194 Management of the Postoperative Cardiac Surgical Patient
 The Cardiac Surgery Patient in the Intensive Care Unit
The Cardiac Surgery Patient in the Intensive Care Unit
History of Cardiac Surgery Linked to the History of Intensive Care
The development of modern cardiac surgery has been intimately related to the development of the ICU. This relationship has worked in both directions. Until the 1950s, cardiac surgery was limited to control of traumatic injuries and the closed repair of valves. Development of the extracorporeal pump oxygenator in 1953 by Gibbon ushered in the era of open-heart surgery.1 Heart valve replacement then became possible. Subsequently, in the 1960s, coronary artery bypass grafting (CABG) for ischemic heart disease was developed and rapidly popularized.2
Several studies have demonstrated that risk-adjusted mortality rates after CABG vary significantly among surgeons and hospitals and that mortality is related both to the number of surgeries performed by each surgeon and the total volume of procedures performed at the hospital.3–5 For high-risk surgical patients, survival is also related to the characteristics of the ICU care.6
The Changing Epidemiology of Cardiac Surgery
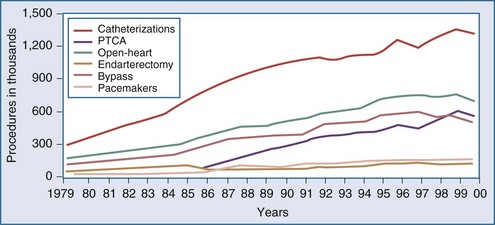
Over the last decade, the population of patients treated with cardiac surgery has changed dramatically. Advances in cardiology including reperfusion therapy, angioplasty, stenting, and drug-eluting stents, have obviated the need for surgical approaches to treatment except for particularly complex problems or after failure of other less invasive modalities. In the year 2000, 561,000 patients in the United States underwent percutaneous transluminal coronary angioplasty (PTCA), an increase of 262% relative to 1987. In the same year, 314,000 patients underwent CABG. Multiyear trends, represented in Figure 194-1, show a leveling off and subsequent decrease in the overall number of patients undergoing CABG.7 The recently developed sirolimus-coated coronary stent has been associated with even better results.8 Studies comparing the use of stents versus CABG for left main disease have found no significant difference in rates of death or of the composite endpoint of death, Q-wave infarction, or stroke between patients receiving stents and those undergoing CABG. However, stenting, even with drug-eluting stents, was associated with higher rates of target-vessel revascularization than was CABG.9
Alternative Techniques for Cardiac Surgery
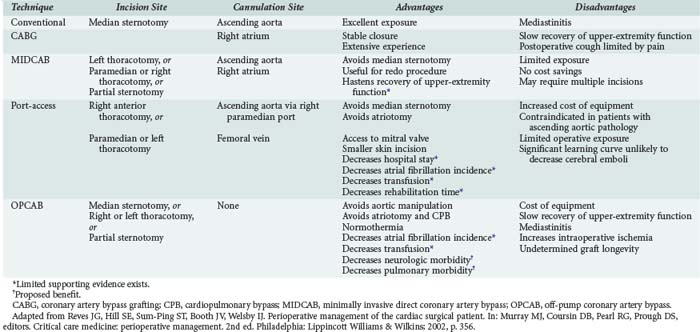
The increasing age of patients undergoing cardiac surgery and the relatively high incidence of adverse effects related to cardiopulmonary bypass (CPB) in these patients have led to the development of less invasive cardiac surgical techniques. These techniques are intended to decrease postoperative morbidity, reduce hospital length of stay, reduce costs, and hasten recovery of lifestyle (Table 194-1). Three major techniques have been proposed.
Minimally invasive direct coronary artery bypass (MIDCAB) differs from conventional CABG mainly in the type of incision used for access. In place of the conventional median sternotomy, access is obtained via a left or right thoracotomy, a parasternal incision, or a partial sternotomy. The proposed benefit of such an approach is the reduction in morbidity related to median sternotomy. This proposed advantage has not been demonstrated. MIDCAB grafting is a challenging technique and should be performed only in selected patients with favorable coronary anatomy. Both bare metal and drug-eluting stenting have been shown to be inferior to MIDCAB for proximal left anterior descending (LAD) coronary artery lesions, owing to higher reintervention rates with similar results in mortality and morbidity.4,10
Off-pump coronary artery bypass (OPCAB) is performed on a beating heart without benefit of CPB. The proposed benefit of this procedure is reduction of morbidity related to hypothermia and CPB. The procedure is undertaken using partial to full heparinization. Extubation may be achieved earlier in these patients because they do not require rewarming and are less coagulopathic. A subset of patients cannot tolerate the extent of retraction of the heart required for the surgery and need to be urgently placed on CPB. These patients may suffer ischemic myocardial injury and require support with inotropes or intraaortic balloon pumping (IABP) during the postoperative period. A retrospective study of 1398 patients showed that use of the OPCAB technique for multivessel myocardial revascularization in high-risk patients significantly reduced the incidence of perioperative myocardial infarction (MI) and other major complications, length of stay in the ICU, and mortality.11 In a single-center non-randomized registry, the incidence of major cardiac events were similar in OPCAB versus sirolimus-eluting stents in diabetic patients with multivessel disease.12
A third method of minimally invasive cardiac surgery is the port-access technique. This operation entails obtaining access for CPB with the use of endovascular catheters. This allows surgery to be performed using CPB via either a left or right thoracotomy. The technique is particularly useful for mitral valve replacement through a right thoracotomy and for redo CABG (avoiding the complications associated with repeat sternotomy). The port-access technique has been shown to be safe and is associated with shorter lengths of stay, reduced transfusion requirements, fewer infections, decreased incidence of renal failure, and less atrial fibrillation when compared with conventional techniques.13 In outcome data using propensity score analysis for mitral valve repair, minimally invasive repair had similar results to open repair. There was an increase in cross-clamp and bypass times, but early outcome was similar.14 Widespread adoption of this technique has been limited by the technical complexity of placing the required catheters, which requires both extra time and a specially trained and skilled operative team.
Organization of the Postoperative Cardiac Surgery Unit
Guidelines developed by the American Heart Association and the American College of Cardiology outline the requirements for cardiac surgical ICUs.15 These include the development of protocol-driven care, a minimum number of cardiac surgical ICU beds that is half the number of surgeries performed per week, and one-to-one nursing care during the first night in the unit. ICU coverage by a dedicated intensivist has been shown to improve outcomes in other types of major surgery and should be recommended after cardiac surgery as well.6
 Separation from Cardiopulmonary Bypass and the End of Surgery
Separation from Cardiopulmonary Bypass and the End of Surgery
Cardiopulmonary Bypass
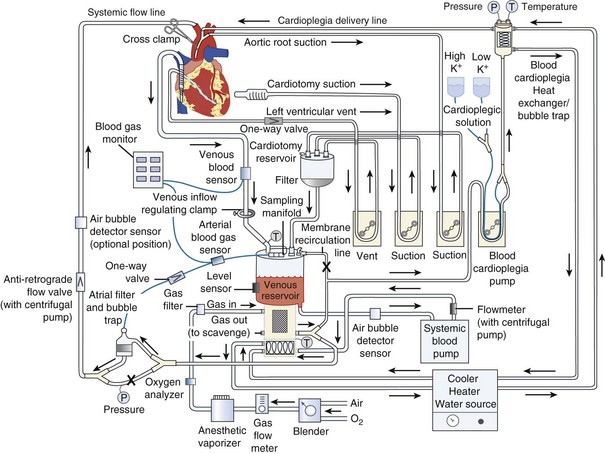
The goal of CPB is to separate the heart and lungs from the systemic circulation so that the heart can be arrested while the surgical repair is constructed. Blood is drained from the right side of the heart, either by gravity or with vacuum assistance, via a cannula in the right atrium directly or via a cannula in the femoral vein that is advanced into the right atrium. The blood is collected in a reservoir and then pumped through an oxygenator that contains a membrane where the blood is oxygenated and carbon dioxide is removed (Figure 194-2). The perfusionist controls both the fraction of inspired oxygen and the rate of oxygen flow through the circuit, thereby controlling the patient’s arterial oxygen and carbon dioxide levels, respectively. The treated blood then passes through an air filter and is returned to the patient via an arterial cannula placed in either the ascending aorta or the femoral artery. The perfusionist controls the amount of flow provided to the patient (i.e., CO). Mild to moderate systemic hypothermia (28°C-34°C) is used during bypass to minimize oxygen consumption by both the body and the brain. After adequate CPB is established, an aortic cross-clamp is applied to the ascending aorta, between the aortic cannula and the heart. The interval when the cross-clamp is applied is referred to as “ischemic” time, because no blood is circulated through the heart during this period. The heart is arrested by infusion of a high-concentration potassium solution into the native coronary arteries (antegrade cardioplegia) via a cannula placed between the aortic cross-clamp and the heart. Cardioplegia may also be given “backwards,” through the venous system of the myocardium (retrograde cardioplegia) via a catheter placed in the coronary sinus. Potassium is used as the arresting agent because it stops the heart from beating and minimizes myocardial oxygen consumption.
 Monitoring the Postoperative Cardiac Surgery Patient
Monitoring the Postoperative Cardiac Surgery Patient
Hemodynamic Monitoring
All patients admitted to the ICU after cardiac surgery will have their blood pressure continuously monitored using an intraarterial line. This is usually placed in either a radial or femoral artery. Accuracy of the measurements depends on strict attention to calibration, leveling, and removal of air from the tubing. After CPB, femoral arterial pressure may more accurately reflect central aortic pressures,16 but this problem has usually resolved by the time the patient arrives in the ICU. If the radial artery is cannulated, the hand should be examined for signs of ischemia.17 Vascular complications of femoral arterial lines are extremely rare, but femoral catheters may be associated with an increased incidence of infection.18
Central venous access is required in all patients for drug administration and hemodynamic monitoring. In the low-risk patient, a CVP catheter may be all that is needed, particularly if echocardiography is available as a backup. Pulmonary artery catheters have the advantage of allowing measurement of pulmonary artery occlusion pressure (PAOP), thermodilution, and CO, as well as sampling of the mixed venous blood saturation (SvO2). Use of the pulmonary artery catheter remains controversial. Improved outcome due to use of a pulmonary artery catheter for monitoring of cardiac surgical patients has not been demonstrated.19 Some studies showed an increased risk of death or adverse outcome when treatment was guided by the use of a pulmonary artery catheter.20,21 However, many of these studies have been criticized on methodological grounds, and use of the catheter in cardiac surgery remains widespread.22 Current guidelines recommend use of the pulmonary artery catheter in high-risk patients undergoing surgery in an appropriate practice setting.23 Such a setting is one in which the physician and nursing staff are familiar with the catheter and trained to properly interpret the information obtained. If echocardiography is readily available, it is possible to manage even high-risk patients using a CVP catheter.
Electrocardiography
Continuous ECG monitoring allows detection of arrhythmias. If an arrhythmia is detected, a 12-lead ECG should be obtained, and serum electrolyte concentrations should be measured. Treatment of arrhythmias should be carried out using established protocols.24 If a malignant arrhythmia occurs, myocardial ischemia should be considered as a possible precipitating cause.
Monitoring of trends in ST-segment elevation or depression allows early detection of postoperative myocardial ischemia. Although transient ST-segment changes are relatively common and of unclear significance, persistent changes should be investigated by obtaining a 12-lead ECG and measuring circulating levels of creatine kinase myocardial band (CK-MB), troponin-T, or troponin-I.25,26 If ischemia is strongly suspected, then echocardiography followed by coronary angiography should be considered. Findings from these studies may indicate the need for further coronary revascularization.
Echocardiography in the Intensive Care Unit
TEE is being used as a tool to facilitate decision making in the management of critically ill patients, including cardiac surgical patients. In the cardiac surgical ICU, this modality may have a particularly high yield when it is used to establish the cause of postoperative hypotension.27 In one large series, a new diagnosis was established or an important pathology was excluded in 45% of TEE examinations performed in the ICU. Pericardial tamponade was diagnosed in 34 cases (11%) and excluded in 36 cases (12%). Other diagnoses included severe left ventricular failure and presence of large pleural effusions. The results of TEE had an impact on therapy in 220 cases (73%) by leading to a change of pharmacologic treatment and/or fluid administration, reoperation, or a decision that reoperation was unnecessary.28
 Clinical Manifestations of the Postbypass Period
Clinical Manifestations of the Postbypass Period
The Normal Course
Patients are typically admitted to the ICU intubated and ventilated. Sedation with a short-acting agent, typically propofol, is continued until the patient is ready for extubation. Once hemodynamic stability is ascertained and chest tube drainage is judged to be under control, the patient is allowed to awaken. There is no need for prolonged weaning from mechanical ventilation. A short trial of spontaneous ventilation is sufficient to determine whether respiration will be adequate without mechanical support. The rapid shallow breathing index (RSBI) has been shown to be a sensitive way to assess the likelihood of successful extubation.29 The RSBI is calculated by dividing the respiratory rate (in breaths per minute) by the tidal volume (in liters). A value of lower than 105 predicts successful extubation. Chest tubes are commonly removed on the first postoperative day. The pulmonary artery catheter, if present, is discontinued, and the patient may be transferred to a step-down unit.
Fast-tracking of cardiac surgical patients refers to a comprehensive program designed to reduce both length of stay and hospital costs.30,31 As a part of this program, multiple anesthetic techniques designed to allow earlier postoperative extubation have been proposed, studied, and shown to be safe. These techniques may allow extubation in the OR.32 The key to proper use of this technique is patient selection. Although the criteria are expanding, patients with unstable angina or a high degree of congestive heart failure are generally not appropriate candidates for fast-tracking. In a retrospective review comparing 4020 patients undergoing cardiac surgery with a conventional anesthetic versus 3969 patients with a fast-track anesthetic, the fast-track group had shorter extubation times, shorter ICU or PACU stays, and a lower incidence of low cardiac output syndrome.
Low Cardiac Output
Preload
In some cases, low preload is not caused by absolute hypovolemia but by relative or distributional hypovolemia. CPB and subsequent rewarming may lead to vasodilatation and a subsequent hypotension. Intravascular volume expansion may be required to maintain perfusion. An acceptable alternative is administration of a low dose of vasopressor such as phenylephrine or norepinephrine to maintain an adequate perfusion pressure. Recently, vasopressin in doses between 0.01 and 0.1 units/min has been demonstrated to be effective in this situation.33,34 Vasodilatation is usually a transient problem that resolves during the first several hours after separation from CPB. Continued vasodilatation after this period should prompt a search for another cause, particularly infection.
Pump Failure
Either or both ventricles may fail postoperatively. Decreased myocardial contractility may be caused by impaired preoperative function, inadequate revascularization at surgery, post-CPB reperfusion injury, or perioperative myocardial ischemia or MI. The incidence of infarction is approximately 5% in large series.35 Preoperative myocardial function and the adequacy of revascularization at surgery should be clear from the history. Determination of circulating levels of CK-MB or troponin postoperatively can provide evidence of perioperative ischemia or infarction.25,26 Often, diminished contractility after operation is caused by inadequate myocardial protection during surgery. Decreased myocardial contractility secondary to inadequate myocardial protection usually resolves within the first 24 hours postoperatively. ECG changes are nonspecific.
Persistent new myocardial dysfunction associated with ECG changes and echocardiographic evidence of new wall-motion abnormalities should raise suspicion that the problem is an occluded graft and MI. Measurements of CK-MB in serum are of limited usefulness because levels of this enzyme are commonly elevated after surgery due to manipulation of the heart and incision of the atria, structures that are rich in the enzyme. If CK-MB levels are very high, greater than 80 mg/dL, then perioperative MI is likely.36 Cardiac troponins are more specific for the diagnosis of perioperative infarction. A comparison of CK-MB, troponin-T, and troponin-I showed that a troponin-I level of greater than 5 µg/L was the most accurate indicator of MI, being superior to either troponin-T or CK-MB.37 Elevated serum concentrations of troponin-I are associated with a cardiac cause of death and with major postoperative complications.38 In addition, troponin-T concentrations measured after surgery are an independent predictor of in-hospital death after cardiac surgery.26 If ischemia or MI is diagnosed, the patient may be taken for angiography or re-exploration and revascularization.
Rate and Rhythm
Bradycardia can lead to ventricular distention, increasing wall tension, and decreasing coronary perfusion pressure, factors that can promote development of ischemia and failure. HR of 80 to 90 appears to be optimal, allowing adequate filling and preventing overdistention but not causing rate-related ischemia. Bradycardia can be corrected by pacing. In general, epicardial pacing wires are left in place after chest closure and are attached to an external pacemaker in the immediate postoperative period. If the dysrhythmia is sinus bradycardia, atrial pacing is usually optimal. The second most common cause of bradyarrhythmia after cardiac surgery is atrioventricular dissociation. The combination of atrial and ventricular leads allows atrioventricular pacing for management of disassociation. Synchronization of the atrioventricular interval between 0.1 and 0.225 second optimizes CO.39
Atrial fibrillation is the most common tachyarrhythmia. It occurs in 10% to 35% of patients after cardiac surgery, usually on the second or third postoperative day. Postoperative atrial fibrillation is associated with increased morbidity and mortality and with longer, more expensive hospital stays.40 The Multicenter Study of Perioperative Ischemia (McSPI) group examined 2417 patients undergoing CABG with or without concurrent valvular surgery.41 The overall incidence of postoperative atrial fibrillation was 27%. Independent predictors of postoperative atrial fibrillation included advanced age, male sex, a past history of atrial fibrillation, a past history of congestive heart failure, and a pre-CPB heart rate greater than 100 beats/min. Surgical practices such as pulmonary vein venting, bicaval venous cannulation, postoperative atrial pacing, and longer cross-clamp times also were identified as independent predictors of postoperative atrial fibrillation. Patients who developed postoperative atrial fibrillation had longer lengths of stay, both in the ICU and in the ward, compared with patients who did not develop the complication.
Although premature ventricular contractions (PVCs) are common, sustained ventricular arrhythmias are far less frequent. Severe ventricular arrhythmias occurring after cardiac surgery are related to ischemia, hypoxemia, hypovolemia, electrolyte abnormalities, the effects of vasoactive drugs, or an underlying preexisting cardiomyopathy.42 In a series of 2100 cardiac operations, only 16 patients (0.8%) developed ventricular fibrillation or a sustained ventricular tachycardia during the interval from 3 days to 3 weeks after surgery. Ten of these patients had undergone valve surgery.43 Prognosis in these patients is dependent on the preoperative ventricular prognosis; it is excellent in those with good function. In those with a left ventricular ejection fraction of less than 40%, the mortality rate may be as high as 75%.44
Tamponade
Tamponade refers to the hemodynamic consequences of a collection of blood or other fluid in the pericardial sac. In postsurgical patients, the presentation of tamponade may be subtle and differ significantly from classic descriptions. Equilibration of filling pressures typically is not seen. More commonly, patients present with isolated elevation of right atrial pressure due to compression of the right atrium and superior vena cava. After cardiac surgery, as many as 66% of pericardial fluid collections are loculated posterior effusions.45
Bleeding from the atrial cannulation site is a common cause of tamponade. As the pressure on the right atrium increases, ventricular filling is impaired, and CO decreases. Diagnosis of tamponade is made difficult by the high overall frequency of pericardial effusions after surgery. Echocardiographic studies have shown that moderate effusions are present in 30% of patients on the eighth postoperative day, with 2% of patients having large effusions.46
If time permits, the diagnosis of tamponade can be confirmed with the use of echocardiography. Although effusions are common, signs of compression or collapse of either atrium or of the right ventricle are diagnostic.47–49 It is important to remember that the diagnosis may be made on clinical suspicion alone, and that treatment should not be withheld to await confirmation. Once tamponade is diagnosed, volume transfusion may temporize the situation. Pericardiocentesis is not effective in this situation, and prompt re-exploration for hemostasis and evacuation of clot is indicated.
Respiratory Complications
ALI and ARDS are rare complications after cardiac surgery, CPB, and blood transfusion. In one retrospective study of 3278 cardiac surgical patients, only 13 (0.4%) developed ARDS during the postoperative period. The mortality rate associated with this complication was 15%. Another study reported a much higher mortality rate (70%).50 The patients who developed ARDS were more likely than their matched controls to have had previous cardiac surgery. During the postoperative period, patients with ARDS received more blood products and developed shock more frequently than patients without ARDS.51
Nosocomial pneumonia can complicate any ICU stay. Patients who require mechanical ventilation for longer than 48 hours are at particular risk. These pneumonias are usually caused by aspiration of oral or gastric secretions into the lungs. The incidence of nosocomial pneumonia can be reduced by diligent mouth care to prevent pooling of secretions and elevation of the head of the bed to greater than 30 degrees. Nosocomial pneumonia carries a mortality rate of 24% to 50% and warrants appropriate broad-spectrum antimicrobial chemotherapy.52 The antibiotic prescription can be tailored once the results of sputum cultures are available.
Diaphragmatic dysfunction is usually caused by cold-induced injury of the phrenic nerve due to application of ice slush to the heart as part of the cardioplegia regimen. This complication occurs in up to 2% of patients undergoing cardiac surgery with topical hypothermia; more rarely, it can occur even if topical cooling was not applied.53,54 While the patient is being ventilated with positive pressure, this injury will not be apparent. If preoperative pulmonary function was normal, unilateral diaphragmatic paralysis usually is well tolerated. Pulmonary function can be severely compromised, however, if pulmonary problems were present preoperatively or, in rare instances, if bilateral diaphragmatic injury occurs.55 These patients are at increased risk for development of nosocomial pneumonia, failure to wean from the ventilator, and death. Diaphragmatic dysfunction usually resolves spontaneously within 3 to 4 months.
Continued Bleeding
Continued bleeding is a common problem and requires immediate and aggressive management before the onset of further complications. The reasons for continued bleeding are often multifactorial and include inadequate surgical hemostasis, platelet dysfunction, coagulopathy, and inadequate heparin reversal. Often these factors occur in combination. Patients undergoing valve replacement are at increased risk.56
Multiple clotting abnormalities are possible, most of which result either directly or indirectly from the use of CPB.57 The tubing, blood reservoir, and oxygenator membrane are all foreign surfaces that can activate the clotting cascade. Because the pump must be primed with either normal saline or lactated Ringer’s solution, the priming process leads to substantial dilution of all blood components including red cells, platelets, and clotting factors. After CPB, the platelet count is decreased, and the remaining platelets are functionally deranged.58,59 There is sequestration of platelets in the liver, spleen, and in the CPB circuit itself. Systemic fibrinolysis due to activation of this system by the CPB circuit occurs.
Renal Dysfunction
Mild renal dysfunction is a common postoperative event. One multicenter study demonstrated significant worsening of renal function in 7% of patients undergoing myocardial revascularization.60 Approximately 1% of patients with postoperative acute renal failure (ARF) require renal replacement therapy. These patients have increased morbidity and mortality. Development of ARF can prolong ICU length of stay as much as fivefold.60
A multicenter study of 2222 patients undergoing CABG identified five independent preoperative predictors of renal dysfunction: age 70 to 79 years or age 80 to 95 years, congestive heart failure, previous myocardial revascularization, type 1 diabetes mellitus, or preoperative serum glucose levels exceeding 300 mg/dL and preoperative serum creatinine levels of 1.4 to 2.0 mg/dL. Independent perioperative factors that exacerbated risk were CPB lasting 3 hours or longer and various measures of ventricular dysfunction.60 The predominant predisposing factor appears to be low CO. This factor may be exacerbated by concurrent use of vasopressors such as phenylephrine.61
Renal dysfunction tends to follow one of three main patterns.62 Abbreviated ARF is a transient event, most probably related to intraoperative renal ischemia. The serum creatinine concentration can be expected to peak on day 4 after surgery. Overt ARF occurs when the duration of the predisposing insult, usually low CO, is longer. The serum creatinine concentration peaks at a higher level than with abbreviated ARF and then decreases over a period of several weeks. Protracted ARF occurs when a second insult, commonly sepsis or hypotension, is superimposed on the resolving renal function. This event triggers a further, often irreversible, decrease in renal function.
Neurologic Complications
Neurologic sequelae of CPB range from subtle neurocognitive deficits (appearing in up to 80% of patients) to stroke. In order to estimate the relative risks of neurologic sequelae associated with various clinical factors, a logistic regression model was applied to prospectively collected data from 273 patients enrolled at 24 American medical centers.63 Adverse cerebral outcomes occurred in 16% of patients and were almost equally divided between type I outcomes (8.4%; 5 cerebral deaths, 16 nonfatal strokes, and 2 new transient ischemic attacks) and type II outcomes (7.3%; 17 new cases of intellectual deterioration persisting at hospital discharge and 3 cases of newly diagnosed seizure disorder). Resource utilization for these patients was significantly increased; median ICU stay was prolonged from 3 days to 6 to 8 days. Total duration of hospitalization was increased by 50% (type II, P = .04) to 100% (type I, P < .001). After discharge from the acute care setting, specialized care was required for 69% of the patients with adverse neurologic sequelae. Risk factors for type I outcomes related primarily to embolic phenomena including proximal aortic atherosclerosis, intracardiac thrombus, and intermittent clamping of the aorta during surgery. Risk factors for type II outcomes included, in addition to these factors, a preoperative history of endocarditis, alcohol abuse, perioperative dysrhythmia, poorly controlled hypertension, and low CO after CPB.
Gastrointestinal Complications
Acute abdominal complications are relatively rare after cardiac surgery. If they do occur, they are associated with extremely high rates of morbidity and mortality. One prospective study of 1116 patients undergoing CPB found that abdominal complications occurred in 23 (2.1%). Ten of these patients underwent subsequent abdominal surgery, and 20 died. Early complications occurred on postoperative days 6 and 7 and consisted of bowel ischemia or hepatic failure. These complications are probably related to perioperative hypotension and low CO.64 Late complications consisted of pseudomembranous colitis, cholecystitis, pancreatitis, and rupture of a septic spleen.65
Mild transient increases in circulating levels of hepatocellular enzymes are common after surgery. These changes are generally of no consequence; however, increased serum transaminase levels, if sustained or very high (e.g., serum alanine aminotransferase concentration greater than 500 IU/L), may represent evidence of severe ischemic injury of the liver. Severe ischemic liver injury after cardiac surgery carries a high mortality and is strongly associated with low CO and increased filling pressures, suggesting that liver ischemia is induced by a combination of decreased perfusion and congestion.66
 Management of Common Postoperative Problems
Management of Common Postoperative Problems
Optimization of Cardiac Output
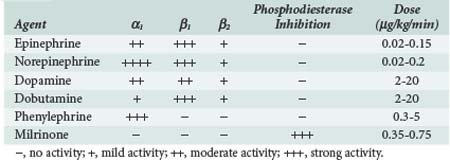
If CO or blood pressure remains low despite intravascular volume resuscitation, then it is necessary to institute inotropic or vasopressor support. No single agent is optimal in all cases. Rather, selection of the agent should be based on the suspected cause of low CO or hypotension and knowledge of the pharmacologic effects of the various inotropic and vasopressor drugs that are available (Table 194-2). If the primary cause of hypotension appears to be vasodilatation, administration of a vasoconstrictor (e.g., phenylephrine, norepinephrine, vasopressin) is indicated. If hypotension is related to inadequate ventricular ejection, then inotropic therapy with a β-adrenergic agent should be instituted. Epinephrine, norepinephrine, dopamine, and dobutamine are all reasonable choices. In patients with chronic systolic dysfunction, response to these agents may be impaired. Chronically elevated levels of circulating catecholamines deplete myocardial norepinephrine stores and down-regulate expression of myocardial β-adrenergic receptors. In these patients, tachyphylaxis to β-adrenergic agonists can develop rapidly. Addition of a phosphodiesterase inhibitor such as amrinone or milrinone is often effective in these patients.67,68 In all cases, agents should be titrated to achieve adequate perfusion.
Mechanical Support of the Circulation
Ventricular assist devices (VADs) are more effective than IABP for maintaining CO. Either the left ventricle, the right ventricle, or both can be supported with VADs. Currently, VADs may be used either as a bridge to transplantation or as a bridge to recovery. Either situation assumes that the VAD is a time-limited intervention. There are some data to support the view that resting the heart through the use of a VAD can allow some recovery of acutely injured myocytes, permitting eventual withdrawal of mechanical support. One case series showed that when VAD was used as a bridge to recovery, 66% of patients were eventually able to wean from support and be discharged home.69 If the heart is chronically diseased, there is little hope of recovery, and the VAD serves to support the patient until transplantation becomes possible.69,70
Ongoing clinical trials are investigating the use of VADs as definitive therapy rather than as a bridge to transplantation. Implantation of these devices may increase the long-term survival of patients with end-stage heart failure.71
Correction of Arrhythmias
Atrial fibrillation is the most commonly encountered arrhythmia after cardiac surgery. Prophylactic use of β-adrenergic blockers reduces the incidence of postoperative atrial fibrillation, and they should be administered after cardiac surgery to all patients unless specific contraindications are present.72 Prophylactic treatment with amiodarone and atrial overdrive pacing should be considered for patients who are at high risk for postoperative atrial fibrillation (e.g., those with a history of previous atrial fibrillation or mitral valve surgery).40,73
If atrial fibrillation develops after cardiac surgery, the intensivist needs to determine whether the primary strategy should be to control the ventricular rate or to restore normal sinus rhythm. If atrial fibrillation is associated with hemodynamic instability or anticoagulation is contraindicated, rhythm management using electrical cardioversion or amiodarone is preferred.74,75 Overdrive pacing using atrial pacing wires also can be effective. The appropriate strategy for most stable patients may be control of ventricular rate, because most will spontaneously revert to sinus rhythm within 8 weeks after discharge.76,77 Appropriate agents to achieve ventricular rate control include intravenous or oral β-adrenergic blockers or calcium channel blockers. All patients with atrial fibrillation persisting for longer than 24 to 48 hours should be anticoagulated unless there is a specific contraindication. Long-term outcomes are similar regardless of whether the rate-control strategy or the rhythm-control strategy is selected.78,79
Postoperative ventricular arrhythmias should be treated immediately according to current Advanced Cardiac Life Support (ACLS) protocols.24 Any postoperative ventricular arrhythmia should prompt a search for an underlying cause. Importantly, ischemia should be ruled out. Patients with sustained ventricular arrhythmias should undergo electrophysiologic testing before long-term antiarrhythmic therapy is instituted. The implantable cardioverter-defibrillator (ICD) device has been shown to be superior to drug therapy for patients with hemodynamically significant arrhythmias.80
Correction of Coagulopathy
Postoperative coagulopathy can promote bleeding and accumulation of blood in the chest or pericardial cavity. Aggressive measures must be used to correct the coagulopathy. A systemic approach to the evaluation and treatment of continued bleeding is needed; one such approach is outlined in Table 194-3. Hypothermia can contribute to coagulopathy. Therefore, profoundly hypothermic ICU patients must be actively rewarmed with the use of a warm air device. Laboratory evaluation of suspected coagulopathy should include measurements of platelet count, prothrombin time (PT), APTT, ACT, and bleeding time.
TABLE 194-3 Evaluation and Treatment of Postoperative Coagulopathy
| Coagulation Test | Normal Range | Suggested Treatment |
|---|---|---|
| Body temperature | — | If less than 35.5°C, the patient should be actively rewarmed. |
| Prothrombin time (PT) | 11-13.3 sec | Administer fresh-frozen plasma. |
| Partial thromboplastin time (PTT) | 21-32 sec | Consider additional protamine.* |
| Platelets | 140,000-440,000/µL | If <100,000, transfuse platelets. |
| Fibrinogen | 150-360 mg/dL | If <100, transfuse cryoprecipitate. |
| Bleeding time | 2.5-9.5 min | If prolonged and platelet count is normal, consider platelet dysfunction, and treat with desmopressin acetate (DDAVP) and/or cryoprecipitate. |
| Activated coagulation test (ACT) | 90-120 sec | Consider additional protamine.* |
Postoperative Bleeding
Bleeding that continues after correction of coagulopathy needs to be aggressively treated. Venous bleeding in the chest can be partially controlled by application of positive end-expiratory pressure (PEEP).81,82
Re-exploration is associated with increased morbidity and mortality. However, this increased mortality and morbidity may be partially explained by delays in the decision to re-explore that lead to avoidable open-chest resuscitations in the ICU.56,83,84
Postoperative Renal Failure
The cornerstone of prevention and treatment of renal failure in the cardiac surgical patient is the maintenance of adequate renal perfusion. This goal is best achieved by optimizing circulating blood volume and CO. Multiple pharmacologic regimens for renal protection have been described. Dopamine at low “renal” doses (1-3 µg/kg/min) has been used. The rationale for this strategy is that dopamine activates type 1 dopaminergic (DA1) receptors, leading to renal artery dilation, natriuresis, and diuresis. However, numerous human studies have failed to show that low-dose dopamine prevents renal failure or improves survival.85 Even low doses of dopamine increase CO, and this may be the basis for any increase in urine output observed.86 Fenoldapam87 and dopexamine88 are DA1 receptor antagonists that also have been proposed as renal protective agents and used with mixed success.89
Loop diuretics such as furosemide have been proposed as renal protective agents, not only because of their ability to produce diuresis and natriuresis, but also because these drugs may reduce medullary tubular oxygen consumption. Mannitol, an osmotic diuretic, been used to prevent development of ARF. Neither mannitol nor furosemide has been shown to improve outcome for patients with ARF.60 Indeed, these drugs may be deleterious because of their ability to promote diuresis and thus exacerbate hypovolemia and inadequate renal perfusion. Some success has been reported with the combination of mannitol, furosemide, and dopamine.90 Infusion of a solution containing these three agents promoted diuresis in patients with acute postoperative ARF and adequate CO and significantly decreased the need for dialysis in the majority of patients.88 Early administration of this solution in ARF caused early restoration of renal function to normal or baseline status.90
The failure of pharmacologic means of preventing and treating renal failure has led to interest in other methods. Early and intensive use of continuous venovenous hemofiltration achieved a better than predicted outcome in a series of 65 consecutive patients with severe ARF who underwent cardiac operations.91
Glucose Control
Recent studies have shown that tight control of blood glucose level in the ICU is associated with an increase in morbidity and mortality (Table 194-4). Hyperglycemia and insulin resistance are common in critically ill patients, even those who have not previously had diabetes. Results of a prospective randomized controlled study92 in which 6104 critically ill adult patients were randomly assigned to receive either intensive insulin therapy (maintenance of blood glucose concentration between 80 and 108 mg/dL) or conventional treatment (infusion of insulin to keep blood glucose level 180 mg/dL or less) showed that at 3 months, the intensive insulin therapy group had an increase in ICU mortality, with an increase in hypoglycemic episodes in the treatment group.
TABLE 194-4 Protocol for Blood Sugar Control in the Postoperative Period
| Decision to initiate IV insulin ↓ If BG <200 mg/dL, begin D5 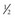 NS at 60-100 mL/h NS at 60-100 mL/hIf BG >300 mg/dL, give stat dose of IV insulin, 0.1 U/kg body weight ↓ Initiate an hourly rate (total daily dose of insulin divided by 24) For patients who have never taken insulin, give 0.02 U/kg body weight per hour* ↓ Check BG hourly and adjust according to table below Recheck BG hourly ↓ If in desirable range (101-150 mg/dL), continue to check BG every 2 h and adjust as necessary |
| Current BG (mg/dL) | Previous BG (mg/dL) |
|---|---|
| <60 60-80 81-100 101-150 151-200 201-250 251-300 301-400 >400 | |
| <60 | Withhold drip and give 1 ampule of 50% glucose; check BG every 30 min until >100 mg/dL, then reinitiate drip at 50% of previous rate |
| 60-80 | Withhold drip; check BG every 30 min until >100 mg/dL, then reinitiate drip at 50% of previous rate |
| 81-100 | ↓ Rate by 1 U/h No change ↓ Rate by 25% or 0.5 U/h† ↓ Rate by 25% or 1 U/h† ↓ Rate by 50% or 2 U/h† |
| 101-150 | No change ↓ Rate by 25% or 1 U/h† |
| 151-200 | ↑ Rate by 1 U/h ↑ Rate by 0.5 U/h ↑ Rate by 25% or 1 U/h† No change ↓ Rate by 25% or 1 U/h† |
| 201-250 | ↑ Rate by 25% or 2 U/h† ↑ Rate by 25% or 1 U/h† ↑ Rate by 1 U/h No change |
| 251-300 | ↑ Rate by 33% ↑ Rate by 25% ↑ Rate by 25% ↑ Rate by ↑ Rate by ↑ Rate by No or 2.5 U/h† or 1.5 U/h† or 1 U/h† 1 U/h† 1.5 U/h† 25% or 2 U/h† change |
| 301-400 | ↑ Rate by 40% or 3 U/h† |
| >400 | ↑ Rate by 50% or 4 U/h† |
| Before discontinuing insulin infusion: | |
| Ensure that patient is able to tolerate oral intake | |
| Write orders for alternative glycemic management | |
| Precede discontinuation by 1-2 h with subcutaneous dose of very rapid or rapid insulin. If patient has never taken insulin, use a dose equal to twice the hourly rate of IV insulin. Otherwise, use the dose of insulin or oral agent given before surgery/admission. | |
BG, blood glucose concentration; D5 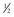 NS, 5% dextrose in half-normal saline; IV, intravenous; U, units.
NS, 5% dextrose in half-normal saline; IV, intravenous; U, units.
* For patients undergoing major surgery (e.g., cardiothoracic surgery, transplantation), higher doses may be necessary.
Copyright © 2003 by Joslin Diabetes Center. All rights reserved. These Guidelines are the property of Joslin Diabetes Center and are protected by copyright. Any reproduction of this document which omits Joslin’s name or copyright notice is prohibited. This document may be reproduced for personal use only. It may not be distributed or sold. It may not be published in any other format (e.g., book, article, Web site) without the prior, written permission of Joslin Diabetes Center, Communications Department, 617-732-2695.
Mechanical Ventilation
A small number of patients develop ALI or ARDS. In a large prospective trial of medical and surgical patients with ARDS or ALI, it was clearly beneficial to employ a lung-protective strategy or mechanical ventilation, limiting tidal volume to 6 mL/kg.93 No such study has been performed in cardiac surgical patients, but it seems reasonable to adopt the same guidelines. These recommendations apply only to patients with established ALI/ARDS; use of low tidal volumes has not been shown to be effective when used prophylactically.
Patients with ALI or ARDS typically require increasing levels of PEEP to support oxygenation. The effect of PEEP on ventricular output is controversial. There is evidence that the application of PEEP up to 30 cm H2O decreases CO by reducing ventricular preload and displacing the interventricular septum toward the left, which restricts left ventricular filling.94 Other studies have not supported this view. When adult patients with normal preoperative respiratory status were randomly assigned to treatment with graded degrees of PEEP between 0 and 10 cm H2O during mechanical ventilatory support, there were no significant differences in cardiac index among the groups.95 It is likely that the effects of PEEP on the circulation are widely variable among patients and that the appropriate strategy is upward titration of PEEP under close monitoring.
 Outcomes of Cardiac Surgery
Outcomes of Cardiac Surgery
Increasingly, health care is being driven by outcome data. Cardiac surgery has been one of the leading specialties in this field. It is difficult to assess results from crude mortality data, because these do not take into account case complexity and differing preoperative risks among patients. Crude comparisons of death rates can be misleading and may encourage surgeons to practice risk-averse behavior. Death rates should be stratified by risk. It is, however, possible to make some generalizations. Among low-risk patients undergoing CABG, mortality rates lower than 2% are achievable.96 Higher mortality rates are to be expected in selected subgroups of patients with major preoperative risk factors (e.g., poor ventricular function, advanced age, comorbid conditions) or major operative risk factors (e.g., reoperative surgery, complex operations).
A prospective cohort of 27,239 consecutive patients undergoing isolated CABG was examined to determine risk factors for hospital mortality. After adjustment for patient and disease characteristics, the following comorbid conditions were found to be related to postoperative mortality: diabetes, vascular disease, chronic obstructive pulmonary disease, peptic ulcer disease, and dialysis-dependent renal failure.97
Cardiac surgery is being performed more frequently in patients 80 years of age and older. In one study, the 30-day mortality rate for patients age 65 to 75 years was 3.4%, and for those older than 80 years of age it was 13.5%. Older patients had longer ICU and postoperative lengths of stay. Total direct costs were $4818 higher in the octogenarian group. Although emergency operations and complex procedures carry high risks for octogenarians and increasing costs for society, most of these patients can be offered operation with short-term morbidity, mortality, and resource use that only modestly exceed those of younger patients.98 Once discharged from the hospital, older patients report a high quality of life.99
Overall, fewer than 10% of cardiac surgical patients spend more than 48 hours in the ICU. Most survive and eventually report improved functional status and a reasonable quality of life.100,101
Key Points
American Heart Association. Heart disease and stroke statistics—2003 update. Dallas, TX: AHA; 2003.
Bashour CA, Yared JP, Ryan TA, et al. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit Care Med. 2000;28:3847-3853.
Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA guidelines for coronary artery bypass graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;34:1262-1347.
These are up-to-date guidelines for management of the cardiac surgical intensive care unit.
Jacka MJ, Cohen MM, To T, Devitt JH, Byrick R. The use of and preferences for the transesophageal echocardiogram and pulmonary artery catheter among cardiovascular anesthesiologists. Anesth Analg. 2002;94:1065-1071.
Montes FR, Sanchez SI, Giraldo JC, et al. The lack of benefit of tracheal extubation in the operating room after coronary artery bypass surgery. Anesth Analg. 2000;91:776-780.
van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
1 Gibbon JHJr. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171-185. passim
2 Garrett HE, Dennis EW, DeBakey ME. Aortocoronary bypass with saphenous vein graft: Seven-year follow-up. JAMA. 1973;223:792-794.
3 Hannan EL, O’Donnell JF, Kilburn HJr, et al. Investigation of the relationship between volume and mortality for surgical procedures performed in New York State hospitals. JAMA. 1989;262:503-510.
4 Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
5 Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
6 Pronovost PJ, Jenckes MW, Dorman T, et al. Organizational characteristics of intensive care units related to outcomes of abdominal aortic surgery. JAMA. 1999;281:1310-1317.
7 American Heart Association. Heart Disease and Stroke Statistics—2003 Update. Dallas, TX: AHA; 2003.
8 Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773-1780.
9 Ki BaeSung, et al. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
10 Thiele H, Neumann-Schniedewind P, et al. Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J Am Coll Cardiol. 2009;53(25):2324-2331.
11 Al-Ruzzeh S, Nakamura K, Athanasiou T, et al. Does off-pump coronary artery bypass (OPCAB) surgery improve the outcome in high-risk patients?: A comparative study of 1398 high-risk patients. Eur J Cardiothorac Surg. 2003;23:50-55.
12 Briguori C, Condorelli G, et al. Comparison of coronary drug-eluting stents versus coronary artery bypass grafting in patients with diabetes mellitus. Am J Cardiol. 2007;100(8):1330.
13 McCreath BJ, Swaminathan M, Booth JV, et al. Mitral valve surgery and acute renal injury: Port access versus median sternotomy. Ann Thorac Surg. 2003;75:812-819.
14 Suri RM, Schaff HV, et al. Thoracoscopic versus open mitral valve repair: a propensity score analysis of early outcomes. Ann Thoracic Surg. 2009;88(4):1190.
15 Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA Guidelines for Coronary Artery Bypass Graft Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1991 Guidelines for Coronary Artery Bypass Graft Surgery). American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;34:1262-1347.
16 Mohr R, Lavee J, Goor DA. Inaccuracy of radial artery pressure measurement after cardiac operations. J Thorac Cardiovasc Surg. 1987;94:286-290.
17 Slogoff S, Keats AS, Arlund C. On the safety of radial artery cannulation. Anesthesiology. 1983;59:42-47.
18 Safdar N, Kluger DM, Maki DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, non-cuffed central venous catheters: Implications for preventive strategies. Medicine (Baltimore). 2002;81:466-479.
19 Tuman KJ, McCarthy RJ, Spiess BD, et al. Effect of pulmonary artery catheterization on outcome in patients undergoing coronary artery surgery. Anesthesiology. 1989;70:199-206.
20 Connors AFJr, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276:889-897.
21 Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5-14.
22 Jacka MJ, Cohen MM, To T, et al. The use of and preferences for the transesophageal echocardiogram and pulmonary artery catheter among cardiovascular anesthesiologists. Anesth Analg. 2002;94:1065-1071.
23 Practice guidelines for pulmonary artery catheterization: An updated report by the American Society of Anesthesiologists Task Force on Pulmonary Artery Catheterization. Anesthesiology. 2003;99:988-1014.
24 American Heart Association. ACLS Provider Manual. Dallas, TX: AHA; 2001.
25 Greenson N, Macoviak J, Krishnaswamy P, et al. Usefulness of cardiac troponin I in patients undergoing open heart surgery. Am Heart J. 2001;141:447-455.
26 Januzzi JL, Lewandrowski K, MacGillivray TE, et al. A comparison of cardiac troponin T and creatine kinase-MB for patient evaluation after cardiac surgery. J Am Coll Cardiol. 2002;39:1518-1523.
27 Reichert CL, Visser CA, Koolen JJ, et al. Transesophageal echocardiography in hypotensive patients after cardiac operations: Comparison with hemodynamic parameters. J Thorac Cardiovasc Surg. 1992;104:321-326.
28 Schmidlin D, Schuepbach R, Bernard E, et al. Indications and impact of postoperative transesophageal echocardiography in cardiac surgical patients. Crit Care Med. 2001;29:2143-2148.
29 Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445-1450.
30 Royse CF, Royse AG, Soeding PF. Routine immediate extubation after cardiac operation: A review of our first 100 patients. Ann Thorac Surg. 1999;68:1326-1329.
31 Sviecevic V, Nierich AP, et al. Fast track anesthesia and cardiac surgery; a retrospective study of 7998 patients. Anesth Analg. 2009;108:727-733.
32 Montes FR, Sanchez SI, Giraldo JC, et al. The lack of benefit of tracheal extubation in the operating room after coronary artery bypass surgery. Anesth Analg. 2000;91:776-780.
33 Argenziano M, Chen JM, Choudhri AF, et al. Management of vasodilatory shock after cardiac surgery: Identification of predisposing factors and use of a novel pressor agent. J Thorac Cardiovasc Surg. 1998;116:973-980.
34 Morales DL, Gregg D, Helman DN, et al. Arginine vasopressin in the treatment of 50 patients with postcardiotomy vasodilatory shock. Ann Thorac Surg. 2000;69:102-106.
35 Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217-225.
36 Lee ME, Sethna DH, Conklin CM, et al. CK-MB release following coronary artery bypass grafting in the absence of myocardial infarction. Ann Thorac Surg. 1983;35:277-279.
37 Bonnefoy E, Filley S, Kirkorian G, et al. Troponin I, troponin T, or creatine kinase-MB to detect perioperative myocardial damage after coronary artery bypass surgery. Chest. 1998;114:482-486.
38 Lasocki S, Provenchere S, Benessiano J, et al. Cardiac troponin I is an independent predictor of in-hospital death after adult cardiac surgery. Anesthesiology. 2002;97:405-411.
39 Durbin CGJr, Kopel RF. Optimal atrioventricular (AV) pacing interval during temporary AV sequential pacing after cardiac surgery. J Cardiothorac Vasc Anesth. 1993;7:316-320.
40 Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061-1073.
41 Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: Predictors, outcomes, and resource utilization. Multicenter Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300-306.
42 Huikuri HV, Yli-Mayry S, Korhonen UR, et al. Prevalence and prognostic significance of complex ventricular arrhythmias after coronary arterial bypass graft surgery. Int J Cardiol. 1990;27:333-339.
43 Brembilla-Perrot B, Villemot JP, Carteaux JP, et al. Postoperative ventricular arrhythmias after cardiac surgery: Immediate- and long-term significance. Pacing Clin Electrophysiol. 2003;26:619-625.
44 Pinto RP, Romerill DB, Nasser WK, et al. Prognosis of patients with frequent premature ventricular complexes and nonsustained ventricular tachycardia after coronary artery bypass graft surgery. Clin Cardiol. 1996;19:321-324.
45 Chuttani K, Tischler MD, Pandian NG, et al. Diagnosis of cardiac tamponade after cardiac surgery: Relative value of clinical, echocardiographic, and hemodynamic signs. Am Heart J. 1994;127:913-918.
46 Pepi M, Muratori M, Barbier P, et al. Pericardial effusion after cardiac surgery: Incidence, site, size, and haemodynamic consequences. Br Heart J. 1994;72:327-331.
47 Bateman T, Gray R, Chaux A, et al. Right atrial tamponade complicating cardiac operation: Clinical, hemodynamic, and scintigraphic correlates. J Thorac Cardiovasc Surg. 1982;84:413-419.
48 Torelli J, Marwick TH, Salcedo EE. Left atrial tamponade: Diagnosis by transesophageal echocardiography. J Am Soc Echocardiogr. 1991;4:413-414.
49 Schwartz SL, Pandian NG, Cao QL, et al. Left ventricular diastolic collapse in regional left heart cardiac tamponade: An experimental echocardiographic and hemodynamic study. J Am Coll Cardiol. 1993;22:907-913.
50 Christenson JT, Aeberhard JM, Badel P, et al. Adult respiratory distress syndrome after cardiac surgery. Cardiovasc Surg. 1996;4:15-21.
51 Milot J, Perron J, Lacasse Y, et al. Incidence and predictors of ARDS after cardiac surgery. Chest. 2001;119:884-888.
52 Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
53 Curtis JJ, Nawarawong W, Walls JT, et al. Elevated hemidiaphragm after cardiac operations: Incidence, prognosis, and relationship to the use of topical ice slush. Ann Thorac Surg. 1989;48:764-768.
54 Diehl JL, Lofaso F, Deleuze P, et al. Clinically relevant diaphragmatic dysfunction after cardiac operations. J Thorac Cardiovasc Surg. 1994;107:487-498.
55 Kohorst WR, Schonfeld SA, Altman M. Bilateral diaphragmatic paralysis following topical cardiac hypothermia. Chest. 1984;85:65-68.
56 Unsworth-White MJ, Herriot A, Valencia O, et al. Resternotomy for bleeding after cardiac operation: A marker for increased morbidity and mortality. Ann Thorac Surg. 1995;59:664-667.
57 Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76:1680-1697.
58 Friedenberg WR, Myers WO, Plotka ED, et al. Platelet dysfunction associated with cardiopulmonary bypass. Ann Thorac Surg. 1978;25:298-305.
59 Salzman EW. Blood platelets and extracorporeal circulation. Transfusion. 1963;80:274-277.
60 Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med. 1998;128:194-203.
61 Badr KF, Ichikawa I. Prerenal failure: A deleterious shift from renal compensation to decompensation. N Engl J Med. 1988;319:623-629.
62 Myers BD, Moran SM. Hemodynamically mediated acute renal failure. N Engl J Med. 1986;314:97-105.
63 Wolman RL, Nussmeier NA, Aggarwal A, et al. Cerebral injury after cardiac surgery: Identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke. 1999;30:514-522.
64 Christenson JT, Schmuziger M, Maurice J, et al. Postoperative visceral hypotension the common cause for gastrointestinal complications after cardiac surgery. Thorac Cardiovasc Surg. 1994;42:152-157.
65 Mierdl S, Meininger D, Dogan S, et al. Abdominal complications after cardiac surgery. Ann Acad Med Singapore. 2001;30:245-249.
66 Raman JS, Kochi K, Morimatsu H, et al. Severe ischemic early liver injury after cardiac surgery. Ann Thorac Surg. 2002;74:1601-1606.
67 Butterworth JF, Royster RL, Prielipp RC, et al. Amrinone in cardiac surgical patients with left-ventricular dysfunction: A prospective, randomized placebo-controlled trial. Chest. 1993;104:1660-1667.
68 Royster RL, Butterworth JF, Prielipp RC, et al. Combined inotropic effects of amrinone and epinephrine after cardiopulmonary bypass in humans. Anesth Analg. 1993;77:662-672.
69 Dekkers RJ, FitzGerald DJ, Couper GS. Five-year clinical experience with Abiomed BVS 5000 as a ventricular assist device for cardiac failure. Perfusion. 2001;16:13-18.
70 Wassenberg PA. The Abiomed BVS 5000 biventricular support system. Perfusion. 2000;15:369-371.
71 Nose Y. Implantable total artificial heart developed by Abiomed gets FDA approval for clinical trials. Artif Organs. 2001;25:429.
72 Kowey PR, Taylor JE, Rials SJ, Marinchak RA. Meta-analysis of the effectiveness of prophylactic drug therapy in preventing supraventricular arrhythmia early after coronary artery bypass grafting. Am J Cardiol. 1992;69:963-965.
73 Daoud EG, Strickberger SA, Man KC, et al. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. N Engl J Med. 1997;337:1785-1791.
74 Van der Lugt JT, Mattioni T, Denker S, et al. Efficacy and safety of ibutilide fumarate for the conversion of atrial arrhythmias after cardiac surgery. Circulation. 1999;100:369-375.
75 Ommen SR, Odell JA, Stanton MS. Atrial arrhythmias after cardiothoracic surgery. N Engl J Med. 1997;336:1429-1434.
76 Elahi M, Hadjinikolaou L, Galinanes M. Incidence and clinical consequences of atrial fibrillation within 1 year of first-time isolated coronary bypass surgery. Circulation. 2003;108(Suppl 1):II207-II212.
77 Lee JK, Klein GJ, Krahn AD, et al. Rate-control versus conversion strategy in postoperative atrial fibrillation: Trial design and pilot study results. Card Electrophysiol Rev. 2003;7:178-184.
78 Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834-1840.
79 Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
80 A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576-1583.
81 Ilabaca PA, Ochsner JL, Mills NL. Positive end-expiratory pressure in the management of the patient with a postoperative bleeding heart. Ann Thorac Surg. 1980;30:281-284.
82 Zurick AM, Urzua J, Ghattas M, et al. Failure of positive end-expiratory pressure to decrease postoperative bleeding after cardiac surgery. Ann Thorac Surg. 1982;34:608-611.
83 Moulton MJ, Creswell LL, Mackey ME, et al. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J Thorac Cardiovasc Surg. 1996;111:1037-1046.
84 Anthi A, Tzelepis GE, Alivizatos P, et al. Unexpected cardiac arrest after cardiac surgery: Incidence, predisposing causes, and outcome of open chest cardiopulmonary resuscitation. Chest. 1998;113:15-19.
85 Holmes CL, Walley KR. Bad medicine: Low-dose dopamine in the ICU. Chest. 2003;123:1266-1275.
86 Corwin HL, Lisbon A. Renal dose dopamine: Long on conjecture, short on fact. Crit Care Med. 2000;28:1657-1658.
87 Caimmi PP, Pagani L, Micalizzi E, et al. Fenoldopam for renal protection in patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2003;17:491-494.
88 Dehne MG, Klein TF, Muhling J, et al. Impairment of renal function after cardiopulmonary bypass is not influenced by dopexamine. Ren Fail. 2001;23:217-230.
89 Lisbon A. Dopexamine, dobutamine, and dopamine increase splanchnic blood flow: What is the evidence? Chest. 2003;123(5 Suppl):460S-463S.
90 Sirivella S, Gielchinsky I, Parsonnet V. Mannitol, furosemide, and dopamine infusion in postoperative renal failure complicating cardiac surgery. Ann Thorac Surg. 2000;69:501-506.
91 Bent P, Tan HK, Bellomo R, et al. Early and intensive continuous hemofiltration for severe renal failure after cardiac surgery. Ann Thorac Surg. 2001;71:832-837.
92 NICE-SUGAR Study InvestigatorsFinfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
93 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301-1308.
94 Jardin F, Farcot JC, Boisante L, et al. Influence of positive end-expiratory pressure on left ventricular performance. N Engl J Med. 1981;304:387-392.
95 Michalopoulos A, Anthi A, Rellos K, Geroulanos S. Effects of positive end-expiratory pressure (PEEP) in cardiac surgery patients. Respir Med. 1998;92:858-862.
96 Bridgewater B, Grayson AD, Jackson M, et al. Surgeon specific mortality in adult cardiac surgery: Comparison between crude and risk stratified data. BMJ. 2003;327:13-17.
97 Clough RA, Leavitt BJ, Morton JR, et al. The effect of comorbid illness on mortality outcomes in cardiac surgery. Arch Surg. 2002;137:428-432. discussion 432-3
98 Avery GJ2nd, Ley SJ, Hill JD, et al. Cardiac surgery in the octogenarian: Evaluation of risk, cost, and outcome. Ann Thorac Surg. 2001;71:591-596.
99 Fruitman DS, MacDougall CE, Ross DB. Cardiac surgery in octogenarians: Can elderly patients benefit? Quality of life after cardiac surgery. Ann Thorac Surg. 1999;68:2129-2135.
100 Treasure T, Holmes L, Loughead K, Gallivan S. Survival and quality of life in patients with protracted recovery from cardiac surgery: Can we predict poor outcome? Eur J Cardiothorac Surg. 1995;9:426-431. discussion 431-2
101 Bashour CA, Yared JP, Ryan TA, et al. Long-term survival and functional capacity in cardiac surgery patients after prolonged intensive care. Crit Care Med. 2000;28:3847-3853.
102 DeLaria GA, Tyner JJ, Hayes CL, Armstrong BW. Heparin-protamine mismatch: A controllable factor in bleeding after open heart surgery. Arch Surg. 1994;129:944-950. discussion 950-1