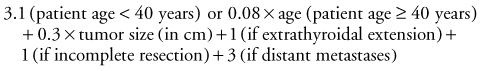CHAPTER 124 Management of Thyroid Neoplasms
Thyroid neoplasms represent almost 95% of all endocrine tumors, although they are relatively uncommon, accounting for approximately 2.5% of all malignancies. In 2008, the estimated annual incidence of thyroid cancer in the United States was 37,340 cases, and approximately 1590 patients (4.3%) were expected to die from thyroid cancer.1 The incidence of thyroid cancer has been steadily increasing over the past 2 decades, and thyroid cancer has the fastest increasing incidence of all major cancers in the United States (approximately 4% increase annually).2 Mortality rates have remained stable, and evidence suggests that improved detection has primarily contributed to the increased incidence.
Although thyroid cancer is rare, the incidence of thyroid nodules is significantly greater, affecting approximately 4% to 7% of the U.S. population.3 Although most of these nodules are benign, the challenge is to identify the approximately 5% of patients with a malignant lesion. A subset of thyroid cancers is particularly aggressive with a potential for devastating morbidity. No reliable indicators are currently available to determine which patients will develop aggressive or recurrent disease, although risk categories based on clinical and pathologic criteria yield important prognostic information.
Most thyroid carcinomas are well-differentiated tumors of follicular cell origin.4,5 These lesions are histologically defined as papillary carcinoma, follicular carcinoma, and Hürthle cell carcinoma. A survey of 53,856 patients described the overall incidence of thyroid cancer in the United States.5 In this report, approximately 79% of cases were papillary carcinoma, 13% were follicular carcinoma, and approximately 3% were Hürthle cell carcinoma. A small proportion (6%) of patients with these lesions have a family history of thyroid cancer. Medullary thyroid carcinoma (MTC), which arises from parafollicular C cells, accounts for about 4% of thyroid carcinomas. Approximately 30% of patients with these lesions have a strong genetic contribution. Anaplastic carcinomas, lymphoma, and metastatic disease constitute a small portion of thyroid malignancies.
The most common presentation of a thyroid cancer is the development of a thyroid mass or nodule. Assessment of the lesion requires a careful history, physical examination, fine-needle aspiration cytology (FNAC), and perhaps imaging studies. With correct diagnosis and management, most patients with well-differentiated thyroid carcinomas have an excellent prognosis. The 10-year disease-specific mortality rate is less than 7% for papillary thyroid cancer and less than 15% for follicular thyroid cancer.5–8 Controversy regarding the treatment of thyroid carcinomas and the extent of thyroidectomy to be performed arises because of the indolent course of most thyroid cancers. Interventions for thyroid cancer have been difficult to evaluate because of the long follow-up and the large number of patients needed to determine differences in survival. The morbidity that may accompany any aggressive intervention needs to be balanced with the generally good prognosis of patients with thyroid cancer.
Surgical Anatomy and Embryology
The thyroid medial anlage derives from the ventral diverticulum of the endoderm from the first and second pharyngeal pouches at the foramen cecum.9,10 The diverticulum descends from the base of the tongue to its adult pretracheal position through a midline anterior path with the primitive heart and great vessels during weeks 4 to 7 of gestation. The proximal portion of this structure retracts and degenerates into a solid, fibrous stalk; persistence of this tract can lead to the development of a thyroglossal duct cyst with variable amounts of associated thyroid tissue. The lateral thyroid primordia arise from the fourth and fifth pharyngeal pouches and descend to join the central component. Parafollicular C cells arise from the neural crest of the fourth pharyngeal pouch as ultimobranchial bodies and infiltrate the upper portion of the thyroid lobes.11 Because of the predictable fusion of the ultimobranchial bodies to the medial thyroid anlage, C cells are restricted to a zone deep within the middle to upper third of the lateral lobes.12
The thyroid gland is composed of two lateral lobes connected by a central isthmus, weighing 15 to 25 g in adults. A thyroid lobe usually measures about 4 cm in height, 1.5 cm in width, and 2 cm in depth. The superior pole lies posterior to the sternothyroid muscle and lateral to the inferior constrictor muscle and the posterior thyroid lamina. The inferior pole can extend to the level of the sixth tracheal ring. Approximately 40% of patients have a pyramidal lobe that arises from either lobe or the midline isthmus and extends superiorly (Fig. 124-1).
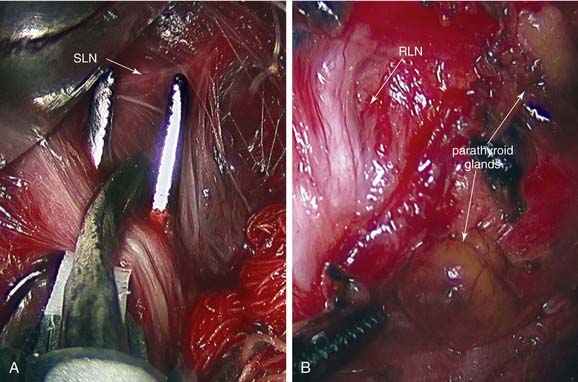
Blood supply to and from the thyroid gland involves two pairs of arteries, three pairs of veins, and a dense system of connecting vessels within the thyroid capsule. The inferior thyroid artery arises as a branch of the thyrocervical trunk (Fig. 124-2). This vessel extends along the anterior scalene muscle, crossing beneath the long axis of the common carotid artery to enter the inferior portion of the thyroid lobe. Although variable in its relationship, the inferior thyroid artery lies anterior to the recurrent laryngeal nerve (RLN) in approximately 70% of patients.13 The inferior thyroid artery is also the primary blood supply for the parathyroid glands.
The superior thyroid artery is a branch of the external carotid artery and courses along the inferior constrictor muscle with the superior thyroid vein to supply the superior pole of the thyroid. This vessel lies posterolateral to the external branch of the superior laryngeal nerve (SLN) as the nerve courses through the fascia overlying the cricothyroid muscle. Care should be taken to ligate this vessel without damaging the SLN. Occasionally, arteria thyroidea ima may arise from the innominate artery, carotid artery, or aortic arch, and supply the thyroid gland near the midline.13 Many veins within the thyroid capsule drain into the superior, middle, and inferior thyroid veins, leading to the internal jugular or innominate veins. The middle thyroid vein travels without an arterial complement, and division of this vessel permits adequate rotation of the thyroid lobe to identify the RLN and parathyroid glands.
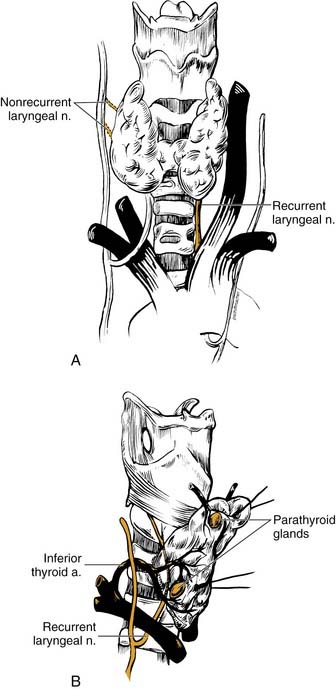
The RLN provides motor supply to the larynx and some sensory function to the upper trachea and subglottic area. Careful management of thyroid carcinomas requires a thorough knowledge of the course of the RLN (see Fig. 124-2). During development, the RLN is dragged caudally by the lowest persisting aortic arches. On the right side, the nerve recurs around the fourth arch (subclavian artery), and on the left side, the nerve recurs around the sixth arch (ligamentum arteriosum).
The right RLN leaves the vagus nerve at the base of the neck, loops around the right subclavian artery, and returns deep to the innominate artery back into the thyroid bed approximately 2 cm lateral to the trachea. The nerve enters the larynx between the arch of the cricoid cartilage and the inferior cornu of the thyroid cartilage. The left RLN leaves the vagus at the level of the aortic arch, and loops around the arch lateral to the obliterated ductus arteriosus. The nerve returns to the neck posterior to the carotid sheath and travels near the tracheoesophageal groove along a more medial course than the right RLN. The nerve crosses deep to the inferior thyroid artery approximately 70% of the time and often branches above the level of the inferior thyroid artery before entry into the larynx.14 The RLN travels underneath the inferior fibers of the inferior constrictor (i.e., the cricopharyngeus muscle) and behind the cricothyroid articulation to enter the larynx. A “nonrecurrent” laryngeal nerve may rarely occur on the right side and enters from a more lateral course (see Figs. 124-2 and 124-4C).15 Typically, an aberrant retroesophageal subclavian artery (arteria lusoria) or other congenital malformation of the vascular rings is present.
The SLN arises beneath the nodose ganglion of the upper vagus and descends medial to the carotid sheath, dividing into an internal and external branch about 2 cm above the superior pole of the thyroid.16 The internal branch travels medially and enters through the posterior thyrohyoid membrane to supply sensation to the supraglottis. The external branch extends medially along the inferior constrictor muscle to enter the cricothyroid muscle. Along its course, the nerve travels with the superior thyroid artery and vein. The nerve typically diverges from the superior thyroid vascular pedicle about 1 cm from the thyroid superior pole.
Proper management of the parathyroid glands during thyroid surgery is crucial to avoid hypoparathyroidism. The superior parathyroid glands are derived from the fourth pharyngeal pouch, whereas the inferior counterparts originate from the third pharyngeal pouch. The parathyroid glands are caramel-colored glands weighing 30 to 70 mg. The subtle distinction of tan and yellow coloration permits differentiation from adjacent fatty tissue, although with trauma, the glands can become mahogany in color. Four parathyroid glands exist in 80% of patients, and at least 10% of patients have more than four glands.17 The glands are situated on the undersurface of the thyroid gland in predictable locations. The superior glands are located at the level of the cricoid cartilage, usually medial to the intersection of the RLN and the inferior thyroid artery.17 The inferior glands are more variable in location than their superior counterparts. These glands may be on the lateral or posterior surface of the lower pole. In many patients, the position of the parathyroid glands on one side is similar to the other side and should be a useful guide.
Molecular Basis for Thyroid Neoplasms
Alterations noted in the development of thyroid carcinomas include changes in total cellular DNA content. The loss of chromosomes, or aneuploidy, has been noted in 10% of all papillary carcinomas, but is present in 25% to 50% of all patients who die as a result of these lesions.18 Similarly, the development of follicular adenomas is associated with a loss of the short arm of chromosome 11 (11p), and transition to a follicular carcinoma seems to involve deletions of 3p, 7q, and 22q.19,20 Loss of heterozygosity involving multiple chromosomal regions is much more prevalent in follicular adenomas and carcinomas than in papillary carcinomas.21
Several oncogenes, altered genes that contribute to tumor development, have been identified in early thyroid tumor progression. Mutations in the thyroid-stimulating hormone (TSH) receptor and G-protein mutations are found in hyperfunctioning thyroid adenomas.22 These changes can lead to the constitutive activation of cell-signaling pathways, such as the adenylate cyclase–protein kinase A system. Point mutations of the G-protein Ras found in thyroid adenomas and multinodular goiters are believed to be an early mutation in tumor progression.23 Somatic Ras mutations are associated with follicular adenomas, and to a lesser extent with follicular carcinomas. The resultant activation of the phosphatidylinositol 3′-kinase (PI3K) signal transduction pathway and AKT, a PI3K-related serine/threonine kinase, also seems to be specific to follicular thyroid carcinoma.24
Other genetic changes have also been associated with certain types of thyroid carcinoma. Mutations within the mitogen-activated protein kinase (MAPK) pathway are involved in malignant transformation to papillary thyroid cancer. Additionally, rearrangements or activation of RET or BRAF proto-oncogenes, which can also activate MAPK, are often found in papillary thyroid cancer.25 Gene rearrangements involving tropomycin-receptor-kinase A, also known as neurotropic tyrosine receptor kinase type 1, a receptor for nerve growth factor, are associated with papillary carcinomas. Mutations in MET/hepatic growth factor have been linked to papillary and poorly differentiated thyroid carcinomas. Other growth factors, such as fibroblast growth factors, epidermal growth factor, and vascular endothelial growth factor, and their cognate receptors may have increased expression in thyroid tumors and contribute to tumor progression.
Different types of galectin, a carbohydrate-binding protein, seem to be differentially expressed in papillary and anaplastic carcinomas, and may be useful in distinguishing benign from malignant thyroid lesions.26,27 In Cowden’s disease (familial goiter and skin hamartomas), inactivating mutations of the phosphatase and tensin homolog (PTEN) gene have been identified.28 PTEN may inhibit phosphorylation and kinase activity of AKT1, leading to the development of follicular adenomas and carcinomas.24 The PAX8/PPARγγ1 (peroxisome proliferator-activated receptor) rearrangement seems to be unique to follicular thyroid carcinoma.29 PAX8 is expressed at high levels during thyroid development, and the PAX/PPARγγ1 gene product seems to function as a dominant negative, blocking the activation of wild-type PPARγγ1. Mutations in the tumor-suppressor gene p53, a transcriptional regulator, seem to be involved in insular thyroid carcinomas and the progression from papillary to anaplastic carcinoma.30,31
The role of mutations of the RET oncogene in the development of papillary carcinoma and MTC has been extensively studied.32 Located on chromosome 10, RET codes for a transmembrane tyrosine kinase receptor that binds glial cell line–derived neurotrophic factor. During embryogenesis, RET protein is normally expressed in the nervous and excretory systems. Abnormalities in RET expression result in developmental defects, including the disruption of the enteric nervous system (Hirschsprung’s disease). Presumably, RET gene mutations result in the activation of the Ras/JNK/ERK1/2 signaling pathways, resulting in further genomic instability and prevention of entry into the apoptotic pathway.33
MTC and pheochromocytoma arise from neural crest cells containing RET point mutations. These point mutations have been well documented in patients with familial MTC, multiple endocrine neoplasia (MEN) type IIA, and MEN IIB.34,35 The aggressiveness of the MTC that develops is linked to the specific RET mutation identified.36 Somatic mutations of ret are also found in approximately 25% of sporadic MTCs. Many of these are identical to the codon 918 mutation found as a germline mutation in MEN IIB, although other codons are more infrequently involved.37
Rearrangements of the RET gene by fusion with other genes also create transforming oncogenes. Although more than 10 rearrangements have been described, three oncogene proteins—RET/PTC1, RET/PTC2, and RET/PTC3—account for most of the rearrangements found in papillary thyroid cancers, and are more frequently associated with childhood thyroid carcinomas.38 Not all patients with papillary carcinomas express a RET/PTC gene however.39 There are marked geographic differences, and the gene rearrangement is strongly associated with radiation exposure. After the Chernobyl nuclear disaster, 66% of the papillary thyroid cancers removed from affected patients had RET/PTC1 or RET/PTC3 rearrangements.40 The RET/PTC3 rearrangement is most commonly associated with a “solid” follicular variant of papillary thyroid carcinoma, whereas RET/PTC1 is associated more often with the classic or diffuse sclerosing variants of papillary thyroid cancer.41,42
Risk Factors and Etiology
Although the specific molecular events related to the development of thyroid carcinomas remain incompletely defined, several patient and environmental factors have been closely examined. Women are three times more likely than men to develop differentiated thyroid cancer, and two times more likely to have anaplastic thyroid cancer. The median age at diagnosis is 47 years, with a peak in women at 45 to 49 years and in men at 65 to 69 years.2 Epidemiologic studies have not shown a clear association between dietary iodine and thyroid carcinomas.43 Also, there does not seem to be a simple relationship between benign goiter and well-differentiated thyroid carcinomas. Although papillary thyroid carcinomas are not associated with goiter, follicular and anaplastic thyroid carcinomas occur more commonly in areas of endemic goiter. Additionally, two particularly important risk factors, exposure to radiation and a family history of thyroid cancer, have been studied extensively.
Exposure to ionizing radiation increases patient risk for the development of thyroid carcinoma.44,45 Ionizing radiation exposure is the only established environmental risk factor for thyroid cancer.46 Low-dose ionizing radiation treatments (<2000 cGy) were used in the treatment of “enlarged thymus” to prevent “sudden crib death,” enlarged tonsils and adenoids, acne vulgaris, hemangioma, ringworm, scrofula, and other conditions. The risk increases linearly from 6.5 to 2000 cGy, and typically has a latency period lasting 10 to 30 years. Although higher doses of ionizing radiation typically lead to the destruction of thyroid tissue, patients with Hodgkin’s disease who receive 4000 cGy also have a higher incidence of thyroid cancer. Palpable thyroid nodularity may be present in 17% to 30% of patients exposed to ionizing radiation.47 A patient with a history of radiation exposure who presents with a thyroid nodule has a 50% chance of having a malignancy.48 Of these patients with thyroid cancer, 60% have cancer within the nodule, and the remaining 40% have cancer located in another area of the thyroid. The thyroid carcinoma tends to be papillary and frequently multifocal. There is also a higher risk of cervical metastases.
Similarly, patients exposed to radiation from nuclear weapons and accidents have a higher incidence of thyroid cancer. Children near the Chernobyl nuclear power facility had a 60-fold increase in thyroid carcinoma after the nuclear accident in 1986.49 Most of these children were infants at the time of the accident, and many of these cases developed without the typical latency period. The thyroid gland seems to be particularly vulnerable to ionizing radiation in children and yet relatively insensitive in adults. In the life span study of atomic bomb survivors in Hiroshima and Nagasaki, the risk of thyroid cancer was associated with patient age at the time of the bombings.50 The risk was greatest for individuals younger than 10 years, and no increased incidence of thyroid cancer was seen in individuals older than 20 years at the time of exposure.
Finally, familial and genetic contributions need to be fully evaluated. A patient with a family history of thyroid carcinoma may require specific diagnostic testing. Approximately 6% of patients with papillary thyroid cancer have familial disease. Papillary thyroid cancer occurs with increased frequency in certain families with breast, ovarian, renal, or central nervous system malignancies.51 Gardner’s syndrome (familial colonic polyposis) and Cowden’s disease are associated with well-differentiated thyroid carcinomas. Patients with a family history of MTC, MEN IIA, or MEN IIB warrant evaluation for the RET point mutation.
Tumor Staging and Classification
TNM Classification
The American Joint Commission on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC) adopted a tumor-node-metastasis (TNM) classification system (Table 124-1). In this system, patient age at presentation influences the clinical staging of a thyroid carcinoma. Of patients with stage I disease, 82% had a 20-year survival of nearly 100%, whereas the 5% of patients with stage IV disease experienced a 5-year survival of only 25%.52
| Primary Tumor (T) | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor = 2 cm in greatest dimension, limited to thyroid |
| T2 | Tumor >2 cm and = 4 cm in greatest dimension, limited to thyroid |
| T3 | Tumor >4 cm in greatest dimension, limited to the thyroid or |
| Any tumor with minimal extrathyroid extension (e.g., extension to sternothyroid muscle or perithyroid soft tissues) | |
| T4a | Tumor of any size extending beyond the thyroid capsule to invade subcutaneous soft tissues, larynx, trachea, esophagus, or recurrent laryngeal nerve |
| T4b | Tumor invades prevertebral fascia or encases carotid artery or mediastinal vessels |
| All Anaplastic Carcinomas Are Considered T4 Tumors | |
| T4a | Intrathyroidal anaplastic carcinoma—surgically resectable |
| T4b | Extrathyroidal anaplastic carcinoma—surgically unresectable |
| Regional Lymph Nodes (N) | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis |
| N1a | Metastasis to level VI (pretracheal, paratracheal, and prelaryngeal/Delphian lymph nodes) |
| N1b | Metastasis to unilateral, bilateral, or contralateral cervical or superior mediastinal lymph nodes |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| Stage Grouping | ||
|---|---|---|
| Age <45 Years | Age ≥45 Years | |
| Papillary/Follicular | ||
| Stage I | Any T any N M0 | T1 N0 M0 |
| Stage II | Any T any N M1 | T2 N0 M0 |
| Stage III | T3 N0 M0 | |
| T1-T3 N1a M0 | ||
| Stage IVA | T4a N0 M0 | |
| T4a N1a M0 | ||
| T1-4a N1b M0 | ||
| Stage IVB | T4b any N M0 | |
| Stage IVC | Any T any N M1 | |
| Medullary | ||
| Stage I | T1 N0 M0 | |
| Stage II | T2 N0 M0 | |
| Stage III | T3 N0 M0 | |
| T1-3 N1a M0 | ||
| Stage IVA | T4a N0 M0 | |
| T4a N1a M0 | ||
| T1-4a N1b M0 | ||
| Stage IVB | T4b any N M0 | |
| Stage IVC | Any T any N M1 | |
| Anaplastic | ||
| Stage IVA | T4a any N M0 | |
| Stage IVB | T4b any N M0 | |
| Stage IVC | Any T any N M1 | |
From American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002.
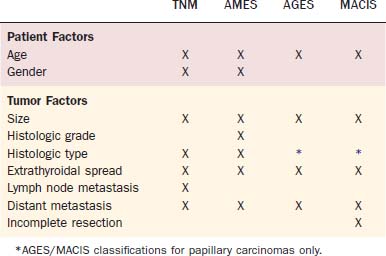
AMES
In the AMES system, patient age, the presence of metastases, extent of tumor invasion, and tumor size were used to stratify patients into low-risk and high-risk groups (Table 124-2). Low-risk patients were young (men, <41 years old; women, <51 years old) without distant metastases and all older patients without extrathyroidal papillary carcinoma, without major invasion of the tumor capsule by follicular carcinoma or with a primary tumor less than 5 cm in diameter. In a review of 310 patients from 1961-1980, low-risk patients (89%) had a mortality of 1.8% compared with a mortality rate of 46% in high-risk patients (11%). Recurrence in low-risk patients was 5%, and in high-risk patients was 55%.53 In DAMES, nuclear DNA content was added to the AMES system to improve risk-stratification for papillary thyroid carcinoma.54
AGES and MACIS
In the original AGES system, age at diagnosis, histologic tumor grade, extent of disease at presentation, and tumor size were used to calculate a prognostic score.55 Because of the infrequent practice of tumor grading, a more recent modification of the system eliminated histologic tumor grade and incorporated metastasis and extent of resection. The MACIS system accounts for metastasis, age at diagnosis, completeness of surgical resection, extrathyroidal invasion, and tumor size.56 The MACIS score is calculated as follows:
Other risk-classification systems with similar diagnostic criteria have been described.57–59 Although numerous multivariable prognostic scoring systems have been developed, none is universally accepted. Additionally, none of these classifications has shown clear superiority, and application of these systems to a single population has shown incompatible findings compared with the original studies.59,60 These systems do not apply to patients with poorly differentiated and more aggressive thyroid carcinomas.
Nevertheless, some general conclusions can be drawn from these studies regarding the prognosis of patients with well-differentiated thyroid carcinomas. Low risk for tumor recurrence and disease-specific mortality is noted in patients who are younger at diagnosis, have smaller primary tumors that lack extrathyroidal extension or regional/distant metastases, and have complete gross resection of disease at the initial surgery. Delay in treatment negatively affects prognosis. The most significant overall indicator of a poor prognosis is distant metastases, however, especially to bone.6 Although a single risk-classification strategy is unavailable, these criteria should guide physicians to use therapeutic strategies that are directed toward the particular disease and risk for an individual patient, rather than applying a general treatment strategy for all patients with a particular form of thyroid carcinoma. More recent management guidelines from the American Thyroid Association (ATA) have recommended use of the AJCC/UICC staging system for all patients with differentiated thyroid cancer.
Evaluation of a Thyroid Nodule
The incidence of thyroid nodular disease is quite high, spontaneously occurring at a rate of 0.08% per year starting in early life and extending into the eighth decade.47 Although thyroid nodules represent a wide spectrum of disease, most are colloid nodules, adenomas, cysts, and focal thyroiditis, with only a few (5%) being carcinoma. With a lifetime incidence of 4% to 7%, the annual incidence of thyroid nodules in the United States is about 0.1%, which is approximately 300,000 new nodules each year.61,62 Most of these nodules are benign and do not require removal. With approximately 37,000 new thyroid cancers each year, about 1 in 20 new thyroid nodules contains carcinoma, however, and approximately 1 in 200 nodules is lethal. The challenge in treating patients with thyroid nodules is to identify the patients with malignant lesions and to balance the potential morbidity of treatment with the aggressiveness of their disease.
Clinical Assessment: History and Physical Examination
Numerous findings should raise suspicion of malignancy in a patient presenting with a thyroid nodule. Younger and older patients are more likely to have a malignant thyroid nodule. Patients younger than 20 years have an approximately 20% to 50% incidence of malignancy when presenting with a solitary thyroid nodule.63 Nodular disease is more common in older patients, usually men older than 40 years and women older than 50 years. Although children may present with more advanced disease and even cervical metastases, malignancy in older patients has a considerably worse prognosis. Men often have more aggressive malignancies than women, but the overall incidence of thyroid nodules and malignancy is higher in women.
The physical examination of a patient with a thyroid nodule begins with careful palpation of the thyroid to assess the lesion. One should determine whether the lesion is solitary or the dominant nodule in a multinodular gland, although the risk of carcinoma in either setting is the same.3,48 Having the patient swallow assists in the examination because nonthyroid pathology does not typically elevate with the thyroid during swallowing. Palpable nodules are typically 1 cm or larger. Smaller nodules can be found incidentally on radiographic studies for other reasons and may be monitored. Lesions greater than 1 cm in size warrant a complete workup. The firmness of the nodule may be associated with an increased risk of carcinoma by twofold to threefold.64 Nodules greater than 2 cm in diameter and solid lesions have an increased incidence of harboring carcinoma. The evaluation of larger lesions also requires more caution because the rate of false-negative results during FNAC also increases.65
Further assessment of the patient may reveal the extent of involvement of a thyroid lesion. Palpable cervical nodes adjacent to the thyroid nodule increase the suspicion for malignancy, and may be the only presenting sign of a thyroid carcinoma. Adenopathy may be present, however, in a patient affected by Hashimoto’s thyroiditis, Graves’ disease, or infection.66,67 Large lesions can potentially shift the larynx and trachea within the neck. The mobility of the nodule relative to the laryngotracheal complex and adjacent neck structures should be evaluated. Malignant lesions are more likely to be fixed to the trachea, esophagus, or strap muscles.
Despite the importance of the initial clinical assessment, the history and physical examination are unreliable in predicting carcinoma. Many of the clinical signs of malignancy are manifest late in the course of disease. Additionally, many of these same findings may be caused by events associated with benign disease (e.g., hemorrhage into a benign nodule). The clinical assessment should provide a justification and a context for the interpretation of diagnostic studies, such as FNAC. Of particular note would be any patient and thyroid nodule features that might be concerning for aggressive carcinoma behavior (Table 124-3).
Table 124-3 Risk Factors for Aggressive Behavior of Well-Differentiated Thyroid Carcinomas
| History |
| Age |
| Younger—<20 yr |
| Older |
| Men—>40 yr |
| Women—>50 yr |
| Gender |
| Male > female |
| History of radiation exposure/therapy |
| Family history of thyroid carcinoma |
| Physical Examination |
| Hard, fixed lesion |
| Rapid growth of mass |
| Pain |
| Lymphadenopathy |
| Vocal cord paralysis |
| Aerodigestive tract compromise |
| Dysphagia |
| Stridor |
| Histopathologic Factors* |
| Size (>4 cm) |
| Extrathyroidal spread |
| Vascular invasion |
| Lymph node metastasis |
| Distant metastasis |
| Histologic type |
| Tall-cell variant of papillary carcinoma |
| Follicular carcinoma |
| Hürthle cell carcinoma |
Diagnostic Studies
Laboratory Studies
Most patients who present with a thyroid nodule are euthyroid. The finding of hypothyroidism or hyperthyroidism tends to shift the workup away from thyroid carcinoma to a functional disorder of the thyroid gland, such as Hashimoto’s thyroiditis or a toxic nodule.68 Although many thyroid hormone tests are available, few are needed in the initial patient evaluation. TSH measurement serves as an excellent screening test. Full thyroid function tests can be performed if the TSH level is abnormal.
Measurement of thyroglobulin is generally not performed initially because thyroglobulin is secreted by normal and malignant thyroid tissue, and it is not recommended in the 2006 ATA guidelines.69 Levels of thyroglobulin cannot differentiate between benign and malignant processes, unless levels are extremely high, as in metastatic thyroid cancer. Antithyroglobulin antibodies can also interfere with the assay. Thyroglobulin levels may be useful in studying patients who have undergone total thyroidectomy for well-differentiated thyroid cancer.
Fine-Needle Aspiration Cytology
FNAC has become the procedure of choice in the evaluation of thyroid nodules.69 The findings are highly sensitive and specific, although the accuracy of FNAC is related to the skill of the aspirator and the experience of the cytopathologist.70 The procedure is minimally invasive and may be performed quickly with little patient discomfort. In contrast to large-bore needle biopsies such as the Tru-cut or Vim-Silverman needle, there are fewer complications. With the advent of this technique, the number of patients requiring surgery has decreased by 35% to 75%, and the cost in managing patients with thyroid nodules has been substantially reduced.71–73 Also, the yield of malignancies has almost tripled in patients who have had thyroid surgery after FNAC.73,74 The accuracy of FNAC diagnosis of papillary carcinoma is 99% with a false-positive rate of less than 1%.75
FNAC should be one of the initial steps in the surgical evaluation of a thyroid nodule. Approximately 15% of all aspirates are inadequate or nondiagnostic, largely because of the sampling from cystic, hemorrhagic, hypervascular, or hypocellular colloid nodules. Repeat aspiration of such a nodule is crucial because a nondiagnostic finding should never be interpreted as a negative finding for carcinoma. Surgical diagnoses after repeated nondiagnostic aspirations revealed malignant nodules in 4% of women and 29% of men.76 Nodules that are difficult to localize and nodules that have yielded nondiagnostic aspirates on previous attempts may benefit from ultrasound-guided aspiration. FNAC is increasingly being performed with ultrasound guidance to improve diagnostic accuracy and yield. Cystic nodules with multiple nondiagnostic FNAC studies require close observation or surgical excision. Also, surgery should be more strongly considered for a solid nodule that is cytologically nondiagnostic.69
Successful FNAC categorizes a nodule into the following groups: benign, malignant, or suspicious. In 60% to 90% of nodules, FNAC reveals a benign or “negative” diagnosis. The likelihood of malignancy (false-negative rate) is 1% to 6%.71,77 The diagnosis of malignancy, particularly papillary (including follicular variant), medullary, and anaplastic carcinomas and lymphomas, can be determined in about 5% of nodules. The likelihood of a false-positive finding is less than 5%.71,77 Frequently, false-positive results occur because of difficulties in interpreting cytology in patients with Hashimoto’s thyroiditis, Graves’ disease, or toxic nodules. A benign cytology is a macrofollicular lesion or a colloid adenomatous nodule. The remaining “suspicious” samples are composed of lesions that contain abnormal follicular epithelium with varying degrees of atypia. This finding needs to be evaluated in the context of patient history and physical findings that may be suggestive of malignancy. A complete report of the FNAC detailing specimen adequacy and pathologic findings is crucial, and efforts have been made to standardize this information.78,79
Follicular neoplasms cannot be classified by FNAC alone. The presence of hypercellular, microfollicular arrays with minimal colloid increases the concern for carcinoma. The differentiation between follicular adenoma and follicular carcinoma depends on the histologic finding of capsular or vascular invasion, which requires evaluation of the entire thyroid nodule. Occasionally, patients with a diagnosis of follicular neoplasm on FNAC have an iodine-123 (123I) thyroid scan. If the suspicious nodule is “cold,” surgery is indicated. If the nodule is hyperfunctioning compared with the surrounding thyroid, surgery can be avoided. Overall, 20% of nodules diagnosed as follicular neoplasms by FNAC contain thyroid carcinomas.80
Similarly, Hürthle cell (oxyphilic) neoplasms can be difficult to evaluate. The presence of Hürthle cells in an aspirate may indicate an underlying Hürthle cell adenoma or carcinoma, but these cells can also be present in thyroid disorders, such as multinodular goiter and Hashimoto’s thyroiditis. Carcinomas can be found in 20% of nodules identified as follicular and oxyphilic neoplasms.81 Because of the risk of underlying carcinoma in these cases, surgery is recommended.
Imaging
A systematic ultrasound examination can be extremely valuable in the assessment of a patient with thyroid cancer, including color and power Doppler examination of the thyroid, specific nodules, and lymph nodes.82,83 Examination of the nodal basins should be bilateral and include the jugular, submandibular, supraclavicular, paratracheal, and suprasternal regions. These studies may detect cervical nodes that may contain early clinically occult metastatic disease that would not otherwise have been included in a surgical dissection.69,84 Characteristics of lymph nodes suspicious for metastatic deposits include loss of the fatty hilum, increased vascularity, rounded node configuration, hypoechogenicity of a solid nodule, and microcalcifications.83,85,86 Ultrasonography is also useful in the evaluation of cervical lymph nodes in patients with a history of thyroid cancer who present with adenopathy or increasing thyroglobulin levels. These studies are not useful, however, in the evaluation of substernal extent of disease or the involvement of adjacent structures.
In a patient with multiple thyroid nodules, FNAC should be performed in conjunction with a diagnostic ultrasound study. Aspiration of the largest or “dominant” nodule alone may miss a thyroid malignancy. In the presence of two or more thyroid nodules larger than 1 to 1.5 cm, nodules with a suspicious ultrasound appearance should be aspirated preferentially. If none of the nodules has suspicious ultrasound characteristics, and multiple sonographically similar coalescent nodules are present, aspiration of the largest nodule only is reasonable.69
Currently, there is no role for ultrasonography in screening asymptomatic patients for thyroid nodules. Preoperative ultrasound evaluation of the lateral cervical lymph nodes is recommended for all patients with papillary and Hürthle cell thyroid cancer before initial thyroidectomy because operative management may be altered in 20% of patients.87 In addition, intraoperative ultrasound examination may be useful in the localization of nonpalpable lesions in the thyroid bed or nodal metastases.
Computed tomography (CT) and MRI scans are usually unnecessary in the evaluation of thyroid tumors except for fixed or substernal lesions. Although these studies are not as effective as ultrasonography in the evaluation of thyroid nodules, they are more reliable in evaluating the relationship of the thyroid lesion to adjacent neck structures, such as the trachea and esophagus. These studies are useful in determining substernal extension, identifying cervical and mediastinal adenopathy, and evaluating possible tracheal invasion.88 Anatomic imaging should be obtained when visceral compartment invasion is suspected and for localization in patients with nodal disease. Also, CT or MRI can supplement ultrasound imaging, which cannot visualize the regions behind the sternum, trachea, and esophagus. Caution must be exercised in the use of iodine-containing contrast material in patients with multinodular goiter if a hyperthyroid state is suspected, and in patients with well-differentiated thyroid cancer. In the latter group, iodinated contrast media preclude the use of postoperative radioactive iodine therapy for 2 to 3 months. Finally, MRI is more accurate than a CT scan in distinguishing recurrent or persistent thyroid tumor from postoperative fibrosis.
Thyroid Isotope Scanning
Radionuclide scanning with 123I or technetium 99m (99mTc) sestamibi assesses the functional activity of a thyroid nodule and the thyroid gland. Nodules that retain less radioactivity than the surrounding thyroid tissue are termed “cold,” nonfunctioning, or hypofunctional. These cold nodules are thought to have lost functions of fully differentiated thyroid tissue and to be at increased risk of containing carcinoma. In a meta-analysis of patients with scanned nodules that were surgically removed, 95% of all nodules were cold.66,67 The incidence of malignancy in cold nodules was 10% to 15% compared with only 4% in hot nodules.
With the evolution of FNAC, radionuclide scanning is not routinely performed in the evaluation of a thyroid nodule. More frequently, “cold” nodules are detected in patients during evaluation for hyperthyroid disorders. Patients who present initially with a thyroid nodule, however, and are found to be hyperthyroid on preliminary thyroid function testing should have radionuclide scanning to differentiate between a toxic nodule and Graves’ disease and a nonfunctioning nodule. Also, after indeterminate FNAC, 123I thyroid scan should be considered. Surgical treatment should be contemplated if a concordant autonomously functioning nodule is not seen.69
Rational Approach to Management of a Thyroid Nodule
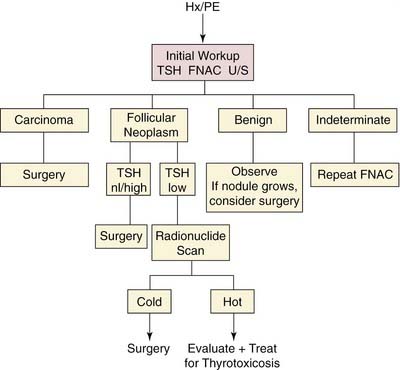
Numerous diagnostic algorithms have been proposed for the evaluation of a thyroid nodule (Fig. 124-3).70,89 Evaluation generally begins with a thorough history and physical examination to identify significant risk factors. Surgery may be deemed appropriate based solely on high-risk factors, such as age, sex, history of radiation exposure, rapid nodule growth, upper aerodigestive tract symptoms, and fixation.
Baseline TSH screening determines the diagnostic course. Patients with hyperthyroidism (suppressed serum TSH level) should receive radionuclide scanning to determine the presence of a toxic “hot” nodule, or Marine-Lenhart syndrome, or Graves’ disease with a concomitant “cold” nodule.90 A patient with hypothyroidism (elevated serum TSH level) should be appropriately treated by an endocrinologist, and then FNAC should be performed. Most patients are euthyroid (normal serum TSH level), and FNAC should be performed. Ultrasound examination can provide valuable diagnostic information, especially in the selection of a nodule for biopsy in a patient with multiple nodules, and may facilitate FNAC. In a patient with a thyroid malignancy, evaluation of the nodal basins may detect early clinically occult disease and alter surgical management. Patients with cytologic findings that are diagnostic or strongly suggestive of malignancy should be referred to a surgeon for removal of the lesion.
Review of Thyroid Neoplasms
Thyroid Adenoma
Pathology
The revised histologic classification of thyroid tumors divides epithelial tumors into the categories of follicular adenoma and other rare tumors (Table 124-4). Follicular adenomas are the most common benign thyroid lesions. Atypical follicular adenomas may show atypical microscopic features, including excess cellularity, increased mitotic figures, and necrotic foci. Although most of these lesions are benign, they may metastasize even in the absence of microinvasion.91
Table 124-4 World Health Organization Revised Histologic Classification of Thyroid Tumors
|
I. Epithelial tumors
|
From Hedinger C, ed. Histological Typing of Thyroid Tumours. 2nd ed. Berlin: Springer-Verlag; 1988.
Management and Prognosis
Thyroid nodules that are determined to be benign require follow-up because of a low false-negative rate (approximately 5%) with FNAC.92,93 Nodule growth alone is not an indication of malignancy, but growth is an indication for repeat biopsy. The 2006 ATA guidelines recommend serial clinical examination for easily palpable benign nodules at 6- to 18-month intervals.69 All other benign nodules should be followed with serial ultrasound examinations 6 to 18 months after initial FNAC. Patients with nodules that remain stable in size may have subsequent examinations at longer time intervals. Patients with evidence of nodule growth should have repeat FNAC, preferably with ultrasound guidance.
Autonomously hyperfunctioning thyroid adenomas are usually anatomically and functionally stable. Although most patients do not develop thyrotoxicosis, 20% of patients with lesions greater than 3 cm may develop thyrotoxicosis. Surgery and radioiodine therapy can be used to manage these lesions, although many physicians prefer surgery for patients younger than 40 years. These patients may require preoperative medications to control thyrotoxic symptoms. The lesions are typically removed with a unilateral thyroid lobectomy. The remaining thyroid tissue typically returns to normal function after several months. Ethanol injection has become increasingly common, especially in Europe, to manage these lesions.94
Thyroid Cyst
Clinical Presentation
Although a thyroid cyst is not a specific diagnosis, this entity is frequently encountered in clinical practice. Approximately 15% to 25% of all thyroid nodules are cystic or have a cystic component.47 The presence of a cyst does not signify a benign lesion because papillary carcinomas and parathyroid tumors may manifest with cystic masses. Papillary carcinoma may be present in 14% to 32% of all cystic nodules, although most of these lesions are benign adenomas or colloid nodules.95,96
Pathology
A thyroid cyst can result from congenital, developmental, or neoplastic causes.95 Many cysts result from intranodular ischemia causing tissue necrosis and liquefaction. True epithelial-lined cysts are rare. Occasionally, parathyroid or thyroglossal duct cysts can be mistaken for thyroid nodules. A parathyroid cyst contains high parathyroid hormone levels within the clear fluid, and a thyroglossal duct cyst contains columnar epithelium. These lesions may also be differentiated by ultrasound imaging.
Management and Prognosis
When encountered during FNAC, a thyroid cyst should be drained completely. This procedure may prove curative in most simple cysts, although one or two additional drainage procedures may be required. If a cyst persists after three drainage attempts or reaccumulates quickly, the suspicion for carcinoma should increase. Brown fluid withdrawn from a cyst may represent old hemorrhage into an adenoma, but red fluid is more suspicious for carcinoma.64 Clear, colorless fluid may be withdrawn from a parathyroid cyst and can be assessed for parathyroid hormone.97 In suspicious cases, the surgeon and patient should consider ultrasound-guided FNAC to sample a solid component of the lesion or a unilateral thyroid lobectomy to obtain a definitive diagnosis. Because of the potential for thyroid carcinoma in cystic lesions, surgical excision for diagnosis is preferable to the injection of sclerosing agents.
Papillary Carcinoma
Clinical Presentation
Papillary carcinoma is the most common form of thyroid malignancy, accounting for 60% to 70% of all thyroid cancer.58,98 This lesion typically occurs in patients 30 to 40 years old, and is more common in women, with a female-to-male ratio of 2 : 1. This ratio has decreased steadily over the past 40 years as the incidence in men has increased.99 Papillary carcinomas are the predominant thyroid malignancy in children (75%). Although children more commonly present with advanced disease, including cervical and distant metastases, their prognosis remains quite favorable.
Most cases of papillary carcinoma occur spontaneously. Patients with a history of low-dose radiation exposure tend to develop papillary carcinomas (85% to 90%).100 These lesions also are more common in patients with Cowden’s syndrome (familial goiter and skin hamartomas), Gardner’s syndrome (familial colonic polyposis), and familial polyposis. Only 6% of papillary carcinomas are associated with familial disease.
Papillary carcinoma can be classified into three categories based on size and extent of the primary lesion.101,102 Minimal carcinoma or occult carcinoma/microcarcinoma tumors are 1.5 cm or smaller in size, and show no evidence of invasiveness through the thyroid capsule or to cervical lymph nodes. These lesions are typically nonpalpable and are usually incidental findings during operative or autopsy examination. Intrathyroid tumors are greater than 1.5 cm in diameter, but are confined to the thyroid gland with no evidence of extrathyroid invasion. Extrathyroid tumors extend through the thyroid capsule to involve the surrounding viscera. This latter form of papillary carcinoma is associated with substantial morbidity and decreased survival.7,101
Most patients present with a slow-growing, painless mass in the neck and are often euthyroid. Often, the primary lesion is confined to the thyroid gland, although 30% of patients may have clinically evident cervical nodal disease.103,104 Histologic studies have shown the strong lymphotropic nature of papillary carcinoma, leading to multifocal disease within the thyroid and regional lymphatics. Microscopic disease has been identified in the cervical nodes of 50% to 80% of patients and in the contralateral lobe in 80% of patients with papillary carcinoma at the time of surgery.105 The significance of this microscopic disease is unclear, however, because clinical recurrences in the neck and in the contralateral lobe occur in less than 10% of patients.106 More likely, the prevalence of microscopic disease suggests that most papillary carcinomas have an indolent course that only occasionally becomes clinically evident. Definite predictors of the clinical course for papillary carcinoma are not well defined, however.
Advanced disease may be associated with symptoms of local invasion, including dysphagia, dyspnea, and hoarseness. Occasionally, cervical nodal involvement may be more apparent than the thyroid nodule. Distant metastases, especially to the lungs, are more commonly encountered in children, although 10% of all patients may ultimately develop distant disease.81
Pathology
On gross examination, papillary carcinoma is firm, white, and not encapsulated. The lesion tends to remain flat on sectioning, rather than bulging the way normal thyroid tissue or benign nodular lesions do. Macroscopic calcifications, necrosis, or cystic changes may be readily apparent.107
Histologically, these lesions arise from thyroid follicular cells and contain papillary structures that consist of a neoplastic epithelium overlying a true fibrovascular stalk.14 Cells are cuboidal with a pale, abundant cytoplasm. Large, crowded nuclei with folded and grooved nuclear margins may have intranuclear cytoplasmic inclusions. Prominent nucleoli account for the “Orphan Annie eye” appearance. Laminated calcium densities, psammoma bodies, are likely the remnants of necrotic calcified neoplastic cells and are present in 40% of cases.
Although a follicular component may predominate, lesions with any papillary features behave clinically as papillary carcinomas. The designation of “papillary carcinoma” includes mixed papillary follicular carcinoma and the follicular variant of papillary carcinoma. A more unfavorable prognosis is associated with certain histologic forms of papillary carcinoma, including diffuse sclerosing and tall-cell variants.14,108 The tall-cell variant is characterized by well-formed papillae covered by cells that are twice as tall as they are wide. The rarer columnar cell variant is characterized by the presence of prominent nuclear stratification.109
Papillary carcinomas have a strong tendency for lymphatic spread within the thyroid and to local lymph nodes in the paratracheal and cervical regions. The tendency for intraglandular spread may lead to the multifocal disease often present in patients. Discrete lesions may be due to de novo formation, however, especially in patients previously exposed to ionizing radiation.110
Local invasion occurs in 10% to 20% of these tumors, leading to involvement of the overlying strap muscles, laryngeal and tracheal framework, RLNs, pharynx, and esophagus. This extension may evolve from the primary lesion or from extracapsular extension of metastatic nodes. Angioinvasion is a clear harbinger of increased risk for recurrence and worse prognosis.58 A coexisting lymphocytic thyroiditis has been correlated with decreased recurrence and better overall prognosis.
Management and Prognosis
Most patients with papillary carcinoma do well regardless of treatment. Prolonged survival, even with recurrent disease, has led to controversy regarding the extent of thyroidectomy for patients with well-differentiated thyroid carcinomas (see section on extent of surgery under surgical management and technique). A balance must be achieved between an effective surgical treatment for these malignancies and the potential morbidity of this surgery. Numerous studies have attempted to categorize patients by their risk factors and to justify more aggressive surgical intervention for high-risk patients (see section on tumor staging and classification). The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology provides specific recommendations for evaluation and management of well-differentiated thyroid carcinomas.111
When patients present with biopsy-proven disease or indications of disease in both lobes, total or near-total thyroidectomy is the procedure of choice. Additionally, patients stratified into high-risk categories in any of the classification schemes previously described (see section on tumor staging and classification) would probably benefit from a more extensive surgical procedure that includes paratracheal lymph node dissection. This procedure would permit possible thyroid hormone suppression therapy and radioiodine ablation of remaining disease.
Multifocal disease is present in 80% of patients in some reports.104,108 This percentage may represent de novo multicentric tumor formation or intraglandular metastasis. The prevalence of multifocal disease lends credence to the argument for more complete surgical removal of the thyroid gland in patients with papillary thyroid cancer. Patients with partial thyroidectomy had higher local recurrence rates and increased pulmonary and cervical metastases.33,112 Controversy remains, however, because this increased local recurrence did not compromise disease survival in some studies.53,113
Generally, invasive tumors are associated with a compromise in survival. Woolner and colleagues107 reviewed 1181 thyroid cancer patients and found that no patient died of papillary cancer when the lesion was less than 1.5 cm in size. Only 3% of patients died when the lesion was larger but remained intrathyroid. Mortality increased to 16% of patients when extrathyroid disease was present.
After total thyroidectomy, patients may be monitored by following thyroglobulin levels, which should remain undetectable, and by neck ultrasonography to evaluate the central and lateral cervical compartments. Any increase in thyroglobulin levels is suspicious for disease recurrence and requires appropriate screening. Approximately 12% of patients with papillary carcinoma are not cured by initial treatment, leading to a prolonged clinical course.114 Recurrent disease may occur after many years, involving the thyroid bed (5% to 6%), regional lymphatics (8% to 9%), or distant sites (4% to 11%).115 Successful treatment of recurrence varies by site of involvement and the patient’s initial risk classification.
Local recurrence is a serious complication and is associated with a disease-related mortality of 33% to 50%.115 Typically, patients with nodal recurrence fare better than patients with tumor recurrence in the thyroid bed or distant sites. Studies have been inconsistent regarding the impact of cervical metastases on survival. Patients older than 40 years may have clinically evident nodal disease in 36% to 75% of cases and overall increased mortality.6,99 Some studies suggest prognosis is better with more cervical node involvement.56,116 Although the role of cervical metastasis in survival may be controversial, there is an association with an increased recurrence rate, especially in elderly patients.114,116,117 Generally, lymph node metastases do not seem to have an impact on overall survival in most patients with low-risk and intermediate-risk disease.101,118 Cervical recurrences occur in 20% of patients with low-risk disease, however, and 59% of patients with high-risk disease.53,119 The continuing debate regarding extent of lymphadenectomy at the time of thyroidectomy reflects a shift in focus from overall survival to recurrence-free survival.
Because of these findings and the overall prevalence of microscopic cervical disease with uncertain prognostic implications, management of cervical metastasis tends to be conservative. There is no role for elective neck dissection in the clinically disease-free neck, especially considering the effectiveness of radioiodine therapy in ablating microscopic disease.106 The central compartment from hyoid bone to mediastinum between the internal jugular veins (levels VI and VII) should be carefully inspected and palpated. The 2006 ATA management guidelines recommend considering routine central compartment (level VI) neck dissection for patients with papillary thyroid carcinoma and suspected Hürthle cell carcinoma, although postoperative radioiodine therapy may provide an alternative approach.69
Elective dissection of the pretracheal and ipsilateral paratracheal nodes may be reasonable, especially in patients with high-risk factors. Additionally, elective removal may potentially decrease nodal recurrence in this region and the need for reoperation. In patients with palpable or visible neck disease, a comprehensive neck dissection (levels II-V) should be performed, as opposed to selective cervical lymph node excision or “berry-picking.” Although level I is seldom involved, metastatic disease is frequently noted on histologic examination of nodal levels II through V.120 In addition, because the incidence of contralateral subclinical metastasis is less than 20%, elective treatment of the contralateral neck is not advocated.
The presence of distant metastasis is associated with a worse prognosis. Approximately 10% of patients with papillary thyroid carcinoma develop distant metastasis at some point during their disease course.81 Most commonly, the lungs are involved, although bone sites and the central nervous system may also be affected.
In nearly every study, patient age at the time of diagnosis is an important prognostic variable.6,116,121 Older patients (especially >40 years old) with papillary carcinoma have a worse prognosis. Extrathyroidal invasion seems to be more common in older patients. The prognosis for men younger than 40 years is comparable to women of the same age. Overall survival is worse for men, however, and the risk of death from papillary thyroid carcinoma may be twice as great.53,116 In the last 40 years, the increase in incidence of papillary thyroid carcinoma in men has decreased the gender ratio of men to women with the disease from 1 : 4 to 1 : 2.99
Children fare better with this disease. Among patients younger than 15 years, 90% show cervical metastasis at some time during their disease course.122 Twenty percent of children may present with pulmonary metastases.123 Neither cervical nor pulmonary metastases seem to have any impact on survival, however. Perhaps these differences may be related to biologic differences in the disease process or between age groups.
Finally, the tall-cell variant of papillary thyroid carcinoma is different from other forms of this disease. A review of patients with tall-cell variant papillary carcinoma showed a more aggressive natural history in all age groups and a worse prognosis.124
Follicular Carcinoma
Clinical Presentation
Follicular carcinomas represent 10% of thyroid malignancies. The mean age of presentation is 50 years compared with 35 years mean age of patients with papillary carcinoma. Women more commonly have this lesion, with a female-to-male ratio of 3 : 1.125 These lesions occur more frequently in iodine-deficient areas, especially areas of endemic goiter. Follicular carcinomas have been correlated with pregnancy and with certain HLA subtypes (DR1, DRw, and DR7). Also, a rare form of familial follicular carcinoma is reported in patients with dyshormonogenesis. The overall incidence of follicular carcinoma in decreasing in the United States.
In contrast to papillary carcinoma, follicular thyroid carcinomas are less likely to metastasize via lymphatic pathways (found in <10% of patients).88 More commonly, follicular carcinomas spread through local extension and hematogenous spread. Often, the presence of cervical lymph node disease indicates significant local disease and visceral invasion.99,126 Distant metastasis is also more common in follicular cancers than papillary cancers, especially at presentation.127,128 A pathologic bone fracture may be the initial presentation of follicular carcinoma. Other common sites include liver, lung, and brain.
Pathology
Follicular thyroid carcinoma tends to manifest as solitary, encapsulated lesions. Cytologic analysis of follicular neoplasms reveals small follicular arrays or solid sheets of cells.14 The follicular structures have lumens that do not contain colloid, and the overall architectural pattern depends on the degree of tumor differentiation. Increased cellularity may increase the suspicion for carcinoma, but cytology alone is insufficient to distinguish between a follicular adenoma and carcinoma.
Histologic findings are necessary to distinguish benign and malignant lesions. Malignant lesions are differentiated by the identification of capsular invasion and potential microvascular invasion of vessels along the tumor capsule.91,129 Complete capsular evaluation must be performed. Frozen-section analysis is often inadequate, and definitive diagnosis requires complete assessment of permanent sections.
The degree of capsular invasion is important for patient prognosis. Follicular carcinomas can be divided into two broad categories. Minimally invasive tumors show evidence of invasion into, but not through, the tumor capsule at one or more sites. These lesions do not exhibit small-vessel invasion. Frankly invasive tumors show invasion through the tumor and often exhibit vascular invasion.130 Tumor infiltration and invasion may be apparent at surgery, with tumor present in the middle thyroid or jugular veins.
Many other factors have been investigated as means to differentiate between adenomas and carcinomas. To date, no molecular markers have been clinically useful. DNA ploidy varies in adenomas and carcinomas with considerable overlap.131 Aneuploid follicular carcinomas are noted to behave in a more aggressive manner.
Management and Prognosis
Patients diagnosed with a follicular lesion by FNAC should have a thyroid lobectomy with isthmectomy performed. The pyramidal lobe, if present, should be included in the resection. As described previously, cytologic findings alone cannot determine the presence of adenoma or carcinoma. Intraoperative frozen-section analysis is not helpful because of the incomplete assessment of the tumor capsule. Frozen sections should be analyzed, however, to confirm gross evidence of adjacent cervical lymphadenopathy. A total thyroidectomy is recommended if carcinoma is identified. In patients with a clinical suspicion for follicular carcinoma, the tendency is to perform a more complete resection. Total thyroidectomy is performed in older patients with a nodule greater than 4 cm in size diagnosed by FNAC as follicular neoplasm. In these patients, the risk of carcinoma is approximately 50%.112 Additional factors that may favor a total thyroidectomy as the initial surgical procedure include a family history of thyroid carcinoma, history of radiation exposure, and patient preference.69
A diagnosis of follicular carcinoma after a thyroid lobectomy usually necessitates a completion thyroidectomy. Patients with minimally invasive follicular cancer have a very good prognosis, and the initial thyroid lobectomy may be sufficient treatment. Invasiveness of follicular carcinoma correlates directly with decreased survival, however. In patients with invasive follicular carcinomas, many surgeons tend toward completion and total thyroidectomy to permit radioiodine scanning for detection and ablation of metastatic disease. More aggressive surgical intervention does not improve survival because invasiveness already indicates the increased likelihood of distant metastasis. Neck dissection is performed if cervical lymphadenopathy is present. When cervical disease is present, consultation with the pathologist is warranted because the patient may have a follicular variant of papillary thyroid carcinoma. Elective neck dissections are unwarranted because nodal involvement is unlikely.10
The recurrence rate after initial management is approximately 30%.132 Recurrence is related to the degree of invasiveness of the initial lesion, not the extent of initial thyroid surgery. Minimally invasive disease behaves similarly to follicular adenoma and is typically cured with conservative surgical procedures (thyroid lobectomy).133 Recurrence of minimally invasive follicular carcinoma is approximately 1%. About 15% of patients with recurrent or metastatic disease can be cured. The prognosis of these patients relates to the site of recurrence and the patient’s initial risk stratification. Survival outcomes are significantly poorer in patients with capsular invasion and angioinvasion.134 Patients with cervical node recurrence have a 50% cure rate, whereas patients with distant metastases have a cure rate of about 9%.115,132
Overall, 5-year survival is 70% and decreases to 40% at 10 years for patients with follicular carcinoma. The presence of distant metastasis diminishes 5-year survival to 20%.135 Factors that worsen prognosis include age older than 50 years at presentation, tumors greater than 4 cm in size, higher tumor grade, marked vascular invasion, extrathyroidal invasion, and distant metastasis at the time of diagnosis.10 Extrathyroidal invasion beyond the capsule and into the thyroid parenchyma and local structures is the key factor decreasing patient survival. Risk stratification shows marked differences in patient survival. In one study, the low-risk group had a 5-year survival rate of 99% and a 20-year survival rate of 86%. In the high-risk group, the 5-year survival rate was only 47%, and this decreased to a 20-year survival rate of 8%.125
The prognosis for patients with follicular carcinoma has typically been reported to be worse than for patients with papillary carcinoma. Some reports that matched age, sex, and stage at time of diagnosis suggest that patients with papillary and follicular carcinomas have similar survival patterns.6,8 The poor prognosis of patients with follicular carcinoma may be related to the increased number of patients who present at an older age and a more advanced disease stage. Also, in contrast to papillary carcinoma, mortality is directly related to recurrence in patients with follicular carcinoma.99
Hürthle Cell Tumor
Clinical Presentation
Hürthle cell neoplasms are typically diagnosed by FNAC. Approximately 20% of these lesions are malignant. Similar to follicular lesions, histologic criteria are required to diagnose carcinomas. Hürthle cell carcinomas represent approximately 3% of all thyroid malignancies. The mean age of presentation for patients with Hürthle cell carcinoma may be older than for follicular carcinoma.80,136 Hürthle cell carcinomas are more aggressive than follicular carcinomas. They are often multifocal and bilateral at presentation. These malignancies also are more likely to metastasize to cervical nodes and distant sites.137
Management and Prognosis
Postoperative management should include TSH suppression and thyroglobulin monitoring, and periodic ultrasound evaluation of the central and lateral cervical compartments. A 99mTc scan may be useful for detecting persistent local or metastatic disease. A 123I scan and ablation can be performed to remove any residual normal thyroid tissue to allow for better surveillance. This therapy is unlikely to be effective in tumor ablation, however, because few (approximately 10%) Hürthle cell carcinomas uptake radioiodine.138
Overall, survival rates for Hürthle cell carcinoma are significantly worse than for follicular thyroid cancer. The number of patients who die of Hürthle cell carcinoma is greater than the number who die of papillary or follicular carcinoma.139 Additionally, Hürthle cell carcinoma is associated with the highest incidence of distant metastases among the well-differentiated thyroid carcinomas.140
Medullary Thyroid Carcinoma
Clinical Presentation
MTC shows an intermediate behavior between well-differentiated thyroid cancers and anaplastic carcinomas. Women and men are equally affected by MTCs.141 Patients usually present with a neck mass associated with palpable cervical lymphadenopathy (≤20%).142 Local pain is more common in these patients, indicating the presence of local invasion, and may be associated with dysphagia, dyspnea, or dysphonia. MTC may manifest along with papillary thyroid carcinoma because related mutations in RET are present in both diseases. Although MTC spreads initially to cervical nodes, distant metastases may be found in the mediastinum, liver, lung, and bone, and are present in 50% of patients at diagnosis.143
Most (70%) MTCs are spontaneous unifocal lesions in patients 50 to 60 years old without an associated endocrinopathy.142 The remaining 30% of cases affecting younger patients are familial. These hereditary MTCs are inherited as autosomal dominant traits with nearly 100% penetrance. MTC in these patients is preceded by multifocal C-cell hyperplasia and leads to disease that is multicentric and bilateral in 90% of cases.144,145 Familial MTC is not associated with any other endocrine pathology. Two forms of MEN syndrome are associated with MTC. Patients with MEN IIA exhibit MTC, pheochromocytoma, and hyperparathyroidism.144,146 Patients with MEN IIB have a marfanoid body habitus and may have MTC, pheochromocytoma, or mucosal neuromas. Although penetrance for MTC approaches 100% in these patients, expression of other features varies.145,147
FNAC diagnosis of MTC is confirmed by elevated serum calcitonin. These patients also should have testing for mutation of the RET proto-oncogene. Genetic screening has replaced provocative pentagastrin-stimulation testing. Careful screening for hereditary diseases is also necessary when a patient is diagnosed with MTC. Hyperparathyroidism can be assessed by serum calcium levels and appropriate imaging studies. Patients should also be screened for the presence of a pheochromocytoma with 24-hour urinary levels for catecholamines and metanephrines, and should undergo abdominal MRI. An undiagnosed pheochromocytoma could lead to an intraoperative hypertensive crisis and death. Additionally, the detection of any hereditary form of MTC in a patient should lead to family screening. Affected family members can often be identified and treated at earlier stages of disease with improved survival.148,149
Pathology
MTC originates from parafollicular C cells of neuroectodermal origin.150 The cells descend to join the thyroid gland proper and are concentrated mainly in the lateral portions of the superior poles. Most MTC lesions are located in the middle and upper thyroid poles. In patients with hereditary forms of MTC, the disease is often multifocal. Grossly, the tumor is solid and firm, and has a gray cut surface. The lesion is nonencapsulated, but well circumscribed.
These lesions are composed of sheets of infiltrating neoplastic cells that are heterogeneous in shape and size. The cells are separated by collagen, amyloid, and dense irregular calcification. The amyloid deposits are likely polymerized calcitonin and are virtually pathognomonic for MTC, although not all MTCs contain amyloid.151 More aggressive tumors typically have increased mitotic figures, nuclear pleomorphism, and areas of necrosis. Immunohistochemistry for calcitonin and CEA are useful diagnostic studies.
Management and Prognosis
Specific guidelines were recently published by the American Thysoid Association regarding the management of MTC.151a
Because of the frequent involvement of cervical nodes, initial surgical management should include bilateral central compartment neck dissection. When central compartment nodes are involved, or when palpable lateral cervical nodes are present, treatment including an ipsilateral or bilateral comprehensive neck dissection (levels II-V) should be considered. When the primary lesion is greater than 1 cm (>0.5 cm for MEN IIB patients), or when central compartment lymph node metastases are present, elective ipsilateral comprehensive neck dissection should be considered because nodal metastases may be present in more than 60% of these patients.111,152,153 The superior mediastinal lymph nodes (level VII) should be routinely removed as well.
Children with any of the genetic disorders leading to MTC need to be treated aggressively. Typically, a total thyroidectomy should be performed by the time the patient is 2 to 3 years old, or before C-cell hyperplasia occurs. Removal of the thyroid gland should prevent development of MTC in these patients and improve survival. MTC has been diagnosed in MEN IIB patients 7 months old, however.154
After surgery, patients require close follow-up and monitoring of serum calcitonin and CEA levels. Calcitonin is more sensitive for detecting persistent or recurrent disease, but CEA levels seem to be predictive for survival.153 Increasing or persistent calcitonin levels should increase suspicion for residual or recurrent disease. Localization studies should be performed to identify potential sites of disease involvement. Tumor debulking for metastatic disease or local recurrence can decrease symptoms of flushing and diarrhea, and may reduce the risk of death resulting from recurrent central neck disease.143,155 MTCs do not respond to radioiodine therapy or TSH suppression therapy, however, because of their parafollicular C-cell origin.149 External-beam radiation therapy (EBRT) has been controversial for patients with positive tumor margins or unresectable tumor, and there is no effective chemotherapy regimen.
Prognosis for patients with MTC is directly related to disease stage. The overall 10-year survival rate is 61% to 75%, but decreases to 45% if cervical nodes are involved.149,156 The best outcome is for patients with familial MTC, then MEN IIA, sporadic disease, and MEN IIB.
Anaplastic Carcinoma
Clinical Presentation
Anaplastic thyroid carcinomas are one of the most aggressive malignancies, with few patients surviving 6 months beyond initial presentation.62,157 These lesions represent less than 5% of all thyroid carcinomas.158 These tumors affect patients 60 to 70 years old; presentation before age 50 years is extremely rare. Women are more commonly affected than men, with a ratio of 3 : 2. Of these malignancies, 80% may occur with a coexisting carcinoma and may represent transformation of a well-differentiated thyroid cancer.157,159
Typically, patients have a long-standing neck mass that enlarges rapidly. This sudden change is often accompanied by pain, dysphonia, dysphagia, and dyspnea. Often the mass is quite large and fixed to the tracheolaryngeal framework, resulting in vocal cord paralysis and tracheal compression. More than 80% have jugular lymph node involvement at the time of presentation, and greater than 50% have systemic metastases.160 Most patients die of superior vena cava syndrome, asphyxiation, or exsanguination.
Pathology
In the case of anaplastic thyroid carcinoma, the gross specimen shows areas of necrosis and macroscopic invasion of surrounding tissues, often with lymph node involvement. Microscopically, sheets of cells with marked heterogeneity are present. Spindle, polygonal, and giant, multinucleated cells are present with occasional foci of differentiated cells. These cells do not produce thyroglobulin, do not transport iodine, and do not express thyroid hormone receptors.157 These findings can often be established on FNAC, although a formal biopsy is occasionally necessary to exclude a diagnosis of lymphoma.
Management and Prognosis
Management of anaplastic carcinoma is extremely difficult, requiring a multidisciplinary approach and close consultation with the patient and family. Surgical debulking may be performed for palliation. Initial therapy should ensure airway protection with a tracheostomy and nutritional support. All treatment forms are disappointing, and median survival is 2 to 6 months.158,161 One current treatment protocol involves doxorubicin, hyperfractionated radiation therapy, and potential surgical debulking.162,163 Although survival beyond 2 years is only 12%, this is one of the only regimens currently available for these patients.
Retrospective evaluation of various treatment strategies identified a subset of patients who have had long-term survival.5,86,164 Independent prognostic variables include resectability of local disease, absence of distant metastasis at diagnosis, and adjuvant treatment with radiation therapy. Additionally, many long-term survivors had small areas of anaplastic foci within well-differentiated carcinoma.
Other Forms of Thyroid Cancer
Insular Thyroid Carcinoma
Insular carcinoma was named for the clusters of cells that contain small follicles resembling pancreatic islet cells.165 These tumors are very rare and manifest as an independent lesion or concomitantly with papillary or follicular thyroid carcinomas. These cells stain with thyroglobulin antibodies, but not for calcitonin. Typically, capsular and vascular invasion is present at the time of diagnosis.
These lesions are very aggressive when compared with follicular and papillary carcinoma, and seem to have an increased recurrence and mortality rate when present as an independent process.166 Insular carcinoma located within follicular or papillary thyroid cancer does not seem to affect the clinical course adversely, however. Many insular thyroid carcinomas are able to concentrate radioiodine.
Lymphoma
Primary thyroid lymphoma is unusual and represents less than 5% of all thyroid malignancies.167 Women are more commonly affected at a ratio of 3 : 1, and lymphoma typically manifests in patients older than 50 years. Patients may present with symptoms similar to anaplastic carcinoma, although the rapidly enlarging mass is often painless. Symptoms may also include regional adenopathy, dysphagia, and vocal cord paralysis caused by RLN invasion. Many affected patients are clinically hypothyroid or already receiving thyroid replacement therapy for conditions such as Hashimoto’s disease.168 Non-Hodgkin B-cell type lymphoma is most common, although Hodgkin’s disease and plasmacytomas occur.169 Thyroid lymphoma can arise as part of a generalized lymphomatous condition because many of these patients have Hashimoto’s disease. A current hypothesis as to why this occurs is chronic antigenic lymphocyte stimulation that results in lymphocyte transformation.
Patients typically respond rapidly to chemotherapy, especially CHOP (cyclophosphamide, hydroxydaunomycin, vincristine [Oncovin], and prednisone).170,171 Combined radiation and chemotherapy regimens have also been developed and have been promising. Thyroidectomy and nodal resection may be considered to alleviate symptoms of airway obstruction in patients who do not respond rapidly to treatment, but surgical options are not primary treatment modalities.
Metastatic Carcinoma
The thyroid is a rare site for metastases from other cancers. Metastasis can occur, however, from primary lesions in the kidney, breast, lung, and skin (i.e., melanoma). The most common metastatic tumor to the thyroid is from a hypernephroma. Also, approximately 3% of bronchogenic carcinomas metastasize to the thyroid, but account for 20% of all metastases to the thyroid.172
Typically, the history and physical examination identify the source of the metastasis. FNAC is performed for definitive diagnosis. Thyroidectomy may be considered for palliation, especially when the primary lesion is very slow growing (e.g., renal cell carcinoma).173
Squamous Cell Carcinoma
Squamous cell carcinoma of the thyroid is very rare, representing less than 1% of thyroid cancers.174 Older patients are most commonly affected, and the disease can progress rapidly with local invasion and metastasis. During the workup, metastasis from another site within the upper aerodigestive tract needs to be excluded. Early detection and aggressive surgical treatment seems to represent the best option for palliation and cure. As with other squamous cell carcinomas of the head and neck, radiation therapy is probably important, although not well characterized.175
Surgical Management and Technique
Approach to the Thyroid Gland
Through blunt dissection, the thyroid lobe is swept anteromedially to the tracheolaryngeal framework (Fig. 124-4A). The middle thyroid vein should be identified; division of this vessel improves lateral exposure. The cricoid and trachea should be identified in the midline, and continued mobilization is achieved by sweeping dorsally all tissue along the posterolateral border of the thyroid lobe. Meticulous hemostasis should be maintained to facilitate identification of the SLN, RLN, and parathyroid glands.
Identification of the RLN is best achieved through an inferior approach in a space defined by Lore and colleagues176 as the RLN triangle. The triangle is bounded by the trachea medially, the carotid sheath laterally, and the undersurface of the retracted inferior thyroid pole superiorly. Careful dissection in this area parallel to the course of the RLN should safely identify the nerve (see Fig. 124-4B). A thyroid goiter or unusually large thyroid mass can potentially displace the nerve. The RLN, in these cases, can become fixed to and splay across the undersurface of the enlarged thyroid lobe. Great care needs to be taken in these situations, and identification of the nerve may require a superior approach, identifying the RLN at its entry into the larynx.
When identified, the RLN should be followed to its laryngeal entry at the level of the cricoid cartilage, passing under or through Berry’s ligament and entering the larynx deep to the inferior constrictor muscle. The nerve may divide into multiple branches before entering the larynx.177 The most difficult portion of the operation is typically in dealing with the dissection where the recurrent nerve passes through Berry’s ligament. The RLN is in close proximity to the thyroid, tethered down by the ligament. Bleeding can occur at this site and should be controlled by gentle pressure before identification of the nerve to avoid injury. Use of electrocautery in this region should be strictly avoided. A small portion of thyroid tissue may be embedded with the ligament, accounting for a remnant of thyroid tissue left after total thyroidectomy. Although controversy exists regarding routine use of intraoperative RLN monitoring, it may be useful if nerve identification or handling or both are anticipated to be more difficult.178–180 Examples of such situations include large tumors or goiters, paratracheal node disease or dissection, and reoperative cases in the thyroid bed or central compartment.
All vessels are ligated and divided on the capsule to reduce the risk of parathyroid devascularization (Fig. 124-5). Ischemic parathyroids and parathyroids situated anteriorly on the thyroid gland or removed with the thyroid lobe should be examined. Biopsy specimens should be obtained and confirmed by frozen-section examination. The parathyroid glands can be minced into pieces of 1 mm3 and reimplanted in the ipsilateral sternohyoid muscle or sternocleidomastoid muscle with a silk suture or clip to mark the reimplantation site.
Special Surgical Situations
Management of Regional Lymphatics
Management of the neck in patients with well-differentiated thyroid carcinomas is typically conservative. Cervical node disease is rare in follicular carcinomas, but more common in papillary carcinomas. There is currently no role for elective neck dissection in cases of follicular carcinoma. Current ATA guidelines recommend consideration of central compartment (level VI) neck dissection for patients with papillary thyroid carcinoma and suspected Hürthle cell carcinoma.69 If disease is present by palpation or visible inspection, patients with papillary or Hürthle cell thyroid carcinoma have a concurrent central compartment lymph node dissection with total thyroidectomy or the completion thyroid lobectomy.
When palpable lateral cervical nodes are present, and metastatic disease is confirmed by FNAC, a selective neck dissection, including levels II through V, should be performed. The submandibular and submental nodes (level I) are rarely involved and should be dissected only when there is clinically positive disease in this region.105,181 Cervical involvement is frequently ipsilateral to the primary thyroid lesion.182 Extension of the collar incision superior to the mastoid tip provides adequate exposure of the lateral neck if palpable disease is present. Careful inspection and dissection should be performed along the spinal accessory nerve because this is a frequent site of metastatic thyroid disease.10 A more extensive modified radical neck dissection may be necessary to remove gross disease. Radioiodine ablation is an important adjunctive therapy for the management of cervical disease in these patients.
In patients with MTC, lymph node metastasis can occur in 81% of cases.183 Retrospective analysis revealed a 10-year survival rate of 67% for patients with MTC treated with neck dissection versus 43% for patients who were not treated with neck dissection.152 Palpable cervical disease necessitates central compartment and comprehensive neck dissection. In patients with clinically negative neck disease, surgical treatment should include total thyroidectomy, central compartment dissection, and elective, ipsilateral comprehensive neck dissection. Dissection of the contralateral neck in all patients should be considered, especially when the primary thyroid lesion is 2 cm or greater in size because bilateral neck dissection may be necessary to maximize regional control.183
Intrathoracic Goiter
Less than 1% of patients may have a thyroid gland that is partially or completely intrathoracic.184,185 In most of these patients, the intrathoracic goiter can be removed via a collar incision in the neck without resorting to a sternotomy. The vascular supply, typically originating in the neck, is identified, ligated, and divided. Division of the isthmus may facilitate mobilization of the substernal goiter from beneath the sternum. Large sutures can be placed deeply into the goiter to facilitate traction and blunt dissection to permit delivery of the thyroid through the neck.
Recurrent Laryngeal Nerve Invasion
Surgical situations necessitating the sacrifice of the RLN are uncommon. If preoperative vocal cord paralysis is present, and carcinoma invasion is seen intraoperatively, the nerve may be sacrificed. More commonly, the RLN should be dissected free of gross disease. Among patients with well-differentiated thyroid carcinomas, there is no survival difference between patients with RLN sacrifice and patients treated postoperatively with radioiodine for gross disease left on the nerve.171 Because of the lack of response by MTC to postoperative radioiodine treatment, RLN sacrifice must be considered to achieve complete removal of gross disease.
Extended Surgical Resection
Surgical treatment of thyroid carcinomas should remove all gross disease, especially in patients with MTC. Fixation to the thyroid cartilage or trachea may require partial-thickness to full-thickness removal of those structures (Fig. 124-6). A thyroid cartilage lamina can be removed without major morbidity if the internal thyroid perichondrium is left intact. The trachea can be partially resected and repaired to permit en bloc tumor removal. Primary anastomosis can be performed for resections involving up to four tracheal rings.186 Additionally, tracheal shaving can be performed, leaving the internal mucosa intact. Isolated full-thickness defects can be repaired with composite mucosal-cartilage grafts from the nasal septum. In patients with more extensive skeletal involvement, a partial laryngectomy may be required and has shown improvements in survival.187,188 Total laryngectomy should be performed in only the most extreme cases of extensive intraluminal invasion, substantial cricoid involvement, or bilateral RLN dysfunction. Typically, this procedure would be done after the failure of radioiodine treatment, EBRT, or both. Pharyngeal and esophageal local invasion typically requires resection of the immediate area and primary closure (Fig. 124-7).
Reoperative Thyroid Surgery
Reoperative surgery has been associated with a higher incidence of complications than initial thyroid surgery. Scarring and fibrosis from prior surgery may cause difficulty in identifying tissue planes, resulting in an increased risk of injury to the RLN. Potential injury or devascularization of the parathyroid glands on one side of the neck creates increased concern for preservation of the glands on the contralateral side. Older series reported a high incidence of complications in reoperative surgery.106,189 More recent reports indicate, however, that the risk of permanent hypoparathyroidism or RLN injury is less than 2%.190–193
The effectiveness of FNAC has also directed surgeons to perform the appropriate surgery for a particular disease. The presence of a follicular or Hürthle cell adenoma should direct a surgeon to perform a unilateral thyroid lobectomy, avoiding exploration of the contralateral lobe. In cases of carcinoma, reoperation is safer because the contralateral lobe has been left undisturbed. When a patient has significant comorbidities that may preclude reoperation, the contralateral side can be addressed at the initial surgery by performing a total or near-total thyroidectomy. FNAC that shows papillary carcinoma prepares a surgeon for total thyroidectomy or more comprehensive surgery for MTC. Although intraoperative frozen-section analysis is controversial, frozen-section analysis in conjunction with cytologic examination can be valuable for cases diagnosed on preoperative FNAC as suspicious for papillary thyroid carcinoma.194,195 Finally, ultrasound evaluation of the thyroid bed and lateral neck provides improved preoperative assessment of the extent of disease and facilitates surgical planning. Even in patients with palpable lymph node recurrences, ultrasound evaluation of the lateral and central compartments may change the extent of the surgical procedure in 40% of patients undergoing reoperative surgery.87
Before reoperation, the vocal cords should be visualized to identify any potential RLN injury. Imaging studies can be useful in cases of disease recurrence to identify local invasion or cervical disease and potential involvement of the RLN or parathyroid glands. During surgery, the RLN should be followed from a previously undissected area into the surgical bed. The surgeon should be familiar with numerous techniques to identify the RLN. A lateral approach from the medial border of the sternocleidomastoid, a low anterior neck approach, and a medial approach to the superior pole of the thyroid have been described.192 Intraoperative monitoring of the RLN can be helpful, and an electrophysiologic nerve stimulator can facilitate and confirm nerve identification. Visualization of the RLN is crucial, and the use of loop magnification may be helpful to identify the RLN and parathyroid glands.
The surgical specimen needs to be inspected carefully, and biopsy specimens of potential parathyroid tissue should be obtained. Identified parathyroid tissue should be reimplanted into the sternocleidomastoid muscle. All parathyroid tissue should be preserved when possible. The combination of parathyroid preservation in situ and autotransplantation of devascularized parathyroid tissue significantly reduces the risk of permanent hypoparathyroidism.196 Parathyroid tissue that has been reimplanted functions even when other parathyroid glands have been left intact and in the absence of hypocalcemia.197
Extent of Surgery
The large body of literature regarding thyroid carcinoma and its treatment has created a wide variety of terms for describing the extent of thyroid tissue removal. Most surgeons agree that a subtotal excision of a thyroid nodule is unacceptable, and that the minimum amount of thyroid removed should be a lobectomy and isthmectomy. Some surgeons have advocated in the past a near-total thyroidectomy that preserves the posterior portion of the gland on one side to avoid injury to the RLN and at least one parathyroid gland. A total thyroidectomy involves the complete removal of both thyroid lobes, although 123I scans may reveal 2% to 5% residual tissue.198
Proponents of a more conservative surgical approach suggest that thyroid lobectomy and isthmectomy is sufficient treatment for most patients (>80%). These patients would be categorized as low-risk in the different classification schemes (see section on tumor staging and classification). Thyroid lobectomy and isthmectomy are simpler to perform and less time-consuming than a total thyroidectomy. The overall risk of morbidity from RLN, SLN, and parathyroid gland injury is less. Finally, compared with total thyroidectomy, this conservative approach does not adversely affect prognosis and survival.
Numerous studies support the conservative approach. In the AGES classification, low-risk patients with papillary carcinoma had the same 2% 25-year mortality rate regardless of whether they were treated with thyroid lobectomy or total thyroidectomy.55 A study by Shah and associates58 based risk classifications on the AJCC staging system. There was no difference in 20-year survival based on surgical treatment for patients with intrathyroidal tumors of less than 4 cm. Patients treated with total thyroidectomy had an increased risk of complications, however.
Although survival differences are not found, several studies report an increased incidence of local recurrence in patients treated with only a thyroid lobectomy.199,200 Some controversy exists regarding the relationship of local recurrence to disease survival, especially with papillary carcinomas.121,193 Finally, patients categorized as high-risk in these classification systems are treated with near-total or total thyroidectomy and radioiodine ablation therapy.
A more aggressive surgical approach favors total thyroidectomy in most cases and lobectomy for small, single lesions. Proponents of this approach favor total thyroidectomy as a better oncologic operation. Removal of the entire thyroid gland encompasses potential extracapsular extension and multicentric lesions. Morbidity of this procedure is low with good technique and an experienced surgeon.191 Several studies showed improved survival and decreased local or distant recurrence.101,114,201 Cautionary studies also show the risk of aggressive disease even in patients determined to be low-risk in the various classification schemes.202,203
Minimally Invasive Thyroidectomy
Over the past decade, surgical approaches have been developed to permit thyroid surgery through smaller incisions.204,205 Some of these approaches advance to the thyroid bed from remote incisions in the axilla or chest.206,207 In addition to improved cosmesis and decreased postoperative discomfort, these approaches have afforded surgeons superior visualization of critical structures such as the SLN through the use of endoscopic instruments (Fig. 124-8). In the transcervical approach, a surgical drain is often not required, and many patients are discharged after adequate same-day postoperative observation and instruction.208 Implementation of these approaches has been careful with an emphasis on providing equal or improved operative outcomes relative to traditional open approaches.209,210
Special Treatment Situations
Children
In children, nodular thyroid disease is uncommon, with an incidence of 0.2% to 1.8%.211 Most neck masses occurring in children are due to congenital or inflammatory causes. Malignant neck masses may be due to lymphoproliferative disorders, sarcomas, or thyroid carcinomas. Less than 6% of pediatric neck tumors are thyroid malignancies.212 Approximately 10% of all thyroid carcinomas occur in patients younger than 21 years.213
Most children with thyroid neoplasms present with an asymptomatic mass within either the thyroid or the lateral neck. The lesions are often asymptomatic and frequently noted by a parent or pediatrician during a routine physical examination. A complete history should include special attention to family history and radiation exposure. The physical examination should carefully evaluate potential fixation of the thyroid gland to surrounding neck structures. Vocal cord mobility should be documented by indirect fiberoptic nasolaryngoscopy. The central compartment and lateral necks also should be carefully palpated. Lymph node metastases are present in more than 50% of children with differentiated thyroid cancer.214 Local extension was reported in 18%. In addition, 15% of patients have distant metastasis, frequently identified with postoperative radioiodine.215,216 A chest radiograph may be normal or may show a fine interstitial reticular pattern, potentially mistaken for miliary tuberculosis. Children with extensive pulmonary involvement may present with dyspnea on exertion.
Although the most common cause of a solitary thyroid nodule in children is a follicular adenoma (68.9%), malignancies are found in 16% to 25% of the nodules.217,218 The most common malignant histologic type is papillary carcinoma, present in more than 80% of these cases. In contrast to thyroid carcinoma in adults, papillary carcinomas are usually follicular in pattern, and tall-cell and columnar-cell variants do not occur in children.219,220 Hürthle cell carcinomas may manifest with multiple lesions and a papillary architecture as a familial syndrome.221 MTCs constitute about 10% of pediatric thyroid carcinomas, and all family members need to be carefully evaluated for possible familial MTC or MEN syndromes.
Management of a child with thyroid carcinoma must balance the potential morbidity associated with treatment against the low probability that the patient will die of this disease. The primary goal of surgery is to remove gross disease completely at the primary and regional metastatic sites. A total or near-total thyroidectomy is recommended.213,214,222,223 Multifocal disease is present in 10% to 30% of patients. In addition, a total or near-total thyroidectomy allows the use of serum thyroglobulin to monitor disease recurrence, and facilitates the use of radioiodine to identify and treat distant metastases. More recent studies have shown a low incidence of permanent hypoparathyroidism and RLN injury after total thyroidectomy. The recurrence rate is lower for patients undergoing total thyroidectomy than for patients undergoing lesser procedures.224
Children often present with cervical nodal metastases requiring therapeutic lymphadenectomy. In patients with nodal disease, the paratracheal and pretracheal lymph nodes are involved in 90% of cases.32 The metastatic deposits are frequently compressive rather than infiltrative with respect to adjacent neck structures. Radical neck dissection is rarely necessary. Patients should have central compartment dissections when cervical disease is present, rather than simple node plucking. Care should be taken to identify and preserve the blood supply to the parathyroid glands. Also, lateral neck disease requires a comprehensive lateral neck dissection (levels II-V).
Although children and adolescents commonly present with more advanced disease than adults, their prognosis remains excellent. In several series, young patients have a 10-year survival near 100% and a 2- to 5-year survival rate near 90%.225 Relapse is most common in the cervical lymphatics, and is more common in children than in adults. Recurrence seems to be more common in younger patients and patients with papillary histology.226 Cervical disease can be surgically excised, and pulmonary recurrence is responsive to radioiodine therapy.227
Pregnancy
Because thyroid neoplasms occur more commonly in women and often during their childbearing years, physicians occasionally need to manage this disease in pregnant women. The incidence of thyroid nodules during pregnancy has been reported as 2% to 10%.228,229 Management of these patients must protect the well-being of the mother and fetus, and minimize the risks of preterm labor or abortion. A crucial difference in thyroid nodule management during pregnancy and breastfeeding is the absolute contraindication for the use of radioactive iodine.230
During pregnancy, the thyroid gland physiology is markedly altered. The gland can increase significantly in size because of a relative iodine insufficiency and hormonal changes.231,232 These physiologic and hormonal changes may increase the incidence of thyroid nodules or cause the enlargement of existing thyroid nodules.
The presence of any discrete thyroid nodule should be evaluated by FNAC.233 Some authors argue that a pregnant patient beyond 20 weeks of gestation should have FNAC deferred until after the pregnancy to reduce patient anxiety.229,234 These authors argue that treatment would likely occur after surgery, and they advocate FNAC only when the nodule shows continued growth or other suspicious features. Other authors advocate immediate FNAC at any stage of pregnancy.230
When FNAC is benign, the patient should be reassured, and ultrasonography can monitor the nodule for possible growth during pregnancy. A follicular neoplasm may be monitored closely or surgically explored. If FNAC is suspicious or shows thyroid carcinoma, surgery is recommended at the safest time possible.228 Thyroid carcinomas identified late in pregnancy should be treated in the immediate postpartum period. Carcinomas diagnosed during the first and early second trimesters may be surgically treated during the 22nd through 26th gestational weeks, when the risk to fetal development and for preterm labor is lowest. The patient can be reassured that thyroidectomy and even cervical lymphadenectomy at midtrimester do not carry significant risks for the fetus. Maternal complications are similar to nonpregnant patients. A more recent study suggests, however, no difference in the natural history and prognosis of pregnant patients with a thyroid carcinoma diagnosed early in gestation who defer surgery until after labor.235 Most patients would likely experience too much stress and anxiety to have an untreated cancer present throughout gestation.
Complications
Among head and neck surgical procedures, thyroid surgery is very safe. Mortality rates are extremely low, and morbidity is relatively low. Generally, serious complications occur in less than 2% of all thyroid cases.10 Complications can typically be divided into nonmetabolic and metabolic complications. Of particular concern are injuries to the RLN and the parathyroid glands.
Superior Laryngeal Nerve Injury
Injury to the external branch of the SLN is believed to be rare, but the exact frequency is unknown. Often disturbance of SLN function is temporary and unrecognized by the patient and the surgeon.236 Injury to the SLN alters function of the cricothyroid muscle. Patients may have difficulty shouting, and singers find difficulty with pitch variation, especially in the higher frequencies. The external branch of the SLN is not often visualized and lies near the superior pole vessels. Adequate exposure of the superior thyroid pole and close ligation of the individual vessels on the thyroid capsule may prevent SLN injury. Voice therapy may help patients to compensate in cases of SLN injury.
Recurrent Laryngeal Nerve Injury
Injury to the RLN has a much greater impact and is more noticeable than SLN injury. The incidence of permanent RLN paralysis is approximately 1% to 1.5% for total thyroidectomy and less for near-total procedures.101,237,238 Temporary dysfunction because of nerve traction occurs in 2.5% to 5% of patients.176 Incidence increases with second and third procedures. RLN injury is also more common in thyroidectomy with neck dissection, although this may reflect more advanced disease states.239 Disease-specific risk factors for permanent nerve damage include recurrent thyroid carcinoma, substernal goiter, and various thyroiditis conditions. Vocal cord function should be evaluated and documented by indirect laryngoscopy, especially in patients who have had previous surgery.
Unilateral RLN injury leads to a vocal cord in the paramedian position, and the voice may be breathy and lack volume. Concurrent injury of the SLN results in a more laterally positioned vocal cord and worsens voice quality and glottic competence.240 Occasionally, patients may have difficulty with aspiration and pneumonia.239
Identification and careful dissection along the course of the RLN decreases the incidence of permanent injury. The surgeon should also be aware of the possibility of a nonrecurrent nerve, most commonly on the right side. If the nerve is transected during surgery, microsurgical repair of the nerve is recommended. Although the repair is unlikely to restore normal function, reanastomosis of the RLN may decrease the extent of vocal cord atrophy.241 Some surgeons advocate anastomosis of the ansa hypoglossal nerve to the distal end of the severed RLN to prevent laryngeal synkinesis and possible vocal cord hyperadduction.242,243
Hypocalcemia
Transient symptomatic hypocalcemia after total thyroidectomy occurs in approximately 7% to 25% of cases, but permanent hypocalcemia is less common (0.4% to 13.8%).244,245 The risk of hypoparathyroidism is related to the size and degree of invasion of the tumor, pathology, and extent of procedure and surgeon experience.55,246 Changes in serum calcium levels are often transient and may not always be related to parathyroid gland trauma or vascular compromise.
Parathyroid autotransplantation may be considered when the risk of postoperative hypocalcemia is increased.247,248 These situations may include thyroid carcinoma requiring total thyroidectomy with central neck dissection, en bloc resections that require removal of the parathyroid glands, and reoperation after previous thyroid or parathyroid surgery. Autotransplantation is commonly performed in the sternocleidomastoid muscle, although various locations, including the brachioradialis muscle of the nondominant forearm, have been used.249
Typically, serum calcium levels are measured in the immediate postoperative period and the next morning for patients with a total or completion thyroidectomy. Patients should have a stable or increasing serum calcium level. Patients undergoing a thyroid lobectomy do not usually require serum calcium monitoring. Findings that should be worrisome for hypoparathyroidism include hypocalcemia, hyperphosphatemia, and metabolic alkalosis. PTH levels may also be measured to predict potential hypocalcemia.250
Postoperative Management and Special Considerations
Thyroid Hormone Replacement
After total or completion thyroidectomy, exogenous supplementation of thyroid hormone is necessary to prevent symptomatic hypothyroidism.6,77 To counteract potential trophic effects of TSH that could facilitate recurrence or progression of well-differentiated thyroid carcinomas, long-term supplementation with levothyroxine is monitored to suppress TSH to below-normal levels (<0.1 mU/L in high-risk patients and 0.1 to 0.5 mU/L in low-risk patients).67 Patients receiving suppressive therapy have a lower recurrence rate and improved survival.251,252
Radioactive Iodine Treatment
Radiolabeled iodine has been used for more than 40 years to ablate normal thyroid tissue and to treat residual tumor and metastases.253 The 131I isotope emits β particles that penetrate and destroy tissue within a 2-mm zone. Patients classified as high risk with papillary thyroid carcinoma and most patients with follicular carcinoma are considered for treatment.127 When administered as part of the initial therapy, postoperative 131I treatment contributes to decreased recurrence and disease-specific mortality.254–256 Despite poor radioiodine uptake in Hürthle cell and medullary carcinomas, these patients are often treated to provide any possible benefit.
Elevated TSH levels are necessary to enhance uptake of iodine by thyroid cancer cells. A TSH greater than 30 mU/L is associated with increased radioiodine uptake in tumors.257 Patients are taken off thyroid hormone suppression therapy for 2 to 4 weeks before scanning and placed on a low-iodine diet.69 Additionally, the administration of exogenous recombinant human TSH has proved safe and effective for stimulating radioiodine uptake and for detecting serum thyroglobulin in patients undergoing evaluation for thyroid cancer persistence and recurrence.258,259
Whole-body diagnotic scans stage the patient and determine the need and potential benefit of radioiodine therapy. The phenomenon termed stunning may occur, however, when scanning doses of 131I induce follicular cell damage. Poor uptake by thyroid remnant and metastases of subsequent therapeutic 131I doses reduces the potential benefits of this therapy.260 Pretreatment scans are now performed with small doses of 131I (2 to 3 mCi) or 123I. The physical properties of 123I permit better image quality and decrease possible stunning of functioning thyroid cells by the β particle emission of 131I261; this permits the maximal benefit of 131I in ablation after the diagnostic procedure.262
After the diagnostic scan, therapeutic ablative doses of 131I can be given. Typically, 100 mCi of 131I is given for uncomplicated cases with only thyroid bed uptake. Although a lower dose of 30 mCi has commonly been used, studies have shown this is not as effective. Current guidelines recommend employing the minimum activity necessary to achieve successful thyroid remnant ablation, particularly for low-risk patients.69 Patients with uptake in cervical nodes, distant metastases, or more aggressive tumor histology (e.g., tall-cell, insular, columnar-cell carcinoma) receive 100 to 200 mCi.263,264 Doses greater than 200 mCi have not been shown to be more effective in most cases.
No clear evidence exists regarding the superiority of 131I delivery by empiric fixed amounts, quantitative tumor dosimetry, or blood/body dosimetry.265 In addition, there are not enough outcome data currently to recommend recombinant human TSH–mediated 131I therapy for patients with metastatic disease. Such use of recombinant human TSH may be indicated, however, in patients with underlying comorbidities who would be at risk because of iatrogenic hypothyroidism. Post-treatment scans are typically performed approximately 1 week after radioactive iodine therapy to visualize potential metastases.
Diverse alternatives and protocols exist regarding the use of radioiodine therapy. Surgeons who favor total thyroidectomy for most thyroid carcinomas argue that removal of normal tissue enhances radioiodine ablation therapy. Proponents of more conservative treatment suggest that radioiodine therapy can be used to remove even a remaining thyroid lobe before whole-body scanning for residual tumor or metastases. The 2006 ATA guidelines recommend against the use of radioactive iodine ablation instead of completion thyroidectomy.69 Continued work in this area is aimed at improving patient outcomes and decreasing disease recurrence.
External-Beam Radiotherapy and Chemotherapy
Because of the effectiveness of surgery and radioiodine treatment for most thyroid carcinomas, experience with EBRT and chemotherapy is more limited. EBRT seems to improve local control of well-differentiated carcinomas, especially when used in combination with doxorubicin.182,216,266 The effect of EBRT on survival is uncertain, however.266,267 Palliation of patients with distant metastasis of well-differentiated carcinomas, especially to the bone, seems to be improved with EBRT.182 Current recommendations support consideration of EBRT for patients older than 45 years with grossly visible extrathyroidal extension at the time of surgery and a high likelihood of microscopic residual disease.69 EBRT also may be employed for patients with gross residual tumor unlikely to respond to further surgery or radioactive iodine.
Limited success with EBRT in combination with doxorubicin/cisplatin has been noted with anaplastic carcinoma.86,268 This disease remains uniformly fatal, however, and palliation through local control and airway protection are the only realistic goals. Patients with metastatic medullary carcinoma may benefit from EBRT and chemotherapy to decrease local recurrence.269,270 Typically, patients with regional nodal metastases recognized during surgery receive postoperative EBRT. Generally, the poorer outcomes for patients with medullary and anaplastic carcinomas have not been significantly altered by EBRT and chemotherapy. Overall, there is no role for the routine adjunctive use of chemotherapy with differentiated thyroid cancer.69 Based on improved understanding of the biologic basis for thyroid cancer development and progression, numerous novel targeted-therapy agents, including kinase and angiogenesis inhibitors, are currently in clinical trials.271 A more recent phase II clinical trial showed some promising results with the BRAF inhibitor sorafenib in patients with iodine-refractory metastatic disease.272
Follow-up Management
Patients treated for thyroid carcinomas require long-term follow-up and monitoring. Approximately 30% of patients with differentiated thyroid carcinomas have tumor recurrences within several decades, and 66% of these recurrences occur within the first decade after initial treatment.6 Various staging systems for thyroid cancer, including the AJCC/IUCC system, were developed to predict risk for death, not recurrence.69,273,274 After initial surgery and remnant ablation, low-risk patients have no local or distant metastases, complete resection of all macroscopic tumor, no locoregional tumor invasion, no aggressive histology, no vascular invasion, and no 131I uptake outside the thyroid bed in the first post-treatment whole-body radioiodine scan. Intermediate-risk patients have microscopic invasion of tumor into the perithyroidal soft tissues at initial surgery, aggressive histology, or vascular invasion. High-risk patients have macroscopic tumor invasion, incomplete tumor resection, distant metastases, or radioiodine uptake beyond the thyroid bed on the post-treatment scan done after thyroid remnant ablation.
In addition to regular physical examination, thyroid hormone and TSH levels are monitored to ensure adequate suppression. Thyroglobulin levels should be closely monitored, and diagnostic radioiodine scanning should be performed. These tests should be performed annually for the first 2 years and then every 5 years for 20 years.275 Typically, thyroglobulin levels should be less than 2 ng/mL after total thyroidectomy and radioiodine ablation therapy (<3 ng/mL if the patient is off thyroid replacement therapy). Increasing serum thyroglobulin levels are highly sensitive (97%) and specific (100%) for thyroid cancer recurrence.276 Elevation of thyroglobulin levels warrants repeat radioiodine scanning and therapy. More recent studies have shown the sensitivity of serum thyroglobulin measurements for predicting thyroid cancer recurrence after a patient has received two injections of recombinant human TSH.277 Depending on risk classification, additional follow-up procedures vary. The 2006 ATA guidelines recommend measurement of serum thyroglobulin and a cervical ultrasound examination in low-risk patients approximately 12 months after initial treatment with surgery and radioiodine ablation, but not additional whole-body radioiodine scans.69 In intermediate-risk and high-risk patients, cervical ultrasound examination and diagnostic whole-body radioiodine scans are recommended every 6 to 12 months.
Patients with MTCs require serial measurements of calcitonin and CEA. Suspected recurrences may also be detected with pentagastrin-stimulation test. See the section on medullary thyroid carcinoma for further details.
American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:1-33.
Cognetti DM, Pribitkin EA, Keane WM. Management of the neck in differentiated thyroid cancer. Surg Oncol Clin N Am. 2008;17:157-173.
Fish SA, Langer JE, Mandel SJ. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37:401-417.
Frates MC, Beson CB, Charboneau JW, et al. Management of thyroid nodules detected at US. Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2005;237:794-800.
Grubbs EG, Rich TA, Li G, et al. Recent advances in thyroid cancer. Curr Probl Surg. 2008;45:156. 250
Kloos RT, Eng C, Evans DB, et al. American Thyroid Association Guideline Task Force. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565-612.
Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539-547.
Thyroid carcinoma. NCCN Clinical Practice Guidelines in Oncology. V.2.2007.
Tuttle RM, Leboeuf R, Martorella AJ. Papillary thyroid cancer: monitoring and therapy. Endocrinol Metab Clin North Am. 2007;36:753-758.
1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. Cancer J Clin. 2008;58:71-96.
2. Surveillance Epidemiology and End Results (SEER) Program. 2006.
3. Mazzaferri EL. Thyroid cancer in thyroid nodules: finding a needle in the haystack. Am J Med. 1992;93:359-362.
4. Hay ID, Klee GG. Thyroid cancer diagnosis and management. Clin Lab Med. 1993;13:725-734.
5. Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995. Cancer. 1998;83:2638-2648.
6. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418-428.
7. Frazell EL, Foote FWJr. Papillary cancer of the thyroid: a review of 25 years of experience. Cancer. 1958;11:895-922.
8. Donohue JH, Goldfien SD, Miller TR, et al. Do the prognoses of papillary and follicular thyroid carcinomas differ? Am J Surg. 1984;148:168-173.
9. Hayes B, Anthony A, Kersham R. Anatomy and development of the thyroid gland. Ear Nose Throat J. 1985;64:10.
10. Sessions RB. Cancer of the Thyroid Gland. Philadelphia: Lippincott-Raven; 1999.
11. Copp DH, Cockcroft DW, Kueh Y. Calcitonin from ultimobranchial glands of dogfish and chickens. Science. 1967;158:924-925.
12. Wolfe HJ, DeLellis RA, Voelkel EF, et al. Distribution of calcitonin-containing cells in the normal neonatal human thyroid gland: A correlation of morphology with peptide content. J Clin Endocrinol Metab. 1975;41:1076-1081.
13. Hollingshead W. Anatomy of the endocrine glands. Surg Clin North Am. 1958;39:1115-1140.
14. Rosai J. Tumors of the Thyroid Gland. Washington, DC: AFIP; 1992.
15. Henry JF, Audiffret J, Denizot A, et al. The nonrecurrent inferior laryngeal nerve: review of 33 cases, including two on the left side. Surgery. 1988;104:977-984.
16. Lennquist S, Cahlin C, Smeds S. The superior laryngeal nerve in thyroid surgery. Surgery. 1987;102:999-1008.
17. Wang C. The anatomic basis of parathyroid surgery. Ann Surg. 1976;183:271-275.
18. Sozzi G, Bongarzone I, Miozzo M, et al. Cytogenetic and molecular genetic characterization of papillary thyroid carcinomas. Genes Chromosomes Cancer. 1992;5:212-218.
19. Fagin JA. Molecular pathogenesis of human thyroid neoplasms. Thyroid Today. 1994;18:1-6.
20. Kitamura Y, Shimizu K, Tanaka S, et al. [Genetic alterations in thyroid carcinomas]. Nippon Ika Daigaku Zasshi. 1999;66:319-323.
21. Sobrinho-Simoes M, Preto A, Rocha AS, et al. Molecular pathology of well-differentiated thyroid carcinomas. Virchows Arch. 2005;447:787-793.
22. Williams ED. Mechanisms and pathogenesis of thyroid cancer in animals and man. Mutat Res. 1995;333(1-2):123-129.
23. Namba H, Rubin SA, Fagin JA. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol. 1990;4:1474-1479.
24. Ringel MD, Hayre N, Saito J, et al. Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res. 2001;61:6105-6111.
25. Grubbs EG, Rich TA, Li G, et al. Recent advances in thyroid cancer. Curr Probl Surg. 2008;45:156-250.
26. Faggiano A, Talbot M, Baudin E, et al. Differential expression of galectin 3 in solid cell nests and C cells of human thyroid. J Clin Pathol. 2003;56:142-143.
27. Herrmann ME, LiVolsi VA, Pasha TL, et al. Immunohistochemical expression of galectin-3 in benign and malignant thyroid lesions. Arch Pathol Lab Med. 2002;126:710-713.
28. Harach HR, Soubeyran I, Brown A, et al. Thyroid pathologic findings in patients with Cowden disease. Ann Diagn Pathol. 1999;3:331-340.
29. Kroll TG, Sarraf P, Pecciarini L, et al. PAX8-PPAR gamma1 fusion oncogene in human thyroid carcinoma. Science. 2000;289:1357-1360.
30. Fagin JA, Matsuo K, Karmakar A, et al. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179-184.
31. Takeuchi Y, Daa T, Kashima K, et al. Mutations of p53 in thyroid carcinoma with an insular component. Thyroid. 1999;9:377-381.
32. Frankenthaler RA, Sellin RV, Cangir A, et al. Lymph node metastasis from papillary-follicular thyroid carcinoma in young patients. Am J Surg. 1990;160:341-343.
33. Takahashi M, Asai N, Iwashita T, et al. Molecular mechanisms of development of multiple endocrine neoplasia 2 by RET mutations. J Intern Med. 1998;243:509-513.
34. Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA. 1996;276:1575-1579.
35. Santoro M, Carlomagno F, Romano A, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science. 1995;267:381-383.
36. Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539-547.
37. Wohllk N, Cote GJ, Bugalho MM, et al. Relevance of RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab. 1996;81:3740-3745.
38. Vecchio G, Santoro M. Oncogenes and thyroid cancer. Clin Chem Lab Med. 2000;38:113-116.
39. Takahashi M. Oncogenic activation of the ret protooncogene in thyroid cancer. Crit Rev Oncog. 1995;6:35-46.
40. Santoro M, Grieco M, Melillo RM, et al. Molecular defects in thyroid carcinomas: role of the RET oncogene in thyroid neoplastic transformation. Eur J Endocrinol. 1995;133:513-522.
41. Santoro M, Thomas GA, Vecchio G, et al. Gene rearrangement and Chernobyl related thyroid cancers. Br J Cancer. 2000;82:315-322.
42. Thomas GA, Bunnell H, Cook HA, et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab. 1999;84:4232-4238.
43. Williams ED, Doniach I, Bjarnason O, et al. Thyroid cancer in an iodide rich area: a histopathological study. Cancer. 1977;39:215-222.
44. Becker FO, Economou SG, Southwick HW, et al. Adult thyroid cancer after head and neck irradiation in infancy and childhood. Ann Intern Med. 1975;83:347-351.
45. Duffy BJJr, Fitzgerald PJ. Cancer of the thyroid in children: a report of 28 cases. J Clin Endocrinol Metab. 1950;10:1296-1308.
46. Nagataki S, Nystrom E. Epidemiology and primary prevention of thyroid cancer. Thyroid. 2002;12:889-896.
47. Rojeski MT, Gharib H. Nodular thyroid disease: evaluation and management. N Engl J Med. 1985;313:428-436.
48. Daniels GH. Thyroid nodules and nodular thyroids: a clinical overview. Compr Ther. 1996;22:239-250.
49. Malone J, Unger J, Delange F, et al. Thyroid consequences of Chernobyl accident in the countries of the European Community. J Endocrinol Invest. 1991;14:701-717.
50. Prentice RL, Kato H, Yoshimoto K, et al. Radiation exposure and thyroid cancer incidence among Hiroshima and Nagasaki residents. Natl Cancer Inst Monogr. 1982;62:207-212.
51. McTiernan A, Weiss NS, Daling JR. Incidence of thyroid cancer in women in relation to known or suspected risk factors for breast cancer. Cancer Res. 1987;47:292-295.
52. DeGroot LJ, Kaplan EL, Straus FH, et al. Does the method of management of papillary thyroid carcinoma make a difference in outcome? World J Surg. 1994;18:123-130.
53. Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947-953.
54. Pasieka JL, Zedenius J, Auer G, et al. Addition of nuclear DNA content to the AMES risk-group classification for papillary thyroid cancer. Surgery. 1992;112:1154-1159.
55. Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088-1095.
56. Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050-1057.
57. Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma. A study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033-1041.
58. Shah JP, Loree TR, Dharker D, et al. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164:658-661.
59. Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012-1021.
60. Hannequin P, Liehn JC, Delisle MJ. Multifactorial analysis of survival in thyroid cancer: pitfalls of applying the results of published studies to another population. Cancer. 1986;58:1749-1755.
61. Gharib H. Management of thyroid nodules: another look. Thyroid Today. 1997;20:1.
62. Leeper RD. Thyroid cancer. Med Clin North Am. 1985;69:1079-1096.
63. McHenry C, Smith M, Lawrence AM, et al. Nodular thyroid disease in children and adolescents: a high incidence of carcinoma. Am Surg. 1988;54:444-447.
64. Sadler G. Thyroid and parathyroid. In: Schwartz S, editor. Principles of Surgery. New York: McGraw-Hill; 1999:1661-1713.
65. Miller JM, Kini SR, Hamburger JI. The diagnosis of malignant follicular neoplasms of the thyroid by needle biopsy. Cancer. 1985;55:2812-2817.
66. Ashcraft MW, Van Herle AJ. Management of thyroid nodules, I: History and physical examination, blood tests, x-ray tests, and ultrasonography. Head Neck Surg. 1981;3:216-230.
67. Ashcraft MW, Van Herle AJ. Management of thyroid nodules, II: Scanning techniques, thyroid suppressive therapy, and fine needle aspiration. Head Neck Surg. 1981;3:297-322.
68. Ahuja S, Ernst H. Hyperthyroidism and thyroid carcinoma. Acta Endocrinol (Copenh). 1991;124:146-151.
69. Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109-142.
70. Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553-559.
71. Baloch ZW, Sack MJ, Yu GH, et al. Fine-needle aspiration of thyroid: an institutional experience. Thyroid. 1998;8:565-569.
72. Bisi H, de Camargo RY, Longatto Filho A. Role of fine-needle aspiration cytology in the management of thyroid nodules: review of experience with 1,925 cases. Diagn Cytopathol. 1992;8:504-510.
73. Hamburger JI. Consistency of sequential needle biopsy findings for thyroid nodules: management implications. Arch Intern Med. 1982;147:97-99.
74. Pepper GM, Zwickler D, Rosen Y. Fine-needle aspiration biopsy of the thyroid nodule: results of a start-up project in a general teaching hospital setting. Arch Intern Med. 1989;149:594-596.
75. Caruso D, Mazzaferri EL. Fine needle aspiration in the management of thyroid nodules. Endocrinologist. 1991;1:194-202.
76. McHenry CR, Walfish PG, Rosen IB. Non-diagnostic fine needle aspiration biopsy: a dilemma in management of nodular thyroid disease. Am Surg. 1993;59:415-419.
77. Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993;118:282-289.
78. Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. Mod Pathol. 1996;9:710-715.
79. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
80. Grant CS. Operative and postoperative management of the patient with follicular and Hurthle cell carcinoma. Do they differ? Surg Clin North Am. 1995;75:395-403.
81. Callender D. Cancer of the Thyroid, 3rd ed. Philadelphia: Saunders; 1996.
82. Frates MC, Benson CB, Doubilet PM, et al. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003;22:127-131.
83. Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US. Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794-800.
84. Kouvaraki MA, Shapiro SE, Fornage BD, et al. Role of preoperative ultrasonography in the surgical management of patients with thyroid cancer. Surgery. 2003;134:946-954.
85. Price DC. Radioisotopic evaluation of the thyroid and the parathyroids. Radiol Clin North Am. 1993;31:991-1015.
86. Tan RK, Finley RK3rd, Driscoll D, et al. Anaplastic carcinoma of the thyroid: a 24-year experience. Head Neck. 1995;17:41-47.
87. Stulak JM, Grant CS, Farley DR, et al. Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg. 2006;141:489-494.
88. Clark OH. Thyroid nodules and thyroid cancer: surgical aspects. West J Med. 1980;133:1-8.
89. Tyler DS, Winchester DJ, Caraway NP, et al. Indeterminate fine-needle aspiration biopsy of the thyroid: identification of subgroups at high risk for invasive carcinoma. Surgery. 1994;116:1054-1060.
90. Chandramouly B, Mann D, Cunningham RP, et al. Marine-Lenhart syndrome: Graves’ disease with poorly functioning nodules. Clin Nucl Med. 1992;17:905-906.
91. Lang W, Georgii A, Stauch G, et al. The differentiation of atypical adenomas and encapsulated follicular carcinomas in the thyroid gland. Virchows Arch A Pathol Anat Histol. 1980;385:125-141.
92. Carmeci C, Jeffrey RB, McDougall IR, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998;8:283-289.
93. Ylagan LR, Farkas T, Dehner LP. Fine needle aspiration of the thyroid: a cytohistologic correlation and study of discrepant cases. Thyroid. 2004;14:35-41.
94. Ferrari C. Value of ethanol injection in the treatment of the autonomous thyroid nodule. J Endocrinol Invest. 1995;18:465-467.
95. de los Santos ET, Keyhani-Rofagha S, Cunningham JJ, et al. Cystic thyroid nodules: the dilemma of malignant lesions. Arch Intern Med. 1990;150:1422-1427.
96. Rosen IB, Provias JP, Walfish PG. Pathologic nature of cystic thyroid nodules selected for surgery by needle aspiration biopsy. Surgery. 1986;100:606-613.
97. Harvey HK. Diagnosis and management of the thyroid nodule: an overview. Otolaryngol Clin North Am. 1990;23:303-337.
98. Jossart GH, Clark OH. Well-differentiated thyroid cancer. Curr Probl Surg. 1994;31:933-1012.
99. Cady B, Sedgwick CE, Meissner WA, et al. Changing clinical, pathologic, therapeutic, and survival patterns in differentiated thyroid carcinoma. Ann Surg. 1976;184:541-553.
100. Schneider AB, Favus MJ, Stachura ME, et al. Incidence, prevalence and characteristics of radiation-induced thyroid tumors. Am J Med. 1978;64:243-252.
101. Mazzaferri EL, Young RL. Papillary thyroid carcinoma: a 10 year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511-518.
102. Woolner LB, Lemmon ML, Beahrs OH, et al. Occult papillary carcinoma of the thyroid gland: a study of 140 cases observed in a 30-year period. J Clin Endocrinol Metab. 1960;20:89-105.
103. Breaux GPJr, Guillamondegui OM. Treatment of locally invasive carcinoma of the thyroid: how radical? Am J Surg. 1980;140:514-517.
104. McConahey WM, Hay ID, Woolner LB, et al. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1986;61:978-996.
105. Noguchi S, Murakami N. The value of lymph-node dissection in patients with differentiated thyroid cancer. Surg Clin North Am. 1987;67:251-261.
106. Tollefsen HR, Shah JP, Huvos AG. Papillary carcinoma of the thyroid: recurrence in the thyroid gland after initial surgical treatment. Am J Surg. 1972;124:468-472.
107. Woolner LB, Beahrs OH, Black BM, et al. Classification and prognosis of thyroid carcinoma: a study of 885 cases observed in a thirty year period. Am J Surg. 1961;102:354-387.
108. LiVolsi VA. Papillary neoplasms of the thyroid: pathologic and prognostic features. Am J Clin Pathol. 1992;97:426-434.
109. Rosai J. Papillary carcinoma. Monogr Pathol. 1993;35:138-165.
110. Favus MJ, Schneider AB, Stachura ME, et al. Thyroid cancer occurring as a late consequence of head-and-neck irradiation: evaluation of 1056 patients. N Engl J Med. 1976;294:1019-1025.
111. Sherman SI, Angelos P, Ball DW, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2007;5:568-621.
112. Lang W, Choritz H, Hundeshagen H. Risk factors in follicular thyroid carcinomas: a retrospective follow-up study covering a 14-year period with emphasis on morphological findings. Am J Surg Pathol. 1986;10:246-255.
113. Crile GJr, Antunez AR, Esselstyn CBJr, et al. The advantages of subtotal thyroidectomy and suppression of TSH in the primary treatment of papillary carcinoma of the thyroid. Cancer. 1985;55:2691-2697.
114. DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414-424.
115. Rossi RL, Nieroda C, Cady B, et al. Malignancies of the thyroid gland: the Lahey Clinic experience. Surg Clin North Am. 1985;65:211-230.
116. Mazzaferri EL, Young RL, Oertel JE, et al. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore). 1977;56:171-196.
117. Sellers M, Beenken S, Blankenship A, et al. Prognostic significance of cervical lymph node metastases in differentiated thyroid cancer. Am J Surg. 1992;164:578-581.
118. Rossi RL, Cady B, Silverman ML, et al. Current results of conservative surgery for differentiated thyroid carcinoma. World J Surg. 1986;10:612-622.
119. Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241-260.
120. Pingpank JFJr, Sasson AR, Hanlon AL, et al. Tumor above the spinal accessory nerve in papillary thyroid cancer that involves lateral neck nodes: a common occurrence. Arch Otolaryngol Head Neck Surg. 2002;128:1275-1278.
121. Cady B, Rossi R, Silverman M, et al. Further evidence of the validity of risk group definition in differentiated thyroid carcinoma. Surgery. 1985;98:1171-1178.
122. Hayles AB, Kennedy RL, Beahrs OH, et al. Management of the child with thyroidal carcinoma. JAMA. 1960;173:21-28.
123. Exelby PE, Frazell EL. Carcinoma of the thyroid in children. Surg Clin North Am. 1969;49:249-259.
124. Prendiville S, Burman KD, Ringel MD, et al. Tall cell variant: an aggressive form of papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2000;122:352-357.
125. Brennan MD, Bergstralh EJ, van Heerden JA, et al. Follicular thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1991;66:11-22.
126. Kahn NF, Perzin KH. Follicular carcinoma of the thyroid: an evaluation of the histologic criteria used for diagnosis. Pathol Annu. 1983;18(Pt 1):221-253.
127. Harness JK, Thompson NW, McLeod MK, et al. Follicular carcinoma of the thyroid gland: trends and treatment. Surgery. 1984;96:972-980.
128. Young RL, Mazzaferri EL, Rahe AJ, et al. Pure follicular thyroid carcinoma: impact of therapy in 214 patients. J Nucl Med. 1980;21:733-737.
129. Yamashina M. Follicular neoplasms of the thyroid: total circumferential evaluation of the fibrous capsule. Am J Surg Pathol. 1992;16:392-400.
130. Woolner LB. Thyroid carcinoma: pathologic classification with data on prognosis. Semin Nucl Med. 1971;1:481-502.
131. Johannessen JV, Sobrinho-Simoes M, Lindmo T, et al. The diagnostic value of flow cytometric DNA measurements in selected disorders of the human thyroid. Am J Clin Pathol. 1982;77:20-25.
132. Maxon HR3rd, Smith HS. Radioiodine-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am. 1990;19:685-718.
133. Silverman M. Pathology of Thyroid and Parathyroid Glands. Philadelphia: Saunders; 1991.
134. van Heerden JA, Hay ID, Goellner JR, et al. Follicular thyroid carcinoma with capsular invasion alone: a nonthreatening malignancy. Surgery. 1992;112:1130-1136.
135. Crile GJr, Pontius KI, Hawk WA. Factors influencing the survival of patients with follicular carcinoma of the thyroid gland. Surg Gynecol Obstet. 1985;160:409-413.
136. Evans HL, Vassilopoulou-Sellin R. Follicular and Hurthle cell carcinomas of the thyroid: a comparative study. Am J Surg Pathol. 1998;22:1512-1520.
137. Thompson NW, Dunn EL, Batsakis JG, et al. Hurthle cell lesions of the thyroid gland. Surg Gynecol Obstet. 1974;139:555-560.
138. el-Naggar AK, Batsakis JG, Luna MA, et al. Hurthle cell tumors of the thyroid: a flow cytometric DNA analysis. Arch Otolaryngol Head Neck Surg. 1988;114:520-521.
139. Tallini G, Carcangiu ML, Rosai J. Oncocytic neoplasms of the thyroid gland. Acta Pathol Jpn. 1992;42:305-315.
140. Ruegemer JJ, Hay ID, Bergstralh EJ, et al. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67:501-508.
141. Hazard JB. The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma: a review. Am J Pathol. 1977;88:213-250.
142. Chong GC, Beahrs OH, Sizemore GW, et al. Medullary carcinoma of the thyroid gland. Cancer. 1975;35:695-704.
143. Ellenhorn JD, Shah JP, Brennan MF. Impact of therapeutic regional lymph node dissection for medullary carcinoma of the thyroid gland. Surgery. 1993;114:1078-1081.
144. DeLellis RA. Multiple endocrine neoplasia syndromes revisited: clinical, morphologic, and molecular features. Lab Invest. 1995;72:494-505.
145. Raue F, Frank-Raue K, Grauer A. Multiple endocrine neoplasia type 2: clinical features and screening. Endocrinol Metab Clin North Am. 1994;23:137-156.
146. Moley JF. Medullary thyroid cancer. Surg Clin North Am. 1995;75:405-420.
147. Goodfellow PJ, Wells SAJr. RET gene and its implications for cancer. J Natl Cancer Inst. 1995;87:1515-1523.
148. Bergholm U, Adami HO, Bergstrom R, et al. Clinical characteristics in sporadic and familial medullary thyroid carcinoma: a nationwide study of 249 patients in Sweden from 1959 through 1981. Cancer. 1989;63:1196-1204.
149. Saad MF, Ordonez NG, Rashid RK, et al. Medullary carcinoma of the thyroid: a study of the clinical features and prognostic factors in 161 patients. Medicine (Baltimore). 1984;63:319-342.
150. Sessions RB, Harrison L, Forastiere A. Tumors of the Salivary Glands and Paragangliomas, 5th ed. Philadelphia: Lippincott-Raven; 1996.
151. Stepanas AV, Samaan NA, Hill CSJr, et al. Medullary thyroid carcinoma: importance of serial serum calcitonin measurement. Cancer. 1979;43:825-837.
151a. Kloos RT, Eng C, Evans DB, et al. American Thyroid Association Guideline Task Force. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19:565-612.
152. Block MA. Surgical treatment of medullary carcinoma of the thyroid. Otolaryngol Clin North Am. 1990;23:453-473.
153. Donovan D, Gagel RF. Medullary Thyroid Carcinoma and the Multiple Endocrine Neoplasia Syndromes, 2nd ed. Philadelphia: Lippincott-Raven; 1997.
154. O’Riordain DS, O’Brien T, Crotty TB, et al. Multiple endocrine neoplasia type 2B: more than an endocrine disorder. Surgery. 1995;118:936-942.
155. Wells SAJr, Baylin SB, Gann DS, et al. Medullary thyroid carcinoma: relationship of method of diagnosis to pathologic staging. Ann Surg. 1978;188:377-383.
156. Kakudo K, Carney JA, Sizemore GW. Medullary carcinoma of thyroid: biologic behavior of the sporadic and familial neoplasm. Cancer. 1985;55:2818-2821.
157. LiVolsi VA, Merino M. Pathology of Thyroid Tumors. Philadelphia: Saunders; 1987.
158. Nel CJ, van Heerden JA, Goellner JR, et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 82 cases. Mayo Clin Proc. 1985;60:51-58.
159. Kapp DS, LiVolsi VA, Sanders MM. Anaplastic carcinoma following well-differentiated thyroid cancer: etiological considerations. Yale J Biol Med. 1982;55(5-6):521-528.
160. Demeter JG, De Jong SA, Lawrence AM, et al. Anaplastic thyroid carcinoma: risk factors and outcome. Surgery. 1991;110:956-961.
161. Venkatesh YS, Ordonez NG, Schultz PN, et al. Anaplastic carcinoma of the thyroid: a clinicopathologic study of 121 cases. Cancer. 1990;66:321-330.
162. Kim JH, Leeper RD. Treatment of locally advanced thyroid carcinoma with combination doxorubicin and radiation therapy. Cancer. 1987;60:2372-2375.
163. Tennvall J, Lundell G, Hallquist A, et al. Combined doxorubicin, hyperfractionated radiotherapy, and surgery in anaplastic thyroid carcinoma: report on two protocols. The Swedish Anaplastic Thyroid Cancer Group. Cancer. 1994;74:1348-1354.
164. Voutilainen PE, Multanen M, Haapiainen RK, et al. Anaplastic thyroid carcinoma survival. World J Surg. 1999;23:975-978.
165. Carcangiu ML, Zampi G, Rosai J. Poorly differentiated (“insular”) thyroid carcinoma: a reinterpretation of Langhans’ “wuchernde Struma.”. Am J Surg Pathol. 1984;8:655-668.
166. Burman KD, Ringel MD, Wartofsky L. Unusual types of thyroid neoplasms. Endocrinol Metab Clin North Am. 1996;25:49-68.
167. Sirota DK, Segal RL. Primary lymphomas of the thyroid gland. JAMA. 1979;242:1743-1746.
168. Tsang RW, Gospodarowicz MK, Sutcliffe SB, et al. Non-Hodgkin’s lymphoma of the thyroid gland: prognostic factors and treatment outcome. The Princess Margaret Hospital Lymphoma Group. Int J Radiat Oncol Biol Phys. 1993;27:599-604.
169. Salhany KE, Pietra GG. Extranodal lymphoid disorders. Am J Clin Pathol. 1993;99:472-485.
170. Devine RM, Edis AJ, Banks PM. Primary lymphoma of the thyroid: A review of the Mayo Clinic experience through 1978. World J Surg. 1981;5:33-38.
171. Rasbach DA, Mondschein MS, Harris NL, et al. Malignant lymphoma of the thyroid gland: a clinical and pathologic study of twenty cases. Surgery. 1985;98:1166-1170.
172. Ivy HK. Cancer metastatic to the thyroid: a diagnostic problem. Mayo Clin Proc. 1984;59:856-859.
173. Green LK, Ro JY, Mackay B, et al. Renal cell carcinoma metastatic to the thyroid. Cancer. 1989;63:1810-1815.
174. Meissner WA, Adler A. Papillary carcinoma of the thyroid: A study of the pathology of two hundred twenty-six cases. AMA Arch Pathol. 1958;66:518-525.
175. Tubiana M, Haddad E, Schlumberger M, et al. External radiotherapy in thyroid cancers. Cancer. 1985;55(9 Suppl):2062-2071.
176. Lore JMJr, Kim DJ, Elias S. Preservation of the laryngeal nerves during total thyroid lobectomy. Ann Otol Rhinol Laryngol. 1977;86(6 Pt 1):777-788.
177. Nemiroff PM, Katz AD. Extralaryngeal divisions of the recurrent laryngeal nerve: Surgical and clinical significance. Am J Surg. 1982;144:466-469.
178. Snyder SK, Hendricks JC. Intraoperative neurophysiology testing of the recurrent laryngeal nerve: plaudits and pitfalls. Surgery. 2005;138:1183-1191.
179. Yarbrough DE, Thompson GB, Kasperbauer JL, et al. Intraoperative electromyographic monitoring of the recurrent laryngeal nerve in reoperative thyroid and parathyroid surgery. Surgery. 2004;136:1107-1115.
180. Robertson ML, Steward DL, Gluckman JL, et al. Continuous laryngeal nerve integrity monitoring during thyroidectomy: does it reduce risk of injury? Otolaryngol Head Neck Surg. 2004;131:596-600.
181. Marchetta FC, Sako K, Matsuura H. Modified neck dissection for carcinoma of the thyroid gland. Am J Surg. 1970;120:452-455.
182. Tubiana M, Schlumberger M, Rougier P, et al. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer. 1985;55:794-804.
183. Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg. 1999;229:880-887.
184. Mack E. Management of patients with substernal goiters. Surg Clin North Am. 1995;75:377-394.
185. Shaha AR, Alfonso AE, Jaffe BM. Operative treatment of substernal goiters. Head Neck. 1989;11:325-330.
186. Grillo HC, Zannini P. Resectional management of airway invasion by thyroid carcinoma. Ann Thorac Surg. 1986;42:287-298.
187. Ballantyne AJ. Resections of the upper aerodigestive tract for locally invasive thyroid cancer. Am J Surg. 1994;168:636-639.
188. Shelton VK, Skolnik EM, Berlinger FG, et al. Laryngotracheal invasion by thyroid-carcinoma. Ann Otol Rhinol Laryngol. 1982;91(4 Pt 1):363-369.
189. Beahrs OH, Vandertoll DJ. Complications of secondary thyroidectomy. Surg Gynecol Obstet. 1963;117:535-539.
190. Chao TC, Jeng LB, Lin JD, et al. Completion thyroidectomy for differentiated thyroid carcinoma. Otolaryngol Head Neck Surg. 1998;118:896-899.
191. Kupferman ME, Mandel SJ, DiDonato L, et al. Safety of completion thyroidectomy following unilateral lobectomy for well-differentiated thyroid cancer. Laryngoscope. 2002;112(7 Pt 1):1209-1212.
192. Moley JF, Lairmore TC, Doherty GM, et al. Preservation of the recurrent laryngeal nerves in thyroid and parathyroid reoperations. Surgery. 1999;126:673-677.
193. Pasieka JL, Thompson NW, McLeod MK, et al. The incidence of bilateral well-differentiated thyroid cancer found at completion thyroidectomy. World J Surg. 1992;16:711-716.
194. Basolo F, Baloch ZW, Baldanzi A, et al. Usefulness of Ultrafast Papanicolaou-stained scrape preparations in intraoperative management of thyroid lesions. Mod Pathol. 1999;12:653-657.
195. Rodriguez JM, Parrilla P, Sola J, et al. Comparison between preoperative cytology and intraoperative frozen-section biopsy in the diagnosis of thyroid nodules. Br J Surg. 1994;81:1151-1154.
196. Lo CY, Lam KY. Postoperative hypocalcemia in patients who did or did not undergo parathyroid autotransplantation during thyroidectomy: a comparative study. Surgery. 1998;124:1081-1086.
197. Sierra M, Herrera MF, Herrero B, et al. Prospective biochemical and scintigraphic evaluation of autografted normal parathyroid glands in patients undergoing thyroid operations. Surgery. 1998;124:1005-1010.
198. Park HM, Park YH, Zhou XH. Detection of thyroid remnant/metastasis without stunning: an ongoing dilemma. Thyroid. 1997;7:277-280.
199. Grant CS, Hay ID, Gough IR, et al. Local recurrence in papillary thyroid carcinoma: is extent of surgical resection important? Surgery. 1988;104:954-962.
200. Segal K, Friedental R, Lubin E, et al. Papillary carcinoma of the thyroid. Otolaryngol Head Neck Surg. 1995;113:356-363.
201. Schlumberger M, Tubiana M, De Vathaire F, et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960-967.
202. Attie JN, Bock G, Moskowitz GW, et al. Postoperative radioactive iodine evaluation of total thyroidectomy for thyroid carcinoma: reappraisal and therapeutic implications. Head Neck. 1992;14:297-302.
203. Chonkick GD, Petti GHJr. Treatment of thyroid carcinoma. Laryngoscope. 1992;102:486-491.
204. Miccoli P, Berti P, Conte M, et al. Minimally invasive surgery for thyroid small nodules: preliminary report. J Endocrinol Invest. 1999;22:849-851.
205. Miccoli P, Berti P, Raffaelli M, et al. Comparison between minimally invasive video-assisted thyroidectomy and conventional thyroidectomy: a prospective randomized study. Surgery. 2001;130:1039-1043.
206. Takami HE, Ikeda Y. Minimally invasive thyroidectomy. Curr Opin Oncol. 2006;18:43-47.
207. Palazzo FF, Sebag F, Henry JF. Endocrine surgical technique: endoscopic thyroidectomy via the lateral approach. Surg Endosc. 2006;20:339-342.
208. Terris DJ, Moister B, Seybt MW, et al. Outpatient thyroid surgery is safe and desirable. Otolaryngol Head Neck Surg. 2007;136:556-559.
209. Lombardi CP, Raffaelli M, Princi P, et al. Safety of video-assisted thyroidectomy versus conventional surgery. Head Neck. 2005;27:58-64.
210. Lai SY, Walvekar RR, Ferris RL. Minimally invasive video-assisted thyroidectomy: expanded indications and oncologic completeness. Head Neck. 2008;30:1403-1407.
211. Rallison ML, Dobyns BM, Keating FRJr, et al. Thyroid nodularity in children. JAMA. 1975;233:1069-1072.
212. Kirkland RT, Kirkland JL, Rosenberg HS, et al. Solitary thyroid nodules in 30 children and report of a child with a thyroid abscess. Pediatrics. 1973;51:85-90.
213. Buckwalter JA, Gurll NJ, Thomas CGJr. Cancer of the thyroid in youth. World J Surg. 1981;5:15-25.
214. Harness JK, Thompson NW, McLeod MK, et al. Differentiated thyroid carcinoma in children and adolescents. World J Surg. 1992;16:547-553.
215. Goepfert H, Dichtel WJ, Samaan NA. Thyroid cancer in children and teenagers. Arch Otolaryngol. 1984;110:72-75.
216. Simpson WJ, Panzarella T, Carruthers JS, et al. Papillary and follicular thyroid cancer: impact of treatment in 1578 patients. Int J Radiat Oncol Biol Phys. 1988;14:1063-1075.
217. Hung W. Solitary thyroid nodules in 93 children and adolescents: a 35-years experience. Horm Res. 1999;52:15-18.
218. Khurana KK, Labrador E, Izquierdo R, et al. The role of fine-needle aspiration biopsy in the management of thyroid nodules in children, adolescents, and young adults: a multi-institutional study. Thyroid. 1999;9:383-386.
219. Harach HR, Williams ED. Childhood thyroid cancer in England and Wales. Br J Cancer. 1995;72:777-783.
220. Tronko MD, Bogdanova TI, Komissarenko IV, et al. Thyroid carcinoma in children and adolescents in Ukraine after the Chernobyl nuclear accident: statistical data and clinicomorphologic characteristics. Cancer. 1999;86:149-156.
221. Katoh R, Harach HR, Williams ED. Solitary, multiple, and familial oxyphil tumours of the thyroid gland. J Pathol. 1998;186:292-299.
222. Danese D, Gardini A, Farsetti A, et al. Thyroid carcinoma in children and adolescents. Eur J Pediatr. 1997;156:190-194.
223. Vassilopoulou-Sellin R, Goepfert H, Raney B, et al. Differentiated thyroid cancer in children and adolescents: clinical outcome and mortality after long-term follow-up. Head Neck. 1998;20:549-555.
224. Welch Dinauer CA, Tuttle RM, Robie DK, et al. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf). 1998;49:619-628.
225. Gorlin JB, Sallan SE. Thyroid cancer in childhood. Endocrinol Metab Clin North Am. 1990;19:649-662.
226. La Quaglia MP, Corbally MT, Heller G, et al. Recurrence and morbidity in differentiated thyroid carcinoma in children. Surgery. 1988;104:1149-1156.
227. Vassilopoulou-Sellin R, Klein MJ, Smith TH, et al. Pulmonary metastases in children and young adults with differentiated thyroid cancer. Cancer. 1993;71:1348-1352.
228. Hay ID. Nodular thyroid disease diagnosed during pregnancy: how and when to treat. Thyroid. 1999;9:667-670.
229. Mazzaferri EL. Evaluation and management of common thyroid disorders in women. Am J Obstet Gynecol. 1997;176:507-514.
230. Morris PC. Thyroid cancer complicating pregnancy. Obstet Gynecol Clin North Am. 1998;25:401-405.
231. Burrow GH. Thyroid status in normal pregnancy. J Clin Endocrinol Metab. 1990;71:274-275.
232. Glinoer D, Lemone M. Goiter and pregnancy: a new insight into an old problem. Thyroid. 1992;2:65-70.
233. Rosen IB, Korman M, Walfish PG. Thyroid nodular disease in pregnancy: current diagnosis and management. Clin Obstet Gynecol. 1997;40:81-89.
234. Doherty CM, Shindo ML, Rice DH, et al. Management of thyroid nodules during pregnancy. Laryngoscope. 1995;105(3 Pt 1):251-255.
235. Moosa M, Mazzaferri EL. Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab. 1997;82:2862-2866.
236. Ward PH, Berci G, Calcaterra TC. Superior laryngeal nerve paralysis an often overlooked entity. Trans Sect Otolaryngol Am Acad Ophthalmol Otolaryngol. 1977;84:78-89.
237. Beahrs OH. Complications of surgery of the head and neck. Surg Clin North Am. 1977;57:823-829.
238. Flynn MB, Lyons KJ, Tarter JW, et al. Local complications after surgical resection for thyroid carcinoma. Am J Surg. 1994;168:404-407.
239. Netterville JL, Aly A, Ossoff RH. Evaluation and treatment of complications of thyroid and parathyroid surgery. Otolaryngol Clin North Am. 1990;23:529-552.
240. Dedo HH. The paralyzed larynx: an electromyographic study in dogs and humans. Laryngoscope. 1970;80:1455-1517.
241. Boles R, Fritzell B. Injury and repair of the recurrent laryngeal nerves in dogs. Laryngoscope. 1969;79:1405-1418.
242. Crumley RL, Izdebski K. Voice quality following laryngeal reinnervation by ansa hypoglossi transfer. Laryngoscope. 1986;96:611-616.
243. Tucker HM. Reinnervation of the unilaterally paralyzed larynx. Ann Otol Rhinol Laryngol. 1977;86(6 Pt 1):789-794.
244. Beahrs OH. Complications in Thyroid and Parathyroid Surgery. Philadelphia: Saunders; 1979.
245. Bourrel C, Uzzan B, Tison P, et al. Transient hypocalcemia after thyroidectomy. Ann Otol Rhinol Laryngol. 1993;102:496-501.
246. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: a “normal” finding in Finland: a systematic autopsy study. Cancer. 1985;56:531-538.
247. Abboud B, Sargi Z, Akkam M, et al. Risk factors for postthyroidectomy hypocalcemia. J Am Coll Surg. 2002;195:456-461.
248. Lo CY, Lam KY. Routine parathyroid autotransplantation during thyroidectomy. Surgery. 2001;129:318-323.
249. Brunt LM, Sicard GA. Current status of parathyroid autotransplantation. Semin Surg Oncol. 1990;6:115-121.
250. Noordzij JP, Lee SL, Bernet VJ, et al. Early prediction of hypocalcemia after thyroidectomy using parathyroid hormone: an analysis of pooled individual patient data from nine observational studies. J Am Coll Surg. 2007;205:748-754.
251. Pujol P, Daures JP, Nsakala N, et al. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab. 1996;81:4318-4323.
252. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med. 2002;34(7-8):554-564.
253. Samaan NA, Schultz PN, Hickey RC, et al. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714-720.
254. Tsang RW, Brierley JD, Simpson WJ, et al. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375-388.
255. Mazzaferri EL. Thyroid remnant 131I ablation for papillary and follicular thyroid carcinoma. Thyroid. 1997;7:265-271.
256. Taylor T, Specker B, Robbins J, et al. Outcome after treatment of high-risk papillary and non-Hurthle-cell follicular thyroid carcinoma. Ann Intern Med. 1998;129:622-627.
257. Edmonds CJ, Hayes S, Kermode JC, et al. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. 1977;50:799-807.
258. Haugen BR, Pacini F, Reiners C, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877-3885.
259. Meier CA, Braverman LE, Ebner SA, et al. Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study). J Clin Endocrinol Metab. 1994;78:188-196.
260. Leger FA, Izembart M, Dagousset F, et al. Decreased uptake of therapeutic doses of iodine-131 after 185-MBq iodine-131 diagnostic imaging for thyroid remnants in differentiated thyroid carcinoma. Eur J Nucl Med. 1998;25:242-246.
261. Mandel SJ, Shankar LK, Benard F, et al. Superiority of iodine-123 compared with iodine-131 scanning for thyroid remnants in patients with differentiated thyroid cancer. Clin Nucl Med. 2001;26:6-9.
262. Shankar LK, Yamamoto AJ, Alavi A, et al. Comparison of 123I scintigraphy at 5 and 24 hours in patients with differentiated thyroid cancer. J Nucl Med. 2002;43(1):72-76.
263. Beierwaltes WH. The treatment of thyroid carcinoma with radioactive iodine. Semin Nucl Med. 1978;8:79-94.
264. Beierwaltes WH, Nishiyama RH, Thompson NW, et al. Survival time and “cure” in papillary and follicular thyroid carcinoma with distant metastases: statistics following University of Michigan therapy. J Nucl Med. 1982;23:561-568.
265. Robbins RJ, Schlumberger MJ. The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005;46(Suppl 1):28S-37S.
266. Brunt LM, Wells SAJr. Advances in the diagnosis and treatment of medullary thyroid carcinoma. Surg Clin North Am. 1987;67:263-279.
267. Samaan NA, Schultz PN, Hickey RC. Medullary thyroid carcinoma: prognosis of familial versus sporadic disease and the role of radiotherapy. J Clin Endocrinol Metab. 1988;67:801-805.
268. De Besi P, Busnardo B, Toso S, et al. Combined chemotherapy with bleomycin, Adriamycin, and platinum in advanced thyroid cancer. J Endocrinol Invest. 1991;14:475-480.
269. Rougier P, Parmentier C, Laplanche A, et al. Medullary thyroid carcinoma: prognostic factors and treatment. Int J Radiat Oncol Biol Phys. 1983;9:161-169.
270. Samaan NA, Yang KP, Schultz P, et al. Diagnosis, management, and pathogenetic studies in medullary thyroid carcinoma syndrome. Henry Ford Hosp Med J. 1989;37(3-4):132-137.
271. Sherman SI. Early clinical studies of novel therapies for thyroid cancers. Endocrinol Metab Clin North Am. 2008;37:511-524.
272. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714-4719.
273. Schlumberger M, Berg G, Cohen O, et al. Follow-up of low-risk patients with differentiated thyroid carcinoma: a European perspective. Eur J Endocrinol. 2004;150:105-112.
274. Rouxel A, Hejblum G, Bernier MO, et al. Prognostic factors associated with the survival of patients developing loco-regional recurrences of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:5362-5368.
275. Szanto J, Vincze B, Sinkovics I, et al. Postoperative thyroglobulin level determination to follow up patients with highly differentiated thyroid cancer. Oncology. 1989;46:99-104.
276. Ozata M, Suzuki S, Miyamoto T, et al. Serum thyroglobulin in the follow-up of patients with treated differentiated thyroid cancer. J Clin Endocrinol Metab. 1994;79:98-105.
277. Wartofsky L. Using baseline and recombinant human TSH-stimulated Tg measurements to manage thyroid cancer without diagnostic (131)I scanning. J Clin Endocrinol Metab. 2002;87:1486-1489.