Chapter 6 Maternal Physiologic and Immunologic Adaptation to Pregnancy
 Normal Values in Pregnancy
Normal Values in Pregnancy
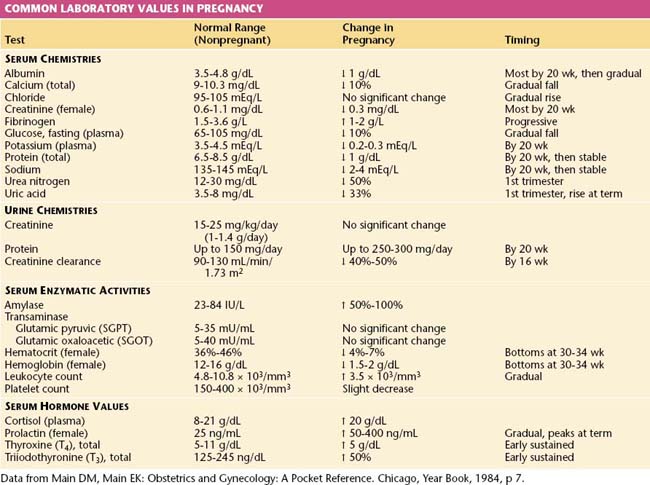
The normal values for several hematologic, biochemical, and physiologic indices during pregnancy differ markedly from those in the nonpregnant range and may also vary according to the duration of pregnancy. These alterations are shown in Table 6-1.
 Cardiovascular System
Cardiovascular System
CARDIAC OUTPUT
The hemodynamic changes associated with pregnancy are summarized in Table 6-2. Retention of sodium and water during pregnancy accounts for a total body water increase of 6 to 8 L, two thirds of which is located in the extravascular space. The total sodium accumulation averages 500 to 900 mEq by the time of delivery. The total blood volume increases by about 40% above nonpregnant levels, with wide individual variations. The plasma volume rises as early as the 6th week of pregnancy and reaches a plateau by about 32 to 34 weeks’ gestation, after which little further change occurs. The increase averages 50% in singleton pregnancies and approaches 70% with a twin gestation. The red blood cell mass begins to increase at the start of the second trimester and continues to rise throughout pregnancy. By the time of delivery, it is 20% to 35% above nonpregnant levels. The disproportionate increase in plasma volume compared with the red cell volume results in hemodilution with a decreased hematocrit reading, sometimes referred to as physiologic anemia of pregnancy. If iron stores are adequate, the hematocrit tends to rise from the second to the third trimester.
| Parameter | Amount of Change | Timing |
|---|---|---|
| Arterial blood pressures | ||
| Systolic | ↓ 4-6 mm Hg | All bottom at 20-24 wk, then rise gradually to prepregnancy values at term |
| Diastolic | ↓ 8-15 mm Hg | |
| Mean | ↓ 6-10 mm Hg | |
| Heart rate | ↑ 12-18 beats/min | 1st, 2nd, 3rd trimesters |
| Stroke volume | ↑ 10%-30% | 1st and 2nd trimesters, then stable until term |
| Cardiac output | ↑ 33%-45% | Peaks in early 2nd trimester, then stable until term |
Data from Main DM, Main EK: Obstetrics and Gynecology: A Pocket Reference. Chicago, Year Book, 1984, p 18.
 Respiratory System
Respiratory System
RESPIRATORY MECHANICS IN PREGNANCY
The changes in lung volume and capacities associated with pregnancy are detailed in Table 6-3. Assessment of mechanical changes during pregnancy reveals that the diaphragm at rest rises to a level of 4 cm above its usual resting position. The chest enlarges in transverse diameter by about 2.1 cm. Simultaneously, the subcostal angle increases from an average of 68.5 degrees to 103.5 degrees during the latter part of gestation. The increase in uterine size cannot completely explain the changes in chest configuration because these mechanical changes occur early in gestation.
| Test | Definition | Change in Pregnancy |
|---|---|---|
| Respiratory rate | Breaths/minute | No significant change |
| Tidal volume | The volume of air inspired and expired at each breath | Progressive rise throughout pregnancy of 0.1-0.2 L |
| Expiratory reserve volume | The maximum volume of air that can be additionally expired after a normal expiration | Lowered by about 15% (0.55 L in late pregnancy compared with 0.65 L postpartum) |
| Residual volume | The volume of air remaining in the lungs after a maximum expiration | Falls considerably (0.77 L in late pregnancy compared with 0.96 L postpartum) |
| Vital capacity | The maximum volume of air that can be forcibly inspired after a maximum expiration | Unchanged, except for possibly a small terminal diminution |
| Inspiratory capacity | The maximum volume of air that can be inspired from resting expiratory level | Increased by about 5% |
| Functional residual capacity | The volume of air in lungs at resting expiratory level | Lowered by about 18% |
| Minute ventilation | The volume of air inspired or expired in 1 min | Increased by about 40% as a result of the increased tidal volume and unchanged respiratory rate |
Data from Main DM, Main EK: Obstetrics and Gynecology: A Pocket Reference. Chicago, Year Book, 1984, p 14.
 Renal Physiology
Renal Physiology
RENAL BLOOD FLOW AND GLOMERULAR FILTRATION RATE
Renal plasma flow and the glomerular filtration rate (GFR) increase early in pregnancy, with maximum plateau elevations of at least 40% to 50% above nonpregnant levels by mid-gestation, and then remain unchanged to term. As was true for cardiac output, renal blood flow and GFR (clinically measured as the creatinine clearance) reach their peak relatively early in pregnancy, before the greatest expansion in intravascular and extracellular volume occurs. The elevated GFR is reflected in lower serum levels of creatinine and urea nitrogen, as noted in Table 6-1.
 Placental Transfer of Nutrients
Placental Transfer of Nutrients
Amino acids are actively transported across the placenta, making fetal levels higher than maternal levels. Glucose is transported by facilitated diffusion, leading to rapid equilibrium with only a small maternal-fetal gradient. Glucose is the main energy substrate of the fetus although amino acids and lactate may contribute up to 25% of fetal oxygen consumption. The degree and mechanism of placental transfer of these and other substances are summarized in Table 6-4.
| Function | Substance | Placental Transfer |
|---|---|---|
| Glucose homeostasis | Glucose | Excellent—”facilitated diffusion” |
| Amino acids | Excellent—active transport | |
| Free fatty acids (FFA) | Very limited—essential FFA only | |
| Ketones | Excellent—diffusion | |
| Insulin | No transfer | |
| Glucagon | No transfer | |
| Thyroid function | Thyroxine (T4) | Very poor—diffusion |
| Triiodothyronine (T3) | Poor—diffusion | |
| Thyrotropin-releasing hormone (TRH) | Good | |
| Thyroid-stimulating immunoglobulin (TSI) | Good | |
| Thyroid-stimulating hormone (TSH) | Negligible transfer | |
| Propylthiouracil | Excellent | |
| Adrenal hormones | Cortisol | Excellent transfer and active placental conversion of cortisol to cortisone beginning at mid-pregnancy∗ |
| ACTH | No transfer | |
| Parathyroid function | Calcium | Active transfer against gradient |
| Magnesium | Active transfer against gradient | |
| Phosphorus | Active transfer against gradient | |
| Parathyroid hormone | Not transferred | |
| Immunoglobulins | IgA | Minimal passive transfer |
| IgG | Good—both passive and active transport from 7 wk gestation | |
| IgM | No transfer |
∗ At mid-gestation, placental 11-β hydroxysteroid dehydrogenase converts cortisol to cortisone.
Data from Main DM, Main EK: Obstetrics and Gynecology: A Pocket Reference. Chicago, Year Book, 1984, p 37.
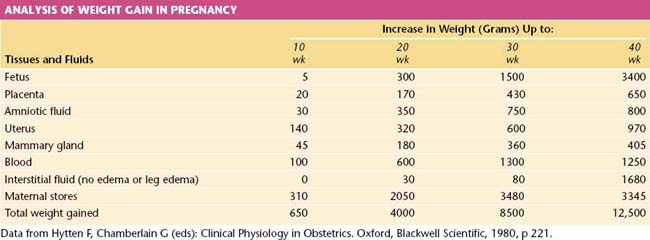
 Weight Gain in Pregnancy
Weight Gain in Pregnancy
The average weight gain in pregnancy uncomplicated by generalized edema is 12.5 kg (28 lb). The components of this weight gain are indicated in Table 6-5. The products of conception constitute only about 40% of the total maternal weight gain.
 Placental Transfer of Oxygen and Carbon Dioxide
Placental Transfer of Oxygen and Carbon Dioxide
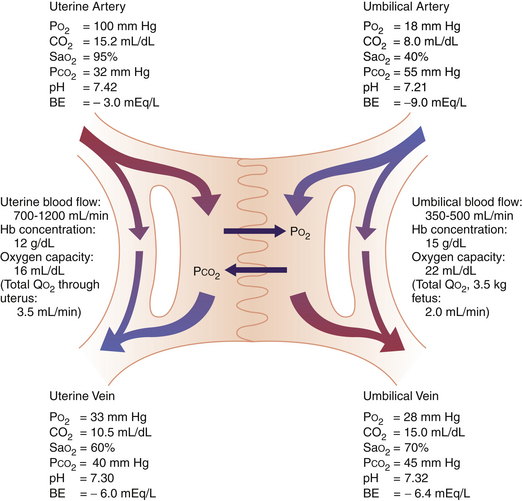
FETAL OXYGENATION
Figure 6-1 depicts the anatomic distribution of uterine and umbilical blood flow and O2 transfer across the placenta. A maternal shunt, which describes the fraction of blood shunted to the myoendometrium and is estimated to constitute 20% of uterine blood flow, is depicted. Similarly, a fetal shunt, which supplies blood to the placenta and fetal membranes and accounts for 19% of umbilical blood flow, is shown. The maternal-to-fetal PO2 and PCO2 gradients are calculated from measurements of gas tensions in the uterine and umbilical arteries and veins. The umbilical vein of the fetus, like the pulmonary vein of the adult, carries the circulation’s most highly oxygenated blood. The umbilical venous PO2 of about 28 mm Hg is relatively low by adult standards. This relatively low fetal tension is essential for survival in utero because a high PO2 initiates physiologic adjustments (e.g., closure of the ductus arteriosus and vasodilation of the pulmonary vessels) that normally occur in the neonate but would be harmful in utero.
FETAL AND MATERNAL HEMOGLOBIN DISSOCIATION CURVES
Most of the oxygen in blood is carried by hemoglobin in red blood cells. The maximum amount of oxygen carried per gram of hemoglobin, that is, the amount carried at 100% saturation, is fixed at 1.37 mL. The hemoglobin flow rates depend on blood flow rates and hemoglobin concentration. The uterine blood flow at term has been estimated at 700 to 1200 mL/min, with about 75% to 88% of this entering the intervillous space. The umbilical blood flow has been estimated at 350 to 500 mL/min, with more than 50% going to the placenta (see Figure 6-1).
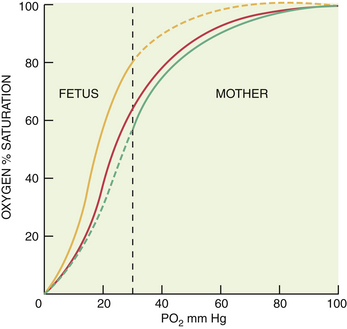
The affinity of hemoglobin for oxygen, which is reflected as the percent saturation at a given oxygen tension, depends on chemical conditions. As is illustrated in Figure 6-2, when compared with that in nonpregnant adults, the binding of oxygen by hemoglobin is much greater in the fetus under standard conditions of PCO2, pH, and temperature. In contrast, maternal affinity is lower under these conditions, with 50% of hemoglobin saturated with O2 at a PO2 of 26.5 mm Hg (P50) for mother compared with 20 mm Hg for the fetus.
In vivo, the greater fetal temperature and lower pH shift the O2-dissociation curve to the right, while the lower maternal temperature and higher pH shift the maternal curve to the left. As a result, the O2-dissociation curves for the fetal and maternal blood are not too dissimilar at the site of placental transfer. Maternal venous blood probably has an O2 saturation of about 73% and a PO2 of about 36 mm Hg, and the corresponding values for blood in the umbilical vein are about 63% and 28 mm Hg. As the only source of O2 for the fetus, blood in the umbilical vein has a higher O2 saturation and PO2 than blood in the fetal circulation (Figure 6-3). In the presence of a low fetal arterial PO2, fetal oxygenation is maintained by a high rate of blood flow to fetal tissues, which is supported by a very high cardiac output. This feature, along with the lower P50 of fetal blood, results in normal O2 delivery to fetal organs.
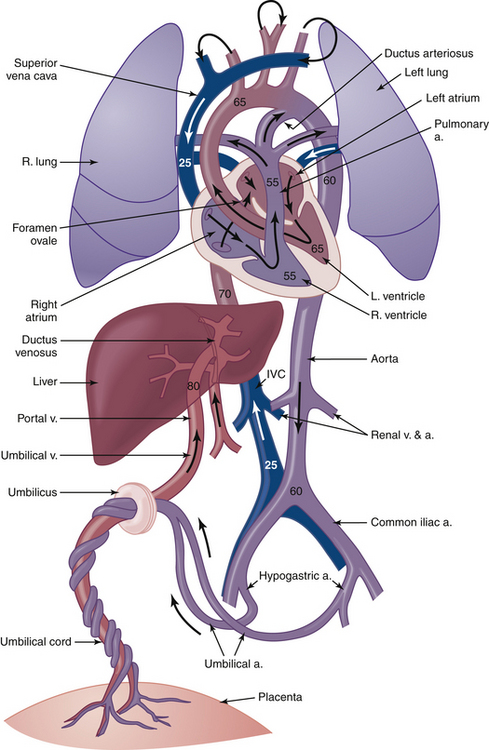
 Fetal Circulation
Fetal Circulation
Several anatomic and physiologic factors must be noted in considering the fetal circulation (Table 6-6; see Figure 6-3).
| Fetal Structure | From/To | Adult Remnant |
|---|---|---|
| Umbilical vein | Umbilicus/ductus venosus | Ligamentum teres hepatis |
| Ductus venosus | Umbilical vein/inferior vena cava (bypasses liver) | Ligamentum venosum |
| Foramen ovale | Right atrium/left atrium | Closed atrial wall |
| Ductus arteriosus | Pulmonary artery/descending aorta | Ligamentum arteriosum |
| Umbilical artery | Common iliac artery/umbilicus | Superior vesical arteries; lateral vesicoumbilical ligaments |
Data from Main DM, Main EK: Obstetrics and Gynecology: A Pocket Reference. Chicago, Year Book, 1984, p 34.
 Immunology of Pregnancy
Immunology of Pregnancy
Fineman J.R., Clyman R., Heymann M.A. Fetal cardiovascular physiology. In: Creasy R.K., Resnick R., Iams J.D., editors. Maternal-Fetal Medicine. Principles and Practice. 5th ed. Philadelphia: WB Saunders; 2004:169-180.
Kahn D.A., Koos B.J. Maternal physiology during pregnancy. In: Decherney A.H., Nathan L., Goodwin T.M., Laufer N., editors. Current Diagnosis and Treatment. Obstetrics & Gynecology. 10th ed. New York: McGraw-Hill; 2007:149-158.
Koos B.J. Breathing and sleep states in the fetus and at birth. In: Marcus C.L., Carroll J.L., Donnelly D.F., Loughlin G.M., editors. Sleep and Breathing in Children. 2nd ed. New York: Informa Healthcare; 2008:1-17.
Terness P., Kallikourdis M., Betz A., et al. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am J Reprod Immunol. 2007;58:238.

 Homeostasis of Maternal Energy Substrates
Homeostasis of Maternal Energy Substrates Other Endocrine Changes
Other Endocrine Changes