CHAPTER 126 Management of Thyroid Eye Disease (Graves’ Ophthalmopathy)
In 1835, Graves described a clinical syndrome that included symptoms such as hypermetabolism, diffuse enlargement of the thyroid gland, and exophthalmos. Although others also had recognized this entity, Graves defined the thyroid as playing a central role in the disease. Graves’ disease is now recognized as a multisystem disorder characterized by one or more of the following: (1) hyperthyroidism associated with diffuse hyperplasia of the thyroid gland, (2) infiltrative ophthalmopathy (leading to exophthalmos), and (3) infiltrative dermopathy (localized pretibial myxedema). More recent work has helped to establish Graves’ disease as an autoimmune process targeted at the thyroid-stimulating hormone (TSH) receptor in the thyroid.1–3 In addition, retro-ocular fibroblasts have been found to play a key role in the development and progression of the ophthalmopathy seen in some patients with Graves’ disease.4,5
Pathophysiology
Graves’ Disease
Current theory describing the development of Graves’ disease involves autoreactive T cells, which arise through an escape from clonal deletion, through failure of suppressor T-cell activity, or through molecular mimicry to become reactive to TSH receptors.6,7 Most of the T cells responsible for the reaction are located in the thyroid gland itself. Subsequent thyroid damage from any etiology (e.g., chronic thyroiditis, radiotherapy, smoking, drugs) results in the release of the thyroid autoantigen (TSH receptors). As the autoimmune process amplifies, T lymphocytes are activated, and humoral immunity produces antibodies to the TSH receptor that are stimulatory, resulting in hyperthyroidism. In some patients, underlying chronic thyroiditis may dramatically reduce thyroid reserve, or TSH receptor–blocking antibodies may be present, resulting in “euthyroid” patients with Graves’ disease.
Graves’ Ophthalmopathy
The extraocular muscles are the site of the most clinically evident changes in patients with Graves’ ophthalmopathy. Although the muscles are enlarged on computed tomography (CT) scan, the myocytes themselves appear fairly normal histopathologically.8 There is an associated intense proliferation of perimysial fibroblasts and dense lymphocytic infiltration. Early reports of circulating autoantibodies against eye muscle antigen in sera from patients with Graves’ ophthalmopathy led to the theory that the disease was a result of an autoimmune response directed against the extraocular eye muscle fibers.9 This theory began to lose favor as study of these autoantibodies proved them to be neither tissue-specific nor disease-specific. The lack of histologic evidence of cytotoxicity against eye muscle in vivo also argues against this theory.
Attention has now focused on the retrobulbar fibroblast as playing a key role in the pathogenesis of Graves’ ophthalmopathy. These fibroblasts have several capabilities that place them at the center of the changes seen in the eye. They secrete a range of glycosaminoglycans (predominantly hyaluronate), the deposition of which is a hallmark of Graves’ ophthalmopathy and causes interstitial edema as a result of their intensely hydrophilic nature. These cells can also produce major histocompatibility complex (MHC) class II molecules, heat shock proteins, and lymphocyte adhesion molecules, which allow them to act as target and effector cells in the ongoing immune process in patients with Graves’ ophthalmopathy.10 In addition, autoantibodies against fibroblast antigens have been found in most patients with Graves’ ophthalmopathy. These antibodies share some characteristics with TSH receptor antibodies (TRAbs).5 More recently, probes to TSH receptor messenger RNA (mRNA) have labeled mRNA within the retrobulbar fibroblasts of patients with Graves’ ophthalmopathy.6 The “fibroblast antigen” may be similar to all or part of the TSH receptor, and it represents a shared thyroid-eye antigen. Such an antigenic similarity would explain the immune cross-reactivity between these two seemingly unrelated tissue sites.
Lymphocytes also are active in the ongoing immune process of Graves’ ophthalmopathy. Orbital lymphocyte infiltrates have been found to be primarily T cells, including CD4+ (T helper cells) and CD8+ (T suppressor and cytotoxic cells). Cytokines released by T cells have been shown to induce fibroblast proliferation and collagen and glycosaminoglycan deposition. Grubeck-Loebenstein and colleagues11 cultured retrobulbar suppressor and cytotoxic T cells out of tissues removed at the time of orbital decompression, and found them capable of targeting the retrobulbar fibroblasts. Interactions with fibroblasts resulted in pronounced T-cell cytokine production and fibroblast proliferation without evidence of fibroblast cytotoxicity. The T cell–retrobulbar fibroblast interaction may be responsible for the clinical manifestations of Graves’ ophthalmopathy.
Epidemiology and Etiology
An accurate estimate of the prevalence of Graves’ ophthalmopathy is difficult to determine and depends in part on the diagnostic criteria used to define the presence of ophthalmopathy. “Lid lag” and “stare” are nonspecific signs and can be seen with thyrotoxicosis stemming from etiologies other than Graves’ disease. In an exhaustive review of the literature, Burch and Wartofsky6 found an incidence of ophthalmopathy in patients with Graves’ disease of 10% to 25% if these nonspecific signs were excluded, and 30% to 45% if lid findings were included as diagnostic criteria. When intraocular pressure on upgaze or CT findings also were included, the incidence increased to nearly 70%. The most severe form of Graves’ ophthalmopathy with optic nerve involvement and visual impairment occurs in only 2% to 5% of patients with Graves’ disease.12,13
Effects of Genetics and Sex
The role of genetic predisposition and MHC antigen patterns in patients with Graves’ ophthalmopathy has been extensively studied, but remains poorly characterized.6 Ethnicity seems to play some role; Tellez and coworkers14 found that European patients with Graves’ disease were six times more likely to develop ophthalmopathy than Asian patients with Graves’ disease. More important are the influences of sex on the development of Graves’ disease and the associated ophthalmopathy. A strong 3 : 1 female-to-male preponderance exists for Graves’ disease,15,16 which decreases to about 2 : 1 for patients with Graves’ ophthalmopathy. Overall, male patients with Graves’ disease have a higher incidence of ophthalmopathy that is more severe and tends to develop later in life.6
Effects of Tobacco
Several studies have reported an increased incidence of goiter in tobacco smokers compared with nonsmokers, attributing this to thiocyanate, a known goitrogen that is present in inhaled tobacco smoke.17,18 Studies have also reported an association between smoking and the incidence and severity of Graves’ ophthalmopathy.14,19 This relationship was not found in patients with other forms of thyroid disease, and suggests the tobacco effects are specific to Graves’ disease. It is possible that the decrease in female preponderance in Graves’ ophthalmopathy, especially in the more severe forms, may be a reflection of the higher incidence of smoking among male patients, rather than a true sex difference.6
Effects of Thyroid Status
The role of thyroid hormonal status in the development and severity of Graves’ ophthalmopathy has been particularly difficult to ascertain because of the overlap of thyrotoxicosis with antithyroid therapy in these patients. Thyrotoxicosis alone is thought to have little direct effect on the autoimmune process. It serves as a poor marker for disease severity because the prevalence and course of hyperthyroidism correlate poorly with that of Graves’ ophthalmopathy.6 An improvement in eye status with maintenance of euthyroidism during antithyroid therapy may be more a reflection of improving immune function than a decreased circulating thyroid hormone.
Elevated circulating TSH levels seem to promote eye disease in patients with Graves’ disease. Hamilton and colleagues20 have reported an increased incidence of progressive ophthalmopathy with hypothyroidism, which followed antithyroid therapy. Tamaki and associates21 described a marked improvement in eye status and a reduction of circulating TRAb in two patients receiving thyroid hormone replacement during antithyroid therapy. The mechanism by which elevated TSH achieves its influence is unclear, but it may serve to up-regulate TSH receptors (apparent autoantigens; discussed later) in thyrocytes22 and possibly lymphocytes.23
Natural History
On summarizing the available literature on the natural history of Graves’ ophthalmopathy, Burch and Wartofsky6 noted the disease tended to progress through a phase of rapid progression (6 to 24 months), followed by a prolonged plateau phase with subsequent slow but incomplete regression of eye changes. Lid retraction and soft tissue changes, such as chemosis and eyelid edema, tended to be short-lived with improvement or resolution over 1 to 5 years (60% to 90%). Ophthalmoplegia resolved incompletely and less rapidly, although 30% to 40% of patients showed some improvement in ocular motility without specific therapy. Proptosis is the eye finding least likely to improve or resolve spontaneously (10%). Trobe24 reviewed 32 patients with untreated Graves’ optic neuropathy and found that vision improved spontaneously in most, but 21% had a final visual acuity of 20/100 or worse, with 5 patients progressing to near blindness. Facing potential loss of sight, it is not surprising that attempts at medical and surgical intervention, some of them heroic, have occurred for the past 80 years.
Clinical Features
Thyroid Disease
A patient with Graves’ ophthalmopathy most commonly visits an endocrinologist for management of thyroid disease or an ophthalmologist for evaluation of eye complaints. A thorough history, examination, and high level of suspicion are required to make the diagnosis. Classically, the patient is experiencing hyperthyroidism, and the expected hypermetabolic findings are present at the time of presentation with eye disease. In a review of more than 800 cases from the literature, Burch and Wartofsky6 found, however, that 20% of patients presented with eye disease before any manifestation of hyperthyroidism, 39% presented concurrently with thyroid disease and eye disease, and 41% presented with eye disease after clinical hyperthyroidism already was evident. In 80% of the patients in whom both diseases eventually manifested, both became clinically evident within 18 months of each other, although some patients may never present with thyroid disease and eye disease, or many years may separate the two presentations.
Pretibial Myxedema
Pretibial myxedema, or thyroid dermopathy, is the localized thickening of the skin, usually in the pretibial area. It occurs in 4.3% of patients with Graves’ disease, but at a higher rate (12% to 15%) in patients with Graves’ ophthalmopathy,25,26 and usually is a late manifestation. Conversely, almost all patients with pretibial myxedema have Graves’ ophthalmopathy, although the dermopathy may precede the ophthalmopathy. Symptomatic lesions consist of shiny, erythematous-to-brown plaques, nodules, or areas of nonpitting edema, which most commonly occur in the anterior or lateral aspects of the leg or at sites of old or recent trauma. Involvement of other body sites is rare. Almost all patients have high circulating levels of TRAbs, although the true pathogenesis of the dermopathy is not understood. Pretibial myxedema usually is of cosmetic importance only, but if the feet or hands become massively swollen, it can cause functional difficulties.
Graves’ Ophthalmopathy
As described previously, Graves’ disease occurs more commonly in women and over a broad age range (16 to 81 years), with a mean age in the fifth and sixth decades.27–29 Eye involvement is bilateral in most patients with Graves’ disease, although 5% to 14% of patients have unilateral disease depending on the method of detection.6 With careful testing (i.e., CT scan), 50% to 90% of these patients show changes in both eyes. In contrast, major asymmetry in the extent of eye involvement is common. Graves’ ophthalmopathy remains the most common etiology of “unilateral” proptosis in adults.30
Ophthalmopathy Classification
The spectrum of eye changes ranges from eyelid retraction (resulting in the appearance of a “stare”), to proptosis, corneal exposure and ulceration, diplopia, and loss of vision. A clinical classification system for eye involvement by Graves’ disease was proposed by Werner in 1969,31 approved by the American Thyroid Association (ATA), and subsequently modified in 1977.32 The ATA’s detailed classification is shown in Table 126-1. This classification is strictly clinical and has been helpful for reporting purposes. The disease does not progress systematically through the classes, and may skip one or more classes entirely. In addition, the classification system has been criticized for not considering disease activity (stable or rapidly progressing), which is crucial for making patient treatment decisions.33
Table 126-1 Detailed Classification of Eye Changes of Graves’ Disease
| Classes | Grades | Ocular Symptoms and Signs |
|---|---|---|
| 0 | No signs or symptoms | |
| I | Only signs, no symptoms (signs limited to upper lid retraction and stare, with or without lid lag and proptosis) | |
| II | Soft tissue involvement with symptoms and signs | |
| o | Absent | |
| a | Minimal | |
| b | Moderate | |
| c | Marked | |
| III | Proptosis ≥3 mm in excess of upper normal limit, with or without symptoms | |
| o | Absent | |
| a | 3- to 4-mm increase over upper normal | |
| b | 5- to 7-mm increase | |
| c | ≥8 mm increase | |
| IV | Extraocular muscle involvement (usually with diplopia, other symptoms or signs) | |
| o | Absent | |
| a | Limitation of motion at extremes of gaze | |
| b | Evident restriction of motion | |
| c | Fixation of a globe or globes | |
| V | Corneal involvement (primarily a result of lagophthalmos) | |
| o | Absent | |
| a | Stippling of cornea | |
| b | Ulceration | |
| c | Clouding, necrosis, perforation | |
| VI | Sight loss (caused by optic nerve involvement) | |
| o | Absent | |
| a | Disk pallor or choking, or visual field defect: vision 20/20 to 20/60 | |
| b | Same, but vision 20/70 to 20/200 | |
| c | Blindness (i.e., failure to perceive light, vision <20/200) |
In response to these deficiencies and several other proposed classification schemes, an international ad hoc committee representing the American, European, Asia-Oceanic, and Latin America Thyroid Associations recommended in 1992 a new characterization of Graves’ ophthalmopathy (Table 126-2). This system is recommended for use in attempting objective clinical assessment and documenting disease activity, as in clinical studies. The older ATA classification system is still used for educational purposes and clinical evaluation.
Table 126-2 Characterization of Graves’ Ophthalmopathy: Recommendations of an International Ad Hoc Committee*
| Category of Disease | Objective Criteria Monitored |
|---|---|
| Eyelid | Maximal lid fissure width |
| Upper lid to limbus distance | |
| Lower lid to limbus distance | |
| Cornea | Exposure keratitis assessed by rose bengal or fluorescein staining (indicate presence or absence) |
| Extraocular muscles | Single binocular vision in central 30 degrees of vision (indicate presence or absence, with or without prisms) |
| One or more of the following measurement techniques | |
| Maddox rod test | |
| Alternate cover test | |
| Hess chart measurements | |
| Lancaster red-green test | |
| Optional | |
| Intraocular pressure in downward gaze | |
| CT or MRI | |
| Proptosis | Exophthalmometer reading (CT or MRI measurement may also be used for measurement) |
| Optic nerve | Visual acuity |
| Visual fields | |
| Color vision | |
| Activity score | Sum of 1 point each for any of the following |
| Spontaneous retrobulbar pain | |
| Pain with eye movement | |
| Eyelid erythema | |
| Eyelid edema or swelling | |
| Conjunctival injection | |
| Chemosis | |
| Caruncle swelling | |
| Patient self-assessment | Satisfaction with the following (indicate change with therapy in each using a scale such as greatly improved, improved, unchanged, worse, much worse) |
| Appearance | |
| Visual acuity | |
| Eye discomfort | |
| Diplopia |
* Consensus of an 18-member ad hoc committee comprising representatives from the American, European, Asia-Oceanic, and Latin America Thyroid Associations.
From Classification of eye changes of Graves’ disease. Thyroid. 1992;2:235.
Eye Findings
Lid lag and the appearance of a stare are seen in the mildest form (ATA class I disease) of eye involvement by Graves’ disease. This involvement is thought to occur initially as the result of an increased sympathetic sensitivity to catecholamines as seen with patients with hyperthyroidism.34 As the disease progresses, and the lymphocytic inflammatory reaction infiltrates the extraocular muscles and orbital fat, the fibroblasts proliferate and deposit glycosaminoglycans, predominantly hyaluronic acid.35,36 The resulting muscle and fat enlargement combines with interstitial edema to cause an increase in intraocular pressure.
Intraocular pressure is increased in primary gaze (straight ahead) and even more so in upward gaze (supraduction). This increase in intraocular pressure can lead to a misdiagnosis of glaucoma, with a subsequent delay in appropriate therapy.28 Over time, increases in intraocular pressure also produce conjunctival chemosis, excessive lacrimation, periorbital edema, and photophobia (ATA class II disease).
As enlargement of orbital muscle and fat progresses, the volume of the orbital contents increases. The orbital cavity has four fixed bony walls with an average volume of 26 mL.37 In healthy individuals, the globe takes up 30% of this volume, with retrobulbar and peribulbar structures taking up the remaining 70% of the volume. With nowhere else to expand, an increase of only 4 mL in the volume of the orbital contents results in 6 mm of proptosis (ATA class III disease).
In its most severe form, Graves’ ophthalmopathy involves the optic nerve to impair vision (ATA class VI disease). Optic nerve involvement typically manifests as a painless gradual loss of visual acuity or visual field,12 although it can occur precipitously over days to weeks. Although originally thought to be caused by ischemia or venous congestion of the nerve as a result of increased intraocular pressure, there now is convincing evidence to support crowding and compression of the optic nerve at the orbital apex by the enlarged extraocular muscles as the etiology of nerve dysfunction.38
Optic nerve function is measured in several ways, one or all of which may be impaired. In one study of 31 patients with optic nerve involvement,28 visual acuity was 20/25 or worse in 100% of eyes; color vision was decreased in 64%; and visual fields were decreased in 70%, with inferior scotomata and cecocentral scotomata defects most common. Impaired visual fields or color vision also may be found in patients with normal visual acuity.27
Clinical Evaluation
Differential Diagnosis
Graves’ ophthalmopathy presents a spectrum of clinical manifestations that are reminiscent of other clinical entities. Eye changes range from minimal—requiring a detailed eye examination or CT scan for identification—to dramatic, disfiguring, and vision-threatening changes that eclipse the manifestations of the underlying thyroid disease. The high prevalence of asymmetric eye involvement may also lead the clinician to suspect a unilateral disease process, rather than a systemic one. Although the differential diagnosis for proptosis is extensive (Table 126-3), most other disease entities have only superficial similarities to Graves’ ophthalmopathy and can be quickly ruled out. Most importantly, the clinician should maintain a high degree of suspicion if the diagnosis of Graves’ ophthalmopathy is to be made in a timely fashion.
Thyroid Function
A full endocrinology workup is essential in the diagnosis and management of Graves’ disease. Laboratory testing should include thyroid function tests and a TSH level. In some apparently euthyroid patients, more detailed dynamic testing of thyroid function may be required to uncover thyroid dysfunction. These studies include the suppression of radioactive iodine uptake with triiodothyronine (T3) to assess for non–TSH-mediated thyroid stimulation, the thyrotropin-releasing hormone stimulation test to determine the presence of low-grade suppression of the hypothalamic-pituitary axis, and TSH stimulation tests of thyroid reserve. Serologic evaluation for evidence of thyroid autoimmunity also can be performed, including microsomal antibody, thyroglobulin antibody, and TRAb assays. Overall, with sufficient scrutiny, most, if not all, patients with euthyroid ophthalmopathy can be shown to have some degree of thyroid dysfunction.6
Imaging Studies
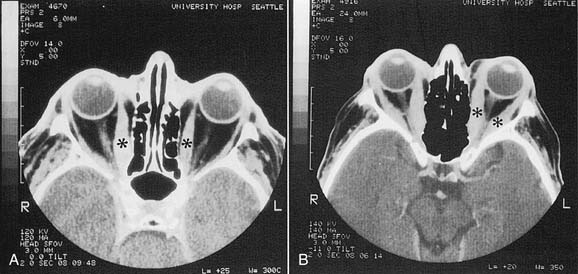
CT scans of the orbit can be helpful in the diagnosis of Graves’ ophthalmopathy in euthyroid patients, and are essential if surgical intervention is being considered. Typical findings include a twofold to eightfold enlargement of the extraocular muscle bodies, sparing the tendinous portions (Fig. 126-1). The changes are bilateral in 90% of patients, although asymmetry in the extent of involvement is the rule. The medial and inferior rectus muscles are involved most commonly, although any or all of the muscles may be enlarged.39 The orbital and extraocular muscle volume can be estimated using CT images.40–42 Although estimates of extraocular muscle volume have correlated with the presence of optic neuropathy, subsequent studies have not found a correlation between muscle volume estimates and the severity of optic neuropathy or the effectiveness of decompression,28,43 limiting the usefulness of these estimates.
Ultrasound (orbital echography) has been reported to be reliable and effective in evaluating extraocular muscle size.44 Although not as beneficial in the initial diagnosis and surgical planning as CT scan in patients with Graves’ disease, it is proposed as an inexpensive, noninvasive method for monitoring response to therapy (steroids or radiation).
Magnetic resonance imaging (MRI) of the orbits has proved excellent for the evaluation of the soft tissues of the orbit. Extraocular muscle volume can be readily estimated from a single coronal MRI section.45 Studies have suggested that T2-weighted MR images may provide a sensitive measure of active inflammation in the orbit, giving much needed information on disease activity.46,47 MRI provides little detail of the bony anatomy of the orbit, which is required if surgical intervention is being considered.
Nuclear medicine studies, such as single photon emission computed tomography (SPECT) imaging using technetium 99m diethylenetriaminepentaacetic acid and gallium 67, have also been used to image the orbit. These studies give reliable information about the level of disease activity within the extraocular muscles.48,49
Management of Graves’ Ophthalmopathy
A multispecialty team approach for the treatment of patients with Graves’ disease and Graves’ ophthalmopathy is recommended because of the multiple organ systems involved and the various diagnostic and therapeutic modalities needed to provide optimal care of this complex entity. Team members from endocrinology, radiology, nuclear medicine, radiotherapy, ophthalmology, otolaryngology, head and neck surgery, and neurosurgery are involved to varying degrees. A coordinating member of the team (usually the endocrinologist for Graves’ disease and the ophthalmologist for Graves’ ophthalmopathy) is crucial for maintaining records, tracking disease progression, and providing continuity of care for long periods.50
Medical Therapy
Thyroid Therapy
The management of the thyroid in patients with Graves’ disease is controversial, and a complete discussion is beyond the scope of this chapter. The impact of thyroid management on Graves’ ophthalmopathy is only now becoming clear. As previously discussed, the correlation between the severity of hyperthyroidism and the prevalence or course of Graves’ ophthalmopathy is poor at best. For patients with thyrotoxicosis, the thyroid may be managed with antithyroid drugs (i.e., propylthiouracil and methimazole), radioactive iodine ablation (iodine 131), or thyroidectomy (usually subtotal). Reports on the effect of each of these modalities on the development or progression of ophthalmopathy are conflicting in the literature, although in a more recent prospective study, Tallstedt and colleagues51 randomly assigned 168 patients into groups receiving antithyroid drugs, radioactive iodine ablation, or surgery. There was no difference in the rate of new-onset or progression of existing ophthalmopathy between the antithyroid drug and surgery groups (10% to 16%), but the radioactive iodine ablation group showed a higher incidence (33%) of these problems.
One possible mechanism for the increase in patients with ophthalmopathy receiving radioactive iodine ablation includes transient hypothyroidism and the release of thyroid antigens, promoting an acceleration of the autoimmune process. In support of this hypothesis, investigators have found that TRAb levels briefly decrease and then show a sustained increase after iodine 131 ablation for Graves’ disease.52 After thyroidectomy, however, TRAb levels showed a transient increase followed by a gradual decrease.53 Potentially least concerning were TRAb levels, which gradually declined after antithyroid therapy alone. It would seem that radioactive iodine ablation of the thyroid may not be the best option for the management of thyrotoxicosis in patients with Graves’ disease. As mentioned previously, thyroid hormone replacement and suppression during antithyroid drug therapy or after radioactive iodine or surgical ablation (before the onset of measurable hypothyroidism) seem to result in a decrease in the prevalence or progression of ophthalmopathy in patients with Graves’ disease and should be considered.21
Steroid Therapy
High-dose corticosteroids (prednisone, 80 to 100 mg/day) are commonly used as the first-line management of more severe Graves’ ophthalmopathy. The administration of high doses is maintained for 2 to 4 weeks, followed by a slow taper over several months. Patients experience rapid relief of pain, erythema, and conjunctival edema, and improved vision. Trobe and associates13 reported a 48% success rate after 2 months of therapy. Steroids may be only temporizing, however, with recurrence of visual loss on taper. Improvement in proptosis and ophthalmoplegia may also be seen with corticosteroid therapy, but generally less so, and these conditions are more likely to recur on steroid withdrawal. The multiple adverse effects of steroid therapy are well known and include glucose intolerance, weight gain, psychosis, peptic ulcer disease, and osteoporosis with vertebral fracture. Corticosteroid therapy should be considered temporizing, while either regression and stabilization of disease or definitive therapy is awaited.
Immunosuppression with cyclosporine also has been evaluated for patients with Graves’ ophthalmopathy and has been found to be less effective than prednisone in single-agent therapy.6 The beneficial effects of both drugs seem to be additive, and maintenance cyclosporine therapy may be corticosteroid-sparing. Careful drug-level monitoring is necessary to avoid nephrotoxicity. Other common adverse effects include hypertension, liver enzyme elevation, gum hypertrophy, and paresthesias. Plasmapheresis to remove circulating antibodies and other immunomodulatory drugs have also been used, but the results suggest further evaluation before widespread use.
Radiotherapy
Radiotherapy to the orbit has been used for more than 85 years for patients with Graves’ ophthalmopathy. Traditionally, radiotherapy is accomplished by delivering 20 Gy in 10 fractions of 2 Gy over 2 weeks. The fractions are delivered to a field just behind the lateral canthus to spare the cornea and lens. In a 1989 review of the literature, Sautter-Bihl and Heinze54 found an overall good to excellent response in 35% to 92% of patients, and improvement of impaired visual acuity in 33% to 85% of patients treated with orbital radiation. The mechanism of action of radiation to the orbit is thought to be in large part an effect on the lymphocytes infiltrating the orbital muscles and fat during the inciting stages of the disease. Patients treated early in the course of the disease with pronounced soft tissue involvement are most likely to benefit. Proptosis, ophthalmoplegia, and optic neuropathy are less responsive, and patients with long-standing stable disease are unlikely to benefit. The portion of the radiation dose delivered to the lens is less than 5% and has not been reported to be harmful.
More recent studies have cast considerable doubt on the efficacy of radiotherapy for Graves’ ophthalmopathy. In 2003, Gerling and colleagues55 found no difference in the documented improvement when patients were treated with very low dose radiotherapy (2.4 Gy) and more standard doses (16 Gy). In another study, improvement in symptoms and clinical findings was not different between patients treated with steroids alone compared with patients treated with steroids plus 24 Gy of radiotherapy.56 In a prospective randomized trial treating one orbit of each patient with 20 Gy of radiotherapy, Gorman and coworkers57 were unable to show any significant benefit over the nontreated orbit at 1 year. Long-term follow-up in an uncontrolled fashion also found no benefit from the radiotherapy.58 Because radiotherapy has potential long-term adverse effects, its usefulness in Graves’ ophthalmopathy should be questioned.
Surgical Therapy
Indications for surgical decompression of the orbit have evolved over the years and have included cosmetic management of proptosis, lid lag, and stare, and the management of optic neuropathy.27,34,59,60 New-onset strabismus after orbital decompression has been reported in 30% to 64% of patients, suggesting that patient selection should be done with caution.2,60 As a result, many centers perform orbital decompression primarily in the setting of optic nerve involvement combined with failure to respond or inability to tolerate steroid therapy, or for the relapse of symptoms with the taper of steroid therapy.12,28 Radiotherapy is generally reserved for patients who refuse, cannot tolerate, or fail surgical decompression, although some authors still advocate primary radiotherapy.61,62
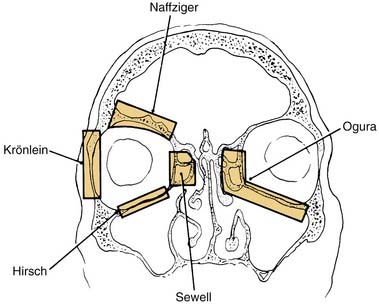
The goal of surgical decompression of the orbit is simply to expand the bony orbital confines to make room for the increased volume of the orbital contents. Surgical decompression was first described by Dollinger,63 who advocated removal of the lateral orbital wall for decompression into the temporal fossa (Krönlein’s procedure). Twenty years later, Naffziger64 reported removal of the orbital roof with decompression into the anterior cranial fossa via a transcranial approach. Decompression into the paranasal sinuses was first advocated by Sewell,65 who described decompression into the ethmoid air cells, and Hirsch,66 who later reported inferior decompression into the maxillary sinus by removal of the orbital floor. Walsh and Ogura67 combined the inferior and medial approaches into a single transantral decompression of two orbital walls using the Caldwell-Luc approach (Fig. 126-2). This approach is extracranial, decompresses two walls of the orbit into the largest empty space, and allows gravity to aid in the expansion of orbital contents into the paranasal sinuses. For these reasons, it has become the most widely used technique.
Predicting the results of transantral decompression in patients with severe Graves’ ophthalmopathy remains a challenge. In a retrospective analysis of 428 patients, Fatourechi and colleagues68 reported that young men with long-standing eye symptoms were likely to have more severe initial proptosis. Only the severity of the initial proptosis and the longer postoperative follow-up period correlated with greater recession of proptosis after decompression. Failure of corticosteroid or orbital radiotherapy did not affect the degree of recession of proptosis or improvement in visual acuity. A greater degree of recession of proptosis postoperatively was associated with better visual acuity, but also with a greater likelihood of persistent strabismus. Patient satisfaction with postoperative eye appearance was associated only with procedures performed for cosmetic indications.
Transantral Orbital Decompression
SURGICAL TECHNIQUE
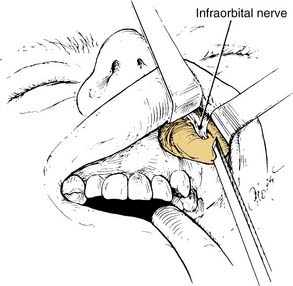
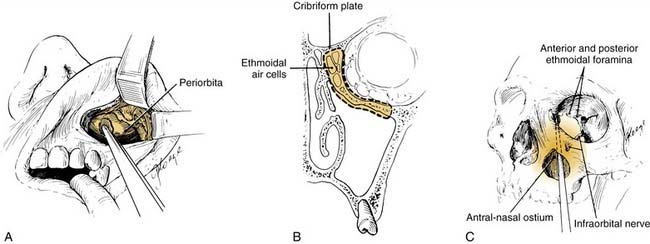
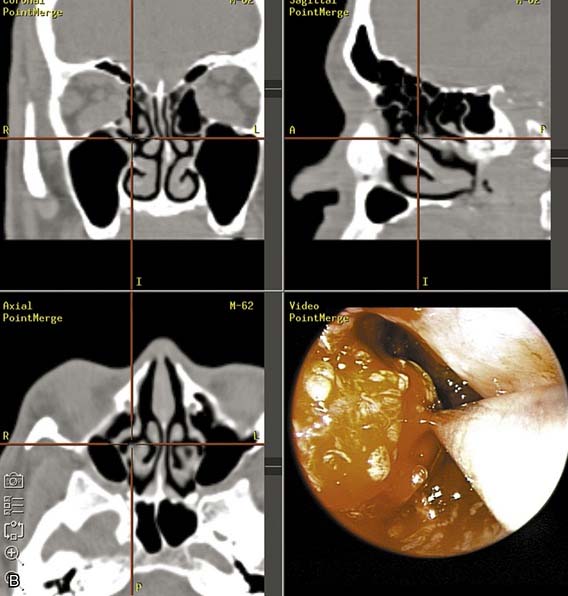
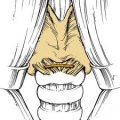
The transantral decompression (Walsh-Ogura method) of the orbital floor and medial wall is performed during general anesthesia with an oral Ray endotracheal tube secured in place. The eyes are protected with topical ointment and are not taped to allow examination of the pupil during the procedure. Intravenous broad-spectrum antibiotics and high-dose corticosteroids (dexamethasone, 8 to 10 mg) are administered before the surgery. A curved sublabial incision with a standard Caldwell-Luc antrostomy and ethmoidectomy is performed (Fig. 126-3). Care is taken to identify and preserve the inferior orbital nerve in its bony canal. An extensive ethmoidectomy should be performed, while preserving the lamina papyracea, middle turbinate insertion, and fovea ethmoidalis (Fig. 126-4). The remaining mucosa is carefully stripped from the maxillary sinus roof, keeping in mind that the inferior orbital nerve is partially or completely dehiscent in 29% of patients.69
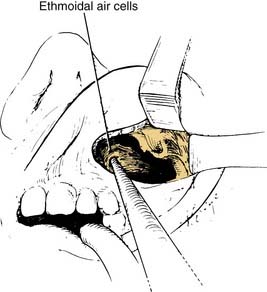
Figure 126-4. Ethmoidal cells are entered with a curette and subsequently removed by curettage and bone-biting forceps.
The bone of the maxillary sinus roof (orbital floor) medial to the infraorbital nerve is carefully removed (Fig. 126-5). This maneuver can be performed using a drill with cutting or diamond burrs or with small osteotomes, as long as the underlying periorbita is not violated. Some surgeons find the operating microscope useful at this stage of the procedure. The lamina papyracea (medial orbital wall) is gently fractured medially and removed up to the posterior ethmoid neurovascular bundle, preserving the periorbita. A No. 12 scalpel blade is used to make incisions in the posterior-to-anterior direction through the periorbital fascia, which allows the immediate herniation of orbital fat through the incision and into the sinuses (Fig. 126-6). This process is begun medial and superior to avoid the loss of visualization as the fat herniates from the orbit. The number of incisions required is determined intraoperatively by assessing the degree of residual proptosis after each incision.
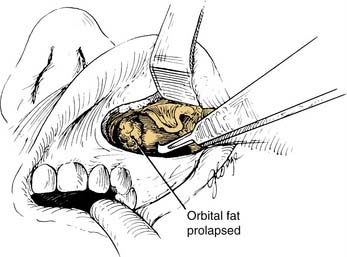
Figure 126-6. Radial incisions in the intact periorbita with a scalpel, permitting prolapse of orbital fat into the sinuses.
Calcaterra and Thompson34 recommended operating on the more severe eye first with planned incomplete recession because an additional 1 to 2 mm of recession develops during the first 3 months after surgery. The less severe eye then is decompressed to match the position of the first eye. Four to six incisions in the periorbita usually are adequate.70 Finally, a large nasoantral window is created, and the sinus is packed with a large Penrose drain coated in antibiotic ointment, which is brought out through the nose for removal the next day. The sublabial incision is closed with absorbable suture. The patient is given antibiotics and corticosteroids intravenously overnight and then orally after discharge from the hospital.
Immediately after surgery, all patients (unless it is medically contraindicated) are given high-dose corticosteroids with a slow taper. The rate of steroid taper is determined by the clinical response to the surgery. In one study, steroid use was discontinued in 80% of patients within 2 months of surgery.28
SURGICAL OUTCOME
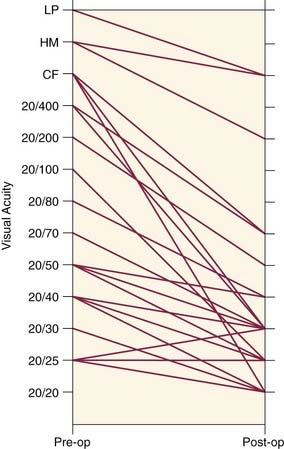
The results of transantral orbital decompression surgery depend on the indications for the operation. For patients with optic neuropathy, Walsh-Ogura decompression was effective in improving vision in 92% of patients (Fig. 126-7). Equally important, these patients had stabilization of disease with successful taper of steroid therapy. Major improvement in proptosis of 1 to 12 mm can be achieved (Fig. 126-8A), with an average improvement of 3.4 to 5.3 mm.28,45,51,60 This decrease in proptosis is of functional and cosmetic benefit and is not seen with either medical therapy or radiotherapy. Major reduction in intraocular pressure (100% of patients) (Fig. 126-8B), improvement of extraocular motility (36%), and improvement of strabismus (47%) are other benefits of decompression.
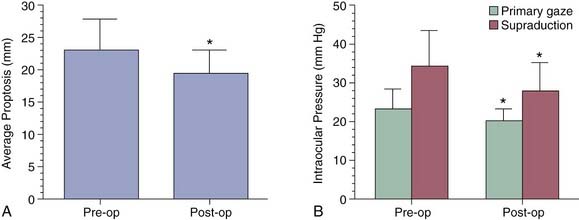
Figure 126-8. A, Preoperative (left) and postoperative (right) average proptosis for 36 eyes undergoing decompression (see Fig. 126-7). B, Intraocular pressure in the same eyes in primary gaze and upward gaze before and after decompression surgery. *P < .05.
Transantral decompression failed to stabilize the disease in 3% to 8% of patients who underwent surgery for severe ophthalmopathy.28,60 In this setting, repeat CT scanning with coronal cuts can be helpful to assess the adequacy of the decompression, and to decide between further surgery using the same approach or via a lateral or superior approach,71 or proceeding with radiotherapy.
The earlier the diagnosis of Graves’ optic neuropathy is made and intervention initiated, the better the outcome will be. When preoperative visual acuity was limited to detecting hand motion or worse, decompression provided little improvement in vision (see Fig. 126-7).28 When visual acuity allowed counting fingers or better, all patients experienced a favorable outcome.
Ocular dysmotility and strabismus remain a major problem in surgical patients despite decompression. Extraocular motility was impaired (primarily in supraduction) in 92% of patients undergoing decompression; only one third showed improvement, and 9% experienced a worsening of motility postoperatively.28 Strabismus (diplopia) was present preoperatively in 67% to 85% of patients and in 71% to 80% of patients postoperatively.28,60 When the eye disease was stable, 70% of patients underwent extraocular muscle surgery to correct diplopia, and some were able to be corrected with prisms alone. One long-term follow-up evaluation (average 8.8 years) of 355 patients with decompression reported only 17% having double vision most or all of the time.60
Transantral decompression for cosmetic indications should be evaluated carefully because it carries the risk of lower patient acceptance of adverse effects than when vision is threatened. Fatourechi and colleagues59 reviewed the outcomes of 34 patients who underwent transantral decompression for cosmesis and noted a dramatic improvement in proptosis (average 5.2 mm), but no major change in asymmetry between the eyes. Postoperative diplopia developed in 73% of patients who did not have diplopia preoperatively. Half of the patients underwent subsequent extraocular muscle surgery and eyelid surgery. On long-term follow-up evaluation (average 12 years after surgery) of 29 patients, 82% reported they were satisfied with their current eye status. Decompression surgery for cosmetic indications may be appropriate in a select group of patients who are willing to undergo subsequent eye muscle surgery for diplopia.
SURGICAL COMPLICATIONS
Patients uniformly complain of hypoesthesia in the distribution of the inferior orbital nerve immediately after decompression as a result of intraoperative stretching of the nerve with retraction. This hypoesthesia resolves spontaneously over several months in greater than 95% of patients. In one large series, Garrity and associates60 reported other infrequent complications, including sinusitis (4%), lower eyelid entropion (9%), cerebrospinal fluid leaks (3%), and frontal lobe hematoma (0.2%). Blindness from nerve injury or orbital hemorrhage is rare, but does occur.48,72 Although theoretically possible, orbital infection has not been reported.
Modifications of Transantral Orbital Decompression
Transantral orbital decompression has held up well to the test of time since its description in 1957. Only in the past several years have advances in surgical technique and instrumentation led to the description of major modifications to the Walsh-Ogura operation. A transorbital approach using a modified blepharoplasty (subciliary) incision provides excellent exposure to the anterior orbital floor and decreases the risk to the infraorbital nerve.12 This approach also provides access for a lateral orbital wall decompression without disrupting the orbital rim or canthal tendon, providing a three-wall decompression.73 This method is reported to have a lower incidence of postoperative strabismus, although the reason for this is unclear. Other authors have reported using the transconjunctival approach for the antral-ethmoidal decompression.72,74 The major criticism of these anterior approaches is limited exposure to the medial orbit, which can restrict the degree of decompression around the orbital apex as is required in patients with optic neuropathy.
Endoscopic Orbital Decompression
With the rapid development and dissemination of endoscopic instrumentation and techniques for transnasal paranasal sinus surgery, it is not surprising that this technology has been applied to include antral-ethmoidal decompression of the orbit. Despite concerns about adequate anterior orbital floor access, Kennedy and coworkers75 reported equivalent results with transantral decompression and endoscopic decompression. Reports of an average of 3.2- to 5.1-mm reduction of proptosis with an endoscopic decompression of the medial and inferior orbital walls compare well with the traditional approach.76–78 Metson and Samaha77 reported a modification to the standard endoscopic decompression that preserves a horizontal sling of periorbita to support the globe position without limiting the degree of decompression. This modification has resulted in a significant decrease in the incidence of new-onset or worsening diplopia after surgery.
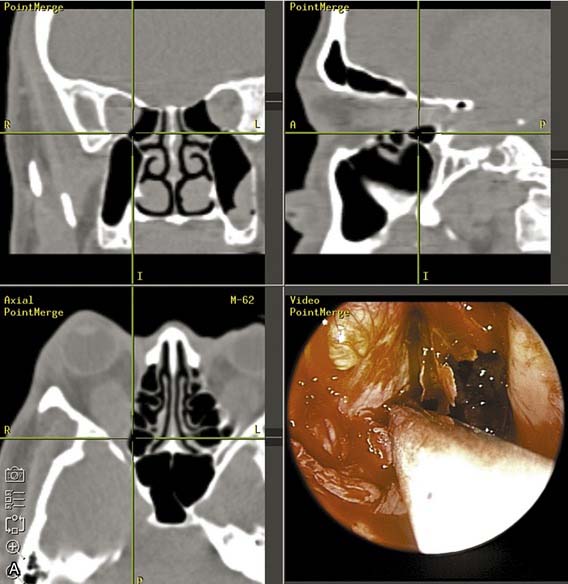
Advances in image-guided surgery have resulted in improved intraoperative localization during endoscopic sinus surgery. This technology has found a role in endoscopic orbital decompression surgery as well. The use of the CT-guided technology allows a safer and more aggressive decompression of the orbital apex, orbital nerve (if indicated), and superior medial orbital wall. The inferior orbital nerve also can be easily identified and preserved. Equally helpful is the ability to assess and measure the degree of orbital content decompression into the sinuses intraoperatively using measurements on the CT scans (Fig. 126-9).
Combined Endoscopic and Open Approach to Orbital Decompression
For a more thorough optic nerve decompression, Khan and associates79 have described a combined transconjunctival-endoscopic decompression of the orbit. The addition of a small medial external skin incision also aided the retraction of the orbital contents to allow the dissection of the medial wall beyond the posterior ethmoidal neurovascular bundle up to the optic canal. Experience with 72 orbital decompressions in 41 patients resulted in an average reduction of proptosis of 3.65 mm, an 89% improvement in vision, and minimal complications.80 Other authors have combined the endoscopic orbital decompression with a percutaneous lateral orbital wall decompression via the upper lid crease resulting in additional decompression (≤6.9 mm).76,81 In addition, Metson and colleagues82 have successfully performed combined endoscopic and lateral wall decompressions during local anesthesia, which has the advantage of avoiding general anesthesia and permitting intraoperative monitoring of visual function, crucial for surgery on an only-seeing eye. In this setting, if both eyes are to be decompressed, they are done so in stages.
Balanced Orbital Decompression
In an attempt to reduce the incidence of new-onset diplopia after orbital decompression surgery, several authors have advocated removing the medial and lateral walls of the orbit without decompressing the orbital floor. This “balanced” approach decompresses equally medially and laterally and is less likely to affect the position of the globe.81,83,84 Postoperative new-onset diplopia is reported in 0% to 15% of patients with this technique. The degree of decompression with this two-wall approach can be comparable to the three-wall medial-inferior-lateral decompression if orbital fat is resected at the time of the decompression.81
Combined Endoscopic Orbital and Optic Nerve Decompression
A more recent adjunct to standard endoscopic orbital decompression for dysthyroid ophthalmopathy is endoscopic optic nerve decompression. In certain cases of thyroid ophthalmopathy with progressive optic neuropathy, it has been hypothesized that direct compression of the optic nerve is the cause of visual deterioration. In a study on patients with optic neuropathy, analysis of CT scans showed that patients have increased apical crowding with flattening of the optic nerve at or near the orbital apex from compression by enlarged extraocular muscles compared with controls.38 The compressive forces are thought to result in conduction block and focal demyelination, as the optic nerve remains in its fixed bony canal. Release of the nerve from its bony confines may alleviate the detrimental effects of the compressive forces.85
Previously, surgical approaches for optic nerve decompression were developed for traumatic optic neuropathy and have included transorbital, extranasal transethmoid, transantral, intranasal microscopic, and craniotomy approaches. Advances in endoscopic equipment, in addition to curved diamond burrs and image guidance systems, have allowed for safe endoscopic optic nerve decompression all the way back to the orbital apex. After a standard sphenoethmoidectomy is performed, the sphenoid face is widely opened, and the carotid artery and optic canal bulge are identified on the lateral sphenoid wall. The medial orbital wall is decompressed, and the thick bone overlying the optic canal is thinned with a diamond burr with careful avoidance of the prominence overlying the carotid artery. A microcurette is used to fracture the bone medially and allow for decompression of the optic nerve from its bony canal. Preliminary studies have not shown whether the additional incisure of the optic nerve sheath is necessary for adequate decompression considering the possible damage to optic nerve fibers and the ophthalmic artery, and cerebrospinal fluid leak.85,86
Reports of this technique are very preliminary in the literature. Luxenberger and colleagues86 described four patients in whom a combined endoscopic orbital and optic nerve decompression was performed. In that study, two patients had improved vision, one patient’s vision improved and then deteriorated, and the final patient did not have sufficient ophthalmologic data. In a more recent review of this new technique combining endoscopic optic nerve decompression with standard endoscopic orbital decompression, Pletcher and associates85 noted an improvement in visual acuity in 8 of 10 patients with nontraumatic optic neuropathy, including Graves’ ophthalmopathy.
Other Approaches for Orbital Decompression
A modification of the Walsh-Ogura method called the three-wall orbital decompression refers to the addition of the lateral wall decompression to the standard antral-ethmoidal decompression. This addition can be achieved by any combination of approaches, including a modified blepharoplasty incision, a sublabial or transconjunctival incision plus an anterior orbitotomy, or a bicoronal forehead flap. The advantage of the three-wall decompression is that it allows the further enlargement of the orbital volume by expansion into the temporal fossa and the paranasal sinuses. Metson and colleagues76 were able to increase the recession of proptosis from an average of 3.2 to 5.6 mm with the addition of the lateral decompression to the endoscopic antral-ethmoid decompression. Other authors87,88 prefer the bicoronal approach to the three-wall decompression, even for unilateral decompressions. Although dramatic decompression is reported (average 7.5 mm), a high complication rate can be expected, including infraorbital hypoesthesia (70%), supraorbital hypoesthesia (60%), and frontalis palsy (30%).87
A two-wall decompression of the superior-lateral orbit can be achieved through a bicoronal incision with subsequent frontal craniotomy64,89 or a lateral rim approach.90 This approach allows decompression of the orbital contents into the anterior cranial fossa and temporal fossa. Expansion of orbital contents into the empty paranasal sinuses with the help of gravity would be predicted to provide a more adequate decompression, although in a cadaveric study, Stanley and coworkers91 found the superior-lateral decompression to be as effective as the antral-ethmoidal decompression in withstanding intraocular pressure increases and in allowing orbital recession. The craniotomy approach has the advantages of direct access to the optic canal for superior decompression and of potential additional medial orbital wall decompression via the ethmoid sinuses. The orbital rim approach has the advantage of less dural exposure and the ability to advance the superior orbital rim with plates, screws, and bone grafts to increase orbital volume further.90 Because the superior-lateral decompression is a more involved procedure with the violation of the cranial cavity, regardless of the approach, most centers reserve it for patients in whom antral-ethmoidal decompression has failed.
Classic descriptions of orbital decompression have always referred to the removal of one or more orbital bony walls. Trokel and associates62 have reported successful decompression of the orbit strictly by the removal of orbital fat via superior and inferior orbitotomies without any bone removal. This procedure was reserved primarily for cosmetic indications in patients with inactive disease. An average reduction in proptosis of 1.8 mm was achieved, although patients with large amounts of fat seen by CT scan and with greater preoperative proptosis had more dramatic reductions. There were no new long-term motility problems commonly reported with bony orbital decompression for cosmesis, which may be an important decompression option for cosmetic indications in an attempt to reduce postoperative strabismus and the need for subsequent eye muscle surgery.
Ancillary Procedures
Extraocular Muscle Surgery
Extraocular motility problems resulting from Graves’ ophthalmopathy are not likely to resolve spontaneously with disease regression and are mostly refractory to medical management. Extraocular motility problems are also the manifestation of eye involvement most likely to develop or worsen after orbital decompression.92 Although disturbing, this is not surprising because irreversible extraocular muscle fibrosis is seen late in the disease. In one study, 70% of patients with decompression ultimately underwent eye muscle surgery for strabismus after the eye disease was stable.60
The indications for eye muscle surgery are diplopia in the primary and reading positions, with the goal being to restore single vision in these positions. It is unlikely that eye muscle surgery would achieve single vision in all positions because of the baseline restrictive ophthalmoplegia induced by the disease. Dyer93 reviewed 290 patients with Graves’ ophthalmopathy requiring eye muscle surgery, and found 59% required a single procedure, 30% required two procedures, and 12% required three or more procedures to achieve single vision in the primary and reading positions.
The timing of eye muscle surgery is crucial for predictable results. Patients not requiring orbital decompression for Graves’ ophthalmopathy should manifest stable disease with corticosteroid administration for 6 months to avoid subtle changes in the muscles over time. If these patients have serious proptosis, orbital decompression should be considered before eye muscle surgery, which may exacerbate the proptosis and corneal problems.93 In addition, a decompression is likely to alter the position of the globes and disturb the gaze, and it should be completed before eye muscle surgery. Because recession of the globe is progressive for several months after an orbital decompression, it is advisable to delay eye muscle surgery for 2 to 3 months.
Eyelid Surgery
Surgery on the eyelids may be required early or late in the management of Graves’ ophthalmopathy. Early in the disease, a lateral tarsorrhaphy may be needed urgently to provide corneal coverage and protection as a temporizing measure while medical therapy is instituted. Late in the course of the disease, eyelid surgery may be indicated for cosmesis and corneal protection in patients with permanent lid retraction. Multiple upper eyelid procedures have been described, including excision or recession of Müller’s muscle, levator aponeurosis transection or recession with or without scleral grafts, and levator myotomy.6 Similar procedures have been described for the lower lid. In the overall rehabilitation of the eye, eyelid surgery should be delayed for 1 year after control of hyperthyroidism and at least 6 months after eye disease stabilizes.
Burch HB, Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747.
Girod DA, Orcutt JC, Cummings CW. Orbital decompression for the preservation of vision in Graves’ ophthalmopathy. Arch Otolaryngol Head Neck Surg. 1993;119:229.
Calcaterra TC, Thompson JW. Antral-ethmoidal decompression of the orbit in Graves’ disease: 10-year experience. Laryngoscope. 1980;90:1941.
Fatourechi V, Bergstralh EJ, Garrity JA, et al. Predictors of response to transantral orbital decompression in severe Graves’ ophthalmopathy. Mayo Clin Proc. 1994;69:841.
Forbes G, Gorman CA, Brennan MD, et al. Ophthalmopathy of Graves’ disease: computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol. 1986;7:651.
Garrity JA, Fatourechi V, Bergstralh EJ, et al. Results of transantral orbital decompression in 428 patients with severe Graves’ ophthalmopathy. Am J Ophthalmol. 1993;116:533.
Gorman CA, Garrity JA, Fatourechi V, et al. The aftermath of orbital radiotherapy for grave ophthalmopathy. Ophthalmology. 2002;109(11):2100.
Hallin ES, Feldon SE, Luttrell J. Graves’ ophthalmopathy, III: effect of transantral orbital decompression on optic neuropathy. Br J Ophthalmol. 1988;72:683.
Hirsch O. Surgical decompression for malignant exophthalmosis. Arch Otolaryngol Head Neck Surg. 1950;51:325.
Kennedy DW, Goodstein ML, Miller NR, et al. Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg. 1990;116:275.
Khan JA, Wagner DV, Tiojanco JK, et al. Combined transconjunctival and external approach for endoscopic orbital apex decompression in Graves’ disease. Laryngoscope. 1995;105:203.
Lindberg JV, Anderson RL. Transorbital decompression: indications and results. Arch Ophthalmol. 1981;99:113.
MacCarty CS, Kenefick TP, McConahey WM, et al. Ophthalmopathy of Graves’ disease treated by removal of roof, lateral walls, and lateral sphenoid ridge: review of 46 cases. Mayo Clin Proc. 1970;45:488.
Metson R, Shore JW, Gliklich RE, et al. Endoscopic orbital decompression under local anesthesia. Otolaryngol Head Neck Surg. 1995;113:661.
Metson R, Dallow RL, Shore JW. Endoscopic orbital decompression. Laryngoscope. 1994;104:950.
Metson R, Samaha M. Reduction of diplopia following endoscopic orbital decompression: the orbital sling technique. Laryngoscope. 2002;112:1753.
Naffziger H. Progressive exophthalmos following thyroidectomy: its pathology and treatment. Ann Surg. 1931;94:582.
Pletcher SD, Sindwani R, Metson R. Endoscopic orbital and optic nerve decompression. Otolaryngol Clin North Am. 2006;39:943.
Schaefer SD, Soliemanzadeh P, Della Rocca DA. Endoscopic and transconjunctival orbital decompression for thyroid-related orbital apex compression. Laryngoscope. 2003;113:508.
Shorr N, Neuhaus RW, Baylis HA. Ocular motility problems after orbital decompression for dysthyroid ophthalmopathy. Ophthalmology. 1982;89:323.
Trokel S, Kazim M, Moore S. Orbital fat removal: decompression for Graves’ orbitopathy. Ophthalmology. 1993;100:674.
Unal M, Leri F, Konuk O, et al. Balanced orbital decompression combined with removal in Graves’ ophthalmopathy: do we really need to remove the third wall? Ophthalm Plast Reconstr Surg. 2003;19:112.
Walsh TE, Ogura JH. Transantral orbital decompression for malignant exophthalmos. Laryngoscope. 1957;67:544.
Weisman RA, Osguthorpe JD. Orbital decompression in Graves’ disease. Arch Otolaryngol Head Neck Surg. 1994;120:831.
Werner SC. Modification of the classification of the eye changes of Graves’ disease: recommendations of the ad hoc committee of the American Thyroid Association. J Clin Endocrinol Metab. 1977;44:203.
1. Kosugi S, Akamizu T, Takai O, et al. The extracellular domain of the TSH receptor has an immunogenic epitope reactive with Graves’ sera but unrelated to receptor function as well as epitopes having different roles for high affinity TSH binding, the activity of thyroid stimulating antibodies. Thyroid. 1991;1:321.
2. Nagy EV, Burch HB, Mahoney K, et al. Graves’ IgG recognizes linear epitopes in the human thyrotropin receptor. Biochem Biophys Res Commun. 1992;188:28.
3. Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocrinology. 1988;9:106.
4. Bahn RS, Gorman CA, Woloschak GE, et al. Human retroocular fibroblasts in vitro: a model for the study of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1987;65:665.
5. Bahn RS, Heufelder AE. Retroocular fibroblasts: important effector cells in Graves’ ophthalmopathy. Thyroid. 1992;2:89.
6. Burch HB, Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747.
7. Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;165:575.
8. Hufnagel TJ, Hickey WF, Cobbs WH, et al. Immunohistochemical, ultrastructural studies of the exenterated orbital tissues of a patient with Graves’ disease. Ophthalmology. 1984;91:1411.
9. Kodama K, Sikorska H, Bandy-Dafoe P, et al. Demonstration of a circulating autoantibody against soluble eye-muscle antigen in Graves’ ophthalmopathy. Lancet. 1982;2:1353.
10. Heufelder AE, Bahn RS. Modulation of Graves’ orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Invest Ophthalmol Vis Sci. 1994;35:120.
11. Grubeck-Loebenstein B, Trieb K, Sztankay A, et al. Retrobulbar cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognized autologous fibroblasts. J Clin Invest. 1994;93:2738.
12. Lindberg JV, Anderson RL. Transorbital decompression: indications and results. Arch Ophthalmol. 1981;99:113.
13. Trobe JD, Glaser JS, Laflamme P. Dysthyroid optic neuropathy: clinical profile, rationale for management. Arch Ophthalmol. 1978;96:1199.
14. Tellez M, Cooper J, Edmonds C. Graves’ ophthalmopathy in relation to cigarette smoking and ethnic origin. Clin Endocr (Oxf). 1992;36:291.
15. Marcocci C, Bartalena L, Bogazzi F, et al. Studies on the occurrence of ophthalmopathy in Graves’ disease. Acta Endocr (Copenh). 1989;120:473.
16. Teng CS, Smith BR, Clayton B, et al. Thyroid-stimulating immunoglobulins in ophthalmic Graves’ disease. Clin Endocr (Oxf). 1977;6:207.
17. Christensen SB, Ericsson UB, Janzon L, et al. Influence of cigarette smoking on goiter formation, thyroglobulin, and thyroid hormone levels in women. J Clin Endocrinol Metab. 1984;58:615.
18. Ericsson UB, Lindgarde F. Effects of cigarette smoking on thyroid function, the prevalence of goitre, thyrotoxicosis and autoimmune thyroiditis. J Clin Endocrinol Metab. 1991;229:67.
19. Bartalena L, Martino E, Marcocci C, et al. More on smoking habits and Graves’ ophthalmopathy. J Endocrinol Invest. 1989;12:733.
20. Hamilton RD, et al. Ophthalmopathy of Graves’ disease: a comparison between patients treated surgically and patients treated with radioiodine. Mayo Clin Proc. 1967;42:812.
21. Tamaki H, Amino N, Iwatani Y, Miyai K, et al. Improvement of infiltrative ophthalmopathy in parallel with a decrease of thyroid-stimulating antibody activity in two patients with hypothyroid Graves’ disease. J Endocr Invest. 1989;12:47.
22. Huber GK, Concepcion ES, Graves PN, Davies TF, et al. Positive regulation of human thyrotropin receptor mRNA by thyrotropin. J Clin Endocrinol Metab. 1991;72:1394.
23. Francis T, Burch HB, Cai WY, et al. Lymphocytes express thyrotropin receptor specific mRNA as detected by the PCR technique. Thyroid. 1991;1:223.
24. Trobe JD. Optic nerve involvement in dysthyroidism. Ophthalmology. 1981;88:488.
25. Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves’ disease (pretibial myxedema). Medicine (Balt). 1994;107:257.
26. Kriss JP. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409.
27. DeSanto LW. The total rehabilitation of Graves’ ophthalmopathy. Laryngoscope. 1980;90:1652.
28. Girod DA, Orcutt JC, Cummings CW. Orbital decompression for the preservation of vision in Graves’ ophthalmopathy. Arch Otolaryngol Head Neck Surg. 1993;119:229.
29. Warren JD, Spector JG, Burde R. Long-term follow-up and recent observations on 305 cases of orbital decompression for dysthyroid orbitopathy. Laryngoscope. 1989;99:35.
30. Calcaterra TC, Hepler RS, Hanafee WN. The diagnostic evaluation of unilateral exophthalmos. Laryngoscope. 1974;2:231.
31. Werner SC. Classification of the eye changes of Graves’ disease. J Clin Endocrinol Metab. 1969;29:982.
32. Werner SC. Modification of the classification of the eye changes of Graves’ disease: recommendations of the ad hoc committee of the American Thyroid Association. J Clin Endocrinol Metab. 1977;44:203.
33. Van Dyk HJL. Orbital Graves’ disease: a modification of the “NO SPECS” classification. Ophthalmology. 1981;88:479.
34. Calcaterra TC, Thompson JW. Antral-ethmoidal decompression of the orbit in Graves’ disease: 10-year experience. Laryngoscope. 1980;90:1941.
35. Riley FD. Orbital pathology in Graves’ disease. Mayo Clin Proc. 1972;47:974.
36. Sergott RC, Glaser JS. Graves’ ophthalmopathy: a clinical and immunologic review. Surv Ophthalmol. 1981;26:1.
37. Gorman CA. The presentation and management of endocrine ophthalmopathy. Clin Endocrinol Metab. 1978;7:67.
38. Neigel JM, Rootman J, Belkin RI, et al. Dysthyroid optic neuropathy: the crowded orbital apex syndrome. Ophthalmology. 1988;95:1515.
39. Jelks GW, Jelks EB, Ruff G. Clinical and radiographic evaluation of the orbit. Otolaryngol Clin North Am. 1988;21:13.
40. Feldon SE, Weiner JM. Clinical significance of extraocular muscle volumes in Graves’ ophthalmopathy: a quantitative computed tomography study. Arch Ophthalmol. 1982;100:1266.
41. Forbes G, Gorman CA, Brennan MD, et al. Ophthalmopathy of Graves’ disease: computerized volume measurements of the orbital fat and muscle. AJNR Am J Neuroradiol. 1986;7:651.
42. Hallin ES, Feldon SE. Graves’ ophthalmopathy, I: simple CT estimates of extraocular muscle volume. Br J Ophthalmol. 1988;72:674.
43. Hallin ES, Feldon SE, Luttrell J. Graves’ ophthalmopathy, III: effect of transantral orbital decompression on optic neuropathy. Br J Ophthalmol. 1988;72:683.
44. Erickson BA, Harris GJ, Lewandowski MF, et al. Echographic monitoring of response of extraocular muscles to irradiation in Graves’ ophthalmopathy. Int J Radiat Oncol Biol Phys. 1995;31:651.
45. Szucs-Farkas Z, Toth J, Balazs E, et al. Using morphologic parameters of extraocular muscles for diagnosis and follow-up of Graves’ ophthalmopathy: diameters, areas, or volumes? AJR Am J Roentgenol. 2002;179:1005.
46. Hosten N, Sander B, Cordes M, et al. Graves’ ophthalmopathy: MR imaging of the orbit. Radiology. 1989;172:759.
47. Just M, Kahaly G, Higer HP, et al. Graves’ ophthalmopathy: role of MR imaging in radiotherapy. Radiology. 1991;179:187.
48. Galuska L, Leovey A, Szucs-Farkas Z, et al. SPECT using 99m Tc-DTPA for the assessment of disease activity in Graves’ ophthalmopathy: a comparison with the results from MRI. Nucl Med Commun. 2002;23:1211.
49. Konuk O, Atasever T, Unal M, et al. Orbital gallium-67 scintigraphy in Graves’ ophthalmopathy. Thyroid. 2002;12:603.
Terwee C, et al. Long-term effects of Graves’ ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146:751.
51. Tallstedt L, Lundell G, Tørring O, et al. Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. N Engl J Med. 1992;326:1733.
52. Bech K, Madsen N. Influence of treatment with radioiodine and propylthiouracil on thyroid stimulating immunoglobulins in Graves’ disease. Clin Endocrinol (Oxf). 1980;13:417.
53. Bech K, Feldt-Rasmussen U, Bliddal H, et al. The acute changes in thyroid stimulating immunoglobulins, thyroglobulin, and thyroglobulin antibodies following subtotal thyroidectomy. Clin Endocrinol (Oxf). 1982;16:235.
54. Sautter-Bihl ML, Heinze HG. Radiotherapy of Graves’ ophthalmopathy. Dev Ophthalmol. 1989;20:139.
55. Gerling J, Kommerell G, Henne K, et al. Retrobulbar irradiation for thyroid-associated orbitopathy: double-blind comparison between 2.4 and 16 Gy. Int J Radiat Oncol Biol Phys. 2003;55:182.
56. Ohtsuka K, Sato A, Kawaguchi S, et al. Effect of steroid pulse therapy with and without orbital radiotherapy on Graves’ ophthalmopathy. Am J Ophthalmol. 2003;135:285.
57. Gorman CA, Garrity JA, Fatourechi V, et al. A prospective, randomized, double-blind, placebo controlled study of orbital radiotherapy for Graves’ ophthalmopathy. Ophthalmology. 2001;108:1523.
58. Gorman CA, Garrity JA, Fatourechi V, et al. The aftermath of orbital radiotherapy for grave ophthalmopathy. Ophthalmology. 2002;109:2100.
59. Fatourechi V, Garrity JA, Bartley GB, et al. Graves’ ophthalmopathy: results of transantral orbital decompression performed primarily for cosmetic indications. Ophthalmology. 1994;101:938.
60. Garrity JA, Fatourechi V, Bergstralh EJ, et al. Results of transantral orbital decompression in 428 patients with severe Graves’ ophthalmopathy. Am J Ophthalmol. 1993;116:533.
61. Pigeon P, Orgiazzi J, Berthezene F, et al. High voltage radiotherapy and surgical orbital decompression in the management of Graves’ ophthalmopathy. Horm Res. 1987;26:172.
62. Trokel S, Kazim M, Moore S. Orbital fat removal: decompression for Graves’ orbitopathy. Ophthalmology. 1993;100:674.
63. Dollinger J. Die drickentlastung der augenhokle durch entfernung der ausseren obitalwand bei hochgradigen exophthalmus und koneskutwer hornhauter kronkung. Dtsch Med Wochenschr. 1911;37:1888.
64. Naffziger H. Progressive exophthalmos following thyroidectomy: its pathology and treatment. Ann Surg. 1931;94:582.
65. Sewell EC. Operative control of progressive exophthalmosis. Arch Otolaryngol Head Neck Surg. 1936;24:621.
66. Hirsch O. Surgical decompression for malignant exophthalmosis. Arch Otolaryngol Head Neck Surg. 1950;51:325.
67. Walsh TE, Ogura JH. Transantral orbital decompression for malignant exophthalmos. Laryngoscope. 1957;67:544.
68. Fatourechi V, Bergstralh EJ, Garrity JA, et al. Predictors of response to transantral orbital decompression in severe Graves’ ophthalmopathy. Mayo Clin Proc. 1994;69:841.
69. Kent KJ, Merwin GE, Rarey KE. Margins of safety with transantral orbital decompression. Laryngoscope. 1988;98:815.
70. Weisman RA, Savino PJ. Management of endocrine orbitopathy. Otolaryngol Clin North Am. 1988;21:93.
71. Fatourechi V, Garrity JA, Bartley GB, et al. Orbital decompression in Graves’ ophthalmopathy associated with pretibial myxedema. J Endocrinol Invest. 1993;16:433.
72. Kulwin DR, Cotton RT, Kersten RC. Combined approach to orbital decompression. Otolaryngol Clin North Am. 1990;23:381.
73. Antoszyk JH, Tucker N, Codere F. Orbital decompression for Graves’ disease: exposure through a modified blepharoplasty incision. Ophthalmic Surg. 1992;23:516.
74. Weisman RA, Osguthorpe JD. Orbital decompression in Graves’ disease. Arch Otolaryngol Head Neck Surg. 1994;120:831.
75. Kennedy DW, Goodstein ML, Miller NR, Zinreich SJ. Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg. 1990;116:275.
76. Metson R, Dallow RL, Shore JW. Endoscopic orbital decompression. Laryngoscope. 1994;104:950.
77. Metson R, Samaha M. Reduction of diplopia following endoscopic orbital decompression: the orbital sling technique. Laryngoscope. 2002;112:1753.
78. Wee DT, Carney AS, Thorpe M, Wormald PJ. Endoscopic orbital decompression for Graves’ ophthalmopathy. J Laryngol Otol. 2002;116:6.
79. Khan JA, Wagner DV, Tiojanco JK, Hoover LA. Combined transconjunctival and external approach for endoscopic orbital apex decompression in Graves’ disease. Laryngoscope. 1995;105:203.
80. Schaefer SD, Soliemanzadeh P, Della Rocca DA. Endoscopic and transconjunctival orbital decompression for thyroid-related orbital apex compression. Laryngoscope. 2003;113:508.
81. Unal M, Leri F, Konuk O, Hasanreisoğlu B. Balanced orbital decompression combined with removal in Graves ophthalmopathy: do we really need to remove the third wall? Ophthalm Plast Reconstr Surg. 2003;19:112.
82. Metson R, Shore JW, Gliklich RE, Dallow RL. Endoscopic orbital decompression under local anesthesia. Otolaryngol Head Neck Surg. 1995;113:661.
83. Kacker A, Kazim M, Murphy M, et al. “Balanced” orbital decompression for severe Graves’ orbitopathy: technique with treatment algorithm. Otolaryngol Head Neck Surg. 2003;128:228.
84. Vaseghi M, Tarin TT, Levin PS, Terris DJ. Minimally invasive orbital decompression for Graves’ ophthalmopathy. Ann Otol Rhinol Laryngol. 2003;112:57.
85. Pletcher SD, Sindwani R, Metson R. Endoscopic orbital and optic nerve decompression. Otolaryngol Clin North Am. 2006;39:943.
86. Luxenberger W, Stammberger H, Jebeles JA, et al. Endoscopic optic nerve decompression: the Graz experience. Laryngoscope. 1998;108:873.
87. Leatherbarrow B, Lendrum J, Mahaffey PJ. Three wall orbital decompression for Graves’ ophthalmopathy. Eye. 1991;5:456.
88. van der Wal KG, de Visscher JG, Boukes RJ, Smeding B. Surgical treatment of proptosis bulbi by three-wall orbital decompression. J Oral Maxillofac Surg. 1995;53:140.
89. MacCarty CS, Kenefick TP, McConahey WM, Kearns TP. Ophthalmopathy of Graves’ disease treated by removal of roof, lateral walls, and lateral sphenoid ridge: review of 46 cases. Mayo Clin Proc. 1970;45:488.
90. Elisevich K, Allen L, Bite U, Colcleugh R. Decompression for dysthyroid ophthalmopathy via the orbital rim approach, technical note. J Neurosurg. 1994;80:580.
91. Stanley RJ, McCaffrey TV, Offord KP, DeSanto LW. Superior and transantral orbital decompression procedures: effects on increased intraorbital pressure and orbital dynamics. Arch Otolaryngol Head Neck Surg. 1989;115:369.
92. Shorr N, Neuhaus RW, Baylis HA. Ocular motility problems after orbital decompression for dysthyroid ophthalmopathy. Ophthalmology. 1982;89:323.
93. Dyer JA. Ocular muscle surgery. In: Gorman CA, Campbell RJ, Dyer JA, editors. The Eye and Orbit in Thyroid Disease. New York: Raven Press, 1984.