Management of Vascular Anomalies of the Face
Introduction
For the purpose of this chapter, the term vascular lesion or vascular anomaly is used to define a variety of cutaneous neoplasms and malformations originating from blood vessels and related structures. Owing to major differences in prognosis and treatment strategies, it is imperative to accurately differentiate between these various abnormalities. Vascular lesions can be divided into two main groups: vascular tumors and vascular malformations. Overall, vascular anomalies affect approximately 8% to 10% of births worldwide, which is nearly 400,000 new cases per year in the United States alone.1
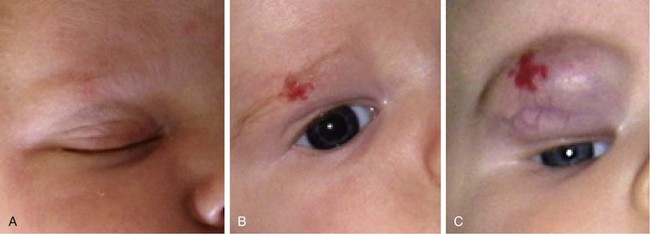
Infantile hemangiomas, the most common vascular anomaly, are the most common benign tumor occurring in infancy and childhood and are present in about 2% of neonates. They are true tumors exhibiting the features of all neoplasms, such as increased mitosis and hyperplasia. Although up to 30% of these tumors may be present at birth, the majority become apparent in the first weeks of life. Infantile hemangiomas have a unique and characteristic natural history.1,2 During the first few months of life, they increase in size by proliferation (hyperplasia) and involve skin, mucosa, and subcutaneous tissues to variable degrees. Proliferation typically ends within the first 4 to 6 months and is followed by a quiescent plateau before regression begins. Rarely, growth may continue for 12 to 14 months after birth (Fig. 28-1).
The end of the proliferative phase marks the beginning of the involutional phase. During this phase, which may last years, the hemangioma undergoes varying amounts of regression in size and replacement with fibrofatty tissue. Approximately 30% to 40% of infantile hemangiomas involute to a cosmetically and functionally acceptable point and do not require any treatment. Necessary intervention is sought by the remaining majority of patients. Cutaneous hemangiomas may involve papillary dermis (superficial), papillary and reticular dermis, subcutaneous tissue (deep), or both skin and subcutaneous tissue (compound). They are most commonly focal, well-defined growths or diffuse, involving dermatome-like segments of skin. There are sites of predilection on the face (midface, nose, lips) for the development of hemangiomas.3
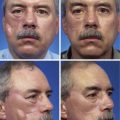
After involution of hemangiomas is complete, superficial hemangiomas leave atrophic redundant skin manifesting variable degrees of telangiectasia, whereas deep hemangiomas leave a residual mass of fibrofatty tissue. Involuting compound hemangiomas show varying degrees of all these features (Fig. 28-2).
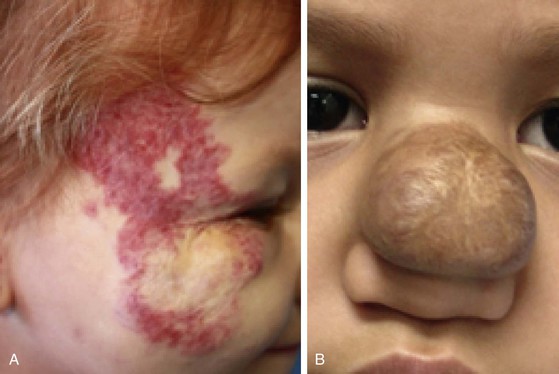
FIGURE 28-2 Examples of involuting (A) and fully involuted (B) infantile hemangiomas. Note the residual telangiectasias, expanded skin, fibrofatty residual mass effect, and textural changes.
Treatment of infantile hemangiomas is dependent on multiple factors including the stage of its natural history, the anatomic location of the tumor, and the age of the child.4 Accurate diagnosis and treatment of infantile hemangiomas are entirely dependent on clinical history and physical examination. Imaging studies are rarely required and are of limited importance.
Congenital hemangiomas are completely formed and present at birth and are very different from infantile hemangiomas in their natural history and prognosis. Rapidly involuting congenital hemangiomas appear fully formed at birth, do not proliferate, and regress within the first year of life or do not (non–rapidly involuting hemangiomas).5 There are other, rarer vascular tumors that are beyond the scope of this chapter.1 Terms such as strawberry or capillary angioma and cavernous hemangioma are of historic and folkloric interest and should not be used in communicating about these abnormalities. More than 60% of infantile hemangiomas occur on the head and neck, predominantly in white children and somewhat less commonly in those of African or Asian descent. For unclear reasons, female neonates are more likely to be affected than male neonates in a 3 to 5 : 1 ratio. Infantile hemangiomas are more common in premature infants. The increased prevalence in premature neonates correlates with decreasing gestational age and birth weight.6,7 A plethora of new knowledge links infantile hemangiomas and placenta tissue. Many of the characteristic molecular markers of infantile hemangioma blood vessels are expressed uniquely by normal fetal microvessels. In particular, GLUT-1 (glucose transporter protein) is exclusively demonstrated by infantile hemangioma and placenta but not by other vascular anomalies or other normal tissues. There is also a striking similarity between the infantile hemangioma mRNA transcriptome and that of placenta. The leading hypothesis concerning this relationship is that fetal placental or precursor cells metastasize from the placenta to the baby and become implanted in areas of high flow, such as the head and neck. Little is known about the mechanism of this process; however, the link between placental and hemangioma cells is irrefutable.8
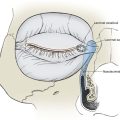
In contrast to infantile hemangiomas, vascular malformations (Fig. 28-3) are always present at birth, although they may not be apparent. They enlarge by hypertrophy, never proliferate, and never involute. They are true developmental anomalies, not neoplasms. Their rate of hypertrophy and hence their functional and cosmetic significance are extremely variable. Vascular malformations may originate from capillaries, veins, venules, lymphatics, arterioles, or any combination of these structures. Similar to hemangiomas, vascular malformations may involve skin, subcutaneous tissues, and mucosa. Like hemangiomas, they may be superficial, deep, compound, focal, or diffuse.9
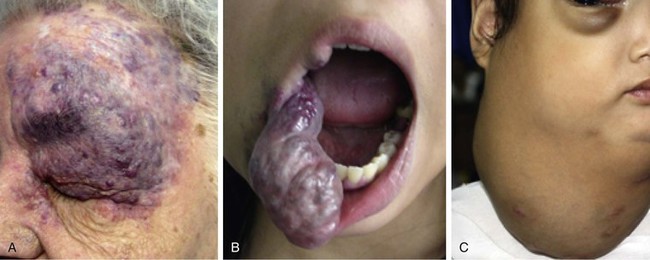
FIGURE 28-3 Examples of vascular malformations. A, Capillary malformation (port-wine stain). B, Venous malformation. C, Lymphatic malformation.
Venular malformations, known as port-wine stains, are made up of ectatic postcapillary venules. Although they may be manifested as flat pink macules at the beginning of life, they usually darken and thicken with advancing age, forming a cobblestone appearance as the dermal vessels continue to dilate under constant hydrostatic pressure. Their distribution patterns often correspond to dermatomes, and the presumed etiology is deficient or ineffective postcapillary venule innervation.10 Although this is the presumed mechanism, no definitive evidence exists to prove it.
Venous malformations are composed of ectatic veins. They are usually observed in the lips, tongue, floor of mouth, and buccal spaces. Patients suffering from venous malformations frequently complain of swelling with dependency, pain, limitations in the function of the affected region, and cosmetic deformity. Superficial venous malformations are visible as purple masses. Deeper lesions are manifested as bluish or colorless subcutaneous masses.
Arteriovenous malformations are rare vascular lesions originating from arteriovenous channels that failed to regress during fetal development.9 A palpable mass with an obvious well-developed arterial supply and dilated tortuous veins are typical features. A murmur may be heard or a thrill may be palpated over the mass. Malformations must be differentiated from arteriovenous fistulas, which are usually precipitated by trauma.
In contrast to dealing with infantile hemangiomas, imaging studies are important in assisting with establishment of an accurate diagnosis and planning of treatment of vascular malformations. Magnetic resonance imaging, angiography, and ultrasonography are important diagnostic tools, and the particulars of differentiating the different types of vascular malformations by radiographic studies have been well described.11
Treatment
Infantile Hemangiomas
There is no accepted consensus on the treatment of infantile hemangiomas, although the prevailing trend is to intervene rather than to follow the old dictum of benign neglect (“leave it alone, it will go away”). This is supported by the fact that a significant number of hemangiomas incompletely involute regardless of how long patients are willing to wait. Incomplete involution often results in a cosmetic deformity necessitating treatment because of the high social impact of facial differences. In addition, the psychology literature has well-documented the impact of physical appearance on the development of self-image of children between the ages of 2 and 5 years.12,13 Therefore, early treatment of hemangiomas is justified. There are numerous treatment options available. The goal of treatment is to restore to the child as much as possible normal appearance and function. This ideally should be accomplished by the age of 3 years and no later than elementary school age. Treatment of hemangiomas depends on the anatomic location and functional and cosmetic impairment the neoplasm is causing. The depth of tissue involvement, the presence of complicating factors (ulceration, visual axis impingement), and whether the hemangioma is proliferating or involuting also influence the approach to treatment.
An important decision to make in treatment of hemangiomas is whether to intervene and what treatment modality to use. This decision is best made by asking the question, Can the surgeon obtain a result with available treatment modalities that is as effective as allowing the hemangioma to follow its natural course? If the answer is yes, intervention is justified. If the answer is no, then observation is continued until a predetermined point of re-evaluation. Serial observation is an active treatment option and is very different from telling parents of children afflicted with hemangiomas to wait an indeterminate number of years for the hemangioma to “go away.” In addition to observation, during the proliferative period, treatment may include medical therapy, laser treatment, surgical excision, or a combination of these treatments.
Superficial cutaneous hemangiomas may be treated with a yellow light laser, such as the flashlamp-pumped pulsed dye laser (PDL; 595-nm wavelength). The goal is to achieve complete resolution of superficial lesions thinner than 1.5 mm. With use of the PDL, lightening and retardation of growth may be accomplished with thicker lesions in preparation for other treatment modalities. If the lesion is compound, the superficial component may be treated with the laser first in preparation for surgical excision of the deep component. The PDL is very useful in assisting the healing of ulcerating hemangiomas and preventing further scarring from tissue destruction while waiting for other interventions.14
Rapidly proliferating hemangiomas that pose a serious functional or cosmetic concern may be treated with medical therapy. Before 2008, corticosteroids were the first line of medical therapy. Since then, propranolol has become the first drug of choice.15,16 Like corticosteroids, propranolol seems to be most useful during the proliferative phase. There is some reason to think, however, that it may be useful during involution as well. Whereas steroids exerted their effect through blunting of neovasculogenesis, propranolol seems to have an effect by vasoconstriction, inhibiting vasculogenesis and promoting apoptosis.17 Much work needs to be done to determine the exact role for propranolol in the treatment algorithm.
Hemangiomas that are life-threatening or do not respond to propranolol or steroid therapy can be treated with other medications.15
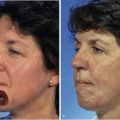
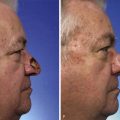
Surgical management is integral to the overall treatment algorithm of infantile hemangioma.2 Historical misgivings and misconceptions about the operability of hemangiomas have been supplanted by experience and a better understanding of these tumors. Surgical planes exist between hemangiomas and surrounding structures and can be created between the superficial and deep components or within the deep component (Fig. 28-4) of hemangiomas. Hemangiomas are solid tumors with few isolated feeding vessels. Therefore, meticulous technique and the routine use of unipolar and bipolar microneedle electrocautery devices make dissection virtually bloodless. Conservatism is critical in resecting facial tissue involved with hemangioma in children. The use of flaps and grafts is avoided as much as possible. The goal is to resect sufficient tissue and still be able to achieve primary closure of the wound. When this is not possible because of size or location of the tumor, serial excision has proved helpful as with other benign tumors.18 The author avoids the use of flaps and grafts in small children except in the most severe, complicated cases in which additional scars resulting from use of sophisticated techniques are justified. To preserve contour or to set the stage for an additional resection, subtotal excision of the deep component of a hemangioma is commonly performed. To minimize the potential psychological sequelae on a child afflicted with a hemangioma, every effort is made to obtain the best possible functional and cosmetic result before the child reaches school age. The author’s threshold for excision of nasal tip and periorbital hemangiomas is lower than for other sites because of the obvious potential for severe functional and cosmetic impairment.19 Recently, the early treatment of nasal hemangiomas has been supported by the best available evidence. Treatment is geared to obtaining the best possible result by the social and developmental milestones previously described. The superficial component of compound hemangiomas is treated with photocoagulation if it cannot be entirely resected together with the deep portion.
Capillary Malformations
Laser photocoagulation is the preferred method of treating capillary and venular malformations (port-wine stains). Safe treatment parameters are used for initial PDL treatments to evaluate the clinical response and to set the stage for further treatments.20 Port-wine stains are typically treated every 6 weeks to a point of improvement that is acceptable to the patient and surgeon. Because the underlying vessel abnormalities are not cured, patients are prepared for the necessity of occasional re-treatment if the port-wine stain darkens during ensuing years. Photocoagulation of a thickened or “cobblestoned” port-wine stain can be performed with a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser in noncontact mode. Surgical excision of hypertrophic port-wine stains or associated venous malformations is a useful adjunct in management of selected lesions. Adjacent skin flaps, tissue expansion, microvascular tissue transfer, and other reconstructive techniques are all useful for replacement of the involved tissues.20,21
Venous Malformations
Venous malformations may be treated with laser photocoagulation, sclerotherapy, or surgical resection, depending on the depth, extent, and location of the lesion (Fig. 28-5). A superficial lesion or the superficial component of a compound malformation is best treated with Nd:YAG laser photocoagulation as described before. Laser photocoagulation diminishes the vascularity of the overlying skin or mucosa, which may then be preserved during surgical resection of the deeper component. The deep component of the malformation is subsequently carefully resected because of the risk of bleeding due to extremely fragile ectatic vessels.
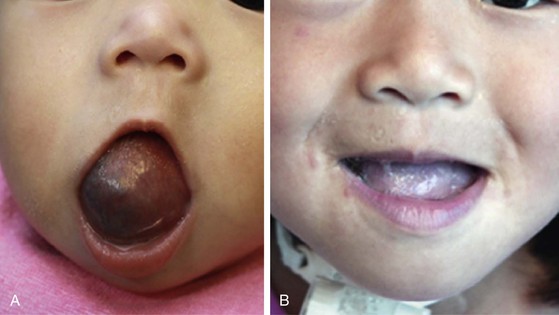
FIGURE 28-5 Venous malformation of the tongue before (A) and after (B) sclerotherapy and interstitial and transmucosal Nd:YAG laser treatment.
Sclerotherapy and embolization are the primary modalities of treatment of venous malformations. They are also useful as preoperative and postoperative adjunctive treatment. Sclerotherapy is accomplished by percutaneous puncture into the venous malformation with fluoroscopic guidance. An irritant, such as alcohol or bleomycin, is injected into the malformation to promote clotting, inflammation, and eventually fibrosis of the lesion. Sclerotherapy may require repeated treatments to maintain control of the malformation and usually is not considered curative as the lesion may eventually re-expand. Sclerosing agents, such as absolute alcohol, sodium tetradecyl, sodium morrhuate, polidocanol, sclerosant foam, and ethanolamine, have been used to treat venous malformations. The amount of sclerosing agent injected depends on the agent employed and the extent of the venous malformation. Treatments are spaced at 4- to 6-week intervals. Because veins in the head and neck region lack valves, injection of sclerosing agents into malformations located in the superior and central third of the face may risk cavernous sinus thrombosis and should be done by an interventional radiologist under image guidance.22
Arteriovenous Malformations
Treatment with lasers, steroids, or irradiation has not been effective in the management of arteriovenous malformations. Complete surgical excision is difficult and accompanied by morbidity. If the malformation is resectable, combined treatment is appropriate and consists of highly selective embolization followed by resection within the following 24 to 48 hours. The natural progression of arteriovenous malformations is inexorable growth over time. Therefore, the main goal of surgery is complete eradication with surgical margins free of involvement to prevent recurrence. The sacrifice of structures involved by an arteriovenous malformation (e.g., mandible, facial nerve, muscles of mastication) may be a necessary part of the treatment. The surgical and anesthetic team must be prepared to replace blood loss with blood products, using Cell Saver technology in the more difficult cases. Resection and reconstruction of these and other related malformations are more akin to traditional head and neck cancer procedures than those for resection of infantile hemangiomas. Because of the associated morbidities and low likelihood of cure, interventional radiology procedures are the mainstay of treatment for most.9 Research and advances in medical therapy are likely to be the source for curative treatment in the future for these lesions.
Lymphatic Malformations
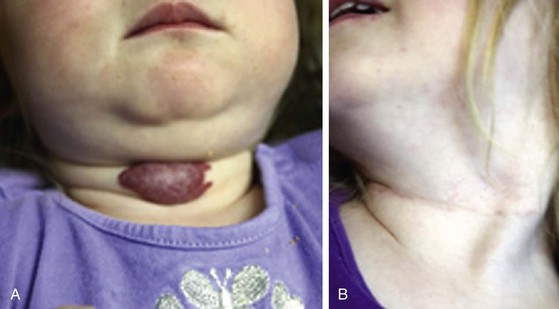
Surgical excision has been the preferred treatment modality for lymphatic malformations that are focal and discrete. However, interventional therapy is supplanting this approach because of the lower morbidity. Because of the difficulty of distinguishing involved tissue from normal tissue, complete resection of lymphatic malformations with microcystic infiltrating features is not always possible. Malformations with well-defined macrocystic features are more likely to be completely resected. Superficial mucosal lymphatic malformations may be treated with a carbon dioxide laser using 20-W continuous mode until sufficient depth of destruction is obtained. The wound is then left to heal by secondary intention. Extensive lesions involving both mucosa and underlying soft tissue may require a combined approach. Recurrence after “total” resection of macrocystic malformations is probably due to the failure to completely remove infiltrating lymphatic tissue at the interface of resected tissue and normal-appearing tissue. Prenatal diagnosis of large cervical lymphatic malformations allows appropriate management at birth, including airway control. OK-432 (a lyophilized mixture of a low-virulent group A Streptococcus pyogenes incubated with penicillin G) has been successively used to treat lymphatic malformations but is not approved for general use in the United States. OK-432 has been used extensively in Europe and Japan, with results showing up to 96% complete response of macrocystic malformations. Bleomycin has also been used with similar results and is the sclerosant of choice in the United States. Overall, the literature continues to report good results with sclerotherapy or surgery in patients with macrocystic disease only. Microcystic disease, especially if it is extensive, is likely to require multiple therapies to keep the disease in check.11,23
References
1. Marler, J, Mulliken, J. Vascular anomalies—classification, diagnosis and natural history. Facial Plast Surg Clin North Am. 2001; 9:495.
2. Hochman, M, Adams, D, Reeves, T. Current knowledge and management of vascular anomalies, I. Hemangiomas. Arch Facial Plast Surg. 2011; 13:135.
3. Waner, M, North, P, Scherer, KA, et al. The non-random distribution of facial hemangiomas. Arch Dermatol. 2003; 139:869.
4. William, EF, Stanislaw, P, Dupree, M. Hemangiomas in infants and children: an algorithm for intervention. Arch Facial Plast Surg. 2000; 2:103.
5. Krol, A, MacArthur, CJ. Congenital hemangiomas. Arch Facial Plast Surg. 2005; 7:307.
6. Powell, TG, West, CR, Pharoah, PO, et al. Epidemiology of strawberry hemangioma in low birthweight infants. Br J Dermatol. 1987; 116:635.
7. Amir, J, Mezker, A, Krikler, R, Reisner, SH. Strawberry hemangioma in preterm infants. Pediatr Dermatol. 1986; 3:331.
8. Phung, TL, Hochman, M. Pathogenesis of infantile hemangiomas. Facial Plast Surg. 2012; 28:554.
9. Hochman, M, Adams, D, Reeves, T. Current knowledge and management of vascular anomalies, II. Malformations. Arch Facial Plast Surg. 2011; 13:425.
10. Smollen, BR, Rosen, S. Port wine stains: a disease of altered neural modulation of blood vessels? Arch Dermatol. 1986; 122:177.
11. Balakrishnan, K, Perkins, J. Management of head and neck lymphatic malformations. Facial Plast Surg. 2012; 28:596.
12. Lande, RG, Crawford, PM, Ramsey, B. Psychosocial impact of vascular birthmarks. Facial Plast Surg Clin North Am. 2001; 9:561.
13. Williams, EF, Hochman, M, Rodgers, BJ, et al. A psychological profile of children and families afflicted with hemangiomas. Arch Facial Plast Surg. 2003; 5:220.
14. Thomas, RT, Hornug, R, Manning, S, et al. Hemangiomas of infancy: treatment of ulceration. Arch Facial Plast Surg. 2005; 7:312.
15. Blei, F. Medical management of vascular anomalies. Facial Plast Surg. 2012; 28:575.
16. Drolet, BA, Frommelt, PC, Charin, K, et al. Initiation and use of propranolol for infantile hemangiomas: report of a consensus conference. Pediatrics. 2013; 131:128.
17. Storch, CH, Hoeger, PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010; 163:269.
18. Kulbersh, J, Hochman, M. Serial excision of facial hemangiomas. Arch Facial Plast Surg. 2011; 13:199.
19. Hochman, M, Mascareno, A. Management of nasal hemangiomas. Arch Facial Plast Surg. 2005; 7:295.
20. Kelly, KM, Choi, B, McFarlane, S. Distribution and analysis of treatment for port wine stains. Arch Facial Plast Surg. 2005; 7:287.
21. Ortiz, A, Nelson, JS. Port-wine stain laser treatment and novel approaches. Facial Plast Surg. 2012; 28:611.
22. Richter, G, Braswell, L. Management of venous malformations. Facial Plast Surg. 2012; 28:603.
23. Banieghbal, B, Davies, MR. Guidelines for the successful treatment of lymphangioma with OK-432. Eur J Pediatr Surg. 2003; 13:103.