Chapter 10 Management of the Patient with Laryngitis
Introduction
Dysphonia is a problem that afflicts millions of people on an annual basis.1 Its impact on quality of life varies from patient to patient depending upon the underlying laryngeal abnormality and whether the voice difficulties are episodic or chronic. The literature base is replete with research on many different benign and malignant causes of dysphonia. However, the possible role of allergy in the pathogenesis and expression of vocal pathologies has not been extensively investigated, despite the fact that allergic diseases of both the upper and lower respiratory subsystems are among the most prevalent illnesses regularly treated by physicians throughout the world.
Inasmuch as the larynx is an integral component of the unified airway,2,3 it is not unreasonable to suggest that it is as susceptible as other juxtaposed respiratory structures to the development of adverse allergy manifestations. The sparse data base on the potential causal relationship between allergy and vocal pathologies provides a rich opportunity for theoretical discussions and seminal research on this subject. Prospective outcomes of these endeavors may prove valuable to medical and health science practitioners from various subspecialties who frequently evaluate and manage patients with allergies.
Unified Airway
Both Hurwitz2 and Grossman3 discussed the frequent coexistence of upstream and downstream respiratory tract inflammatory conditions, including transient and chronic laryngopharyngeal involvement. More recently, deBenedictis and Bush demonstrated that these allergic manifestations represent a continuum of inflammation throughout this integrated tract of respiratory organs.4 They further suggested that arbitrary separation of the airway into upper and lower subdivisions ignores the inherent anatomic and physiological coupling of this single integrated system.
A considerable amount of research has been conducted in the past couple of decades focusing on the possible causal interrelationships between sinusitis, rhinitis, and lower respiratory tract functional abnormalities.5–10 These investigators have reported that as much as 90% of all patients with asthma also suffer from rhinosinusitis; and that approximately 25% of those with allergic rhinitis experience occasional hyperreactive lower respiratory tract symptoms, such as pulmonary congestion, shortness of breath, spasmodic coughing, and throat clearing due to perceptions of excessive endolaryngeal mucous accumulation. These findings have bolstered support for the model of a unified airway; one common system of organ linkages that is subject to widespread, simultaneous, and reactive (i.e., delayed) allergic manifestations. To date, debate continues within the clinical and scientific communities regarding how these allergy-induced interrelationships are driven. Additionally, the specific role of the phonation subsystem in this inflammatory loop remains unclear, particularly with respect to whether it assumes a largely passive or reactive role, or whether its phylogenetic anatomic position enforces a physiologically more active and integrative role during times of allergic and nonallergic common airway inflammation.11
Phonation Subsystem Anatomy and Physiology
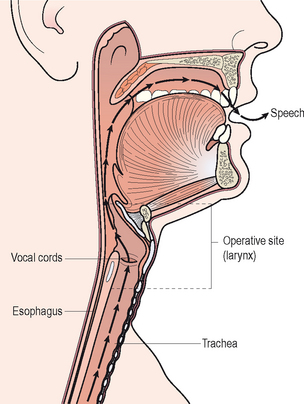
All mammals possess a larynx. Figure 10.1 illustrates that this complex organ is a component of the upper airway and it is suspended in the anterior neck by a sling of extrinsic muscles, ligaments, and specialized joints in the approximate vicinity of the fourth, fifth, and sixth cervical vertebrae. It functions as a biologic valve for: (1) breathing, (2) airway protection during swallowing, (3) coughing to help clear bodily secretions and any foreign particles from the tracheobronchial tree and endolarynx, (4) bowel evacuation, (5) heavy lifting, and (6) childbirth. These functions occur involuntarily or reflexively. The vocal folds within the larynx, in concert with downstream expiratory efforts, can also be voluntarily recruited to vibrate and produce vocalizations known as voice. Over the course of early childhood, humans learn how to coordinate respiratory airflow dynamics, biomechanical vocal fold activities, and upstream articulatory adjustments for the purposes of generating various speech sounds to communicate their thoughts and wishes.
▪ Laryngeal Skeleton
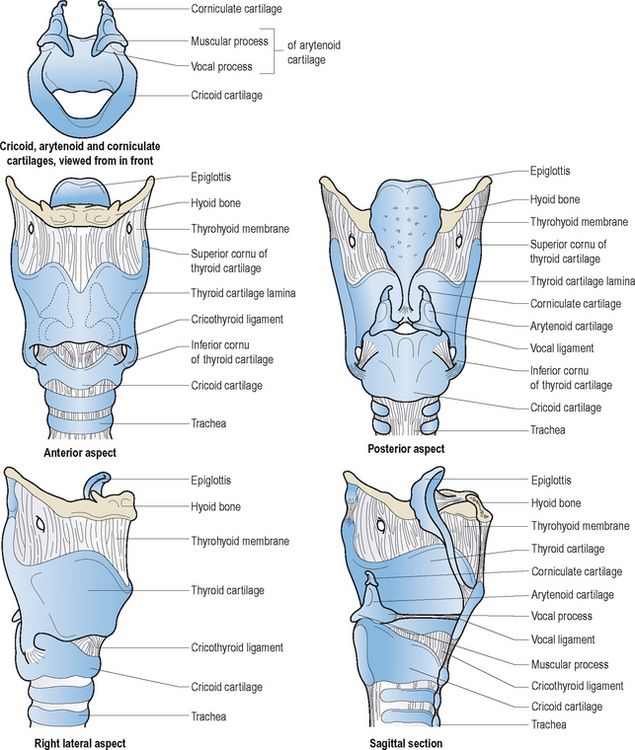
The larynx is composed of three pairs of small cartilages (arytenoid, corniculate, cuneiform) and three large unpaired cartilages (thyroid, cricoid, epiglottis). Figure 10.2 illustrates these structures and their interconnecting membranes and ligaments. The trachea directly links the larynx with the lungs. There are two synovial articulations or laryngeal joints: cricothyroid and cricoarytenoid. Hinge-like action of the former paired joints increases the anteroposterior length of the vocal folds. This adjustment results in increased tension and reduced cross-sectional mass of these structures; most notably influential during pitch variations in speech and song. The primary action of the latter joints is rocking motion of the arytenoids; anteromedial action is of paramount importance for vocal fold adduction, and posterolateral action is essential for vocal fold abduction. There is little evidence in the scientific literature to support classic textbook descriptions of rotary and gliding motions of the arytenoid cartilages during voice production or other valving activities.
▪ Laryngeal Muscles
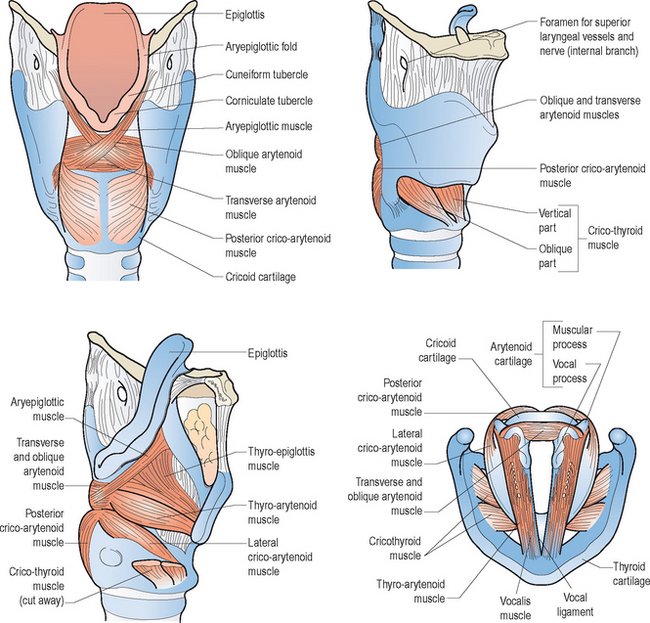
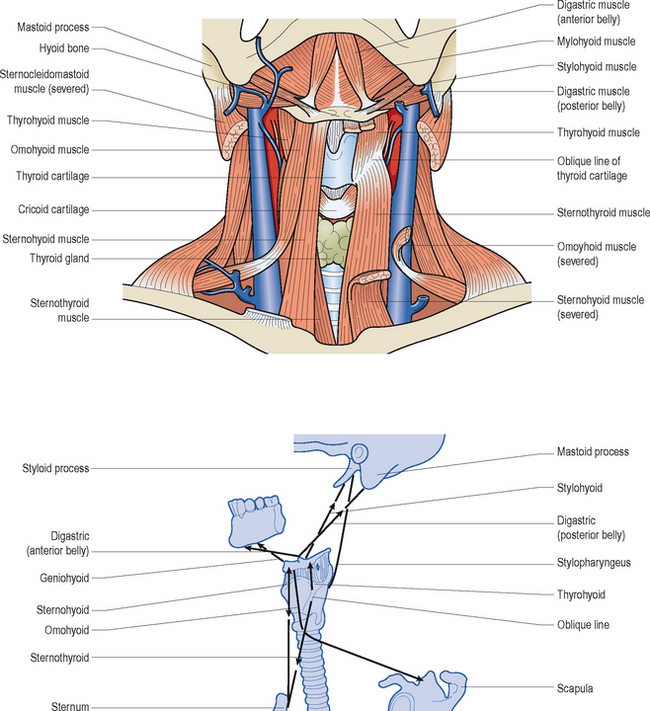
Figures 10.3 and 10.4 illustrate the various intrinsic and extrinsic muscles of the larynx, respectively. Suffice it to say that the true vocal folds arise from or are components of the thyroarytenoid muscle bundles. The intrinsic group of muscles work harmoniously to open, close, tense, and relax the vocal folds during breathing, swallowing, and speaking. Although this entire group probably works in a coordinated and collective manner to achieve these movements; for ease of review, specific functions can be attributed to individual components. That is, contractions of the thyroarytenoid, lateral cricoarytenoid, and interarytenoid muscles generally contribute to vocal fold adduction. As noted earlier, the cricothyroid muscles chiefly lengthen and tense the vocal folds, which decreases their cross-sectional mass. Posterior cricoarytenoid muscle contractions are critical for vocal fold abduction, associated with deep breaths and cessation of vibrations during running speech to accommodate the demands of voiceless consonant production and to terminate voicing at the completion of an utterance.
▪ The Vocal Folds
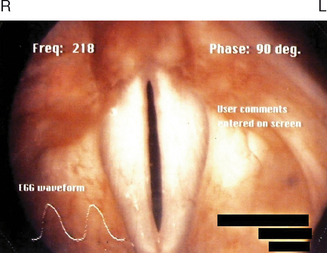
Figure 10.5 demonstrates the normally white and glistening appearances of the true vocal folds within the framework of the thyroid cartilage. The space between the vocal folds is called the glottis, and it varies in its anterior and posterior dimensions during various biological and phonatory behaviors. The vocal folds consist: of (1) an outermost layer of mucosa and stratified, nonkeratinizing squamous epithelium, known as the cover, and (2) deeper layers, which contain the aforementioned thyroarytenoid muscle fibers, as well as high density fibroblasts and elastic and collagenous tissues. Immediately deep to the cover there exists a potential space known as Reinke’s area, which consists mainly of amorphous material with few fibroblasts or elastic tissue. Later in the chapter, we will discuss the biomolecular make-up of the endolarynx and true vocal folds, particularly as it pertains to the presence or absence of mast cells and eosinophils, which normally mediate allergic inflammation in other components of the respiratory subsystem.
Hirano described the cover-body concept of vocal fold vibration.12 His elaborate explanations more than 30 years ago have been instrumental in the development of modern phonosurgical procedures for benign vocal fold pathologies. Because Reinke’s space possesses a gelatinous consistency, it enables fluid vibratory motion of the cover over the vocal fold body (thyroarytenoid muscle fibers) during phonation. Detailed appraisal of such activity can be achieved in the clinical setting using laryngovideostroboscopy. This quasi-slow motion imaging technique reveals traveling waves of mucosa from the inferior to superior surface of the vocal folds. Scarred or fibrotic vocal folds, as a result of invasive benign or malignant pathologies, do not produce normal mucosal waves.
▪ Peripheral Laryngeal Innervation
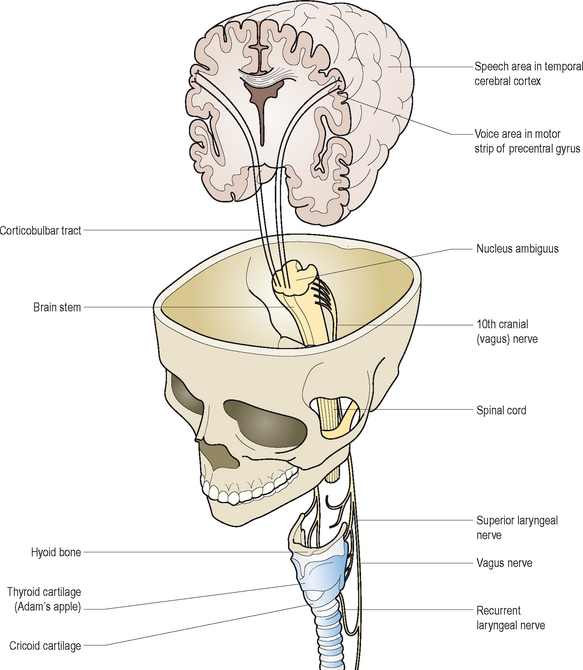
Volitional voice production depends upon a complex loop of neural interactions between the central nervous system, peripheral nervous system, and various respiratory and speech musculature. Kotby et al13 described a six-level hierarchy of laryngeal neuromuscular integration. Of these interrelated segments, the higher levels generally function to activate, inhibit, and modulate output of the lower levels for purposeful voice production. Some of the lower level activities are organized into reflex pathways. Based on a top-down model of control, conceptual programming of speech and voice occurs at the highest level. At the cerebellar level below, movement adjustments are coordinated, and errors are detected and corrected for accurate performance. The pyramidal or upper motor neuron system serves the next level of function as the primary initiator of all muscular contractions through synapses with motor nuclei of all cranial and spinal nerves normally involved in speech production. The extrapyramidal level functions as an ongoing, automatic and subconscious regulator of all sensorimotor outputs and underlying muscle tone, via complex loop circuitry between the central and peripheral nervous system. The vestibular–reticular level helps to activate and regulate motor inputs to and sensory outputs from the cranial and spinal nerves (lower motor neurons) and the muscles responsible for speech and voice production. The lower motor neurons represent the lowest level of this integrated system. They form motor units within the muscle tissues they innervate to stimulate muscle contractions for volitional movement purposes.
The vagus or Xth cranial nerve pair arise from the medulla on the brain stem. They descend into the neck through the jugular foramen, and distribute three primary branches that help control and regulate voice and speech activities: (1) pharyngeal nerve, (2) superior laryngeal nerve, and (3) recurrent laryngeal nerve. The first of these branches provides nerve fibers to the pharynx and most of the soft palate. The second branch contains an internal and external laryngeal nerve component. The former one enters the larynx and divides into two additional branches, both of which contain sensory fibers from the mucous membranes that line the endolarynx above the vocal folds, and from neighboring muscles’ spindles and stretch receptors. The external branch is the chief motor nerve supply of the cricothyroid (pitch changing) muscle and inferior constrictor muscles of the pharynx. The recurrent laryngeal nerve or third primary branch of the vagus nerve, descends past the larynx and then loops back up to provide motor innervation to all of the other intrinsic laryngeal muscles. Whereas the right recurrent nerve loops under the ipsilateral subclavian artery en route to the larynx, the left one descends more inferiorly in the chest, deep to and winding under the arch of the aorta, before it ascends in the tracheoesophageal groove to enter the larynx behind the cricothyroid joint on either side. In addition to its widespread motor inputs, it supplies sensory filaments to the mucous membranes within the lining of the subglottis, immediately below the vocal folds. These fibers transmit afferent output from these tissues as well as stretch receptors in the surrounding musculature.
Mechanoreceptors mediated by the recurrent and superior laryngeal nerves are abundantly located within the mucosal lining, muscles, and joints of the larynx. These sensory elements influence respiratory and vegetative reflexes, and they contribute to what may be termed the intrinsic laryngeal monitoring system, as they relay oscillating discharges to the lower brain stem in response to air pressure fluctuations that occur during voice production. Polysynaptic loops are then formed with the motor neuron pools of the vagus nerve at this level to establish the so-called tonic servo-reflex system of the larynx.14 Figure 10.6 offers a schematic representation of these hierarchical neurologic pathways associated with voice production. For more detailed reviews of the neurologic substrates of phonation, the reader is referred to other sources.15,16
▪ Voice Production
As shown in Figure 10.1, vocal fold vibrations during speech efforts send a traveling wave of acoustic energy upstream through the vocal tract. This pathway, which includes the oral and nasal cavities, acts as a resonating chamber to enhance, absorb, and reflect the sounds generated into distinctive voice qualities. The infraglottal tract consists of the respiratory musculature, lungs, trachea, and immediate subglottis. These structures function as a collective power source for phonation by providing ongoing airflow dynamics required to drive vocal fold vibrations during speech efforts. The vibratory activity itself is largely a passive act. That is to say, at the start of a vibratory cycle the vocal folds are volitionally preset in an adducted position at the midline of the glottis. Assuming the lungs have been supplied with a sufficient amount of inspired air in preparation for speech, pressure increases within the trachea with expiratory effort to generate upstream airflow to induce vocal fold vibrations and voice. When subglottic pressure exceeds the level of resistance created by the adducted vocal folds, a puff of air is emitted into the vocal tract. This momentary break in the glottal seal initiates the vacuumous Bernoulli effect. This aerodynamic phenomenon results from increases in the velocity of air molecules passing through the narrow glottic inlet. As this occurs, air pressure between the vocal folds decreases, which, in turn, induces glottic closure to complete the vibratory cycle (closed–open–closed). This wave is assisted by intrinsic laryngeal myoelastic properties. Sustained phonation depends upon adequate intrinsic laryngeal muscle and elastic glottal closing forces, and sufficient and continuous infraglottal airflow support to initiate and drive vocal fold vibrations.
▪ Parameters of Voice
Quality, loudness, and pitch are the primary parameters of human voice. Quality represents the overall timbre or pleasantness of voice. The rhythm and symmetry of vocal fold vibrations, and the adequacy of glottal closure, significantly influences vocal quality. Irregular motion and glottal incompetence during the closed phases of vibration usually result in escapes of unphonated air, which distorts the voice signal. Hoarse, harsh, raspy, breathy, wet-gurgly, spasmodic, and tremorous, are common terms used to classify vocal quality disorders. The speech diagnostic terms hyponasality and hypernasality are sometimes used within this context of vocal quality disturbances. However, these disorders result from upstream velopharyngeal and nasal cavity disturbances.
Evaluating the Allergic Patient with Voice Complaints
▪ Team Approach
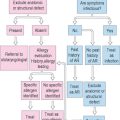
A team approach to the evaluation and treatment of patients with suspected laryngeal allergic sequelae is usually most successful. Members in this effort should minimally include an allergy physician with a background in otolaryngology or immunology, a speech-language pathologist with expertise in the area of vocal pathologies, and a nurse practitioner. The diagnosis of chronic allergic laryngitis should be considered for patients who present with histories of upper respiratory allergies and co-occurring phonation subsystem disturbances, such as globus sensations, excessive laryngeal mucus and reactive throat clearing and coughing behaviors, dry-itchy throat, and voice difficulties. With this clinical population, the history component of the examination usually produces the most indispensable diagnostic data. In this vein, the exploration of antecedent events or triggers of allergy symptoms almost always proves to be of paramount importance to accurate diagnoses, as does the time course or seasonality of such complaints. Whether the patient suffers from any co-occurring diseases, such as asthma, otitis media, sinusitis, and chronic acid reflux, should be evaluated because these conditions can exacerbate the underlying allergy. Prior or current use of medical or complementary therapies to treat these problems should be factored into the differential diagnostic and subsequent management equations.
In the following segment of the chapter commonly employed qualitative and quantitative phonation subsystem evaluation techniques are described in detail. Most of the procedures discussed are not routinely performed by physicians, unless they have undergone training in the area of otolaryngology, or they have worked closely with medical colleagues or speech-language pathologists with such expertise. Notwithstanding this limitation, it is not unfeasible to suggest that virtually all of the techniques below can be easily learned by the inquiring and determined physician, regardless of his or her medical subspecialty background. Those clinicians who prefer to send the dysphonic patient to a laryngologist should, at the very least, achieve a working knowledge of the rationale for and diagnostic benefits of all of these testing procedures. Acquiring this understanding will foster communication with the practitioners to whom the patient is referred, and such information will facilitate comprehensive diagnostic and treatment discussions.
▪ History of the Dysphonia and Initial Examiner Impressions
The origin and course of abnormal voice signs and symptoms are of vital importance to differential diagnosis and management. Initially, the examiner should render a perceptual impression of the overall severity of dysphonia that the patient exhibits during the history-taking process. The types of underlying disturbances in vocal quality, pitch, and loudness should be noted, and each perceived difficulty should be behaviorally defined and rated with respect to degree of impairment. Box 10.1 provides a rating form that may prove helpful for such purposes.
The examiner should employ a logical sequence of questions to evaluate the background of the problem. First, questions regarding how long the dysphonia has existed, and whether it varies in degree from day to day, should be asked. If there is a previous history of dysphonia, ascertain what types of treatments may have been rendered in the past to improve the problem. Second, the examiner should note whether the dysphonia characteristics vary during the interview. Third, whether the patient has determined the possible cause, or can link the voice difficulties to specific times or events, are important factors that must be explored. For example, has there been a recent exacerbation of allergic symptomatology that might account for the voice difficulty, owing to significantly associated coughing or throat clearing behaviors, which may have resulted in vocal fold trauma. Fourth, the patient should be asked whether the difficulty has improved at all since the onset. If so, it would be important to inquire as to what such improvement might be attributed. Fifth, if the patient reports that there are times in the day when the voice is better or worse, these fluctuating abilities should be discussed to try to determine possible causal conditions. Sixth, the astute examiner should always ask whether there have been days since the onset of dysphonia when the voice was completely normal for long periods of time. Seventh, it is essential to rule out significant comorbid medical problems for which the allergic patient may have been treated, and to which the dysphonia may be fully or partially attributed. For example, recent intubation anesthesia, laryngeal trauma, thyroid or neurologic disease, illness, or injury, and severe laryngopharyngeal reflux should be considered as possible causes, either acting alone or in combination with the allergy history. Eighth, determine if the patient abuses the vocal folds; excessive throat clearing, coughing, yelling, smoking, limited water intake and substantial consumption of diuretic beverages, regular use of inhaled corticosteroids, and routine use of decongestant medications are prime examples of behaviors that can provoke vocal fold swellings and generalized signs and symptoms of laryngitis. Ninth, inquire as to whether the patient is suffering from any type of swallowing difficulty. A significant degree of laryngitis can cause odynophagia and glottal incompetence, which can result in aspiration symptoms. Aspiration usually elicits coughing reactions. Coughing can exacerbate existing laryngeal swellings, which increase the swallowing difficulties. This vicious cycle is not uncommon, and it needs to be broken to restore nutritional balance and relieve the patient of potentially deleterious pulmonary side effects. Tenth, if the patient sounds stridorous at rest, during exertion, or both, auscultation of the upper airway with a stethoscope on the larynx to confirm the possible presence of laryngeal airflow difficulty can be diagnostically valuable. Stridor is usually associated with anatomic or physiological glottal obstruction, as may occur with severe vocal fold swellings, large ball-valving glottic or subglottic lesions or stenosis, or bilateral abductor vocal fold paralysis. Stridor may act alone to cause dyspnea, or it may occur in combination with downstream (e.g., asthma; COPD) or upstream (e.g., allergic rhinitis; rhinosinusitis) airway diseases. Table 10.1 provides a synopsis of these steps for easy reference.
TABLE 10.1 Exploring the history of dysphonia
| Examination steps | Objectives |
|---|---|
The initial impressions rendered by the examiner, and answers to the various questions posed to the patient, provide indispensable data ultimately required to formulate a differential diagnosis of the dysphonia and a possible treatment plan for this specific problem. Acute onset dysphonia can usually be tracked to a specific recent event, injury, or illness. Profound yelling at a ballgame, prolonged intubation during a surgical procedure, direct laryngeal trauma in an accident, laryngeal anaphylaxis or non-IgE-mediated allergic laryngitis secondary to substantial antigen exposure, neck or thoracic surgery that normally places the recurrent laryngeal nerve at risk for either stretch neurapraxia or resection injury, repetitive intubation-extubation abrasion of the vocal folds, stroke, Guillain–Barré syndrome, and closed head injury are some of the most common potential etiologies of sudden voice difficulties. Not infrequently, if the resultant laryngeal abnormality causes significant glottal incompetency, the patient may also suffer from aspiration symptoms and a more complicated clinical course. The prognosis for spontaneous recovery of normal voice within a relatively short period of time without phonation subsystem medical intervention largely depends upon the underlying etiology of the acute dysphonia.
▪ Voice Sampling
Contextual speech and voice characteristics will be automatically obtained during the history-taking process. In addition to the collection of these important data, the examiner should request the patient to perform specific tasks. First, instruct the patient to take a deep breath and then prolong the vowel /a/ for as long and steady as possible. This maximum phonation time (MPT) task is usually measured in seconds, and the patient’s mean performance over two trials should be calculated and encircled on the aforementioned rating form (Box 10.1). Normally, adults should be able to generate at least 14 seconds of MPT; children can usually normally sustain voice for at least 10 seconds. Abnormal performance is often attributable to glottal incompetence and consequential air wastage during the phonatory effort. Downstream pulmonary system limitations may also cause reduced MPT, owing to insufficient vital capacity or forced expiratory volume, as may occur in the asthmatic patient. Second, ask the patient to sing up and down a musical scale at his or her most comfortable pitch level. Assess the number of notes that can be sung, as well as the associated voice features throughout the task. Patients with dysphonia often exhibit difficulty raising or lowering pitch from their habitual level, because this activity requires finite vocal fold stretching and relaxing adjustments, respectively. Swellings or lesions involving these structures typically restrict their flexibility and cause pitch production limitations, along with disturbed quality and volume parameters. Third, ask the patient to cough sharply to assess the force of glottal closure during this abrupt vocal fold behavior. Significantly weak or breathy coughing often signifies glottal incompetence, and usually strongly correlates with the dysphonia characteristics. Patients who cannot generate volitionally adequate coughing activity, regardless of the cause, may be at risk for aspiration and pulmonary infection because physiological integrity of the glottal valve is of paramount biologic importance during swallowing and tracheobronchial mucus clearing.
▪ Voice Symptom Questionnaire
Box 10.2 is a questionnaire that can be used to determine the extent to which a patient exhibits and experiences specific signs and symptoms that collectively may be manifestations of allergy and causally related to vocal fold abuses and voice difficulties.
▪ Examination of the Larynx
Flexible or Rigid Fiberoptic Endoscope (Stroboscopy)
If examination of the larynx is the primary objective, use of a rigid endoscope is recommended. This instrument usually affords more enhanced pixel resolution and optical amplification for visual appraisal and video recording purposes than its flexible endoscope counterpart. However, if the exam focus is on the entire vocal tract, and possibly the immediate subglottis, then use of a flexible scope will be necessary. Images obtained with this type of instrument are narrow field and usually are not visually robust; use of a three-chip camera and video monitor interface can improve these inherent limitations. The well-equipped clinical facility may include an examination cart containing a laryngovideostroboscopy system, which affords quasi-slow motion images of vocal fold vibrations, mucosal wave dynamics, and overall laryngeal anatomy. This unit usually includes a rigid laryngoscope, strobe light source, and a single or multiple chip camera. Some advanced systems include powerful digital computer connections that materially enhance data analyses, storage, and retrieval. All of these endoscopic approaches to the laryngeal examination are expensive to conduct. Even without the video (stroboscopy) interface, the flexible or rigid scopes and light source can cost as much as US$5000.00 If a camera, video monitor, video recorder, and color printer are added for more comprehensive evaluation options, the price tag can easily exceed US$10 000.00 The low-end videostroboscopy systems may cost more than US$25 000.00 The advanced systems are priced at US$60 000.00 and up. Such costs often prove prohibitive for many practitioners, particularly those whose practices do not include patients with nonallergy-related vocal pathologies. The value of such technology in the differential diagnosis of voice disorders has been discussed by several clinical researchers.17,18
▪ Acoustic Analysis
Whereas perceptual impressions of dysphonia are vital to the differential diagnosis and treatment plan, computerized measures of the voice abnormality provide the means to quantify the levels of impairment of each voice parameter, using normal voice referents for baseline comparisons. Coupled to perceptual speech ratings, quantitative voice analyses, obtained prior to and following specific treatments, can provide objective evidence of notable improvement. Certainly, from an academic point of view, such data are considered indispensable to discussions of the suggested benefits of alternative treatments for different types of vocal pathologies.
▪ Speech Aerodynamic Testing
Commercially available systems enable comprehensive evaluations of subglottal pressure, glottal resistance, and transglottal airflow rate during speech activities, using indirect or noninvasive instrumentation methodologies. Most systems include an anesthesia-like face mask that is coupled to differential airflow and intraoral air pressure transducers. This hardware unit is interfaced with a computer platform and specialized software so that detailed quantitative analyses of the aerodynamic properties underlying voice and speech production can be achieved. Speech scientists and voice therapists might argue that the study of phonation would be incomplete without in-depth examination of this energy source or power supply for vocal fold vibrations. The minimal level of subglottal pressure required to drive voice is 5 cmH2O/5 s. In the speaker with normal voice, transglottal airflow rate averages 100 ml/s through the length of an utterance, and the compression force between the vocal folds at the onset of and during voice production (i.e., glottal resistance) averages between 35 and 50 cmH2O/lps. Patients with vocal fold pathologies that cause glottal incompetence and air wastage during phonation often exhibit higher than normal subglottal pressure and airflow rate values, owing to increased respiratory efforts to compensate for such difficulties. Limited MPT levels are also common in these individuals. Patients with obstructive glottal or subglottal lesions, and those who suffer from downstream pulmonary subsystem disease (e.g., asthma, COPD, neuromuscular illness/injury, lung cancer), struggle with abnormally low subglottal pressure levels, reduced transglottal airflow rates, and high levels of glottal resistance; those with adductor spasmodic dysphonia or severely strained-tense voice production habits exhibit similar speech aerodynamic disturbances. The cost factor associated with this technology, and the time involved in collecting these types of data, prove prohibitive and unjustifiable for most clinical practitioners. The most prevalent application of such instrumentation occurs in speech physiology laboratories in academic and specialized clinical settings for the purposes of advancing the knowledge base on this subject via scientific research investigations.
▪ Fiberoptic Endoscopic Examination of Swallowing (FEES)
Patients who present with allergy-related dysphonia may also complain of occasional, but usually mild odynophagia, globus sensations, and general swallowing difficulties. The possible role of gastroesophageal and laryngopharyngeal reflux disease must be considered in the differential diagnosis of such patients, as these conditions have been linked to the pathogenesis of certain types of dysphonia.19 Additionally, laryngopharyngeal edema secondary to oral allergy syndrome may be of etiologic significance.20–22 This condition occurs frequently in patients with food sensitivities,23–25 which can, albeit rarely, cause anaphylactic shock because the upper aerodigestive tract contains a widespread population of mast cells. These mediators of inflammation can contribute to cross-reactivity between certain pollen inhalant antigens to which a patient may be allergic, and specific fruits or vegetables with structurally similar antibodies. When the patient ingests such foods an allergic reaction is elicited that may resemble the signs and symptoms that occur secondary to pollen exposure. Even if the response is mild in degree, threshold swelling and associated pharyngeal and laryngeal hypersensitivity may cause the aforementioned dysphagia signs and symptoms.
Comprehensive history taking and physical examination are essential procedures in the diagnosis of oral allergy syndrome. In particular, clinical investigation of dysphagia can be facilitated using a simple in-office technique, such as FEES. A flexible endoscope with camera attachment, compatible light source, video monitor, and video recorder are required equipment for this procedure. Once the tip of the scope is positioned for simultaneous viewing of the valleculae, posterior pharyngeal wall, perilaryngeal folds and boundaries, endolarynx, and piriform sinuses the patient can be administered various foods (with green food coloring for easy identification) for swallowing analyses. Whereas this technique does not enable evaluation of the oral phase of swallowing or possible distal spread of aspirated material, it provides excellent images of: (1) abnormal levels of standing secretions in the valleculae, endolarynx, and/or piriform sinuses, (2) abnormal degrees of pharyngeal edema/erythema, laryngeal edema/erythema, or both, (3) premature bolus spillage into and abnormal retention within the valleculae, (4) bolus penetration into the endolaryngeal cavity, and frank and delayed violation of the glottis, (5) reflexive coughing (or lack thereof) in response to observed penetration and suspected aspiration, (6) abnormal retention of bolus in the postcricoid area or piriform sinuses with possible delayed spillage anteriorly into the endolarynx (aspiration), and (7) esophageal reflux. Clinically significant findings, which strongly correlate with the patient’s chief swallowing complaints, often help to explain the difficulties and may provide clues for effective management strategies. Additionally, if aspiration is suspected with FEES, referral to radiology for a modified barium swallow (i.e., cookie swallow) study (+/− esophagram) is indicated to verify the problem and help regulate oral feeding decisions. Table 10.2 offers a synopsis of the various clinical examination techniques discussed in this section.
TABLE 10.2 Sequential evaluation of dysphonia with suspected allergy
| Clinical evaluation technique | Purpose |
|---|---|
 flow (transglottal airflow), Ps (subglottal pressure), and Rg (glottal resistance)
flow (transglottal airflow), Ps (subglottal pressure), and Rg (glottal resistance)Benign Laryngeal Pathologies
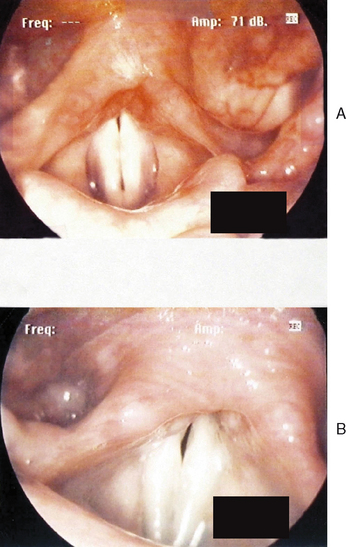
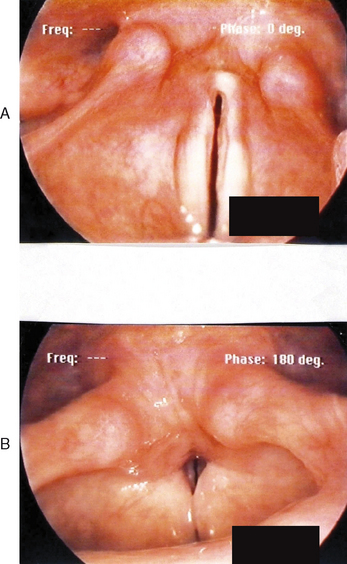
The most common cause of dysphonia is self-induced, hyperfunctional vocal fold misuse or abuse. Common types of misuse include: (1) yelling or screaming, (2) unusually excessive speaking, especially at loud levels, (3) frequent singing, (4) chronic throat clearing, coughing, or both, and (5) tense, strained, and hard attack vocalizations. Salespeople, coaches, preachers, teachers, trial lawyers, professional speakers and singers, choir participants, factory workers, emotionally unstable individuals, and those with chronic allergies and associated postnasal drainage and sticky-thick endolaryngeal mucus accumulation are all prone to habitual expression of one or more of these maladaptive behaviors. The end result of such aggressive vocalizations and associated strong collision forces of the vocal folds is diffuse inflammation of these structures. This condition may be transient or persistent, its onset may be acute or gradual, and the severity of the resultant dysphonia may vary from mild to severe both within and between patients. The most common laryngeal pathologies secondary to misuse factors include bilateral vocal fold polyposis or Reinke’s edema, discrete vocal fold polyps (hemorrhagic), edematous or fibrotic vocal fold nodules, submucosal vocal fold cysts, and contact ulcers on the medial aspects of the vocal processes. Some patients also exhibit signs of ventricular phonation, often caused by subconscious recruitment of the false vocal folds to compensate for the ill-effects of true vocal fold lesions. This behavior, known as plica ventricularis, is usually counterproductive because the voice produced is rather cacophonous. In general, misuse vocal pathologies result in variable degrees of hoarse, breathy, raspy, harsh, strained, low pitch, monopitch, soft volume, and monoloud dysphonia characteristics.
Common types of laryngeal abuses include: (1) smoking, (2) regular use of inhaled corticosteroids for asthma, (3) frequent use of antihistamine (decongestant) medications for allergies, (4) excess caffeine consumption, and (5) limited H2O intake. Various systemic, neurologic, and traumatic conditions may also inadvertently result in laryngeal abuse. These include: (1) laryngopharyngeal reflux, (2) asthma, (3) allergies, (4) severe upper respiratory infection, (5) thyroid disease, (6) presbylaryngis, (7) sarcoidosis, (8) lupus, (9) laryngeal trauma, (10) laryngeal joint arthritis, and (11) various neurologic diseases or injuries, including focal laryngeal dystonia (spasmodic dysphonia), essential tremor, stroke, cerebral palsy, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and recurrent laryngeal nerve paralysis.
The latter condition has received considerable attention in the last two decades.26–29 It is generally characterized by interruptive, paradoxical, paroxysmal, or episodic vocal fold movement behaviors at rest and during phonatory efforts. Many patients with these signs and symptoms suffer from psychogenic or emotional disorders, including Münchausen’s syndrome and conversion reactions. Others exhibit these adventitious laryngeal musculature contractions, in the absence of growths or lesions, as a result of severe upper respiratory viral infections, asthma, reflux disease, allergies, and certain neurologic pathophysiological mechanisms.30 Dependent use of systemic and inhaled corticosteroids and bronchodilators for asthma has been linked to episodic paroxysmal laryngospasms and transient dysphonia. In some patients, a large majority of whom also use medications to treat co-occurring allergies, mild vocal fold edema and erythema occur as a result of mucosal inflammatory reactions to the steroid. In others, moderate degrees of vocal fold mucosa thickening and bowing are observed. Fortunately, few patients exhibit dramatic laryngeal pathologies, such as vocal fold leukoplakia, granuloma formations, and endolaryngeal candidiasis.31 Because laryngopharyngeal reflux has also been causally linked to many of these pathologies, differentiating the pathogenesis of these signs in patients suffering from asthma and reflux disease can be very difficult, especially if the examiner is unfamiliar with steroid inhaler laryngitis. The patient who also struggles with co-occurring allergies presents an even more challenging clinical portrait. The extent to which each condition and treatment modality contributes, either alone or synergistically, to these irritable larynx phenomena remains unclear to date. In general, abuse vocal pathologies, regardless of etiology, result in variable degrees of hoarse, breathy, strained-strangled, spasmodic, tremorous, pitch and loudness outburst, and reduced range of pitch and loudness voice disturbances.
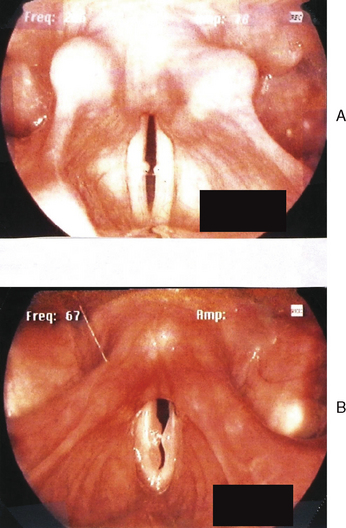
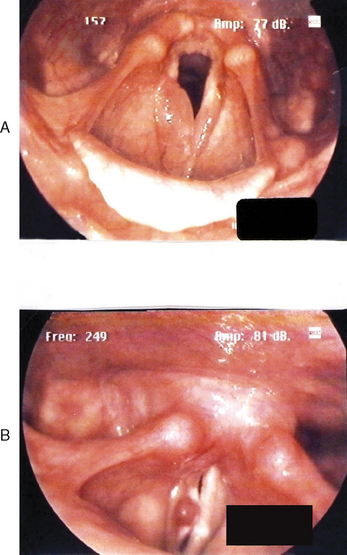
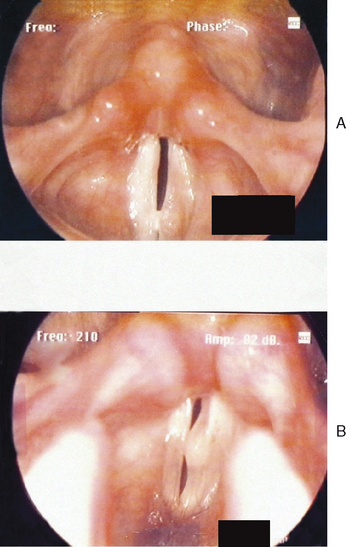
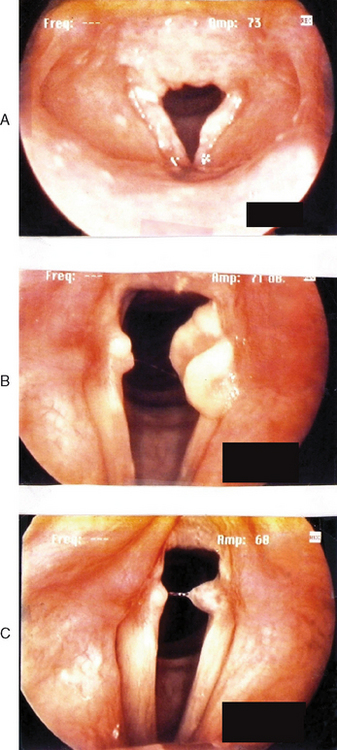
Figures 10.7–10.12 were obtained with a high resolution camera and videolaryngostroboscopy system. They represent examples of some of the benign vocal pathologies mentioned above. Type specific allergy-related laryngeal abnormalities will be illustrated in the next section of the chapter. Because of space limitations, malignant laryngeal neoplasms will not be presented here. Table 10.3 summarizes the most common benign causes of dysphonia and their consequential abnormal anatomic and pathophysiological laryngeal effects.
| Common causes | Resultant pathology | Dysphonia features |
|---|---|---|
| Misuse | ||
CA = cricoarytenoid; CT = cricothyroid; VF = vocal fold; LPR = laryngopharyngeal reflux; URI = upper respiratory infection.
Allergic Laryngitis: Scientific Evidence Versus Clinical Observations
▪ Scientific Background
There has been a great deal of debate over the past forty years regarding the effects of allergy on the larynx. Ostensibly, such discussions center on whether laryngeal disturbances noted in some allergic individuals represent: (1) local end-organ responses, or (2) manifestations of systemic and multiple end-organ sequelae.2,32,33 The scientific literature on this subject is not replete with extensive and well-controlled investigations. The empirical data base to date has not demonstrated an unequivocal cause effect relationship between antigen exposure and pathophysiological laryngeal reactions in patients with either seasonal or perennial allergies. However, several clinical researchers have suggested a high correlation between allergy and laryngeal symptoms, such as chronic throat irritation and soreness and laryngitis.
The immunological mechanisms responsible for upper airway inflammation in allergic patients have been well documented. However, those cellular processes that may also induce chronic laryngeal inflammation, edema, and associated dysphonia in these same individuals after antigen provocation are poorly understood. Despite clinical observations and anecdotes by many physicians and speech-language pathologists suggesting a possible causal relationship between allergic disease and various types of benign vocal pathologies, there have been few human investigations in this area, and animal models of chronic laryngeal inflammation have produced limited data.
Chadwick suggested that both upper and lower airway allergic inflammation can induce varying degrees of primary and secondary biomolecular and biomechanical laryngeal disturbances.34 Corey et al described two primary forms of allergic laryngitis: (1) acute, IgE-mediated, inflammation (anaphylactic), and (2) chronic, cyclic (delayed), non-IgE-mediated inflammation.35 In the former category, there is rapidly developing and generally severe edema of the loose areolar tissue matrix of the laryngeal vestibule or inlet. In addition to airway compromise, inspiratory stridor, globus sensations, lingual and uvula swellings, nasal congestion, hoarseness, and dysphagia may occur concurrently and exacerbate breathing difficulty symptoms. Certain venoms, food items such as peanuts and shellfish, drugs, and insect bites have been causally linked to acute allergic sequelae. Fortunately, these pronounced respiratory and phonatory subsystem reactions do not commonly occur in the general population of allergic individuals.
Chronic or delayed symptoms and signs of possible allergic laryngeal reactions have been reported to include odynophagia, episodic straining to produce voice, transient throat clearing, coughing, hoarseness, and vocal fold edema.36–38 It is important to note that these symptoms are also commonly seen in laryngopharyngeal reflux, and current findings linking eosinophils to gastroesophageal reflux may suggest commonality in mechanisms between reflux and allergic rhinitis.37,39 Whether these two conditions share a common, chronic inflammatory relationship has yet to be determined.
At a cellular level the primary two leukocyte populations involved in allergic inflammation are mast cells and the eosinophil.40 Mast cells are basophilic and contain coarse cytoplasmic granules. The primary substance contained in these granules is histamine, a potent proinflammatory vasoactive amine. In addition to histamine, mast cell granules contain the anticoagulant heparin. These large cells are located throughout the sinonasal tract mucosa, within most loose connective tissue of the body, and along the path of blood vessels. Mast cells act as primary mediators of the acute-phase allergic response, and may be activated by inhaled, ingested, and topical allergens. On exposure to a previously sensitized antigen, mast cells degranulate, releasing their contents into the tissues locally. In addition to the effects of histamine, inhaled irritants often cause swelling of the nasal and bronchial mucosa secondary to release of reactive neuropeptides such as substance P.39 Acute allergic reactions are a function of the inflammatory changes that occur in response to these mediators.
Light and electron microscopic studies of the human larynx have revealed an abundance of mast cells and substance P within the epiglottis and immediate subglottis; neither the squamous epithelium nor the associated neurons of the vocal folds contain such cells or neuropeptide properties.39,40 Two different mast cell phenotypes have been recently demonstrated in human laryngeal mucosa: (1) mast cells containing tryptase alone, and (2) mast cells containing tryptase and chymase.41 Similar phenotypes have been documented to exist in abundance in the nasal and bronchial mucosa.42–44 From a pathophysiological point of view, these mast cell populations are most evident in anatomic regions of the larynx where edematous conditions most commonly occur. Consistent with the lack of mast cells at the level of the glottis, no known primary allergenic reactions or eosinophilic infiltrates have been documented within the vocal folds that would help explain transient swellings of these structures in some individuals with allergic rhinitis. Several investigators have hypothesized the presence of alternative causative mechanisms in this clinical population, such as chronic throat clearing or coughing to evacuate perceived postnasal drainage or viscous mucus accumulation within the hypopharynx or larynx.11,33,36–38 If and when these secondary behaviors occur, they may result in vocal fold trauma, edema, excessive intrinsic laryngeal muscle tension levels, and variable degrees of intermittent or persistent dysphonia. Notwithstanding these theoretical considerations, the true incidence of so-called allergic laryngitis is unknown, and the actual pathogenesis of abnormal laryngeal signs and symptoms in individuals with known allergies remains elusive to date. However, several recent investigations have reinforced earlier research findings and have begun to shed additional revealing light on this controversial subject.
Up until about 10 years ago, the scientific literature data base on allergy-related dysphonia was rather sparse, speculative, and inconclusive. With the recent advent of high technology laryngeal and respiratory subsystem examination equipment, the possible causal relationship between allergy and voice difficulties has been more comprehensively studied. Earlier investigators suggested that inhalant and food allergens occasionally provoke pale edema of the vocal folds and associated chronic laryngitis and dysphonia, which subside with removal of the inciting agents along with topical or oral corticosteroids and immunotherapy.45–49 Some of these researchers described the differential vocal fold anatomy secondary to acute (anaphylactic) reactions versus chronic allergic laryngeal symptoms. In acute cases, diffuse edema and erythema of the entire larynx was observed, involving the epiglottis, true and ventricular vocal folds, and the immediate subglottis. In chronic cases, inflammation was confined to the true folds without significant erythema. None of these authors included both subjective and objective laryngeal imaging, perceptual, acoustic, and speech aerodynamic measurements to substantiate their suggestions that patients exhibit a parallel correlation between allergy symptom exacerbation with antigen exposure and increasing voice difficulties.
Corey et al acknowledged the paucity of epidemiologic data on the existence and prevalence of allergic diseases that affect the larynx, other than evidence on laryngeal anaphylaxis and edema secondary to IgE-mediated antigen exposure.33 These authors reviewed over 200 videostroboscopic examinations of the larynx obtained from patients with voice complaints. Symptoms described by those individuals with histories of nasal allergies in addition to dysphonia included limited pitch range, frequent throat clearing, postnasal drip, chronic cough, and globus sensations. Mild vocal fold edema, sticky-thick endolaryngeal secretion accumulation, mildly erythematous arytenoids, and hyperactive laryngeal reflexes were among the most notable observations. Although many of these pathophysiological conditions have also been causally linked to laryngopharyngeal reflux,1,40 thick, viscid mucous strands within the larynx that bridge the vocal folds have not been shown to be common side effects of acid reflux disease in the absence of allergies.
Naito et al studied 30 individuals diagnosed with chronic laryngeal allergy on the basis of skin testing for inhalant allergens and laryngeal examinations.50 The primary complaints of these patients were persistent globus sensations and nonproductive coughing spells. Laryngeal examinations demonstrated abnormally pale, glistening, and edematous arytenoid mucosa. Approximately 90% of these individuals experienced significant symptomatic improvement with oral H1 antihistamines. The authors suggested several criteria for the diagnosis of laryngeal allergy, including: (1) history of allergic disease, supported by positive skin or in vitro allergy testing, (2) foreign body sensation, itching of the larynx, and/or persistent dry, nonproductive cough, (3) glistening, pale edema, primarily involving the arytenoid mucosa, and (4) normal findings on chest and sinus X-rays and pulmonary function testing.
Within the past decade several researchers have combined the technology of videostroboscopy, speech aerodynamic, and acoustic analysis for quantitative evaluations of the phonation subsystem in patients with perennial and food allergies.51,52 These authors identified the coexistence of irregular glottic edema, excessive and sticky endolaryngeal mucous secretions, and dysphonia in allergic subjects. Whether the sticky-thick mucous accumulation clinging to the vocal folds originated from the membranes of the nasal cavity, larynx, tracheobronchial tree, or combinations thereof, was not clarified by these authors. Reflux disease and associated vocal abuses were also examined. Whereas many of the allergic subjects showed laryngeal signs of laryngopharyngeal reflux, a very small subset actually reported underlying symptoms of reflux. A complex interrelationship between allergy, reflux disease, vocal abuse, and dysphonia was suggested by these authors. Additionally, pulmonary function studies were performed on a small subset of allergic patients, and approximately 25% of these individuals exhibited abnormal spirometry findings, which were considered possible manifestations of previously diagnosed asthma or mild lower respiratory system allergy-related hyperreactivity.
Lack53 and Cohn et al54 reviewed the importance of considering allergic factors in the etiology equation of all chronic inflammatory processes of the unified airway. These researchers suggested that allergic rhinitis and associated postnasal drainage can specifically result in pharyngitis and laryngitis; and that these allergic inflammatory reactions can be amplified if acute viral/bacterial sinusitis develops. That laryngeal inflammation causes dryness, which in turn provokes itching or tickling sensations and reactive throat clearing and coughing, was discussed by these investigators. As mentioned earlier, these abusive behaviors mechanically traumatize the vocal folds, and they can lead to mucosal tears, hemorrhaging, and Reinke’s edema. Persistent abuse can also result in widespread supraglottal swelling and erythema. To overcome any associated voice difficulty, patients may inadvertently bear down on phonation to drive more proficient speech production.55 This compensatory strategy may cause the development of habitual muscle tension dysphonia, which can aggravate the underlying vocal pathology and trigger a vicious cycle of interrelated, abnormal laryngeal biomechanical and biomolecular activities and changes. Chadwick acknowledged that distinguishing the direct laryngeal effects of allergic disease from the adverse influences of nonallergic conditions, such as vocal misuse and vocal fold abuses in the same patient, can be very difficult.34 He suggested that studies focused on demonstrating a possible causal relationship between allergy and laryngeal pathology must ferret out and evaluate all collateral medical and behavioral factors via careful subject selection methods. This approach would enable researchers to specify the individual or synergistic pathophysiological sequelae of the many different conditions that may adversely affect laryngeal form and function.
Other clinical researchers addressed these concerns and experimental recommendations, by conducting three separate prospective studies of patients who tested positive for allergies to the perennial dust mite antigen Dermatophagoides pteronyssinus. In the first two investigations,56,57 placebo-controlled research protocols were employed, in which one group of subjects was challenged with an active antigen for D. pteronyssinus, and the other group was exposed to a placebo suspension. At the outset of the first study, the researchers hypothesized that because the mucosa throughout the respiratory tract is contiguous, oral inhalation of a dust mite antigenic suspension in allergic individuals would elicit type I, IgE-mediated responses within the larynx. Results demonstrated that low dose antigen challenge was inadequate to stimulate clinically significant laryngeal responses.
The second experiment utilized higher doses of the antigen. Moderate to severe signs of respiratory dysfunction (i.e., shortness of breath, chest tightness, bronchospasms, wheezing, coughing, throat clearing, and reduced FEV1 levels) were experienced by the first two subjects who received the antigen solutions. These unexpected pulmonary side effects forced the researchers to terminate the experiment prematurely, with accrual of only three subjects.
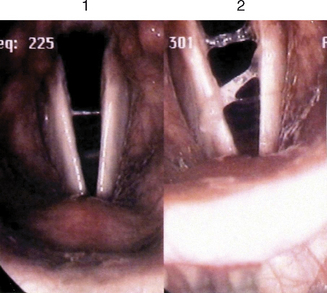
Figure 10.13 is a representative example of one of the most salient laryngeal reactions to the high dose antigen challenge in the experimental subjects. Note from this videostroboscopy photograph the production of a significant amount of sticky-viscous endolaryngeal secretions immediately following such direct exposure, as compared to the corollary baseline image. Vicious throat clearing reactions were prominent and likely contributed to the mild vocal fold edema noted and exacerbating dyspnea symptoms. What could not be explained with certainty was whether these secretions originated in the larynx, or if they were coughed upstream from the lower airway as a consequence of concomitant bronchospasms.
The third investigation addressed the frequent co-occurrence of reflux disease in allergic patients, and the extent to which this comorbid problem might potentially influence or camouflage any laryngeal abnormalities that may be observed in these individuals.58 To control for this anticipated complication, all prospective control (nonallergic) and experimental (dust-allergic) subjects with histories of gastroesophageal or extraesophageal (i.e., laryngopharyngeal) reflux diagnoses and treatments were disqualified from study participation. Results revealed that on more than 15 different comparative phonation and respiration subsystem analyses, there were no significant differences between the two groups of subjects. That is, all subjects exhibited normal phonation and respiration subsystem characteristics.
Several Japanese researchers demonstrated type 1 hypersensitivity reactions in the larynx using animal models. In one study, guinea pigs were sensitized with ovalbumin, and laryngeal inflammation was stimulated with passive cutaneous anaphylaxis.59 Significant populations of eosinophilic and basophilic cells were observed in the sensitized laryngeal mucosa of these animals. In addition, ovalbumin-specific hyposensitization decreased with extent of this cellular infiltration of the larynx. In a separate study, Brown Norway rats that were sensitized to Japanese cedar pollen, a major seasonal antigen in Japan, were examined.60 Results revealed a significant increase in esoinophilic infiltrate in the laryngeal mucosa of these sensitized rats when compared with a control population. These animal studies supported the concept that certain forms of laryngeal edema in humans may result from inhalant antigen exposure in allergic individuals.
▪ Clinical Observations
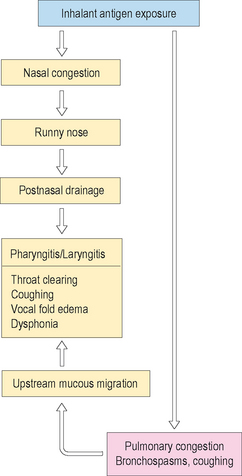
Figure 10.14 summarizes a proposed symptomatic train of interrelated unified airway responses to substantial inhalant antigen exposure in the allergic patient. These abnormal signs and symptoms may occur acutely or as late phase reactions, depending upon the intensity of the inciting antigen and the severity of the patient’s allergy. Note from this figure that initially the patient may simultaneously experience nasal and pulmonary congestion. A common side effect of the former reaction is a runny nose, which often leads to postnasal drainage into the pharyngeal and laryngeal cavities. This downstream mucous discharge may result in localized mild inflammation, itchy and tickling throat sensations, and a globus feeling. Concurrently, underlying pulmonary congestion induces bronchospasms, which cause shortness of breath, vigorous coughing activities, and decreased respiratory support for speech production. Clearance of the tracheobronchial tree of excessive mucous production occurs with coughing, which induces upward migration of the sticky mucus secretions into the larynx. Volitional and reflexive throat clearing and coughing occurs in response to these accumulated secretions in the upper airway. These behaviors result in vocal fold edema, which exacerbates the mild laryngeal swelling caused by postnasal drainage. Vocal fold edema contributes to voice difficulties and vocal fatigue. If exposure continues to a sufficient degree, the cycle repeats itself, and becomes vicious until broken by elimination of the inciting antigen, bodily adaptation, and/or pharmacologic intervention.
Managing Laryngeal Symptoms in the Allergic Patient
▪ Acute Laryngitis
Anaphylaxis
Acute, fulminant, life-threatening edema of the larynx can be IgE- and non-IgE-mediated. Certain foods, inhalants, plants, and insect or animal bites are allergenic; responses by sensitive patients are usually severe and IgE mediated. It is important to note, however, that less severe non-IgE-mediated acute reactions to these allergens can also occur.34 In either case, following exposure the patient may initially present with lip swelling and decreased sensation; associated drooling may be evident as the reaction progresses. Moderate to severe responses include tongue swelling, dysphagia, and difficulty breathing. Initial airway distress may be characterized by progressive pulmonary congestion, labored and rapid respiration, significant shortness of breath, and inhalatory stridor. Laryngeal examination typically demonstrates generalized supraglottal edema and muffled voice quality. Severe signs and symptoms almost always require emergent management to stabilize the airway. Treatment consists of oxygen per mask and epinephrine (adrenaline) injection of 0.3 ml of 1 : 1000 solution subcutaneously. IV steroid injections, consisting of hydrocortisone 80–100 mg, or dexametasone (10 mg) should be administered as soon as IV access is obtained. Topical epinephrine can be given via inhalation, if needed. For supplemental support, IV or IM antihistamines and H1 and H2 blockers may be rendered to counteract any additional allergic responses. Special attention should be given to those individuals with acute laryngeal edema that may be due to abnormal metabolic-mediated allergenic drug reactions (e.g., patients on ACE inhibitor or blocker medication).11 Unlike patients with IgE-mediated reactions, these types of patients do not respond as well to epinephrine; they may, instead, require aggressive airway management if significant airway obstruction occurs before the steroids, H1 and H2 blocker treatments have had a chance to be effective.
Infectious Laryngitis
Viral infections are clearly the most common causes of acute laryngeal inflammation. The most common forms include parainfluenza virus, rhinovirus, adenovirus, and general influenza viruses.61 Prior to full symptom onset, the patient may experience mild postnasal drainage, subtle dysphonia, and frequent throat clearing in response to perceived peri- and endolaryngeal mucous accumulation. As the viral infection progresses, these symptoms worsen; voice becomes barely audible, and fever usually ensues within 24 hours. Patients with an associated productive cough and discolored sputum should be treated appropriately with antibiotics. For patients who present with clear sputum, the treatment of choice is expectorant medication, use of a humidifier, and total voice rest. Nonproductive, dry-hacking coughing can be treated with over-the-counter cough syrups or prescription-strength cough suppressants, such as dextromethorphan, codeine hydrocodone, or benzozoate. Topical nasal steroid sprays or nasal antihistamines are generally helpful for postnasal drainage symptoms. Decongestants should be used sparingly in patients with substantial nasal congestion and recalcitrant nasal drainage. This cautionary note is especially applicable to patients with histories of high blood pressure, as decongestants can exacerbate this condition. Additionally, the use of antihistamine and decongestant medication by any patient can result in significant drying of the mucous membranes of the entire vocal tract, which can adversely affect underlying laryngeal inflammation. This predictable anticholinergic sequela is usually counterproductive, and most often associated with first generation antihistamines. Significant sedative effects are also experienced by those who use these drugs. Even topical decongestants should be recommended with extreme caution (i.e., maximum of 48–72 hours of prescribed use) to prevent rebound rhinitis medicamentosum. Antibiotics are prescribed when patients exhibit one or more of the following bacterial infection signs and symptoms: fever, chills, yellow and purulent sputum, and exudative appearing tonsils, posterior pharyngeal wall, epiglottis, or vocal folds. In such cases, augmented penicillin or a second generation macrolides is usually quite effective. For patients with penicillin allergy, telithromycin or clarithromycin serve as excellent substitutes. Antibiotic therapy should be continued for a sufficient period to eradicate the infection.
▪ Chronic Laryngitis
Vocal Misuse
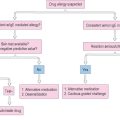
Coaches, preachers, teachers, factory workers, singers, sports enthusiasts, and others who frequently exhibit excessively loud voice behaviors are prone to chronic inflammation of the vocal folds. In many cases, the unusually hard vocal fold collision forces associated with yelling or aggressive speech activities can convert from general swelling into discrete lesions, such as nodules or hemorrhagic polyps. Hoarse-harsh vocal quality and reduced pitch range are the most salient abnormalities. Occasionally, patients complain of co-occurring extrinsic laryngeal and temporomandibular joint muscle tension and tenderness. These symptoms may vary in severity from day to day, but they often persist in some form for many months. First line treatment in most cases is complete cessation of the inciting patterns of voice abuse. In severe cases, strict voice rest may be indicated for a period of 10 days to 2 weeks. Use of microphones, whistles, sign language, and other compensatory communication strategies are all effective methods of reducing vocal fold stress when the patient is permitted to begin using voice again. A formal voice therapy program is typically recommended at this time, focusing on: (1) the specific cause of the chronic laryngitis, (2) rationale for voice conservation, (3) importance of adequate daily hydration, (4) steam inhalation, and (5) specific voice production exercises. This program usually consists of between six and ten 1-hour sessions over the course of 2 months. Patients who continue to struggle with the aforementioned symptoms, despite these conservative treatment methods, may require phonosurgical intervention for persistent vocal fold lesions, followed by a short stint of additional voice therapy. Patients with co-occurring allergies will also continue with their pharmacologic and medical therapies throughout this treatment program.
Laryngopharyngeal Reflux
This condition is very common in the general population, and it often co-occurs in patients with allergies. Acting alone or in combination with allergy, reflux disease can cause substantial laryngitis. When acid material chronically enters the hypopharynx it can ultimately induce locoregional tissue ulcerations, erythema, and inflammation, as previously described.19 Unlike patients with gastroesophageal reflux, those with laryngopharyngeal reflux do not typically complain of heartburn and indigestion, which are symptomatic of esophagitis. Rather, they experience variable degrees of sore throat, coughing, odynophagia, and dysphonia symptoms. Although reflux events can only be definitively identified using multiple probe pH monitoring systems, in most cases the diagnosis is made empirically during the clinical examination on the basis of history, presenting signs and symptoms, and previously positive responses to nonprescription antacids. Initial treatments may include educational materials regarding the disease entity, and proper eating habits. Neither food nor drink is permitted within 2 hours of bedtime; girdles and tight fitting clothes are discouraged, as are smoking and alcohol use. Excessive use of caffeine and spicy food are similarly prohibited. Throat clearing in response to acid or sour taste sensations is also discouraged. Instead, patients should be instructed to take a drink of water and swallow several times for relief. Additionally, they should be encouraged to stifle or suppress all vigorous coughing activities to reduce vocal fold stress and general laryngeal inflammatory reactions. All other forms of vocal abuse or misuse should be discouraged as well. The previously mentioned cough suppressant medications may prove helpful. Patients with persistent reflux symptoms, irrespective of having employed significant behavioral modifications, are good candidates for pharmacologic intervention. Depending upon the severity of the laryngeal signs and symptoms, either once or twice daily proton pump inhibitor therapy is prescribed (e.g., esomeprazole magnesium, rabeprazole sodium, lansoprazole); in severe cases, an H2 blocker (e.g., ranitidine) can be added prior to bedtime. Surgical intervention for laryngopharyngeal reflux is preserved for rare instances of obstructive granuloma formation on the vocal processes of the arytenoid cartilages, the interarytenoid tissue bridge, or both. Even in these cases, an 8- to 12-week trial program of the aforementioned behavioral and pharmacologic therapies is recommended first, whenever possible. Patients with co-occurring allergies should continue on their allergy medications in the usual way.
Allergies
From a pharmacologic management approach, the use of antihistamines theoretically makes sense for allergic patients with chronic symptoms that do not significantly improve with behavioral modifications alone. As mentioned earlier, first generation antihistamine drugs (e.g., chloropheniramine, diphenhydramine) produce substantial anticholinergic effects. Consequently, these medications are generally contraindicated, owing to their potential drying effects on the laryngeal mucosa; friable vocal fold covers secondary to local dehydration are at risk for injurious side effects during voice activities. If antihistamine therapy is necessary to relieve overall significant allergy symptoms, second generation alternatives (e.g., loratidine, fexofenadine, desloratidine) are recommended because they may produce fewer adverse laryngeal and sedative side effects. Decongestants may improve nasal congestion symptoms, but their sympathomimetic sequelae may prove counterproductive to the professional voice user. It is also tempting to treat chronic laryngeal inflammation with topical corticosteroid medications. However, as discussed earlier, inhaled corticosteroids have been shown to cause adverse or irritable vocal fold phonatory biomechanical disturbances. Whereas allergy immunotherapy may hold promise as an effective laryngeal allergy treatment method, substantial clinical research in this area of investigation is required to confirm this hypothesis. Additionally, further studies are indicated relative to the pathophysiological mechanisms that may be involved in individuals with allergies who present with signs and symptoms of laryngeal inflammation.
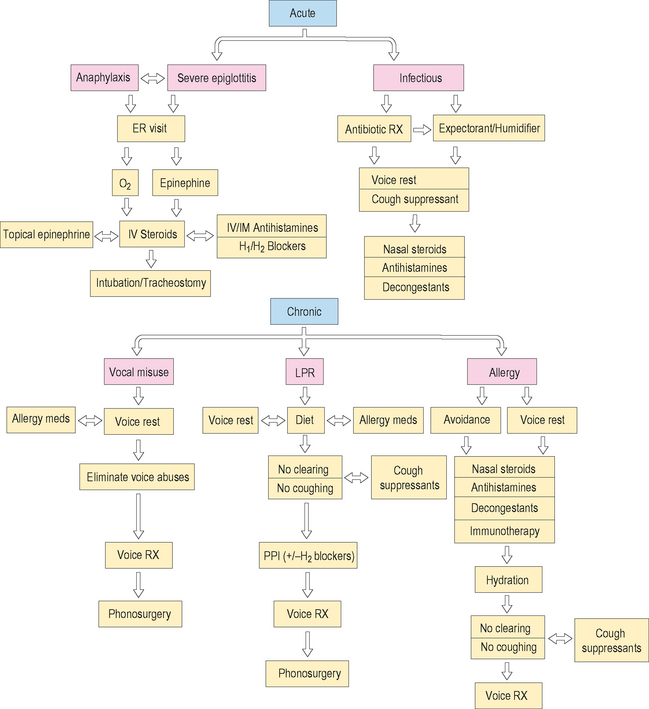
Ultimately, the development of unequivocal treatment algorithms for patients with acute or chronic laryngitis secondary to allergy and related disorders will depend upon numerous prospective, randomized clinical treatment trials from several cooperative groups of researchers, in order to accrue a sufficient number of patients. Figure 10.15 illustrates a synopsis of the possible treatment alternatives for acute and chronic laryngitis in the allergic patient.
Summary and Conclusions
For completeness, discussions were rendered regarding the pathophysiological phonation subsystem effects of other conditions that frequently co-occur in patients with allergies, such as reflux disease, asthma, irritable larynx syndrome, and chronic voice abuse behaviors. This information was included to emphasize that during the evaluation work-up of the patient with allergic disease, the probable complex coexistence of several aerodigestive and airway disorders must be explored and, when necessary, factored into the differential diagnosis and ultimate treatment plan. The central theme of the chapter focused on the importance of cooperative interactions of physicians and allied health clinicians from different subspecialties in order to ensure comprehensive and successful treatment outcomes with this multivaried clinical population. With such objectives in focus, these practitioners were cautioned that when patients with allergic diseases present with complaints of intermittent or chronic voice difficulties, these problems should not be dismissed as completely unrelated to the allergy. Diagnostic conclusions that the two conditions are not causally interrelated may prove premature in many cases; a causative association may be more likely in these individuals than a simple relationship of coexistence. Finally, the need for carefully controlled research investigations of the primary and secondary laryngeal effects of allergy and commonly associated disorders was demonstrated, with hopes of stimulating additional scientific inquiry in this area of interest.
1 Koufman J. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101(suppl 53):1-78.
2 Hurwitz B. Nasal pathophysiology impacts bronchial reactivity in asthmatic patients with allergic rhinitis. J Asthma. 1997;34:427-431.
3 Grossman J. One airway, one disease. Chest. 1997;11(suppl):11S-16S.
4 de Benedictis F, Bush A. Rhinosinusitis and asthma: epiphenomenon or causal association? Chest. 1999;115:550-556.
5 Smith JM. Epidemiology and natural history of asthma, allergic rhinitis, and atopic dermatitis (eczema). In: Middleton JE, Reed CE, Ellis EF, et al, editors. Allergy principles and practice, 2nd ed. St Louis: Mosby; 1983:771-803.
6 Braman SS, et al. Airway hyperresponsiveness in allergic rhinitis:a rsik factor for asthma. Chest. 1987;91:671-674.
7 Slavin RG, et al. Sinusitis and bronchial asthma. J Allergy Clin Immunol. 1980;66:250-257.
8 Fokkens W, et al. The interactions between upper and lower airways and allergic rhinitis. The nose 2000 and beyond: International Rhinologic Society programs and abstracts. Biennial meeting of the International Rhinologic Society, Washington, DC; Sep. 21–25, 2000. Washington, DC:A-158(abs.).
9 Irvin CG. Sinusitis and asthma: an animal model. J Allergy Clin Immunol. 1992;90:521-533.
10 Voegels R, et al. Can endoscopic nasal surgery improve asthma symptoms? The nose 2000 and beyond:International Rhinologic Society programs and abstracts. Biennial meeting of the International Rhinologic Society. Washington, DC; Sep. 21–25, 2000. Washington, DC:A-30(abs.).
11 Chadwick SJ. The pharynx and larynx. In: Krouse JH, et al, editors. Allergy and immunology: an otolaryngic approach. Philadelphia: Lippincott Williams & Wilkins; 2002:249-270.
12 Hirano M. Phonosurgery. Basic and clinical investigations. Otologia (fukuoka). 1975;21:239-442.
13 Kotby MN, et al. Electromyography and neurography in neurolaryngology. J Voice. 1992;6:159-187.
14 Dworkin JP, et al. Use of topical lidocaine in the treatment of muscle tension dysphonia. J Voice. 2000;14:567-574.
15 Titze I. Principles of voice production. New Jersey: Prentice-Hall, 1994.
16 Blitzer A, et al. Neurologic disorders of the larynx. New York: Thieme, 1992.
17 Dworkin JP, et al. Videostroboscopy, mirror, and fiberoptic laryngoscopy: objective comparisons. J Med Speech-Lang Path. 2004;12:99-107.
18 Sataloff RT, Spiegel JR, Hawkshaw MJ. Strobovideolaryngoscopy: results and clinical value. Ann Otol Rhinol Laryngol. 1991;100:725-727.
19 Koufman JA. Laryngopharyngeal reflux 2002: a new paradigm of airway disease. ENT J. 2002;81:2-7.
20 Patorello EA, et al. Mechanisms in adverse reactions to food: the mouth and pharynx. Allergy. 1995;50:41-44.
21 Amlot PL, et al. Oral allergy syndrome: symptoms of IgE mediated hypersensitivity to foods. Clin Allergy. 1987;17:33-42.
22 Ortolani C, et al. The oral allergy syndrome. Ann Allergy. 1988;61:47.
23 Bircher AJ, et al. IgE to food allergens are highly prevalent in patients allergic to pollens, with and without symptoms of food allergy. Clin Exp Allergy. 1994;24:367.
24 Ebner C, et al. Common epitopes of birch, pollen, and apples: studies by Western and Northern blot. J Allergy Clin Immunol. 1991;88:588.
25 Eriksson NE. Clustering of foodstuffs in food hypersensitivity and inquiry study in pollen-allergic patients. J Allergol Immunol Pathol. 1984;12:28.
26 Andrianopoulos MV, Gallivan GJ, Gallivan KH. PVCM, PVCD, EPL, and irritable larynx syndrome: What are we talking about and how do we treat it? J Voice. 2000;14:607-619.
27 Gallivan GJ, Hoffman L, Gallivan KH. Episodic paroxysmal laryngospasm: voice and pulmonary assessment and management. J Voice. 1996;10:93-105.
28 Koufman JA. The differential diagnosis of paradoxical vocal cord movement. Visible Voice. 1994;3:3.
29 Morrison M, Rammage L, Emami AJ. The irritable larynx syndrome. J Voice. 1999;13:447-455.
30 Martin RJ, et al. Paradoxical vocal cord motion in presumed asthmatics. Semin Resp Med. 1987;8:332-338.
31 DelGaudio JM. Steroid inhaler laryngitis. Arch Otolaryngol Head Neck Surg. 2002;128:677-681.
32 Cohn JR, Spiegel JR, Sataloff RT. Vocal disorders in the professional voice user: the allergist’s role. Ann Allergy Asthma Immunol. 1995;74:363-376.
33 Corey JP. Allergy for the laryngologist. Otol Clin North Am. 1998;31:422-426.
34 Chadwick SJ. Allergy and the contemporary laryngologist. Otol Clin North Am. 2003;36:957-988.
35 Corey JP, Gungor A, Karnell M. Allergy for the laryngologist. Otol Clin North Am. 1998;31:189-205.
36 Alimov AL. The clinical symptomatology in the diagnosis of allergy in acute and chronic laryngitis. Otol Rhinol Laryngol. 1968;30:71-75.
37 Hogan SP, Rothenberg ME. Review article: the eosinophil as a therapeutic target in gastrointestinal disease. Alimen Pharmacol Ther. 2004;20:1231-1240.
38 Baroody FM. Allergic rhinitis: broader disease effects and implications for management. Otolaryngol Head Neck Surg. 2003;128:616-631.
39 Domeji S, et al. Similar distribution of mast cells and substance P and calcitonin gene-related peptide-immunoreactive nerve fibers in the adult human larynx. Ann Otol Rhinol Laryngol. 1996;105:825-831.
40 Ishi J, et al. Local eosinophilia of the nose, the larynx, and the trachea in rats sensitized with Japanese cedar pollen. Arerugi. 1997;46:1251-1257.
41 Ishida H, et al. Immunohistochemical study on distribution of mast cell phenotypes in human laryngeal mucosa: evidence for laryngeal type I allergy. Ann Otol Rhinol Laryngol. 2005;114:139-143.
42 Shanahan F, et al. Human lung mast cells: distribution and abundance of histochemically distinct subpopulations. Int Arch Allergy Appl Immunol. 1987;83:329-331.
43 Irani AM, Schwartz LB. Mast cell heterogeneity. Clin Exp Allergy. 1989;19:143-155.
44 Okuda M, Ohtsuka H, Kawabori S. Basophil leukocytes and mast cells in the nose. Eur J Respir Dis Suppl. 1983;64:7-15.
45 Pang LQ. Allergy of the larynx, trachea, and bronchial tree: Symposium on Allergy and Otolaryngology. Otol Clin North Am. 1974;7:719-734.
46 Williams RI. Allergic laryngitis. Ann Otol Rhinol Laryngol. 1972;81:558-564.
47 Sala E, et al. Occupational laryngitis with immediate allergic or immediate-type specific chemical hypersensitivity. Clin Otolaryngol. 1996;21:42-48.
48 Rea WJ. Elimination of oral food challenge reaction by ingestion of food extracts: a double blind study. Arch Otolaryngol. 1984;110:248.
49 Rea WJ. Environmental aspects of ear, nose and throat disease. Part 1. J Clin Ecol Otorhinol. Allergy. 1979;41:41.
50 Naito K, et al. Laryngeal allergy: a commentary. Eur Arch Otorhinolaryngol. 1999;256:455-457.
51 Dixon HS. Allergy in laryngeal disease. Otol Clin North Am. 1992;25:239-249.
52 Jackson-Menaldi CA, et al. Allergies and vocal fold edema:a preliminary report. J Voice. 1999;13:113-122.
53 Lack G. New perspectives in pediatric allergic rhinitis and comorbid disorders. J Allergy Clin Immunol. 2001;108:S9-15.
54 Cohn JR, Sataloff RT, Branton C. Responsive asthma-related voice dysfunction to allergen immunotherapy: a case report of confirmation by methacholine challenge. J Voice. 2001;15:558-560.
55 Cohn JR, et al. Airway reactivity-induced asthma in singers (arias). J Voice. 1991;5:332-337.
56 Reidy PM, Dworkin JP, Krouse JH. Laryngeal effects of antigen stimulation challenge with perennial allergen Dermatophagoides pteronyssinus. Otol Head Neck Surg. 2003;128:455-462.
57 Dworkin JP, et al. Effects of sequential Dermatophagoides pteronyssinus antigen stimulation on the anatomy and physiology of the larynx. ENT J (in press).
58 Carron M, et al, The effect of dust mite allergies on the phonation subsystem. Paper at COSM 2007, San Diego.
59 Iwae S, Ishida H, Amatsu M. Laryngeal type I allergy in sensitized guinea pig. Larynx Jpn. 1995;7:1-6.
60 Lidegram M, et al. Mast cells in the laryngeal mucosa of the rat: a quantitative and immunohistochemical study at the light and electron microscopic levels. Acta Anat (Basel). 1996;157:135-143.
61 Turnidge J. Responsible prescribing for upper respiratory tract infections. Drugs. 2001;61:2065-2077.