Chapter 6 Management of the Patient with Ocular Allergy
Epidemiology/Prevalence/Burden
▪ Acute Allergic Conjunctivitis
Seasonal (SAC) and perennial (PAC) allergic conjunctivitis are the most common forms of allergic conjunctivitis, SAC representing approximately half of the cases of ocular allergy.1 Perennial allergic conjunctivitis is identical to SAC, but with year-round symptoms. In SAC, the allergens are the pollens, and in PAC dust, mite feces, animal dander, and feathers are the common provocative antigens.2
Allergic conjunctivitis may affect individuals of all ages, social strata, and gender. Atopic ocular disorders are common but underdiagnosed. Most epidemiologic studies lack objective testing data to describe the prevalence and natural history of these diseases in an accurate manner, primarily centering on seasonal and perennial allergic conjunctivitis. These vary among geographic regions and are more common in warmer climates. The prevalence of SAC is reported to be as high as 33% in certain populations.3 SAC occurs in a large proportion of people afflicted by seasonal rhinitis. The seasonal influence on the appearance and disappearance of the symptoms is obvious from the history, and a positive family history of atopy is obtained in about 70% of patients with SAC.2
▪ Chronic Allergic Conjunctivitis
Giant Papillary Conjunctivitis
Giant papillary conjunctivitis (GPC) is a well-known complication of chronic contact lens use, but may also be observed in patients after cataract surgery, ocular prosthesis wearing, extruding scleral buckle retinal detachment surgery material, and corneal foreign bodies.4 A retrospective study performed by Donshik and Porazinski5 from 1993 to 1997, reported that 21.27% of contact lens users developed GPC. Most of these patients replaced their lenses at 4 weeks or longer and had previous history of allergy. The authors did not find a relationship between GPC and sex, age, average daily wearing time, contact lens material, or fitting characteristics of the lens. Unfortunately, the epidemiologic data on GPC is limited and outdated. The contact lens industry has made great advances in lens material as well as in cleaning solutions, which should reduce the prevalence of GPC. Strict FDA guidelines regarding lens replacement are now implemented, possibly decreasing complications in contact lens users.
Vernal Keratoconjunctivitis
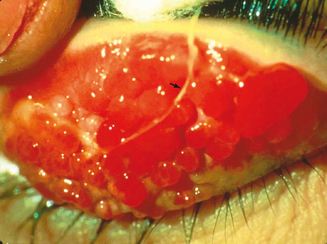
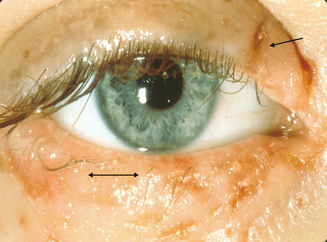
Vernal keratoconjunctivitis (VKC) is a chronic allergic conjunctival inflammatory disorder frequently associated with secondary keratopathy. The characteristic hallmark of VKC is the presence of giant papillae, usually in the upper tarsal conjunctiva, but in some cases in the conjunctiva at the corneoscleral limbus (Figure 6.1). It is a disease predominantly of young men, with pronounced seasonal (spring) influence, probably secondary to vernal allergens, but perennial forms exist as well.2 VKC may be differentiated from SAC in that increased disease activity may also occur in summer and SAC patients never develop the giant papillae so characteristic of VKC. A long-term follow-up study performed by Bonini reports that approximately 23% of patients have a perennial form of VKC and that 60% have additional recurrences during winter.6 He found that almost 16% of patients with longstanding seasonal VKC developed chronic, perennial inflammation after an average of 3 years, implying that persistent disease is most likely to develop in this population.
A personal or family history of atopy is usually uncovered in VKC patients, and in many cases, specific allergens to which the patient is sensitive can be determined by history and by scratch and prick allergen skin testing. One particularly notorious provocative allergen in patients with VKC is the house dust mite and its feces.2
Vernal keratoconjunctivitis has a worldwide distribution, with pronounced regional variations and prevalence. It is most common in the Mediterranean region and Central and South America, and is rare in North America and Northern Europe.2 VKC may represent as much as 3% of serious ophthalmic disease in some regions,7 whereas in Northern Europe and North America, the prevalence is about 1 in 5000 cases of eye disease.8
VKC has been reported to affect patients from 1 month old to more than 70 years of age, but at least 50% of the patients in most reported series are between 5 and 25 years of age. In most patients, the disease resolves spontaneously within 10 years of onset. Regrettably, however, the improperly treated patient may be blind by the time this happens.2
In a study by Dart,9 78% of 120 patients with VKC developed the disorder before the age of 16 years. Dart found that the corneal complications of VKC in this population occurred almost exclusively in patients with palpebral or mixed palpebral and limbal VKC. He found no differences in serum or tear IgE levels among VKC patients with the various forms of the disease; the VKC patients did have higher than normal levels of IgE, and specific IgE to cat dander and to house dust mites was detected. This finding agrees with other studies which suggest that this disorder is not solely IgE-mediated.2 Twenty-seven percent of the study population lost vision because of VKC, and Dart commented, “therapeutic complications are also common, and may lead to blindness.”
Atopic Keratoconjunctivitis
Hogan10 defined atopic keratoconjunctivitis in 1952 as allergic keratoconjunctivitis occurring in association with atopic dermatitis (eczema). It is estimated that between 25% and 40% of patients with atopic dermatitis have some type of ocular complication.11,12 Chronic allergic conjunctivitis with superficial punctate keratitis was the most frequent clinical presentation.12 This represents a substantial number of people who are at risk of bilateral blinding corneal complications from this complex inflammatory disorder. AKC most frequently occurs in men. It typically presents in the late teen years or early twenties, rarely before puberty, and may persist until the fourth or fifth decade of life.1 A recent study shows that childhood-onset adult AKC patients have greater ocular surface epithelial damage due to the effects of prolonged inflammation.13 It is this keratopathy which produces such profound disability for patients with AKC (Figure 6.2).
Relevant Anatomy and Physiology
The conjunctiva is a thin, transparent, vascularized mucous membrane that covers the posterior surface of the eyelids, reflects forwards on the eye at the fornix, to cover the anterior sclera. Anatomically, the conjunctiva is composed of a bulbar and a palpebral component. The bulbar conjunctiva is a thin, semitransparent, colorless tissue that covers the sclera up to the corneoscleral junction, the limbus. The palpebral conjunctiva, a thick, opaque, red tissue, is further divided into marginal, tarsal, and orbital zones. The marginal zone exhibits minimal keratinization and is located between the skin and the conjunctiva. The tarsal conjunctiva is a relatively smooth fibrous layer that gives the eyelid its characteristic shape, evident upon eyelid eversion (Figure 6.3).
Pathophysiology
The pathogenesis of ocular allergic diseases involves multiple steps. Allergic conjunctivitis represents a pure type 1 hypersensitivity response.2 Although the pathogenesis of GPC, VKC, and AKC involves more complex immunoinflammatory reaction, a type 1 hypersensitivity response has also been implicated in each of these disorders.14 Histopathologic characterization of inflamed conjunctiva shows similar cellular infiltrate among the atopic ocular disorders, consisting predominantly of eosinophils and lymphocytes.2 Studies illustrate the importance of the relationship between adhesion molecules and chemokines in the recruitment of leukocytes in the ocular allergic inflammatory response. With an advancing knowledge of the mechanisms involved in leukocyte recruitment into ocular inflammatory sites, more definitive treatment strategies are currently under investigation.
▪ Acute Allergic Conjunctivitis
Pathophysiologic features of SAC and PAC are prototypic type 1 anaphylactic hypersensitivity reactions. In sensitized individuals the allergen crosslinks IgE antibodies on the surface of mast cells and basophils, resulting in the degranulation of these cells with release of mediators including histamine and kinins. Patients with SAC and PAC have elevated levels of IgE in their tears and serum and pollen specificity has been demonstrated in both diseases.15
Seasonal allergic conjunctivitis is accompanied by migration of mast cells into the conjunctival epithelium,16 and by an increase in tear levels of the mast cell neutral protease tryptase.17 Challenging sensitized individuals reproduces ocular allergy features of increased tear levels of the inflammatory mediators histamine, all products of mast cell degranulation, and prostaglandin D2 and leukotrienes C4 and D4, newly produced mediators.18 Subsequent studies reveal that mast cells store and secrete a range of multifunctional cytokines including interleukin 4 (IL-4), IL-5, IL-6, IL-8, and tumor necrosis factor alfa (TNF-α).19 The release of these chemokines suggests that the mast cell has the capacity of upregulating local allergic responses, as well as organizing the participation of other inflammatory cells. IL-4 regulates the expression of VCAM-1 (vascular cell adhesion molecule 1) which is involved in the selective recruitment of eosinophils from the microvasculature,20 a critical event in the blinding ocular allergies, VKC and AKC.
As previously noted, mast cells (MC) are critical components of acute allergic disorders. Anderson and colleagues investigated the expression of inflammatory cytokines between the two known subsets of mast cells, MCTC and MCT,21 which are classified on the basis of neutral protease content.22 They found that MCTC had preferential expression of IL-4 and IL-13, suggesting that this subset is capable of regulating IgE, at least locally, through IL-4 “driven” humoral immunity. On the other hand, patients with SAC had low MCT expression of IL-5 and IL-6, consistent with previous reports that show high levels of IL-5 are necessary to activate eosinophils in chronic allergic disease.21
Studies show that there is a correlation between the levels of adhesion molecules and the different types of inflammatory cell infiltrate in allergic eye disease. Adhesion molecules help anchor circulating eosinophils to the endothelium with subsequent extravasation. All types of adhesion molecules (E-selectin, intercellular adhesion molecule (ICAM-1), and VCAM-1) are expressed in increased amounts only in active allergic eye disease, and not, for instance, in out of season SAC.23 There is a positive correlation between ICAM-1 and E-selectin levels and the degree of granulocyte and lymphocyte conjunctival infiltrate, and VCAM-1 expression with eosinophil levels.24 Therefore, the specific pattern of granulocyte and lymphocyte infiltrate is due to the relative concentration rather the selective recruitment of the different adhesion molecules.23 Out of season symptoms in patients with SAC and PAC, which tend to be milder, are associated with an increased number of CD45RO memory cells and EG2-positive cells.25
Human eotaxin-1, otherwise known as CC chemokine ligand 11 (CCL11), and its receptor CCR3, have been shown to be upregulated in tears of individuals with SAC26 and AKC with severe corneal damage,27 and in the mucus of patients with VKC.28 Eotaxin-1 is an important protein in inflammatory conditions because it helps recruit eosinophils to the site of inflammation, leading to their activation,29 and succeeding release of cytokines. Among these is IL-13, whose functions include promoting IgE class switching, regulation of cell-mediated immunity, inhibition of eosinophil apoptosis, and possibly mediation of tissue fibrosis.30 It also targets Th2 lymphocytes and mast cells, promoting the allergen-driven production of IL-4 and IL-5,31 upregulation of adhesion molecules, and persistence of the inflammatory process.
▪ Chronic allergic conjunctivitis
Giant Papillary Conjunctivitis
In 1974, Spring32 reported that 78 of 170 soft contact lens wearers developed an allergic reaction on the upper tarsal conjunctiva, presenting with complaints of contact lens intolerance and excessive mucus production. Allansmith and coworkers33 more definitively described this disorder and called it giant papillary conjunctivitis because of the appearance of papillae in the upper tarsal conjunctiva; these papillae grew larger when the condition remained untreated. Most recently, in 2005, Chang and colleagues found that GPC changes are not limited to the upper tarsal conjunctiva. Patients diagnosed with GPC associated with ocular prosthesis had a honeycomb pattern consistent with giant papillae in both upper and lower tarsal conjunctiva.34
GPC appears to develop as a result of tarsal conjunctival sensitization to environmental allergenic material present on the surface of the contact lens, coupled with the trauma to the upper tarsal conjunctiva associated with the excursion of the eyelid over the soft lens at each blink, an event that occurs about 8000 times each day.2 Irritation secondary to trauma to the tarsal conjunctiva leads to the release of neutrophil chemotactic factor and other inflammatory mediators.35 Scanning electron microscopy studies show that within 8 hours of wear, the contact lens is coated with material composed of mucus, protein, bacteria, cells, cell debris, and air-borne pollutants.36
The chemistry of the contact lens is another important element in the development of GPC, as well as edge design, surface properties, fitting characteristics, and replacement cycle.37 Nevertheless, patients need to be aware that the condition may occur with any type of lens, especially if they are replaced at intervals greater than 3 weeks.
Various inflammatory mediators contribute to the development of GPC. Leukotriene C4 (LTC4), known to be released by eosinophils, is elevated in the tear fluid of these patients. Patients who wear contact lenses have decreased levels of decay-accelerating factor (DAF),38 a complement-activation inhibiting protein, leading to increased inflammation secondary to increased complement upregulation. It is interesting to find higher tear levels of eotaxin in contact lens users,39 previously noted to be increased in SAC, AKC, and VKC. In fact, Maschos found that eotaxin levels were proportional to the severity of GPC, suggesting that this molecule could be involved in the formation of the papilla associated with GPC.
Biopsy of the conjunctival papillae discloses mast cells in the conjunctival epithelium and substantia propria, eosinophils in the same sites, and occasionally basophils in the conjunctival epithelium or substantia propria. Mast cell participation in GPC is substantially greater than would first appear to be the case on the basis of light microscopic observations.2 Ultrastructural studies show many more mast cells than can be observed by light microscopy, with ultrastructural evidence of mast cell degranulation.40 This high content of mast cells correlates with past findings associating GPC with atopy.
The tarsal conjunctiva, overlying the giant papilla, is thickened and irregular, with many indippings to the underlying stroma.1 The epithelium over the atypical portions of the papillae may show localized reduction of the goblet cell population, whereas in the interpapillary crypts, mucus-secreting elements seem to be hyperplastic.41
Vernal Keratoconjunctivitis
The histopathologic and immunopathologic characteristics of the tissues affected with VKC have led some authorities42,43 to conclude that VKC is not a pure type 1 Gell and Coombs hypersensitivity reaction, but rather a combination of both type 1 and type 1V reactions.2 Immunohistochemical studies show that the mononuclear cells are rich in helper (CD4) T cells and that the cytokines produced by the inflammatory cells are, among other things, inducing abnormal expression of class 2 HLA glycoproteins on conjunctival epithelium and stromal cells.42 In these CD4 areas of VKC biopsies, there is an increased level of IL-5, but not of IL-2, confirming Th2 rather than Th1 influence.44 Recent studies also reveal an increased expression of adhesion molecules. These promote the recruitment of inflammatory cells as well as the interaction between lymphocytes, antigen-presenting cells, and epithelial cells.45
The histopathology of the conjunctival papillae discloses not only the cells typically associated with allergic reactions (mast cells and eosinophils), but also large collections of mononuclear cells, fibroblasts, and newly secreted collagen, leading to the characteristic conjunctival findings.2 Leukotrienes may be largely responsible for chemosis, conjunctival injection, and increased mucus secretions seen in VKC. They may induce conjunctival vasodilation, edema, hyperemia, and epithelial gland glycoprotein secretion.46–48
Current research proposes that eosinophil cationic protein (ECP), released by eosinophils, is influential in the pathogenesis of palpebral VKC. Pucci and colleagues49 observed a direct correlation between the number and size of giant tarsal papillae and ECP serum levels. They postulate that serum ECP is a reliable marker of disease activity in patients with palpebral and mixed VKC. On the other hand, patients with bulbar VKC had lower ECP and total IgE serum concentrations, suggesting “IgE sensitization is a predisposing factor for tarsal rather than limbal VKC.”49
Another constituent of eosinophil granules is major basic protein (MBP). At high levels, MBP can be cytotoxic,50 whereas at lower concentrations it contributes to histamine release from mast cells.51 Extracellular deposition of MBP has been observed in VKC, AKC, and GPC,52–54 leading to neutrophil activation and tissue injury through the release of lysosomal enzymes, neutrophil oxidase, and superoxide anion.55
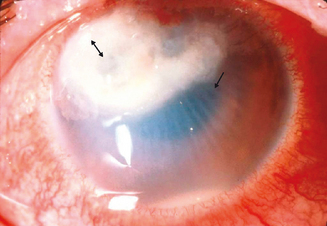
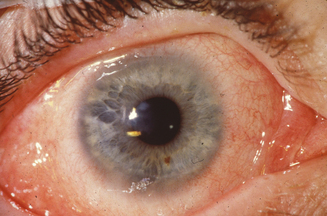
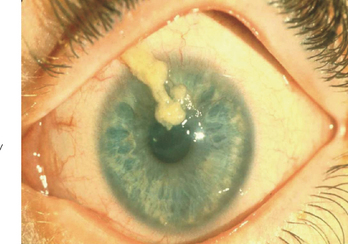
The limbal form of VKC was first described by Arlt56 in 1846, predating the description by von Graefe57 of the palpebral form by 25 years. This form is characterized by the presence of large papillae in the conjunctiva at the corneoscleral limbus, with associated collections of inflammatory cells rich in eosinophils at the apices of the limbal papillae, the so-called Horner–Trantas dots.2 In especially severe forms of limbal VKC, the steady accumulation of inflammatory cells may result in formation of a frank mound on the peripheral cornea (Figure 6.4). This complex immunologic process seems to be coordinated with the help of the chemokine receptor CXCR3, reported to predominate in this type of disorder.58
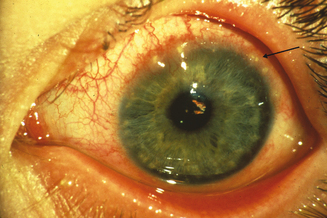
Figure 6.4 Limbal vernal keratoconjunctivitis. Note the white-opaque perilimbal Horner–Trantas dots (arrow).
Conjunctival remodeling in VKC is characterized by epithelial hyperplasia, angiogenesis, and deposition of extracellular matrix components in the substantia propia.59,60 Abu El-Asrar et al61 ascertained significant overexpression of α3– and α6-integrin subunits, epidermal growth factor (EGFR), vascular endothelial growth factor (VEGF), transforming growth factor beta (TGF-β), basic fibroblast growth factor (bFGF), and platelet derived growth factor (PDGF) in VKC lesions. Abnormal keratinocyte deposition has been associated with suprabasal integrin expression.62 TGF-β, bFGF, and PDGF stimulate fibroblast proliferation, while VEGF influences angiogenesis and vascular permeability.63–65 Taken together, it is not surprising to find such profound conjunctival alterations in this disease.
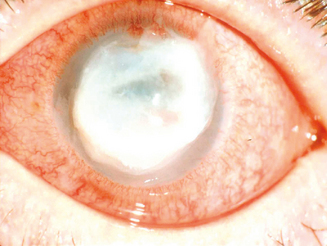
The keratopathy of vernal keratoconjunctivitis typically begins as a diffuse superficial punctate keratitis. If the inflammation continues with an outpouring of inflammatory mediators, especially those from eosinophils, into the tear film and with associated epithelial toxicity and possibly conspiracy from the mechanical effects of the large papillae, a frank epithelial defect appears next.2 These defects have been termed shield ulcers because of their position and morphology (Figure 6.5). They are present in approximately 3–11% of patients with VKC.6 Epithelial defects are trophic, defying the therapeutic strategies that usually are successful in healing corneal abrasions or epithelial defects. The longer such trophic defects persist, the higher is the likelihood of eventual stromal ulceration, secondary microbial infestation, and permanent corneal scarring (Figure 6.6). In chronic advanced cases, the inflammatory material is deposited in the form of opaque white or yellow “plaque.”1
Failure of corneal ulcer or plaque re-epithelialization may be attributed to the overexpression of matrix metalloproteinases (MMPs) by resident corneal cells,66 as well as to deposition of MBP at the ulcers.52 Corneal sensitivity, tear film break-up time (BUT) values, and MUC5AC mRNA expression was significantly lower in atopic patients with corneal ulcers, as reported by Dogru et al.67 It should be noted that MUC5AC is part of the mucinous component of the tear film, promoting tear adhesion to the corneal surface. They believe there is an inverse relationship between MUC5AC expression and the degree of ocular surface inflammation in atopic eyes with allergy.
At a molecular level, corneal damage in VKC may result from various sources. The extracellular matrix is an important component of tissue remodeling. Matrix metalloproteinases (MMPs), mediators of collagen degradation and inflammatory cell migration, and tissue inhibitors of MMPs (TIMP), have to be in equilibrium in order to achieve normal corneal healing. Leonardi68 found increased tear levels and activity of MMP-1 and -9 in patients with VKC, leading to altered homeostasis and tissue damage. In fact, they found a direct relationship between MMP-9 activity and clinical findings, including corneal involvement and giant papillae formation. MMP-1, also known as collagenase, is activated by mast cell chymase.69 Ebihara et al70 observed increased levels of chymase in the tears of VKC patients, further substantiating Leonardi’s findings.
Atopic Keratoconjunctivitis
Atopic keratoconjunctivitis was defined by Hogan10 in 1952 as allergic keratoconjunctivitis occurring in association with atopic dermatitis (eczema). This definition, although imprecise, is in common usage and connotes the patients with the most severe form of atopic ocular disease seen in association with eczema.2 The argument by some physicians that other types of atopic conjunctivitis, such as chronic allergic conjunctivitis or perennial atopic conjunctivitis, also are atopic ocular diseases and therefore can be confused with atopic keratoconjunctivitis is not a constructive one. This is particularly true in view of the fact that in those latter disorders, keratitis or significant keratopathy is not part of the clinical picture. Corneal disease is, however, typical of patients with atopic keratoconjunctivitis.2
Ocular surface disease in patients with atopic dermatitis is characterized by decreased goblet cells and conjunctival squamous metaplasia, which seem to worsen with increased number of flare-ups.67 Reduced goblet cell counts contribute to tear instability by limiting the secretion of mucin, specifically MUC5AC,71 a phenomena present in VKC also.
Atopic individuals have a defect in suppressor T cells responsible for regulating IgE production to antigens, in addition to other genetically governed abnormalities which set the stage for atopic or inappropriate responses to environmental allergens.1 Type 1 hypersensitivity is only one of the mechanisms in the pathogenesis of AKC, in fact it is widely accepted that Th2 cells play an important role as well. Some of the immunopathologic characteristics of AKC specimens are similar to those of cicatricial pemphigoid and ocular rosacea, emphasizing that fibroblast activation, proliferation, and production of cicatrization may result from a variety of chronic conjunctival inflammatory disorders in which T cells, macrophages, and mast cells collaborate.72 Matsuura et al corroborated the systemic predilection towards a type 2 immune response and the associated infiltration of type 2 T cells into the local ocular site, without undermining the presence of type 1 T cells in chronic allergic inflammation.73 Another mechanism by which Th2 cells may predominate in atopic disease is through preferential apoptosis of their counterpart, Th1 cells.74
Recent studies focus on the characterization of Th1 and Th2 cytokines in chronic ocular allergy. Leonardi75 detected IL-4 and interferon gamma (IFN-γ) in tears of individuals with AKC, but found that only IFN-γ levels correlated with disease activity, specifically with corneal involvement. Upregulated IFN-γ levels seemed to go in hand with increased expression of ICAM-1 on conjunctival fibroblasts and the secretion of IL-6 and IL-8.75 In 2005, Okada et al76 contributed to Leonardi’s hypothesis. They noticed that when activated by IL-4 and TNF-α, corneal fibroblasts expressed ICAM-1 and VCAM-1 and increased eotaxin production, all leading to continued eosinophil adhesion to these molecules and persistent allergic keratopathy.
Perforin is a plasma membrane protein that plays an essential role in T-cell cytotoxicity. Ambach and colleagues77 demonstrated that atopic patients have a reduced number of perforin-containing CD8+ cytotoxic lymphocytes, leading us to conclude that this is one of the many mechanisms by which these individuals are likely to develop inflammation.
The histopathologic findings of the conjunctiva of patients with AKC are characterized by mast cell and eosinophil invasion of the epithelium, epithelial pseudotubule formation, and increased goblet cell presence.1 Mast cells and eosinophils along with a chronic mononuclear cell infiltration are also prominent in the substantia propria.72 Conjunctival biopsies reveal high mRNA levels of IL-3, IL-4, and IL-5.78 Patients with VKC or AKC have higher levels of conjunctival IL-4 producing CD4+ T cells than patients with acute allergic conjunctivitis.73
Giant papillary formation is essential in the pathogenesis of AKC, as well as in VKC. Conjunctival histopathologic examination of the excised papillae shows elevated number of goblet cells, inflammatory leukocytes, and fibrotic tissue, along with evidence of angiogenesis.79,80 Asano-Kato81 showed increased production of VEGF by conjunctival fibroblasts when stimulated by Th2 cytokines, TGF-β1, and IL-1β. These findings led to the conclusion that conjunctival fibroblasts may be influential in exacerbating inflammation and tissue remodeling, paving the way towards papillary formation.81
Yamagami and coworkers evaluated the tarsal conjunctival giant papillae which are typically seen in patients with AKC and atopic dermatitis and/or asthma.82 They found that the predominant chemokine receptor genes were CXCR4 and CCR4. Giant papillae showed increased IL-4 and IL-13 levels in association with high CCR4 gene expression. Eotaxin and its receptor, CCR3, were also detected, but at lower levels. These findings are relevant to atopic disease because CC-receptors are important for eosinophil migration.83
Diagnosis
▪ Acute Allergic Conjunctivitis
Physical Examination
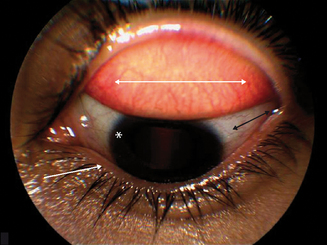
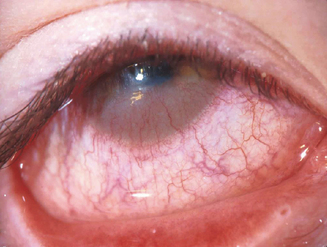
Signs of ocular inflammation, even during a time of maximal symptoms, usually are unimpressive. The eye may not be obviously inflamed. Indeed, the conjunctiva may appear totally white and quiet.2 Further inspection by slit lamp biomicroscopy, however, often reveals mild edema of the bulbar conjunctiva and signs of inflammation in both upper and lower tarsal conjunctiva (Figure 6.7). The conjunctivae are usually mildly injected and edematous leading to a “milky” appearance. Increased mucus is frequently observed in the preocular tear film and in the inferior fornix. Fine papillary hypertrophy of the upper tarsal conjunctiva may occur.84 Additionally venous congestion in the skin of the lids can cause the appearance of dark circles around the eyes termed “allergic shiner.” Many individuals also have concurrent nasal symptoms.85 The cornea is characteristically unaffected, an important sign which differentiates acute allergic conjunctivitis from the chronic forms, which usually involve corneal alterations.
Laboratory Evaluation
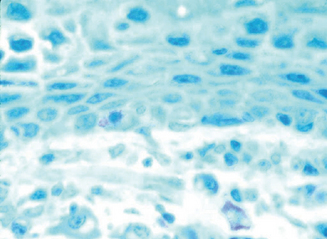
The most appropriate way to diagnose allergic conjunctivitis should not only focus on clinical signs and symptoms, but also should include allergen-skin prick test, IgE tear levels, and conjunctival biopsy with specific attention to the presence of mast cells and eosinophils, particularly in the epithelium (Figure 6.8). Most studies indicate that serum IgE levels are not necessary.86 Measuring tear-specific inflammatory markers may be useful for the diagnosis and management of ocular allergy. Proposed markers include: ECP, IL-4, IL-5, and eotaxin.87 A trained allergist is most valuable in the evaluation of these patients, especially with the implementation of new diagnostic markers.
▪ Chronic Allergic Conjunctivitis
Giant Papillary Conjunctivitis
Physical Examination
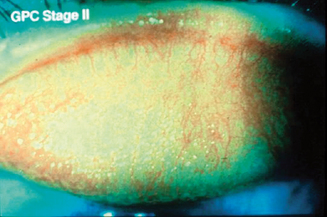
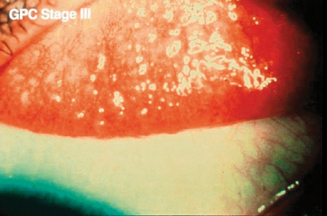
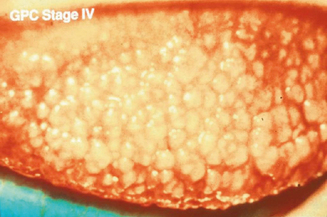
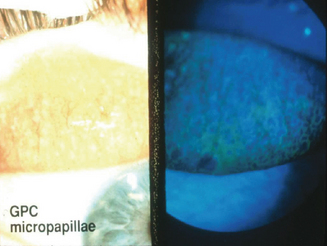
Some degree of ocular itch may be present, and examination of the upper tarsal conjunctiva discloses conjunctival hyperemia and tarsal papillae greater than 1 mm in diameter.2 The geographic extent of the papillary response and the size of the papillae, as well as the presence or absence of epithelial erosions on the apices of the papillae, are important features guiding therapy2. Staging is based on the extent of the papillary response, the size of the papillae, and evidence of apical epithelial erosions (Figures 6.9–6.11). Two percent fluorescein dye instilled into the preocular tear film, with subsequent eversion of the upper eyelid and examination of the tarsal conjunctiva with cobalt blue-filtered light, facilitates the recognition of low-profile papillae because the fluorescein dye outlines the macropapillae as it lies in the valleys at their bases (Figure 6.12). The dye also shows stained epithelial defects at the apices of macropapillae.2
Patients who use hydrogel soft contact lenses usually have papillary changes involving the entire tarsal conjunctiva, especially over the fold of the everted eyelid. In rigid contact lens users, the inflammatory reaction is concentrated at the junction where the edge of the lens meets the tarsal conjunctiva. These opposing patterns support the hypothesis that mechanical irritation is an essential component in GPC.88
Laboratory Evaluation
Conjunctival biopsy of the papilla shows increased mast cell density over the epithelium, and eosinophils in the conjunctival epithelium and substantia propria. Epithelial irregularity and hyperplasia and increased mucus-secreting glands are common findings. Analysis of tear fluid composition reveals elevated IgG, IgM, IgE, as well as complement factors such as C3, factor B, and C3 anaphylatoxin.88 Eotaxin and leukotrienes (LTC4) are also increased, while lactoferrin and decay accelerating factor are diminished. These novel markers show promise as diagnostic tools.
Vernal Keratoconjunctivitis
History
The predominant symptom of VKC is itching. As a rule, the patient spontaneously complains of profound itching. Excessive tearing, mucus production, photophobia, burning, and foreign body sensation are common symptoms. Eyelid irritation is uncommon, but blepharospasm has been reported. Patients often complain of frequent conjunctival redness after exposure to nonspecific stimuli.89 Past diagnosis of an atopic condition, such as asthma and eczema, are present in the vast majority of affected individuals, as well as a positive family history. Seasonal variations may be useful to differentiate VKC from atopic keratoconjunctivitis, as is the age of the individual.
Physical Examination
The signs are mostly confined to the conjunctiva and cornea. Eyelid skin and margins are relatively uninvolved compared to AKC. As previously noted, the “hallmark” finding in VKC is the cobblestone-like giant papillae, found in the upper tarsal conjunctiva in the palpebral form of the disease or at the limbus in bulbar VKC (Figure 6.1). In palpebral VKC, papillae markedly increase the mass of the upper lid, making ptosis an additional presenting finding.
The bulbar conjunctiva is usually injected and edematous. Inflammation of the bulbar conjunctiva is variable, but a ropy, lardaceous thread can be found in the inferior fornix (Figure 6.13). Limbal changes may be prominent, especially in heavily pigmented patients. The limbus and perilimbal conjunctiva may be thickened and edematous, forming a gelatinous-like hypertrophy.1 Limbal nodules and Trantas’ dots, composed of eosinophils and dead epithelial cells, may be observed. These limbal changes may result in pannus and superficial neovascularization of the cornea.1 It is interesting to note that the degree of conjunctival injection and edema correlate with the severity of corneal complications, while the height of the papilla and the amount of mucous discharge had no effect on them.90
The most important and vision threatening complications of VKC occur in the cornea, usually in the form of mild epithelial keratitis, or in more severe cases “shield ulcers.”1 The characteristic shield ulcer of the VKC is typically oval or pentagonal, superficial, and superiorly located with grayish opacification of the bed and elevated margins91 (Figure 6.5). As previously discussed, these are indolent and may take months to re-epithelialize.
Patients with VKC may also develop the “mucous fishing syndrome” because of this elastoid, irritating mucous thread, with the result that an especially ocularly pernicious conspiracy between these two problems is established.2 Vernal keratoconjunctivitis has been associated with keratoconus, atopy, and atopic cataract. Common complications also involve steroid-induced glaucoma and cataracts.
Laboratory Evaluation
VKC is usually diagnosed solely on clinical signs and symptoms, cinched with the patient’s past medical and family history of atopy. Total and specific IgE determination, as well as skin tests, is not considered useful because about half of the patients will have negative results.89 In cases where the diagnosis is equivocal, a conjunctival scraping will show an increased eosinophilic infiltrate in the epithelium. Deposition of collagen within the substantia propria results in conjunctival thickening. Histochemical analysis of mast cells will be positive for chymase and tryptase. Serum tear levels of tryptase, chymase, MMP-1, and MMP-9 will be elevated in some patients, while mucin levels may be significantly low. Elevated serum ECP levels seem to correlate with disease activity in the palpebral form of VKC.
Atopic Keratoconjunctivitis
Physical Examination
The most common signs of AKC are conjunctival injection, upper tarsal giant papillae, and blepharitis. Cicatrizing conjunctivitis may develop with chronic conjunctival inflammation, and lid dermatitis and chronic blepharitis with lid thickening and meibomian gland dysfunction are typical.2 Lids are often red, macerated with crusting and scaling (Figure 6.14). The inferior forniceal conjunctiva is most commonly affected, in contrast to VKC, leading to formation of symblepharon, adhesions between the bulbar and palpebral conjunctiva, in the most severe scenarios. Papillary hypertrophy of the inferior tarsal conjunctiva may also be observed.1
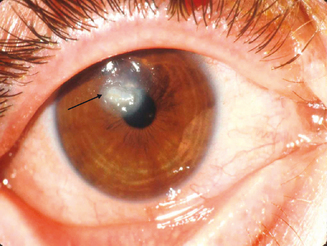
Punctate epithelial keratitis is an early corneal finding which may progress into persistent epithelial defects.1 These defects are often complicated by herpes simplex or Staphylococcus aureus superinfections.11 Limbal Tantra’s dots and anterior stromal scarring are present in some patients. Loss of vision occurs because of corneal scarring and neovascularization92 (Figure 6.15). Keratoconus, retinal detachment, and posterior subcapsular cataract formation, even in the absence of prior steroid treatment, may be associated with this condition.93,94
Laboratory Evaluation
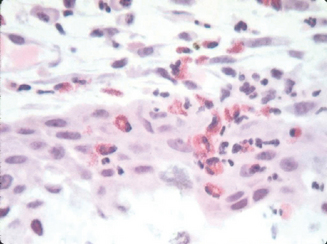
Total serum IgE levels and eosinophil tear levels are almost always elevated in patients with AKC, even when there is no marked evidence of disease activity. On the other hand, radioallergosorbent test (RAST), skin tests, and IgE antibody tests are negative to common allergens in a considerable number of individuals,94 making them poor diagnostic instruments. Conjunctival scrapings will show increased numbers of eosinophils (Figure 6.16). Conjunctival sac cultures and staphylococcal enterotoxin A- and B-specific IgE antibodies in tears should be evaluated if there is suspicion of bacterial superinfection.87 Conjunctival biopsies are reserved for patients in whom there is a suspicion of ocular cicatriceal pemphigoid, due to symblepharon formation. These will have positive basement membrane antibodies and complement deposition.
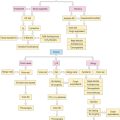
Treatment
▪ Nonpharmacologic Therapy
Allergen Avoidance
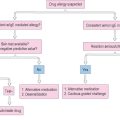
The most effective way to treat allergic ocular disorders is through allergen avoidance, hence the importance of identifying potential offenders. Policing the patient’s environment and scrupulously cleaning it of all potential allergen provocateurs is critical to the long-term stability of patients with allergic disease. Involvement by an expert allergist is essential. The allergist has the most expertise in detecting potential allergens, and can provide the patient with specific instructions for environmental control procedures. These include implementing specific air-conditioning units, furnace air-filtering devices, air purifiers, mattress and bedding material alterations, and specific housecleaning techniques. He or she deals with issues relating to existing carpeting and pets, the elimination of which is sometimes essential, particularly in the more severe forms of allergy. All of these methods are employed to try to reduce the surfaces on which the allergens can accumulate.2
The allergist is the best resource for expert skin testing and identification of the allergens responsible for provoking acute episodes, and can determine whether the use of systemic antihistamines and the embarkation on the lengthy road of desensitization immunotherapy are appropriate.2 When deemed necessary, this allergist should perform not only the appropriate patch, scratch, and prick tests as well as serum RAST, but also the environmental detective work and motivational and educational work necessary for a successful environmental control program. The wisdom, importance, and usefulness of this component of the patient’s care cannot be overemphasized.2
▪ Pharmacologic Therapy
Topical Antihistamines (H1-receptor Antagonists)
Topical antihistamines may be helpful, temporarily, in patients with mild disease, but because these agents competitively inhibit only one mediator liberated by the mast cells, they are not the best choice for long-term therapy.2 In patients presenting with exclusive ocular allergy, these agents are preferred over oral antihistamines because they cause fewer side effects. Emedastine difumarate (Emadine) and levocabastine (Livostin) are selective H-receptor antagonists, although the latter has been shown to be H1 receptor selective. Blockage of the histamine receptor has been shown to downregulate the expression of adhesion molecules, specifically ICAM-1, thereby reducing the chemotaxis of inflammatory mediators.95 Both are used four times a day on the affected eye.
Mast Cell Stabilizers
Common mast cell stabilizers include cromolyn sodium, lodoxamide, nedocromil sodium, and pemirolast potassium. Cromolyn sodium 4% (Opticrom, Crolom) inhibits histamine and SRS-A (slow-releasing substance of anaphylaxis) release from mast cells. Lodoxamide tromethamine 0.1% (Alomide) stabilizes mast cells by preventing calcium influx upon antigen stimulation and decreasing vascular permeability. They do not exhibit intrinsic anti-inflammatory, antihistamine, or vasoconstrictive effects. Nedocromil sodium 2% (Alocril) interferes with the release of leukotrienes and platelet activating factor from mast cells. Pemirolast potassium 0.1% (Alamast) interferes with mast cell degranulation and mast cell stabilizer in both early and late phases. A meta-analysis of various randomized double-blinded controlled clinical trials revealed the effectiveness of mast cell stabilizers over placebo in the treatment of allergic conjunctivitis.96 There was insufficient evidence to recommend one agent over the other.
Antihistamines/Mast Cell Stabilizers
Olopatadine (Patanol), epinastine (Elestat), and ketotifen (Zaditor) are part of this group of medications. Olopatadine hydrochloride 0.1% (Patanol) is a selective H1 histamine receptor antagonist and less specific mast cell stabilizer. Clinical trials describe a decrease in redness and pruritus in patients that used olopatadine 0.1% versus levocabastine 0.05% ophthalmic solution.97 In pediatric patients with allergic conjunctivitis, once daily olopatadine was successful in decreasing ocular symptoms without serious adverse effects.98 Olopatadine has been shown to reduce lid swelling. Ketotifen fumarate 0.025% (Zaditor) is a relatively selective, noncompetitive histamine antagonist (H1-receptor) and mast cell stabilizer with documented decreased chemotaxis and activation of eosinophils. Studies show it is at least as effective as levocabastine, sodium cromoglicate, nedocromil, and olopatadine,99–101 and that when combined with oral desloratadine it improved the overall antiallergic efficacy of both medications.102 At a molecular level, it diminishes the production of eotaxin and the expression of CD29, the common chain of the β1 integrins, by epithelial cells.103 In SAC patients, ketotifen and olopatadine effectively reduced the expression of CAMs and inflammatory markers on the conjunctival surface cells.104 Epinastine hydrochloride 0.05% (Elestat) and azelastine hydrochloride 0.05% (Optivar) are both antihistamine and mast cell stabilizers. These agents offer the convenience of twice daily administration, increasing patient compliance to the medication.
Nonsteroidal Anti-inflammatory Drugs (NSAIDs)
NSAIDs inhibit the production of prostaglandins and thromboxanes by blocking the action of cyclooxygenase. They do not affect leukotrienes or other products of the lipoxygenase pathway, and therefore they will not alleviate all signs and symptoms associated with SAC. They are especially useful for the vasodilatation and edema findings. Ketorolac tromethamine (Acular) and bromfenac sodium (Bromfenac) are topical NSAIDs used to treat ocular symptoms. The latter was found to be as safe and effective as pemirolast potassium for the treatment of allergic conjunctivitis.105 Both are used up to four times a day on the affected eye.
Corticosteroids
Corticosteroids are potent pharmacologic agents which block both cyclooxygenase and lipoxygenase pathways through their action on the first step of the arachidonic acid pathway, phospholipase inhibition. Steroids reduce circulating eosinophils through inhibition of the potent inflammatory transcription factor NF-κB and through the induction of eosinophil apoptosis.106,107 Increased IL-12 levels, which promote eosinophil apoptosis, are observed after steroidal therapy.108 They are very effective, but nonspecific anti-inflammatory drugs whose long-term use has been associated with serious adverse effects. These include: delayed corneal epithelium wound healing, local immunosuppression with possible superinfection, elevation of intraocular pressure (IOP), and cataract formation. These deleterious side effects are the main reason why steroid use has to be closely monitored by a trained ophthalmologist. Slit lamp examination and intraocular pressure measurement have to be included in the follow-ups of any patient that is using topical steroids. They should be prescribed for a short amount of time and for refractory cases in which conventional treatment was unsuccessful.
Rimexolone, medrysone, and fluorometholone are relatively weak topical steroids whose potency is associated with fewer ocular side effects. By contrast, prednisolone acetate is a very potent steroid with a higher incidence of side effects. Loteprednol etabonate (Lotemax 0.05% and Alrex 0.02%) has a safer safety profile and is rapidly metabolized once it enters the anterior chamber of the eye. It is a topical ester steroid drop that has been reported to have a decreased risk of elevating IOP. Recent studies report that 0.02% loteprednol is relatively safe for long-term treatment of SAC and PAC,109 and more effective than placebo for SAC.110 Even so, it is recommended that topical steroids should only be prescribed by ophthalmologists in a regularly monitored setting. When indicated, the usual regimen begins with instillation four times a day for 1 week, followed by a slow tapering of one drop per week until discontinuation.
Allergen Immunotherapy
Systemic desensitization immunotherapy is indicated in the patient who has striking sensitivity to a limited number of allergens. Performing desensitization immunotherapy on a patient with ocular allergy is not easy, however, and some features of this practice are different from the typical practice of desensitization immunotherapy in the patient with allergies not affecting the eyes.2
▪ Treatment of Specific Disorders
Acute Allergic Conjunctivitis
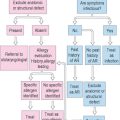
Treatment of SAC should comprise the following steps: environmental controls, antihistamines/mast cell-stabilizing agents throughout the patient’s known allergy seasons, systemic antihistamines when environmental allergen exposure is unavoidable, topical antihistamine, and desensitization immunotherapy.2
Management begins with the avoidance of known allergens. Iced artificial tears or cold compresses may be enough to relieve mild symptoms. Pharmacotherapy is based on the severity of the clinical signs and symptoms. Mild allergic conjunctivitis may be treated with a topical over-the-counter antihistamine/vasoconstrictor agent or with the more effective second-generation topical H1-histamine antagonists.111 Topical antihistamines, usually in combination with decongestants, are also frequently used on an “as necessary” basis. Mast cell stabilizers should be used prophylactically and on a regular basis for effectiveness. Approved combinations of antihistamines/mast cell stabilizers are convenient in reducing the number of prescription eye drops used by the patient, especially in the younger ones. These are to be used twice daily. In severe cases, short-term topical steroids may be considered for 1–2 weeks, keeping in mind the serious side effects associated with long-term use. In patients with significant nasal symptoms, oral antihistaminics can also be added. It is important to note that follow-up visits should be scheduled in accordance with the severity of the disease, its etiology, and choice of treatment agents.
Chronic Allergic Conjunctivitis
Giant Papillary Conjunctivitis
The goal of treatment in GPC is resolution of symptoms and restoration of functional use of contact lenses or ocular prosthetics. Although removal of the responsible foreign body is the definitive treatment, and while that may be appropriate for exposed sutures or scleral buckles, complete discontinuation of contact lenses or ocular prosthetics may be met with some degree of resistance from patients. Fortunately, contact lens wear does not need to be completely discontinued to minimize the symptoms of GPC.2
Significant reduction in the signs and symptoms may be achieved by modifying the contact lens care routine. Patient education regarding proper contact lens hygiene is of utmost importance. It involves vigorous daily cleaning with a soft contact lens cleaning agent, hydrogen peroxide sterilization, lens storage in preserved saline solution, and protein enzyme treatment at least twice a week.1 Disinfecting solutions that contain chemical preservatives should be discontinued. Patients should be aware of the increased incidence of GPC with increased interval wear (more than 4 weeks), meaning that they should replace their lenses as often as possible. Contact lens wearing time should be kept to 50 hours per week, or less if the patient expects to remain a successful contact lens wearer for many years.1
If the patient does not experience significant relief by modifying his/her contact lens hygiene regimen, it may be necessary to use a different polymer material or modify the contact lens edge design. Prescribing a new lens of different polymer and edge design modifies the conjunctival trauma and allergen adherence profile in comparison with the original lens that resulted in the GPC.1 Converting a patient from soft daily-wear contact lenses to disposable or daily-disposable soft contact lenses may prevent the accumulation of proteinaceous deposits, which may be the antigenic stimulus for GPC. Rigid gas permeable contact lenses may provide further relief from symptoms if disposable lenses do not provide adequate response because of the decreased proclivity of the rigid gas permeable contact lenses to develop adherent deposits and coatings.2
Pharmacologic treatment of GPC is usually reserved for patients that did not achieve comfort with the techniques that were recently mentioned. It includes the use of mast cell stabilizers, topical corticosteroids, antihistamines, and combined agents similar to those in the other immunologic conjunctival disorders discussed previously. Most topical treatments require that the lens be removed prior to using the drop and be replaced at least 10 minutes after. As always, care must be taken when using topical corticosteroids; a pulsed regimen is recommended to minimize adverse reactions.2 If topical medications are unsuccessful, the patient will have to weigh the benefits of using contact lenses versus the symptoms they are causing. Laser vision correction may ultimately be the only choice for some individuals.
Vernal Keratoconjunctivitis
Although VKC is usually a self-limited disease, the complications arising from improper treatment are sight-threatening. The first step in treating VKC is allergen avoidance, important in the long-term control of the disease. Combined antihistamines/mast cell stabilizers are the mainstay of the pharmacological treatment because they affect both the acute and long-term inflammatory reaction mediators associated with the pathogenesis of the disorder. Olopatadine 0.1% has been shown to be effective in relieving the signs and symptoms of VKC. Studies show that it also reduces the number of goblet cells, therefore decreasing the amount of mucus discharge.112
Topical steroids may be used in the treatment of acute initial disease or to control breakthrough attacks of highly active VKC inflammation after the patient has encountered a stimulating allergen. Because of their potential side effects, topical steroids should be prescribed at the lowest effective concentration and for the shortest duration possible.2 Pulse therapy involves the administration of a low-potency topical steroid four times daily for 2 days, with subsequent tapering to three times daily for the succeeding 2 days, twice daily for the 2 days after that, once daily for an additional 3 days, and subsequent discontinuation thereafter.2 Supratarsal injection of corticosteroid is a safe, effective modality for managing recalcitrant VKC,113 although this should be performed by skilled ophthalmologists. Systemic corticosteroids are usually not warranted in the treatment of mild-moderate VKC.
Additional pharmacologic agents may be used to provide varying degrees of relief. Vasoconstrictors and topical antihistamines may reduce hyperemia and acute symptoms, but are not effective in severe cases on a long-term basis. Adjunctive ocular therapy may also be required for secondary infection, for extreme mucous production and mucous plaque formation on shield ulcers, and for persistent epithelial defect. N-acetylcysteine, a mucolytic agent used four times a day at 10–20% concentration, may decrease mucous production, while bandage contact lenses may aid heal epithelial defects. The key to healing a persistent epithelial defect is control of the associated inflammation.
Several reports have shown that topical cyclosporin (Restasis) may be effective in reducing some of the signs and symptoms of VKC without significant adverse effects.114 It is effective as an adjunct therapy to reduce the use of corticosteroids in AKC.115 Since it causes significant irritation and discomfort symptoms, patient compliance may be a problem. The immunomodulatory effects of cyclosporin on both cellular and humoral responses make it especially useful in VKC.1 It is especially useful in patients exhibiting conjunctival remodeling because it reduces conjunctival fibroblast proliferation rate and IL-1β production.116 Several reports show that patients with VKC may also benefit from oral antihistamines, aspirin, and oral montelukast. In severe vision-threatening cases, hospital admission and a course of intravenous or oral steroids may be considered.
Atopic Keratoconjunctivitis
Treatment of patients with AKC is similar to that of VKC, in that it includes controlling the environment, avoidance of allergens, and may require both topical and systemic medications to provide symptomatic relief.2 As with VKC, topical vasoconstrictors and antihistamines may provide very limited, short-term relief; they are not the mainstay of treatment.
It has been proposed that systemic cyclosporin may be useful in controlling AKC through its inhibition of IL-2, with resultant reduction in T-cell activation. The downside of this treatment, as with any immunomodulating therapy, is the need for regular monitoring of blood counts and liver function tests. Tacrolimus ointment shows promise in treating eyelid eczema in AKC patients.117
▪ Surgical Care
Severe cases of corneal shield ulcer may require superficial keratectomy to promote epithelial regeneration. Generally, shield ulcers are chronic conditions that are often refractory to conventional therapy. There have been reports of excimer laser phototherapeutic keratectomy (PTK) being used to remove fibrin deposits on the Bowman layer and theoretically facilitate epithelial healing.2 Limbal cell transplants in patients with severe ocular surface disease have been successful in patients in which previous amniotic membrane graft alone did not restore the ocular surface.118
Other surgical procedures such as cryoablation of giant papillae or surgical removal of papillae with mucosal grafting generally are not required, but they may be helpful in extremely advanced cases.2 Simple resection of papillae combined with topical cyclosporin and intraoperative mitomycin-C after excision of the papillae have shown promising results.119,120 Most recently, CO2 laser ablation seems to be safe and effective for removing giant papillae.121 Remember that since VKC is a self-limited disease, extensive reconstructive surgery may not have an acceptable risk–benefit ratio.
Penetrating keratoplasty may be executed in cases of AKC with severe corneal scarring or thinning, with great attention in controlling ocular surface inflammation.
▪ Experimental Therapy
Selectin Antagonist: sPSGL-1
P-selectin glycoprotein ligand-1 (PSGL-1) is expressed on the surface of most leukocytes and is a counter-receptor for P-selectin.122 It has been shown to bind E- and L-selectin,123 transmembrane adhesion receptors that mediate the initial rolling interaction between leukocytes and the vascular endothelium prior to extravasation.124 A soluble, extracellular form of human PSGL-1 (sPSGL-1) has been shown to bind all members of the selectin family.122,123 This shows promise in disrupting the inflammatory cycle by impeding leukocyte adhesion and diapedesis.
Integrin Antagonist: anti-ICAM-1 mAB/anti-LFA-1 mAB
It has been discussed that leukocyte activation requires prior upregulation of intracellular adhesion molecules. The roles of ICAM-1 and its counter-receptor LFA-1 were examined in a mouse model of allergic conjunctivitis.125 Blockage of either molecule reduced the clinical signs and inhibited inflammatory infiltration into the conjunctiva. This effect was synergistic when inhibiting both molecules, suggesting the possible therapeutic role of adhesion molecule blockage.
Cytokine Antagonist: IL-1Ra
IL-1Ra is a member of the IL-1 family, known to regulate host defenses by competitively inhibiting IL-1 activity.126 Recombinant IL-1Ra was administered to a mouse model of allergic conjunctivitis, with resultant decrease in clinical signs when compared to controls.127 There was an associated reduction of degranulated mast cells, decline in eosinophil infiltrate, and a reduction of transcription of allergen-induced chemokines.
CCR3 Chemokine Receptor Antagonist
Mouse models of allergic conjunctivitis have been used to verify the efficacy of certain drugs. Oral administration of CCR3 antagonist ablates both the early and late-phase reactions in the mouse model, as shown recently by Nakamura et al.128 It stabilized the mast cell in vivo, leading to reduced eosinophilia and neutrophilia.
Transglutaminase Inhibitors
It has been established that steroids reduce inflammation by inhibiting phospholipase A2 (PLA2). Recent studies demonstrate that PLA2 is activated by transglutaminase 2 (TGase2) and that the latter’s expression is increased in various inflammatory disease such as celiac and Crohn’s disease.129–131 Through an animal model, Sohn and colleagues show that inhibition of TGase2 reversed the inflammation of allergic conjunctivitis.132
Anti-IgE Monoclonal Antibody
In several clinical controlled trials, anti-IgE monoclonal antibody (omalizumab) resulted in effectivly reducing asthma-related symptoms, decreasing corticosteroid use, and improving quality of life of asthmatic patients.133 It has been proposed that IgE-mediated diseases, including allergic conjunctivitis, would benefit from this drug’s inhibition of acute and late-phase reactions.
Abelson MB, editor. Allergic diseases of the eye. Philadelphia: WB Saunders, 2000.
Abu El-Asrar AM, Al-Mansouri S, Tabbara KF, Missotten L, Geboes K. Immunopathogenesis of conjunctival remodeling in vernal keratoconjunctivitis. Eye. 2006;20:71-79.
American Academy of Ophthalmology. Conjunctivitis, preferred practice pattern. San Francisco: American Academy of Ophthalmology, 2003.
Bonini S. Atopic keratoconjunctivitis. Allergy. 2004;59(suppl 78):71-73.
Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. 2004;18:345-351.
Butrus S, Portela R. Ocular allergy; diagnosis and treatment. Ophthalmol Clin North Am. 2005;18:485-492.
Calonge M, Enriquez-de-Salamanca A. The role of the conjunctival epithelium in ocular allergy. Curr Opin Allergy Clin Immunol. 2005;5:441-445.
Donshik PC, Porazinski AD. Giant papillary conjunctivitis in frequent-replacement contact lens wearers; a retrospective study. Trans Am Ophthalmol Soc. 1999;97:205-216. discussion 216–220.
Foster CS. Immunologic disorders of the conjunctiva, cornea, and sclera. Principles and practices of ophthalmology, 2nd edn., Philadelphia: WB Saunders; 1999:803-828.
Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793-805.
Leonardi A. In-vivo diagnostic measurements of ocular inflammation. Curr Opin Allergy Clin Immunol. 2005;5:464-472.
Leonardi A, Curnow SJ, Zhan H, Calder VL. Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures. Clin Exp Allergy. 2006;36:777-784.
Martin A, Gomez Demel E, Gagliardi J, et al. Clinical signs and symptoms are not enough for the correct diagnosis of allergic conjunctivitis. J Invest Allerg Clin Immunol. 2003;13:232-237.
Okada N, Fukagawa K, Takano Y, et al. The implications of the upregulation of ICAM-1/VCAM-1 expression of corneal fibroblasts on the pathogenesis of allergic keratopathy. Invest Ophthalmol Vis Sci. 2005;46:4512-4518.
Owen CG, Shah A, Henshaw K, et al. Topical treatments for seasonal allergic conjunctivitis; systematic review and meta-analysis of efficacy and effectiveness. Br J Gen Pract. 2004;54:451-456.
Spencer WH, Zimmerman LE. Conjunctiva; Third Ed. Ophthalmic Pathology; An Atlas and Textbook. vol 1. 1985. W.B. Saunders Company, Philadelphia, PA. 109-228.
Stern ME, Siemasko KF, Niederkorn JY. The Th1/Th2 paradigm in ocular allergy. Curr Opin Allergy Clin Immunol. 2005;5:446-450.
Strauss EC, Foster CS. Atopic ocular disease. Ophthalmol Clin North Am. 2002;15:1-5.
Trocme SD, Leiferman KM, George T, et al. Neutrophil and eosinophil participation in atopic and vernal keratoconjunctivitis. Curr Eye Res. 2003;26:319-325.
1 Akpek AK. Ocular allergy. Massachusetts Eye Research and Surgery Institute’s clinical immunology case reports 1998;3:1. Online. Available at: http://www.uveitis.org/medical/articles/case/Allergy.html. Accessed March 23, 2006.
2 Foster CS. Immunologic disorders of the conjunctiva, cornea, and sclera. Principles and practices of ophthalmology, 2nd edn., Philadelphia: WB Saunders; 1999:803-828.
3 Du Toit G. Clinical allergy images. Curr Allergy Clinical Immunol. 2005;18:148-150.
4 Friedlander MH. Conjunctivitis of allergic origin; clinical presentation and differential diagnosis. Surv Ophthalmol. 1993;38(suppl):105-114.
5 Donshik PC, Porazinski AD. Giant papillary conjunctivitis in frequent-replacement contact lens wearers; a retrospective study. Trans Am Ophthalmol Soc. 1999;97:205-216. discussion 216–220.
6 Bonini S, Lambiase A, Marchi S, et al. Vernal keratoconjunctivitis revisited; a case series of 195 patients with long-term follow-up. Ophthalmology. 2000;107:1157-1163.
7 Beigleman MN. Vernal conjunctivitis. Los Angeles: University of Southern California Press, 1950.
8 Jones BR, Andrews BE, Henderson WG, Schofield PB. The pattern of conjunctivitis at Morefield’s during 1956. Trans Ophthalmol Soc UK. 1957;77:291-305.
9 Dart JKG. The epidemiology of vernal keratoconjunctivitis. Proceedings of the Second Fisons International Ophthalmology Workshop. Bollington, Cheshire, UK: Pennine, 1989;26-37.
10 Hogan MJ. Atopic keratoconjunctivitis. Trans Am Ophthalmol Soc. 1952;50:265-281.
11 Jay JL. Clinical features and diagnosis of adult atopic keratoconjunctivitis and the effect of treatment with sodium cromoglycate. Br J Ophthalmol. 1981;65:335-340.
12 Garrity JA, Liesegang TJ. Ocular complications of atopic dermatitis. Can J Ophthalmol. 1984;19:21-24.
13 Onguchi T, Dogru M, Okada N, et al. The impact of the onset time of atopic keratoconjunctivitis on the tear function and ocular surface findings. Am J Ophthalmol. 2006;141:569-571.
14 Smolin G. Ocular allergy. The Third Annual Thygeson Lecture. Presented at the Ocular Microbiology and Immunology Group. Cornea. 1998;17:253-256.
15 Sainte Laudy J, Couturier P, Basset-Stheme D. Importance of the lacrimal levels (total IgE, specific IgE, and albumin) for the study of allergic conjunctivitis. Allerg Immunol. 1994;26:95-96.
16 Morgan SJ, Williams JH, Walls AF, Church MK, Holgate ST, McGill JI. Mast cell numbers and staining characteristics in the normal and allergic human conjunctiva. J Allergy Clin Immunol. 1991;87:111-116.
17 Butrus SI, Ochsner KI, Abelson MB, Schwartz LB. The level of tryptase in human tears. Ophthalmology. 1990;97:1678-1683.
18 Proud D, Sweet J, Stein P, et al. Inflammatory mediator release on conjunctival provocation of allergic subjects with allergen. J Allergy Clin Immunol. 1990;85:896-905.
19 Moller A, Lippert U, Lessmann D, et al. Human mast cells produce IL-8. J Immunol. 1993;151:3853-3855.
20 Schleimer RP, Stervinsky SA, Kaiser J, et al. IL-4 induces adherence of human eosinophils and basophils but not neutrophils to endothelium. J Immunol. 1992;148:1086-1092.
21 Anderson DF, Zhang S, Bradding P, McGill JI, Holgate ST, Roche WR. The relative contribution of mast cell subsets to conjunctival Th2-like cytokines. Invest Ophthalmol Vis Sci. 2001;42:995-1001.
22 Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464-4468.
23 McGill J. Conjunctival cytokines in ocular allergy. Clin Exp Allergy. 2000;30:1355-1357.
24 Bacon AS, Baddeley S, Metz D, Lightman SL, McGill JI, Holgate ST. Upregulation of adhesion molecules in allergic eye disease and relationship to leukocyte infiltration. Invest Ophthalmol Vis Sci. 1998;39:322-330.
25 Di Gioacchino M, Cavallucci E, Di Sciascio MB, et al. Increase in CD45RO+ cells and activated eosinophils in chronic allergic conjunctivitis. Immunobiology. 2000;201:541-551.
26 Eperon S, Sauty A, Lanz R, Leimgruber A, Lurati F, Guex-Crosier Y. Eotaxin-1 (CCL11) up-regulation in tears during seasonal allergic conjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2004;242:966-970.
27 Fukagawa K, Nakajima T, Tsubota K, Shimmura S, Saito H, Hirai K. Presence of eotaxin in tears of patients with atopic keratoconjunctivitis with severe corneal damage. J Allergy Clin Immunol. 1999;103:1220-1221.
28 Leonardi A, Jose PJ, Zhan H, Calder VL. Tear and mucus eotaxin-1 and eotaxin-2 in allergic keratoconjunctivitis. Ophthalmology. 2003;110:487-492.
29 Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment; implications for human disease. Mol Med Today. 2000;6:20-27.
30 Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425-456.
31 Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005-2007.
32 Spring TF. Reaction to hydrophilic lenses. Med J Aust. 1974;1:499.
33 Allansmith MR, Korb DR, Greiner JV, et al. Giant papillary conjunctivitis in contact lens wearers. Am J Ophthalmol. 1977;83:697-708.
34 Chang WJ, Tse DT, Rosa RH, Huang A, Johnson TE, Schiffman J. Conjunctival cytology features of giant papillary conjunctivitis associated with ocular prostheses. Ophthal Plast Reconstr Surg. 2005;21:39-45.
35 Elgebaly SA, Donshik PC, Rahhal F, Williams W. Neutrophil chemotactic factor in the tears of giant papillary conjunctivitis patients. Invest Ophthalmol Vis Sci. 1991;32:208-213.
36 Fowler SA, Allansmith MR. Evolution of soft contact lens coatings. Arch Ophthalmol. 1980;98:95-99.
37 Donshik PC. Contact lens chemistry and giant papillary conjunctivitis. Eye Contact Lens. 2003;29(1 suppl):S37-39. discussion S57–59, S192–194.
38 Szczotka LB, Cocuzzi E, Medof ME. Decay-accelerating factor in tears of contact lens wearers and patients with contact lens-associated complications. Optom Vis Sci. 2000;77:586-591.
39 Moschos MM, Eperon S, Guex-Crosier Y. Increased eotaxin in tears of patients wearing contact lenses. Cornea. 2004;23:771-775.
40 Henriquez AS, Baird RS, Korb DR, Allansmith MR. Histology of hard and soft contact lens-associated giant papillary conjunctivitis. Ann Ophthalmol. 1980;12:929-933.
41 Allansmith MR, Korb DR, Greiner JV. Giant papillary conjunctivitis induced by hard or soft contact lens wear; quantitative histology. Trans Am Acad Ophthalmol Otolaryngol. 1978;85:766-778.
42 Bahn AK, Fujikawa LS, Foster CS. T-cell subsets and Langerhans cells in normal and diseased conjunctiva. Am J Ophthalmol. 1982;94:205-212.
43 Allansmith MR. Vernal conjunctivitis. Duane T, editor. Clinical ophthalmology, vol 4. New York: Harper & Row, 1978.
44 Maggi E, Biswas P, Del Prete G, et al. Accumulation of Th-2-like helper T cells in the conjunctiva of patients with vernal conjunctivitis. J Immunol. 1991;146:1169-1174.
45 Abu el-Asrar AM, Geboes K, al-Kharashi S, Tabbara KF, Missotten L, Desmet V. Adhesion molecules in vernal keratoconjunctivitis. Br J Ophthalmol. 1997;81:1099-1106.
46 Bisgaard H, Olsson P, Bende M. Effect of leukotriene D4 on nasal mucosal blood flow, nasal airway resistance and nasal secretion in humans. Clin Allergy. 1986;16:289-297.
47 Bisgaard H, Ford-Hutchinson AW, Charleson S, Taudorf E. Production of leukotrienes in human skin and conjunctival mucosa after specific allergen challenge. Allergy. 1985;40:417-423.
48 Woodward DF, Ledgard SE. Effect of LTD4 on conjunctival vasopermeability and blood–aqueous barrier integrity. Invest Ophthalmol Vis Sci. 1985;26:481-485.
49 Pucci N, Novembre E, Lombardi E, et al. Atopy and serum eosinophil cationic protein in 110 white children with vernal keratoconjunctivitis; differences between tarsal and limbal forms. Clin Exp Allergy. 2003;33:325-330.
50 Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979;123:2925-2927.
51 O’Donnell MC, Ackerman SJ, Gleich GJ, Thomas LL. Activation of basophil and mast cell histamine release by eosinophil granule major basic protein. J Exp Med. 1983;157:1981-1991.
52 Trocme SD, Kephart GM, Allansmith MR, Bourne WM, Gleich GJ. Conjunctival deposition of eosinophil granule major basic protein in vernal keratoconjunctivitis and contact lens-associated giant papillary conjunctivitis. Am J Ophthalmol. 1989;108:57-63.
53 Trocme SD, Kephart GM, Bourne WM, Buckley RJ, Gleich GJ. Eosinophil granule major basic protein deposition in corneal ulcers associated with vernal keratoconjunctivitis. Am J Ophthalmol. 1993;115:640-643.
54 Trocme SD, Leiferman KM, George T, et al. Neutrophil and eosinophil participation in atopic and vernal keratoconjunctivitis. Curr Eye Res. 2003;26:319-325.
55 Moy JN, Gleich GJ, Thomas LL. Noncytotoxic activation of neutrophils by eosinophil granule major basic protein. Effect on superoxide anion generation and lysosomal enzyme release. J Immunol. 1990;145:2626-2632.
56 Arlt F. Physiologisch und pathologisch anatomische Bemerkungen über die Bindehaut des Auges. Prager Vierteljahrschrift. 1846;4:73.
57 von Graefe A. Klinische Vorträge über Augenheilkunde. Germany: Hirschberg, 1871;21.
58 Abu El-Asrar AM, Struyf S, Al-Mosallam AA, Missotten L, Van Damme J, Geboes K. Expression of chemokine receptors in vernal keratoconjunctivitis. Br J Ophthalmol. 2001;85:1357-1361.
59 Abu El-Asrar AM, Van den Oord JJ, Geboes K, Missotten L, Emarah MH, Desmet V. Immunopathological study of vernal keratoconjunctivitis. Graefe’s Arch Clin Exp Ophthalmol. 1989;227:374-379.
60 Abu El-Asrar AM, Meersschaert A, Al-Kharashi SA, Missotten L, Geboes K. Immunohistochemical evaluation of conjunctival remodelling in vernal keratoconjunctivitis. Eye. 2003;17:767-771.
61 Abu El-Asrar AM, Al-Mansouri S, Tabbara KF, Missotten L, Geboes K. Immunopathogenesis of conjunctival remodelling in vernal keratoconjunctivitis. Eye. 2006;20:71-79.
62 Caroll JM, Romero R, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957-968.
63 Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039.
64 Kovacs E, Di Pietro L. Fibrogenic cytokines and connective tissue production. FASEB J. 1994;8:854-861.
65 Bonner JC, Osornio-Vargas AR, Badgett A, Brody AR. Differential proliferation of lung fibroblasts induced by the platelet-derived growth factor -AA, -AB, and -BB isoforms secreted by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1991;5:539-547.
66 Fini ME, Parks WC, Rinehart WB, et al. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996;149:1287-1302.
67 Dogru M, Okada N, Asano-Kato N, et al. Atopic ocular surface disease; implications on tear function and ocular surface mucins. Cornea. 2005;24(8 suppl):S18-S23.
68 Leonardi A, Brun P, Abatangelo G, Plebani M, Secchi AG. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44:3052-3058.
69 Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18:1707-1715.
70 Ebihara N, Funaki T, Takai S, Miyazaki M, Fujiki K, Murakami A. Tear chymase in vernal keratoconjunctivitis. Curr Eye Res. 2004;28:417-420.
71 Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379-388.
72 Foster CS, Rice BA, Dutt JE. Immunopathology of atopic keratoconjunctivitis. Ophthalmology. 1991;98:1190-1196.
73 Matsuura N, Uchio E, Nakazawa M, et al. Predominance of infiltrating IL-4-producing T cells in conjunctiva of patients with allergic conjunctival disease. Curr Eye Res. 2004;29:235-243.
74 Akdis M, Trautmann A, Klunker S, et al. T helper (Th) 2 predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector Th1 cells. FASEB J. 2003;17:1026-1035.
75 Leonardi A, Fregona IA, Plebani M, Secchi AG, Calder VL. Th1- and Th2-type cytokines in chronic ocular allergy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1240-1245.
76 Okada N, Fukagawa K, Takano Y, et al. The implications of the upregulation of ICAM-1/VCAM-1 expression of corneal fibroblasts on the pathogenesis of allergic keratopathy. Invest Ophthalmol Vis Sci. 2005;46:4512-4518.
77 Ambach A, Bonnekoh B, Gollnick H. Perforin hyperreleasability and depletion in cytotoxic T cells from patients with exacerbated atopic dermatitis and asymptomatic rhinoconjunctivitis allergica. J Allergy Clin Immunol. 2001;107:878-886.
78 Metz DP, Hingorani M, Calder VL, Buckley RJ, Lightman SL. T-cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol. 1997;100(6 Pt 1):817-824.
79 Spencer WH, Zimmerman LE. Conjunctiva. 3rd edn. Ophthalmic pathology; an atlas and textbook. vol 1. 1985. WB Saunders, Philadelphia, PA. 109-228.
80 Volker HE, Naumann GOH. Conjunctiva. Pathology of the eye, 1st edn., Berlin: Springer-Verlag; 1985:249-316.
81 Asano-Kato N, Fukagawa K, Okada N, et al. TGF-beta1, IL-1beta, and Th2 cytokines stimulate vascular endothelial growth factor production from conjunctival fibroblasts. Exp Eye Res. 2005;80:555-560.
82 Yamagami S, Ebihara N, Amano SY. Chemokine receptor gene expression in giant papillae of atopic keratoconjunctivitis. Mol Vis. 2005;11:192-200.
83 Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793-805.
84 Donshik PC. Allergic conjunctivitis. Int Ophthalmol Clin. 1988;28:294-302.
85 Marks MB. Stigmata of respiratory tract allergies. Kalamazoo: Upjohn, 1977;12.
86 Martin A, Gomez Demel E, Gagliardi J, et al. Clinical signs and symptoms are not enough for the correct diagnosis of allergic conjunctivitis. J Invest Allerg Clin Immunol. 2003;13:232-237.
87 Leonardi A. In-vivo diagnostic measurements of ocular inflammation. Curr Opin Allergy Clin Immunol. 2005;5:464-472.
88 Weissman BA, Yeung KK. Giant papillary conjunctivitis. Online. Available at: http://www.emedicine.com/oph/topic87.htm. Accessed April 2006.
89 Bonini S, Coassin M, Aronni S, Lambiase A. Vernal keratoconjunctivitis. Eye. 2004;18:345-351.
90 Tanaka M, Dogru M, Takano Y, et al. The relation of conjunctival and corneal findings in severe ocular allergies. Cornea. 2004;23:464-467.
91 Jones BR. Vernal keratitis. Trans Ophthalmol Soc UK. 1961;81:215-228.
92 Foster CS, Calonge M. Atopic keratoconjunctivitis. Ophthalmology. 1990;97:992-1000.
93 Tuft SJ, Kemeny DM, Dart JKG, Buckley RJ. Clinical features of atopic keratoconjunctivitis. Ophthalmology. 1991;98:150-158.
94 Bonini S. Atopic keratoconjunctivitis. Allergy. 2004;59(suppl 78):71-73.
95 Bielory L, Lien KW, Bigelsen S. Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis. Drugs. 2005;65:215-228.
96 Owen CG, Shah A, Henshaw K, et al. Topical treatments for seasonal allergic conjunctivitis; systematic review and meta-analysis of efficacy and effectiveness. Br J Gen Pract. 2004;54:451-456.
97 Abelson MB, Greiner JV. Comparative efficacy of olopatadine 0.1% ophthalmic solution versus levocabastine 0.05% ophthalmic suspension using the conjunctival allergen challenge model. Curr Med Res Opin. 2004;20:1953-1958.
98 Abelson MB, Gomes PJ, Vogelson CT, et al. Clinical efficacy of olopatadine hydrochloride ophthalmic solution 0.2% compared with placebo in patients with allergic conjunctivitis or rhinoconjunctivitis; a randomized, double-masked environmental study. Clin Ther. 2004;26:1237-1248.
99 Ganz M, Koll E, Gausche J, et al. Ketotifen fumarate and olopatadine hydrochloride in the treatment of allergic conjunctivitis; a real-world comparison of efficacy and ocular comfort. Adv Ther. 2003;20:79-91.
100 Greiner JV, Minno G. A placebo-controlled comparison of ketotifen fumarate and nedocromil sodium ophthalmic solutions for the prevention of ocular itching with the conjunctival allergen challenge model. Clin Ther. 2003;25:1988-2005.
101 Greiner JV, Michaelson C, McWhirter CL, Shams NB. Single dose of ketotifen fumarate 0.025% vs 2 weeks of cromolyn sodium 4% for allergic conjunctivitis. Adv Ther. 2002;19:185-193.
102 Crampton HJ. Comparison of ketotifen fumarate ophthalmic solution alone, desloratadine alone, and their combination for inhibition of the signs and symptoms of seasonal allergic rhinoconjunctivitis in the conjunctival allergen challenge model; a double-masked, placebo- and active-controlled trial. Clin Ther. 2003;25:1975-1987.
103 Martin AP, Urrets-Zavalia J, Berra A, et al. The effect of ketotifen on inflammatory markers in allergic conjunctivitis; an open, uncontrolled study. BMC Ophthalmol. 2003;3:2.
104 Avunduk AM, Tekelioglu Y, Turk A, Akyol N. Comparison of the effects of ketotifen fumarate 0.025% and olopatadine HCl 0.1% ophthalmic solutions in seasonal allergic conjunctivities; a 30-day, randomized, double-masked, artificial tear substitute-controlled trial. Clin Ther. 2005;27:1392-1402.
105 Miyake-Kashima M, Takano Y, Tanaka M, et al. Comparison of 0.1% bromfenac sodium and 0.1% pemirolast potassium for the treatment of allergic conjunctivitis. Jpn J Ophthalmol. 2004;48:587-590.
106 Schleimer RP, Beck L, Schweibert L. Inhibition of inflammatory cell recruitment by glucocorticoides; cytokines as primary targets. In: Schleimer RP, Busse WW, O’Byrne P, editors. Inhaled glucocorticoids in asthma. Mechanisms and clinical actions. New York: Marcel Dekker; 1997:203-238.
107 Meagher LC, Cousin JM, Seckl JR, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422-4428.
108 Nutku E, Zhuang Q, Soussi-Gounni A, Aris F, Mazer BD, Hamid QJ. Functional expression of IL-12 receptor by human eosinophils; IL-12 promotes eosinophil apoptosis. Immunology. 2001;167(2):1039-1046.
109 Ilyas H, Slonim CB, Braswell GR, Favetta JR, Schulman M. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004;30:10-13.
110 Shulman DG, Lothringer LL, Rubin JM, et al. A randomized, double-masked, placebo-controlled parallel study of loteprednol etabonate 0.2% in patients with seasonal allergic conjunctivitis. Ophthalmology. 1999;106:362-369.
111 American Academy of Ophthalmology. Conjunctivitis, preferred practice pattern. San Francisco: American Academy of Ophthalmology, 2003.
112 Corum I, Yeniad B, Bilgin LK, Ilhan RJ. Efficiency of olopatadine hydrochloride 0.1% in the treatment of vernal keratoconjunctivitis and goblet cell density. Ocul Pharmacol Ther. 2005;21:400-405.
113 Singh S, Pal V, Dhull CS. Supratarsal injection of corticosteroids in the treatment of refractory vernal keratoconjunctivitis. Indian J Ophthalmol. 2001;49:241-245.
114 Pucci N, Novembre E, Cianferoni A, et al. Efficacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol. 2002;89:298-303.
115 Hingorani M, Moodaley L, Calder VL, et al. A randomized, placebo-controlled trial of topical cyclosporine A in steroid-dependent atopic keratoconjunctivitis. Ophthalmology. 1998;105:1715-1720.
116 Leonardi A, Borghesan F, DePaoli M, Plebani M, Secchi AG. Procollagens and inflammatory cytokine concentrations in tarsal and limbal vernal keratoconjunctivitis. Exp Eye Res. 1998;67:105-112.
117 Nivenius E, van der Ploeg I, Jung K, Chryssanthou E, van Hage M, Montan PG. Tacrolimus ointment vs steroid ointment for eyelid dermatitis in patients with atopic keratoconjunctivitis. Eye advance online publication. May 5, 2006. 10.1038/sj.eye.6702367.
118 Sangwan VS, Murthy SI, Vemuganti GK, Bansal AK, Gangopadhyay N, Rao GN. Cultivated corneal epithelial transplantation for severe ocular surface disease in vernal keratoconjunctivitis. Cornea. 2005;24:426-430.
119 Fujishima H, Fukagawa K, Satake Y, et al. Combined medical and surgical treatment of severe vernal keratoconjunctivitis. Jpn J Ophthalmol. 2000;107:1157-1163.
120 Tanaka M, Takano Y, Dogru M, et al. A comparative evaluation of the efficacy of intraoperative mitomycin C use after the excision of cobblestone-like papillae in severe atopic and vernal keratoconjunctivitis. Cornea. 2004;23:326-329.
121 Belfair N, Monos T, Levy J, Mnitentag H, Lifshitz T. Removal of giant vernal papillae by CO2 laser. Can J Ophthalmol. 2005;40:472-476.
122 Sako D, Chang XJ, Barone KM, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179-1186.
123 Strauss EC, Larson KA, Brenneise I, et al. Soluble P-selectin glycoprotein ligand-1 inhibits ocular inflammation in a murine model of allergy. Invest Ophthalmol Vis Sci. 1999;40:1336-1342.
124 Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Ann Rev Physiol. 1995;57:827-872.
125 Whitcup SM, Chan CC, Kozhich AT, Magone MT. Blocking ICAM-1 (CD54) and LFA-1 (CD11a) inhibits experimental allergic conjunctivitis. Clin Immunol. 1999;93:107-113.
126 Strauss EC, Foster CS. Atopic ocular disease. Ophthalmol Clin North Am. 2002;15:1-5.
127 Keane-Myers AM, Miyazaki D, Liu G, Dekaris I, Ono S, Dana MR. Prevention of allergic eye disease by treatment with IL-1 receptor antagonist. Invest Ophthalmol Vis Sci. 1999;40:3041-3046.
128 Nakamura T, Ohbayashi M, Toda M, Hall DA, Horgan CM, Ono SJ. A specific CCR3 chemokine receptor antagonist inhibits both early and late phase allergic inflammation in the conjunctiva. Immunol Res. 2005;33:213-221.
129 Cordella-Miele E, Miele L, Mukherjee AB. A novel transglutaminase-mediated post-translational modification of phospholipase A2 dramatically increases its catalytic activity. J Biol Chem. 1990;265:17180-17188.
130 Bruce SE, Bjarnason I, Peters TJ. Human jejunal transglutaminase; demonstration of activity, enzyme kinetics and substrate specificity with special relation to gliadin and celiac disease. Clin Sci. 1985;68:573-579.
131 D’Argenio G, Biancone L, Cosenza V, et al. Transglutaminases in Crohn’s disease. Gut. 1995;37:690-695.
132 Sohn J, Kim TI, Yoon YH, Kim JY, Kim SY. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J Clin Invest. 2003;111:121-128.
133 D’Amato G. Role of anti-IgE monoclonal antibody (omalizumab) in the treatment of bronchial asthma and allergic respiratory diseases. Eur J Pharmacol. 2006;533(1–3):302-307.