19:959.
139 Wollmann JC. The history of sclerosing foams. Dermatol Surg. 2004;30:694.
140 Cabrera J, Cabrera Garcia-Olmedo JR. Nuevo metodo de esclerosis en las varicas tronculares. Patol Vasc. 1995;4:55.
141 Monfreux A. Traitement sclérosant des troncs saphéniens et leurs collatérales par la méthode MUS. Phlebologie. 1997;50:351.
142 Guex J-J. Foam sclerotherapy: an overview of use for primary venous insufficiency. Semin Vasc Surg. 2005;18:25.
a Product description on hydroxypolyethoxydodecane, Dexo SA Pharmaceuticals, France, May 1985; product insert for Aethoxysklerol, Kreussler & Co GmbH, Wiesbaden-Biebrich, Germany, 1985.
b Henschel O: Sclerosing of varicose veins sclerotherapy with Aethoxysklerol-Kreussler (product booklet), Kreussler & Co GmbH, Wiesbaden-Biebrich, Germany.
a Correspondence received from Laboratories Ondee Ltee, 280 Milice Longueuil, Montreal, Canada (1986).
a Correspondence received from Laboratories Ondee Ltee, 280 Milice Longueuil, Montreal, Canada (1986).
a Sclérémo product information from Laboratories E. Bouteille, 7 Rue des Belges, Limoges 8100, France (1987).
a Product information from Glaxo Pharmaceuticals, Research Triangle Park, N.C. (1989).
b Ethamolin injection, 5%; product information from Glaxo Pharmaceuticals Inc. (December 1988).
a STD injection product data sheet from STD Pharmaceutical Products, Hereford, England (1977).
b Sotradecol, Bioniche Pharma Group, Casla, Co. Galway, Ireland.
c Thromboject product information from Omega, Montreal, Canada (rev 10/87).
a Analyses were performed at the Bioniche Pharma Group Limited Quality Control Laboratory, Inverin, County Galway, Ireland.
a Henschel O: Sclerosing of varicose veins sclerotherapy with Aethoxysklerol-Kreussler (product booklet), Kreussler & Co GmbH, Wiesbaden-Biebrich, Germany.
b Kreussler Pharma: ‘Expert Information on Aethoxysklerol’, July 1993.
a Dexo SA Pharmaceuticals, France: product description on hydroxypolyethoxydodecane. Received with correspondence, May 1985.
b Product information (July 1993) from Kreussler Pharma, Wiesbaden, D-65203, Germany.
c Henschel O: Sclerosing of varicose veins sclerotherapy with Aethoxysklerol-Kreussler (product booklet), Kreussler & Co GmbH, Wiesbaden-Biebrich, D-6202, Postfach 9105, Germany.