Chapter 148 Management of Tuberculous Infections of the Nervous System
Infection of the central nervous system (CNS) by Mycobacterium tuberculosis is invariably secondary to a primary focus elsewhere in the body. The avium, bovine, and atypical mycobacteria are rarely isolated from the nonimmunocompromised host. The primary sites are usually pulmonary, bone, and gastrointestinal tract; genitourinary sites are less common. The incidence of CNS tuberculosis (TB) is a reflection of the overall incidence of TB in a population. A third of the global population (i.e., 2 billion people) harbors the M. tuberculosis bacillus and is at risk of developing active disease. It is still a leading cause of mortality from a single infectious disease.1 The epidemiology of the disease has been greatly affected by the advent of the human immunodeficiency virus (HIV);2–5 emergence of multidrug-resistant TB (MDR TB) and extensively drug-resistant strains of TB (XDR TB); widespread intravenous drug abuse; inadequate case detection, diagnosis, and therapy; collapse of health infrastructures due to economic crisis and war; and continuing poverty.
The health burden related to TB has prompted the implementation of World Health Organization’s (WHO’s) Stop TB Strategy, which includes the following goals: (1) TB incidence should fall by 2015, (2) prevalence and death rates should be halved by 2015 from the baseline of 1990, (3) at least 70% of incident smear-positive cases should be detected and treated in the Direct Observation of Treatment (DOTS) program, and (4) at least 85% of detected incident smear-positive cases should be successfully treated.6 The WHO has also been publishing an annual report on global control of TB since 1997 to provide a comprehensive and up-to-date assessment of the TB epidemic and progress in controlling the disease. The trends suggest that neither the prevalence nor the death rate would be halved in Africa and Europe by 2015. An estimated 37% of incident TB cases are not being treated in the DOTS program, 96% of incident case with MDR TB are not being diagnosed and treated according to guidelines, the majority of HIV-positive TB cases do not know their HIV status, and of those who do, the majority do not have access to antiretroviral therapy.1
In the United States,7 the average decline of 5% per year in the TB case rate plateaued, and from 1986 to 1992 there was an increase in the number of reported cases.4 Subsequent to aggressive management of TB, the average annual percentage decline in TB was 7.3% per year during the period from 1993 to 2000 and 3.8% during the period from 2000 to 2008, along with a 75% reduction in drug resistance.8
HIV and TB Coinfection
An estimated 9.27 million new cases of TB (15% of which were among HIV–positive patients) were reported in 2007, with Asia and Africa accounting for almost 90% of them. There were 1.75 million deaths, 25% in HIV-positive individuals. There is a clear synergistic relation between HIV and TB. Being HIV-positive increases the likelihood of developing TB 20 to 37 times.1,9 In Asia and the Pacific region, as much as 40% to 70% of HIV patients have TB.10 The tubercle bacillus has been demonstrated to enhance the replication of HIV by transcriptional activation.11,12 The increased susceptibility and accelerated natural history of TB with HIV infection leads to more rapid creation of drug resistance.
TB of the nervous system, which merits the attention of a neurosurgeon, occurs in several forms, and more than one form may be present in the same individual (Table 148-1). This chapter deals only with tuberculomas, tuberculous meningitis (TBM), and tuberculous spinal arachnoiditis. Pott’s disease of the spine and tubercular encephalopathy are extensively described in orthopedic and neurologic texts. The significant threat to life and/or a risk of subsequent severe handicap merits the classification of all CNS-related TB as severe extrapulmonary TB.
| Anatomic Area | Manifestation |
|---|---|
| Intracranial Tuberculosis | |
| Parenchymal | Tuberculoma |
| Abscess | |
| Tubercular encephalopathy | |
| Meningeal | Chronic meningitis |
| Calvarial | Osteomyelitis |
| Spinal Tuberculosis | |
| Vertebral | Pott’s disease of the spine |
| Meningeal | Arachnoiditis |
| Parenchymal | Tuberculoma |
Tuberculomas
Incidence
The incidence of tuberculomas in India, which comprised 20% to 30% of all intracranial space-occupying lesions in the 1950s and 1960s, has declined since 1980.13–16 Although TB is widely prevalent in Nigeria17 and Taiwan,18 tuberculomas are rare. Tuberculomas are increasingly reported in industrialized nations and account for 1% to 2% of all intracranial lesions.19–22
Location
Tuberculomas can occur at any site in the brain, most commonly the various lobes and the cerebellum. Unusual sites of tuberculomas include the dura mater,20–23 subdural space, orbital fissure, intraventricular, brain stem,21,24–26 pituitary gland,27–31 and hypothalamus.32 Spinal intramedullary tuberculomas are rare.33–39
Pathologic Features
The typical mature tuberculoma is a solid, creamy white, well-defined, avascular mass with multiple nubbins encased in a firm gliotic capsule and extending into and compressing the surrounding brain. The cut section is pale yellow with an often gritty caseating central core.40 The immature form consists of multiple small tubercles, some with caseating or liquefied centers dispersed within an edematous brain. Severe edema, possibly caused by an allergic response,40 may surround these tubercles. Tuberculomas vary in size from 1.5 to 8 cm. Giant tuberculomas can occupy an entire cerebral hemisphere,41 and many adhere to the dura. The dural attachment can be tenuous or so firm that the tumor resembles a meningioma.
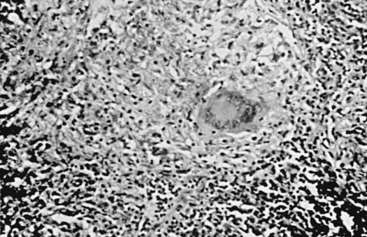
Microscopically, the central zone of caseous necrosis is surrounded by tuberculous granulation tissue consisting of epithelioid cells, Langerhans giant cells, and some lymphocytes, polymorphonuclear leukocytes, and plasma cells (Fig. 148-1). Acid-fast bacilli, although sparse, are usually present in both layers. The brain surrounding a tuberculoma may show degenerated axons and nerve cells, thrombosed vessels, and occasionally, swollen astrocytes and oligodendroglial cells. The changes in the small vessels can lead to microhemorrhages or microinfarcts, and these areas may coalesce.42 Smaller satellite tuberculomas may surround the main mass.
Tuberculomas can take several unusual forms,43,44 representing the spectrum of inflammatory reaction: (1) incipient tuberculoma, which may appear as an irregular, fleshy, gray cortical mass with associated meningeal tuberculomatosis or even grape-like clusters of tuberculoma along cerebral vessels; (2) subdural cyst overlying an intracerebral tuberculoma; (3) cystic tuberculoma; (4) tubercular abscess; (5) extensive edematous encephalopathy without a tuberculoma; (6) severe cerebral edema with a small, “inconsequential” tuberculoma; and (7) rarely, tuberculoma that has spread transdurally to the calvarium.
TB is a classic example of a disease the resistance to which is mediated by cellular immunity. The nature of the immunologic compromise in HIV, with its major effect on cellular immunity, increases host susceptibility to TB and abscess formation. Chronic inflammatory granulomas seen in immunocompetent patients are less common in patients with HIV.45 Organisms belonging to the Mycobacterium avium–intracellulare complex are the most common cause of systemic bacterial infection in patients with HIV and have been demonstrated in 50% of such patients coming to autopsy.46 Tubercular abscess is a distinct entity from a tuberculoma with central liquefaction. The abscess has a wall of chronic inflammatory cells without tubercular granulomas, and the “pus” contains a large number of acid-fast bacilli.
The liquefaction produced by hydrolytic enzymes released from brain tissue is thought to allow tubercle bacilli to proliferate, leading to abscess formation. Enzyme inhibitors from dead bacilli and necrotic tissue in caseous material have been reported to prevent liquefaction in tuberculous lesions.47 The vessels in the reactive border zone of tuberculomas show marked proliferation of the basement membrane into several concentric layers associated with fragmentation. This basement membrane, consisting mainly of glycoproteins, may act as a newly formed antigen, initiating a cellular antibody reaction that results in vasculitis and brain damage.48
Clinical Features
Tuberculoma is a disease of the young, with 70% of patients younger than 30 years. However, it is uncommon in children under 4 years of age. Both sexes are equally affected.40,49
The signs and symptoms of tuberculomas resemble those of other intracranial space-occupying lesions. As they enlarge gradually, the clinical picture is one of a slowly progressive lesion, although in at least 50% of patients, the symptoms are less than 6 months in duration. Features helpful in distinguishing tuberculomas from other brain tumors are constitutional symptoms, such as weight loss, fever, or malaise; a history of active or known TB elsewhere in the body; close contact with a patient with an open case of TB; a high frequency of seizures, even in association with a cerebellar lesion; a positive result on the Mantoux test; and an increased sedimentation rate. Infants and young children may have an enlarging head. The clinical diagnosis is often presumptive. Pyrexia is variable and may not be present in more than 20% to 25% of patients,49 and the Mantoux test result may be negative.40 The clinical course may uncommonly show spontaneous remissions and relapses.24 Clinical evidence of an active focus of TB, such as the lungs and lymph glands, may be present in only 33% of patients40 and in approximately 10% of close relatives. Rare signs include scalp swelling, cerebrospinal fluid (CSF) rhinorrhea, features of a pituitary tumor,27–31 unilateral proptosis, and trigeminal neuralgia.40 The clinical picture may be confusing when multiple lesions are present. Intramedullary tuberculomas with no evidence of extracranial TB are clinically indistinguishable from intramedullary tumors; the diagnosis may be suspected on magnetic resonance imaging (MRI) and is usually established at surgery.
Extrapulmonary manifestations, particularly CNS involvement, are frequently seen in patients with HIV.50 Seizures, headaches, and an altered mental state are common presentations, but fever is often absent. The infection is usually a reactivation of latent TB.51
The clinical setting of a rare but well-described paradoxical response to antituberculous drugs52–56 has been reviewed by Hejazi and Hassler.54 Most of these patients were young adults in whom inoperable intracranial tuberculomas located in high-risk regions had developed while they were receiving adequate antitubercular therapy. Frequently, intracranial tuberculomas develop or enlarge at a stage when systemic TB is responding to therapy. This paradoxical response is attributed to the load of antigenically active dead bacteria in a setting of a hypersensitive cell-mediated response. In this group of patients, associated TBM was a common feature. In TBM, symptoms of increased intracranial pressure (ICP) and development of focal neurologic signs, such as motor weakness, cerebellar signs, field defects, visual compromise, and behavioral problems in children, necessitate a search for expanding tuberculomas.
Radiographic Features
An abnormal chest radiograph is a pointer to the diagnosis of a tuberculoma.57–60 More sensitive is a computed tomography (CT) scan of the chest in picking up a tubercular involvement. Calcification occurs in fewer than 6% of tuberculomas and is rarely extensive or dense,41 with the striking exceptions of the Inuit (Eskimos) and North American Indians, in whom nearly 60% of tuberculomas are known to have calcifications.46 Calcification does not indicate an inactive lesion. Cerebral angiography invariably reveals an avascular mass, although surface tuberculomas adherent to the dura may show some peripheral vascularity.41,60 An associated vascular spasm may be seen that is ascribed to tuberculous vasculitis. These angiographic findings may also be seen on magnetic resonance angiography.
Computed Tomography and Magnetic Resonance Imaging
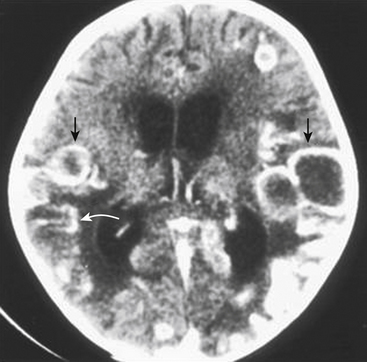
Reviews from Africa,61 Asia,58–65 the West,65 and the Middle East59 have pointed out that most tuberculomas are similar in appearance on CT (Fig. 148-2). The CT scan image of a tuberculoma is characterized by (1) a lesion that appears isodense with the brain or slightly hyperdense and enhances strongly with contrast, revealing a dense, unbroken ring of enhancement; (2) in some cases, an enhancing disc or nodular mass with a regular or irregular margin; and (3) combinations of rings and discs, which may coalesce. Uncommonly, tuberculomas may present as a nonenhancing lesion or even a strongly enhancing lesion that is indistinguishable from a meningioma. Welchman61 described the rather rare target sign, wherein a central focus of calcification and occasional enhancement is surrounded by a peripheral ring of contrast enhancement. The target sign is not specific for CNS TB and may lead to an erroneous diagnosis of TB.66
Multiplicity is common in CT scans of patients with tuberculomas. Bhargava and Tandon57,58 found that 50% to 60% of cases may demonstrate multiple lesions.
Microtuberculomas
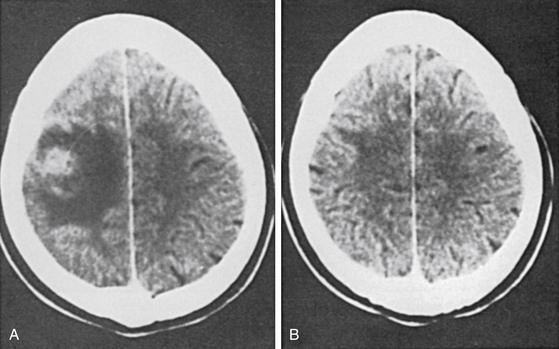
The CT scan also picks up small lesions that are less than 1.5 cm in size, disc-like or ring shaped, and single or multiple; have slightly increased attenuation that enhances with contrast; and are surrounded by disproportionately extensive low-attenuating white matter edema. Bhargava and Tandon58 labeled them microtuberculomas. Careful review of these cases suggests that not all of those lesions are tuberculous in etiology. Indian neurologists and neurosurgeons encounter these lesions frequently in children and young adults, but reports have come from other countries as well. The patients usually present with focal epilepsy and no neurologic deficit. Although some of these cases are definitely tuberculomas, as proved by biopsy, others result from a variety of causes.67–75
Goulatia et al.70 suggested that edema and increased vascular permeability due to seizures may be responsible for the CT appearance. Chandy et al.68,69 found cysticercosis as the most common cause, and Ahuja et al.67 noted that 12 of their 38 patients were seropositive for cysticercosis and two were seropositive for TB. On CT scan, tubercular lesions tend to be larger than 20 mm, more frequently irregular in outline, with a midline shift. Cysticercus cysts, in contrast, tend to be smaller than 20 mm, with a regular outline and no midline shift.73 When first seen, they could represent tuberculomas, abscesses, cysticercus granulomas, focal meningoencephalitis, astrocytomas, or metastases. A prospective study of the predictive value of CT diagnosis of intracranial TB concluded that “although the sensitivity of CT in the diagnosis of intracranial tuberculomas is 100%, and its specificity is 85.7%, the positive predictive value is only 33%.” The low positive predictive value of making a diagnosis of intracranial TB based on CT alone has been cited as a reason for obtaining histologic confirmation by open or stereotactic biopsy.74 Nearly 30% to 40% of these lesions may regress, either spontaneously or as a result of anticonvulsant drugs alone (Fig. 148-3).
Magnetic Resonance Imaging
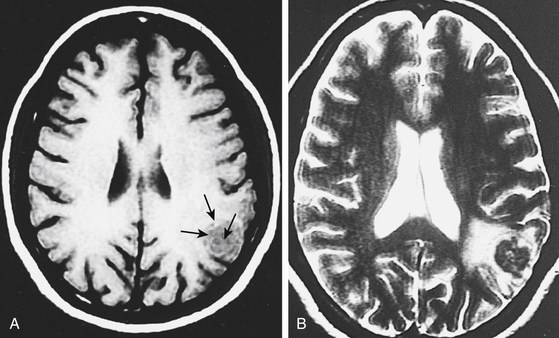
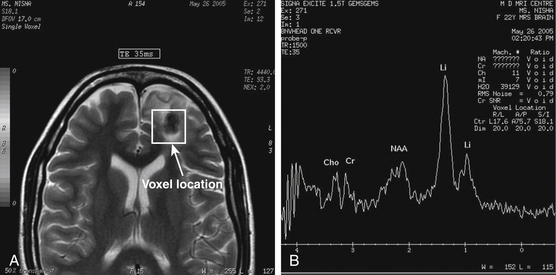
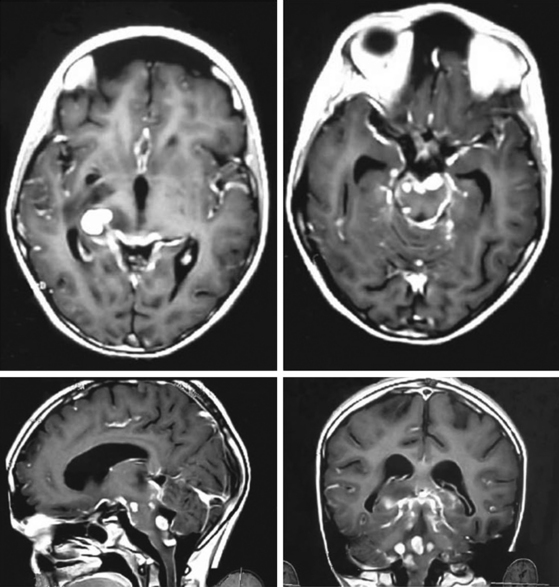
In a review of 100 consecutive cases of tuberculoma, Wasay et al. described the finding of a hypointense core surrounded by a hyperintense periphery as the most common signal characteristic on T2-weighted images; in T1-weighted images, the core was isointense with a hypointense rim (Fig. 148-4).65 This hyperintense signal on T2-weighted images made lesions stand out even when there was only minimal central liquefaction.76 On comparing MRI signal intensities with histologic results, Kim et al.77 noted that the hyperintense and hypointense rims on T1-weighted images corresponded to layers of collagenous fibers and inflammatory cellular infiltrate, respectively, whereas the central zone consisted of caseation necrosis and cellular infiltrate. T2-weighted images did not discriminate among the various layers. Gupta et al.78 found that granulomas that consisted predominantly of macrophages and gliosis were hypointense on T2-weighted images. This characteristic hypointensity of intraparenchymal tuberculomas is not found in most other space-occupying lesions.22 When the histologic pattern was one of marked cellular infiltration, with minimal gliosis, the appearance was hyperintense on T2-weighted images. In vivo proton magnetic resonance spectroscopy in hypointense lesions shows a marked increase in lipids compared with normal brain parenchyma79,80 (Fig. 148-5). In comparison to neurocysticercosis, the magnetic resonance spectroscopy of tuberculomas shows more choline and less creatinine.81–83 Although the course of TB is more fulminant in the patient with HIV, the imaging findings are similar to those in nonimmunosuppressed patients.84
Spinal intramedullary tuberculomas appear isointense or hypointense on T1-weighted images, and on T2-weighted images, the lesion is isointense, hypointense, or hyperintense, surrounded by a ring of hyperintensity because of the edema that commonly accompanies these lesions. On contrast enhancement, there is rim or nodular enhancement (Fig. 148-6).
TB of the pituitary gland is rare, and clues to a tubercular etiology include intense contrast enhancement, meningeal enhancement, and a thick pituitary stalk.28,29 Pachymeningeal TB typically is isointense on T1-weighted images and isointense to hypointense on T2-weighted images.20,23
Medical Treatment
Antituberculous Drugs
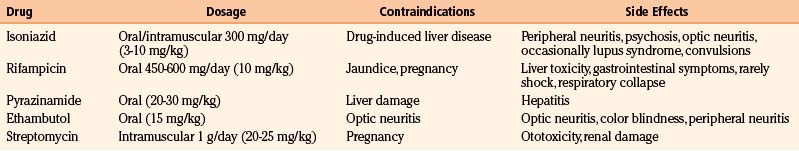
The drugs usually prescribed nearly always belong to a group of five antibiotics known to be effective in the treatment of extracranial TB (Table 148-2). The first-line agents most commonly used are isoniazid, rifampicin (rifampin), and pyrazinamide, all of which are bactericidal. Ethambutol, a bacteriostatic drug, or streptomycin, in children too young to be monitored for visual acuity, is included in the initial treatment regimen if there is a possibility of drug resistance.85
The optimal duration of treatment is not definite because, apart from early trials with streptomycin,86 there is only one controlled trial in the treatment of intracranial tuberculomas or TBM. Rajeswari et al.87 tested the efficacy of a short-course chemotherapy in the treatment of brain tuberculoma in 108 patients and concluded that a 9-month course was effective. This is in marked contrast to the treatment of pulmonary TB, which is based on data obtained from well-controlled trials. Guidelines for treating severe extrapulmonary TB suggest an initial 2 months of four drugs followed by 4 to 6 months with isoniazid and rifampicin.88 In practice, such guidelines are not commonly followed for CNS TB. At present, four drugs are administered for the initial 3 or 4 months, and two drugs are given for an additional 14 to 16 months.49,85 Occasionally, drug treatment may have to be prescribed in larger doses for 18 months to 3 years for symptomatic intracranial tuberculomas developing during treatment of TBM.89,90
Transient disturbance in liver function is often observed in patients taking a combination of isoniazid and rifampicin. This needs to be monitored at regular intervals. The incidence of serious liver disturbance appears to be higher in Asians.91 Pyridoxine (10 mg/day) is invariably added to prevent peripheral neuropathy due to isoniazid intake.
Most intracranial tuberculomas resolve with medical therapy.49,61–64,91 The clinical and radiographic improvements are a result of the reduction in the size of the tuberculoma and the perilesional edema. Regardless of their size, lesions usually start to regress after 4 to 6 weeks, and most tuberculomas resolve within 12 to 14 months of treatment. In approximately one third of cases, telltale evidence of the lesion consists of an area of calcification or sometimes just a speck of low attenuation.49 Some ring lesions change their character and become disc-like or nodular on treatment. In general, patients with increased ICP are slower to respond than those with seizures alone.
Medical treatment may occasionally result in liquefaction of the center of the lesion without any reduction in size.26 In some patients, the tuberculoma may either show no change or increase in size on use of antituberculous drugs (described earlier).54,56,92 Tuberculomas seem to enlarge and compress the surrounding brain without causing the destruction usually associated with a malignant tumor; as a result, they can resolve with minimal residual deficits. The treatment of TB in HIV-positive patients is the same as for those who are HIV negative with the exception that thioacetazone is contraindicated in the HIV-positive patients.
Drug Resistance
Drug resistance of M. tuberculosis has been recognized since the early days of streptomycin therapy. More recently, there has been an emergence of MDR TB, defined as TB that is resistant to the two most effective first-line therapeutic drugs, isoniazid and rifampicin. There are also virtually untreatable strains of MDR TB labeled as XDR TB that are also resistant to the most effective second-line therapeutic drugs: fluoroquinolones and at least one of three injectable second-line drugs used to treat TB (amikacin, kanamycin, or capreomycin). Because of the limited responsiveness of XDR TB to available antibiotics, mortality rates are similar to the preantibiotic era. The mechanism is by chromosomal mutation with emergence of resistant clones on the backdrop of inadequate drug therapy. The incidence of acquired multidrug resistance (i.e., resistance to both isoniazid and rifampicin) ranges from 0% to 48%.93,94 The WHO estimates that there are nearly 0.5 million new cases of MDR TB, which accounts for 5% of the total of 9 million new TB cases. The highest rate is in Baku, the capital of Azerbaijan, where 22.3% of new cases were MDR TB.95 High rates were reported from New York City,96 Estonia, and Latvia. A subsequent report of a decline in the prevalence of drug resistance in New York97 and in the United States as a whole,98 as well as the two Baltic countries,95 highlights the effectiveness of a strong TB program and the need for continuous surveillance of drug resistance.99
Second-line drugs include the bactericidal drugs, fluoroquinolones, amikacin, kanamycin, ethionamide, capreomycin, prothionamide, and the bacteriostatic cycloserine. Guidelines for treating MDR TB involve the use of at least four new drugs never used by the patient, including a fluoroquinolones and at least one of the three injectable drugs (amikacin, kanamycin, or capreomycin). Resistance to pyrazinamide and ethambutol is less likely, and they can be included in the initial treatment. The initial treatment is for at least 6 months, followed by a continuation phase of 12 to 18 months with the three most active and best tolerated drugs.88 Patients with organisms resistant to rifampicin and isoniazid have a high rate of treatment failure. Patients with HIV infections not only are more prone to TB but also are more susceptible to drug-resistant TB.100–102 Such patients require a longer duration of therapy and may still die of TB despite optimal treatment.102
Surgery
An appropriate craniotomy or craniectomy is performed over the site of the lesion. Perioperative ultrasonography and image guidance are useful for accurate localization of small, deep-seated lesions. A clear plane of cleavage40,41 exists between the firm, avascular tuberculoma and the edematous brain. The edema is usually not as pronounced as that associated with metastatic deposits. Tuberculous lesions are often on the cortical surface and adherent to the overlying dura. Although dural adhesions are usually separable with ease, the dural attachment at times can be extremely vascular, resembling that of meningiomas.41 After the tumor surface is identified, it is removed piecemeal from within the confines of the granuloma. The ultrasonic aspirator is a useful aid in decompression. Where the center is liquefied or necrotic, aspiration of the contents is sufficient; no attempt should be made to excise the capsule. Subcortical lesions are approached through a small corticectomy with preservation of as many vessels as possible. Parts of the tuberculoma adherent to major vessels, venous sinus, or brain stem are left in situ. The practice of frontal and temporal lobectomy or excision of edematous brain is seldom necessary to achieve decompression. Antitubercular chemotherapy is mandatory even after a complete excision of a tuberculoma. After several months of administration of antituberculous drugs, the lesion may be tough in consistency and resistant to curetting.
CT- or MRI-guided stereotactic biopsy and aspiration constitute the preferred mode of diagnosis and treatment for (1) deep-seated lesions, such as those in the thalamus or basal ganglia, and (2) tubercular abscesses or tuberculomas with a liquefied center that can be readily decompressed by this method.62 Atypical lesions also merit a stereotactic instead of an open biopsy. Although stereotactic biopsy can be quite safe,25,103 because of cost considerations, a trial with antituberculous therapy is a worthwhile alternative in patients with strong circumstantial evidence of tubercular etiology, reserving surgery only for those lesions that continue to grow despite antituberculous drugs. Stereotactic biopsy may also be a procedure of choice for patients with so-called single, small lesions, described earlier, if they fail to resolve on antiepileptic therapy.
Chiasmal decompression may be indicated for a suprasellar tuberculoma developing during treatment for TBM. Brain stem tuberculomas are a rarity and seldom require surgical decompression; they may be sampled for biopsy specimen if the diagnosis is in doubt.25 We have some reservations regarding the safety of biopsy of brain stem lesions, which can be extremely firm. A ventriculoperitoneal shunt may be required for a tuberculoma that causes hydrocephalus, resulting either from obstruction of the CSF pathway or from associated TBM. Tuberculomas of the pituitary gland are rare lesions,27–31 and a diagnosis is frequently made only after surgery. The postoperative course can be stormy with features of panhypopituitarism, such as diabetes insipidus, hypothermia, and hypotension.
Results
Initial reports of mortality ranged from 10% to 27% for intracranial tuberculomas, but the results have improved dramatically in recent years. Harder et al.59 reported no deaths in 20 cases. In our experience of 50 consecutive cases,49 1 patient died in the hospital and 1 died 2 years after treatment, probably as a result of infection with a drug-resistant organism. Both of these patients had multiple tuberculomas and markedly elevated ICP. Numerous reports have been published of patients with deep-seated, inaccessible lesions and lesions in the brain stem who have had excellent recoveries.
Tuberculous Meningitis
TBM is a major cause of morbidity and mortality in countries where pulmonary TB is still common. TBM is a disease of childhood whose highest incidence is in the first 3 years of life.104 An increasing incidence in adults has been reported, and in our experience, adults account for 50% of all patients. The incidence of TBM is five times higher in patients with HIV.105
The major neurosurgical interest in TBM is the occurrence of hydrocephalus, tuberculomas, and rarely, chiasmal and spinal arachnoiditis. In the acute stage, increased ICP is related to the general inflammatory process, increased CSF proteins, and impaired CSF absorption. When the disease becomes subacute or chronic, the inflammatory basal exudates extend along small proliferating blood vessels into the brain substance, leading to a border zone encephalitis associated with diffuse or focal ischemic changes due to vasculitis. Larger vessels, commonly the internal carotid artery siphon, its bifurcation, proximal segments of the middle cerebral artery, and sometimes the anterior cerebral artery, may get involved, leading to occlusion and infarction. The affected artery shows changes of periarteritis, massive subintimal fibrosis with narrowing or obstruction.106,107
Hydrocephalus is almost invariable in children who survive for more than 4 to 6 weeks and is most often caused by blockage of the basal cisterns and the sylvian fissures by tubercular exudates.108–110 In more chronic phases, hydrocephalus is caused by vascular adhesive arachnoiditis. In some cases, hydrocephalus may be caused by obstruction at the outlet of the fourth ventricle and less commonly by obstruction at the level of the aqueduct, either as a result of circumferential narrowing of the brain stem by exudates or of an intraluminal tuberculoma. Obstruction of CSF circulation in TBM often occurs at multiple sites.104 TBM may rarely be followed by the development of syringomyelia despite appropriate chemotherapy.111
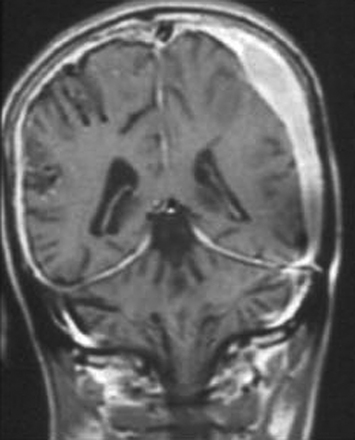
As the disease becomes more chronic, the exudates become firm and organized. The clinical evidence of meningitis disappears, leaving behind thickened, localized, hard, fibrotic leptomeninges that may form a plaque-like cover over the cerebral hemispheres, posterior fossa, foramen magnum, or spinal cord. The disease, although localized, is still active and can cause progressive symptoms.112 This condition is increasingly being recognized as a manifestation of CNS TB20,23 (Fig. 148-7) and must be differentiated from idiopathic hypertrophic pachymeningitis.
Problems of Diagnosis
In many patients, the diagnosis of TBM still poses considerable difficulties. Examination of CSF is often inconclusive, tubercle bacilli being found on direct smears in no more than 10% to 15% of initial samples. A notable exception was the report of Kennedy and Fallon,113 who isolated M. tuberculosis in 83% of 52 patients. The gold standard in the diagnosis of TB remains culturing in a Lowenstein-Jensen medium, the BACTEC radiometric system, a mycobacterial growth indicator tube, or luciferase reporter mycobacteriophage assays. The main limitation is the time taken for culture (2-6 weeks). The BACTEC 460 radiometric system can detect mycobacteria as early as 5 to 10 days.114 As an alternative to bacteriologic methods, several newer techniques have been used to diagnose TB that are faster. These include nucleic acid amplification methods and serologic tests.
Serologic tests include enzyme-linked immunosorbent assay to detect M. tuberculosis antigen 5,115–117 38-kD antigen,115 antigen 60 immunoglobulin G,118 and lipoarabinomannan antigen.116,119,120 In general, serologic tests for HIV-related TB are disappointing.115,119 Although specificity is very high, no single test gives 100% sensitivity. Future research is being directed toward identifying the best possible combination of antigens for the serodiagnosis of TB or a combination of serodiagnosis and polymerase chain reaction (PCR) to improve sensitivity.121 In the liposomal agglutination card test, a cocktail of cell wall–associated antigens incorporated onto the surface of liposomal particles react with antibodies in clinical samples to produce a blue agglutination. This test, with a sensitivity of 94%, specificity of 98.3%, low cost, and speed, makes it useful for screening large populations for active disease.122 Similarly, the Assure TB rapid test123 based on a solid phase immunochromatographic assay, relies on a cocktail of antigens.
Infinitesimal quantities of tuberculostearic acid, a component of M. tuberculosis detected by gas–liquid chromatography in CSF, are considered diagnostic of TBM.124–126 Diagnosis of TB in HIV-infected patients poses special problems. The preponderance of extrapulmonary forms with the very low number of tubercle bacilli in the available test samples makes detection difficult. The resemblance of TB to some opportunistic infections in HIV-infected patients has popularized molecular diagnosis in these patients.
On the whole, the clinical validity of nucleic acid amplification methods to detect M. tuberculosis remains controversial because, although the tests are rapid and sensitive, they remain inferior to culture with regard to sensitivity and specificity.127–130 In specific clinical situations such as nonpurulent meningitis diagnosed based on cytology and biochemistry of CSF, in which the immediate diagnosis of TB is essential, such methods are used, overriding considerations of cost and sensitivity. While the sensitivity and specificity is acceptable in smear-positive specimens, it is not so for smear-negative specimens.131 When nucleic amplification results are negative, the test has to be repeated with a fresh specimen or with material obtained from fluid culture systems after 1 to 2 weeks of incubation to maximize the sensitivity. In tropical countries, CSF cultures tend to be less sensitive than nucleic acid amplification methods. Nguyen et al.132 from Vietnam reported on 99 cases of confirmed or probable TBM based on clinical features of TBM and response to antitubercular treatment. They found that PCR had a sensitivity of 32%, culture of 17%, and microscopy of 1%. The Indian story has been similar, with positive culture rates of approximately 15% in the best of circumstances.133–134 To arrive at an early diagnosis of a disease in which any delay involves higher mortality and morbidity, Ahuja et al.134 defined a set of criteria based on clinical features, CSF examination, CT findings, and the presence of extraneural TB. Seventy-six patients suspected of having TBM were divided into definite, highly probable, probable, and possible TBM categories based on the criteria. The validity of the criteria was tested using information from bacterial isolation, PCR test for TB, response to therapy, and autopsy. On antituberculous therapy, 91% of the patients with highly probable and 66% with probable TB improved.
Advances in molecular diagnostic tests for evaluating drug resistance need to be mentioned.128,135 Molecular data are available for rifampicin, streptomycin, and isoniazid, but genetic data for other first-line drugs are partially understood136 and for second-line drugs are not available. Mutations identified in the gene encoding the ribonucleic acid polymerase B subunit directly confer rifampicin resistance to M. tuberculosis. The genetic resistance mechanism to isoniazid is more complicated and appears to be based on more than one molecular variant.137,138 Although identifying strains resistant to rifampicin is possible with first-generation methods including PCR, the more widely used procedure involves nucleic acid hybridization with oligonucleotide probes.139–141 While genotypic assays are rapid, their limitations are that they detect only known mutations, thus reducing their sensitivity. In addition, tests may not be sensitive enough to detect small drug-resistant populations. Until details of genes conferring resistance are known for all first-line drugs, growth-dependent methods will remain the gold standard for determining drug susceptibility.
Imaging
Computed Tomography
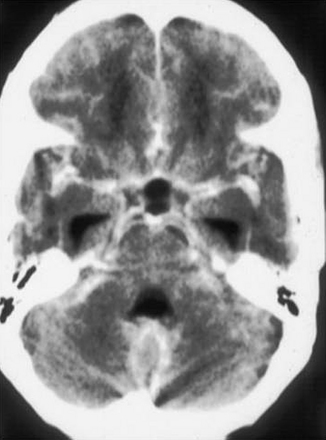
Although normal scan results may be seen in the early stages of TBM, the following features may be demonstrable in the course of the illness: exudates in the basal cisterns or sylvian fissures, hydrocephalus, infarcts, tuberculomas, gyral and meningeal enhancement, and edema in the white matter109,142–144 (Fig. 148-8). The exudate enhancement is irregular and is unlike the sharply defined enhancement of circulating blood in normal vessels in the fissures and cisterns. Hydrocephalus is seen in more than one third of cases in the first scan and becomes more frequent as the disease progresses.145
In most cases, associated periventricular lucency indicates transependymal CSF flow caused by elevated ICP and is therefore an important sign of impending deterioration. Bullock and Van Dellen142 pointed out that in TBM, periventricular lucency is likely to be a result of spread of the inflammatory process, making it an unreliable sign of elevated intraventricular pressure.
Magnetic Resonance Imaging
No consistent or characteristic signal abnormality attributed to meningeal inflammation or basal cistern exudate has been described (Fig. 148-9). When hydrocephalus is present, CSF is forced transependymally. This interstitial accumulation of CSF is seen as bilateral, rather uniform periventricular areas of increased intensity. MRI is more sensitive in demonstrating early infarcts than is CT.146
Medical Treatment
There is evidence that bacille Calmette–Guérin vaccination offers some protection against TBM.147,148 Once meningitis is established, drug therapy similar to that for tuberculomas is initiated (Table 148-2). Short-course chemotherapy is well established for treatment of pulmonary TB but not for extrapulmonary disease. Goel et al.149 reviewed 35 cases of TBM in which chemotherapy was given for periods of less than 2 years. Short-term therapy was associated with recrudescence of TBM and, in some cases, with the development of deep cerebral infarcts and permanent neurologic deficit.
In a critical reappraisal of the literature on adjunctive corticosteroid therapy in TB, Dooley et al.150 concluded steroids did not reduce the efficacy of adequate antimycobacterial therapy and appeared to offer significant short- and long-term benefits in TBM. Several randomized trials of steroids in TBM have appeared in the literature. In the first prospective, randomized, controlled trial of dexamethasone in TBM, Kumarvelu et al.151 concluded that dexamethasone appeared to be a useful adjunct, especially in patients with severe disease. A similar prospective, randomized, controlled trial of steroids in TBM in 141 consecutive children concluded that the survival rate and intellectual outcome were significantly better with steroids. There was enhanced resolution of basal exudates and tuberculomas on serial CT scanning. However, ICP and the incidence of basal ganglia infarction remained unchanged.152 In a randomized, double-blind trial involving 59 adults with TBM, prednisolone was not found to be beneficial in patients with poor neurologic status, increased ICP, and cranial nerve palsies.153 A controlled trial on 545 adults with TBM demonstrated that dexamethasone improved survival but did not improve disability.154 It is not clear how steroids work. Steroids do not appear to act by attenuating immunologic mediators of inflammation.155
In the absence of anticonvulsants, seizures occurred in less than 10% of children in the first 3 months. Patwari et al.156 advocated that all children with focal seizures and those with generalized tonic–clonic seizures and tonic spasms manifesting more than once during hospitalization or associated with abnormal CT or electroencephalographic findings be given long-term anticonvulsants. Children without seizures and those with generalized tonic–clonic seizures before hospitalization or not more than one seizure during the first week of hospitalization and without abnormal CT or electroencephalographic findings were not given long-term anticonvulsants. Close follow-up is essential, especially when anticonvulsant therapy has been withheld.
Surgery
Cairns157 first advocated ventricular decompression during the acute stage of TBM. Since then, numerous procedures have been tried, and reports have conclusively documented the efficacy of ventriculoatrial or ventriculoperitoneal shunts for this condition.146,157–160 The fear of spreading tubercle bacilli through the shunt is unfounded. Hydrocephalus may resolve with medical treatment alone; however, surgical diversion of CSF is indicated when hydrocephalus is associated with symptomatic elevated ICP.
After a shunt is inserted, a progressive reduction in size of the ventricles occurs, but the ventricles may not return to normal size.109 A low-pressure shunt appears to be best suited to these patients. Infrequently, separate shunts are required for each lateral ventricle if a CSF block is present at the level of the foramen of Monro. Alternatively, an endoscopic fenestration of septum pellucidum eliminates the need for two ventricular ends. Loculations within ventricles could similarly be fenestrated to reduce the number of shunts required. Where the obstruction is at the aqueduct or the fourth ventricular outlet on imaging, it is tempting to perform a third ventriculostomy, but the surgeon must keep in mind that CSF frequently is obstructed at multiple sites, and bypassing one obstruction may simply uncover another. In acute TBM, the combination of an inflamed, opaque, tubercle-studded third ventricular floor and exudates in the underlying subarachnoid space makes the procedure risky and prone to failure. The success rate is quite variable, ranging from 41% to 77%. Whether it is a third ventriculostomy or an endoscopic fenestration of septate, the results would be better when performed following 4 weeks or more of anti-TB treatment or when the disease has been quiescent.161–164 Rarely, optochiasmal arachnoiditis may be responsible for the development of visual deterioration and may indicate a need for decompression of the optic nerves and chiasm.92 Cerebral tuberculomas can develop insidiously during treatment of TBM,52–56 and the patient may die as a result of elevated ICP. These tuberculomas tend to occur in deep structures, making surgical access difficult and hazardous. Steroids may be of help during the crisis.54
Results
The prognosis of TBM depends on the delay in treatment, the patient’s level of consciousness, the presence and degree of exudates, and the presence of hydrocephalus and cerebral infarcts.109,160–168 After TBM, there is frequent marked, generalized impairment of cognitive and motor development.169 Palur et al.160 reviewed 114 patients with TBM and hydrocephalus who underwent shunt surgery and followed them for a period ranging from 6 months to 13 years (mean 45.6 months). They described a grading score at admission based on sensorium and neurologic deficit, which was found to correlate statistically significantly with the outcome (p < 0.001) The grading system has since been modified to include the Glasgow Coma Scale to improve reproducibility170 (Table 148-3). The utility of this grading system has been ratified by other researchers.171,172
Table 148-3 Modified Vellore Grading of TBM and Hydrocephalus
| Grade | Description |
|---|---|
| I | GCS 15 Headache, vomiting, fever with or without neck stiffness, no neurologic deficit |
| II | GCS 15 Neurologic deficit present |
| III | GCS 9-14 Neurologic deficit may or may not be present |
| IV | GCS 3-8 Neurologic deficit may or may not be present |
GCS, Glasgow Coma Scale score.
Early shunt surgery is advocated for patients in grades I and II. For patients in grade III, surgery may be performed either without a trial of external ventricular drainage or when an improvement in sensorium occurs after such a trial. All patients in grade IV should undergo external ventricular drainage, and only those who show a significant change in their neurologic status within 24 to 48 hours of drainage should undergo shunt surgery. There would be a few patients in this group who show no improvement on drainage yet may show improvement after shunt surgery.173
Nadvi et al. compared two groups of 15 patients each, one with TBM and the other whose patients were also HIV positive, and concluded that the mortality and outcome following shunt surgery was significantly worse in patients who were HIV positive even though at initial presentation they had a better sensorium and fewer neurologic deficits. None of the HIV-positive patients had a good recovery, and there were no survivors in grades III and IV. They concluded that all patients of TBM who were HIV positive should be given a trial of CSF drainage, and only those who show an improvement should undergo shunt surgery.174
Tuberculous Spinal Arachnoiditis
Tuberculous spinal arachnoiditis usually occurs as a result of a spread of meningitis from within the cranium during the course of treatment, while the disease is still active, or after a variable period of months to years after the disease has “burnt out.”175,176 Sometimes the disease may start primarily in the spinal meninges because of rupture into the subarachnoid space of a superficial spinal tuberculoma.177,178 Rarely, the disease occurs as a result of a direct transdural spread in spinal caries.179
The maximal involvement is in the thoracic and thoracolumbar region, with longitudinal extensions ranging from a few segments to the entire cord. The disease is more marked posterior to the cord, and it may be difficult to distinguish the meninges from the cord. The meninges may become thickened and hard, whereas the cord is atrophied, soft, and edematous, with one or more visible tuberculomas on the surface. As the exudates organize, the spinal cord or roots get entrapped, producing a myeloradiculopathy.180
Imaging
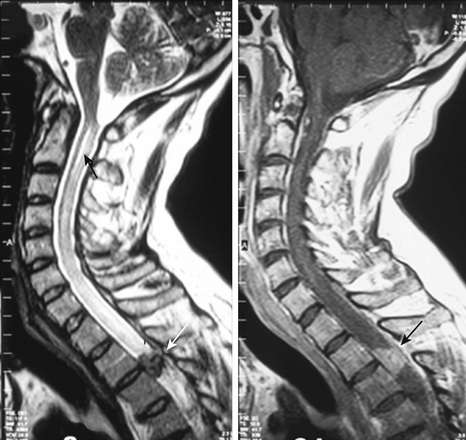
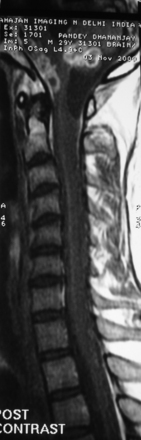
Tuberculous arachnoiditis on myelography shows an irregular thecal sac; nodularity; thickening of the nerve roots, with clumping of the roots to one another and the thecal sac; and CSF block. These findings are reflected on MRI scans. In addition, there are CSF loculations and an increase in CSF intensity on T1-weighted images, leading to loss of cord–CSF interface or a shaggy outline. Cord involvement is seen in more than 80% of cases in the form of increased intensity on T2-weighted images and cord cavitation. With contrast, there is meningeal enhancement in 80% of cases (Fig. 148-10), although enhancement of the roots and cord is less frequently seen. Associated findings of tuberculous spondylitis, basal exudate, and intracranial granulomas are additional clues to the diagnosis.181–183
Treatment
It is generally accepted that steroids are a useful adjunct in treating patients threatened with paraplegia. Intrathecal steroids may help in further resolution of the exudate. Gourie-Devi and Satish Chandra184 have advocated intrathecal hyaluronidase to lyse the adhesions. Recurrence is common, and available treatments are not very effective. The outcome of surgical intervention is quite unpredictable, and it may be offered as a treatment option if the diagnosis is in doubt, in localized lesions, or if imaging suggests a cyst at the expected clinical level.
Ahuja G.K., Behari M., Prasad K., et al. Disappearing CT lesions in epilepsy: Is tuberculosis or cysticercosis the cause? J Neurol Neurosurg Psychiatry. 1989;52:915-916.
Ahuja G.K., Mohan K.K., Prasad K., Behari M. Diagnostic criteria for tuberculous meningitis and their validation. Tuber Lung Dis. 1994;75:149-152.
Centers for Disease Control and Prevention. Plan to combat extensively drug-resistant tuberculosis: recommendations of the Federal Tuberculosis Task Force. MMWR Recomm Rep. 2009 Feb 13;58(RR-3):1-43.
Centers for Disease Control and Prevention: Trends in tuberculosis—United States, 2008. MMWR. March 20, 2009.
Chandy M.J., Rajshekhar V.R., Ghosh S., et al. Single small enhancing CT lesions in Indian patients with epilepsy: clinical, radiological and pathological considerations. J Neurol Neurosurg Psychiatry. 1991;54:702-705.
Cho S.N., Brennan P.J. Tuberculosis: diagnostics. Tuberculosis. 2007;87:S14-S17.
Global Tuberculosis Control Report, 2009 http://www.who.int/tb/publications/global_report/2009/pdf/full_report.pdf Available from URL
Gupta R.K., Jena A., Singh A.K., et al. Role of magnetic resonance (MR) in the diagnosis and management of intracranial tuberculoma. Clin Radiol. 1990;41:120-127.
Gupta R.K., Pandey R., Khan E.M., et al. Intracranial tuberculomas: MRI signal intensity correlation with histopathology and localised proton spectroscopy. Magn Reson Imaging. 1993;11:443-449.
Kim T.K., Chang K.H., Kim C.J., et al. Intracranial tuberculoma: comparison of MR with pathologic findings. AJNR Am J Neuroradiol. 1995;16:1903-1908.
Kumarvelu S., Prasad K., Khosla A., et al. Randomised controlled trial of dexamethasone in tuberculous meningitis. Tuber Lung Dis. 1994;75:203-207.
Mathew J.M., Rajshekhar V., Chandy M.J. Shunt surgery for poor-grade patients with tuberculous meningitis and hydrocephalus: effect of response to external drainage and other factors on long term outcome. J Neurol Neurosurg Psychiatry. 1998;65:115-118.
Nadvi S.S., Nathoo N., Annamalai K., et al. Role of cerebrospinal fluid shunting for human immunodeficiency virus-positive patients with tuberculous meningitis and hydrocephalus. Neurosurgery. 2000;47:644-649.
Palur R., Rajshekhar V., Chandy M.J., et al. Shunt surgery for hydrocephalus in tuberculous meningitis: a long-term follow up study. J Neurosurg. 1991;74:64-69.
Patwari A.K., Aneja S., Ravi R.N., et al. Convulsions in tuberculous meningitis. J Trop Pediatr. 1996;42:91-97.
Rajeswari R., Sivasubramanian S., Balambal R., et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57(4):368-374.
Ramdurg S.R., Gupta D.K., Suri A., et al. Spinal intramedullary tuberculosis: a series of 15 cases. Clin Neurol Neurosurg. 2009;111(2):115-118. Feb
Shah N.S., Pratt R., Armstrong L., Robison V., Castro K.G., Cegielski J.P. Extensively drug-resistant tuberculosis in the United States, 1993-2007. JAMA. 2008;300(18):2153-2160.
Sharma M.C., Arora R., Mahapatra A.K., et al. Intrasellar tuberculoma—an enigmatic pituitary infection: a series of 18 cases. Clin Neurol Neurosurg. 2000;102(2):72-77.
Thwaites Nguyen G.E.D.B., Nguyen H.D., Hoang T.Q., Do T.T., et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741-1751.
World Health Organization. The Global Plan to Stop Tuberculosis. Geneva, 2001 (WHO/CDS/STB/2001.16).
World Health Organization. Treatment of Tuberculosis. Guidelines for National Programmes, 3rd ed, 2003 WHO/CDS/TB/2003
World Health Organization. The WHO/IUALTD global report on anti-tuberculosis drug resistance in the world. Fourth global report, 2008 http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf Available from URL
Zhang Y., Nakata K., Weiden M., Rom W.N. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995;5:2324-2331.
1. Global Tuberculosis Control Report, 2009 http://www.who.int/tb/publications/global_report/2009/pdf/full_report.pdf Available from URL
2. Schurmann D., Nightingale S.D., Bergmann F., Ruf B. Tuberculosis and HIV infection: a review. Infection. 1997;25:274-280.
3. Lesprit P., Zagdanski A.M., de La Blanchardiere A., et al. Cerebral tuberculosis in patients with the acquired immunodeficiency syndrome (AIDS): report of 6 cases and review. Medicine (Baltimore). 1997;76:423-431.
4. Centers for Disease Control and Prevention. Tuberculosis final data: United States 1986. MMWR Morb Mortal Wkly Rep. 1988;36:817-820.
5. Pitchenick A.E., Cole C., Russel B., et al. Tuberculosis, atypical mycobacteriosis and the acquired immunodeficiency syndrome among Haitian and non Haitian patients in South Florida. Ann Intern Med. 1984;101:641-645.
6. World Health Organization. The Global Plan to Stop Tuberculosis. Geneva, 2001 (WHO/CDS/STB/2001.16).
7. Davidson P.T. Tuberculosis: new views of an old issue. N Engl J Med. 1985;312:1514-1515.
8. Centers for Disease Control and Prevention. Trends in tuberculosis—United States, 2008. MMWR. Morbidity and Mortality Weekly Report. March 20, 2009.
9. Centers for Disease Control and Prevention. Plan to combat extensively drug-resistant tuberculosis: recommendations of the Federal Tuberculosis Task Force. MMWR Recomm Rep. 2009 Feb 13;58(RR-3):1-43.
10. Narain J.P., Lo Y.R. Epidemiology of HIV-TB in Asia. Indian J Med Res. 2004;120:277-289.
11. Zhang Y., Nakata K., Weiden M., Rom W.N. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995;5:2324-2331.
12. Toossi Z. Virology and immunologic impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146-1155.
13. Lalitha V.S., Dastur D.K. Tuberculosis of the central nervous system. II: brain tuberculomas vis-à-vis intracranial space-occupying lesions, 1953-1978. Neurol India. 1980;28:202-206.
14. Mathai K.V., Chandy J. Tuberculous infection of the nervous system. Clin Neurosurg. 1966;14:145-177.
15. Ramamurthi B., Ramamurthi R., Vasudevan M.C., et al. The changing face of tuberculomas. Ann Acad Med. 1993;22:852-855.
16. Tandon P.N. Nervous system tuberculomas and their surgical management. Neurol Infect Epidemiol. 1996;1:109-117.
17. Ohaegbulam S.C., Amuta J., Saddeqi N. Tuberculoma of the central nervous system in eastern Nigeria. Tubercle. 1979;60:163-166.
18. Tang L.M., Chee C.Y., Cheng S.Y., et al. Neurological complications of tuberculous meningitis. In: Sixth Asian and Oceanian Congress of Neurology Abstracts. Amsterdam: Excerpta Medica; 1983:93-94.
19. Gropper M.R., Schulder M., Sharan A.D., Cho E.S. Central nervous system tuberculosis: medical management and surgical indications. Surg Neurol. 1995;44:378-384.
20. Parney I.F., Johnson E.S., Allen P.B. “Idiopathic” cranial hypertrophic pachymeningitis responsive to antituberculous therapy: case report. Neurosurgery. 1997;41:965-971.
21. Gropper M.R., Schulder M., Duran H.L., Wolansky L. Cerebral tuberculosis with expansion into brainstem tuberculoma: report of two cases. J Neurosurg. 1994;81:927-931.
22. Kioumehr F., Dadsetan M.R., Rooholamini S.A., Au A. Central nervous system tuberculosis: MRI. Neuroradiology. 1994;36:93-96.
23. Goyal M., Sharma A., Mishra N.K., et al. Imaging appearance of pachymeningeal tuberculosis. AJR Am J Roentgenol. 1997;169:1421-1424.
24. Mahanta A., Kalra L., Maheshwari M.C., et al. Brain stem tuberculoma: an unusual presentation. J Neurol. 1982;227:249-253.
25. Rajshekhar V., Chandy M.J. Tuberculomas presenting as isolated intrinsic brainstem masses. Br J Neurosurg. 1997;11:127-133.
26. Pandya S.K., Desai A.D., Dastur H.M. Caseative liquefaction within brain stem tuberculoma under drug therapy with simultaneous regression of cerebral tuberculomas. Neurol India. 1982;30:121-128.
27. Choudhary C., Mehta V.S., Roy S. Tuberculoma of anterior pituitary: a case report. Neurol India. 1986;34:341-344.
28. Ashkan K., Papadopoulos M.C., Casey A.T., et al. Hypophyseal tuberculoma: report of two cases. Acta Neurochir (Wien). 1997;139:523-525.
29. Ranjan A., Chandy M.J. Intrasellar tuberculoma. Br J Neurosurg. 1994;8:179-185.
30. Brooks P.H., Dumlao J.S., Bronsky D., Waldstein S.S. Hypophysial tuberculoma with hypopituitarism. Am J Med. 1973;54:777-781.
31. Sharma M.C., Arora R., Mahapatra A.K., et al. Intrasellar tuberculoma—an enigmatic pituitary infection: a series of 18 cases. Clin Neurol Neurosurg. 2000;102(2):72-77.
32. Indira B., Panigrahi M.K., Vajramani G., et al. Tuberculoma of the hypothalamic region as a rare cause of hypopituitarism: a case report. Surg Neurol. 1996;45:347-350.
33. Jena A., Banerji A.K., Tripathi R.P., et al. Demonstration of intramedullary tuberculomas by magnetic resonance imaging: a report of two cases. Br J Radiol. 1991;64:555-558.
34. Rhoton E.L., Ballinger W.E.Jr., Quisling R., Sypert G.W. Intramedullary spinal tuberculoma. Neurosurgery. 1988;22:733-736.
35. Sanchez Pernaute R., Berciano J., Rebollo M., Pascual J. Intramedullary tuberculoma of the spinal cord with syringomyelia. Neuroradiology. 1996;38(suppl 1):105-106.
36. Gupta V.K., Sharma B.S., Khosla V. Intramedullary tuberculoma: report of two cases with MRI findings. Surg Neurol. 1995;44:241-243.
37. Hanci M., Sarioglu A.C., Uzan M., et al. Intramedullary tuberculous abscess: a case report. Spine. 1996;21:766-769.
38. Citow J.S., Ammirati M. Intramedullary tuberculoma of the spinal cord: case report. Neurosurgery. 1994;35:327-330.
39. Ramdurg S.R., Gupta D.K., Suri A., et al. Spinal intramedullary tuberculosis: a series of 15 cases. Clin Neurol Neurosurg. 2009;111(2):115-118. Feb
40. Dastur H.M.. Tuberculoma, Vinken P.J., Bruyn G.W., editors, Handbook of Clinical Neurology, New York, Elsevier, 1975;vol 18:413-426.
41. Ramamurthi B., Varadarajan M.G. Diagnosis of tuberculomas of the brain. J Neurosurg. 1961;18:1-7.
42. Dastur D.K. Neurosurgically relevant aspects of pathology and pathogenesis of intracranial and intraspinal tuberculosis. Neurosurg Rev. 1983;6:103-110.
43. Sinh G., Pandya S.K., Dastur D.K. Pathogenesis of unusual intracranial tuberculomas and tuberculous space-occupying lesions. J Neurosurg. 1968;29:149-159.
44. Sandhyamani S., Roy S., Bhatia R. Tuberculous brain abscess. Acta Neurochir (Wien). 1981;59:247-256.
45. Farrar D.J., Flanigan T.P., Gordon N.M., et al. Tuberculous brain abscess in a patient with HIV infection: case report and review. Am J Med. 1997;102:297-301.
46. Armstrong F.B., Edwards A.M. Intracranial tuberculoma in native races of Canada, with special reference to symptomatic epilepsy and neurologic features. CMAJ. 1963;89:56-65.
47. Dannenberg A.M.Jr., Sugimoto M. Liquefaction of caseous foci in tuberculosis. Am Rev Respir Dis. 1976;113:257-259.
48. Dastur D.K., Dave U.P. Further observations on the fine structure of blood vessels in neurotuberculosis: possible significance of vasculitis with proliferated basement membranes. Adv Neurol. 1978;20:577-589.
49. Tandon P.N., Bhargava S. Effect of medical treatment on intracranial tuberculoma: a CT study. Tubercle. 1985;66:85-97.
50. Martinez Vazquez C., Bordon J., Rodriguez Gonzalez A., et al. Cerebral tuberculoma: a comparative study in patients with and without HIV infection. Infection. 1995;23:149-153.
51. Bishberg E., Sunderam G., Reichman L.B., Kapila R. Central nervous system tuberculosis with the acquired immunodeficiency syndrome and its related complex. Ann Intern Med. 1986;105:210-213.
52. Alame T., Keller K., Michel O., Sergysels R. Hyperthermia occurring with paradoxical development of cerebral tuberculomas. Respiration. 1996;63:381-383.
53. Borah N.C., Maheshwari M.C., Mishra N.K., et al. Appearance of tuberculoma during the course of TB meningitis. J Neurol. 1984;231:269-270.
54. Hejazi N., Hassler W. Multiple intracranial tuberculomas with atypical response to tuberculostatic chemotherapy. Infection. 1997;25:233-239.
55. Lebas J., Malkin J.E., Coquin Y., et al. Cerebral tuberculomas developing during treatment of tuberculous meningitis. Lancet. 1980;2:84.
56. Malik G.M., Mubarik M., Basu J.A., et al. Paradoxical expansion of cerebral tuberculomas during therapy for Pott’s spine. J R Soc Med. 1996;89:643-644.
57. Bhargava S., Tandon P.N. Intracranial tuberculomas: a CT study. Br J Radiol. 1980;53:935-945.
58. Bhargava S., Tandon P.N. CNS tuberculosis: lessons learned from CT studies. Neurol India. 1980;28:207-212.
59. Harder E., Al-Kawl M.Z., Carney P. Intracranial tuberculomas: conservative management. Am J Med. 1983;74:570-576.
60. Tandon P.N., Pathak S.N. Tuberculosis of the central nervous system. In: Spillane J.D., editor. Tropical Neurology. London: Oxford University Press; 1973:51-62.
61. Welchman J.M. Computerized tomography of intracranial tuberculoma. Clin Radiol. 1979;30:567-573.
62. Bhatia R., Tandon P.N., Misra N.K. Inflammatory lesions of the basal ganglia and thalamus: review of twenty-one cases. Neurosurgery. 1986;19:983-988.
63. Vengsarkar U.S., Pisipaty R.P., Parekh B., et al. Intracranial tuberculoma and the CT scan. J Neurosurg. 1986;64:568-574.
64. Teoh R., Humphries M.J., Hoare R.D., O’Mahony G. Clinical correlation of CT changes in 64 Chinese patients with tuberculous meningitis. J Neurol. 1989;236:48-51.
65. Wasay M., Kheelani B.A., Moolani, et al. Brain CT and MRI findings in 100 consecutive patients. J Neuroimaging. 2003;13:240-247.
66. Bargallo J., Berenguer J., Garcia Barrionuevo J., et al. The “target sign”: is it a specific sign of CNS tuberculoma? Neuroradiology. 1996;38:547-550.
67. Ahuja G.K., Behari M., Prasad K., et al. Disappearing CT lesions in epilepsy: is tuberculosis or cysticercosis the cause? J Neurol Neurosurg Psychiatry. 1989;52:915-916.
68. Chandy M.J., Rajshekhar V.R., Ghosh S., et al. Single small enhancing CT lesions in Indian patients with epilepsy: clinical, radiological and pathological considerations. J Neurol Neurosurg Psychiatry. 1991;54:702-705.
69. Chandy M.J., Rajshekhar V., Prakash S., et al. Cysticercosis causing single small CT lesions in Indian patients with epilepsy. Lancet. 1989;1:390-391.
70. Goulatia R.K., Verma A., Mishra N.K., Ahuja G.K. Disappearing CT lesions in epilepsy. Epilepsia. 1987;28:523-527.
71. Rajshekhar V. Etiology and management of single small CT lesion in patients with seizures: understanding a controversy. Acta Neurol Scand. 1991;84:465-470.
72. Rajshekhar V., Chandy M.J. Validation of diagnostic criteria for solitary cerebral cysticercus granuloma in patients presenting with seizures. Acta Neurol Scand. 1997;96:76-81.
73. Rajshekhar V., Haran R.P., Prakash G.S., Chandy M.J. Differentiating solitary small cysticercus granuloma and tuberculomas in patients with epilepsy: clinical and computerised tomographic criteria. J Neurosurg. 1993;78:402-407.
74. Selvapandian S., Rajshekhar V., Chandy M.J., Idikula J. Predictive value of computer tomography–based diagnosis of intracranial tuberculomas. Neurosurgery. 1994;35:845-850.
75. Wadia R.S., Makhale C.N., Kelkar A.V., Grant K.B. Focal epilepsy in India with special reference to lesions showing ring or disc-like enhancement on contrast computed tomography. J Neurol Neurosurg Psychiatry. 1987;50:1298-1301.
76. Gupta R.K., Jena A., Singh A.K., et al. Role of magnetic resonance (MR) in the diagnosis and management of intracranial tuberculoma. Clin Radiol. 1990;41:120-127.
77. Kim T.K., Chang K.H., Kim C.J., et al. Intracranial tuberculoma: comparison of MR with pathologic findings. AJNR Am J Neuroradiol. 1995;16:1903-1908.
78. Gupta R.K., Pandey R., Khan E.M., et al. Intracranial tuberculomas: MRI signal intensity correlation with histopathology and localised proton spectroscopy. Magn Reson Imaging. 1993;11:443-449.
79. Gupta R.K., Poptani H., Kohli A., et al. In vivo localized proton magnetic resonance spectroscopy of intracranial tuberculomas. Indian J Med Res. 1995;101:19-24.
80. Gupta R.K., Roy R., Dev R., et al. Finger printing of Mycobacterium tuberculosis in patients with intracranial tuberculomas by using in vivo, ex vivo, and in vitro magnetic resonance spectroscopy. Magn Reson Med. 1996;36:829-833.
81. Saini K.S., Patel A.L., Shaikh W.A., et al. Magnetic resonance spectroscopy in pituitary tuberculoma. Singapore Med J. 2007;48(8):783.
82. Pretell E.J., Martinot C., Garcia H.H., et al. Differential diagnosis between cerebral tuberculosis and neurocysticercosis by magnetic resonance spectroscopy. J Comput Assist Tomogr. 2005;29:112-114.
83. Batra A., Tripathi R.P. Diffusion-weighted magnetic resonance imaging and magnetic resonance spectroscopy in the evaluation of focal cerebral tubercular lesions. Acta Radiol. 2004;45:679-688.
84. Villoria M.F., Fortea F., Moreno S., et al. MR imaging and CT of central nervous system tuberculosis in the patient with AIDS. Radiol Clin North Am. 1995;33:805-820.
85. Bass J.B.Jr., Farer L.S., Hopewell P.C., et al. Treatment of tuberculosis infection in adults and children: American Thoracic Society and The Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994;149:1359-1374.
86. Medical Research Council (Streptomycin in Tuberculosis Trials Committee). Streptomycin treatment of tuberculous meningitis. Lancet. 1948;1:582-596.
87. Rajeswari R., Sivasubramanian S., Balambal R., et al. A controlled clinical trial of short-course chemotherapy for tuberculoma of the brain. Tuber Lung Dis. 1995;76:311-317.
88. World Health Organization. Treatment of Tuberculosis. Guidelines for National Programmes, 3rd ed, 2003 WHO/CDS/TB/2003
89. Lees A.J., MacLeod A.F., Marshall J. Cerebral tuberculomas developing during treatment of tuberculosis meningitis. Lancet. 1980;1:1208-1211.
90. Teoh R., Humphries M.J., O’Mahony G. Symptomatic intracranial tuberculoma developing during treatment of tuberculosis: a report of 10 patients and review of the literature. Q J Med. 1987;241:449-460.
91. Traub M., Colchester A.C.F., Kingsley D.P., Swash M. Tuberculosis of the central nervous system. Q J Med. 1984;53:81-100.
92. Teoh R., Poon W., Humphries M.J., O’Mahony G. Suprasellar tuberculoma developing during treatment of tuberculous meningitis requiring urgent surgical decompression. J Neurol. 1988;235:321-322.
93. Cohn D.I., Bustreo F., Raviglione M.C. Drug resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD global surveillance project. Clin Infect Dis. 1997;24(suppl 1):121-130.
94. Paramasivan C.N. An overview of drug resistant tuberculosis in India. Indian J Tuberc. 1998;45:73-81.
95. World Health Organization. The WHO/IUALTD global report on anti-tuberculosis drug resistance in the world. Fourth global report, 2008 http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf Available from URL
96. Frieden T.R., Sterling T., Pablos-Mendez A., et al. The emergence of drug resistant tuberculosis in New York City. N Engl J Med. 1993;328:521-526.
97. Fujiwara P.I., Cook S.V., Rutherford C.M., et al. Continuing survey of drug resistance tuberculosis, New York City, April 1994. Arch Intern Med. 1997;157:531-536.
98. Shah N.S., Pratt R., Armstrong L., et al. Extensively drug-resistant tuberculosis in the United States, 1993-2007. JAMA. 2008;300(18):2153-2160.
99. W.H.O. Global Tuberculosis Programme and IUATLD: guidelines for surveillance of drug resistance in tuberculosis. Geneva: World Health Organization; 1997. WHO/TB/96 216
100. Angarano G., Carbonara D., Costa D. Drug resistance of Mycobacterium tuberculosis strains isolated from HIV-infected Italian patients: preliminary report from a multicentric study. The Italian Tuberculosis Drug Resistance Study Group. Microbiologica. 1995;18:69-72.
101. Kim S.J., Hong Y.P. Drug resistance of Mycobacterium tuberculosis in Korea. Tuber Lung Dis. 1992;73:219-224.
102. Pablos-Mendez A., Sterling T., Freiden T.R. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276:1223-1228.
103. Rajshekhar V., Chandy M.J. CT-guided stereotactic surgery in the management of intracranial tuberculomas. Br J Neurosurg. 1993;7:665-671.
104. Tandon P.N., Bhatia R., Bhargava S.. Tuberculous meningitis, Vinken P.K., Bruyn G.W., Klawans H.L., editors, Handbook of Clinical Neurology, Amsterdam, Elsevier, 1988;vol 8:195-226.
105. Berenguer J., Moreno S., Laguna F., et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. New Engl J Med. 1992;326:668-672.
106. Dastur D.K., Manghani D.K., Udani P.M. Pathology and pathogenetic mechanisms in neurotuberculosis. Radiol Clin North Am. 1995;33:733-752.
107. Dastur D.K., Wadia N.H. Spinal meningitis with radiculomyelopathy: part II. J Neurol Sci. 1969;8:261-297.
108. Schoeman J., Donald P., van Zyl L., et al. Tuberculous hydrocephalus: comparison of various treatments with regard to ICP, ventricular size and clinical outcome. Dev Med Child Neurol. 1991;33:396-405.
109. Bhargava S., Gupta A.K., Tandon P.N. Tuberculous meningitis: a CT scan study. Br J Radiol. 1982;55:189-196.
110. Rajshekhar V. Management of hydrocephalus in patients with tuberculous meningitis. Neurol India. 2009;57(4):368-374.
111. Fehlings M.G., Bernstein M. Syringomyelia as a complication of tuberculous meningitis. Can J Neurol Sci. 1992;19:84-87.
112. Suwanwela E. Complications of tuberculous meningitis. Proc Aust Assoc Neurol. 1968;3:493-495.
113. Kennedy D.H., Fallon R.J. Tuberculous meningitis. JAMA. 1984;241:264-268.
114. Venkataraman P., Herbert D., Parmasivan C.N. Evaluation of BACTEC radiometric method in the early diagnosis of tuberculosis. Ind J Med Research. 1998;108:120-127.
115. Bothamley G.H. Serological diagnosis of tuberculosis. Eur Respir J Suppl. 1995;20:676-688.
116. Gupta S., Bhatia R., Datta K.K. Serodiagnosis of tuberculosis. J Commun Dis. 1995;27:208-214.
117. Radhakrishnan V.V., Mathai A. Enzyme-linked immunosorbent assay to detect Mycobacterium tuberculosis antigen 5 and antimycobacterial antibody in cerebrospinal fluid of patients with tubercular meningitis. J Clin Lab Anal. 1991;5:233-237.
118. Thakur A., Mandal A. Usefulness of ELISA using antigen A60 in serodiagnosis of neurotuberculosis. J Commun Dis. 1996;28:8-14.
119. Ratanasuwan W., Kreiss J.K., Nolan C.M., et al. Evaluation of the MycoDot test for the diagnosis of tuberculosis in HIV seropositive and seronegative patients. Int J Tuberc Lung Dis. 1997;1:259-264.
120. Park S.C., Lee B.I., Cho S.N., et al. Diagnosis of tuberculous meningitis by detection of immunoglobulin G antibodies to purified protein derivative and lipoarabinomannan antigen in cerebrospinal fluid. Tuber Lung Dis. 1993;74:317-322.
121. Miorner H., Sjobring U., Nayak P., Chandramuki A. Diagnosis of tuberculous meningitis: a comparative analysis of 3 immunoassays, an immune complex assay and the polymerase chain reaction. Tuber Lung Dis. 1995;76:381-386.
122. Bisen PS, Tiwari RP: a diagnostic kit for detecting pulmonary and extrapulmonary tuberculosis. World Intellectual Property Organization. PCT-W02005/080987 A1. An international patent published on 1 September 2005.
123. Houghton R.L., Lode M.J., Dillon D.C., et al. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin Diagnost Lab Immunol. 2002;9(4):883-891.
124. Brooks J.B., Daneshvar M.I., Fast D.M., Good R.C. Selective procedure for detecting femtomole quantities of tuberculostearic in serum and cerebrospinal fluid by frequency-pulsed electron-capture gas–liquid chromatography. J Clin Microbiol. 1987;25:1201-1206.
125. Brooks J.B., Daneshvar M.I., Haberberger R.L., Mikhail I.A. Rapid diagnosis of meningitis by frequency-pulsed electron-capture gas–liquid chromatography detection of carboxylic acids in cerebrospinal fluid. J Clin Microbiol. 1990;28:989-997.
126. Mardh P.A., Larsson P.A., Odham G. Tuberculostearic acid as a diagnostic marker in tuberculous meningitis. Lancet. 1983;1:367-370.
127. Forbes B.A. Critical assessment of gene amplification approaches on the diagnosis of tuberculosis. Immunol Invest. 1997;26:105-116.
128. Roth A., Schaberg T., Mauch H. Molecular diagnosis of tuberculosis: current clinical validity and future perspectives. Eur Respir J. 1997;10:1877-1891.
129. Shankar P., Manjunath N., Mohan K.K., et al. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction. Lancet. 1991;337:5-7.
130. A new diagnostic test for tuberculous meningitis [editorial]. Tubercle. 1985;66:157.
131. American Thoracic Society. Rapid diagnostic test for tuberculosis. What is the appropriate use? American Thoracic Society Workshop. Am J Crit Care Med. 1997;155:1804-1814.
132. Nguyen L.N., Kox L.F., Pham L.D., et al. The potential contribution of the polymerase chain reaction to the diagnosis of tuberculous meningitis. Arch Neurol. 1996;53:771-776.
133. Selvakumar N., Vanajakumar, Thilothammal N., Paramasivan C.N. Isolation of Mycobacterium tuberculosis from cerebrospinal fluid by the centrifugation and filtration methods. Indian J Med Res. 1996;103:250-252.
134. Ahuja G.K., Mohan K.K., Prasad K., Behari M. Diagnostic criteria for tuberculous meningitis and their validation. Tuber Lung Dis. 1994;75:149-152.
135. Pretorius G.S., Sirgel F.A., Schaaf H.S., et al. Rifampicin resistance in Mycobacterium tuberculosis: rapid detection and implications in chemotherapy. S Afr Med J. 1996;86:50-55.
136. Cho S.N., Brennan P.J. Tuberculosis: diagnostics. Tuberculosis. 2007;87:S14-S17.
137. Zhang Y., Heym B., Allen B., et al. The catalase–peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591-593.
138. Banerjee A., Dubnau A., Balasubramanium V., et al. InhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227-230.
139. Victor T.C., Jordaan A.M., Van Rie A., et al. Detection of mutations in drug resistance genes of Mycobacterium tuberculosis by a dot-blot hybridization strategy. Tuber Lung Dis. 1999;79:343-348.
140. Cooksey R.C., Morlock G.P., Glikman S., Crawford J.T. Evaluation of a line probe rpoB mutations in rifampicin-resistant Mycobacterium tuberculosis from New York City. J Clin Microbiol. 1997;35:1281-1283.
141. Giannoni F., Iona Sementilli F., Brunori L., et al. Evaluation of a new line probe assay for rapid identification of gyrA mutation in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2005;49:2928-2933.
142. Bullock M.R.R., Van Dellen J.R. The role of cerebrospinal fluid shunting in tuberculous meningitis. Surg Neurol. 1982;18:274-276.
143. Kingsley D.P.E., Hendrickse W.A., Kendall B.E., et al. Tuberculous meningitis: role of CT in management and prognosis. J Neurol Neurosurg Psychiatry. 1987;50:30-36.
144. Upadhyaya P., Bhargava S., Sundaram K.P., et al. Hydrocephalus caused by tuberculous meningitis: clinical picture, CT findings, and results of shunt surgery. Z Kinderchir. 1983;38:76-79.
145. Patwari A.K., Aneja S., Ravi R.N., et al. Convulsions in tuberculous meningitis. J Trop Pediatr. 1996;42:91-97.
146. Brown B.C., Post M.J.D. Intracranial infections. In: Atlas S.W., editor. Magnetic Resonance Imaging of the Brain and Spine. New York: Raven Press; 1991:520-523.
147. Zodpey S.P., Maldhure B.R., Shrikhande S.N., Tiwari R.R. Effectiveness of bacillus of Calmette–Guérin (BCG) vaccination against tuberculous meningitis: a case-control study. J Indian Med Assoc. 1996;94:338-340.
148. Rodrigues L.C., Diwan V.K., Wheeler J.G. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22:1154-1158.
149. Goel A., Pandya S.K., Satoskar A.R. Whither short course chemotherapy for meningitis? Neurosurgery. 1990;27:418-421.
150. Dooley D.P., Carpenter J.L., Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin Infect Dis. 1997;25:872-887.
151. Kumarvelu S., Prasad K., Khosla A., et al. Randomised controlled trial of dexamethasone in tuberculous meningitis. Tuber Lung Dis. 1994;75:203-207.
152. Schoeman J.F., Van Zyl L.E., Laubscher J.A., Donald P.R. Effect of corticosteroids on intracranial pressure, computed tomographic findings, and clinical outcome in young children with tuberculous meningitis. Pediatrics. 1997;99:226-231.
153. Chotmongkol V., Jitpimolmard S., Thavornpitak Y. Corticosteroid in tuberculous meningitis. J Med Assoc Thai. 1996;79:83-90.
154. Thwaites G., Nguyen D.B., Nguyen H.D., et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741-1751.
155. Cameron P.S., Guy E.T., Nguyen T.H.Q., et al. The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol. 2005;175:579-590.
156. Patwari A.K., Aneja S., Chandra D., Singhal P.K. Long-term anticonvulsant therapy in tuberculous meningitis: a four-year follow-up. J Trop Pediatr. 1996;42:98-103.
157. Cairns H. Neurosurgical methods in the treatment of tuberculous meningitis with a note on some of the unusual manifestations of the disease. Arch Dis Child. 1951;26:373-386.
158. Bhagwati S.N. Ventriculoatrial shunts in tuberculous meningitis with hydrocephalus. J Neurosurg. 1971;35:309-313.
159. Bullock M.R.R., Welchman J.M. Diagnostic and prognostic features of tuberculous meningitis on CT scanning. J Neurol Neurosurg Psychiatry. 1982;45:1098-1101.
160. Palur R., Rajshekhar V., Chandy M.J., et al. Shunt surgery for hydrocephalus in tuberculous meningitis: a long-term follow up study. J Neurosurg. 1991;74:64-69.
161. Figaji A.A., Fieggen A.G., Peter J.C. Endoscopy for tuberculous hydrocephalus. Childs Nerv Syst. 2007;23:79-84.
162. Sing D., Sachdev V., Sing A.K., Sinha S. Endoscopic third ventriculostomy in post-tubercular meningitic hydrocephalus. A preliminary report. Minim Invasive Neurosurg. 2005;48:47-52.
163. Husain M., Jha D.K., Rastogi M., et al. Role of neuroendoscopy in the management of patients with tuberculous meningitis hydrocephalus. Neurosurg Rev. 2005;28:278-283.
164. Chug H., Husain M., Gupta R.K., et al. Surgical outcome of tuberculous meningitis hydrocephalus treated by endoscopic third ventriculostomy: prognostic factors and postoperative neuroimaging for functional assessment of ventriculostomy. J Neurosurg Paediatr. 2009;3:371-377.
165. Kent S.J., Crowe S.M., Yung A., et al. Tuberculous meningitis: a 30-year review. Clin Infect Dis. 1993;17:87-94.
166. Misra U.K., Kalita J., Srivastava M., Mandal S.K. Prognosis of tuberculous meningitis: a multivariate analysis. J Neurol Sci. 1996;137:57-61.
167. Verdon R., Chevret S., Laissy J.P., Wolff M. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis. 1996;22:982-988.
168. Kemaloglu S., Ozkan U., Bukte Y., et al. Timing of shunt surgery in childhood tuberculous meningitis with hydrocephalus. Pediatr Neurosurg. 2002;37:194-198.
169. Schoeman C.J., Herbst I., Nienkemper D.C. The effect of tuberculous meningitis on the cognitive and motor development of children. S Afr Med J. 1997;87:70-72.
170. Mathew J.M., Rajshekhar V., Chandy M.J. Shunt surgery for poor-grade patients with tuberculous meningitis and hydrocephalus: effect of response to external drainage and other factors on long term outcome. J Neurol Neurosurg Psychiatry. 1998;65:115-118.
171. Aggarwal D., Gupta A., Mehta V.S. Role of shunt surgery in paediatric tuberculous meningitis with hydrocephalus. Ind Pediatr. 2005;42:245-250.
172. Singh D., Kumar S. Ventriculoperitoneal shunt in post tubercular hydrocephalus. Ind Pediatr. 1996;33:854-855.
173. Srikantha U., Morab J.V., Sastry S., et al. Outcome of ventriculoperitoneal shunt placement in grade IV tubercular meningitis with hydrocephalus: a retrospective analysis in 95 patients. J Neurosurg Pediatr. 2009;4(2):176-183.
174. Nadvi S.S., Nathoo N., Annamalai K., et al. Role of cerebrospinal fluid shunting for human immunodeficiency virus–positive patients with tuberculous meningitis and hydrocephalus. Neurosurgery. 2000;47:644-649.
175. Rao B.D., Subrahmanyam M.V. Spinal cord complications of tuberculous meningitis. Neurol India. 1962;10:62.
176. Tandon P.N., Misra R.N., Singh B. Post meningitic spinal arachnoiditis. Bull All India Inst Med Sci. 1968;2:88-96.
177. Bawa T.S., Wahi P.L. Spinal tuberculous meningitis. J Ind Med Assoc. 1961;37:449-452.
178. Harbitz F. Tuberculosis of the spinal cord with peculiar changes. JAMA. 1922;78:330-331.
179. Garceau G.J., Brady T.A. Pott’s paraplegia. J Bone Joint Surg Am. 1950;32:87-96.
180. Wadia N.H., Dastur D.K. Spinal meningitis with radiculomyelopathy. Part 1: clinical and radiological features. J Neurol Sci. 1969;8:239-260.
181. Gupta R.K., Gupta S., Kumar S., et al. MRI in intraspinal tuberculosis. Neuroradiology. 1994;36:39-43.
182. Kumar A., Montanera W., Willinsky R., et al. MR features of tuberculous arachnoiditis. J Comput Assist Tomogr. 1993;17:127-130.
183. Sharma A., Goyal M., Mishra N.K., et al. MR imaging of tubercular spinal arachnoiditis. AJR Am J Roentgenol. 1997;168:807-812.
184. Gourie-Devi M., Satish Chandra P. Hyaluronidase as an adjuvant in the management of tuberculous spinal arachnoiditis. J Neurol Sci. 1991;102:105-111.