Chapter 52 Maxillofacial surgical techniques for hypopharyngeal obstruction in obstructive sleep apnea
1 INTRODUCTION
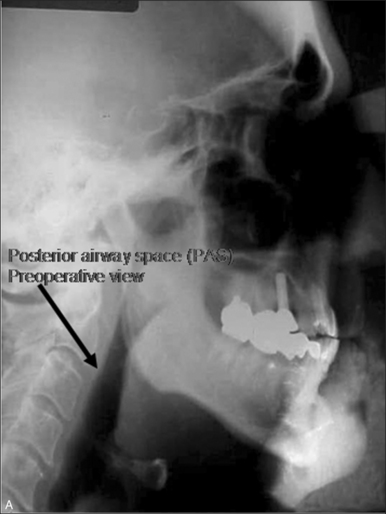
Due to poor patient acceptance of tracheostomy and incomplete treatment of obstructive sleep apnea syndrome (OSA) with palatal surgery, we have explored the use of maxillofacial surgical techniques to alleviate hypopharyngeal obstruction.1,2 The aim of surgical treatment is to alleviate upper airway obstruction and its associated neurobehavioral symptoms and morbidities.3,4 No longer is a 50% reduction in the Apnea/Hypopnea Index (AHI) deemed acceptable. Rather, the objective is to treat to cure (normalization of respiratory events and elimination of hypoxemia) and achieve outcomes that are equivalent to those of continuous positive airway pressure (CPAP).5
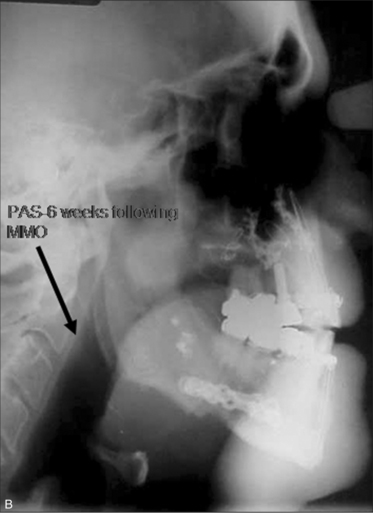
Selecting the appropriate surgery for a patient can be challenging. However, we have created a two-phase surgical protocol (Powell–Riley protocol) as a logically directed plan to treat the specific areas of upper airway obstruction.6,7 It is imperative that a polysomnogram (PSG) be obtained after any surgery to determine efficacy. Genioglossus advancement and hyoid suspension are considered part of phase I surgery. These are conservative maxillofacial techniques which are often combined with nasal and palatal surgery, should multilevel obstruction exist. Phase II surgery refers to maxillomandibular advancement (MMO). This surgery is usually reserved for those patients who have been incompletely treated with phase I surgery and have documented hypopharyngeal obstruction. MMO is the only surgery which physically creates more room for the tongue by advancing both the mandible and maxilla.
2 PATIENT SELECTION
Proper selection of patients is paramount to achieve successful outcomes and to minimize postoperative complications. The presurgical evaluation requires a comprehensive medical history, head and neck examination, fiberoptic nasopharyngolaryngoscopy, radiographic cephalometric analysis and polysomnography (PSG). This systematic approach will aid in determining the severity of OSA and identifying probable anatomic sites of obstruction. Armed with this information, the surgeon can proceed with a site-specific surgical protocol.8
Since CPAP is considered the primary treatment of OSA, all patients should be offered this therapy prior to surgical intervention. While the efficacy of CPAP has been clearly documented, a subset of patients struggle to comply with CPAP therapy.9 In fact, Kribbs et al. reported that only 6% of patients who receive CPAP therapy are able to tolerate the device for at least 7 hours per night on 70% of days.10
Our protocol encourages all patients to use their CPAP for 2 weeks prior to surgery, particularly those patients with severe OSA (AHI > 40). Preoperative CPAP can alleviate the risks associated with sleep deprivation and postobstructive pulmonary edema. Furthermore, we recommend the insertion of a temporary tracheostomy in patients with an AHI > 60 and/or a SaO2<70% who are intolerant of CPAP.11
Despite a thorough preoperative selection process, it is difficult to accurately predict outcomes for an individual patient. While MMO has documented success rates >90%, our studies have demonstrated that a substantial number of patients may not need such extensive surgery.12–14 In fact, approximately 70% of our patients have obviated the need for MMO by undergoing phase I surgery.12 Thus, we employ conservative maxillofacial surgical techniques (genioglossus advancement or hyoid suspension) to treat hypopharyngeal obstruction, before proceeding to MMO. There are cases in which MMO may be the initial surgery, as in non-obese patients with marked mandibular deficiency and a normal palate.
3 MANDIBULAR OSTEOTOMY WITHGENIOGLOSSUS ADVANCEMENT
When the preoperative evaluation implicates the base of tongue (Fujita Type II–III) as the source of airway obstruction, the genioglossus advancement (GA) procedure is indicated. This procedure is a conservative maxillofacial technique since the osteotomy is created without altering the dental occlusion. The rationale for the GA procedure is to place the primary protrusion muscle of the tongue, the genioglossus, under direct tension. This tension resists the collapse of the tongue into the airway that can occur with sleep-induced hypotonia.15 Essentially, the genial tubercle and the attached genioglossus muscle are advanced anteriorly. The degree of advancement depends on the thickness of the anterior mandible and the compliance of the genio-glossus muscle. Less muscle compliance will provide a greater degree of tension. Unfortunately, there is no objective preoperative study to determine the compliance of the genioglossus muscle.
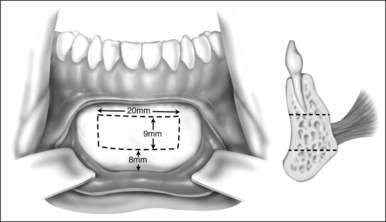
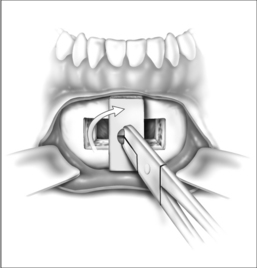
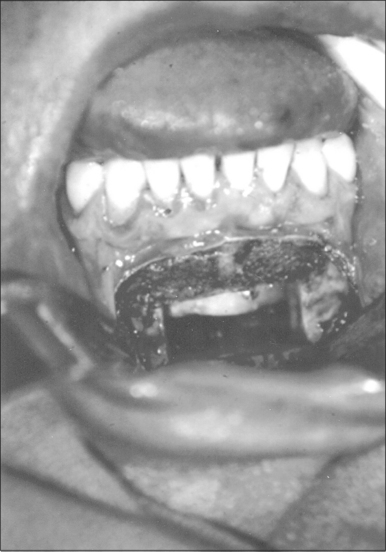
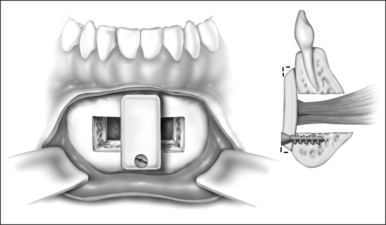
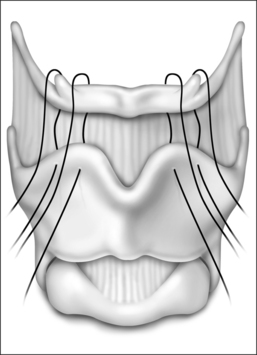
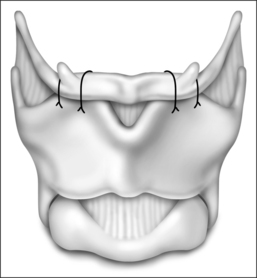
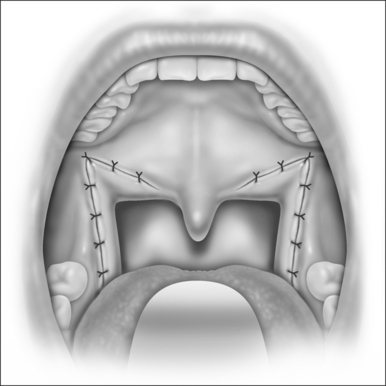
Surgery may be performed under general anesthesia or, in appropriate cases, with the patient under intravenous sedation. An intraoral incision is made in the gingivolabial sulcus, taking care to preserve a cuff of mucosa to facilitate closure and prevent wound dehiscence. The incision extends between the canine dentition. Dissection is performed submucoperiosteally to expose the inferior border of the mandible. The floor of the mouth is palpated to identify the location of the genial tubercle. A rectangular osteotomy is then outlined along the outer cortex of the mandible with a 2-hole sagittal saw blade. The osteotomy is typically 9 mm×20 mm in order to capture the genial tubercle. It is recommended that the superior osteotomy be placed at least 5 mm inferior to the root apices to avoid injury.16 In addition, the inferior osteotomy should be approximately 8 mm above the inferior border of the mandible to avoid a potential fracture (Fig. 52.1). A titanium screw is inserted in the center of the osteotomy to allow manipulation of the fragment (Fig. 52.2). The cuts are then completed through the inner cortex. It is critical to maintain parallel walls while performing the osteotomies to prevent tapering of the inner cortex. The fragment is displaced into the floor of the mouth and hemostasis is achieved (Fig. 52.3). The titanium screw is then grasped and the bony fragment is gently advanced and rotated 90° in either direction (Fig. 52.4). The outer cortex and marrow are removed with the sagittal saw. A 10 mm×2.0 mm titanium screw is used to fix the fragment to the mandible inferiorly (see Fig. 52.4). A pear-shaped cutting burr may be used to contour the bony fragment. Lastly, the wound is irrigated and closed with interrupted absorbable sutures (Fig. 52.5).
4 HYOID MYOTOMY WITH SUSPENSION
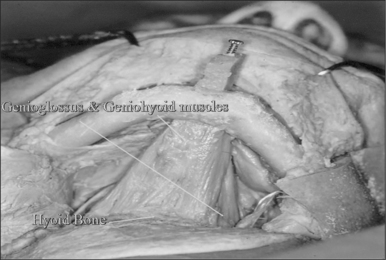
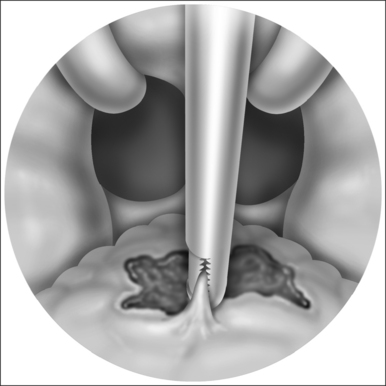
The surgical approach for hyoid suspension is similar to thyroglossal duct excision (Sistrunk procedure). Either general or local anesthesia may be utilized. A horizontal incision is placed at the level of the hyoid bone along a natural skin crease. Dissection is continued to expose the body of the hyoid. The infrahyoid musculature is detached. The stylohyoid ligament may need to be sectioned from the lesser cornu to allow adequate mobility, but the suprahyoid musculature remains intact. The strap muscles are divided in the midline to expose the thyroid cartilage (Fig. 52.6). An Allis clamp is placed on the body of the hyoid and the hyoid complex is advanced anteriorly. Constant traction is maintained on the hyoid bone. Three or four #1-0 permanent sutures are passed around the hyoid and through the superior aspect of the thyroid cartilage to advance and stabilize the hyoid bone (Fig. 52.7). Meticulous hemostasis is achieved. A drain is placed and the wound is closed in layers. Placement of a pressure dressing for 24 hours reduces the incidence of hematoma or seroma formation.
5 MAXILLOMANDIBULAR ADVANCEMENTOSTEOTOMY (MMO)
Diligent planning in the preoperative period is critical to achieve successful outcomes. Consequently, we fashion a methylmethacrylate splint several weeks prior to surgery to maintain proper dental occlusion postoperatively. Furthermore, we ask all patients to provide one unit of autologous blood in case a transfusion is needed. The surgery is performed under general anesthesia via nasotracheal intubation. It is essential that the surgeon be present during induction of anesthesia in case an airway emergency arises. Frequently, sleep apnea patients can be difficult to intubate, so proper communication between the anesthesiologist and surgeon can expedite this process. 1% Lidocaine with 1:100,000 epinephrine is used to inject the maxillary vestibule and the gingivolabial sulcus overlying both the maxillary and mandibular arches. Vasoconstriction of the nasal mucosa is achieved by placing cottonoids soaked with 4% cocaine hydrochloride along the floor of the nose. The table is rotated 180° away from the anesthesiologist and the patient is sterilely prepped and draped.
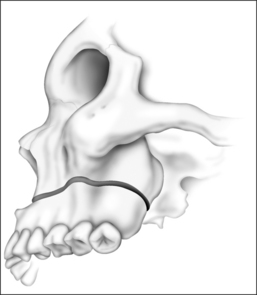
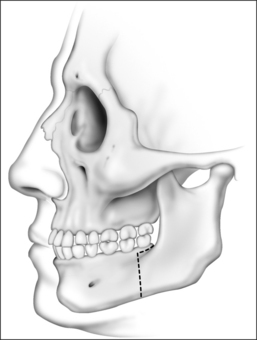
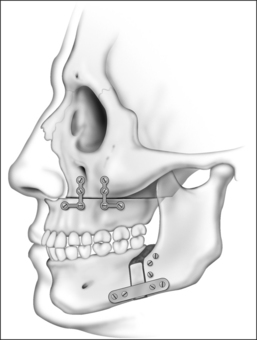
An incision is placed in the gingivolabial sulcus of the maxilla between the second molars. The periosteum is elevated with a periosteal elevator to visualize the root of the zygoma laterally. Care is taken to preserve the infraorbital nerve. A Freer elevator is used to elevate the periosteum of the piriform aperture and floor of the nose. Bleeding commonly occurs while elevating the soft tissue of the piriform aperture and nasal floor. Hemostasis is achieved by placing cottonoids in this dissection plane. The Le Fort I osteo-tomy is then performed. A reciprocating saw is inserted at the level of the piriform aperture and the osteotomy is carried laterally through the zygomatic maxillary crest to the pterygomaxillary suture. The osteotomy must be placed at a vertical height that will avoid the apices of the teeth. A fenestration is created in the anterior maxillary sinus wall with a pear-shaped cutting burr. This is performed at the level of the osteotomy. A thin osteotome is placed through this fenestration to cut the posterior and medial walls of the maxillary sinus. The osteotomy is completed by placing a curved osteotome into the pterygomaxillary suture to separate the maxilla from the pterygoid plates (Fig. 52.8). This process is completed on the contralateral aspect of the maxilla. An osteotome is used to separate the nasal septum from the maxillary crest. A 24-gauge wire is passed through the anterior nasal spine to allow manipulation of the maxilla. Using gentle, constant pressure the maxilla is down fractured. The descending palatine arteries are identified and, if possible, preserved. If necessary, the arteries may be ligated using vascular clips. Riley–Powell distraction forceps are utilized to mobilize the maxilla anteriorly. While mobilizing the attachments of the pterygoid plates, be careful not to tear the mucosa. It is critical to completely mobilize these attachments to allow for the 10–12 mm of advancement that is needed. The maxilla is advanced and rigid fixation is accomplished with two 24-gauge wires and two titanium mini plates (2.0 mm plates) on each side of the maxilla.
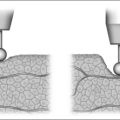
Attention is then turned to the mandible. Advancement is achieved by a sagittal split osteotomy. An incision is made in the mucosa along the external oblique ridge. The incision extends from the third molar to the first molar. Dissection is carried down to the periosteum. The periosteum is reflected to expose the medial and lateral aspects of the mandibular ramus. A V-shaped retractor is used to reflect the periosteum and the muscular attachments of the mandible superiorly to the level of the coronoid process. Subperiosteal dissection continues along the medial border of the ramus in order to identify the lingula. A nerve hook can be used to palpate for the lingula, if it cannot be easily visualized. Identification of the lingula allows one to protect the inferior alveolar neurovascular bundle. A pear-shaped cutting burr is used to create a trough just above and lateral to the lingula (Fig. 52.9). This permits the insertion of a reciprocating saw which carries the sagittal osteotomy to the level of the first molar. Care is taken to stay 5 mm lateral to the molar teeth to prevent injury to the roots. Only the outer cortex is cut with the reciprocating saw. The vertical osteotomy is performed along the inferior border of the mandible at the mesial surface of the first molar with a fissure burr. A channel retractor is very effective in retracting and protecting the soft tissue along the inferior border of the mandible. It is recommended that both the inner and outer cortices of the mandible are cut at the inferior border. Both cortices are cut for approximately 5 mm, at which point the cut only includes the outer cortex as the vertical osteotomy meets the sagittal component (Fig. 52.10). The osteotomy is then completed using osteo-tomes to gently separate the medial and lateral segments. Once again, the inferior alveolar nerve must be identified and preserved. If necessary, the nerve may need to be dissected from its canal to allow advancement of the distal segment. After a sagittal split osteotomy is performed on the contralateral aspect of the mandible, the distal segments can be advanced. The dental splint, which was fashioned preoperatively, is inserted to ensure proper occlusion. IMF is accomplished with 24-gauge stainless steel wires. Rigid fixation is achieved with percutaneous bicortical titanium screws placed in the proximal segments. Additionally, 2.3 mm titanium plates are used to rigidly fix the advanced distal segments of the mandible (Figs 52.11 and 52.12). The IMF wires are released and the patient’s occlusion is evaluated. If the occlusion is not correct, the fixationhardware will need to be readjusted to correct this problem. Previously harvested bone grafts may be placed in theosteotomy sites, if the surgeon deems this necessary.Cranial bone grafts may be used; however, more recently, we have been using bone grafts obtained from the maxilla. This reduces the operative time and potential morbidity associated with harvesting cranial bone grafts.17 All intraoral incisions are closed with interrupted 4-0 Vicryl. The percutaneous incisions are closed with interrupted 5-0chromic. Rubber bands may be placed on the arch bars to limit jaw opening in the early postoperative period. The upper aerodigestive tract is suctioned. A head wrap is placed for 24 hours to prevent hematoma formation. Finally, the anesthesia is reversed but the patient is not extubated until it is clear that they have the ability to maintain their airway.
6 POSTOPERATIVE MANAGEMENT ANDCOMPLICATIONS
Many factors can complicate the postoperative management of OSA patients. Commonly, patients have a medical condition (hypertension, arrhythmias) which increases the risk of surgery. However, preventing airway obstruction remains the priority. Sleep apnea patients already have a compromised airway. The added surgical edema, muscle atonia and respiratory depression from general anesthesia and narcotics further jeopardize the airway. Thus, we have implemented a risk management protocol to ensure patient safety.18,19
Admission to the ICU overnight is indicated for patients with comorbid medical conditions, moderate to severe OSA, multilevel surgery and MMO. Continuous pulse oximetry and hemodynamic monitoring are mandatory. Furthermore, we use CPAP immediately after surgery to reduce edema and prevent REM rebound sleep.19
The requirements for discharge from the hospital include reasonable pain control, adequate oral intake and a patent airway. We perform fiberoptic laryngoscopy on all patients prior to discharge to confirm a stable airway.20 If obstruction is noted, the patient is observed in the hospital until this resolves.
Mandible fractures can occur as a result of genioglossus advancement surgery. Any technique which violates the inferior border of the mandible increases the risk of fracture. While the rectangular osteotomy technique significantly reduces this risk, a fracture can occur if the osteotomy is poorly designed.21 Avulsion of the genioglossus muscle is perhaps the most worrisome complication and may not be correctable. Minor complications, such as wound infection, anesthesia of the teeth or lower lip and root injury requiring endodontic therapy, have incidences rates of 2%, 6% and 1% respectively, at our center.
Hyoid suspension is rarely associated with major complications. While injury to the superior laryngeal or hypoglossal nerve is possible, we have not encountered this problem. Seroma formation can occur; however, the placement of surgical drains and pressure dressings has markedly reduced this complication. Transient dysphagia or aspiration can be observed but it usually resolves within 10 days. If these symptoms persist, removal of the suspension sutures should alleviate this problem.22
Injury to the descending palatine arteries can occur during the Le Fort I osteotomy. Although rare, avascular necrosis of the palate can occur. If there is any indication that the blood supply to the maxilla is inadequate, the surgeon should place the maxilla in its preoperative position. Injury to the inferior alveolar nerve can cause permanent anesthesia of the dentition and perioral region following sagittal split osteotomy. Typically, the anesthesia is transient and self-limited as a result of edema of the neuro-vascular bundle. Dental malocclusion may occur despite the use of a dental splint. Usually this can be managed with occlusal equilibration. More complicated problems may warrant orthodontic treatment or surgical correction.22
7 SUCCESS RATE
As noted previously, genioglossus advancement and hyoid suspension are considered part of phase I surgery. Furthermore, a majority of our patients have multilevel pharyngeal obstruction, which necessitates treating the palate synchronously with the tongue base. Rarely do we perform hyoid suspension or genioglossus advancement as isolated procedures. Exceptions would include those patients who do not have palatal obstruction or those who have previously undergone palatal surgery. Our published data have shown that approximately 70% of patients are cured with phase I surgery.12
Incomplete treatment with phase I surgery is related to persistent hypopharyngeal obstruction. Those patients who have been incompletely treated would then be considered for phase II surgery (MMO). Maxillomandibular advancement osteotomy has documented success rates >90%.12–14 Temperature-controlled radiofrequency of the tongue base or tracheostomy are the only other surgical modalities available if a patient should be incompletely treated with MMO.
1. Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156-177.
2. Riley RW, Powell NB. Maxillofacial surgery and obstructive sleep apnea syndrome. Otolaryngol Clin North Am. 1990;23:809-826.
3. Dincer HE, O’Neill W. Deleterious effects of sleep-disordered breathing on the heart and vascular system. Respiration. 2006;73:124-130.
4. Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034-2041.
5. Riley RW, Powell NB, Guilleminault C. Maxillofacial surgery and nasal CPAP: a comparison of treatment for obstructive sleep apnea syndrome. Chest. 1990;98:1421-1425.
6. Riley R, Powell N, Guilleminault C, et al. Obstructive sleep apnea syndrome: a surgical protocol for dynamic upper airway reconstruction. J Oral Maxillofac Surg. 1993;51:742-747.
7. Powell NB, Riley RW. A surgical protocol for sleep disordered breathing. Oral Maxillofac Surg Clin North Am. 1995;7:345-356.
8. Powell NB, Riley RW, Guilleminault C. Surgical management of sleep-disordered breathing. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practices of Sleep Medicine. 4th edn. Philadelphia: Elsevier Saunders; 2005:1081-1097.
9. Sin DD, Mayers I, Man GC, et al. Long-term compliance rates of CPAP in obstructive sleep apnea: a population-based study. Chest. 2002;121:430-435.
10. Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887-895.
11. Powell N, Riley R, Guilleminault C, et al. Obstructive sleep apnea, continuous positive airway pressure, and surgery. Otolaryngol Head Neck Surg. 1988;99:362-369.
12. Riley R, Powell N, Guilleminault C. Obstructive sleep apnea syndrome: a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg. 1993;108:117-125.
13. Waite PD, Wooten V, Lachner J, et al. Maxillomandibular advancement surgery in 23 patients with obstructive sleep apnea syndrome. J Oral Maxillofac Surg. 1989;47:1256-1261.
14. Hochban W, Brandenburg U, Hermann PJ. Surgical treatment of obstructive sleep apnea by maxillomandibular advancement. Sleep. 1994;17:624-629.
15. Troell RJ, Powell NB, Riley RW. Hypopharyngeal airway surgery for obstructive sleep apnea syndrome. Semin Respir Crit Care Med. 1998;19:175-183.
16. McBride KL, Bell WH. Chin surgery. In: Bell WH, Proffit WR, White RP, editors. Surgical Correction of Dentofacial Deformities. 1st edn. Philadelphia: WB Saunders; 1980:1210-1281.
17. Powell NB, Riley RW. Facial contouring with outer table calvarial bone. Arch Otolaryngol Head Neck Surg. 1989;115:1454-1458.
18. Riley RW, Powell NB, Guilleminault C, et al. Obstructive sleep apnea surgery: risk management and complications. Otolaryngol Head Neck Surg. 1997;117:648-652.
19. Li KK, Powell N, Riley R. Postoperative management of the obstructive sleep apnea patient. Oral Maxillofac Surg Clin N Am. 2002;14:401-404.
20. Li KK, Riley RW, Powell NB, et al. Fiberoptic nasopharyngoscopy for airway monitoring following obstructive sleep apnea surgery. J Oral Maxillofac Surg. 2000;58:1342-1345.
21. Riley R, Powell N, Guilleminault C. Inferior sagittal osteotomy of the mandible with hyoid myotomy–suspension: a new procedure for obstructive sleep apnea. Otolaryngol Head Neck Surg. 1986;94:589-593.
22. Li KK, Riley R, Powell N. Complications of obstructive sleep apnea surgery. Oral Maxillofac Surg Clin N Am. 2003;15:297-304.