Chapter 12 Management of the Patient with Occupational Allergy
Introduction
Occupational allergy causes considerable morbidity and mortality, posing a great burden to society in terms of disability, health care costs, and time off work. In the USA there are an estimated 14.6 million work absence days due to asthma alone annually.1 Some of the common conditions caused by occupational allergies include occupational asthma, hypersensitivity pneumonitis, latex rubber allergy, and allergic contact dermatitis. The greatest challenge is to be able to distinguish occupational allergy from non-work-related allergic conditions. This chapter will focus on the characteristics of occupational allergies that will help to differentiate occupational from non-work-related causes. One of the effective ways to reduce the burden of occupational allergy is to prevent the exposure and sensitization to occupational allergens in susceptible workers, but it is equally important to prevent long-term complications by recognizing and treating these conditions early once they have occurred. Failure to recognize occupational asthma can lead to chronic symptoms, disability, absenteeism from work, and increased health care costs. Similarly delayed detection of hypersensitivity pneumonitis can lead to irreversible pulmonary fibrosis and long-term disability. In this chapter discussion will be limited to occupational asthma, hypersensitivity pneumonitis, latex allergy, and allergic contact dermatitis, which are some of the commonest occupational allergic conditions.
Prevalence
According to the Asthma and Allergy Foundation of America the annual cost of asthma is estimated to be US$18 billion, it is the fourth leading cause of work absenteeism with nearly 15 million missed or lost workdays each year and a total cost of nearly 3 billion in lost productivity.2 Work-related asthma is the most commonly reported occupational lung disease in the USA. In the USA 10–25% of adult asthma is either initiated or aggravated by work, with incidence rates of 29–710 cases per million workers per year.3 In the UK it is estimated occupational asthma accounts for 2–6% of adult asthma,4 and other population-based studies in Europe have estimated it to be between 5% and 10%. The occupations at highest risk for occupational asthma are farmers, painters, plastic workers, cleaners, spray painters, and agriculture workers.5 Latex-induced asthma is an important cause of asthma in health care workers.
The prevalence of hypersensitivity pneumonitis is unknown, due in part to the lack of standard diagnostic criteria and partly because it has so many causes. According to NIOSH Occupational Respiratory Disease Surveillance the number of deaths from hypersensitivity pneumonitis has been increasing from less than 20 per year in 1979 to 57 in 1999.6 Many agents can cause hypersensitivity pneumonitis and its development depends on the size and nature of the antigen, the concentration of the antigen, the duration of exposure, and the frequency of repeated exposures. It is interesting to note that research shows hypersensitivity pneumonitis occurs more frequently in nonsmokers than smokers.7
The prevalence of latex sensitization in the general population ranges between 5% and 10%. Clinical associations have also been found between latex and certain fruits and vegetables such as kiwi, banana, chestnut, avocado, lettuce, tomato, and potato. Because of this, latex allergy may follow fruit or vegetable allergy or vice versa.8 Occupations at highest risk to latex allergy include health care workers (nurses, doctors, dentists, operating room staff, laboratory technicians) and workers in the latex industry. One study reported a prevalence of 11% for positive skin tests to latex in a surgical glove manufacturing plant.9 In another study the prevalence of latex sensitization among health care workers at two hospital sites was found to be 12.1%.8 The amount of latex needed to produce sensitization is unknown, and no safe occupational exposure limit has been established for latex protein.
In the USA skin diseases and disorders accounted for 13% (57 900 cases) of all nonfatal occupational illness cases in private industry reported in 1997. Of these there were 6600 cases of dermatitis that involved time away from work. Workers with dermatitis had a median of 3 days off from work. Service and manufacturing industries each were the cause of 29% of the cases of dermatitis with days away from work. Laborers, fabricators, precision production workers, repair personnel and craft workers are the workers most commonly affected by dermatitis with time away from work.10 In the work setting hands are the most often affected part of the body, either alone or with other parts of the body. The hands are involved in 80–90% of cases of occupational dermatitis.11
Epidemiology
▪ Work-related Asthma
Work-related asthma is classified as either occupational or work-aggravated asthma. Workplace exposures can cause new onset asthma or exacerbate pre-existing asthma. Occupational asthma is defined as new onset asthma, which is caused by workplace exposures. There are two subtypes of occupational asthma, sensitizer-induced asthma that has a latency period and is due to immunologic mechanisms and irritant-induced asthma that has no latency period and occurs after high-intensity exposures to irritating agents. The latency period can vary from weeks to years. Reactive airways dysfunction is a discrete subset of irritant-induced asthma. Pre-existing asthma that is worsened by workplace exposures is called work-aggravated asthma. In this chapter the focus will be on sensitizer-induced asthma, which is characterized by variable airflow limitation or bronchial hyperresponsiveness, or both induced by agents in the workplace.
▪ Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis is an immunologically mediated parenchymal lung disease which involves the alveolar walls and terminal bronchioles. It is caused by inhalation of organic dusts. The clinical picture and pathology of hypersensitivity pneumonitis is the same regardless of the cause. Many different dusts are know to cause hypersensitivity pneumonitis but the mechanism of disease is not clearly understood. Specific IgG antibodies against the dust involved seem to be necessary. However, this is not sufficient on its own to explain the symptoms, as not all inviduals with antibodies develop disease. Repeated exposure to the antigen causes sensitization followed by immunologically mediated damage to the lungs. Many agents can cause hypersensitivity pneumonitis. The major categories of antigen include animal/insect proteins, microbial agents (bacteria, fungi), and low molecular weight agents. The clinical presentation can be acute, subacute, or chronic. Some of the causes of hypersensitivity pneumonitis are listed in Table 12.1.
TABLE 12.1 Some of the causes of hypersensitivity pneumonitis
| Disease | Source of antigen | Antigen |
|---|---|---|
| Bird fancier’s lung | Pigeon, parakeet, dove, duck | Avian proteins |
| Furrier’s lung | Animal pelts/cat hair | Dust from fur/cat hair |
| Farmer’s lung | Moldy hay, silage, grain | Thermophilic actinomycetes |
| Woodworker’s disease | Cedar, oak, mahogany, pine, spruce | Alternaria spp. |
| Tobacco grower’s lung | Tobacco leaves | Tobacco dust |
| Coffee worker’s lung | Coffee bean dust | Coffee dust |
| Bagassosis | Moldy sugar cane | Thermoactinomyces sacchari |
| Malt worker’s lung | Moldy malt | Aspergillus clavatus |
| Cheese worker’s lung | Moldy cheese | Penicillum casei |
| Oyster shell lung | Shell dust | Oyster shell protein |
| Tea grower’s lung | Tea leaves | Tea dust |
| Mushroom worker’s lung | Moldy mushroom | Thermoactinomyces sacchari |
| Pauli’s reagent alveolitis | Laboratory reagent | Pauli’s reagent |
| Pyrethrum lung | Insecticide | Pyrethum |
| Epoxy resin worker’s lung | Adesive, foam, resin | Phthalic anhydride |
| Drug-induced alveolitis | Medications | E.g. sulfasalazine, nitrofurantoin |
| Plastic worker’s lung | Plastic | Trimellitic anhydride |
| Humidifier lung | Contamined forced air system | Saccharopolyspora rectivirgula, Thermoactinomyces vulgaris |
| Chemical worker’s lung | Urethane paint catalyst | Diphenylmethane diisocyanate |
▪ Contact Dermatitis
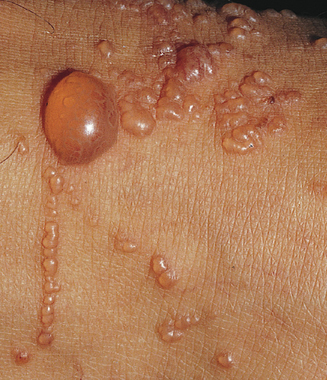
Contact dermatitis can be divided into allergic or irritant types. Up to 80% of cases of contact dermatitis are due to irritants. Irritants cause dermatitis by nonimmunologic reactions whereas allergens cause dermatitis by delayed hypersensitivity immunologic reactions. It is difficult to differentiate the irritant from allergic contact dermatitis clinically. Chemicals are the most important cause of occupational skin disease followed by mechanical, physical, and biological agents. Individuals may develop contact dermatitis to most chemicals if they are exposed to the chemical in sufficient quantities for an adequate period of time. Chemicals cause approximately 53% of work-related dermatitis.10 In humans more than 3000 chemicals have been implicated in contact dermatitis. Poison ivy and oak are the commonest cause of allergic contact dermatitis followed by chrysanthemums and compositae species (Figure 12.1). Horticulturists, farmers, loggers, foresters, florists, and nursery workers are at risk of developing phytodermatitis from contact with allergens from plants.
Anatomy and Physiology
The average adult has approximately 18 square feet of skin, which consists of the outer epidermis and the deeper dermis. The outermost layer of the superficial epidermis is the keratin layer (stratum corneum), which is made up of nonviable anucleate cells. Deep to this layer there are the stratum spinosum and the basal (germinative) layers. The germinative layer produces epidermal cells that move up and transform into the flat, compact, and dehydrated keratinized cells of the stratum corneum. The stratum corneum is a protective barrier of the skin preventing internal tissues from exposure to chemicals, toxins, ultraviolet radiation, bacteria, and extreme temperatures. It is thickest on the palms of the hands and the soles of the feet, so that hand dermatitis mainly affects the dorsal aspect rather than the palms. The keratin layer allows lipophilic chemicals to be absorbed more so than hydrophilic substances. Permeability of the skin is increased if waterproof materials such as gloves cover the skin, and if the skin has abrasions or is inflamed. The deeper layers of the epidermis contain the Langerhans cells, which are dendritic antigen-presenting cells, that are necessary for allergic contact dermatitis to take place. Melanocytes in the epidermis produce the pigment melanin, which protects against ultraviolet radiation. The dermis is mainly made of collagen in a ground substance and this makes the skin strong and flexible. The dermis contains blood vessels, lymphatic drainage, nerves, and epidermal appendages.12
Pathophysiology
▪ Occupational Asthma
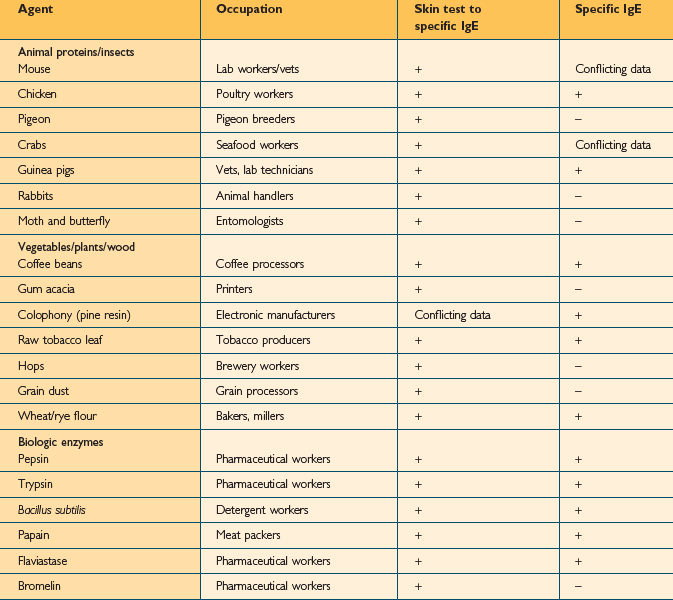
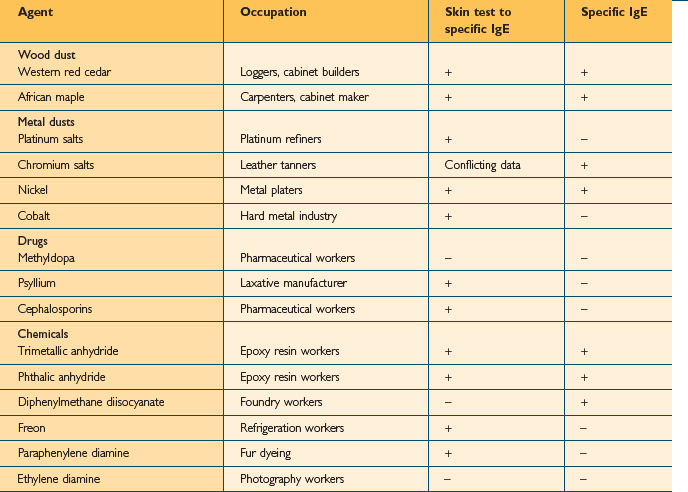
Over 350 agents are known to trigger occupational asthma.3 Sensitizing agents that cause occupational asthma can be divided into high-molecular weight compounds (>5000 Daltons) and low-molecular weight compounds (<5000 Daltons). High molecular weight compounds such as plant proteins act as complete antigens and cause the production of specific IgE antibodies (Table 12.2). The mechanism for low molecular weight compounds is unknown but certain low molecular weight compounds such as acid anhydrides probably act as haptens and bind with proteins to form functional antigens. Many low molecular weight agents, including isocyanate, do not consistently induce specific IgE antibodies (Table 12.3).
The first step in sensitizer-induced occupational asthma is the binding of the antigen with specific IgE receptors on the surface of mast cells, basophils, and possibly macrophages and eosinophils. These cells, after capturing the antigen migrate to the local lymph nodes and interact with naïve T cells (Th0) which differentiates into Th2 cells in the presence of interleukin (IL) 4 and other proinflammatory mediators. This leads to a cascade of events causing an influx of inflammatory cells into the airway and the subsequent release of inflammatory mediators. In addition to this, it stimulates isotype switching of B cells to antibody production of IgG and IgM to IgE. The IgE antibody produced by B cells circulates in the blood attaching to high affinity receptors on mast cells and low affinity receptors on basophils. Once the antigen binds with the IgE there is a release of granule stored mediators such as histamine and eosinophil/neutrophil chemotactic factors. Leukotrienes and prostaglandins are also released from membrane phospholipids. These mediators cause the symptoms of asthma such as edema and bronchoconstriction by affecting vascular permeability, smooth muscle tone, cell migration, and mucus production. Following sensitization, subsequent re-exposure to even minute quantities of allergen causes immediate symptom production which may be severe. The latency period for the development of sensitization can vary from weeks to years. However in order to produce sensitization the antigen concentration and duration of exposure has to be significant.
▪ Allergic Contact Dermatitis
Allergic contact dermatitis is caused by a type 4 hypersensitivity reaction. As with contact irritant dermatitis the reaction occurs at the site where the antigen contacts the skin, but secondary spread can occur if the hands are contaminated with the antigen, spreading it to other areas of the body. There are different phases in the development of allergic contact dermatitis. The first phase is the sensitization phase in which the lipid soluble antigen penetrates the stratum corneum of the epidermis, forms the hapten–protein complex, and reaches the basal layer where it interacts with the Langerhans cells. These cells present the antigen on their surface and move to lymph nodes close to the site of contact.13
In the regional lymph nodes the Langerhans cells display the antigen to naïve Th1 cells. This interaction produces antigen-specific memory Th1 cells that enter the circulation. For hypersensitivity to develop the individual usually needs to be exposed to the antigen for a certain period of time before sensitization occurs, even with very potent antigens. Contact allergens tend to be low molecular weight substances weighing less than 600 Daltons that easily penetrate the stratum corneum and form covalent bonds with carrier proteins in the skin to become fully immunogenic. Individuals vary in their susceptibility to develop hypersensitivity and one cannot predict how susceptible a particular individual is to developing contact allergy. Hypersensitivity usually lasts indefinitely but some individuals may lose the hypersensitivity after prolonged periods of time.14
After initial sensitization when the workers are re-exposed to the antigen the elicitation phase occurs. In the elicitation phase the Langerhans cells present the antigen to the larger pool of Th1 cells in the epidermis, dermis, and local lymph nodes and cause an inflammatory reaction by causing further proliferation of clonal Th1 cells and their return to the initial hapten provoked site to release of inflammatory mediators such as cytokines. This in turn results in the development of skin lesions and symptoms within 12–48 hours of exposure. The delay is due to the time it takes for each of the steps in the elicitation phase, from presentation of the antigen by the Langerhans cells to release of inflammatory mediators. After contact, workers develop pruritus with erythema and edema followed by the appearance of papules, vesicles, and bullae. Subsequently, crusting and weeping may occur, increasing the risk of secondary infection. The symptoms and lesions usually resolve over a period of 3–4 weeks if the offending antigen is removed. If another chemical has a structure that is similar to that of the substance that induced the initial reaction sometimes cross-sensitization occurs causing the elicitation phase to develop with exposure to the second chemical.15
If workers are exposed to antigens, in most cases only a small number develop sensitization because allergic contact dermatitis occurs in genetically susceptible individuals. For example only 5–15% of women and up to 1% of men will become sensitized to nickel in the general population. An exception to this is phytodermatis in workers exposed to poison ivy which has sensitized up to 70%. It may take only 3–4 days for sensitization to develop after exposure to poison ivy. However workers that are continually exposed to an antigen may not develop sensitivity for months or even years, making the diagnosis challenging.16
A common cause of allergic contact dermatitis in landscapers, ranch workers, and outdoor workers is phytodermatitis or plant dermatitis. It is often caused by the Rhus family such as poison ivy, poison oak, and poison sumac. The acute phase is characterized by the appearance of tiny vesicles and weepy encrusted lesions. The lesions are often linear at the site of contact with the plant.
Other common sensitizers include chromate, rubber, nickel, and formaldehyde. Occupations at risk from nickel allergic contact dermatitis include textile workers, electronic workers, mechanics, hairdressers, and machinists. Textile workers, cement workers, mechanics, and printers are at risk of developing chromate allergic contact dermatitis. Chromate dermatitis found in cement workers can take a few years to develop. Some of the common causes and related occupations of allergic contact dermatitis are listed in Table 12.1. (Not all the causes or occupations are listed.)
Diagnosis
It is important to take a complete and very detailed history with any work-related injury or illness, but it is even more so with occupational allergies. Some of the important questions to ask are listed in Box 12.1.
BOX 12.1 Questions to ask to aid the diagnosis of occupational allergy
Questions about symptom timing and type
Questions about past medical conditions
Questions about social history
Questions about hobbies and other nonoccupational activities
▪ Occupational Asthma
Obtaining detailed information on workplace factors associated with occupational allergies is essential. Workplace factors include the presence of worker safety programs, adherence to safety policies, and the presence or absence of effective industrial hygiene measures. Material Safety Data sheets on all the potential chemical exposures should also be obtained. Taking a smoking history is important because smokers usually have higher total IgE concentration and this can increase their predisposition to be sensitized to allergens in the workplace.17
How and when symptoms developed, together with severity of symptoms, should be noted. Inviduals with occupational asthma usually have wheezing, shortness of breath, and coughing while at work or within several hours of leaving work. With persistent exposure the symptoms may become chronic and lose their temporal relationship with work. One should be aware that low molecular weight sensitizers usually cause late phase responses, which can occur hours after the work shift is over. On the other hand high molecular weight sensitizers usually cause early or dual responses. Figure 12.2 shows FEV1 readings taken from individuals who show early, late and dual responses.
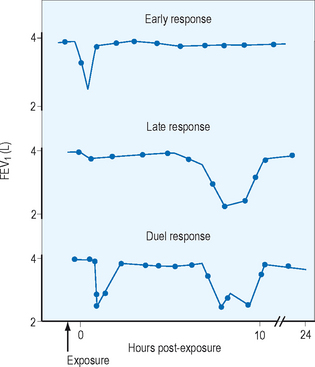
Figure 12.2 Three common patterns of response to inhaled allergen in sensitized workers with asthma.
As with all occupational allergies a full physical exam should be undertaken. However in occupational asthma particular attention should be paid to the respiratory system. The physical exam may detect wheezing but usually the examination is normal if the individual is not in the midst of an acute attack. The nasal, ocular, and oropharyngeal areas should be carefully examined for inflammation. The skin should be examined and cyanosis or clubbing noted. Vital signs as well as any signs of respiratory distress should be noted. The cardiovascular system should be examined to rule out cardiovascular causes of shortness of breath. The differential diagnosis of allergic occupational asthma includes irritant-induced asthma, hypersensitivity pneumonitis, and metal fume fever. Hypersensitivity pneumonitis can cause obstructive symptoms, but it can be easily ruled out with chest X-rays and lung function tests. Metal fume fever causes flu-like symptoms. Heating certain metals such as zinc to their melting point produces metal oxide fumes generating particles from 0.1 to 1 μm in diameter. Inhalation of these metal oxide fumes causes fever and a self-limiting flu-like illness.
▪ Hypersensitivity Pneumonitis
The clinical presentation of hypersensitivity pneumonitis can be acute, subacute, or chronic. The acute form occurs approximately 5–8 hours after intense exposure. Symptoms including fever, chills, dyspnea, chest tightness, and dry cough start abruptly and usually clear within 2–3 days if re-exposure does not occur. In the subacute form symptoms are less severe and appear insidiously over weeks or months. Patients usually have fatigue, anorexia, weight loss, fever, progressive dyspnea, and cough. In both the subacute and acute forms symptoms disappear in days, weeks, or months if the individual is not reexposed to the offending agent. The chronic form can occur in two ways; either by long term low level antigen exposure or if recurrent acute and subacute episodes are not diagnosed and treated. With low level exposure the individual has few symptoms in the early stages but eventually develops dyspnea on exertion with cough, weight loss, malaise, and fatigue. The chronic form can progress to pulmonary fibrosis indistinguishable from the pulmonary fibrosis of other etiologies.
▪ Latex Allergy
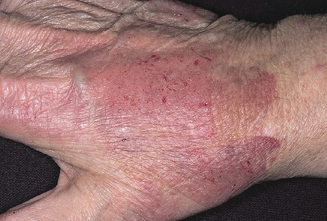
In the assessment of latex allergy a full medical, occupational, and allergy history should be undertaken. The history should include work history, workplace exposures, effect of days off work, atopy or other skin disorders, and hobbies with possible non-work-related exposures. Clinical manifestations of latex allergy include allergic contact dermatitis, irritant contact dermatitis, contact urticaria, rhinoconjunctivitis, asthma, and anaphylaxis. Allergic contact dermatitis is a type 4 hypersensitivity reaction and requires sensitization by repeated exposures producing edema, erythema, pruritus, cracking, and thickening of the skin in exposed areas. Contact dermatitis is due to injury from direct contact with the latex (Figure 12.3). Contact urticaria is a type 1 hypersensitivity reaction causing immediate IgE antibody mediated immune reaction on contact with the latex-protein-releasing mediators that cause a wheal and flare with pruritus. Respiratory, nasal and ocular symptoms can include rhinitis, sneezing, conjunctivitis, and coughing, and are due to an IgE-mediated immediate response. Anaphylaxis is a medical emergency and occurs within seconds of the exposure.
The cause of allergic contact dermatitis cannot be ascertained by the pattern and types of lesions. However a detailed history is paramount to ascertain the cause and select the correct allergens for patch testing. Sometimes incomplete recollection by the worker can hinder obtaining a complete history. Most cases of occupational contact dermatitis occur in the early years of employment and approximately half of the cases occur in workers within 2 years of starting employment.13 In addition to taking a thorough history the physician should carry out a full physical exam placing emphasis on the type of rash, the location and symmetry of the rash, and any other associated symptoms.
Testing
▪ Occupational Asthma
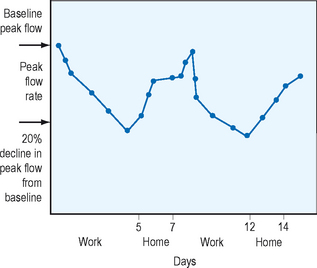
The American Thoracic Society (ATS) defines a 12% improvement in FEV1 after a bronchodilator a significant improvement that indicates airway hyperresponsiveness. Methacholine or histamine challenge tests can detect nonspecific airway hyperresponsiveness in workers who do not show reversibility with bronchodilators but are suspected to have asthma. Methacholine testing is done at the workplace or within 2 hours of the end of the work shift. The negative predictive value of the methacholine challenge test is very high, therefore, a negative test usually excludes occupational asthma. The baseline FEV1 is set using the saline control. The inhaled methacholine concentration is gradually increased, and the test is terminated when the FEV1 falls below 20% or the maximum concentration of methacholine has been reached. The provocative concentration of methacholine that produces a fall in FEV1 by 20% is measured and this is the PC20FEV1. The cutoff value that is diagnostic for asthma varies from center to center, with cutoff values varying as much as 25 mg/ml or less to 8 mg/ml or less. Contraindications to the test include a baseline FEV1 that is less than 70% or the presence of symptomatic asthma. Specific inhalational challenge tests are only performed at certain centers where individuals are challenged with specific agents at levels and conditions that mimic workplace conditions. These tests are potentially dangerous as well as time consuming and should only be performed in patients in which the diagnosis is not clear. If the spirometry shows reversibility or the methacholine challenge test shows hyperresponsiveness then serial peak flow measurements should be performed and a 20% decrease in peak expiratory flow rate (PEFR) in the workplace compared to away from work suggests occupational asthma. The American Thoracic Society recommends peak flow meters capable of providing readings between 100 and 850 L/min for adults. Patients should be instructed in the proper use of the devices. They should stand, inhale fully, put the meter in the mouth and close their lips around it, and then exhale with maximal force. One of the drawbacks of the PEFR is that it is dependent on the effort of the patient and therefore potential falsification of results may occur. Studies using computer chips to store PEFR data have shown that PEFR is inaccurately reported on diaries.18,19 Individuals with suspected occupational asthma are asked to record PEFR four times a day for periods from weeks to months. Figure 12.4 shows the serial peak flow recordings of an individual suffering from occupational asthma. Note the potential variation in patterns with early, delayed, and dual responses.
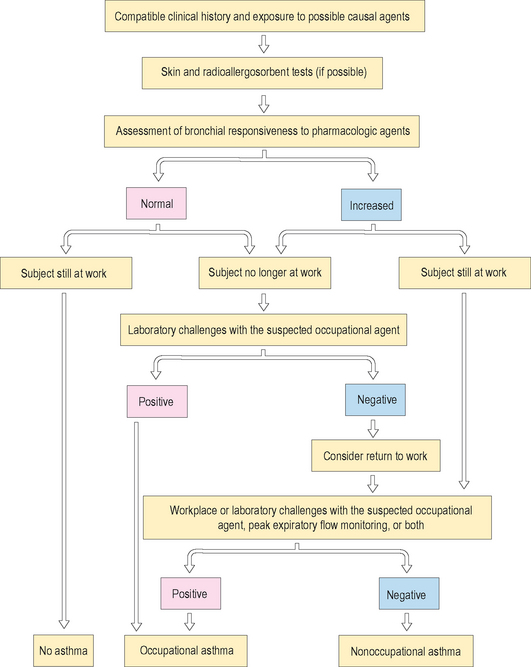
Skin testing with appropriate extracts of the specific agents that cause occupational asthma can help to detect the causal agent. Skin testing is helpful with high molecular weight agents and only a few low molecular weight agents. Atopy is a risk factor mainly for high molecular weight agents. IgE antibodies can also be assayed using the radioallergosorbent test (RAST) or the enzyme-linked immunosorbent assay (ELIZA). It should be noted however that the presence of serum or skin-sensitizing antibody alone does not necessarily mean the symptoms are caused by the antigen. Figure 12.5 shows the clinical algorithm for the clinical investigation of occupational asthma.
▪ Hypersensitivity Pneumonitis
In the acute and subacute forms of hypersensitivity pneumonitis there may be increased neutrophils with lymphopenia. C-reactive protein and the erythrocyte sedimentation rates are usually increased. Serum precipitating IgG antibodies to the specific antigen are positive in individuals with hypersensitivity pneumonitis; however they can also be positive in healthy people who have been exposed in the past. In the acute phase the chest X-ray may show a patchy ground glass appearance while in the subacute form there may be reticulonodular shadowing. In the chronic type there is a reticular pattern, which may progress to honeycombing. High resolution computed tomography (CT) is more sensitive in detecting hypersensitivity pneumonitis than chest X-rays.20 High resolution CT findings include centrilobular micronodules, ground glass opacification, patchy consolidation, and linear densities. Although these suggest hypersensitivity pneumonitis they are not pathognomonic. Pulmonary function tests usually show a restrictive pattern with a reduced FEV1 and FVC; unchanged FEV1/FVC ratio; and a decreased lung diffusion capacity, although some individuals may have an obstructive or mixed pattern. If the offending agent is avoided the abnormalities usually reverse.
Skin tests are not useful in the diagnosis of hypersensitivity pneumonitis. Other diagnostic methods include inhalation challenge tests and lung biopsies. Inhalational challenges can distinguish hypersensitivity pneumonitis from other interstitial lung diseases. However the tests are not standardized and interpretation is often difficult. These tests should only be performed by centers that have experience in performing them. Lung biopsy may help distinguish hypersensitivity pneumonitis from other diseases and open biopsy is preferred because transbronchial biopsy may not produce adequate tissue samples to aid in the diagnosis.
▪ Allergic Contact Dermatitis
The diagnosis of allergic contact dermatitis to antigens other than latex can also be confirmed with a positive patch test. Patch testing is not useful in contact irritant dermatitis but it is the gold standard for diagnosing allergic contact dermatitis. The test carries risk of causing hypersensitivity to the tested substance in the worker. However in the clinic setting only a small percent of workers with contact dermatitis have the patch test done. If there is active dermatitis at the site of testing during an acute attack the patch test should not be done until the acute episode has resolved because of the increased rate of false positives. Another problem with the patch test is that allergens available in the standardized test are those common in domestic and not industrial environments. Industrial chemicals for which there is no standardized test can be patch tested by an experienced allergist or dermatologist. The allergist or dermatologist needs to be well versed in occupational issues as it is essential that the correct concentration of the allergen be used and an adequate number of controls be run. If too little allergen is applied the test will be suboptimal.16
▪ Other Diagnostic Tests
Photoallergic dermatitis can be diagnosed using ultraviolet light and the photo patch. In certain cases specific antibodies to allergens in the blood can be measured using the RAST test. Other skin lesions such as impetigo and secondary infection should be considered in the differential diagnosis of allergic contact dermatitis and should be ruled out by Gram stain and culture of the lesions. If scabies is complicated with impetigo the presentation can be confused with contact dermatitis and scraping for mites is indicated. Other conditions in the differential diagnosis of photoallergic dermatitis include psoriasis, pomphylyx (dishydrosis), lichen simplex chronicus, and atopic dermatitis.21
Treatment
▪ Hypersensitivity Pneumonitis
As with occupational asthma, the mainstay of treatment for hypersensitivity pneumonitis is to avoid exposure to the offending antigen. This can be achieved by removing the individual from the workplace. If this is not possible, using measures such as improved ventilation, engineering controls, administrative controls, or the use of personal protective equipment may lessen the symptoms. Progressive hypersensitivity pneumonitis with severe symptoms is treated with prednisone (1 mg/kg/day) for 1–2 weeks, after which it is tapered over 2–6 weeks until there is clinical improvement. If bronchospasm is present beta agonists are useful. Hypoxemia can be treated with oxygen. Individuals with the acute type usually recover without prednisone. Subacute disease with severe symptoms requires steroids while those with chronic disease may recover just by avoiding the antigen. A trial of prednisone may be useful in those with chronic disease to see whether any reversibility can be obtained. Once the patient with hypersensitivity pneumonitis has reached maximal medical improvement an impairment rating can be done.
▪ Latex Allergy
Latex allergy can be treated by the use of nonlatex gloves or powder-free low protein latex gloves can be used. It should be noted that substituting vinyl gloves for latex gloves involves some sacrifice in barrier protection,8 but nitrile gloves may be a suitable alternative.
▪ Allergic Contact Dermatitis
In the treatment of allergic contact dermatitis the first step is to avoid the exposure that is producing the dermatitis once it has been identified. This is especially important for allergic contact dermatitis because even the smallest exposure to the allergen can produce dermatitis. If removal of the allergen is not possible the worker may have to be moved to a different location or job with no allergen exposure. Acute vesicular eruptions are treated with wet dressings for 24–36 hours using Burrow’s solution, followed by steroid lotion. Colloidal oatmeal baths (Aveeno) may give symptomatic relief of itching. High potency fluorinated topical glucocorticoid creams can be used once the lesions have dried out. Oral antihistamines and topical steroid creams may improve the symptoms and hasten recovery. Secondary infection is not common, but if it occurs oral antibiotics can be used. Topical antibiotics and antihistamines should not be used because of the risk of sensitization.20 If workers have widespread lesions or lesions involving the face or genitalia treatment with oral corticosteroids may be indicated. Treatment with oral corticosteroids needs to be continued for at least 10–14 days, otherwise recurrence of skin lesions can occur. For this reason some of the commercially available prepackaged dose packs should be avoided if they contain inadequate doses or duration of treatment.
In the workplace certain preventive measures can be implemented to prevent contact dermatitis. Pre-employment screening can detect individuals who have conditions that predispose to contact dermatitis, such as hand eczema, atopic dermatitis, and xerosis. These individuals should avoid wet work and exposure to irritants or allergens and should be advised to do dry work. The best method to prevent contact dermatitis is to eliminate or replace the irritating substance or allergen. If this is not possible engineering control such as automation, ventilation, or encapsulating a process can be effective. If engineering controls cannot be used administrative controls such as job rotation can be used. Works can be rotated between wet work and dry work to prevent prolonged exposures to wet work. Personal protective equipment is the last resort. Face shields, aprons, and plastic or synthetic gloves can be worn to prevent contact with irritants and allergens. Barrier creams and after-work creams can also be used; however they have questionable efficacy in protecting against irritants. Some moisturizers when used on normal skin may increase skin susceptibility to irritants. Soap substitutes can provide some protection and after-work creams can also reduce the incidence of contact dermatitis. Workers should be advised to use mild soaps, rinse hands thoroughly to remove the chemicals, and dry the hands well, especially the finger web spaces where moisture can be trapped. Sunscreens can be used in those with photodermatitis.
1 Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma – United States, 1980–1999. MMWR Surv Sum. 2002;51(SS01):1-13.
2 Asthma and Allergy Foundation of America. Available: www.sleep-workplay.com. Accessed 10/08/06.
3 Petsonk EL. Work related asthma and implications for the general public. Environ Health Perspect. 2002;110(suppl 4):569-572.
4 Meredith S. Occupational asthma: measures of frequency from four countries. Thorax. 1996;51:435-440.
5 Kogevinas M, Josef MA, Jordi S, et al. Occupational asthma in Europe and other industrial areas: a population based study. Lancet. 1999;353(9166):1750-1754.
6 NIOSH Occupational Respiratory Disease Surveillance. Available: www.cdc.gov/niosh/topics/surveillance/ords/NationalStatistics/Highlights. Accessed 06/11/2006.
7 Warren CP. Extrinsic allergic alveolitis: a disease commoner in non-smokers. Thorax. 1977;32:567-569.
8 Ahmed DD, Sobczak SC, Yunginger JW. Occupational allergies caused by latex. Immunol Allergy Clin North Am. 2003;23:205-219.
9 Tarlo SM. Occupational asthma caused by latex in a surgical glove manufacturing plant. J Allergy Clin Immunol. 1990;85:626-631.
10 US Bureau of Labor Statistics. Nonfatal occupational illnesses by category of illness, private industry. 1997. US Department of Labor, Washington DC. Available: www.cdc.gov/niosh/pdfs/2002-120.pdf. Accessed 06/13/06.
11 Gawkrodger D. Patch testing in occupational dermatology. Occup Environ Med. 2001;58:823-828.
12 Skin disorders. In: Levy BS, Wegman D, editors. Occupational health – recognizing and preventing work-related disease and injury. 4th edn. Philadelphia: Lippincott Williams and Wilkins; 2000:537-551.
13 English JSC. Current concepts of irritant contact dermatitis. Occup Environ Med. 2004;61:722-726.
14 White I. ABC of work related disorders: occupational dermatitis. Br Med J. 1996;313:487-489.
15 Habif TP. Contact dermatitis and patch testing. In Clinical dermatology, 4th edn., St Louis, MO: Mosby; 2004:81-103.
16 La Dou J. Occupational skin disorders. In Occupational and environmental medicine, 2nd edn., Norwalk, CT: Lange; 1997:272-279.
17 Zetterstrin O. Another smoking hazard: raised serum IgE concentrations and increased risk of occupational allergy. Br Med J (Clin Res Ed). 1981;283(6301):1215-1217.
18 Malo JL, Trudeau C, Ghezzos H, et al. Clinical aspects of allergic disease. J Allergy Clin Immunol. 1995;96:601-607.
19 Quirce S. Peak expiratory flow monitoring is not a reliable method for establishing the diagnosis of occupational asthma. Am J Respir Crit Care Med. 1995;152(3):1100-1102.
20 Lynch DA. Hypersensitivity pneumonitis: sensitivity of high-resolution CT in a population-based study. Am J Roentgenol. 1992;159:469-472.
21 Ferri FF, editor. Ferri’s clinical advisor. 2004. Mosby, Philadelphia. 262.
22 Chang-Yeung M, Lam S. Occupatinal asthma. Am Rev Respir Dis. 1986;133:686-703.
23 Ellenhorn MJ. Respiratory toxicology. In Ellenhorn’s medical toxicology, 2nd edn., St Louis, MO: Williams and Wilkins; 1997:1500-1501.
24 Rabatin JT, Cowl CT. A guide to the diagnosis and treatment of occupational asthma. Mayo Clin Proc. 2001;76:633-640.