Chapter 4 Management of the Patient with Rhinosinusitis
Introduction
Rhinosinusitis is one of the most common and prevalent illnesses throughout the world. Both acute and chronic forms of rhinosinusitis (RS) have been described, and affect patients of all ages. By a variety of reports, the incidence of RS is increasing, and the number of patients diagnosed around the world with RS continues to climb.1 Allergy plays a role in the pathogenesis and expression of symptoms among patients with both acute and chronic RS, although the precise relationship between atopy and the presence of sinus disease can be difficult to define. Due to the heterogeneity seen in acute and chronic RS, consensus panels have been convened to provide clarity to the diagnosis of these common conditions, and to develop guidelines for further research and optimal treatment. This chapter will discuss the various types of RS, diagnostic strategies, and treatment options for both acute and chronic rhinosinusitis.
Prevalence and Burden of Rhinosinusitis
From a variety of sources, it has been noted that RS is increasing in prevalence. In the USA alone it is estimated to affect as many as 31 million patients on an annual basis.2 It accounts for billions of dollars in expenditure, with significant increases in direct and indirect costs noted over a several year period.3 In addition, 15% of the US population is symptomatic from sinusitis for a period of at least 3 months annually.4
While most adults will experience between two and four acute upper respiratory infections annually, less than 2% of these episodes are due to acute bacterial rhinosinusitis (ABRS).5 By calculation, there would therefore be an expected 20000000 cases of ABRS in the USA alone on a yearly basis. RS was estimated to account for 9% of pediatric and 21% of adult prescriptions in the USA annually,6 with over $3.5 billion USD spent in 1999 funds for the treatment of ABRS alone.7
Two recent studies have examined the burden of chronic rhinosinusitis (CRS) on both patient impact and financial cost. Bhattacharyya followed 322 patients with CRS prospectively over a 1-year period.8 During that 1-year period, patients received an average of 2.7 courses of oral antibiotics, and used nasal steroids for over 18 weeks. In addition, patients missed an average of 4.8 days of work related to CRS and had mean medical expenses of US$921. In a second study by Murphy et al, over 200 000 patients in a health maintenance organization (HMO) were surveyed during the 1-year period 1994.9 Approximately 10% of patients in the HMO were identified with diagnoses of CRS during this one year. Among this patient group, CRS was responsible for an average of 2.0 office visits and 5.1 pharmacy fills per patient during 1994. Extrapolating to the general US population, this study estimated the overall direct cost of CRS in 1994 to be US$4.3 billion.
In addition to the clear economic burden of both acute and chronic RS, both of these diseases exert significant impact on patient symptoms and quality of life. Gliklich and Metson assessed this impact, and noted that RS is associated with a more negative quality of life than other common medical diseases such as chronic obstructive pulmonary disease and lower back pain.10
Definition of Rhinosinusitis
The term that is currently accepted by the majority of specialists who manage patients with acute and chronic illnesses affecting the paranasal sinuses is rhinosinusitis. While there is still some debate regarding the use of this term, and while there are individuals who prefer the older term sinusitis, rhinosinusitis is the accepted term agreed to by a consensus group of American medical societies, including the American Academy of Allergy, Asthma and Immunology (AAAAI), the American Academy of Otolaryngic Allergy (AAOA), the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS), the American College of Allergy, Asthma and Immunology (ACAAI), and the American Rhinologic Society (ARS).11 The choice of the term rhinosinusitis implies that the physiological condition of the sinuses correlates closely with the concurrent condition of the nose. Nasal disease, i.e. rhinitis, usually precedes the development of sinus disease, and sinusitis is rare without rhinitis.1,11,12 Given the contiguity of the nasal and sinus mucosa and the interdependency of the nose and sinuses, the term rhinosinusitis (RS) appears to be a more representative name for this process, and will be used in the current chapter.
In 1996, a task force of the AAO-HNS reviewed the current state of knowledge in RS, and drafted a definition statement based upon this expert panel. They defined RS as “a condition manifested by an inflammatory response involving the following: the mucous membranes (possibly including the neuroepithelium) of the nasal cavity and the paranasal sinuses, fluids within these cavities, and/or underlying bone.”1 The key message implied from this definition is that RS is primarily an inflammatory disease, not an infectious disease. Infection is only one type of pathophysiological process that can affect the sinuses. In addition, recognizing the close relationship between the nose and sinuses, a conjoint conference involving the American allergy/immunology and otolaryngology communities concluded that “rhinosinusitis may be a more appropriate term than either rhinitis or sinusitis alone.”12 Similar definitions have been applied by the European allergy and otolaryngology communities.13
In 2003, the Sinus and Allergy Health Partnership (SAHP), a conjoint society made up of representatives from the AAOA, the AAO-HNS, and the ARS, also met to consider the knowledge base around RS, and drafted a similar definition to that offered in 1996. The SAHP defined RS as “a group of disorders characterized by inflammation of the mucosa of the nose and the paranasal sinuses.”14 The 2004 consensus panel decided to “endorse and adopt” this definition for framing future research strategies and diagnostic and treatment methodologies.11 The members of this panel recognized that inflammatory processes simultaneously affect the nose and sinuses, and that the term rhinosinusitis best describes this concurrent process.
Classification of Rhinosinusitis
▪ RS: 1997 Consensus
In the 1997 system, the diagnosis of RS was based purely on patient symptoms. These symptoms were broken down into major and minor factors that were associated with the presence of RS. These major and minor factors are noted in Box 4.1. It is important to note that headache or facial pain are not sufficient symptoms for the diagnosis of RS in the absence of other major or minor factors, primarily nasal in origin. These criteria are useful in assisting the clinician in the consideration of the diagnosis of acute or chronic RS, but do not require the presence of confirmatory findings on either physical examination or radiological imaging to apply a diagnosis. This lack of confirmation was felt to be a weakness in the 1997 document.
BOX 4.1 Factors associated with a diagnosis of rhinosinusitis.
(Adapted from Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117:S1–S7)
a Facial pain/pressure alone does not constitute a suggestive history for rhinosinusitis in the absence of another major nasal symptom or sign.
b Fever in acute sinusitis alone does not constitute a strongly suggestive history for acute rhinosinusitis in the absence of another major nasal symptom or sign.
The 1997 task force also described the temporal course of RS and divided RS further into five categories, three primary categories: acute rhinosinusitis, recurrent acute rhinosinusitis, and chronic rhinosinusitis; and two derivative categories: subacute rhinosinusitis and acute exacerbations of chronic rhinosinusitis. The three primary groupings are often used to delineate the time course of RS in individual patients and to assist in the development of appropriate treatment modalities (Figure 4.1).
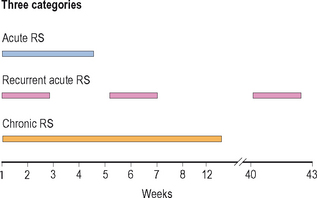
Figure 4.1 Temporal course of rhinosinusitis: acute, recurrent acute, and chronic.
(Adapted with permission from Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 1997;117:S1–S7.)
Acute Rhinosinusitis
Acute rhinosinusitis (ARS) is a very common condition, and generally has a sudden onset of symptoms, often following a viral upper respiratory infection. It is defined as lasting from 1 day to 4 weeks. It is important to distinguish between a viral upper respiratory infection, which is very common, and an acute bacterial rhinosinusitis. The most robust method of distinguishing these two conditions is the temporal course of the illness. Viral symptoms generally peak within 3 or 4 days of onset and then decline in severity. By contrast, acute bacterial RS generally worsens after 5 days and persists longer than 10 days.15 In addition, certain physical signs are suggestive of a diagnosis of acute bacterial RS. Facial erythema or swelling are associated with bacterial disease, although they are uncommon. Maxillary tooth pain is correlated well with the presence of bacterial disease, and when accompanied with fever often confirms the presence of acute bacterial RS.16
Recurrent Acute Rhinosinusitis
Many patients may experience episodes of acute bacterial RS that last 7–10 days and respond rapidly to treatment, yet seem to have frequent recurrent episodes with a similar pattern. Between symptomatic episodes, these patients may be clear of any symptoms of nasal or sinus disease. The frequent recurrent nature of these episodes suggests a separate categorization of disease, which has been called recurrent acute RS. While there are not strict criteria for the diagnosis of this process, some authors have suggested that patients should experience at least four episodes of acute RS within a year, and that intervening periods without symptoms should be at least 8 weeks.1,17 It is not clear whether recurrent acute RS is a distinct pathophysiological entity, reflecting a unique underlying process, or if it simply reflects random variation in the frequency of disease.
Chronic Rhinosinusitis
Chronic rhinosinusitis (CRS) is in many ways a distinct pathophysiological process that reflects the chronic inflammatory nature of the disease. It is not simply acute disease that has not resolved or has progressed into chronic disease. From a temporal standpoint, CRS is generally classified as persistent sinonasal symptoms for at least 12 weeks,11,18 although some systems suggest that 8 weeks may be adequate to classify the disease as CRS.19 CRS likely has numerous mechanisms involved in its pathogenesis, and is probably best considered as a syndrome rather than a unique pathophysiological entity. Recent classification systems make clear distinctions between CRS with the presence of nasal polyps (CRSwNP) and CRS without the presence of nasal polyps (CRSsNP).11,13
▪ RS: 2004 Consensus
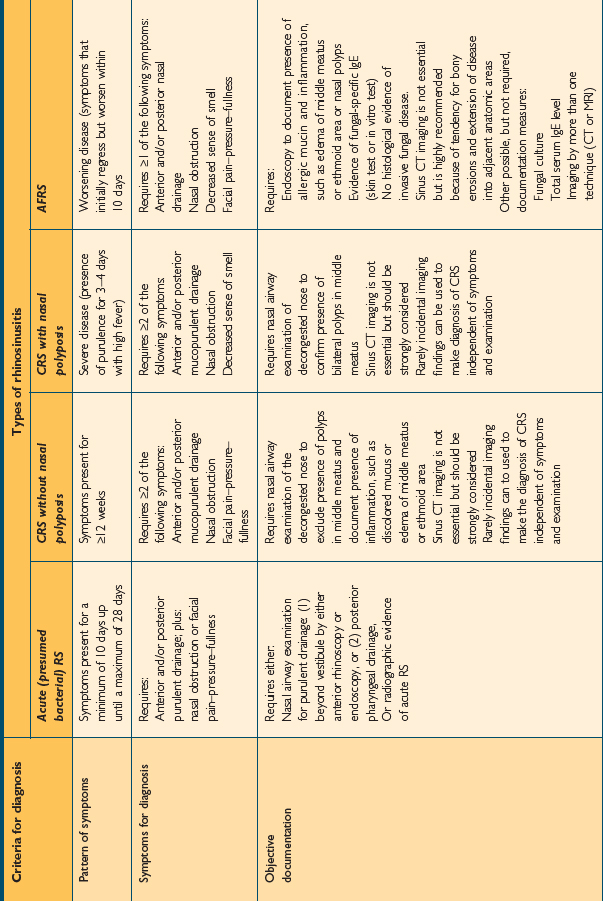
The 2004 consensus document broadens the criteria necessary for the diagnosis of RS. While the document is designed to promulgate diagnostic criteria for research studies and clinical trials, it is practical to include some element of objective evidence into diagnostic practices when managing the patient with presumed RS. This document sets out specific criteria for the diagnosis of various forms of RS based on patient symptoms, the pattern of those symptoms, and the presence of objective documentation. It describes specific definitions for patient care. These consensus criteria are noted in Table 4.1.
TABLE 4.1 Rhinosinusitis consensus definitions for patient care. (Source: Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg 2004;131:S1–S62)
Allergic Fungal Rhinosinusitis
Allergic fungal rhinosinusitis (AFRS) is classically described as a type of CRS with or without polyposis that is associated in its expression and pathogenesis with an IgE-mediated response to one or more families of fungal organisms. These organisms are present in the sinuses of patients with AFRS, and patients will demonstrate an IgE-mediated hypersensitivity reaction to those fungi, either by in vitro specific IgE testing or through skin tests for relevant fungi. AFRS is not diagnosed in the absence of confirmatory IgE testing, despite the presence of fungi on either culture or histopathology. The diagnosis of AFRS requires the presence of nasal and sinus symptoms for at least 12 weeks, including anterior/posterior mucopurulent drainage, nasal obstruction, and facial pain-pressure-fullness. It also requires a nasal airway examination demonstrating allergic mucin and inflammation, with or without nasal polyps. CT imaging is important to demonstrate the extent of the disease. In addition, physical examination and CT imaging should not demonstrate the presence of changes consistent with invasive fungal sinusitis.
Anatomy of the Paranasal Sinuses
Ethmoid Sinuses
The anatomy of the ethmoid sinuses is variable. They can be best viewed as a group of discrete cells separated into anatomic regions by a series of bony partitions. These cells can be grouped into two units: the anterior ethmoid cells, which are numerous and smaller in size, and the posterior ethmoid cells, which are less numerous and larger.
Frontal Sinuses
The sinus chambers are lined with a ciliated pseudostratified columnar respiratory-type epithelium. This mucosa is similar histologically to that seen in the nose and the lower respiratory tract (Figure 4.2). This histological similarity is one reason that inflammatory respiratory diseases commonly affect more than one portion of the respiratory tract simultaneously. This epithelium secretes mucus, which is swept from the interior of the sinuses to the ostia and into the nose by fine cilia on the surface of the mucosal cells. This mucus blanket traps particles in the nose and sinuses, and transports these particles posteriorly into the nasopharynx, where they then can be swallowed. Particulate matter that can affect the nasal and sinus mucosa includes bacteria, viruses, irritants, and allergens.
Figure 4.2 Appearance of the normal respiratory epithelium of the nose and paranasal sinuses.
(Reproduced with permission from Minshall E, Ghaffar O, Cameron L, et al. Assessment by nasal biopsy of long-term use of mometasone furoate aqueous nasal spray in the treatment of perennial rhinitis. Otolaryngol Head Neck Surg 1998;118:648–654.)
Physiology of the Sinuses in Health and Disease
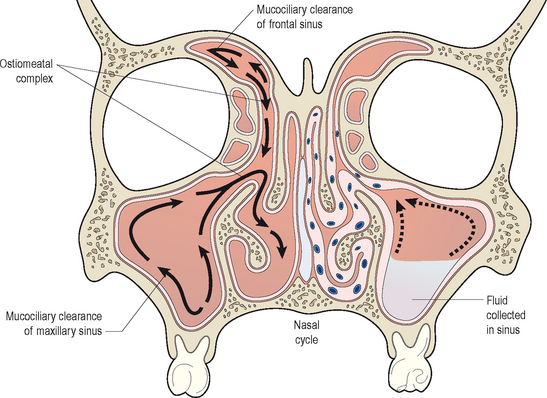
In healthy sinuses, mucus is secreted by goblet cells within the epithelium and transported to the ostia of the sinuses for clearance into the nose. This process for the transport of mucus within the nose and sinuses is known as mucociliary clearance. Through active action of the cilia, mucus is easily moved from the periphery of the sinuses toward the ostia. This mucociliary clearance is generally rapid and efficient, and prevents the accumulation of mucus and particulate debris within the sinus chambers. It is essential for normal sinus health and function (Figure 4.3). Mucociliary clearance patterns within the sinuses are genetically directed to maintain the flow of mucus from the sinuses through the sinus ostium and into the nose itself.20
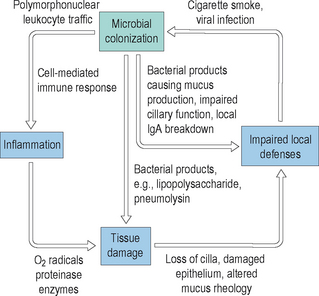
By contrast, when normal mucociliary clearance is inhibited, either through local physiological changes or through anatomic obstruction, the sinuses become diseased. It is clear that ventilation of the sinuses and drainage of mucus out of the sinuses maintains the normal physiology of the sinuses. Disruptions to this normal ventilation and drainage is a central factor in the pathogenesis of acute and chronic rhinosinusitis.21 Many factors can adversely influence the clearance of mucus from the sinuses, and thereby result in acute and chronic changes. Mucociliary clearance can be directly affected by ciliostatic agents, such as tobacco smoke and viral infections. Both irritants such as smoke and viral disease disrupt the cilial layer in the sinuses, resulting in cilial loss and impaired cilial motility. With impairment of the normal clearance mechanisms of the sinus mucosa, mucus stasis occurs and bacterial colonization can begin. As bacteria colonize the sinuses, they can proliferate within the mucus layer and can cause localized acute inflammation. This inflammation will cause tissue edema and ostiomeatal narrowing, which can result in additional injury to the mucosa. In addition, any structural obstruction to the ostia or significant decrease in their diameter will further impair mucociliary clearance and lead to increased bacterial growth. In response to the increased presence of bacteria, normal host mechanisms will recruit neutrophils and other inflammatory cells into the sinuses, resulting in increased local inflammation and secondary injury to the mucosa. An illustration of this inflammatory cycle is displayed in Figure 4.4.
The consequence of this cyclical inflammation is the creation of conditions that support the development of both acute and chronic rhinosinusitis. As also demonstrated in Figure 4.3, in contrast to the normal physiology of the healthy sinuses, in diseased sinuses conditions exist that favor the proliferation of bacteria and the development of symptoms. In diseased sinuses pressure is created from tissue edema, resulting in contact between the mucous membranes of the nose, turbinates, and sinuses. This contact stimulates nerve fibers resulting in localized pain. In addition, bacteria multiply within the chambers of the sinuses, resulting in additional pain as mucosal swelling increases. In addition, fever is common in response to the presence of infection and the host response generated by the infection. It is clear from this model that normal mucociliary clearance is essential in the normal physiological functioning of the sinuses.
Pathophysiology of Acute and Chronic Rhinosinusitis
▪ Acute (Presumed Viral) Rhinosinusitis
Most upper respiratory infections are viral, and are therefore self-limited. These viral infections can be classified as viral rhinosinusitis, but are often referred to as resulting in a common cold. A range of viruses can affect the nose and sinuses, including rhinovirus, adenovirus, and influenza virus. While rhinovirus does not generally cause injury to the nasal epithelium itself, both adenovirus and influenza virus can cause significant mucosal injury.22,23 Viral infections of the nose and sinuses cause an upregulation of a variety of acute inflammatory mediators, including bradykinin, histamine, and interleukins 1, 6, and 8. In addition, viral infections trigger a suppression of the normal immune functions of effector cells such as macrophages and neutrophils.24 As a consequence of this immunosuppressive effect, the mucosa of the nose and sinuses is more easily infected secondarily by bacteria following viral infections.
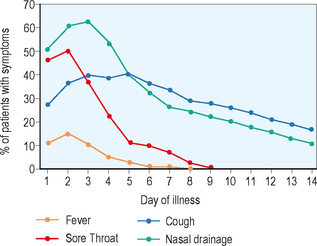
The symptoms of viral rhinosinusitis are triggered by activation of the parasympathetic nervous system and various inflammatory pathways that lead to the symptoms experienced during the common cold: fever, myalgias, rhinorrhea, and pharyngitis. While the more acute symptoms related to viral infections can be resolved within 5 days, some symptoms such as nasal congestion and cough may persist for several weeks following the acute onset of the infection. While most of the acute symptoms of the viral upper respiratory infection will resolve by days 7–10, when symptoms worsen after the first 5–7 days, or when symptoms such as fever and purulent rhinorrhea persist for longer than 7–10 days, the likelihood of the patient having an acute bacterial rhinosinusitis are significantly increased (Figure 4.5).5
▪ Acute Bacterial Rhinosinusitis
As noted here, persistent or worsening upper respiratory symptoms suggest the diagnosis of acute bacterial rhinosinusitis (ABRS). Patients with ABRS will usually present with symptoms of fever, purulent rhinorrhea, nasal congestion, cough, anosmia, and related aural symptoms such as fullness and blockage. ABRS is a bacterial infection of the nose and paranasal sinus mucosa that often is preceded by a viral upper respiratory infection. As mucociliary clearance mechanisms are disrupted by viral infection and the subsequent immune-mediated events that occur from viral disease, the mucosa of the nose and sinuses is increasingly sensitive to infection by bacterial organisms. Ostial obstruction and ciliary dysfunction lead to bacterial proliferation with acute changes of active bacterial infection.
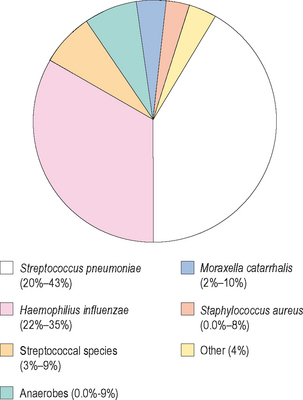
ABRS generally occurs in adults and children as a result of infections due to one of three organisms: Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis.5 The latter organism is primarily seen as a pathogen in children, while the other two organisms can be recovered in patients with ABRS across the lifespan. While the nasopharynx is commonly colonized with these organisms, they do not generally become pathogenic without injury or infection to the normal respiratory epithelium. S. pneumoniae is the most common organism recovered in cases of ABRS, with prevalence rates of 20–43%.5 H. influenzae follows this organism closely in frequency of species recovered, with prevalence rates of 22–35%.5 By contrast, M. catarrhalis is seen in 2–10% of adults with ABRS, but in 15–20% of children with ABRS.5 Other pathogens are less commonly seen, and include other streptococcal species, anaerobic organisms, and Staphylococcus aureus (Figure 4.6).
▪ Chronic Rhinosinusitis
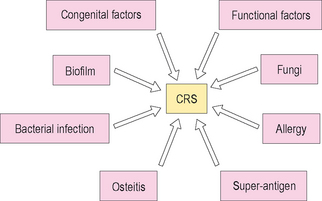
In contrast to ABRS, chronic rhinosinusitis (CRS) is a more variable disease, and is not as well characterized. Issues in precisely defining CRS have limited broad-scale research into this disease, and have impaired understanding of the pathophysiological etiology of this common disease. It is probably best to conceptualize CRS as a syndrome rather than as a distinct pathological entity. This model will allow consideration of the various potential mechanisms that may be active in an individual who presents with symptoms suggestive of CRS (Figure 4.7).
As noted earlier CRS is generally characterized by rhinorrhea, nasal congestion, nasal obstruction, and pain and pressure within the sinuses that persists for longer than 12 weeks in duration. Histopathologically, CRS is distinguished by basement membrane thickening, submucosal fibrosis and edema, goblet cell hyperplasia, and chronic and persistent inflammation.14 CRS is associated with partial occlusion and obstruction of the sinus ostia, which leads to stasis of mucus and secondary bacterial colonization. It is also associated with chronic changes to the nasal and sinus mucosa, resulting in chronic hyperplasia and the development of polypoid disease within the nose and sinuses.25
In patients who have both CRS and IgE-mediated inhalant allergy, a cellular infiltrate is seen within the sinuses. This infiltrate is characterized by the presence of eosinophils and both T and B lymphocytes.26,27 In addition, there is a higher concentration of IL-4, IL-5, and IL-13 in the mucosa of sinuses among patients with both allergic disease and CRS than in normal control subjects.28 The presence of inflammatory chemokines in patients with CRS is associated with the accumulation of inflammatory cells, such as eosinophils, which have often been described as the primary cell involved in the pathogenesis of CRS.29,30
The subepithelial layer of the sinus mucosa consists of bundles of collagen fibers and ground substance.31 With ongoing injury to the mucous membrane of the upper respiratory tract, there is edema of the stroma, resulting in cellular infiltration by eosinophils and other inflammatory cells.32 Eosinophils are activated by a number of mediators in the pathogenesis of CRS, with the release of toxic proteins such as eosinophilic cationic protein (ECP) and major basic protein (MBP).33,34 These and other cellular mediators trigger significant chronic change in the mucosa of the sinuses.
In another model of CRS, Schubert discusses the role of T-cell-mediated inflammation triggered by nonspecific bacterial exotoxins.35 This model has been described as the superantigen hypothesis, and suggests that bacterial exotoxins may exert immune-mediated mucosal damage through activating inflammation through an alternative pathway. These exotoxins, which have been primarily reported with Staph. aureus, bind to a portion of the alpha chain of the human leukocyte antigen major histocompatibility complex II (HLA-II) molecule, at a separate site from the antigen-specific region of the molecule. T-cell mediators are upregulated, including IL-4, IL-5, IL-13, and interferon gamma (IFN-γ), and progressive inflammation can be triggered. In this superantigen model, persistent stimulation from exotoxins can incite chronic changes in the respiratory mucosa, and may be responsible for diseases such as chronic rhinosinusitis, allergic fungal rhinosinusitis, and asthma. Other researchers have also supported the role of superantigen-mediated inflammation in CRS.36,37
Another potential mechanism involved in the pathogenesis of CRS is the role of biofilms. Biofilms are colonies of bacteria and other microbes that cluster together in a state of decreased metabolic activity, yet actively communicate in maintaining the stability and persistence of the colony. They have been found in a variety of chronic and persistent infectious conditions, including dental plaque, indwelling catheters, and implantable medical devices such as heart valves and joint replacements. Bacterial biofilms have been found in numerous patients with CRS over the past few years, yet appear to be rare in the sinus mucosa of individuals without CRS.38,39 Biofilms are able to persist because they form a community of organisms that is extremely resistant to antibiotic treatment, and chronic infection is a common sequela of the presence of these colonies. Since biofilms are extremely resistant to treatment with current medical therapies, surgical debridement of these biofilms is often necessary to eradicate the bacteria and bring the infection under control. The extent to which biofilms are responsible for ongoing inflammation in CRS, as opposed to their role as opportunistic pathogens, is yet to be appreciated.
▪ Allergic Fungal Rhinosinusitis
In the development of AFRS, an atopic host is exposed to one of several types of dematiaceous fungi through normal nasal respiration, which provides the basis for the IgE-mediated immune response. The type of response is not different from that noted in other allergic nasal conditions (see Chapter 3), although the significant degree of polypoid change and ostial occlusion associated with this inflammatory response provokes additional pathophysiological changes. Due to ostial occlusion, fungal proliferation occurs, as does an ongoing robust allergic response to these fungal organisms. Allergic mucin is produced, and the net result of these inflammatory changes is an expansile inflammatory mass that can cause bony remodeling and impingement upon intracranial and intraorbital structures.
Diagnosis of the Patient with Rhinosinusitis
As in most areas in medicine, the most important element of the diagnostic process in evaluating the patient with suspected rhinosinusitis is the history. The 1997 guidelines have been reviewed above, and form a foundation for reaching a presumptive diagnosis of acute or chronic RS. These 1997 guidelines again suggest a list of major and minor factors that would suggest to the clinician the diagnosis of RS. These factors are reproduced in Table 4.1.
Imaging studies
The primary methodology that should be used to image the sinuses is the computed tomographic (CT) scan of the sinuses. CT scanning is rapid and safe, and newer methods have very low exposure to radiation. CT scans allow illustration of the ethmoid sinuses, which can be difficult to visualize on standard plane radiographs, as well as demonstrating the fine anatomy of the sinus ostia as they communicate with the nose. CT scans in the coronal plane are the most valuable for rapidly evaluating the sinuses, as they bring the sinus ostia into visualization most readily. Axial scans can also be useful in evaluating for neoplastic changes in the nose and sinuses, as well as in surgical planning.
Allergy testing
In patients suspected of having significant inhalant allergy concurrent with CRS, allergy testing using either in vitro or in vivo techniques can be useful. Since there is a higher prevalence of allergy among patients with CRS than among matched controls without CRS, it would appear that allergy plays a role in at least the expression of symptoms among these patients. Methods of allergy testing are discussed in Chapter 2.
Treatment of the Patient with Rhinosinusitis
▪ Acute (Presumed Viral) Rhinosinusitis
Patients with presumed viral ARS should be treated with medications to reduce their acute symptoms. Systemic analgesics and antipyretics such as acetaminophen or ibuprofen are frequently prescribed in both adults and children with viral upper respiratory infections. Salicylates should be avoided in suspected viral infections in children due to an association with Reye’s syndrome. In addition, topical and systemic decongestants can be of use on a short-term basis to reduce the nasal blockage that usually accompanies these infections. Oxymetazoline or phenylephrine can be used topically as nasal sprays to reduce congestion and improve nasal airflow. These agents work as topical vasoconstrictors, and rapidly decrease vascular inflow to the nasal turbinates, reducing turbinate bulk and improving nasal ventilation. These agents do have the potential for rebound congestion, and should only be used for brief periods of time lasting 3–4 days at the maximum. Systemic decongestants such as pseudoephedrine and phenylephrine can also be recommended for patient use, although they are not as efficacious in reducing nasal congestion and are often accompanied by significant side effects such as nervousness, palpitations, and insomnia.
▪ Acute (Presumed Bacterial) Rhinosinusitis
As was discussed earlier, the primary pathogens responsible for ABRS are S. pneumoniae and H. influenzae. Other organisms are present in ABRS, including both Staph. aureus and M. catarrhalis, although these organisms are somewhat less common (Figure 4.6). For that reason, when antibiotics are prescribed for treating ABRS, consideration must be given to the microbiology of the disease and the most prevalent organisms.
An additional factor that must be considered in choosing antibiotics for the treatment of ABRS is the patterns of resistance that continue to develop. Penicillin resistance among S. pneumoniae varies around the world, but is generally present in at least 25–30% of specimens cultured. In addition, macrolide resistance to this organism parallels penicillin resistance, and is generally at least 30% in most areas. Further, beta-lactamase producing strains of H. influenzae are also quite common, again in excess of 30% of specimens cultured, and essentially all specimens of M. catarrhalis are beta-lactamase producing. Beta-lactam drugs such as penicillins and cephalosporins are therefore inactive in treating these resistant strains. Antibiotic selection must therefore take into account knowledge of the local resistance rates for these common pathogens, and physicians must monitor the effectiveness of antibiotics prescribed to insure response to therapy.5
While symptomatic medications are used to reduce the pain, fever, and congestion often associated with ABRS, antibiotics are prescribed to eradicate bacteria from the site of infection. By clearing the bacterial burden, antibiotic therapy assists the sinuses in returning to a healthy physiological state, decreases the length of symptoms, allows patients to return to activity more rapidly, and lessens the likelihood of the patient developing a complication of sinusitis such as meningitis. These severe complications, however, are uncommon.5
Based upon a consideration of microbial resistance patterns and the common prevalent pathogens seen in ABRS, the Sinus and Allergy Health Partnership in 2004 published a specific series of recommendations on types of antibiotic medications to be used in the treatment of ABRS. These guidelines are illustrated in Figure 4.8. Amoxicillin, with or without clavulanate, remains a common yet effective treatment for adults and children with ABRS. The addition of clavulate will be of assistance in treating beta-lactamase-producing strains of H. influenzae, which is a common pathogen in children with ABRS. Amoxicillin alone continues to have efficacy in most patients with ABRS, although doses often must be increased to overcome intermediate resistance. The 2004 SAHP guidelines suggest a daily dose of 4 g in adults (2 g orally twice daily), and 90 mg/kg/day in children, given in divided twice-daily doses. Cephalosporin medications are alternative drugs for treating ABRS, and later generation agents have good stability in the presence of beta-lactamase-producing organisms. They are often less effective, however, against S. pneumoniae. Recommended cephalosporins include cefuroxime, cefpodoxime, and cefdinir. In patients with allergies to beta-lactam drugs, alternate agents can be chosen. These include trimethoprim/sulfamethoxazole, doxycycline, and macrolide medications such as clarithromycin and azithromycin.
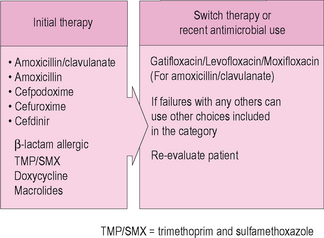
Figure 4.8 Guidelines in the selection of antibiotics for acute bacterial rhinosinusitis.
(Adapted from Anon JB, Jacobs MR, Poole MD, et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg 2004;130:S1–S45.)
In addition to treatment with antibiotics, topical nasal corticosteroid sprays can provide additional benefit in the management of the patient with ABRS. Studies have demonstrated more rapid symptom reduction among patients using topical steroid sprays than those treated with antibiotics alone. Doses of these agents are generally higher than would be used in the treatment of allergic rhinitis, and the drugs are delivered twice daily rather than once daily. Agents that have shown benefit as adjuvants to antibiotic treatment of ABRS include fluticasone propionate and mometasone furoate.40,41
▪ Chronic Rhinosinusitis, with and without Nasal Polyps
As has been discussed previously, chronic rhinosinusitis (CRS) is pathophysiologically a distinct condition from ABRS, not simply the chronic progression of acute bacterial disease. For that reason, treatment of CRSwNP and CRSsNP is much different than the treatment of ABRS. The most appropriate treatment of CRS continues to be unclear in the absence of a uniform agreement concerning its pathogenesis. Most experts will agree, however, that the primary treatment of CRS remains medical therapy, with surgery reserved for patients who fail aggressive, appropriate and maximal medical management.42 Maximal medical management of CRS usually involves the use of broad-spectrum antibiotics to control the acute bacterial component of the patient’s symptoms, as well as topical intranasal corticosteroid sprays, and oral and topical decongestants,14,42 although prospective clinical trials have not been conducted to evaluate these treatment approaches. Appropriate medical therapy, however, appears to control nasal and sinus symptoms in about 80% of patients with CRS.43
Infection
In patients who have had several courses of antibiotics without improvement, one consideration is the presence of an unusual or resistant organism. In those cases, endoscopically guided cultures of the middle meatus can be useful. Culture and sensitivity data from this technique can then be used to guide selection of an appropriate antibiotic. Again, this antibiotic should be considered for use for a period of at least 4 weeks.
Allergy
Allergy can play a significant role in the expression of symptoms among patients with CRS, and can contribute to the pathogenesis of CRS through interacting with other inflammatory mechanisms. Allergy has been shown to be a predictor of the severity of symptoms among patients with CRS, and the treatment of the allergic component of the disease among atopic individuals can be of benefit. Allergy management should include treatment of the patient’s rhinitis with appropriate pharmacotherapy and immunotherapy. A further discussion of allergic rhinitis and its treatment can be found in Chapter 3.
Surgical Treatment
In patients with evidence of significant obstruction of the sinus ostia, or in patients with severe sinonasal polyposis, surgical treatment of the sinuses can be useful. The goals of surgery are to remove the burden of any obstructive tissue, to eliminate any sources of chronic infection, such as diseased bone, and to improve ostial patency, thereby maximizing mucociliary function and ventilation and drainage of the sinuses. Surgery of the sinuses is best conceptualized as a tool in the comprehensive management of patients with CRS, and is most useful when done along with careful preoperative and postoperative medical therapy.
Treatment Recommendations for the Patient with CRS
In order to maximize the reduction in patient symptoms from CRS, a comprehensive strategy for medical management is essential. Since patients with CRS often present to the clinician at the time of an acute exacerbation of CRS, and since bacterial proliferation often triggers these symptomatic exacerbations, antibiotic treatment is usually indicated in the initial treatment. If the patient has significant nasal congestion at the time of this acute exacerbation, a short term course of an oral corticosteroid such as prednisone can be useful. Steroid treatment can rapidly improve patient symptoms of congestion and pressure, and through improving edema and encouraging drainage can allow antibiotic therapy to be more rapid and efficacious in reducing symptoms. In addition to the use of these two oral medications, topical treatment can be effective as well. Nasal saline sprays and irrigations can cleanse the nasal mucosa and rinse thickened mucopurulent secretions out of the nose. In addition, topical nasal corticosteroids can be useful in their local anti-inflammatory effects, and can be continued for extended periods of time after the acute exacerbation is brought under control.
▪ Allergic Fungal Rhinosinusitis
Management of the patient with allergic fungal rhinosinusitis (AFRS) is similar to the approach used with patients in treating CRS with nasal polyps. The primary goal of treatment for AFRS is the elimination of the burden of disease, and as such, surgical therapy is indicated in all patients with AFRS. In contrast to other forms of CRS, surgical intervention is considered an indispensable component of the management of patients with AFRS. Surgical therapy is used as an initial portion of the treatment plan among these patients. The goal of surgical therapy is to eliminate as fully as possible the polypoid disease and underlying fungal material in the nose and sinuses, and to widely open and ventilate the involved sinuses. While external surgical approaches were commonly used in the past, in most cases patients with AFRS can be managed well with intranasal FESS procedures.
Data have also suggested that immunotherapy for fungal antigens can be useful in decreasing the inflammation present in these patients.44,45 A series of studies by Mabry and colleagues have shown improved control among patients treated with immunotherapy for fungal antigens that persist for a significant period of time following treatment. These patients required less medication over the course of their treatment, and had significantly improved quality of life with immunotherapy.
Adjuvant medical therapy may also be of benefit in patients with AFRS. Leukotriene-modifying agents have been used in patients with nasal polyps, both with and without fungal hypersensitivity. Results have been encouraging, although additional study is necessary to clarify their use in AFRS. In addition, one treatment that would theoretically be of benefit in these patients is the use of the monoclonal antibody anti-IgE (omalizumab). Studies to date have not been conducted, but since AFRS is strongly associated with high IgE levels to specific fungi, there could be theoretical benefit in decreasing IgE levels using this approach. Again, studies have not been conducted to explore this potential method of treatment.
Conclusion
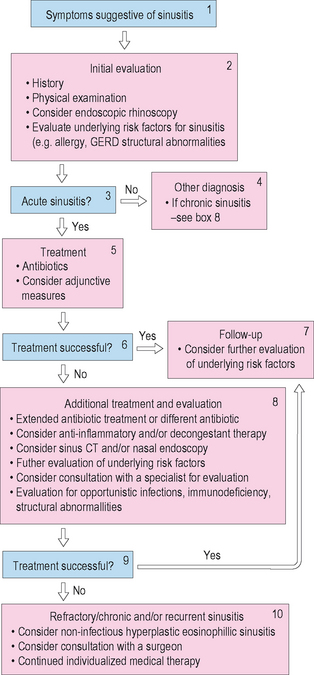
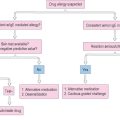
Rhinosinusitis is a common disease that affects a substantial portion of the adult and pediatric population. It has various forms and etiologies, and response to therapy depends upon the type of RS and its underlying pathology. Diagnostic criteria and classification systems for RS have not been uniformly accepted, which can make treatment confusing. One algorithm that can be used in the diagnosis and treatment of the patient with rhinosinusitis is found in Figure 4.9.
1 Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117:S1-S7.
2 International Rhinosinusitis Advisory Board. Infectious rhinosinusitis in adults: classification, etiology and management. Ear Nose Throat J. 1997;76:5-22.
3 Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol. 2004;193:S3-S5.
4 Reilly JS. The sinusitis cycle. Otolaryngol Head Neck Surg. 1990;103:856-862.
5 Anon JB, Jacobs MR, Poole MD, et al. Antimicrobial treatment guidelines for acute bacterial rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130:S1-S45.
6 Scott Levin Prescription Audit from Verispan, L.L.C. January–December 2002.
7 Ray NF, Baraniuk JN, Tharner M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis, and other airway disorders. J Allergy Clin Immunol. 1999;103:408-414.
8 Bhattacharyya N. The economic burden and symptom manifestations of chronic rhinosinusitis. Am J Rhinol. 2003;17:27-32.
9 Murphy MP, Fishman P, Short SO, et al. Health care utilization and cost among adults with chronic rhinosinusitis enrolled in a health maintenance organization. Otolaryngol Head Neck Surg. 2002;127:367-376.
10 Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104-109.
11 Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg. 2004;131:S1-S62.
12 Kaliner AM, Osguthorpe SD, Fireman D, et al. Sinusitis: bench to bedside. J Allergy Clin Immunol. 1997;99:S829-S848.
13 Fokkens W, Lund V, Bachert C, et al. EAACI position paper on rhinosinusitis and nasal polyps executive summary. Allergy. 2005;60:583-601.
14 Benninger M, Ferguson B, Hadley J, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1-S32.
15 Evans FO, Sydnor JB, Moore WEC, et al. Sinusitis of the maxillary antrum. N Engl J Med. 1975;293:735-739.
16 Williams JW, Simel DL. Does this patient have sinusitis? Diagnosing acute sinusitis by history and physical examination. JAMA. 1993;270:1242-1246.
17 International Rhinosinusitis Advisory Board. Infectious rhinosinusitis in adults: classification, etiology, and management. Ear Nose Throat J. 1997;76:1-22.
18 Newman LJ, Platts-Mills TAE, Phillips D, et al. Chronic sinusitis: relationship of computed tomography findings to allergy, asthma, and eosinophilia. JAMA. 1994;271:363-367.
19 Slavin RG, Spector SL, Bernstein IL, et al. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116:S1-S47.
20 Stammberger H. Functional endoscopic sinus surgery: the Messerklinger technique. Philadelphia: BC Decker, 1991.
21 Corren J, Togias A, Bousquet J. Upper and lower respiratory disease. New York: Marcel Dekker, 2003.
22 Makela MJ, Puhakka T, Ruuskanen O, et al. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539-542.
23 Winther B, Gwaltney JMJr, Mygind N, et al. Viral-induced rhinitis. Am J Rhinol. 1998;12:17-20.
24 Greve JM, Davis G, Meyer AM, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839-847.
25 Christodoulopoulos P, Cameron L, Durham S, et al. Molecular pathology of allergic disease II: upper airway disease. J Allergy Clin Immunol. 2000;105:211-223.
26 Hamilos DL, Leung DYM, Wood R, et al. Chronic hyperplastic sinusitis: association of tissue eosinophilia with mRNA expression of granulocyte–macrophage colony stimulating factor and interleukin 3. J Allergy Clin Immunol. 1993;92:39-48.
27 Hamilos DL, Leung DYM, Wood R, et al. Evidence for distinct cytokine expression in allergic versus non-allergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537-544.
28 Al-Ghamdi K, Ghaffar O, Small P, et al. IL-4 and IL-13 expression in chronic sinusitis: relationship with cellular infiltrate and the effect of topical corticosteroid treatment. J Otolaryngol. 1997;26:160-166.
29 Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877-884.
30 Sherris DA, Ponikau JU, Kern EB. Eosinophilic mucin rhinosinusitis. Laryngoscope. 2001;111:1670-1672.
31 Appenroth E, Gunkel AR, Muller H, et al. Activated and non-activated eosinophils in patients with chronic rhinosinusitis. Acta Otolaryngol (Stockh). 1998;118:240-242.
32 Kakoi H, Hirado F. A histological study of formation and growth of nasal polyps. Acta Otolaryngol (Stockh). 1987;103:137-144.
33 Peters MS, Rodriguez M, Gleich GJ. Localisation of human eosinophil granule, major basic protein, eosinophil cationic protein and eosinophil-derived neurotoxin by immunoelectron microscopy. Lab Invest. 1986;54:656-662.
34 Hisamatsu K, Ganbo T, Nakazawa T, et al. Cytotoxicity of human eosinophil granule major basic protein to human nasal sinus mucosa in vitro. J Allergy Clin Immunol. 1990;86:52-63.
35 Schubert MS. A superantigen hypothesis for the pathogenesis of chronic hypertrophic rhinosinusitis, allergic fungal sinusitis, and related disorders. Ann Allergy Asthma Immunol. 2001;87:181-188.
36 Bernstein JM, Ballow M, Schlievert PM, et al. A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol. 2003;17:321-326.
37 Bachert C, Gevaert P, Holtappels G, et al. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607-614.
38 Ferguson BJ, Stolz DB. Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am J Rhinol. 2005;19:452-457.
39 Sanclement JA, Webster P, Thomas J, et al. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope. 2005;115:578-582.
40 Meltzer EO, Charous BL, Busse WW, et al. Added relief in the treatment of acute recurrent sinusitis with adjunctive mometasone furoate nasal spray. The Nasonex Sinusitis Group. J Allergy Clin Immunol. 2000;106:630-637.
41 Dolor RJ, Witsell DL, Hellkamp AS, et al. Comparison of cefuroxime with or without intranasal fluticasone for the treatment of rhinosinusitis. The CAFFS Trial: a randomized controlled trial. JAMA. 2001;286:3097-3105.
42 Calhoun K. Diagnosis and management of sinusitis in the allergic patient. Otolaryngol Head Neck Surg. 1992;107:850-854.
43 McNally PA, White MV, Kaliner MA. Sinusitis in an allergist’s office: analysis of 200 consecutive cases. Allergy Asthma Proc. 1997;18:169-175.
44 Mabry RL, Manning SC, Mabry CS. Immunotherapy in the treatment of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1997;116:31-35.
45 Mabry RL, Marple BF, Mabry CS. Outcomes after discontinuing immunotherapy for allergic fungal sinusitis. Otolaryngol Head Neck Surg. 2000;122:104-105.