93 Mechanical Support in Cardiogenic Shock
An estimated 61.8 million people in the United States have heart disease, among whom 950,000 die annually.1 Of these, 540,000 people suffer myocardial infarctions each year; 193,000 succumb to complications directly related to the infarction. The leading cause of death among hospitalized patients with acute myocardial infarction (AMI) continues to be cardiogenic shock.2 The incidence of cardiogenic shock complicating AMI (approximately 7%) has remained constant over the past 25 years. Accurate statistics on the worldwide utilization of all mechanical support for cardiogenic shock are not known. However, estimates on the use of intraaortic counterpulsation for patients in shock after AMI suggest a rate of use in only 22% of eligible patients.3 The reasons for the apparent underutilization of this readily available modality are not clear. Accordingly, the indications, benefits, and limitations of mechanical cardiac support are outlined in this chapter.
 Historical Background
Historical Background
The evolution of mechanical cardiac support dates to the early 1950s when Gibbon developed the prototype cardiopulmonary bypass (CPB) apparatus.4 In the years following, Lillehei, Kirklin, and others applied the heart-lung machine to facilitate open-heart surgery; their pioneering work and early observations led directly to the development of modern mechanical cardiac support systems.5–7 These surgeons recognized that some patients had improved outcomes after surgery if they were weaned slowly rather than abruptly from CPB support. Their initial publications introduced the concept that left ventricular (LV) decompression and myocardial rest could afford enhanced cardiac recovery after the insult of open-heart surgery. Clinical use of extracorporeal CPB for heart surgery became widespread in the early 1960s. Simultaneously, several groups of investigators were testing means of mechanical cardiac assistance for use outside the operating room for support of patients in cardiogenic shock. The current modes of mechanical support are derivations of those originally developed and include aortic counterpulsation, continuous flow pumps with or without an oxygenator, and pulsatile pumps.
History of Aortic Counterpulsation
The concept of arterial counterpulsation was introduced in 1961 by Clauss and coworkers and involved use of an external “ventricular” chamber that filled with blood from a catheter in the iliac artery8 and was subsequently compressed by a piston. Compression of the “ventricle” was synchronized to either the QRS complex of an electrocardiogram (ECG) or the impulse of a pacemaker, so that a counter pulse of blood was delivered into the arterial system during diastole. It was demonstrated in dogs that cardiac stroke work and LV end-systolic pressures could be substantially reduced with the use of a counterpulsation into the aorta. The following year, Moulopaulus and associates adapted the model to create an intraaortic balloon pump (IABP) that could provide a similar counterpulsation without the need for blood reservoirs.9 The investigators used a balloon that was rapidly inflated and deflated with carbon dioxide during native diastole. The IABP was subsequently adapted and described for clinical use by Kantrowitz and colleagues in 1968.10
The physiologic rationale for the efficacy of the IABP is that balloon deflation provides a rapid, synchronized reduction in impedance (afterload) during isovolemic LV contraction. This is followed by a rapid, synchronized increase in aortic pressure during isovolemic LV relaxation (diastolic augmentation) caused by balloon inflation. In combination, these events achieve two important goals. First, LV systolic unloading directly reduces stroke work, which in turn reduces myocardial oxygen consumption during the cardiac cycle. Second, diastolic augmentation raises arterial blood pressure and provides better coronary arterial perfusion during diastole, yielding increased oxygen delivery to the myocardium. The IABP does not directly move or redistribute blood flow; however, peak diastolic coronary flow velocity can be increased as much as 87% with IABP augmentation and peak diastolic flow velocity by as much as 117%.11 Since introduction into clinical use in 1968, the IABP has remained an important adjunct to supporting patients in cardiogenic shock. Myocardial recovery is promoted by the reduction of cardiac work and the simultaneous increase in myocardial oxygen supply. However, therapeutic success is dependent on the patient having a minimum degree of LV function that, in combination with IABP support, facilitates an adequate cardiac output to sustain end-organ function. When this minimal cardiac output is not met, alternative mechanical cardiac assistance must be considered.
History of Mechanical Assist Devices
The need for effective mechanical cardiac assist devices became apparent in the 1950s during the development of CPB for open-heart surgery. Initial attempts with prolonged postoperative CPB demonstrated that the bypass circuit was damaging to both end-organ function and blood constituents after several hours of use.12 The first attempt at isolated extracorporeal LV support was with a simple roller pump in 1962.13 Subsequently, femoral venous–to–femoral arterial CPB was successfully used by Spencer and colleagues in four patients with postcardiotomy cardiac failure.14
Simultaneous to Spencer and colleagues’ work with extracorporeal systems, DeBakey designed the first intracorporeal LV assist device (LVAD), the DeBakey blood pump.15 This device consisted of a Dacron-reinforced silicone rubber tube with an inner chamber of blood from the left atrium that was connected to the descending thoracic aorta. Pressurized air was instilled into the outer chamber by an external pneumatic controller to compress the inner blood chamber, timed to the R wave of the QRS complex. Blood flow was directed from the left atrium to the descending aorta with the use of ball valves at both the inflow and outflow ends of the device. The DeBakey blood pump was first used in a patient who died 4 days postoperatively of neurologic complications. A remodeled extracorporeal version was subsequently used for postcardiotomy failure in a 37-year-old woman after aortic and mitral valve replacements. The device was needed for 10 days, but the patient survived.16
By 1972, investigators at the Texas Heart Institute had developed a pneumatically driven LVAD designed to be implanted in the abdomen.17 This device had a blood chamber compressed by pulses of air delivered into the pump by a percutaneous driveline. Modern devices have chamber compression that is electrically powered via percutaneous drivelines. Paracorporeal, pneumatically driven devices were a parallel development. Paramount to the evolution of these devices was the sponsorship of the Artificial Heart Program of the National Heart, Lung, and Blood Institute, which was chartered in 1964.
By the 1960s, continuous flow, as compared to pulsatile, pumps were under development.18,19 Over the subsequent 15 years, centrifugal pumps were perfected and introduced into clinical use. These pumps work on the principle of a forced, constrained vortex devised from three magnetic cones.20–22 They have been shown to be useful in a variety of clinical settings where short-term mechanical support is needed and an IABP is inadequate. Several types of small, axial-flow or rotary pumps have also been developed, including some that allow for percutaneous deployment.23–36 These are generally constructed of a magnetically suspended impeller that rotates at extremely fast rates (25,000 to 35,000 rpm). The axial rotary pump technology has some potential advantages over pulsatile devices; they are quite small with few moving parts and do not require a compliance chamber. The latest generation of rotary pump technology utilizes fully magnetically levitated rotors that completely eliminate the need for seals or bearings. This technology reduces the risk of damage to blood elements and may lead to lower rates of thromboembolism.
 Current Mechanical Support Devices
Current Mechanical Support Devices
Counterpulsation/Intraaortic Balloon Pump
Indications
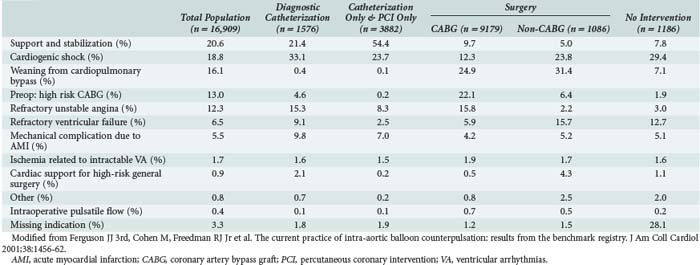
The absolute indications for IABP placement include cardiogenic shock, uncontrolled angina pectoris, acute postinfarction ventricular septal defect or mitral regurgitation, and postcardiotomy left-sided heart failure with low cardiac output. In these settings, IABP should be considered a primary therapy that should not be delayed until noncardiac injury is clinically evident. It is important to recognize that blood pressure alone is not an adequate indication of hemodynamic or cardiac stability. Limb perfusion, renal function, mental status, and even gastrointestinal function need to be considered in the assessment of adequate resuscitation and homeostasis. Additional measurable indices include arterial (SaO2) and mixed venous oxygen saturation (SvO2), acid-base status, urine output, and body temperature. A multivariate analysis of data accrued from 391 postcardiotomy patients requiring IABP demonstrated that epinephrine requirements greater than 0.5 µg/kg/min, a left atrial pressure greater than 15 mm Hg, urine output less than 100 mL/h, and SvO2 less than 60% correlated with mortality.37 These criteria were used to help predict mortality and the need for subsequent mechanical support.
Other relative indications for IABP use include (1) high-risk, catheter-based interventional procedures such as left main coronary artery angioplasty, (2) after unsuccessful attempts at catheter-based intervention in patients with poorly controlled ventricular arrhythmias, and (3) concomitant poor LV function, and (4) in settings of persistent stunned, ischemic myocardium. These are all circumstances in which reduction of LV systolic wall tension and oxygen consumption by the IABP might enhance myocardial recovery after intervention. Conversely, the use of an IABP had no impact on mortality in a population of patients without hemodynamic instability undergoing high-risk angioplasty randomized in a prospective trial reported in 1997.38 More recently, the Benchmark Counterpulsation Outcomes Registry of IABP use in 22,663 patients from 250 hospitals worldwide demonstrated that cardiogenic shock and high-risk angioplasty were the most common indications for utilization of the device.39 Table 93-1 depicts a further characterization of the Benchmark report with respect to indications for use of the IABP and subsequent interventions.40 Nevertheless, despite the widespread use of the IABP in over 150,000 patients worldwide each year,41 no prospective randomized trial has ever demonstrated a survival benefit with IABP use in the patient population undergoing high-risk catheter intervention. In contrast, the SHOCK trial showed that early revascularization of patients with coronary artery disease and shock after an AMI, often facilitated by IABP use (86%), yielded a lower 6-month mortality rate (50%) than with medical therapy alone (63%).2 Additional studies have shown that in patients undergoing urgent or emergent revascularization after an AMI, those supported preoperatively with an IABP had a lower operative mortality than those in whom an IABP was not used (5.3%-8.8% versus 11.8%-28.2%).42,43 These data seem to justify a strategy of aggressive IABP use to facilitate early revascularization in the postinfarction patient.
Technical Considerations
The optimal site of insertion of an IABP is a common femoral artery that can be accessed either percutaneously with the use of a guidewire or by surgical cutdown. Modern intraaortic balloon catheters are available for adults and children according to the appropriate size and length for a given height and weight of the patient. Adult intraaortic balloons have a range in volume filled between 25 and 50 mL, with a standard balloon size holding 40 mL of helium. IABP catheters placed through the femoral artery are positioned so that the tip is just distal to the takeoff of the left subclavian artery in the proximal descending thoracic aorta. Optimally, the tip of the catheter should be positioned with transesophageal echocardiographic (TEE) or fluoroscopic guidance.44 To reduce the diameter of femoral cannulation, a sheathless IABP technique can be utilized and is our preferred method.45
Inflation of the balloon should be timed with closure of the aortic valve (at the dicrotic notch of the aortic pressure tracing) and should be inflated to nearly occlude the descending thoracic aorta. Timing can be synchronized in one of three ways: (1) using an arterial (preferably aortic) pressure tracing in synchrony with the dicrotic notch, (2) using the descent of the R wave on a rhythm tracing, or (3) timed after a ventricular pacing spike when a pacemaker is in use.46–50 The effectiveness of IABP is significantly improved by proper timing of inflation and deflation, which can be difficult when there is an accelerated heart rate, cardiac rhythm disturbances, atrioventricular dyssynchrony, or low mean arterial pressure. IABP timing should be adjusted to maximize diastolic augmentation; hence, deflation should be as late as possible but just before opening of the aortic valve. If this cannot be gauged by the pressure tracing, it can be timed to the onset of the R wave on the ECG tracing or with the use of M-mode echocardiography.51
When femoral arterial cannulation is not desirable because of aortoiliac occlusive disease or extensive peripheral vascular disease, the subclavian artery or the ascending aorta can be utilized.52–56 With either technique, the IABP catheters are advanced antegrade down the descending thoracic aorta so that the balloon tip sits above the level of the diaphragmatic hiatus, and the most proximal end of the balloon is distal to the takeoff of the left subclavian. These antegrade balloons should always be placed with either fluoroscopic or echocardiographic guidance. They should be removed with open arterial repair in all cases.
Complications
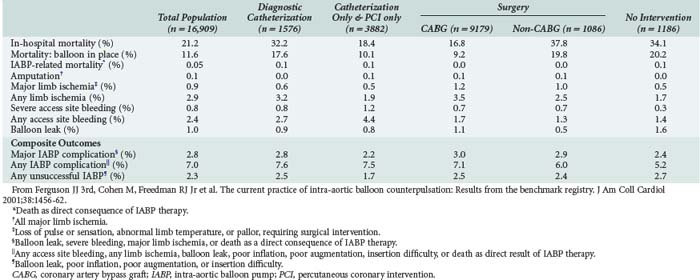
The overall complication rate of IABP utilization is between 5% and 10%. Major complications occur at a rate of about 3% and include severe bleeding, major limb ischemia or amputation, infection, visceral or spinal cord ischemia, and attributable IABP mortality.39,43 A summary of IABP complications as they occur in relation to subsequent percutaneous or operative coronary revascularization from the Benchmark Registry are listed in Table 93-2.40 In this registry, rates of complications were quite low, the most common being access-site bleeding (4.3%) and limb ischemia (2.3%).39 The rates of amputation, stroke, visceral or spinal cord ischemia and IABP-related mortality are all 0.1% or less.39 Intraaortic balloon entrapment is a rare complication.57–59 The incidence of major vascular complications according to the STS National Database (1996-1997) and the Benchmark Registry (1997-1999) is 5.4% and 1.4%, respectively.40,43 Ipsilateral limb ischemia should be immediately addressed after its recognition. This usually requires removal of the IABP, with replacement at another location if it is still indicated. The ischemic limb may require thrombectomy with or without revascularization and fasciotomy.60–66
Outcomes
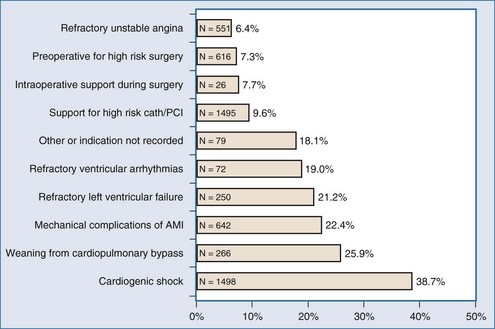
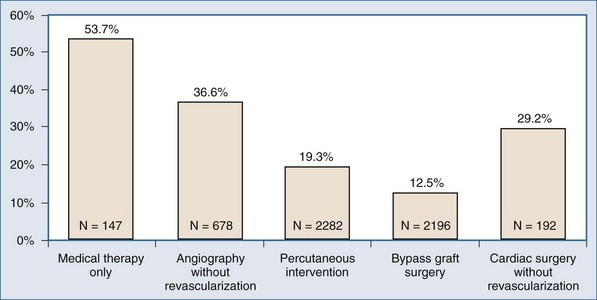
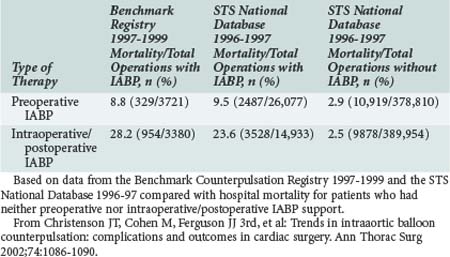
In the absence of prospective randomized data, it is difficult to ascribe outcome secondary to IABP placement. The Second Angioplasty in Myocardial Infarction (PAMI-II) Trial data examined high-risk patients with acute myocardial infarction revascularized by percutaneous intervention only and demonstrated a modest survival advantage at 6 months with the use of periprocedural IABP support.38 When evaluating hospital mortality rates among patients undergoing coronary artery bypass graft (CABG) and/or valve surgery who received preoperative IABP or required intraoperative/postoperative IABP support, it is evident that mortality was significantly lower among patients supported preoperatively, as depicted in Table 93-3.40,43 Hence, there appears to be a survival advantage to earlier IABP support for patients with AMI and cardiogenic shock who need revascularization. In the setting of an acute ventricular septal defect (VSD) or acute mitral regurgitation after an AMI, IABP support can offer a dramatic improvement in the hemodynamic response of the patient.67–71 Figures 93-1 and 93-2 stratify hospital mortality rates associated with IABP use in patients with AMI by principal usage indication or by performance of percutaneous or surgical coronary revascularization. It is clear that the mortality rate of cardiogenic shock after AMI remains high at 39%. However, IABP support combined with revascularization portends a better prognosis than adjunctive IABP use with medical therapy alone.39
TABLE 93-3 Hospital Mortality (Outcome Parameter) for Patients Undergoing Cardiac Surgery Who Either Received Preoperative IABP or Intra-/Postoperative IABP Support
Continuous Flow Pumps
Both roller pumps and centrifugal pumps deliver continuous flow but have other distinct limitations. Roller pumps remain in widespread use for cardiopulmonary support during cardiac surgery; applications outside the operating room have been virtually abandoned for several reasons. Roller pumps are insensitive to changes in arterial line resistance that may cause disruption of the apparatus. They require unobstructed venous flow. The rollers eventually cause spallation of tubing, leading to particle emboli and weakening of the tubing.72 Roller compression causes hemolysis after prolonged use.73 Alternatively, centrifugal pumps are sensitive to both outflow resistance and filling pressure, offering a safer applicability outside the operating room. Centrifugal pumps like the BioMedicus Bio-Pump (Medtronic Corp., Minneapolis, Minnesota) generate a constrained vortex within an acrylic shell that houses concentric magnetic cones. The cones rotate as a magnetic rotary motor spins adjacent to the base of the cones20–22 and can generate very high flows with less trauma to blood cells than roller pumps.73–75
The technology of centrifugal pumps, axial flow pumps, and membrane oxygenators has remarkably improved. Pump durability and reduced blood cell trauma have been demonstrated.19,26,73–76 As a result, considerable experience has accumulated with the use of centrifugal pumps (cardiopulmonary support) for postcardiotomy LV failure, fulminant myocarditis, or cardiogenic shock after AMI.32,77–93 Newer devices have incorporated design modifications that allow for improved pump performance as well as percutaneous application.
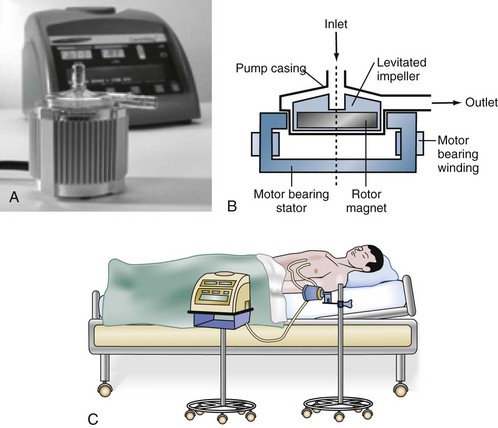
A novel centrifugal blood pump, the CentriMag system, utilizes fully magnetically levitated technology (Figure 93-3) to provide external mechanical circulatory support in a fashion similar to the BioMedicus Bio-Pump. The CentriMag system has many advantages that make it attractive for short-term mechanical support in the acute setting.94 These advantages include ease of implantation, direct outflow cannulation of the ventricle for improved decompression, minimal need for anticoagulation, and less damage to blood elements compared to traditional devices such as the BioMedicus pump. It has been used effectively for uni- or biventricular support in the setting of postcardiotomy cardiac failure as a bridge to decision, recovery, or long-term mechanical circulatory support device. For patients who also require pulmonary support, an oxygenator may be added to the circuit, effectively converting it to an ECMO system.
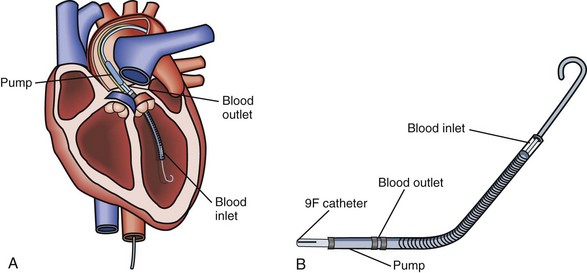
The Tandem Heart System (Cardiac Assist Inc., Pittsburgh, Pennsylvania) is an external centrifugal pump system that allows for percutaneous LV support (Figure 93-4).95 Utilizing a percutaneous venous cannula that crosses the atrial septum, this pump can provide LV assistance without performing a sternotomy. This device has been utilized both for short-term support in the catheterization suite or as a bridge to recovery, more definitive mechanical circulatory support (i.e., implantable device), or transplantation.
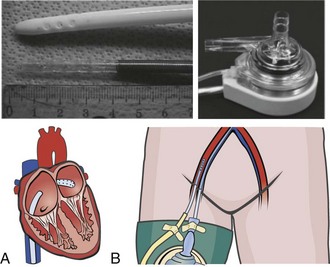
The most recent addition to the armamentarium for acute short-term mechanical circulatory support is the Impella Recover axial flow pump (Abiomed Inc., Danvers, Massachusetts). This device can be placed percutaneously or directly via the open chest during cardiac surgery (Figure 93-5). It has been used for partial support during percutaneous coronary interventions as well as for postcardiotomy cardiogenic shock as a bridge to recovery or a more definitive long-term device.96 Important limitations for the use of this device include aortic stenosis that precludes proper positioning of the device as well as peripheral vascular disease that may make percutaneous deployment impossible or mandate a surgical cutdown for placement. This device cannot be used in patients who have had a previous mechanical aortic valve replacement.
Indications
Short-term cardiopulmonary support for cardiogenic shock has emerged as an important adjunctive therapy. It is a relatively simple means of establishing immediate and complete circulatory support, requiring no additional equipment other than that needed for standard CPB support during cardiac surgery. Cardiopulmonary support can be initiated percutaneously via the common femoral artery and vein. Alternatively, when faced with postcardiotomy LV failure, cardiopulmonary support can facilitate patient stabilization for subsequent transport to a tertiary medical center for VAD placement. Cardiopulmonary support circuits can be converted to longer-term support (beyond 6-8 hours) by upgrading the oxygenator.97,98 A standard microporous hollow-fiber oxygenator (the type used in most CPB circuits) has a lifespan of 6 to 12 hours.99 Changing to a solid-silicone membrane oxygenator (not microporous) will lengthen the lifespan of the cardiopulmonary support circuit up to 21 days; this conversion constitutes extracorporeal membrane oxygenation (ECMO) support. ECMO is generally used in the adult population for periods of 1 to 10 days when there is marked concomitant pulmonary insufficiency and cardiac failure. ECMO is also used for short-term (1-3 days) support when the neurologic status of a patient is unclear and longer-term support (i.e., VAD support) may not be appropriate until this status is clarified. Thus, ECMO can be used as a bridge to a longer-term, pulsatile flow assist device once the suitability of the patient is determined.
Technical Considerations and Complications
Disadvantages to the use of peripheral cardiopulmonary support or ECMO include the greater potential for ipsilateral limb complications, higher rates of hemolysis, the requirement for anticoagulation to prevent thrombosis of the oxygenator and circuit, and failure to adequately decompress the left ventricle.100–106 Inadequate LV decompression with peripheral cardiopulmonary support/ECMO systems may be the mechanism responsible for some treatment failures. Regardless of the etiology of cardiogenic shock, a rested ventricle (i.e., decompressed) has a better chance of recovery than a distended ventricle.
Outcomes
The use of ECMO in the adult population for reasons other than primary cardiac failure with secondary pulmonary insufficiency has limited advantages over conventional therapies.107,108 However, a substantial subset of patients who present with cardiogenic shock and are initially resuscitated with cardiopulmonary support/ECMO survive to revascularization, transplantation, or recovery, with survival rates as high as 75%.77,78,81,82,109–116 ECMO used as a bridge to VAD placement for profound cardiogenic shock (“double bridge” mechanical assistance) can yield survival rates greater than 40%.80 This strategy is pragmatic and offers immediate end-organ support while a subsequent definitive treatment plan can be designed.
Ventricular Assist Devices
Pulsatile Pumps
There is a growing body of evidence suggesting that pulsatile assisted circulation, in the setting of acute cardiogenic shock, offers improved end-organ perfusion and lymphatic flow and is thus beneficial.117–119 VADs that utilize direct cardiac outflow cannulation (VAD inflow) provide better ventricular decompression and rest than peripheral bypass support systems. There are now several mechanical assist devices that achieve these goals, including the extracorporeal ABIOMED AB 5000 (Abiomed) and the paracorporeal Thoratec VAD system (Thoratec Corp., Pleasanton, California). Two other implantable intracorporeal pulsatile VADs that were designed for patients with chronic heart failure may have roles in certain subsets of patients with acute cardiogenic shock. These are the HeartMate LVAS XVE (Thoratec) and the intracorporeal Thoratec VAD system (Thoratec).
Extracorporeal Short-Term Support
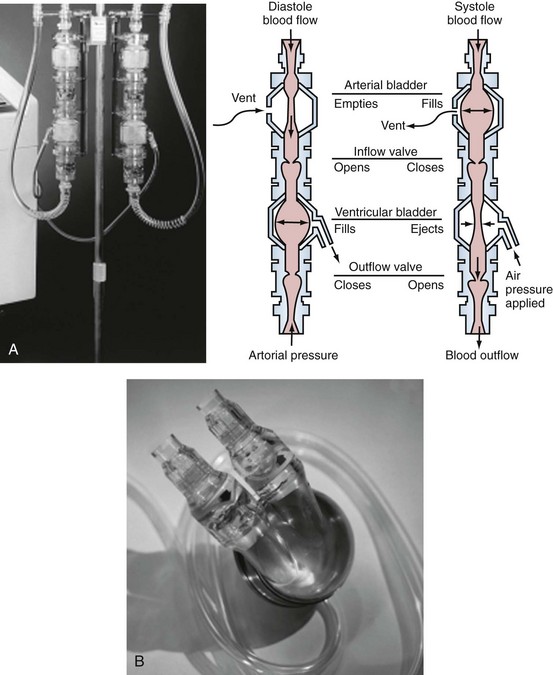
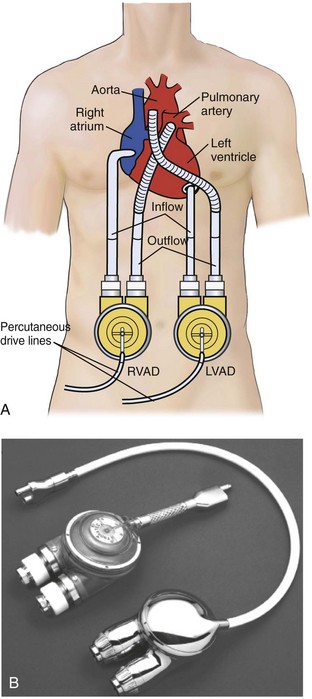
The recently FDA-approved ABIOMED AB 5000 “ventricle” replaces the previously utilized BVS 5000 which was developed in the 1980s and was granted approval for use for postcardiotomy heart failure by the U.S. Food and Drug Administration in 1992.116 Since that time, indications for the device have been broadened to include most patients with either postcardiotomy shock or precardiotomy shock who do not adequately respond to inotropes and an IABP. The ABIOMED system is a pneumatically driven, dual-chamber blood pump that delivers pulsatile flow. ABIOMED inflow cannulas are placed in the left and/or right atrium for univentricular or biventricular support. Outflow cannulas are housed with a Hemashield graft (Meadox Medicals Inc., Oakland California) and are sewn to the aorta and/or pulmonary artery for left-sided and/or right-sided heart support. The pumps, as depicted in Figure 93-6, are extracorporeal (BVS5000) or paracorporeal (AB5000). In the BVS5000, the upper (first) chamber fills passively by gravity, and the lower chamber serves as the pumping chamber. The two chambers are separated by a polyurethane trileaflet inflow valve; the lower chamber is separated from the arterial circulation by an outflow valve that prevents retrograde flow. As the pumping chamber is filled with blood, the surrounding air within the polycarbonate housing is displaced back into the drive console. This is sensed by the console; the console delivers compressed air back into the pumping chamber, which compresses the bladder and forces a pulse of blood into the arterial circulation.120 The stroke volume that results is 70 to 80 mL, with VAD output dependent on the rate of upper-chamber filling. The AB5000 “ventricle” has a single ventricular blood chamber that fills by vacuum assistance from a portable console. Blood is ejected by pneumatic inflation of polyurethane bladder housed within the pump casing. Valves constructed of Angioflex, ABIOMED’s proprietary polyether-based polyurethane plastic, insure unidirectional flow. Typically, flows of 5 L/min are achieved with either of the ABIOMED systems. Both of these devices require anticoagulation, particularly for LV assistance. The BVS5000 is generally useful for short-term (<7-10 days) support because of the increased risk of thromboembolic complications or device malfunction beyond this period. If longer support (2-3 months) is necessary, the ABIOMED pump can be exchanged with AB5000 or converted to a longer-term VAD system such as the Thoratec p-VAD or HeartMate.
ABIOMED cannulation can be achieved either on or off CPB and with or without aortic cross-clamping. However, the condition of the patient is typically unstable, and cannulation—particularly of the pulmonary artery and aorta—may be safer on bypass with a decompressed, supported heart. Access is obtained via median sternotomy, with all cuffed cannulas brought out of the chest through separate subcostal incisions. The cuffs allow soft tissue growth and adherence to reduce the incidence of infection of the cannulas and endocardium. Approximately 6000 ABIOMED VADs have been placed worldwide for precardiotomy or postcardiotomy cardiac failure.118,120–123 Survival and hospital discharge rates have ranged from 20% to 45%, depending on the indication for the ABIOMED and the hemodynamic condition of the patient before surgery.118,120–122 The most common complications directly attributed to this VAD include bleeding, stroke, and infection, with rates of 20% to 40%.118,120,122 Hemolysis is not a common problem.
Paracorporeal Longer-Term Support
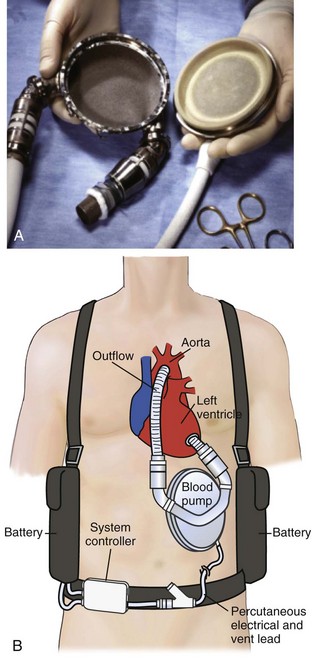
The Thoratec paracorporeal VAD (p-VAD) system is composed of a single chamber with a polyurethane seamless bladder housed in a rigid casing (Figure 93-7).124 VAD inflow cannulas are either atrial or ventricular. Outflow cannulas have a polyester graft attached for direct connection to the aorta or pulmonary artery, similar to the ABIOMED cannulas. There are Bjork-Shiley tilting disc valves at both the inflow and outflow connections to the bladder to ensure unidirectional flow; they require anticoagulation. A pneumatic driveline is connected to the rigid casing and supplies alternating vacuum and pressure to facilitate bladder filling and emptying, respectively. The blood pump can be adjusted to accommodate changing preload and afterload. The pneumatically driven pulses (systole) can be controlled in three different modes: asynchronous, synchronous, and volume. The asynchronous mode maintains a fixed rate, but stroke volume may vary. The synchronous mode provides counterpulsation by timing ejection to the patient’s R wave—this provides both a variable rate and variable stroke volume. The volume mode delivers a fixed stroke volume triggered by bladder filling, but the rate will vary. The volume mode is usually the most practical because the VAD output changes with the patient’s physiologic condition.
The Thoratec p-VAD is similar to the ABIOMED but is more portable and has the potential for outpatient use in patients who are bridging to recovery or transplantation.124–127 Two advantages to the Thoratec p-VAD system are the ability of secure ventricular inflow (VAD) cannulation and the applicability of long-term utilization. LV cannulation provides better ventricular decompression than atrial cannulation.128–132 This is important because LV distention or inadequate decompression will limit ventricular recovery in some patients. Ventricular cannulation also provides better VAD performance and reduces the risk of thrombotic complications, particularly in the setting of AMI.111,128,130,132 Right ventricular cannulation provides similar advantages over right atrial cannulation. However, these advantages may not be manifest if the tricuspid valve is left intact, because the tricuspid leaflets are often in close proximity to the cannulation tip and can obstruct VAD inflow.133 In this situation, the advantages and disadvantages of right atrial versus right ventricular cannulation must be weighed to direct the best approach.
Over 3700 Thoratec p-VADs have been placed worldwide in over 2400 patients134; more than half of these patients received biventricular support. Survival and hospital discharge rates vary widely between 20% and 80%, depending on the etiology of shock and the medical center.77,80,124,131,135–137 Cases of acute fulminant myocarditis with cardiogenic shock are among the best situations for VAD support with the Thoratec p-VAD system, having an 88% recovery-with-discharge rate.77 Complications of the Thoratec p-VAD system are similar to other extracorporeal VAD systems when used for treatment of cardiogenic shock and include infection, stroke, bleeding, and acute renal failure. The rates of these complications vary among different series but range from 10% to 60%.122,124,131,135,137–142 Another device similar to the Thoratec p-VAD and commonly used for paracorporeal long-term support is the Berlin Heart EXCOR device.143,144
Intracorporeal Long-Term Support
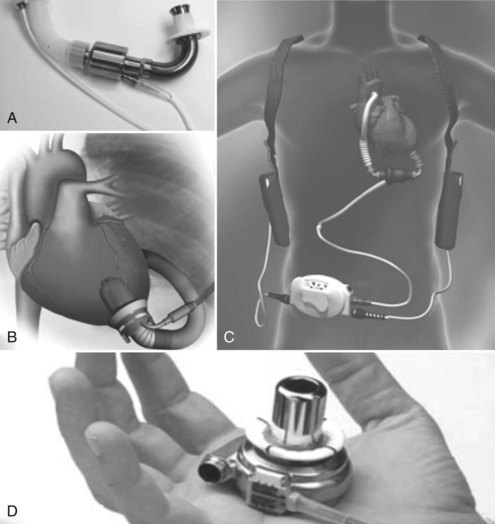
Options for pulsatile intracorporeal long-term support include the Thoratec HeartMate LVAS XVE and the Thoratec implantable VAD (i-VAD). The Heartmate XVE has a fully implanted pusher-plate blood pump with externalized drivelines (Figure 93-8). It uses bioprosthetic porcine valves to ensure unidirectional flow. The HeartMate XVE has a flexible polyurethane diaphragm that pushes against a titanium alloy housing generating a maximum stroke volume of 83 mL. The blood contact surface is textured with polyurethane fibrils on one side and sintered titanium spheres on the housing. Fibrin and cellular components react and bond to the surface, creating a pseudointima, precluding the need for anticoagulation. Antiplatelet therapy is recommended. The i-VAD is an implantable version of the Thoratec p-VAD with identical internal components. The major difference is a smooth polished titanium housing that facilitates implantability (see Figure 93-7). A third device, the Novacor LVAS, is similar in design to the Heartmate XVE LVAS, with a fully implantable pusher plate blood pump and externalized drive lines. It was widely used as a bridge to transplantation in the early experience with LVAD support but is currently not in use and is largely of historic interest.
Both implantable pulsatile systems have variable modes that can generate fixed rates or demand-sensitive rates. Both are approved for use for the treatment of end-stage heart failure, but they may have a selective role for cardiogenic shock. These devices are practical alternatives for use in a “double-bridge” setting with initial resuscitation using a temporary device (i.e., ECMO/cardiopulmonary support or ABIOMED) for stabilization and pulmonary recovery.80,112,113,145,146 Results with these devices have been favorable and, in certain subsets of patients, better than longer-term support with other systems.77,142,147–152 Complications have been similar to other VADs and include bleeding, infection, stroke, thrombotic complications, and renal insufficiency.
NonPulsatile (Continuous Flow) Pumps
There are several miniaturized rotary continuous flow (nonpulsatile) pumps that have been designed for long-term (potentially permanent) mechanical assistance, including the MicroMed-DeBakey pump, the Jarvik 2000, the HeartMate II, and the HeartWare (Figure 93-9).153–164 These devices are being studied in clinical trials for use as a bridge to transplantation, recovery, or permanent replacement therapy.154,163 They are relatively costly, provide isolated LV support, and require specialized training to implant. Consequently, they have not yet received widespread use for acute cardiogenic shock.
 Treatment of Cardiogenic Shock: Algorithm for Mechanical Support
Treatment of Cardiogenic Shock: Algorithm for Mechanical Support
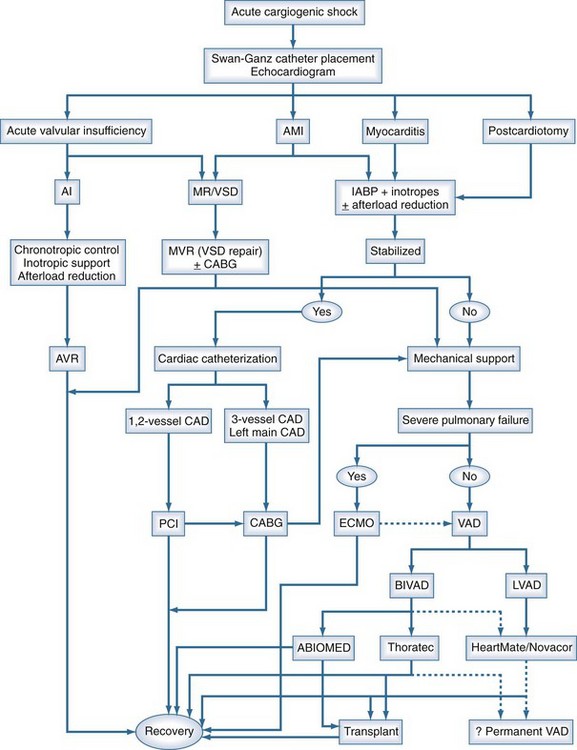
Irrespective of the etiology of cardiogenic shock, the approach toward the initial management of patients should be fairly uniform, and a suggested management algorithm is outlined in Figure 93-10.
Acute fulminant myocarditis usually presents in a previously healthy individual with no history of cardiac disease. Patients with presumed myocarditis who do not stabilize after the insertion of an IABP and concomitant inotropic infusion should be diverted to VAD support expeditiously. A remarkable percentage of these patients will recover if adequately supported during the acute phase of this disease. Short-term to intermediate-term VADs are optimal in these patients because of the ease of their insertion and removal and the anticipation for relatively short-term recovery. Giant cell myocarditis is one exception to this rule, because most patients with this diagnosis will require transplantation.165–168
Cardiogenic shock after AMI requires immediate IABP placement, often with additional pharmacologic support. If a mechanical complication (i.e., severe mitral regurgitation or VSD) has occurred, immediate surgical intervention is usually required. An expeditious cardiac catheterization is reasonable if the patient can be stabilized or placed on percutaneous bypass for the procedure. If no mechanical complication has occurred and the patient has been stabilized with IABP and medical therapy, cardiac catheterization may proceed. The number of diseased arteries usually determines subsequent allocation to percutaneous or surgical revascularization. Patients whose condition is unstable after an AMI, with continued cardiogenic shock despite IABP and inotropic support, should be considered for VAD support (see Figure 93-10).
 Conclusion
Conclusion
Cardiogenic shock remains a lethal problem with a mortality rate as high as 75%.2,169,170 Patients who cannot be stabilized with inotropic support and an IABP should be considered for mechanical assistance with a VAD. The ideal assist device that can be easily placed, is versatile and portable, has minimal risk of complication, offers a normal cardiac output with physiologically equivalent characteristics such as pulsatile flow, and is easily removed does not yet exist. Currently there are three modes of mechanical cardiac assistance that have received widespread use in the patient population with cardiogenic shock: ECMO/cardiopulmonary support, the ABIOMED AB 5000, and the Thoratec p-VAD system. Implantable devices such as the HeartMate LVAS XVE and Heartmate II have occasionally been used in this moribund population but have a more defined role in the subacute and chronic heart failure population.
The use of mechanical assistance for acute cardiogenic shock has facilitated impressive improvements in survival for certain disease cohorts such as those with acute myocarditis, with survival rates over 70%.77 VADs have had a less remarkable impact on patients with postcardiotomy shock or AMI-induced shock,111 but results in these patient populations are improving annually. Inherent to achieving better results is our understanding that patients who present with cardiogenic shock typically have significant underlying comorbidities with multiple-system organ dysfunction and marked derangements in both coagulation and inflammatory mediators that complicate management. They need to be approached by an integrated multidisciplinary team that includes cardiologists, cardiac surgeons, anesthesiologists, critical care specialists, and experienced nursing staff to implement efficient and decisive treatment plans. These integrated systems offer the greatest chance for success. Technologies expand and improve exponentially every year, and it is clear that mechanical assistance will continue to play a pivotal role in the management of these difficult patients.
Key Points
Farrar DJ. The Thoratec ventricular assist device: A paracorporeal pump for treating acute and chronic heart failure. Semin Thorac Cardiovasc Surg. 2000;12:243-250.
Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
Pagani FD, Lynch W, Swaniker F, et al. Extracorporeal life support to left ventricular assist device bridge to heart transplant: a strategy to optimize survival and resource utilization. Circulation. 1999;100:II206-II210.
Samuels LE, Holmes EC, Thomas MP, et al. Management of acute cardiac failure with mechanical assist: experience with the ABIOMED BVS 5000. Ann Thorac Surg. 2001;71:S67-S72. discussion S82-5
Stone GW, Ohman EM, Miller MF, et al. Contemporary utilization and outcomes of intra-aortic balloon counterpulsation in acute myocardial infarction: the benchmark registry. J Am Coll Cardiol. 2003;41:1940-1945.
1 American Heart Association. Heart Disease and Stroke Statistics—2003 Update. Dallas: American Heart Association; 2002.
2 Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634.
3 Anderson RD, Ohman EM, Holmes DRJr, et al. Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: Observations from the GUSTO-I Study. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. J Am Coll Cardiol. 1997;30:708-715.
4 Gibbon JH. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171-185.
5 Dennis C. Certain methods for artificial support of the circulation during intracardiac surgery. Surg Clin North Am. 1956;36:425-436.
6 DeWall RA, Warden HE, Read RC, et al. A simple, expendable, artificial oxygenator for open heart surgery. Surg Clin North Am. 1956;36:1025-1036.
7 Kirklin JW, Dushane JW, Patrick RT, et al. Intracardiac surgery with the aid of a mechanical pump-oxygenator system (Gibbon type): Report of eight cases. Mayo Clin Proc. 1955;30:201-206.
8 Clauss RH, Birtwell WC, Albertal G, et al. Assisted circulation: The arterial counterpulsator. J Thorac Cardiovasc Surg. 1961;41:447-458.
9 Moulopaulus SD, Topoz S, Kolff WJ. Diastolic balloon pumping (with carbon dioxide) in the aorta: A mechanical assistance to the failing circulation. Am Heart J. 1962;63:669-675.
10 Kantrowitz A, Tjonneland S, Freed PS, et al. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203:135-140.
11 Katz ES, Tunick PA, Kronzon I. Observations of coronary flow augmentation and balloon function during intraaortic balloon counter-pulsation using transesophageal echocardiography. Am J Cardiol. 1992;69:1635-1639.
12 Liotta D, Hall CW, Henly WS, et al. Prolonged assisted circulation during and after cardiac or aortic surgery. Am J Cardiol. 1963;12:399-405.
13 Dennis C. Left heart bypass. Mechanical Devices to Assist the Failing Heart. Publication No. 1283. Washington, DC: National Academy of Sciences—National Research Council; 1966. 27
14 Spencer FK, Eiseman B, Trinkle JK, et al. Assisted circulation for cardiac failure following intracardiac surgery with cardiorespiratory bypass. J Thorac Cardiovasc Surg. 1965;49:56-73.
15 Hall CW, Liotta D, Henly WS, et al. Development of artificial intrathoracic circulatory pumps. Am J Surg. 1964;108:685-692.
16 DeBakey ME. Left ventricular bypass pump for cardiac assistance: Clinical experience. Am J Cardiol. 1971;27:3-11.
17 Norman JC, Whalen RL, Daley BDT, et al. An implantable left ventricular assist device (LVAD). Clin Res. 1972;20:855.
18 Saxton GA, Andrews CB. An ideal pump with hydrodynamic characteristics analogous to the mammalian heart. Trans Am Soc Artif Organs. 1960;1:84-86.
19 Olsen DB. The history of continuous-flow blood pumps. Artif Organs. 2000;24:401-404.
20 Dorman F, Bernstein EF, Blackshear PL, et al. Progress in the design of a centrifugal cardiac assist pump with transcutaneous energy transmission by magnetic coupling. Trans Am Soc Artif Intern Organs. 1969;15:441-448.
21 Rafferty EH, Kletschka HD, Wynyard M, et al. Artificial heart II— application of nonpulsatile radially increasing pressure gradient pumping principle. Minn Med. 1968;52:191.
22 Bernstein EF, Consentino LC, Reich S, et al. A compact, low hemolysis, nonthrombogenic system for nonthoracotomy prolonged left ventricular bypass. Trans Am Soc Artif Intern Organs. 1974;20:643-654.
23 Wampler RK, Moise JC, Frazier OH, et al. In vivo evaluation of a peripheral vascular access axial flow blood pump. ASAIO Trans. 1988;34:450-454.
24 Butler KC, Moise JC, Wampler RK. The Hemopump—a new cardiac prosthesis device. IEEE Trans Biomed Eng. 1990;37:193-196.
25 Sato N, Mohri H, Sezai Y, et al. Multi-institutional evaluation of the Tokyo University Ventricular Assist System. ASAIO Trans. 1990;36:M708-M711.
26 Monties JR, Mesana T, Havlik P, et al. Another way of pumping blood with a rotary but noncentrifugal pump for an artificial heart. ASAIO Trans. 1990;36:M258-M260.
27 Konstantinov BA, Dzemeshkevich SL, Rogov KA, et al. Extracorporeal mechanical pulsatile pump and its significance for myocardial function recovery and circulatory support. Artif Organs. 1991;15:363-368.
28 Sasaki T, Takatani S, Shiono M, et al. Development of totally implantable electromechanical artificial heart systems: Baylor ventricular assist system. Artif Organs. 1992;16:407-413.
29 Butler KC, Maher TR, Borovetz HS, et al. Development of an axial flow blood pump LVAS. Asaio J. 1992;38:M296-M300.
30 Ohara Y, Sakuma I, Makinouchi K, et al. Baylor Gyro Pump: a completely seal-less centrifugal pump aiming for long-term circulatory support. Artif Organs. 1993;17:599-604.
31 Rintoul TC, Butler KC, Thomas DC, et al. Continuing development of the Cleveland Clinic-Nimbus total artificial heart. ASAIO J. 1993;39:M168-M171.
32 Wiebalck AC, Wouters PF, Waldenberger FR, et al. Left ventricular assist with an axial flow pump (Hemopump): Clinical application. Ann Thorac Surg. 1993;55:1141-1146.
33 Wouters PF, Sukehiro S, Mollhoff T, et al. Left ventricular assistance using a catheter-mounted coaxial flow pump (Hemopump) in a canine model of regional myocardial ischaemia. Eur Heart J. 1993;14:567-575.
34 Macris MP, Myers TJ, Jarvik R, et al. In vivo evaluation of an intraventricular electric axial flow pump for left ventricular assistance. ASAIO J. 1994;40:M719-M722.
35 Makinouchi K, Ohara Y, Sakuma I, et al. Internal hydraulic loss in a seal-less centrifugal Gyro pump. Artif Organs. 1994;18:25-31.
36 Antaki JF, Butler KC, Kormos RL, et al. In vivo evaluation of the Nimbus axial flow ventricular assist system: Criteria and methods. ASAIO J. 1993;39:M231-M236.
37 Hausmann H, Potapov EV, Koster A, et al. Predictors of survival 1 hour after implantation of an intraaortic balloon pump in cardiac surgery. J Card Surg. 2001;16:72-77.
38 Stone GW, Marsalese D, Brodie BR, et al. A prospective, randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infarction treated with primary angioplasty Second Primary Angioplasty in Myocardial Infarction (PAMI-II) Trial Investigators. J Am Coll Cardiol. 1997;29:1459-1467.
39 Stone GW, Ohman EM, Miller MF, et al. Contemporary utilization and outcomes of intraaortic balloon counterpulsation in acute myocardial infarction: The benchmark registry. J Am Coll Cardiol. 2003;41:1940-1945.
40 Ferguson JJ3rd, Cohen M, Freedman RJJr, et al. The current practice of intraaortic balloon counterpulsation: Results from the Benchmark Registry. J Am Coll Cardiol. 2001;38:1456-1462.
41 Data provided by Datascope Corp., Montvale, NJ, 2003.
42 Creswell LL, Moulton MJ, Cox JL, Rosenbloom M. Revascularization after acute myocardial infarction. Ann Thorac Surg. 1995;60:19-26.
43 Christenson JT, Cohen M, Ferguson JJ3rd, et al. Trends in intraaortic balloon counterpulsation complications and outcomes in cardiac surgery. Ann Thorac Surg. 2002;74:1086-1090. discussion 1090-1
44 Tatar H, Cicek S, Demirkilic U, et al. Exact positioning of intraaortic balloon catheter. Eur J Cardiothorac Surg. 1993;7:52-53.
45 Phillips SJ, Tannenbaum M, Zeff RH, et al. Sheathless insertion of the percutaneous intraaortic balloon pump: An alternate method. Ann Thorac Surg. 1992;53:162.
46 Christenson JT, Simonet F, Badel P, et al. Optimal timing of preoperative intraaortic balloon pump support in high-risk coronary patients. Ann Thorac Surg. 1999;68:934-939.
47 Pantalos GM, Minich LL, Tani LY, et al. Estimation of timing errors for the intraaortic balloon pump use in pediatric patients. ASAIO J. 1999;45:166-171.
48 Nagasaka F, Hasegawa T, Shiono M, et al. New sequential synchronized driving system of intraaortic balloon pumping and left ventricular assist device: Influence on endocardial viability ratio and renal blood flow in their combination. Artif Organs. 1992;16:216-218.
49 Ardire L, Boswell J. Intraaortic balloon pump timing in the patient with hypotension. Focus Crit Care. 1992;19:146-149.
50 Kratz JM. Intraaortic balloon pump timing using temporary myocardial pacing wires. Ann Thorac Surg. 1986;42:120.
51 Minich LL, Tani LY, McGough EC, et al. A novel approach to pediatric intraaortic balloon pump timing using M-mode echocardiography. Am J Cardiol. 1997;80:367-369.
52 H’Doubler PBJr, H’Doubler WZ, Bien RC, et al. A novel technique for intraaortic balloon pump placement via the left axillary artery in patients awaiting cardiac transplantation. Cardiovasc Surg. 2000;8:463-465.
53 McBride LR, Miller LW, Naunheim KS, et al. Axillary artery insertion of an intraaortic balloon pump. Ann Thorac Surg. 1989;48:874-875.
54 Santini F, Mazzucco A. Transthoracic intraaortic counterpulsation: A simple method for balloon catheter positioning. Ann Thorac Surg. 1997;64:859-860.
55 Bonchek LI, Olinger GN. Direct ascending aortic insertion of the “percutaneous” intraaortic balloon catheter in the open chest: Advantages and precautions. Ann Thorac Surg. 1981;32:512-514.
56 Meldrum-Hanna WG, Deal CW, Ross DE. Complications of ascending aortic intraaortic balloon pump cannulation. Ann Thorac Surg. 1985;40:241-244.
57 Luengtaviboon K, Signhathanadgige S, Chartlaorng B, et al. Intraaortic balloon entrapment—a rare complication of intraaortic balloon pump. J Med Assoc Thai. 2002;85(Suppl 1):S153-S155.
58 Horowitz MD, Otero M, de Marchena EJ, et al. Intraaortic balloon entrapment. Ann Thorac Surg. 1993;56:368-370.
59 Millham FH, Hudson HM, Woodson J, et al. Intraaortic balloon pump entrapment. Ann Vasc Surg. 1991;5:381-384.
60 Arafa OE, Pedersen TH, Svennevig JL, et al. Vascular complications of the intraaortic balloon pump in patients undergoing open heart operations: 15-year experience. Ann Thorac Surg. 1999;67:645-651.
61 Busch T, Sirbu H, Zenker D, et al. Vascular complications related to intraaortic balloon counterpulsation: An analysis of ten years experience. Thorac Cardiovasc Surg. 1997;45:55-59.
62 Manord JD, Garrard CL, Mehra MR, et al. Implications for the vascular surgeon with prolonged (3 to 89 days) intraaortic balloon pump counterpulsation. J Vasc Surg. 1997;26:511-515.
63 Barnett MG, Swartz MT, Peterson GJ, et al. Vascular complications from intraaortic balloons: Risk analysis. J Vasc Surg. 1994;19:81-87.
64 Funk M, Ford CF, Foell DW, et al. Frequency of long-term lower limb ischemia associated with intraaortic balloon pump use. Am J Cardiol. 1992;70:1195-1199.
65 Lazar JM, Ziady GM, Dummer SJ, et al. Outcome and complications of prolonged intraaortic balloon counterpulsation in cardiac patients. Am J Cardiol. 1992;69:955-958.
66 Iverson LI, Herfindahl G, Ecker RR, et al. Vascular complications of intraaortic balloon counterpulsation. Am J Surg. 1987;154:99-103.
67 Gacioch GM, Ellis SG, Lee L, et al. Cardiogenic shock complicating acute myocardial infarction: The use of coronary angioplasty and the integration of the new support devices into patient management. J Am Coll Cardiol. 1992;19:647-653.
68 Estrada-Quintero T, Uretsky BF, Murali S, et al. Prolonged intraaortic balloon support for septal rupture after myocardial infarction. Ann Thorac Surg. 1992;53:335-337.
69 Deja MA, Szostek J, Widenka K, et al. Post infarction ventricular septal defect—can we do better? Eur J Cardiothorac Surg. 2000;18:194-201.
70 Park SJ, Nguyen DQ, Bank AJ, et al. Left ventricular assist device bridge therapy for acute myocardial infarction. Ann Thorac Surg. 2000;69:1146-1151.
71 Tavakoli R, Weber A, Brunner-La Rocca H, et al. Results of surgery for irreversible moderate to severe mitral valve regurgitation secondary to myocardial infarction. Eur J Cardiothorac Surg. 2002;21:818-824.
72 Kim WG, Yoon CJ. Roller pump induced tubing wear of polyvinylchloride and silicone rubber tubing: Phase contrast and scanning electron microscopic studies. Artif Organs. 1998;22:892-897.
73 Oku T, Harasaki H, Smith W, et al. Hemolysis: A comparative study of four nonpulsatile pumps. ASAIO Trans. 1988;34:500-504.
74 Moen O, Fosse E, Braten J, et al. Roller and centrifugal pumps compared in vitro with regard to haemolysis, granulocyte and complement activation. Perfusion. 1994;9:109-117.
75 Wheeldon DR, Bethune DW, Gill RD. Vortex pumping for routine cardiac surgery: A comparative study. Perfusion. 1990;5:135-143.
76 Joyce LD, Kiser JC, Eales F, et al. Experience with generally accepted centrifugal pumps: Personal and collective experience. Ann Thorac Surg. 1996;61:287-290.
77 Acker MA. Mechanical circulatory support for patients with acute-fulminant myocarditis. Ann Thorac Surg. 2001;71:S73-S76.
78 Schwarz B, Mair P, Margreiter J, et al. Experience with percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med. 2003;31:758-764.
79 Aiba T, Nonogi H, Itoh T, et al. Appropriate indications for the use of a percutaneous cardiopulmonary support system in cases with cardiogenic shock complicating acute myocardial infarction. Jpn Circ J. 2001;65:145-149.
80 Bowen FW, Carboni AF, O’Hara ML, et al. Application of “double bridge mechanical” resuscitation for profound cardiogenic shock leading to cardiac transplantation. Ann Thorac Surg. 2001;72:86-90.
81 Fujimoto K, Kawahito K, Yamaguchi A, et al. Percutaneous extracorporeal life support for treatment of fatal mechanical complications associated with acute myocardial infarction. Artif Organs. 2001;25:1000-1003.
82 Hata M, Shiono M, Orime Y, et al. Strategy of circulatory support with percutaneous cardiopulmonary support. Artif Organs. 2000;24:636-639.
83 Waldenberger FR, Haisjackl M, Holinski S, et al. Centrifugal pumps as left ventricular assist for coronary revascularization on a beating heart. Artif Organs. 1998;22:698-702.
84 Curtis JJ, Walls JT, Schmaltz RA, et al. Use of centrifugal pumps for postcardiotomy ventricular failure: Technique and anticoagulation. Ann Thorac Surg. 1996;61:296-300.
85 Mair P, Hoermann C, Moertl M, et al. Percutaneous venoarterial extracorporeal membrane oxygenation for emergency mechanical circulatory support. Resuscitation. 1996;33:29-34.
86 El-Banayosy A, Posival H, Minami K, et al. Seven years of experience with the centrifugal pump in patients with cardiogenic shock. Thorac Cardiovasc Surg. 1995;43:347-351.
87 Salvi L, Barbier P, Sisillo E, et al. [Circulatory support with Hemopump in cardiogenic shock secondary to papillary muscle rupture]. Cardiologia. 1995;40:865-868.
88 Lee WA, Gillinov AM, Cameron DE, et al. Centrifugal ventricular assist device for support of the failing heart after cardiac surgery. Crit Care Med. 1993;21:1186-1191.
89 Golding LA, Crouch RD, Stewart RW, et al. Postcardiotomy centrifugal mechanical ventricular support. Ann Thorac Surg. 1992;54:1059-1063.
90 Ishino K, Murakami T, Kawakami S, et al. Mechanical circulatory support with a centrifugal pump after open heart surgery. Acta Med Okayama. 1992;46:141-146.
91 Phillips SJ, Zeff RH, Kongtahworn C, et al. Benefits of combined balloon pumping and percutaneous cardiopulmonary bypass. Ann Thorac Surg. 1992;54:908-910.
92 Lefemine AA, Dunbar J. Simple alternatives using a single centrifugal pump for biventricular assistance in cardiogenic shock. J Biomater Appl. 1990;4:333-361.
93 Graham TR, Chalmers JA. Temporary mechanical ventricular support: Part 1. Br J Hosp Med. 1989;41:420-425.
94 Bhama JK, Kormos RL, Toyoda Y, McCurry KR, Teuteberg J, Siegenthaler MP. Clinical Experience Utilizing the Levitronix CentriMag System for Temporary Right Ventricular Mechanical Circulatory Support. J Heart Lung Transplant. 2008;28:971-976.
95 Thiele H, Lauer B. Hambrecht R, et. al. Reversal of cardiogenic shock by percutaneous left atrio-to-femoral bypass assistance. Circulation. 2001;104:2917-2922.
96 Seyfarth M, Sibbing D. Bauer I, et. al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol.. 2008 Nov 4;52(19):1584-1588.
97 Lazzara RR, Magovern JA, Benckart DH, et al. Extracorporeal membrane oxygenation for adult post cardiotomy cardiogenic shock using a heparin bonded system. ASAIO J. 1993;39:M444-M447.
98 Yamane S, Ohashi Y, Sueoka A, et al. Development of a silicone hollow fiber membrane oxygenator for ECMO application. ASAIO J. 1998;44:M384-M387.
99 Usui A, Murakami F, Ooshima H, et al. A clinical study for the durability of oxygenators on cardiopulmonary support. Artif Organs. 1997;21:772-778.
100 Magliato KE, Kleisli T, Soukiasian HJ, et al. Biventricular support in patients with profound cardiogenic shock: A single center experience. ASAIO J. 2003;49:475-479.
101 Ko WJ, Lin CY, Chen RJ, et al. Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg. 2002;73:538-545.
102 Wu IH, Ko WJ, Chou NK, et al. Extracorporeal membrane oxygenation in treatment of cardiogenic shock caused by acute myocarditis. J Formos Med Assoc. 1998;97:364-366.
103 Ko WJ, Chen YS, Chou NK, et al. Extracorporeal membrane oxygenation in the perioperative period of heart transplantation. J Formos Med Assoc. 1997;96:83-90.
104 Wang SS, Chen YS, Ko WJ, et al. Extracorporeal membrane oxygenation support for postcardiotomy cardiogenic shock. Artif Organs. 1996;20:1287-1291.
105 Muehrcke DD, McCarthy PM, Stewart RW, et al. Extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock. Ann Thorac Surg. 1996;61:684-691.
106 Muehrcke DD, McCarthy PM, Stewart RW, et al. Complications of extracorporeal life support systems using heparin-bound surfaces: The risk of intracardiac clot formation. J Thorac Cardiovasc Surg. 1995;110:843-851.
107 Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295-305.
108 Ramakrishnan N. Eighth World Congress of Intensive and Critical Care Medicine, 28 October-1 November 2001, Sydney, Australia: Harm minimization and effective risk management. Crit Care. 2002;6:89-91.
109 Chen RJ, Ko WJ, Lin FY. Successful rescue of sustained ventricular tachycardia/ventricular fibrillation after coronary artery bypass grafting by extracorporeal membrane oxygenation. J Formos Med Assoc. 2002;101:283-286.
110 Minev PA, El-Banayosy A, Minami K, et al. Differential indication for mechanical circulatory support following heart transplantation. Intensive Care Med. 2001;27:1321-1327.
111 Pennington DG, Smedira NG, Samuels LE, et al. Mechanical circulatory support for acute heart failure. Ann Thorac Surg. 2001;71:S56-S59.
112 Wang SS, Ko WJ, Chen YS, et al. Mechanical bridge with extracorporeal membrane oxygenation and ventricular assist device to heart transplantation. Artif Organs. 2001;25:599-602.
113 Pagani FD, Lynch W, Swaniker F, et al. Extracorporeal life support to left ventricular assist device bridge to heart transplant: A strategy to optimize survival and resource utilization. Circulation. 1999;100:II206-II210.
114 von Segesser LK. Cardiopulmonary support and extracorporeal membrane oxygenation for cardiac assist. Ann Thorac Surg. 1999;68:672-677.
115 Kawahito K, Murata S, Yasu T, et al. Usefulness of extracorporeal membrane oxygenation for treatment of fulminant myocarditis and circulatory collapse. Am J Cardiol. 1998;82:910-911.
116 Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139:302-311.
117 Jett GK. Physiology of nonpulsatile circulation: Acute versus chronic support. ASAIO J. 1999;45:119-122.
118 Samuels LE, Holmes EC, Thomas MP, et al. Management of acute cardiac failure with mechanical assist: Experience with the ABIOMED BVS 5000. Ann Thorac Surg. 2001;71:S67-S72.
119 Taylor KM, Bain WH, Davidson KG, et al. Comparative clinical study of pulsatile and non-pulsatile perfusion in 350 consecutive patients. Thorax. 1982;37:324-330.
120 Jett GK. ABIOMED BVS 5000:Experience and potential advantages. Ann Thorac Surg. 1996;61:301-304.
121 Marelli D, Laks H, Amsel B, et al. Temporary mechanical support with the BVS 5000 assist device during treatment of acute myocarditis. J Card Surg. 1997;12:55-59.
122 Korfer R, el-Banayosy A, Posival H, et al. Mechanical circulatory support: The Bad Oeynhausen experience. Ann Thorac Surg. 1995;59:S56-S62.
123 Chen JM, Spanier TB, Gonzalez JJ, et al. Improved survival in patients with acute myocarditis using external pulsatile mechanical ventricular assistance. J Heart Lung Transplant. 1999;18:351-357.
124 Farrar DJ. The Thoratec ventricular assist device: A paracorporeal pump for treating acute and chronic heart failure. Semin Thorac Cardiovasc Surg. 2000;12:243-250.
125 Dew MA, Kormos RL, Winowich S, et al. Quality of life outcomes in left ventricular assist system inpatients and outpatients. ASAIO J. 1999;45:218-225.
126 Holman WL, Davies JE, Rayburn BK, et al. Treatment of end-stage heart disease with outpatient ventricular assist devices. Ann Thorac Surg. 2002;73:1489-1493.
127 von Segesser LK, Tkebuchava T, Leskosek B, et al. Biventricular assist using a portable driver in combination with implanted devices: preliminary experience. Artif Organs. 1997;21:72-75.
128 Lohmann DP, Swartz MT, Pennington DG, et al. Left ventricular versus left atrial cannulation for the Thoratec ventricular assist device. ASAIO Trans. 1990;36:M545-M548.
129 Chow E, Brown CD, Farrar DJ. Effects of left ventricular pressure unloading during LVAD support on right ventricular contractility. ASAIO J. 1992;38:M473-M476.
130 Holman WL, Bourge RC, Murrah CP, et al. Left atrial or ventricular cannulation beyond 30 days for a Thoratec ventricular assist device. ASAIO J. 1995;41:M517-M522.
131 Arabia FA, Paramesh V, Toporoff B, et al. Biventricular cannulation for the Thoratec ventricular assist device. Ann Thorac Surg. 1998;66:2119-2120.
132 Tevaearai HT, Mueller XM, Jegger D, et al. Atrial, ventricular, or both cannulation sites to optimize left ventricular assistance? ASAIO J. 2001;47:261-265.
133 Kaplon RJ, Qi XS, Andreopoulos FM, et al. Tricuspid valvectomy for right ventricular outflow cannula occlusion with the Thoratec ventricular assist device. J Thorac Cardiovasc Surg. 2001;121:812-813.
134 Data provided by the Thoratec Corporation, Pleasanton, CA, 2003.
135 Leprince P, Combes A, Bonnet N, et al. Circulatory support for fulminant myocarditis: Consideration for implantation, weaning and explantation. Eur J Cardiothorac Surg. 2003;24:399-403.
136 Slaughter MS, Silver MA, Farrar DJ, et al. A new method of monitoring recovery and weaning the Thoratec left ventricular assist device. Ann Thorac Surg. 2001;71:215-218.
137 Reinhartz O, Keith FM, El-Banayosy A, et al. Multicenter experience with the Thoratec ventricular assist device in children and adolescents. J Heart Lung Transplant. 2001;20:439-448.
138 Reiss N, el-Banayosy A, Posival H, et al. Management of acute fulminant myocarditis using circulatory support systems. Artif Organs. 1996;20:964-970.
139 Mehta SM, Aufiero TX, Pae WEJr, et al. Results of mechanical ventricular assistance for the treatment of post cardiotomy cardiogenic shock. ASAIO J. 1996;42:211-218.
140 Mets B, Frumento RJ, Bennett-Guerrero E, et al. Validation of continuous thermodilution cardiac output in patients implanted with a left ventricular assist device. J Cardiothorac Vasc Anesth. 2002;16:727-730.
141 Copeland JG3rd, Smith RG, Arabia FA, et al. Comparison of the CardioWest total artificial heart, the Novacor left ventricular assist system and the Thoratec ventricular assist system in bridge to transplantation. Ann Thorac Surg. 2001;71:S92-S97.
142 Minami K, El-Banayosy A, Sezai A, et al. Morbidity and outcome after mechanical ventricular support using Thoratec, Novacor, and HeartMate for bridging to heart transplantation. Artif Organs. 2000;24:421-426.
143 Potapov EV, Stepanenko A, Kukucka M, Ba Fadhl FH, Qedra N, Weng Y, et al. Prediction of Survival in Patients With Cardiogenic Shock and Multiorgan Failure Treated With Biventricular Assist Device. ASAIO J. 2010 Jun 16. [Epub ahead of print]
144 Drews T, Loebe M, Hennig E, et al. The “Berlin Heart” assist device. Perfusion. 2000;15:387-396.
145 DeRose JJJr, Umana JP, Argenziano M, et al. Improved results for postcardiotomy cardiogenic shock with the use of implantable left ventricular assist devices. Ann Thorac Surg. 1997;64:1757-1762.
146 Robbins RC, Oyer PE. Bridge to transplant with the Novacor left ventricular assist system. Ann Thorac Surg. 1999;68:695-697.
147 Dagenais F, Portner PM, Robbins RC, et al. The Novacor left ventricular assist system: Clinical experience from the Novacor registry. J Card Surg. 2001;16:267-271.
148 Murali S. Mechanical circulatory support with the Novacor LVAS: Worldwide clinical results. Thorac Cardiovasc Surg. 1999;47(Suppl 2):321-325.
149 El-Banayosy A, Arusoglu L, Kizner L, et al. Novacor left ventricular assist system versus Heartmate vented electric left ventricular assist system as a long-term mechanical circulatory support device in bridging patients: A prospective study. J Thorac Cardiovasc Surg. 2000;119:581-587.
150 Portner PM, Jansen PG, Oyer PE, et al. Improved outcomes with an implantable left ventricular assist system: A multicenter study. Ann Thorac Surg. 2001;71:205-209.
151 Strauch JT, Spielvogel D, Haldenwang PL, et al. Recent improvements in outcome with the Novacor left ventricular assist device. J Heart Lung Transplant. 2003;22:674-680.
152 Berman M, Parameshwar J, Jenkins DP, et al. Thoratec implantable ventricular assist device: the Papworth experience. J Thorac Cardiovasc Surg.. 2010;139(2):466-473.
153 Song X, Throckmorton AL, Untaroiu A, et al. Axial flow blood pumps. ASAIO J. 2003;49:355-364.
154 Goldstein DJ. Worldwide experience with the MicroMed DeBakey Ventricular Assist Device as a bridge to transplantation. Circulation. 2003;108:II272-II277.
155 Anderson DW. Blood pumps: Technologies and markets in transformation. Artif Organs. 2001;25:406-410.
156 Noon GP, Morley D, Irwin S, et al. Turbine blood pumps. Adv Card Surg. 2001;13:169-191.
157 Frazier OH, Myers TJ, Westaby S, et al. Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg. 2003;237:631-636.
158 Westaby S, Frazier OH, Beyersdorf F, et al. The Jarvik 2000 Heart. Clinical validation of the intraventricular position. Eur J Cardiothorac Surg. 2002;22:228-232.
159 Cooley DA. Initial clinical experience with the Jarvik 2000 implantable axial-flow left ventricular assist system. Circulation. 2002;105:2808-2809.
160 Siegenthaler MP, Martin J, van de Loo A, et al. Implantation of the permanent Jarvik-2000 left ventricular assist device: A single-center experience. J Am Coll Cardiol. 2002;39:1764-1772.
161 Griffith BP, Kormos RL, Borovetz HS, et al. HeartMate II left ventricular assist system: From concept to first clinical use. Ann Thorac Surg. 2001;71:S116-S120.
162 Burke DJ, Burke E, Parsaie F, et al. The Heartmate II: Design and development of a fully sealed axial flow left ventricular assist system. Artif Organs. 2001;25:380-385.
163 Frazier OH, Myers TJ, Gregoric ID, et al. Initial clinical experience with the Jarvik 2000 implantable axial-flow left ventricular assist system. Circulation. 2002;105:2855-2860.
164 Slaughter MS, Sibieski MA, Tamez D, et al. HeartWare miniature axial-flow ventricular assist device: design and initial feasibility test. Tex Heart Inst J. 2009;36:12-16.
165 Cooper LTJr, Berry GJ, Rizeq M, et al. Giant cell myocarditis. J Heart Lung Transplant. 1995;14:394-401.
166 Davies RA, Veinot JP, Smith S, et al. Giant cell myocarditis: Clinical presentation, bridge to transplantation with mechanical circulatory support, and long-term outcome. J Heart Lung Transplant. 2002;21:674-679.
167 Tsai FC, Marelli D, Laks H, et al. Short-term bridge to heart transplant using the BVS 5000 external ventricular assist device. Am J Transplant. 2002;2:646-651.
168 Marelli D, Kermani R, Bresson J, et al. Support with the BVS 5000 assist device during treatment of acute giant-cell myocarditis. Tex Heart Inst J. 2003;30:50-56.
169 Goldstein DJ, Oz MC. Mechanical support for postcardiotomy cardiogenic shock. Semin Thorac Cardiovasc Surg. 2000;12:220-228.
170 Carnendran L, Abboud R, Sleeper LA, et al. Trends in cardiogenic shock: Report from the SHOCK Study. The SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? Eur Heart J. 2001;22:472-478.