Chapter 17 Maxillofacial Injuries
Many primary care physicians are in the position of initial responsibility for the multicomplex injured patient. Although damage to thoracic or abdominal structures is often more easy to recognize, the ability to identify maxillofacial injuries is important for the complete evaluation of any trauma patient. Although not lethal per se, undiagnosed facial fractures may have potentially lethal complications or may produce contour deformities with or without functional disabilities. Automobile accidents are the most frequent cause of maxillofacial injuries and represent a high-velocity type of injury. Other modes of injury include motorcycle accidents, fistfights, sports, falls, bicycle accidents, and convulsive disorders. Identification of the cause is important, because one third of patients with maxillofacial injuries caused by motor vehicle accidents have associated life-threatening cranial, pulmonary, or intraabdominal injuries. About one third also have accompanying nonlethal injuries, such as extremity fractures or eye loss. On the other hand, patients with maxillofacial injuries secondary to low-velocity causes (assaults or falls) have a markedly decreased incidence of associated injury: life-threatening (4%) and nonlethal (10%).
Associated Injuries
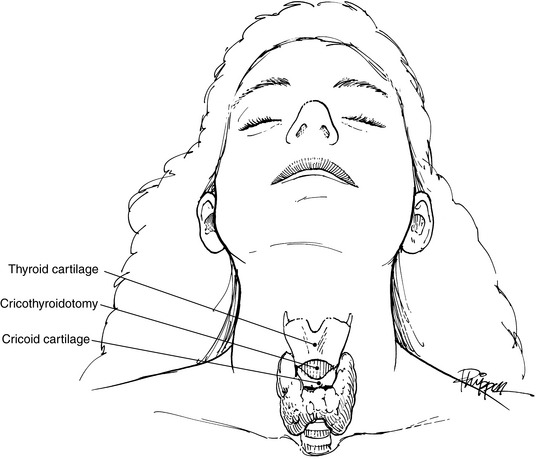
Airway obstruction with subsequent hypoxemia can easily develop in the patient with a maxillofacial fracture. Blood clots, broken teeth or dentures, and foreign bodies, such as dirt or glass, can physically obstruct the airway. The posterior displacement of the tongue secondary to the patient’s position or to a mandibular fracture may occlude the airway. Other potential causes include glossopharyngeal edema and expanding hematoma. In all situations, a patent airway must take immediate priority. Sweeping debris from the oropharynx and mouth by using one’s finger may be a lifesaving technique. Suction, if available, is helpful. Simple traction on a posteriorly displaced tongue by suture or towel clip may alleviate obstruction. If these methods fail, oral intubation must be instituted. If facial edema, facial fractures, or cervical spine fractures prevent oral or nasal intubation, a cricothyroidotomy can be performed through the membrane between the thyroid and cricoid cartilages (Fig. 17-1). This site is a bloodless field, and the procedure can easily be done in the emergency department with only a scalpel. Later, an elective lower tracheotomy can be performed under controlled circumstances in the operating room. A low tracheotomy performed in the emergency department may be very hazardous and should be avoided.
Hemorrhage from open wounds can be controlled most easily by pressure dressings consisting of layers of gauze (Kerlix) and elastic bandages. Occasionally, an active bleeder in a facial wound may need to be clamped and ligated. However, blind clamping of possible bleeding sites is condemned because of the high incidence of iatrogenic complications, such as facial nerve damage. Nasal hemorrhage may require packing. Shock occurs very seldomly from an isolated maxillofacial injury and most commonly results from a thoracic or abdominal injury.
Examination and Diagnosis
An accurate history should be obtained whenever possible from the patient and/or witnesses at the scene of the accident. The type of accident, the patient’s position in the car, the use of safety belts, the mode of impact, and the patient’s condition at the time of injury are all important considerations in the initial assessment. Because alcohol is involved in 50% of automobile accidents, a blood alcohol sample should be drawn. Ingestion of other drugs should be considered by an appropriate drug screen analysis. A review of the patient’s past history should include other illnesses, previous surgery, allergies, and all current medications.
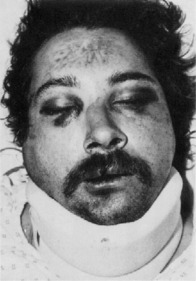
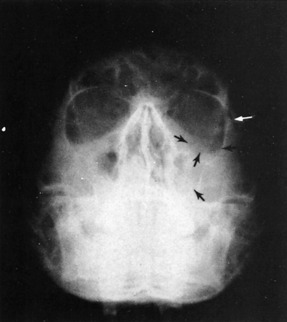
A diagnosis of facial bone injury can be established by three methods: observation, palpation, and radiologic evaluation. Moderate to severe facial edema may mask bony irregularities and asymmetries (Fig. 17-2). After resolution of the edema, facial asymmetry is suggestive of an underlying fracture. Light manual palpation is important in making the initial diagnosis.
The Waters’ view is the single most informative roentgenogram in evaluation of the maxillofacial patient in the emergency department (Fig. 17-3). This study visualizes the floor and rims of the orbits, the walls of the sinuses, the zygomatic bones, the zygomatic arches, and the nasal septum with minimal interference of other bony structures. Opacity of a maxillary sinus suggests hemorrhage as a result of an orbital ridge and/or floor fracture. The Waters’ view requires the cooperation of the patient and a normal cervical spine, however, because the patient must be in the prone position during the examination. If the patient is comatose, uncooperative, or suspected of having a cervical fracture, a reverse Waters’ view with the patient in the supine position is a satisfactory substitute, because it gives almost the same level of detailed information. Other films worth consideration in the emergency department are the submental vertex view of the zygomatic arches and a mandible series. More sophisticated and detailed studies can be obtained later during the hospitalization or on an outpatient basis for isolated facial fractures.
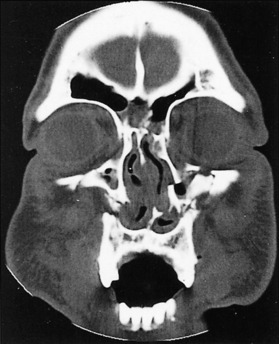
Since the development of computed tomography (CT) scanning, it has become essential for the diagnosis of facial trauma. The CT scan is a more accurate diagnostic study and allows an evaluation of complicated facial fractures before the resolution of edema. Additionally, injuries to the soft tissue structures in the area of trauma can be better evaluated— for example, the optic nerve or orbital herniation of the orbit (Fig. 17-4).
The only disadvantage to the use of the CT scan is seen when artifacts caused by either dental fillings or metal appliances occur. Radiation is not considered a disadvantage; CT exposes the patient to a radiation dosage equal to the amount from linear tomography. Studies suggest that the amount of radiation from either study is less than the amount needed to cause cataracts.
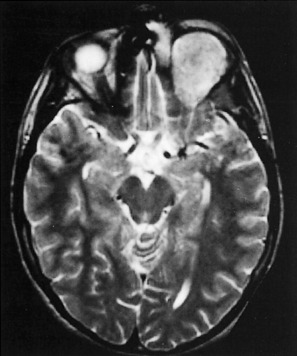
The role of magnetic resonance imaging (MRI) in the craniofacial injury patient is primarily confined to evaluation of soft tissue trauma. When injuries to the ocular structures are noted and disturbances of vision are present, MRI may help localize the site of the injury (Fig. 17-5).
Fractures of the Mandible
Although it is the thickest and heaviest of the facial bones, the mandible is the second-most commonly fractured (after nasal bones). Mandibular fractures may occur as isolated injuries or as components of complex maxillary and mandibular fractures. The most common causes of mandibular fractures are acts of violence that may range from simple falls to motor vehicle accidents. Occasionally, systemic diseases such as hyperparathyroidism and osteomalacia may predis pose to mandibular fractures. Infrequently, benign or malignant tumors, cysts, or osteomyelitis may precipitate such fractures.
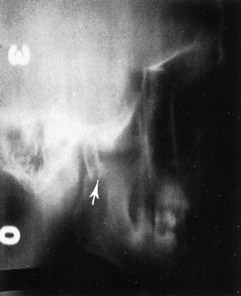
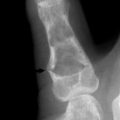
Although the clinical examination most frequently establishes the diagnosis of mandibular fractures, roentgenographic studies more clearly define the direction of the fracture line, the relationship of the teeth to the fracture, and the degree of displacement. Posteroanterior and oblique lateral views of the mandible demonstrate fractures of the body and the angle without difficulty. If available, a Panorex view of the mandible is an excellent and necessary study that shows fractures at any site. Fractures of the temporomandibular joint and condylar area are sometimes difficult to demonstrate on routine mandibular roentgenograms, however, and may require tomograms for the final diagnosis (Fig. 17-6).
The principles of treatment for mandibular fracture include early anatomic reduction of the fracture, immobilization, and control of infection. An isolated mandibular fracture should be reduced at the time of injury. However, if other life-threatening injuries are present, treatment of the mandibular injury may be postponed for 7 to 10 days. All mandibular fractures are considered compound if the slightest displacement is present, and consequently, preoperative and postoperative antibiotics are recommended. Immobilization requires at least the application of arch bars and intermaxillary fixation (IMF) with rubber bands or wires for a minimum of 5 weeks. Rigid fixation with plates or screws is an option. Because IMF is not usually required after rigid stabilization, patients are able to begin a soft diet almost immediately after open reduction. Consequently, the weight loss and compromised oral hygiene present in patients with IMF are markedly reduced. The use of compression plates has also made the treatment of edentulous patients with mandible fractures much simpler.
Fractures of the Maxilla
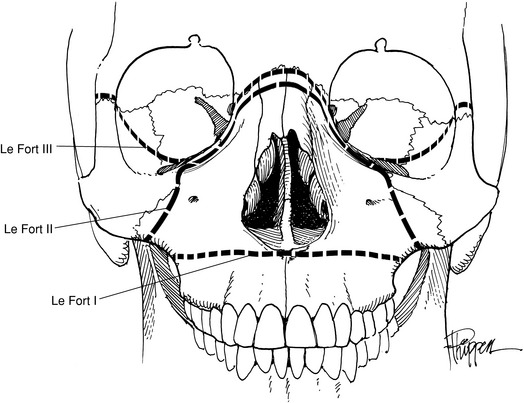
Maxillary fractures can be divided into two groups: vertical and horizontal. Vertical fractures split the palate on either side of the septum. However, the three classic fractures of the maxilla are those horizontal defects described by Le Fort (Fig. 17-7). The Le Fort I (transverse) fracture is a horizontal fracture immediately above the level of the teeth. The Le Fort II fracture has the configuration of a pyramid, with the apex being across the nasal bridge. It extends through the nasal bones, the frontal processes of the maxilla, the lacrimal bones, the inferior rim and floor of both orbits, and the maxillozygomatic suture line. From this last point, the fracture continues posteriorly through the lateral wall of the maxilla and the pterygoid plates into the pterygoid maxillary fossa. The Le Fort III fracture separates the craniofacial complex and extends through the zygomaticofrontal, maxillofrontal, and nasofrontal suture lines, the floors of the orbit, and the ethmoid and sphenoid bones (Fig. 17-8).
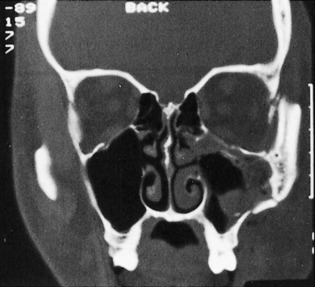
Roentgenographically, the Waters’ view is the most reliable in demonstrating maxillary fractures in the emergency department. For vertical or alveolar fractures, occlusal views are more suitable. CT scans of the facial bones more accurately detail the full extent of the fractures. The study is usually done in the coronal view and shows the amount of the comminution, the degree of rotation, and the extent of displacement of the fracture segments (Figs. 17-9 and 17-10). Additionally, injury to the optic nerve or fat herniation may be visible.
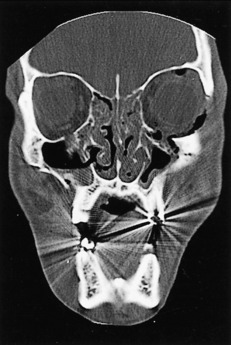
Fig. 17-10 A more posterior CT scan view of the fractures in Fig. 17-9 reveals extensive orbital floor fractures.
Initially, the airway may be compromised by the posterior displacement of the maxillary fractures or by the combination with mandibular fractures. An endotracheal tube is the preferred treatment, although a cricothyroidotomy occasionally may be necessary.
Fractures of the Zygoma
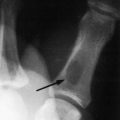
The zygoma articulates with the maxillary, frontal, and temporal bones. Injury to it may cause a separation at the suture lines in an isolated injury or may be combined with fractures of the middle third of the face. If displaced, the zygoma is depressed in the direction of the traumatic force, which most commonly is in the posterior, downward, and medial direction. Although six different groups of malar fractures have been described, fractures of the zygoma are basically either an isolated zygomatic arch fracture (Fig. 17-11) or the “tripod” fracture (Fig. 17-12).
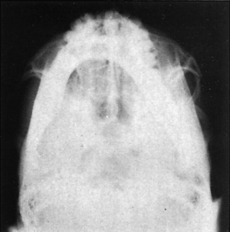
Fig. 17-11 A markedly depressed left zygomatic arch fracture is demonstrated by a submental-vertex view.
Clinically, the patient may complain of pain in the area of the zygomatic arch when attempting to open the mouth. Infraorbital nerve anesthesia may be present in both the ipsilateral cheek and the maxillary gingiva (gingival numbness suggests orbital floor and rim fractures). Diplopia with upward gaze may also be noted.
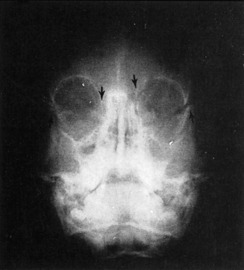
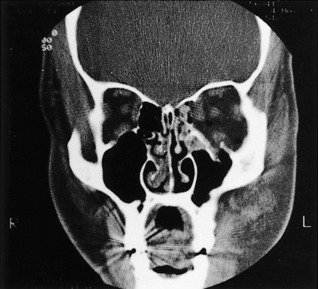
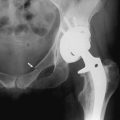
The best roentgenographic studies obtainable from the emergency department are the Waters’ view of the facial bones and the submental-vertex view of the zygomatic arches. Displacement of the malar fragment and/or opacity in the maxillary sinus is readily visible on a Waters’ view. A CT scan of the orbits must be obtained to further delineate the extent of the fractures (Fig. 17-13).
Surgical reduction of the isolated zygomatic arch fracture can be done either intraorally or extraorally on an outpatient basis. After reduction of the bony fragments, stabilization is secured by placement of plates across the fracture lines at two or three sites. Fractures of the zygoma should be reduced within 3 weeks of injury.
Blow-Out Fractures
During examination, the patient may volunteer the presence of diplopia, infraorbital nerve numbness, and possibly, decreased vision. Clinically, periorbital edema and ecchymosis may handicap the initial examination. but the eyelids can usually be pried open to allow a gross examination of vision and light perception. Diplopia may occur as a result of limitation of superior gaze (Fig. 17-14). As the periorbital edema resolves, diplopia may lessen in severity. If the periorbital edema is minimal, enophthalmos may be present.
The most common causes of diplopia in blow-out fractures are entrapment of the inferior rectus muscle, inferior oblique muscle, or periorbital fat herniation. Other causes of immediate diplopia include injury to cranial nerves III, IV, and VI; direct injury or hemorrhage into the extraocular muscles; or displacement of the eyeball into the maxillary sinus. The “traction test” simplifies the differential diagnosis. Topical anesthesia is applied to the conjunctiva. The eyeball is pinched with a fine forceps at the insertion of the inferior rectus muscle and rotated. If rotation of the eyeball cannot be accomplished, entrapment of the inferior rectus muscle is demonstrated. If rotation of the eyeball is achieved, then the diplopia can be traced to one of the other causes.
The most pertinent roentgenographic studies are a Waters’ view initially, followed by a CT scan. A coronal CT scan is the most accurate in diagnosing this defect. However, its use may be limited by the positioning requirements of the patient or by the presence of multiple dental fillings. Abnormal findings include lowering of the orbital floor, orbital fat entrapment in the maxillary sinus (Fig. 17-15), or massive orbital contents displacement into the maxillary sinus.
Nasal Fractures
The pair of nasal bones present at the top of the nose comprise about one third of its length (Fig. 17-16). Each bone attaches to a distal nasal cartilage. The two nasal passages are separated by a cartilaginous septum, the base of which is attached to the nasal crest of the maxilla. The septum also attaches to the ethmoid and vomer.
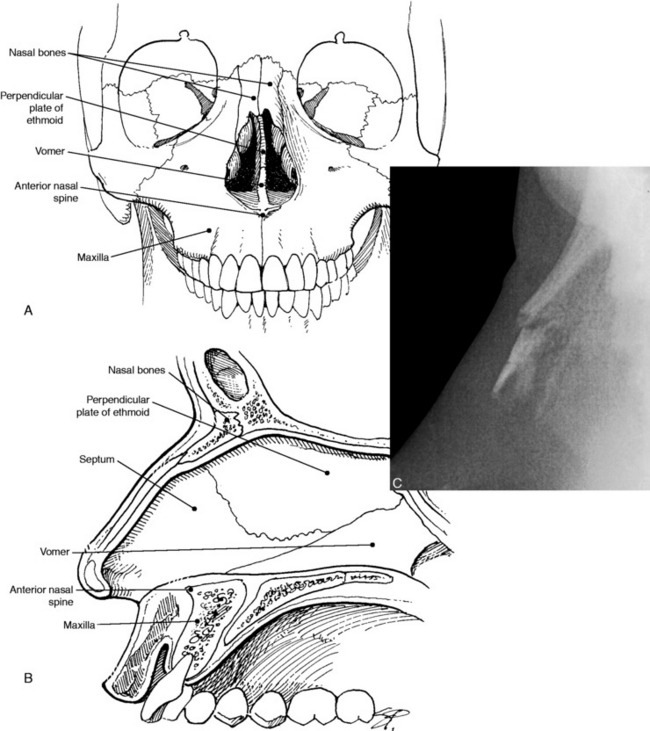
Fig. 17-16 Anterior (A) and lateral (B) views of the nasal bones, septum, and supporting structures. (C)Lateral radiograph showing fracture of the nasal bone.
Late complications of nasal fracture include nasal deformity or airway obstruction. The nasal deformity may be secondary to malunion of the nasal bones or to a septal injury. Malunion usually occurs as a result of the patient’s failure to seek early medical attention. Correcting a nasal fracture after 14 days past the injury is rarely successful and requires rhinoplasty to correct the deformity. Persistent nasal deformity or airway obstruction may be caused by a septal injury and also requires rhinoplasty and possible submucous cartilage resection for correction. In the pediatric age group, rhinoplasties are normally postponed until the age of 15 years to avoid any possible growth disturbances with the development of the nose. In the adult, rhinoplasty can be performed after satisfactory wound healing has occurred, usually about 3 months after the injury.
Facial Fractures in the Pediatric Patient
The diagnosis of facial bone fractures in the pediatric patient is difficult both clinically and radiographically. The child may not understand or be able to answer questions about the injury. Cooperation in the physical examination may also be difficult to obtain, but tenderness to palpation usually can be elicited. The key factor in identifying faciasl bone fractures in the pediatric age group is maintaining a high level of suspicion. Because plain radiographs are notoriously unreliable in the diagnosis, axial and coronal CT scans must be obtained in children when facial fractures are suspect.
Soft Tissue Injury Management
SURGICAL TECHNIQUE
After the wound is anesthetized, it should be treated with an appropriate solution such as povidone–iodine (Betadine) and draped. If any foreign material is still present or suspicion of bacteria seeding is high, local irrigation of the wound is performed. Such irrigation provides mechanical cleansing of the wound. The choice of the solution for irrigation is not as important as the actual cleansing of the wound. The simplest technique for irrigation is the use of a 25-cc syringe with a 22-gauge needle. The amount of irrigation necessary to clean the wound, of course, depends on the level of contamination and tissue injury of the wound. The irrigation may also be supplemented by using a toothbrush or scrub brush to gently remove any embedded particles.
ANATOMIC CONSIDERATIONS
Lip lacerations are common and may completely split either the upper or lower lip. Such injuries requires layer closure for optimal results. The labial mucosa closure can be accomplished with a 5–0 absorbable suture. The orbicularis oris muscle should be approximated with a 4–0 absorbable suture such as Dexon. The dermis is closed with the 5–0 absorbable suture and the skin with a 6–0 nylon suture. For the vermilion portion of the wound, 6–0 silk sutures would be appropriate. If the laceration violates the vermilion cutaneous border, careful approximation of the border should be done to avoid a notching defect. The closure can be accomplished by placing a stitch either just above or below the vermilion cutaneous junction and approximating this point first. Subsequent interrupted sutures can then be placed for final closure.
Tissue adhesives are valuable for use in children or in wounds under low tension (where 5–0 or 6–0 sutures might be used). They should not be used in lacerations at a mucocutaneous junction or in contaminated wounds.
Dingman RO, Natvig P. Surgery of facial fractures. Philadelphia: WB Saunders Co, 1964.
LaFort R. Fractures de la mâchoire superieur. Cong Int Med. 1990:275. C.R.(Paris)
Manson PN. Management of facial fractures. Perspect Plast Surg. 1988;2:1.
Nahum AM. The biomechanics of maxillofacial trauma. Clin Plast Surg. 1975;2:59-64.
Parker MG, Lehman JJr. Management of facial fractures in children. Perspect Plast Surg. 1989;3:1.
Schultz RC. Supraorbital and glabellar fractures. Plast Reconstr Surg. 1970;45:227-233.