Chapter 9 Management of the Patient with Inner Ear Allergy
Introduction
It is likely that there is more to an “allergic” reaction involving the labyrinth than a classic type 1 hypersensitivity reaction. The pathogenesis of immune-mediated inner ear disorders is not known, although autoantibodies, autoreactive T cells, immune-complex deposition, and vasculitis have all been suggested.1 Autoimmune responses involving the inner ear range from the patient with Ménière’s disease who has a clear seasonal component to his or her symptoms, to the patient with a true organ-specific response characteristic of autoimmune inner ear disease (AIED).
The temporal relationship between ingestion of a suspect food and the later development of vertigo or hearing loss in an affected individual, as well as the well-documented evidence of increased circulating immune complexes in the serum of patients with Ménière’s disease, all hint strongly at a type 3 immune-complex-mediated hypersensitivity in some patients with this syndrome.2,3
However, we do not believe that all immune-mediated damage to the inner ear is “allergic,” but rather that there is truly a spectrum, including some who have an organ-specific autoimmune disease involving the labyrinth, which is best treated by steroids or, potentially, other immunosuppressive or immunomodulating medications.
Review of Immunology
The inner ear has little exposure to pathogens and few resident cells that are associated with immunologic function.4 While this led in the past to the assumption that it is immunologically privileged, in a manner similar to that of the brain, more recent research has indicated that inflammation can occur there, and that it is actually more immunoresponsive than the brain. Like the brain, the labyrinth also exhibits a blood–labyrinthine barrier, analogous to the blood–brain barrier, which helps to maintain the ionic characteristics that are unique to the cochlear environment. Also, in a manner similar to the brain, there is no lymphatic drainage from the inner ear. While immunoglobulins are present in the perilymph, the amount is only 1/1000 of that present in the serum.5
The inner ear demonstrates both cellular and humoral immunity. Most leukocytes enter the cochlea via the spiral modiolar vein.6 The innate immunity of the cochlea has been suggested to allow an adaptive local response to antigen challenge.7 Hashimoto et al have suggested that the inner ear may be “primed” by lipopolysaccharides or other viral/bacterial antigens, resulting in the upregulation of interleukin 1β in the fibrocytes of the spiral ligament, which in turn permits the entry of leukocytes. Subsequently, in those individuals having lymphocytes primed to react against inner-ear antigens, an immune response may be initiated, resulting in local inflammation and hearing loss.
Altermatt et al have suggested that the seat of immunoactivity in the inner ear appears to reside in the endolymphatic sac and duct.8 Immunoglobulins G, M, and A, as well as secretory component, are all found in the endolymphatic sac, and numerous plasma cells and macrophages are found in the perisaccular connective tissue.
As well as having an apparent innate immunity, the labyrinth has been found to have active components of allergic reactivity as well. Mast cells have been identified in the perisaccular connective tissue. Following sensitization, IgE-mediated degranulation of the mast cells has resulted in eosinophilic infiltration of the perisaccular connective tissue and the clinical production of endolymphatic hydrops.9
The endolymphatic sac has been shown to be capable of both processing antigen, as well as producing its own local antibody response.10,11 The surgical destruction of the endolymphatic sac or the obliteration of the endolymphatic duct results in a decrease in both antigen and antibody responses.11 The endolymphatic sac has a highly vascular subepithelial space containing numerous fenestrated blood vessels.12 Arterial branches of the posterior meningeal artery supply the endolymphatic sac and duct.13 While the labyrinth is similar to the rest of the central nervous system in being protected by this blood–labyrinthine barrier, the posterior meningeal artery is fenestrated, offering a peripheral portal of circulation. In other parts of the body, fenestrated vessels supplying organs involved in absorption (e.g., kidney, choroid) are especially susceptible to damage by immune complex deposition.
From this abbreviated background of otoimmunology, we shall now review some specific symptom complexes in the labyrinth that may be caused or influenced by an underlying allergic reaction (Box 9.1).
Ménière’s Disease
The senior author’s practice is composed largely of patients diagnosed with Ménière’s disease, whether felt to be related to allergy or not, and this chapter will largely deal with this syndrome. A suggestion that at least some cases of Ménière’s disease may have an allergic component is hardly a new idea. In fact, the first published report of Ménière’s disease felt to be provoked secondary to an allergic reaction was in 1923.14 Both inhalant and food allergies have been linked with symptoms of Ménière’s disease and cochlear hydrops.15 Changes in electronystagmography and electrocochleography recordings have been noted on patients injected with food extracts during provocative food testing.16,17 Additionally, Gibbs et al have reported the production of electrocochleographic changes in patients with known inhalant allergies and Ménière’s disease after nasal provocation by inhalant antigens.18
Many of the clinical characteristics of Ménière’s disease suggest an underlying inflammatory if not autoimmune etiology. Its notorious propensity to wax and wane, becoming active again after long periods of remission, suggests an inflammatory component. It is bilateral in a significant number of cases.19 A delayed Ménière’s-like picture may develop in a normal ear following trauma to the contralateral ear. It is often initially responsive to steroid treatment, although the senior author has noted a tendency towards steroid resistance developing in patients with Ménière’s disease. An increased level of circulating immune complexes has been found to be present in 96% of patients with Ménière’s disease.3
Despite the aforementioned evidence of immune activity, only 30% of patients with Ménière’s disease show evidence of a true autoantibody response to specific anticochlear antibody by western blot assay.20 Tests of abnormal cell-mediated immunity, such as the lymphocyte transfer test and the lymphocyte migration inhibition assay, have either been inconsistent, or have been found to be normal even in patients with known causes of autoimmune dysfunction of the inner ear, such as Cogan’s syndrome.20 It is clear that, in spite of our increasing understanding of inner ear immunoreactivity, we have not yet developed a reliable laboratory marker to “prove” autoimmune or allergic causation in a patient with Ménière’s or other forms of suspected inflammatory hearing loss.
Although the most accurate tests currently available to diagnose an autoimmune abnormality are often normal, there may be other immune-mediated causes for the development of symptoms. With an incidence of 20%, allergy is the most common “autoimmune” disease clinically. In a survey of 734 patients with Ménière’s syndrome, the prevalence of test-confirmed concurrent allergic disease was 41%, twice the incidence in the population in general.21 In a yet more recent survey study, patients reported a 58% rate of history of allergy, and, again, a 41% rate of positive skin or blood test.22 An increased incidence of self-reported migraine as well as allergic rhinitis has been reported in patients with Ménière’s disease as compared to a control group of age and sex-matched patients without Ménière’s attending an otolaryngology clinic.23 The prevalence of migraine in Ménière’s sufferers was 39%, while the rate in the control group was 18%. The prevalence of reported allergy in this study was also higher in those with Ménière’s disease, 51.9%, compared to 23% in the non-Ménière’s group.
This apparent relationship between allergic rhinitis, Ménière’s disease, and migraine is interesting in that all produce symptoms that are recurrent and paroxysmal. Vascular changes have been implicated to play an important role in all three conditions as well. While there are at least as many published “triggers” of migraine as there are of Ménière’s, the pathophysiology appears to be that of vasoconstriction, followed by vasodilation of meningeal vessels, with plasma protein extravasation.24 In a like manner, small ruptures in the membranous labyrinth secondary to changes in the cochlea microvasculature have been found to be associated with endolymphatic hydrops and Ménière’s disease.25,26
An elevated level of circulating immune complexes (CICs) has been reported in patients with allergic rhinitis and asthma, as well as those with Ménière’s disease.3,27 The whole concept of a sudden influx of fluid into the endolymphatic sac, producing a rupture of Reissner’s membrane, and the resulting production of Ménière’s symptoms would be very consistent with the vasodilation, fluid transudation, and inflammatory reaction that are the hallmarks of an allergic reaction.
Adding support to a hypothesized allergic component to symptom production in some patients with Ménière’s disease, Keles et al found total IgE to be elevated in 41.3% of patients with Ménière’s disease, but in only 19.5% of their control group.28 This is in contrast to an earlier study by Stahle et al who found no elevation of total IgE in patients with Ménière’s disease.29 However, adding support to an apparent allergic predisposition in Ménière’s, the Keles et al study also reported significant elevations of Th2 derived IL-4 in the Ménière’s disease group as compared with the controls.28
If we accept that 40% of patients with Ménière’s disease are also allergic, how do we distinguish which patients should undergo diagnostic testing and treatment? Should all patients with Ménière’s disease be tested for allergies? While the authors have reported a prevalence rate of allergy, confirmed by skin and/or in vitro testing in 40% of patients with Ménière’s, the corollary is that the other 60% do not have allergy. A careful history, including family history of allergy, as well as a physical examination specifically looking for other stigmata of allergy is essential in all patients with Ménière’s disease. It is the senior author’s opinion that it is the uncommon patient with Ménière’s and underlying allergy who does not also have other nonotologic physical signs or symptoms suggestive of an allergic diasthesis. An uncommon exception is the patient who has no family or childhood history of allergy, no history of upper or lower respiratory symptoms suggestive of allergy, but who will report a clear-cut relationship between Ménière’s symptom production and either season of the year or ingestion of a food that does not contain natural vasoactive compounds such as caffeine or has a high natural sodium content. Box 9.2 lists the type of symptoms that should alert the clinician to the possibility that an underlying problem with allergies may cause or contribute to Ménière’s disease symptoms in a given patient.
BOX 9.2 Indications for allergy testing in patients with Ménière’s disease
Childhood or past history of allergy
Patient suspects food reaction
Other allergic symptoms (asthma, rhinoconjunctivitis, etc.)
Steroid-dependent symptoms (rule out autoimmune inner ear disease)
There have been several studies from our institution to assess the profile and treatment outcome in patients with Ménière’s disease and allergy treated with specific immunotherapy and dietary elimination. In the first study, 93 patients with Ménière’s disease, diagnosed according to AAO-HNS standards, were tested for allergies.30 Criteria for patients in the testing group included a history of Ménière’s symptoms related to seasons, weather changes, or a suspect food; a known history of allergy; a significant childhood history of allergy; bilaterality of symptoms; or refractoriness to usual methods of treatment. Patients underwent control skin testing with histamine, saline, and glycerine, then the serial endpoint titration (SET) technique skin testing, now referred to as Intradermal Dilutional Testing, or IDT, for inhalant allergens. RAST screening was also performed in selected cases. Patients were asked to keep a food diary for 1 to 2 weeks before undergoing food testing with the Subcutaneous Provocative Food Test. In addition, IgG, IgE, and RAST tests were performed for selected foods.
Nearly a third (32.6%) of the patients felt that a reaction to a food provoked their Ménière’s symptoms. Many patients also felt that their symptoms were related to weather (23.7%) or seasonal changes (47.3%). Nine percent of patients had a history of a known autoimmune disease. Eighty-two percent of patients had a normal total serum IgE (<100 ng/ml). Antigen-specific IgE as measured by SET also tended to be of low levels, with the majority of endpoint dilutions to weed, grass, and tree pollens, as well as dust and mold, occurring in dilution #2 (1–500 w/v). The most common foods identified by provocative food testing (PFT) included wheat, milk, corn, egg, yeast, and soy. Following immunotherapy, 56 of the 90 patients with follow-up (62%) reported a decrease in both frequency and severity of vertigo attacks. Fifty percent also reported an improvement in tinnitus. None were worse.
In a second study, we evaluated the effect of specific allergy immunotherapy and food elimination of suspected food allergens on the course of patients with Ménière’s disease for whom allergy treatment had been recommended.31 Subjects were mailed a questionnaire regarding their symptoms. The 113 patients treated for symptoms of allergy using desensitization and diet showed a significant improvement from pre- to posttreatment, not only in allergy symptoms, but also in Ménière’s symptoms. The patient ratings of frequency, severity, and interference with everyday activities of their Ménière’s symptoms also appeared better after allergy treatment than ratings from the control group of 24 untreated patients. Vertigo control results, using the AAO-HNS classification, were 47.9% Class A or B. Hearing was stable or improved in 61.4%. Results indicate that patients with Ménière’s disease can show improvement in their symptoms of tinnitus and vertigo when receiving specific allergy therapy and suggest that the inner ear may also be the target, directly or indirectly, of an allergic reaction.
Although the above study asked patients to retrospectively rate their pretreatment symptoms, a more recent prospective study had 68 patients complete the questionnaire before treatment and at an average of 23 months follow-up.32 Results were similar, with significant improvement in frequency and severity of vertigo and unsteadiness, and improvement in the AAO-HNS disability scale and a number of quality of life scales (Table 9.1). Statistical analyses strongly suggested that these improvements were independent of medical treatment for Ménière’s disease and were not likely related just to natural history.
TABLE 9.1 Paired comparison of symptoms at initial and follow-up intervals for 68 patients with Ménière’s disease and allergy undergoing immunotherapy and/or diet treatment for allergy32
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
Clearly, a weakness of these studies, as well as other case reports and series suggesting that antigen-specific immunotherapy or dietary food elimination results in an improvement in the symptoms of Ménière’s disease, is that there is no control group receiving saline injections or given diets eliminating foods that were negative on testing. It has been our observation that patients with Ménière’s disease as well as allergic rhinitis are reluctant to commit to months or a year of possible placebo injections to “prove” that immunotherapy may be helpful in improving symptoms of vertigo, hearing loss, and/or tinnitus. For the present, we rely on the use of statistical control as well as research that clearly demonstrates the ability of allergy immunotherapy to downregulate Th2-driven inflammatory responses in patients with allergic rhinitis and asthma.33 If Ménière’s disease is believed to be a chronic condition with an inflammatory component, it is logical that downregulating the production and release of both proinflammatory and vasoactive mediators that promote fluid extravasation and/or retention could be helpful in lessening symptoms. This has certainly been the rationale for the use of steroids and even dietary restriction of salt in patients with Ménière’s. Interestingly, increased levels of sodium have been shown to increase the vasodilatory effect of histamine.34
While there is no universal agreement, the symptoms of Ménière’s disease – vertigo, hearing loss, and tinnitus – are thought to be produced by a sudden influx of fluid into the endolymphatic sac, producing a rupture of Reissner’s membrane in the cochlea. The resulting potassium intoxication of the auditory and vestibular nerves causes the local neurological changes. This increase in fluid could be caused by a sudden cessation of endolymph resorption in the endolymphatic sac, or could be secondary to the rapid production of endolymph in either the stria vascularis or the endolymphatic sac. The site is more likely the endolymphatic sac; the sac is not only capable of secretion as well as absorption, it is also known that the response of the sac to inner ear disturbance actually appears to be an increase in its secretory activity.35
Increasingly, there is evidence that it may also be necessary to have an anatomic variant in order to develop Ménière’s disease. It has been noted that the endolymphatic sac is smaller in patients with Ménière’s disease than in normal controls (Figure 9.1).36 Yet, we also find patients with smaller than average endolymphatic sacs in this group who did not develop Ménière’s disease. We could theorize that perhaps a smaller sac in the presence of a patient with allergy, or some other condition resulting in local inflammation, would predispose that individual to being less able to handle the sudden secretion of endolymph that could be stimulated by an underlying problem with allergy.
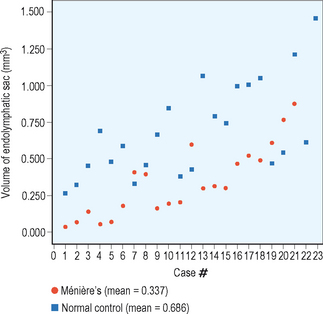
Figure 9.1 Endolymphatic sac volume (mm3) for 21 patients with Ménière’s disease and 23 normal controls.
(Data courtesy of Fred Linthicum, MD, House Ear Institute, 2006.)
There may be different possible mechanisms by which an allergic reaction results in the production of Ménière’s disease symptoms. These have been discussed in detail elsewhere, but are briefly summarized here.31 First, the endolymphatic sac itself could be a target organ of the allergic reaction. The sac’s peripheral and fenestrated blood vessels could allow the entry of an antigen, which would then stimulate mast cell degranulation in the perisaccular connective tissue. The resulting inflammatory mediator release could affect the sac’s filtering capability, resulting in a toxic accumulation of metabolic products, and interfering with hair cell function. Additionally, the fenestrated blood vessels to the sac could be pharmacologically vulnerable to the effects of the vasoactive mediators such as histamine, which are released in an allergic reaction elsewhere in the body. The unique blood supply of the interosseus sac would serve as a portal for these mediators to exert a direct pharmacologic effect. The potent vasodilating effects of histamine or other mediators could affect the resorptive capacity of the sac.
Adding credence to this proposed theory, Yan et al have shown that Waldeyer’s ring in the nasopharynx is the anatomic site of T-cell homing to the endolymphatic sac.37 Intranasal antigen stimulation with KLH in Waldeyer’s ring of systemically sensitized rodents resulted in an antigen-specific reaction in the endolymphatic sac and perilymphatic vessels. From an anatomic standpoint, it is possible that a viral or allergic antigen could be processed in the nasopharynx, with the resulting specific immune reaction occurring at the endolymphatic sac.
A second possible proposed mechanism involves the production of a circulating immune complex, such as a food antigen, which is then deposited through the fenestrated blood vessels of the endolymphatic sac, producing inflammation. As previously noted, an increased incidence of circulating immune complexes in the serum has already been described in both Ménière’s disease and allergic rhinitis. The inflammatory response resulting from the deposition of immune complexes along vascular basement membranes is the hallmark of an immune complex disease. While the binding of the complexes to the cell membranes facilitates their phagocytosis, it also results in the release of tissue-damaging enzymes. This is believed to be the mechanism of unexplained sensorineural hearing loss in patients with a prototype immune-complex-mediated disease, Wegener’s granulomatosis. When the temporal bones of patients with Wegener’s granulomatosis and unexplained sensorineural hearing loss have been studied, the cochlea is found to be normal; the pathology is found in the endolymphatic sac.38
Alternatively, circulating immune complexes may be deposited in the stria, causing the normally intact blood–labyrinthine barrier to leak as a result of increased vascular permeability. In addition to disrupting normal ionic and fluid balance in the extracapillary spaces, this could facilitate the entry of autoantibodies into the inner ear. Harris and Ryan found a 30% incidence of a positive 68-kDa autoantibody in the serum of patients with Ménière’s disease.20 This antibody is presumed to be heat shock protein 70, a constitutive protein which serves to chaperone other endogenous proteins during times of stress or damage to tissue. They also found that some human subjects with a known autoimmune disease have elevated levels of this circulating autoantibody, without evidence of hearing loss, suggesting that the antibody had not entered the inner ear yet. Harris and Sharp theorize that perhaps another factor must be present to facilitate its expression.39 This may well be the role played by a food antigen.
A third mechanism is of a viral antigen/allergic interaction. A predisposing viral upper respiratory infection in childhood, such as mumps, herpes, etc., results in antigenic stimulation of Waldeyer’s ring, and subsequently T-cell homing to the endolymphatic sac,37 where it results in a chronic low-grade inflammation. While this is not enough initially to result in hearing loss or vertigo, it does produce a mild impairment of sac absorption. Later in adult life, “something” in the system then stimulates excess fluid production. Shambaugh theorized that allergies or, less frequently, metabolic abnormalities such as thyroid or hormonal dysfunction, were likely culprits, causing the sac to decompensate with the resulting production of endolymphatic hydrops.40
▪ Treatment
Whether or not the patient presenting with Ménière’s disease is felt to have some allergic component to his or her symptoms, the “usual” treatments for Ménière’s should be instituted. Ménière’s is a potentially disabling condition, and patients with chronic conditions will often benefit by being presented with suggested guidelines to help them gain some measure of control over their symptoms. Low sodium diets in the range of 1500 mg/day of sodium should be recommended to all patients with Ménière’s disease. There is no definite established upper limit of “safe” sodium intake, but most patients with Ménière’s disease can tolerate this amount of sodium without symptom production or making food unnecessarily unpalatable. Although we give patients a handout listing the sodium levels of common foods, it is generally advisable that they purchase a reference dietary guide of sodium levels in foods at a bookstore.
We also recommend that patients with Ménière’s disease avoid significant caffeine intake. Those with poorly controlled symptoms are advised that no caffeine is the best course, while those whose symptoms are well controlled should restrict caffeine to no more than 8 oz of caffeinated beverage per day. Table 9.2 lists common dietary sources of caffeine.
| Caffeine | Content | |
|---|---|---|
| Product | Serving size | Caffeine (mg) |
| Beverages | ||
| Coffee, drip method | 8 oz | 135 |
| Java Water | 16.9 oz | 125 |
| Coffee, instant | 8 oz | 95 |
| Jolt Cola energy drink | 12 oz | 71 |
| Edge 20 bottled water | 8 oz | 79 |
| XTC energy drink | 8 oz | 58 |
| Mountain Dew | 12 oz | 54 |
| Espresso | 1 oz | 40 |
| Coca-Cola Classics and Diet Coke | 12 oz | 46 |
| Sunkist Orange Soda | 12 oz | 40 |
| Pepsi and Diet Pepsi | 12 oz | 36 |
| Tea, black (3-min brew) | 6 oz | 35 |
| Tea, instant | 5 oz | 25 |
| Tea, green | 5 oz | 25 |
| Chocolate milk | 8 oz | 8 |
| Decaffeinated coffee, drip | 8 oz | 3 |
| Decaffeinated coffee, instant | 8 oz | 3 |
| Food | ||
| Ben & Jerry’s no fat coffee fudge frozen yoghurt | 1 cup | 85 |
| Starbuck’s low fat mocha mambo ice-cream | 1 cup | 60 |
| Haagen-Dazs coffee ice-cream | 1 cup | 58 |
| Dannon coffee yoghurt | 8 oz | 45 |
| Hershey’s special dark chocolate bar | 1.5 oz | 31 |
| Baking chocolates | 1oz | 25 |
| Milky Way bar | 2.1 oz | 11 |
| Raisinets | 1.58 oz | 11 |
| Carnation chocolate crunch breakfast bar | 1.34 oz | 7 |
| Jell-O chocolate vanilla swirl pudding | 5.5 oz | 7 |
| Other | ||
| NoDoz, maximum strength | 1 tablet | 200 |
| Excedrin | 2 tablets | 130 |
| Anacin | 2 tablets | 54 |
a Caffeine intake may vary due to plant variety and brand. Sources: Mayo Clinic, International Food Information Council, National Coffee Association, Center for Science in the Public Interest, National Soft Drink Association.
(From Lempert P. Watching caffeine intake? Look beyond coffee. “Before you bite column,” Los Angeles Times, Jan. 1, 2001; S.2 (Section: Health))
Patients with Ménière’s disease should be reassured that they can expect to live out normal lives in spite of their diagnosis. All too many of them have read horror stories on the internet or elsewhere that lead them to believe that the disease will leave them incapacitated, or deaf. The most disabling and frightening symptom of Ménière’s is vertigo, rather than hearing loss. Few patients will actually lose all serviceable hearing. In actuality, the symptom of vertigo is generally more responsive than hearing loss to medical treatment alone, including specific allergy therapy in appropriate patients. In our practice, 20% of Ménière’s disease sufferers do not obtain satisfactory control of vertigo by medical means, and require surgery such as endolymphatic sac surgery or vestibular nerve section, or chemical labyrinthectomy with gentamicin injected intratympanically. Amplification is often advisable. As hearing fluctuation is common, consideration of the use of programmable hearing aids is recommended.
The role of transtympanic steroid injection in the treatment of Ménière’s disease has yet to be defined. The senior author has tried various regimens, and has had best results using the technique recommended by Hamid of a compounded solution of 24 mg/ml of dexamethasone injected transtympanically per week for 3 weeks.41 It has been reported that approximately 30% of patients treated with transtympanic injections of dexamethasone will gain additional benefit over those treated with oral steroids alone.41 Consideration of allergy testing or rheumatologic evaluation for a possible steroid-sparing agent should be considered in the patient who requires frequent treatment with steroid, either oral or transtympanic, for control of their symptoms of hearing loss or vertigo. Although it is our observation that most patients with Ménière’s will obtain some temporary benefit from the use of steroids, steroid dependency is a relative indication for allergy testing in a patient with Ménière’s disease.
There may be a true organ-specific autoimmune response against inner ear antigens. However, most autoimmune diseases are not by themselves “pure” entities, but rather a mixture of different hypersensitivity reactions with the type named being the most predominant. The clinical and histologic evidence suggests that a classic type 1 hypersensitivity reaction, a food antigen type 3 circulating immune complex reaction, or both together may play a role in the production of endolymphatic hydrops.
By the same presumed mechanism described above, allergies can also play a role in the production of cochlear and vestibular hydrops.16
Sudden Sensorineural Hearing Loss
While most cases of sudden sensorineural hearing loss (SSNHL) are idiopathic, viral or vascular factors are often suggested as playing a role. McCabe first hypothesized that at least some cases of idiopathic SSNHL might be immune-mediated in etiology, as they were sometimes associated with other autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, or Wegner’s granulomatosis.42 Some studies have suggested a role for seasonal allergies in some patients with SSNHL, suggesting that viral-induced exacerbations in allergic and other inflammatory disorders might contribute to hearing loss.43,44 Lombardi et al reported a case history of recurrent steroid-responsive “SSNHL” in a male that occurred in April and May for several years which was always accompanied by symptoms of allergic rhinoconjunctivitis.45 Skin prick and in vitro testing confirmed sensitivity to olive and grass pollen, and aggressive prophylactic allergic management the following spring led to a lack of hearing loss.
Eosinophilic Otitis Media
Although not cochlear in origin, eosinophilic otitis media has been reported to sometimes result in sensorineural hearing loss and deafness.46 This entity is an intractable middle ear disease characterized by a characteristic yellow “peanut butter” viscous mucoid effusion containing eosinophils, edematous middle ear mucosa, and adult nasal allergy, often with associated nasal polyposis and asthma.47 While initially termed “allergic otitis media,” it is not always associated with type 1 hypersensitivity, despite the association with eosinophilic inflammation.48 While conductive hearing loss is always associated with this entity, 47% of those affected eventually develop sensorineural hearing loss, and 6% progress to deafness.49 The etiology of the sensorineural hearing loss is not clear, but it has been suggested that inflammatory mediators from activated eosinophils and other inflammatory cells are absorbed through the round window and produce damage to the cochlea.48
Management should be conservative, and largely consists of suctioning secretions on a regular basis. This can be technically challenging at times, as the secretions are unusually tenacious. PE tubes placed to aid aural suctioning will frequently become plugged and are of limited benefit. Systemic steroids will often give a short-lived improvement, with the topical administration of steroid and antibiotics only of marginal use. The risks of steroid toxicity in this chronic condition should be weighed against the benefit obtained.
Surgery is rarely indicated in this entity. Mastoidectomy is not only ineffective, but has been associated with both the development of sensorineural hearing loss and actual ossification of the cochlea. Successful cochlear implantation despite ossification has been reported in patients with eosinophilic otitis media, and should be considered early in those patients who become deaf.48 As the condition itself is intractable, long-term observation must be done in implanted patients because of the possibility of complications including meningitis.
Dizziness
Allergic patients may also complain of vague dizziness rather than true vertigo, or a sensation of floating.50 Typically, these are young adults with a normal neurotologic examination and audiogram. Their symptoms are usually perennial, but their complaint of dizziness is almost always accompanied by other symptoms suggestive of allergy, especially nasal congestion or chronic rhinitis.
The author has had a number of patients referred with a history of vague dizziness, aural fullness with or without associated hearing loss, and tinnitus who are suspected of being allergic but who are ultimately diagnosed as having a patulous (patent) Eustachian tube. While the presentation of chronic dizziness and aural fullness may suggest early endolymphatic hydrops, allergic Eustachian tube dysfunction, or the above-mentioned allergic dizziness, those with a patulous Eustachian tube will characteristically have symptoms that improve when lying in a supine position, or during times of an upper respiratory infection. They will also frequently report autophony and hearing their own breathing. The dizziness in the case of a patulous Eustachian tube is most likely related to a relative positive pressure in the middle ear space transmitted to the inner ear through the round window. Predisposing factors for the development of a patulous Eustachian tube include hormonal changes such as pregnancy and oral contraceptives; weight loss with or without chronic illness, and treatment with estrogen therapy in prostate cancer. A careful history, as well as physical examination of the patient in the sitting position using the microscope to assess tympanic membrane mobility with breathing will usually establish the diagnosis.
Tinnitus
Allergic patients may also complain of tinnitus. Two studies evaluating tinnitus in patients with other allergic symptoms severe enough to warrant skin testing found that nearly 40% of all such patients reported the presence of significant tinnitus.51 In one study, a tinnitus questionnaire was completed by a sample of allergy patients with tinnitus. Results indicated that tinnitus was rated as usually being of moderate or loud intensity, but did not interfere greatly in daily life. In the second study, patients with Ménière’s disease described their tinnitus before and after treatment for allergy.31 Ratings regarding the frequency of occurrence of tinnitus, the severity, and the interference with daily activities after treatment for allergy were significantly better than ratings describing tinnitus before treatment. Tinnitus appeared less severe in the treated patients than in a group of patients who did not receive treatment.
1 Agrup C, Luxon LM. Immune-mediated inner-ear disorders in neuro-otology. Curr Opin Neurol. 2006;19:26-32.
2 Brookes GB. Circulating immune complexes in Ménière’s disease. Arch Otolaryngol Head Neck Surg. 1986;112:536-540.
3 Derebery MJ, Rao VS, Siglock TJ, et al. Ménière’s disease: an immune complex-mediated illness? Laryngoscope. 1991;101:225-229.
4 Harris JP, Keithley EM, Ballenger S. Autoimmune inner ear disease. In: Snow JB, Ballenger JJ, editors. Otorhinolaryngology head and neck surgery. 16th edn. Hamilton, Ontario: BC Decker; 2002:396-407.
5 Harris JP. Immunology of the inner ear: response of the inner ear to antigen challenge. Otolaryngol Head Neck Surg. 1983;91:18-23.
6 Stearns GS, Keithley EM, Harris JP. Development of high endothelial venule-like characteristics in the spiral modiolar vein induced by viral labyrinthitis. Laryngoscope. 1993;103:890-898.
7 Hashimoto S, Billings P, Harris JP, Firestein GS, Keithley EM. Innate immunity contributes to cochlear adaptive immune responses. Audiol Neurootol. 2005;10:35-43.
8 Altermatt HJ, Gebbers JO, Muller C, Arnold W, Laissue JA. Human endolymphatic sac: evidence for a role in inner ear immune defence. ORL J Otorhinolaryngol Relat Spec. 1990;52:143-148.
9 Uno K, Miyamura K, Kanzaki Y, et al. Type I allergy in the inner ear of the guinea pig. Ann Otol Rhinol Laryngol. 1992;101(suppl 157):78-81.
10 Harris JP. Immunology of the inner ear: evidence of local antibody production. Ann Otol Rhinol Laryngol. 1984;93:157-162.
11 Tomiyama S, Harris JP. The role of the endolymphatic sac in inner ear immunity. Acta Otolaryngol. 1987;103:182-188.
12 Wackym PA, Friberg U, Linthicum FHJr, et al. Human endolymphatic sac: morphologic evidence of immunologic function. Ann Otol Rhinol Laryngol. 1987;96:276-281.
13 Gadre AK, Fayad JN, O’Leary MJ, et al. Arterial supply of the human endolymphatic duct and sac. Otolaryngol Head Neck Surg. 1993;108:141-148.
14 Duke WW. Ménière’s syndrome caused by allergy. JAMA. 1923;81:2179.
15 Powers WH. Allergic factors in Ménière’s disease. Trans Am Acad Ophthalmol Otolaryngol. 1973;77:22-29.
16 Powers WH, House WF. The dizzy patient – allergic aspect. Laryngoscope. 1969;79:1330-1338.
17 Viscomi GJ, Bojrab DI. Use of electrocochleography to monitor antigenic challenge in Ménière’s disease. Otolaryngol Head Neck Surg. 1992;107:733-737.
18 Gibbs SR, Mabry RL, Rolnad PS, et al. Electrocochleographic changes after intranasal allergen challenge: a possible diagnostic tool in patients with Ménière’s disease. Otolaryngol Head Neck Surg. 1999;121:283-284.
19 House JW, Doherty JK, Fisher LM, Derebery MJ, Berliner KI. Ménière’s disease: prevalence of contralateral ear involvement. Otol Neurotol. 2006;27:355-361.
20 Harris JP, Ryan AF. Fundamental immune mechanisms of the brain and inner ear. Otolaryngol Head Neck Surg. 1995;112:639.
21 Derebery MJ, Berliner KI. Prevalence of allergy in Ménière’s disease. Otolaryngol Head Neck Surg. 2000;123:69-75.
22 Derebery MJ, Berliner KI. Characteristics, onset and progression in Ménière’s disease. In: Proceedings of the 5th International Symposium Ménière’s disease and Inner Ear Homeostasis, Los Angeles, House Ear Institute, 2005:128–130.
23 Sen P, Georgalas C, Papesch M. Co-morbidity of migraine and Ménière’s disease – is allergy the link? J Laryngol Otol. 2005;119:455-460.
24 Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43(suppl 3):S16-S20.
25 Yazawa Y, Kitano H, Suzuki M, Tanaka H, Kitajima K. Studies of cochlear blood flow in guinea pigs with endolymphatic hydrops. ORL J Otorhinolaryngol Relat Spec. 1998;60:4-11.
26 Kimura RS, Schuknecht HF. Membranous hydrops in the inner ear of the guinea pig after obliteration of the endolymphatic sac. Pract Otorhinolaryngol. 1965;27:343-354.
27 Yang WH, Dorval G, Osterland CK, et al. Circulating immune complexes during immunotherapy. J Allergy Clin Immunol. 1979;63:300-307.
28 Keles E, Godekmerdan A, Kalidag T, et al. Ménière’s disease and allergy: allergens and cytokines. J Laryngol Otol. 2004;118:688-693.
29 Stahle J, Deuschl H, Johansson SG. Ménière’s disease and allergy, with special reference to immunoglobulin E and IgE (reagin) antibody in serum. Int J Equilib Res. 1974;4:22-27.
30 Derebery MJ, Valenzuela S. Ménière’s syndrome and allergy. Otolaryngol Clin North Am. 1992;25:213-224.
31 Derebery MJ. Allergic management of Ménière’s disease: an outcome study. Otolaryngol Head Neck Surg. 2000;122:174-182.
32 Derebery MJ, Berliner KI. Allergic management in Ménière’s disease: a prospective study. Presented at the American Academy of Otolaryngic Allergy annual meeting, Orlando, Florida, September 19, 2003.
33 Yang X. Does allergen immunotherapy alter the natural course of allergic disorders? Drugs. 2001;61:365-374.
34 Dale HH, Laidlaw PP. The physiological action of β-iminazolylethylamine. J Physiol. 1910;41:318-344.
35 Rask-Andersen H, Bredberg G, Stahle J. Structure and function of the endolymphatic duct. In: Vosteen KH, Schuknecht H, Pfaltz CR, et al, editors. Ménière’s disease: pathogenesis, diagnosis and treatment. New York: Thieme-Stratton; 1981:99-109.
36 Hebbar GK, Rask-Andersen H, Linthicum FHJr. Three dimensional analysis of the endolymphatic ducts and sacs in ears with and without Ménière’s disease. Ann Otol Rhinol Laryngol. 1991;100:2215-2225.
37 Yan Z, Wang JB, Gong SS, Huang X. Cell proliferation in the endolymphatic sac in situ after the rat Waldeyer ring equivalent immunostimulation. Laryngoscope. 2003;113:1609-1614.
38 Leone CA, Feghali JG, Linthicum FHJr. Endolymphatic sac: possible role in autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1984;93:208-209.
39 Harris JP, Sharp PA. Inner ear autoantibodies in patients with rapidly progressive sensorineural hearing loss. Laryngoscope. 1990;100:516-524.
40 Shambaugh GEJr, Wiet RJ. The endolymphatic sac and Ménière’s disease. Otolaryngol Clin North Am. 1980;13:585-588.
41 Hamid MA. Intratympanic dexamethasone perfusion in Ménière’s. Presented at the spring meeting of the American Neurotology Society, Palm Desert, CA, May 2001:12.
42 McCabe BF. Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1979;88:585-589.
43 Preyer S. [Effect of weather on the incidence of sudden deafness.]. Laryngorhinootologie. 1996;75:443-446. (in German).
44 Dornhoffer JL, Arenberg JG, Arenberg IK, Shambaugh GEJr. Pathophysiological mechanisms in immune inner ear disease. Acta Otolaryngol Suppl. 1997;526:30-36.
45 Lombardi C, Tansini A, Passalacqua G. Seasonal sensorineural hearing loss associated with allergic rhinitis: a case report. J Allergy Clin Immunol. 2006;117:468-469.
46 Nagamine H, Iino Y, Kojima C, Miyazawa T, Iida T. Clinical characteristics of so called eosinophilic otitis media. Auris Nasus Larynx. 2002;29:19-28.
47 Matssutani S, Kobayashi T, Takasaka T. Eosinophilic otitis media. Otolaryngol Head Neck Surg Tokyo. 1995;67:712-713.
48 Iwasaki S, Nagura M, Mizuta K. Cochlear implantation in a patient with eosinophilic otitis media. Eur Arch Otorhinolaryngol. 2006;263:365-369. Epub 2005 Dec 3.
49 Suzuki H, Matsutani S, Kawase T, et al. Epidemiologic surveillance of “eosinophilic otitis media” in Japan. Otol Jpn. 2004;14:112-117.
50 Powers WH. Allergic phenomena in the inner ear. Otolaryngol Clin North Am. 1971;4:557-564.
51 Derebery MJ, Berliner KI. Allergic aspects of tinnitus. In: Reich FE, Vernon HA, eds. Proceedings of the Fifth International Tinnitus Seminar. Portland, OR; 1995:447.