Chapter 57 Mapping, Disconnection, and Resective Surgery in Pediatric Epilepsy
The incidence of epilepsy is higher in children than adults with about 5% of children experiencing a seizure before the age of 20.1,2 The majority of these (80%) will never have another seizure, and therefore not meet the diagnosis of epilepsy.2 Among children with epilepsy, 20% will be refractory to medical therapy even with the numerous new medications available.3 Epilepsy surgery provides a powerful treatment modality for the subgroup of children with refractory epilepsy who are candidates. Determining appropriate surgical candidates requires a team specialists and a number of diagnostic modalities.
Another important distinction from adult epilepsy is the dynamic, developing nervous system of children. Repetitive seizures and anticonvulsive medications present noxious stimuli that may inhibit brain development.4 Seizures may additionally hamper socialization and school integration, causing deleterious impacts beyond the physiologic.5,6 However, brain plasticity could also benefit the child in recovering from resective or disconnective surgery. While these factors weigh significantly in the decision to pursue surgical treatment and the timing of such treatment, each child and family must assess their particular situation with the advice of the epilepsy team to help them weigh the risks of ongoing epilepsy, the risks of surgery, and the likelihood of seizure control with surgery.
Mapping
Nonoperative Localization
Semiology
The clinical semiology of a seizure provides the first clue to localizing its onset. Penfield described an assortment of mental, sensory, and physical aspects of seizures specific to various brain regions.7 These range from mental phenomena such as déjà vu and ill-defined epigastric rising to olfactory and gustatory auras to motor convulsions. The first manifestation of a given seizure most accurately reflects the area of onset, whereas seizure spread can lead to later involvement of other areas outside to true site of seizure onset.
Seizures of temporal lobe origin are the most likely to have auras. Those originating in the mesial temporal structures classically begin with an ill-defined epigastric sensation accompanied with panic or autonomic disturbance. Neocortical temporal seizures commonly have auditory, visual, or perceptual hallucinations. Head turning, posturing, automatisms, and behavioral arrest are common accompaniments of temporal seizures.
Parietal lobe seizures often present with somatosensory symptoms but may have abdominal symptoms such as nausea or a gustatory sensation. Occipital seizures may be heralded by visual phenomena including scotoma.8
Neuropsychology
The classic neuropsychology workup of an epilepsy patient consists of a variety of standardized tests and questionnaires that establish a profile of the patient’s cognitive, emotional, and behavioral abilities. This testing quantifies the patient’s abilities, deficits, and coping strategies. Such information can localize areas of dysfunctional cortex, which may be the site of seizure onset. Neuropsychology also predicts the deficits likely to be incurred by resection of a given focus and the impact that such deficits will have on the individual’s life.9
In many emerging technologies such as functional magnetic resonance imaging (fMRI), the neuropsychologist plays a vital role in administering verbal and functional tasks and assessing the patient’s cooperation with such tests. As these tests are very dependent on cooperation from the child while in the MRI machine, the neuropsychologist plays a vital role in extracting and interpreting information from an otherwise uncooperative patient.9,10
Electroencephalography
Electrophysiologic data are acquired by placing electrodes on the scalp and recording electrical differences between them. Interictal EEG can reveal epileptiform discharges such as spike and sharp waves, providing clues to the lateralization and localization of seizure onset, but prolonged video EEG provides an electrophysiologic picture of the seizures themselves. Video EEG data can confirm that spells are seizures (as opposed to breath-holding or other nonepileptic spells) and in many instances begin to localize the onset of the seizure. Most centers want to record several typical seizures and will continue monitoring until this is accomplished.11
Ictal video EEG will precisely localize the seizure focus in about a third of patients with temporal lobe epilepsy.12 Precise localization is even less common in those with extratemporal epilepsy where seizures tend to spread rapidly. Rapid spread from the orbitofrontal or posterior parietal cortex can falsely localize to the temporal lobe.13
Dense-array EEG is a newer technology that holds promise for more precise seizure localization. The dense array consists of 256 electrodes held to the head by an elastic mesh, giving substantially more information and spatial resolution than the standard 32-electrode array.14
Imaging
Surgical epilepsy cases are sometimes divided into lesional and nonlesional. Classically lesional cases from well-circumscribed pathologies such as cavernoma, dysembryoplastic neuroectodermal tumors (DNETs), and ganglioglioma have a significantly better prognosis than nonlesional cases. The identification of a structural abnormality on MRI is a strong predictor of localization.15 When the scalp EEG confirms seizure onset from the lesion, localization is strongly suggested, and resection often proceeds without invasive mapping.
The distinction between lesional and nonlesional epilepsies has blurred a bit as improved imaging has revealed cortical dysplasias, focal sclerosis, or other pathologies previously noted only by histology. Higher-field MRI, diffusion tensor imaging, and other MRI methods (e.g., MR spectroscopy and fMRI) may also show abnormalities. Voxel-based MRI postprocessing has been recently shown to help visualize blurred gray–white matter junctions or abnormal extension of cortical bands otherwise not recognizable on MRI.16 A negative MRI may still allow for further surgical planning. In a recent series of pediatric temporal lobectomies, half of those eventually shown to have histologically confirmed mesial temporal sclerosis had normal hippocampus on MRI.17 In such cases, other imaging such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetoencephalography (MEG) can help to further define the extent of the epileptic-onset zone and the functionality within and surrounding the malformation.
PET provides a tomographic image of brain glucose utilization, while SPECT images blood flow. Both can reveal areas of metabolic derangement (hypometabolism interictally and hypermetabolism during a seizure) that can represent a seizure focus.18,19 MEG collects minute magnetic potentials generated by bands of synchronized neural currents, a technique that can reveal epileptic foci as well as functional circuits (e.g., motor units).20 The specific role for these various modalities is not determined and considerable variability exists across programs.21
Invasive Monitoring
In the setting of infiltrative low-grade tumors or focal cortical dysplasia, invasive EEG recording may be used to define the relationship of epilepsy onset to the lesion. This may guide the surgeon to resect epileptogenic tissue beyond the bounds of the imaging abnormality. Such infiltrative tumors and cortical dysplasias can have functional neurologic tissue within them. If these lesions are anatomically near motor or speech cortex, mapping of these functions can further define this relationship. It may be found that a lesion has displaced the motor fibers in such a way that resection can be accomplished while preserving function or an aberrant localization of speech function may be present precluding violation of a typically ineloquent area of cortex.
Some centers approach poorly defined lesions and occult lesions on MRI with MEG, PET, and ECoG alone, avoiding grid placement entirely.22 While good results from this approach22 have been published, it is difficult to directly compare it to the liberal use of prolonged grid monitoring. At our center, both ECoG and extraoperative grid monitoring are a part of the armamentarium. The approach used is determined on a case-by-case basis depending on the information from the preoperative workup and what vital questions remain.
Electrocorticography
A significant amount of information about the epileptogenicity and function of an area of tissue can be obtained from intraoperative electrical monitoring. ECoG data are obtained by placing electrodes on the exposed brain and observing the EEG record for epileptiform discharges (spike and sharp waves).23,24 Anesthetic considerations are important to avoid suppressing or altering the EEG record. Benzodiazepenes should be avoided except at induction or after ECoG. Inhalational agents above 0.5 MAC will dampen the record beyond interpretation. A combination of sevoflourane and dexmetomidate is preferred at our (BO and JGO) institution, but a total intravenous anesthetic is also possible.25
Some functional information can be obtained in the anesthetized patient. A distinct pattern of cortical activity (phase reversal) of evoked-somatosensory responses can localize the central sulcus. Direct stimulation of motor cortex can elicit motor responses that are visualized or recorded on EMG. Awake craniotomy in cooperative older children (sometimes as young as 10) can be used to map language cortex.25 Current is passed through a hand-held bipolar stimulator (Ojemann stimulator) to inhibit an area of cortex while the patient is performing speech tasks. Speech arrest induced during this procedure reflects an area of vital speech function.24
Implanted Electrodes (Grids, Strips, Depths)
Intracranial monitoring electrodes are typically placed in the subdural space but epidural placement is employed at times, and yields comparable information.26,27 Placement below the skull dramatically increases the amplitude of cortical electrical signals collected and diminishes muscle artifact, the main source of noise. Implanted electrodes also greatly increase the spatial resolution of the EEG. A typical grid consists of 64 electrodes on an 8×8 cm array, whereas scalp EEG typically employs 32 electrodes to cover the entire head.
Implanting electrodes additionally allows for prolonged functional mapping outside the operating room by stimulating through the same electrodes.2 By applying current across two neighboring electrodes, the intervening area of cortex (about a centimeter of tissue) is inhibited. Deficits observed can identify the function of the cortex and the deficits likely to be incurred by resection. Lower stimulation of motor cortex may produce muscle contraction rather than inhibition. Many children who cannot tolerate with awake intraoperative mapping will cooperate with extraoperative mapping.30 Additionally, the mapping can take place during complex activities such as drawing, writing, or playing a musical instrument potentially identifying important integrative functions.
Grid electrodes consist of a broad array of contacts that can cover a large area of cortex. They can be particularly helpful in defining an epileptic zone within a lobe or defining the relationship of a lesion to seizure onset. Convexity sites are particularly amenable to grid placement.31 Extraoperative language and motor mapping are typically performed through grids as they give ample coverage of the hand and face motor area as well as the typical language sites.
Strip electrodes consist of a single or double row of electrodes spaced along a flexible strip that can be safely passed along the subdural space. Coverage of the interhemispheric cortex, orbitofrontal cortex, and mesial temporal lobe can be achieved by passing strip electrodes, all locations poorly monitored by scalp recordings.28,32 A strip passed along the undersurface of the temporal lobe will typically place the distal electrode just at the parahippocampal gyrus, providing good monitoring of the mesial temporal structures.33 Placement of strip electrodes requires only a burr hole, allowing for limited monitoring of distant sites, including the contralateral hemisphere without requiring a craniotomy.
Distant and mesial temporal recordings can also be obtained with depth electrodes. These electrodes line a narrow probe that passes directly into the region of interest. Some centers also place depth electrodes directly within or along the deep margins of a lesion infiltrating the white matter (e.g., cortical dysplasia) to evaluate the depth of resection necessary.28,34 These various electrodes are often used in combination to monitor all areas of interest.
Complications
Electrode implantation procedures require two separate operations (occasionally more) with a prolonged hospitalization between. The most common complications are cerebrospinal fluid (CSF) leakage, fever, and infection. Minor CSF leak is common while electrodes are in place, but rarely problematic. While low-grade fevers are common and wound infection much less so, suspicion should remain high in order to recognize and treat infections promptly.35
Strip electrodes have fewer complications than grids, 1% overall compared to 3% to 4%. Strips also appear to be safer than depths. Isolated cases of permanent neurologic deficit and death have been reported.35–39 The risk of complications, as well as the additional hospitalization time, need to be balanced against the information gained by invasive monitoring.
Disconnection
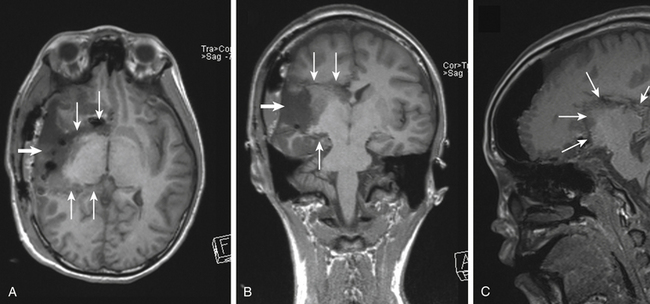
Disconnective operations include hemispherotomy, the modern iteration of hemispherectomy, to isolate a diffusely pathologic, epileptogenic hemisphere and lobar or multilobar disconnection, for pathology that affects multiple lobes (Fig. 57-1).
Trans-Sylvian Hemispheric Disconnection or Functional Hemispherectomy
Hemispheric deafferentation, hemispheric disconnection, hemispherotomy, or functional hemispherectomy are synonyms for surgical procedures that aim to disconnect all cortical structures of one hemisphere from the deeper lying structures of the brain, i.e., the basal ganglia by combination of disconnective steps and additional more or less extensive resective steps. The various terms mirror the development in the last 20 years away from classic anatomic hemispherectomy to procedures that combine more and more disconnective steps with less and less resective steps. In the last 15 years, several procedures have been described40–44 that step by step have replaced anatomic hemispherectomy and Rasmussen’s functional hemispherectomy technique.
Indication
These procedures are primarily indicated in patients with pre-existing unihemispherical damage and typical neurologic deficits such as hemianopia and hemiparesis combined with drug resistant epilepsy. If these cases occur in early infancy, are combined with holohemispheric dysplasias or extensive damage and severe drug resistant seizures they are frequently called “catastrophic epilepsy.” Other typical diagnoses seen in these patients include hemimegalencephaly, multilobar cortical dysplasia, and various disorders of gyration such as polymicrogyria or lisencephaly. Hemimegalencephaly (HME) is a quite rare malformation of the cortical development arising from an abnormal proliferation of anomalous neuronal and glial cells that leads to the hypertrophy of the whole affected cerebral hemisphere. Epilepsy typically presents in infancy with severe, drug-resistant seizures. Many infants with HME undergo hemispherotomy before 2 years of age. HME patients have more operative complications and worse seizure outcomes than other hemispherotomy patients.45,46
Frequently hemispheric damage is due to perinatally acquired brain defects or intrauterine hemorrhagic damage. Perinatal stroke is in many ways an ideal pathology for hemispherotomy as patients typically have little function stored in the affected hemisphere and rarely incur new deficits from the operation. Development and cognition often improve when seizures are controlled and epilepsy medicines are decreased or discontinued. Many of these children have cystic encephalomalacia in continuity with the ventricular system or separated by a thin membrane. This expanded space gives additional surgical access and easier visualization of the deep anatomy that needs to be disconnected, but this anatomy can be distorted. Care must be taken to maintain orientation.
Hemispherotomy may also be considered in diseases such as Sturge-Weber syndrome, a sporadically occurring phakomatosis consisting of unilateral facial port-wine stain in the V1 distribution, glaucoma, and unilateral leptomeningeal angioma. The intracranial angioma causes progressive cortical atrophy and calcification. Epilepsy occurs in 75% to 90% of Sturge-Weber patients, usually presenting in infancy. In some patients, the angioma is focal enough to be resected, but most are candidates for hemispherotomy.47,48
Other pathologies potentially amenable to hemispherotomy include Rasmussen’s encephalitis, and postencephalitic and post-traumatic hemispheric damage. Rasmussen’s encephalitis or chronic focal encephalitis is a chronic T-lymphocyte inflammation that remains localized to one hemisphere. Presentation is usually between 5 and 10 years of age with acute, drastic onset of focal seizures and progressive loss of hemispheric function. Anticonvulsants typically have little success in controlling seizures. Medical treatments to control inflammation, including steroids, intravenous immunoglobulin (IVIG), tacrolimus, and plasmapheresis are the first line of therapy, although their efficacy is limited.49,50
Timing of hemispherotomy has been debated in Rasmussen’s patients particularly. Permanent loss of hemispheric function eventually occurs in a large majority from the disease, prompting recent recommendations for early hemispherotomy. Some evidence supports improved cognitive outcomes with early surgery,46,51 but this must be balanced against the acute loss of hemispheric function from surgery performed before hemispheric dysfunction is fully actualized.
As hemispherotomy disconnects the motor and visual cortices, hemiparesis and visual field defects are inevitable consequences of the operation. The procedure is more appealing if these deficits are already present or if they are an inevitable outcome of the underlying pathology. Postoperative hemiparesis affects the upper extremity more than the lower. Ability to walk is almost always maintained.52,53 Improved development and cognition commonly follow hemispherotomy in cases where seizures stop and anticonvulsants can be weaned.52,54–56
Diagnostics
The most important presurgical diagnostics include MRI and EEG recording of ictal events. MRI may show larger defects, frequently in the distribution of the middle cerebral artery, and malformations of cortical development. MRI findings include gross malformations of the hemisphere, ectopic gray matter, disorders of gyration, extensive postencephalitic damage, or holohemispheric atrophy. In the ideal case all ictal activity can be localized to the affected hemisphere. However, hemispherectomy may be indicated even in the setting of contralateral EEG or even MRI abnormalities. MRI abnormalities on the opposite hemisphere are particularly concerning for predicting poor success.57,58
If the disease has started after the fourth year it is important to demonstrate transfer of language to the other hemisphere for which the intracarotid amobarbital test, or possibly functional MRI,10 is useful. However, the association between speech outcome and hemispherectomy is multifactoral with congenital etiologies having a more favorable outcome, even with age considered.59
Choice of Approach
It has become clear that the disconnective procedures carry the same success rate, may be even higher success rates as those procedures that involve larger resective steps.60,61 In the 10 to 15 years of available follow-up for these disconnective procedures the complications known from anatomic hemispherectomy have not been reported thus far, and in particular no cases of cerebral hemosiderosis, which was reported for earlier procedures. The availability of CT and MRI-scanning has also minimized the potential problem of shunt malfunctions. However, postoperative hydrocephalus is also much rarer in disconnective procedures such as trans-sylvian keyhole deafferentation or trans-sylvian keyhole disconnection, or the alternatives described by others.40,43,44 Other advantages of the trans-sylvian keyhole disconnection procedure favored by us have been confirmed by other series.43,60,62 The keyhole procedures are less well suited for cases with extensive hemispheric malformation such as hemimegalencephaly. In these cases we combine the trans-sylvian approach with a perisylvian window technique such as has been described by Villemure and Mascott.44
Surgical Technique
The patient’s head is brought into a horizontal and slightly downward pointed position. The skin incision starts right before the tragus and can be linear or slightly curvilinear to allow for a craniotomy flap of 4×4 or 5×6 cm, depending on the size of the cranium. This small craniotomy size is possible because one just has to reach in front and behind the corpus callosum, which in children is no longer than 7 cm. Neuronavigation may be helpful to place the craniotomy in an ideal position, that is, the upper border at the level of the corpus callosum and the lower about 1 cm below the level of the M1. The sylvian fissure is opened and the temporal and frontal opercula are dissected away from the insular cortex (if those opercula still exists) and thus the insular cortex is exposed. Due to atrophic processes or postinfarction cyst built-up orientation may be more difficult. The tree of the M2–M3 branches is a good guide, although one should keep in mind that in cases with perinatal infarction, the M2 and M3 branches are smaller in diameter. In some cases the block of basal ganglia, thalamus, and insular cortex is considerably smaller than in healthy brains. If multiple cysts are present, these vascular structures inside the ventricle the choroid plexus are helpful as guides to orientate the surgeon.
Postoperative Care
Complications may be similar to all craniotomies, that is, subdural hemorrhage, epidural hemorrhage, or infection of ventricular space and bone flap. Dreaded complications would occur if the midline is transgressed, particularly in the area of the septum pellucidum where the contralateral fornix is close. Patients frequently develop pyrexia, which may last for a few days or for more than a week and they are usually noninfectious.63 An elevated cell count may also be caused by contamination of CSF with blood.
As with all intraventricular surgeries a certain rate of patients needing a shunt or developing intraventricular cysts or adhesions appears to be unavoidable. Large series of hemispherotomy (20 to 83 patients) report rates of hydrocephalus ranging from 2% to 16%, commonly presenting months after the operation. Hemimegalencephaly and widespread cortical dysplasia patients are more likely to develop hydrocephalus than others. Infections and hemorrhages have also been reported, at times as often as 5% for each.42,53,64,65
A certain degree of deterioration in motor function, especially of the affected hand pincer movement and sometimes in movement of the leg, is unavoidable, whereby ability to walk is typically regained after rehabilitation in those few cases where significantly deterioration in walking ability occurred.52,53
Outcome
Cognitive and developmental outcomes have garnered significant attention recently with quite positive results. An increasing number of studies show improved postoperative development following hemispherotomy as fewer seizures and anticonvulsant medications improve cognitive abilities and social integration.52,54–56 Early surgery has been increasingly recommended to avoid seizures during the critical periods of brain and social development. These points must be balanced against the increased anesthetic and blood loss risks of young children66 and the potential for ongoing development or radiologic revelation of a pathology (as with cortical dysplasia or Sturge-Weber syndrome) that would have argued against hemispherectomy.
Good seizure outcomes have been demonstrated from all forms of hemispheric surgery, with more than two thirds of patients seizure-free. Rasmussen’s encephalitis, Sturge-Weber, and perinatal stroke patients consistently have better seizure outcomes, with reported rates ranging from 73% to 93% seizure-free. Case series of cortical dysplasia and hemimegencephaly are seizure-free between 63% and 80% of the time.53,64,65
In Schramm’s series (mentioned above) of 93 pediatric and juvenile patients with a minimum follow-up of 1 year, Engel class 1 outcome was 88%. Other all-pediatric series have reported similar results.67 Thus, hemispherotomies continue to be a successful type of epilepsy surgery.
Palliative Disconnection—Callosotomy
For children with generalized seizures, and drop attacks in particular, corpus callosotomy is a palliative disconnection consideration. The disconnection will, in theory, prevent the spread and generalization of a seizure. Callostomy has been considered for drop attacks, primary and secondary generalized seizures, and medically refractory mixed seizure types like Lennox-Gastaut.68–72 Traditionally, anterior callosotomy has been the preferred option to avoid disconnection syndromes, but recent reports in the literature suggest that a one-stage complete callosotomy may be a better choice for initial surgical treatment in some patients.73–75
When analyzed by seizure type, atonic spells, myoclonic seizures, and absence seizures appear to be the seizure types most affected by a corpus callosotomy.75–78 Cognitive and psychosocial outcomes have been demonstrated by family surveys and other assessments administered following surgery.79,80 Operative complications of callosotomy can include hydrocephalus, aseptic meningitis, and cerebral edema.75
Resections
Temporal Lobe Resections
The results of pediatric temporal lobe resections are less consistent than adult series and the pathologies more varied. Structural lesions are present in as many as half of pediatric temporal lobectomy candidates.15,81 Hippocampal sclerosis does occur in pediatric patients, but often in association with other neocortical pathology.82 The presence of such dual pathology alters the surgical approach, and the suspicion of such will at times mandate invasive monitoring.
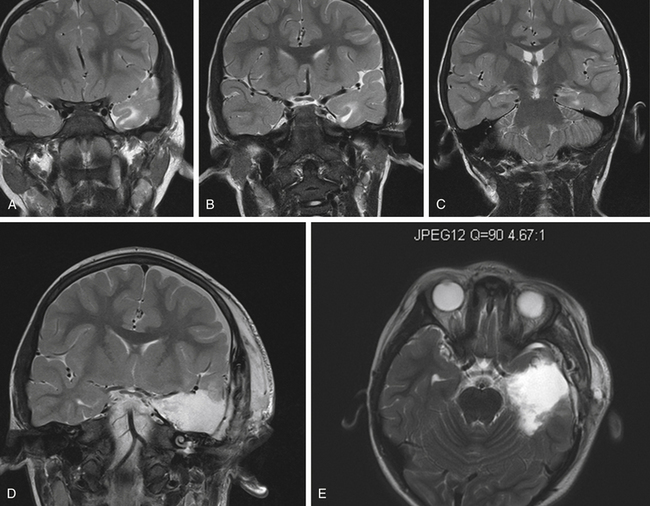
Common temporal lobe pathologies in children include tumors, vascular lesions, and dysplasias. In the subset of patients with a well-circumscribed, neocortical lesion such as DNET, ganglioglioma, or cavernous malformation, the decision must be made whether to resect mesial temporal structures. Lesionectomy alone can yield seizure freedom, but often mesial structures are also involved and may need to be included83–85 typically this is a consideration in the case of a prolonged seizure history. Dysplasia in the anterior temporal lobe can often be seen in the setting of hippocampal changes in the young patient (Fig. 57-2).
Technique
Many different terms are applied to temporal lobectomies. An “aggressive” temporal lobectomy would typically be employed only when extensive dual pathology is present. In what historically might be considered a standard anterior temporal lobectomy, the lateral cortex is resected back 4 cm on the dominant side and 6 cm on the nondominant side.86 Compared to selective procedures, this “standard” temporal lobectomy would be less desirable for cases of isolated medial temporal pathology.87
So-called “selective” approaches cover a variety of strategies. When the lateral temporal lobe is suspected to be pathologic, the resection can be tailored to address the specific imaging or electrophysiologic abnormalities.88,89 Such a “tailored-selective” approach may use ECoG or prolonged monitoring through grids and strips to identify the extent of the epileptic zone when a diffuse lesion is present. In children old enough for consideration of language preservation, functional MRI, invasive mapping, or in some adolescents, awake language mapping may be necessary to identify and preserve speech cortex90 and such considerations may also modify the surgical approach.
In any access to mesial structures, the temporal horn must be accessed, this may be through middle temporal,91 inferior temporal, or basal temporal92,93 in the various “anatomic-selective” approaches that address isolated mesial temporal pathology. Approaches through sylvian fissure access to the hippocampus are also described primarily in adult populations.94,95 The amygdalohippocampectomy can then be performed. While these approaches are often appropriate in adult patients where hippocampal sclerosis is usually isolated and evident on imaging, they are applicable to only the pediatric case that displays these clear preoperative criteria for isolated mesial temporal sclerosis.17 More tailored resections may be indicated, varying the amount of lateral resection, depending on preoperative imaging and other evaluations.
The hippocampal resection is carried back beyond the choroidal point between 1.5 to 3 cm, or until the lateral brain stem is visualized beyond the arachnoid. Direct hippocampal recording has been described to further tailor the operation.96 Care must be taken to maintain a subpial resection as the oculomotor nerve, carotid artery, optic nerves, and brain stem lie medial to the hippocampus.
Complications
Some degree of superior quadrant visual field defect is expected from interruption of Meyer’s loop pathways running through the temporal lobe, even for many selective approaches,93,97,98 but small deficits typically goes unnoticed by the patient and on bedside exam. Dominant temporal lobectomies in rare instances cause severe anomia and more commonly cause a variable decline in verbal ability on neuropsychology testing and difficulty with naming specific proper nouns.99 Good verbal memory preoperatively and a normal MRI are risk factors for decline in language function in adults,100 but the effects in children are less studied. “Selective” procedures appear to have better cognitive outcomes than “standard” temporal lobectomy, but an anatomic-selective versus a tailored-selective approach have not been directly compared. Damage to medial structures can occur, including the brain stem, cranial nerves, or major vessels. Respect for the pial boundary between the temporal lobe and these structures helps to prevent such complications. CSF leak, infection, and hemorrhage complicate temporal lobectomy at similar rates as other craniotomy procedures.101
Outcomes
Outcomes in pediatric patients undergoing temporal lobe surgery for intractable epilepsy are difficult to compare for several reasons. Temporal lobe pathology is less common in pediatric epilepsy series than adult, and the pathologies vary between different series, with some institutions reserving surgery for lesional resections while others take a more aggressive approach. The outcome measures also vary among the reported series. The inclusion of adolescents, who more commonly have mesial temporal sclerosis, can also affect the outcome of a series.
In three recent series of pediatric temporal lobe operations for epilepsy, the rate of Engel class 1 or 2 outcome has varied from 63% to 88.5%.17,102,103 Cortical tumors have the best seizure outcome in most series.103
Mesial temporal sclerosis (MTS) confirmed on pathology also bore a favorable prognosis in a recent multi-institution study.17 MTS was identified histologically in 53% of their series of children younger than 14 years with nontumor temporal lobe pathology. Children with MTS had Engle class 1 or 2 outcome 77% of the time as compared to 57% in those without MTS, a group consisting mostly of cortical dysplasia and gliosis.
Despite the added complexity of dual pathology cases, some series report similar outcomes in cases with preoperatively recognized dual pathology to other children undergoing temporal lobe epilepsy surgery.103 Negative predictors of seizure outcome include developmental delay, multifocal EEG, and multiple seizure types. All of these factors point to diffuse seizure onset.17,103
Extratemporal Resections
Extratemporal epilepsy foci are far more common in children than in adults where mesial temporal sclerosis dominates. Many authors group lateral temporal sources of seizure with extratemporal sources under the category of neocortical epilepsy as distinct form MTS. This taxonomy better reflects the underlying pathology and more starkly divides pediatric from adult epilepsy populations, but when planning a surgical approach we find the geographical categorization of temporal versus extratemporal epilepsy more useful (particularly with the possibility of dual pathology). Extratemporal epilepsy foci occur most commonly in the frontal lobe followed by the parieto-occipital regions.104
Developmental tumors such as gangioglioma, ganglocytoma, and dysembryoplastic neuroectodermal tumor (DNET) most commonly present with seizure. Epilepsy from such lesions has a very high likelihood of intractability, reaching 90% by 10 years after diagnosis.105 Resection of a low-grade tumor provides very good seizure control, with Engle class 1 or 2 in as many as 95% in some series.103 Often after scalp EEG confirmation of the involvement of the lesion in seizures, lesionectomy is undertaken with or without electrocorticography guidance.106,107 Complete resection of the lesion is an independent predictor of good outcome.108
Malformations of cortical development appear on imaging as variations in the depth of sulci, distribution of sulci, thickness of the cortex, or presence of gray matter in the depths of white matter. Focal cortical dysplasia is the most common of these amenable to epilepsy surgery.109 It histologically consists of disorganization of lamellar structure, large neurons, neuronal heterotopias extending into the deep white matter, usually tailing toward the lateral ventricle, and balloon cells with focal gliosis. Cortical dysplasias are most commonly located at extratemporal sites.110 High-resolution MRI or PET may reveal lesions that are otherwise occult.
Vascular malformations including cavernous malformation and arteriovenous malformation (AVM) are a less common cause of pediatric epilepsy. Cavernous malformations are well circumscribed lesions surrounded by an area of hemosiderin-stained brain. For either, lesionectomy outcome resembles that of low-grade tumor resection.111
Technique
When a diffuse lesion such as FCD is identified, the extent of the epileptogenic zone often requires better characterization. Type 2 dysplasias are typically visible on MRI, but PET or MEG may show areas of abnormal metabolism that are more extensive than the visualized malformation. Functional imaging may also reveal type 1 cortical dysplasia which is occult on MRI.112 Several approaches have been taken for cortical dysplasia. Tuberous sclerosis in particular is managed differently across institutions.
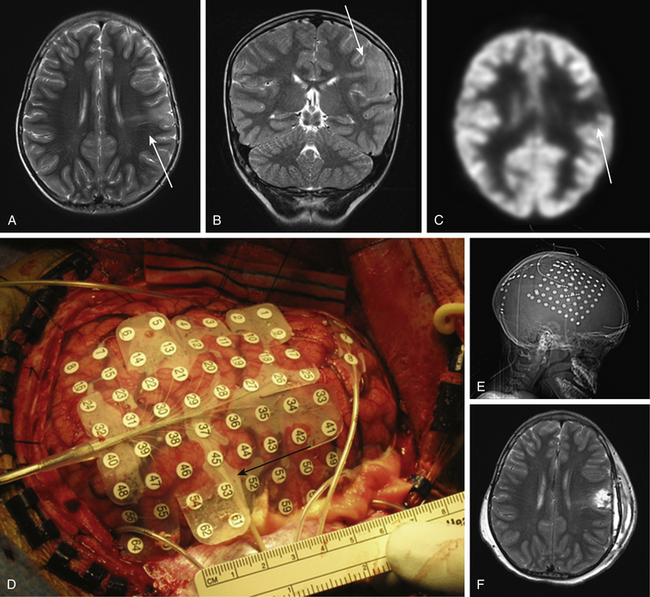
Invasive seizure monitoring and cortical mapping are often employed to tailor the resection of diffuse lesions to include the full seizure focus and avoid important functional tissue (Fig. 57-3). In a two stage approach to mapping such a lesion, a grid is typically placed over the lesion to precisely localize seizure onset. Strip electrodes are useful in monitoring otherwise hard to sample areas such as the interhemispheric fissure, the orbitofrontal cortex, or at times, the contralateral hemisphere. Depth electrodes can define the epileptogenicity of a tail of gray matter extending toward the ventricle from a cortical dysplasia. In some cases, a replacement of the subdural electrodes immediately after an initial resection.
Other groups rely on the preoperative evaluation, including MRI, PET, and/or MEG to identify candidates who undergo a single stage approach. ECoG will give feedback on the presence of epileptiform spikes in given brain areas and the effect of resection on these.113 Outcomes from both strategies are quite similar, although patient selection processes are not the same.
When an epileptic focus is within an area of critical function, multiple subpial transections (MSTs) can offer some reduction of seizures without functional impairment. In this technique, the surface of the cortex is transected along the width of the gyrus. This is thought to disrupt seizure spread while preserving cortical output through descending fibers.114,115
Outcome
Extratemporal resections overall carry a poorer prognosis than temporal lobe resections. As in temporal lobe epilepsy, lesional resection has a better prognosis than cortical dysplasia or nonlesional resections where the boundaries of the epileptogenic zone are poorly defined.116 Many different approaches report similar outcomes and are typically center-specific. Complete resection of MRI-evident lesions is one emerging predictor of outcome.
Multiple subpial transections have shown some benefit, but poorer outcomes than resection. In cases where most of a seizure focus can be resected, MST can be a useful adjunct in nearby functional tissue that is epileptic.117
Conclusion
The pathologies encountered in pediatric epilepsy and the surgical techniques used often differ from adult epilepsy. Mapping is a key component to many pediatric epilepsy cases as children often have diffuse congenital epileptic foci. The resection of such foci is often safe when guided by modern techniques. Technological advances, particularly in imaging, are rapidly changing the field and improving the care of epileptic children. The improved technique of hemispherotomy allows a chance for safe treatment of diffuse hemispheric pathologies not amenable to other approaches.
Boshuisen K., van Schooneveld M.M., Leijten F.S., et al. Contralateral MRI abnormalities affect seizure and cognitive outcome after hemispherectomy. Neurology. 2010;75:1623-1630.
Clarke D.B., Oliver A., Anderman F., et al. Surgical treatment of epilepsy: the problem of lesion/focus incongruence. Surg Neurol. 1996;46:246-585.
Commission on Classification and Terminology of the International League against Epilepsy. proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489-501.
Di Rocco C., Tamburrini G. Sturge-Weber syndrome. Childs Nerv Syst. 2006;22:909-921.
Hallbook T., Ruggieri P., Adina C., et al. Contralateral MRI abnormalities in candidates for hemispherectomy for refractory epilepsy. Epilepsia. 2010;51:556-563.
Hemb M., Velasco T.R., Parnes M.S., et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986-2008. Neurology. 74, 2010. 1786–75
Jonas R., Nguyen S., Hu B., et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology. 2004;62:1712-1721.
Mathern G.W. Challenges in the surgical treatment of epilepsy patients with cortical dysplasia. Epilepsia. 2009;50(suppl 9):45-50.
Mohamed A., Wyllie E., Ruggieri P., et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology. 2001;56:1643-1649.
Morrell F., Whisler W.W., Bleck T.P. Multiple subpial transections: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70:231-239.
Morrison G., Duchowny M., Resnick T., et al. Epilepsy surgery in children: a report of 79 patients. Pediatr Neurosurg. 1992;18:291-297.
Paolicchi J.M., Jayakar P., Dean P., et al. Predictors of outcome in pediatric epilepsy surgery. Neurology. 2000;54:642-647.
Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia. 2008;49:1296-1307.
Schramm J., Behrens E., Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509-515.
Schramm J., Kral T., Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49:891-901.
Shields W.D., Peacock W.J., Roper S.N. Surgery for epilepsy: special pediatric considerations. Neurosurg Clin North Am. 1993;4:301-310.
Sillanpaa M., Falava M., Kaleva O., et al. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715-1722.
Smyth M.D., Limbrick D.D., Ojemann J.G., et al. Outcome following surgery for temporal lobe epilepsy with hippocampal involvement in preadolescent children: emphasis on mesial temporal sclerosis. J Neurosurg. 2007;106(suppl 3 Pediatrics):205-210.
Spencer S.S., Schramm J., Wyler A., et al. Multiple subpial transaction for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43:141-145.
Spooner C.G., Berkovic S.F., Mitchell L.A., et al. New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome. Neurology. 2006;67:2147-2153.
Tanriverdi T., Olivier A., Poulin N., et al. Long-term seizure outcome after corpus callosotomy: a retrospective analysis of 95 patients. J Neurosurg. 2009;110:332-342.
Villemure J.G., Daniel R.T. Peri-insular hemispherotomy in paediatric epilepsy. Childs Nerv Syst. 2006;22:967-981.
Wyler A.R., Ojemann G.A., Lettich E., et al. Subdural strip electrodes for localizing seizure foci in children. J Neurosurg. 1984;60:1195-1200.
Wyllie E. Surgical treatment of epilepsy in pediatric patients. Can J Neurol Sci. 2000;27:106-110.
Wyllie E., Comair Y.G., Kotagal P., et al. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740-748.
1. Hauser W.A., Durland L.T. The epidemiology of epilepsy in Rochester, Minnesota. Epilepsia. 1975;16:1-66.
2. Cowan L.D., Bodensteiner J.B., Leviton A., et al. Prevalence of the epilepsies in children and adolescents. Epilepsia. 1989;30:94-106.
3. National Institutes of Health. Surgery for epilepsy. Consensus Statement. 1990;8:1-20.
4. Sillanpaa M., Falava M., Kaleva O., et al. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715-1722.
5. Holmes G.L. Do seizures cause brain damage? Epilepsia. 1991;32(suppl 5):514-528.
6. O’Leary S.D., Burns T.G., Borden K.A. Performance of children with epilepsy and normal age-matched controls on the WISC-III. Child Neuropsychol. 2006;12:173-180.
7. Penfield W., Jasper H. Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown; 1954.
8. Commission on Classification and Terminology of the International League against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489-501.
9. Lassonde M., Sauerwein H.C., Gallagher A. Neuropsychology: traditional and new methods of investigation. Epilepsia. 2006;47:9-13.
10. Shurtleff H., Warner M., Poliakov A., et al. Functional magnetic resonance imaging for presurgical evaluation of very young pediatric patients with epilepsy. J Neurosurg Pediatr. 2010;5:500-506.
11. Zentner J., Hufnagel A., Ostertun B., et al. Surgical treatment of extratemporal epilepsy: clinical, radiological and histopathologic findings in 60 patients. Epilepsia. 1996;37:1072-1080.
12. O’Brien T.J., Kilpatrick C., Murrie V., et al. Temporal lobe epilepsy caused by mesial temporal sclerosis and temporal neocortical lesions. Brain. 1996;119:2133-2141.
13. Williamson P. Frontal lobe epilepsy: problems in diagnosis and classification. In: Delgado-Escuata A., Halgren E., Bancaud J. Advances in Neurology. New York: Raven Press, 1992.
14. Holmes M.D., Tucker D.M., Quiring J.M. Comparing noninvasive dense array and intracranial electroencephalography for localization of seizures. Neurosurgery. 2010;66:354-362.
15. Shields W.D., Peacock W.J., Roper S.N. Surgery for epilepsy: special pediatric considerations. Neurosurg Clin North Am. 1993;4:301-310.
16. Huppertz H.J., Grimm C., Fauser S., et al. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res,1-2, 2005;67:35–50
17. Smyth M.D., Limbrick D.D., Ojemann J.G., et al. Outcome following surgery for temporal lobe epilepsy with hippocampal involvement in preadolescent children: emphasis on mesial temporal sclerosis. J Neurosurg. 2007;106(suppl 3 Pediatrics):205-210.
18. Theodore W.H., Gaillard W.D. Positron emission tomography in neocortical epilepsies. Adv Neurol. 2000;84:435-446.
19. Kaminska A., Chiron C., Ville D., et al. Ictal SPECT in children with epilepsy: comparison with intracranial EEG and relation to post-surgical outcome. Brain. 2003;126:248-260.
20. Pataraia E., Baumgartner C., Lindinger G., et al. Magnetoencephalography in presurgical epilepsy evaluation. Neurosurg Rev. 2002;25:141-151.
21. Harvey A.S., Cross J.H., Shinnar S. ILAE Pediatric Epilepsy Surgery Survey Taskforce. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146-155.
22. Hemb M., Velasco T.R., Parnes M.S., et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986-2008. Neurology. 74, 2010. 1786–1775
23. Berger M.S., Ghatan S., Haglund M.M., et al. Low grade glioma associated with intractable epilepsy: seizure outcome utilizing electrocorticography during tumor resection. J Neurosurg. 1993;79:62-69.
24. Berger M.S., Ghatan S., Geyer J.R., et al. Seizure outcome in children with hemispheric tumors and associated intractable epilepsy: the role of tumor removal combined with seizure foci resection. Pediatr Neurosurg. 1991;17:185-191.
25. Everett L.L., van Rooyen I.F., Warner M.H., et al. Use of dexmedetomidine in awake craniotomy in adolescents: report of two cases. Paediatr Anaesth. 2006;16:338-342.
26. Goldring S. A method for surgical management of focal epilepsy, especially as it relates to children. J Neurosurg. 1978;49:344-356.
27. Goldring S., Gregorie E.M. Surgical management of epilepsy using epidural recording to localize the seizure focus: review of 100 cases. J Neurosurg. 1984;40:447-466.
28. Adelson P.D., O’Rourke D.K., Albright A.L. Chronic invasive monitoring for identifying seizure foci in children. Neurosurg Clin North Am. 1995;6:491-504.
30. Duchowny M., Jayakar P. Functional cortical mapping in children. Adv Neurol. 1993;63:149-154.
31. Swartz B.E., Rich J.R., Dwan P.S., et al. The safety and efficacy of chronically implanted subdural electrodes: a prospective study. Surg Neurol. 1996;46:87-93.
32. Wyler A.R., Ojemann G.A., Lettich E., et al. Subdural strip electrodes for localizing seizure foci in children. J Neurosurg. 1984;60:1195-1200.
33. Spencer S.S. Depth versus subdural electrode studies for unlocalized epilepsy. J Epilepsy. 1989;2:123-127.
34. Olivier A., Gloor P., Andermann F., et al. The place of stereotactic depth electrode recording in epilepsy. Appl Neurophysiol. 1985;48:395-399.
35. Johnston J.M., Mangano F.T., Ojemann J.G., et al. Complications of invasive subdural electrode monitoring at St. Louis Children’s Hospital, 1994-2005. J Neurosurg. 2006;105:343-347.
36. Hamer H.M., Morris H.H., Mascha E.J., et al. Complications of invasive video–EEG monitoring with subdural grid electrodes. Neurology. 2002;58:97-103.
37. Lee W.S., Lee J.K., Lee S.A., et al. Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol. 2000;54:346-351.
38. Simon S.L., Telfeian A., Duhaime A.C. Complications of invasive monitoring used in intractable pediatric epilepsy. Pediatr Neurosurg. 2003;38:47-52.
39. Onal C., Otsubo H., Araki T., et al. Complications of invasive subdural grid monitoring in children with epilepsy. J Neurosurg. 2003;98:1017-1026.
40. Delalande O., Pinard J., Basevant C. Hemispherotomy: a new procedure for central disconnection. Epilepsia. 1992;33:99-100.
41. Schramm J., Behrens E., Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509-515.
42. Schramm J., Kral T., Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49:891-901.
43. Shimizu H., Maehara T. Modification of peri-insular hemispherotomy and surgical results. Neurosurgery. 2000;47:367-372.
44. Villemure J.G., Mascott C.R. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37:975-981.
45. Di Rocco C., Iannelli A. Hemimegalencephaly and intractable epilepsy: complications of hemispherectomy and their correlations with surgical technique. A report on 15 cases. Pediatr Neurosurg. 2000;33:198-207.
46. Jonas R., Nguyen S., Hu B., et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology. 2004;62:1712-1721.
47. Di Rocco C., Tamburrini G. Sturge-Weber syndrome. Childs Nerv Syst. 2006;22:909-921.
48. Schropp C., Sörensen N., Krauss J. Early periinsular hemispherotomy in children with Sturge-Weber syndrome and intractable epilepsy—outcome in eight patients. Neuropediatrics. 2006;37:26-31.
49. Bien C.G., Granata T., Antozzi C., et al. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain. 2005;128:454-471.
50. Rasmussen T., Olszewski J., Lloyd-Smith D. Focal seizures due to chronic localized encephalitis. Neurology. 1958;8:435445.
51. Rosenblatt B., Vernet O., Montes J.L., et al. Continuous unilateral epileptiform discharge and language delay: effect of functional hemispherectomy on language acquisition. Epilepsia. 1998;39:787-792.
52. Maehara T., Shimizu H., Kawai K., et al. postoperative development of children after hemispherotomy. Brain Dev. 2002;24:155-160.
53. Delalande O., Bulteau C., Dellatolas G., et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60:ONS19-ONS32.
54. Wyllie E. Surgical treatment of epilepsy in pediatric patients. Can J Neurol Sci. 2000;27:106-110.
55. Beardsworth E.D., Zaidel D.W. Memory for faces in epileptic children before and after brain surgery. J Clin Exp Neuropsychol. 1994;16:589-596.
56. Lindsay J., Ounsted C., Richards P. Hemispherectomy for childhood epilepsy: a 36-year study. Dev Med Child Neurol. 1987;29:592-600.
57. Boshuisen K., van Schooneveld M.M., Leijten F.S., et al. Contralateral MRI abnormalities affect seizure and cognitive outcome after hemispherectomy. Neurology. 2010;75:1623-1630.
58. Hallbook T., Ruggieri P., Adina C., et al. Contralateral MRI abnormalities in candidates for hemispherectomy for refractory epilepsy. Epilepsia. 2010;51:556-563.
59. Curtiss S., de Bode S., Mathern G.W. Spoken language outcomes after hemispherectomy: factoring in etiology. Brain Lang. 2001;79:379-396.
60. Cook S.W., Nguyen S.T., Hu B., et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg. 2004;100:125-141.
61. Holthausen H., May T., Adams C. Seizures post hemispherectomy. In: Tuxhorn I., Holthausen H., Boenigk H. Paediatric Epilepsy Syndromes and Their Surgical Treatment. London: John Libbey, 1997.
62. Kestle J., Connolly M., Cochrane D. Pediatric peri-insular hemispherotomy. Pediatr Neurosurg. 2000;32:44-47.
63. Kossoff E.H., Vining E.P., Pyzik P.L., et al. The postoperative course and management of 106 hemidecortications. Pediatr Neurosurg. 2002;37:298-303.
64. Villemure J.G., Daniel R.T. Peri-insular hemispherotomy in paediatric epilepsy. Childs Nerv Syst. 2006;22:967-981.
65. Binder D.K., Schramm J. Transsylvian functional hemispherectomy. Childs Nerv Syst. 2006;22:960-966.
66. Flack S., Ojemann J., Haberkern C. Cerebral hemispherectomy in infants and young children. Paediatr Anaesth. 2008;18:967-973.
67. Limbrick D.D.Jr., Narayan P., Powers A.K., et al. Hemispherotomy: efficacy and analysis of seizure recurrence. J Neurosurg Pediatr. 2009;4:323-332.
68. Fuiks K.S., Wyler A., Hermann B.P., Somes G. Seizure outcome from anterior and complete corpus callosotomy. J Neurosurg. 1991;74:573-578.
69. Gates J.R., Leppik I., Yap J., Gumnint R.J. Corpus callosotomy: clinical and electroencephalographic effects. Epilepsia. 1984;25:308-316.
70. Maehara T., Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001;42:67-71.
71. Mamelak A.N., Barbaro N., Walker J.A., et al. Corpus callosotomy: a quantitative study of the extent of resection, seizure control, and neuropsychological outcome. J Neurosurgery. 1993;79:688-695.
72. Nordgren R.E., Reeves A.G., Viguera A.C., Roberts D.W. Corpus callosotomy for intractable seizures in the pediatric age group. Arch Neurol. 1991;48:364-372.
73. Cukiert A., Burattini J.A., Mariana P.P.. Extended, One-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia2, 2006;47:371-374.
74. Shim K.W., Lee Y.M., Kim H.D. Changing the paradigm of 1-stage total callosotomy for the treatment of pediatric generalized epilepsy. J Neurosurg Pediatr. 2008;2:29-36.
75. Jalilian L., Limbrick D.D., Steger-May K.. Complete versus anterior two-thirds corpus callosotomy in children: analysis of outcome. J Neurosurg Pediatr3, 2010;6:257-66.
76. Jea A., Vachhrajani S., Johnson K.M., Rutka J.T.. Corpus callosotomy in children with intractable epilepsy using frameless stereotactic neuronavigation: 12-year experience at The Hospital for Sick Children in Toronto. Neurosurg Focus3, 2008;25
77. Oguni H., Olivier A., Andermann F., Comair Y.G. Anterior callosotomy in the treatment of medically intractable epilepsies: a study of 43 patients with a mean follow-up of 39 months. Ann Neurol. 1991;30:357-364.
78. Tanriverdi T., Olivier A., Poulin N., et al. Long-term seizure outcome after corpus callosotomy: a retrospective analysis of 95 patients. J Neurosurg. 2009;110:332-342.
79. Gilliam F., Wyllie E., Kotagal P., et al. Parental assessment of functional outcome after corpus callosotomy. Epilepsia. 1996;37:753-757.
80. Yang T.F., Wong T.T., Kwan S.Y., et al. Quality of life and life satisfaction in families after a child has undergone corpus callosotomy. Epilepsia. 1996;37:76-80.
81. Kotagal P., Luders H.O. Recent advances in childhood epilepsy. Brain Dev. 1994;16:1-15.
82. Mohamed A., Wyllie E., Ruggieri P., et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology. 2001;56:1643-1649.
83. Clarke D.B., Oliver A., Anderman F., et al. Surgical treatment of epilepsy: the problem of lesion/focus incongruence. Surg Neurol. 1996;46:246-585.
84. Montes J.L., Rosenblatt B., Farmer J.P., et al. Lesionectomy of MRI detected lesions in children with epilepsy. Pediatr Neurosurg. 1995;22:167-173.
85. Clusmann H., Schramm J., Kral T., et al. Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg. 2002;97:1131-1141.
86. Spencer D.D., Spencer S.S., Mattson R.H., et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;71:667-671.
87. Schramm J. Temporal lobe epilepsy surgery and the quest for optimal extent of resection: a review. Epilepsia. 2008;49:1296-1307.
88. Silbergeld D.L., Ojemann G.A. The tailored temporal lobectomy. Neurosurg Clin North Am. 1993;4:273-281.
89. Adelson P.D. Black PMcL: Temporal lobe resections in children. Neurosurg Clin North Am. 1995;5:521-532.
90. Ojemann G.A., Ojemann J.G., Lettich E., et al. Cortical language localization in left, dominant hemisphere: an electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316-326.
91. Wieser H.G., Yasargil M.G. Selective amygdalohippocampectomy as a surgical treatment of mediobasal limbic epilepsy. Surg Neurol. 1982;17:445-457.
92. Park T.S., Bourgeois B.F., Silbergeld D.L., Dodson W. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy. J Neurosurg. 1996;85:1172-1176.
93. Thudium M.O., Campos A.R., Urbach H., Clusmann H. The basal temporal approach for mesial temporal surgery: sparing the meyer loop with navigated diffusion tensor tractography. Neurosurgery. 2010;67(suppl 2 Operative):385-390.
94. Yasargil M.G., Teddy P.J., Roth P. Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg. 1985;12:93-123.
95. Yasargil M.G., Krayenbühl N., Roth P., et al. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010;112:168-185.
96. McKhann G.M., Schoenfeld-McNeill J., Born D.E., et al. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93:44-52.
97. Egan R.A., Shults W.T., So N., et al. Visual field deficits in conventional anterior temporal lobectomy versus amygdalohippocampectomy. Neurology. 2000;55:1818-1822.
98. Colnat-Coulbois S., Mok K., Klein D., et al. Tractography of the amygdala and hippocampus: anatomical study and application to selective amygdalohippocampectomy. J Neurosurg. 2010;113:1135-1143.
99. Drane D.L., Ojemann G.A., Aylward E., et al. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46:1242-1255.
100. Hermann B.P., Wyler A.R., Sones G., Clement L. Dysnomia after left anterior temporal lobectomy without functional mapping: frequency and correlates. Neurosurgery. 1994;35:52-56.
101. Pilcher W.H., Rusyniak W.G. Complications of epilepsy surgery. Neurosurg Clin North Am. 1993;4:311-325.
102. Terra-Bustamante V.C., Inuzuca L.M., Fernandes R.M.F., et al. Temporal lobe epilepsy surgery in children and adolescents: clinical characteristics and post-surgical outcome. Seizure. 2005;14:274-281.
103. Mittal S., Montes J.L., Farmer J.P., et al. Long-term outcome after surgical treatment of temporal lobe epilepsy in children. J Neurosurg. 2005;103(suppl 5 Pediatrics):401-412.
104. Prats A.R., Morrison G., Wolf A.L. Focal cortical resections for the treatment of extratemporal epilepsy in children. Neurosurg Clin North Am. 1995;6:533-540.
105. Spooner C.G., Berkovic S.F., Mitchell L.A., et al. New-onset temporal lobe epilepsy in children: lesion on MRI predicts poor seizure outcome. Neurology. 2006;67:2147-2153.
106. Chang E.F., Gabriel R.A., Potts M.B., et al. Seizure characteristics and control after microsurgical resection of supratentorial cerebral cavernous malformations. Neurosurgery. 2009;65:31-37.
107. Van Gompel J.J., Rubio J., Cascino G.D., et al. Electrocorticography-guided resection of temporal cavernoma: is electrocorticography warranted and does it alter the surgical approach? J Neurosurg. 2009;110:1179-1185.
108. Paolicchi J.M., Jayakar P., Dean P., et al. Predictors of outcome in pediatric epilepsy surgery. Neurology. 2000;54:642-647.
109. Mathern G.W. Challenges in the surgical treatment of epilepsy patients with cortical dysplasia. Epilepsia. 2009;50(suppl 9):45-50.
110. Hauser W.A., Annegers J.F., Kurland L.T. Prevalence of epilepsy in Rochester, Minnesota. Epilepsia. 1991;32:429-445.
111. Kan P., Van Orman C., Kestle J.R. Outcomes after surgery for focal epilepsy in children. Childs Nerv Syst. 2008;24:587-591.
112. Salamon N., Kung J., Shaw S.J., et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008;71:1594-1601.
113. Wu J.Y., Salamon N., Kirsch H.E., et al. Noninvasive testing, early surgery, and seizure freedom in tuberous sclerosis complex. Neurology. 2010;74:392-398.
114. Morrell F., Whisler W.W., Bleck T.P. Multiple subpial transections: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989;70:231-239.
115. Wyler A.R., Wilkus R.J., Rostad S.W., Vossler D.G. Multiple subpial transections for partial seizures in sensorimotor cortex. Neurosurg. 1995;37:1122-1127.
116. Wyllie E., Comair Y.G., Kotagal P., et al. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740-748.
117. Spencer S.S., Schramm J., Wyler A., et al. Multiple subpial transaction for intractable partial epilepsy: an international meta-analysis. Epilepsia. 2002;43:141-145.