Chapter 11 Management of the Patient with Drug Allergy
Introduction
▪ Epidemiology
Management of a history of adverse reactions to drugs is challenging for both the physician and the patient. Almost all persons will have some sort of adverse drug reaction during their lifetime. Adverse drug reactions are responsible for one in twenty hospital admissions and occur in nearly a fifth of hospitalized patients.1 Fifty-nine percent of drug-related hospitalizations are preventable.2 Patients experiencing adverse drug reactions are more likely to stay in the hospital longer, 10.6 verses 6.8 days in one study, and their hospital costs are over US$5000 higher.3 Adverse drug reactions are responsible for 106 000 deaths per year.4 This makes them the fourth leading cause of death of hospitalized patients, and is even likely a very low estimate given underreporting and misdiagnosis of cause of death.5 This is even more of a tragedy as many of them are preventable and can be avoided through education and correction of systems errors. Any drug can cause an adverse drug reaction, but only a few drugs have been found to cause the majority of severe immune reactions. By becoming familiar with adverse drug reactions, physicians can protect the safety of their patients without unnecessarily withholding vital and recommended treatments.
▪ Risks
Certain medications have been implicated more frequently than others in allergic adverse drug reactions. There are particular risk factors that make an exposure to a drug more likely to lead to a clinically significant reaction (Table 11.1).6 The underlying chemical properties of a drug may make it more likely to become antigenic by reaction with host tissue, for instance by acting as a hapten and complexing with a native protein. Concurrent illness may lead to a drug reaction that would otherwise not occur. For example, almost all cases of Epstein–Barr virus infection treated with a course of amoxicillin lead to a characteristic drug rash.7 This rash does not recur on readministration of the medication when the patient is no longer experiencing their active, primary infection. The molecular weight of a drug is also an important factor. Low molecular weight drugs are likely not immunogenic on their own without haptenizing. This includes most medications. However, newer biologic agents such as monoclonal antibodies, are often complex protein structures with a high molecular weight and are capable of inducing an immune response on their own. The route of administration may make a patient more likely to have a drug reaction. The oral route and the subcutaneous routes are less risky in most cases, versus the IV route of the same medication. When a patient is receiving a repeated course of a medication they may also be more likely to react. The likely mechanism for this type of reaction involves sensitization through antigen presentation to the immune system during the first course (sensitization), followed by faster more severe response with a second dose due to immunologic memory. Patients may also be more likely to experience a drug reaction if they have had a prolonged course at a high dose of a particular drug therapy.
| Risk factor | Proposed mechanism/example |
|---|---|
| Chemical structure |
Atopy significantly increases the risk of both having reactions and having more serious reactions. Female gender is associated with a one-third higher risk of cutaneous reaction verses male counterparts. Extremes of age, the very young and the elderly have fewer allergic drug reactions.6 The mechanisms that predispose an individual to adverse effects are related to the method by which a certain patient’s immune system responds to an antigen and determines what is “foreign,” and to what degree immunologic tolerance exists. Interestingly, HLA genetic polymorphisms are being identified in the population that may make one patient more likely to experience a reaction to a drug verses another patient. This exciting new field of pharmacogenetics may eventually make it economically and clinically feasible to prescreen patients by a laboratory test prior to treatment with medications in order to minimize adverse reactions.7
Classification
One must distinguish between known side effects or symptoms caused by a drug, or toxicity to the drug, termed type A reactions, and those that are more unusual and not anticipated in the general population based on the drug’s properties, termed type B reactions. An example of type A reactions would be tinnitus with high dose aspirin or nausea after taking an antibiotic. Type B reactions include allergic, pseudoallergic, and idiosyncratic drug reactions. Only type B reactions will be discussed in detail below and will be the focus of this text. Approximately 25% of adverse drug reactions can be classified as type B reactions.
▪ Pseudoallergic reactions
These reactions often include symptoms that are indistinguishable clinically from true IgE-mediated allergic reactions, including hives and life-threatening collapse of the respiratory and circulatory systems. Medications commonly implicated in pseudoallergic reactions are listed in Box 11.1. These reactions are usually non-IgE-mediated mast cell or basophil mediator release.8 No previous period of sensitization to the medication is required. Pseudoallergic reactions are typical of drug reactions to opiates, colloids, some nonsteroidal medications, radiocontrast media, and some antibiotics. The treatment of a pseudoallergic reaction is the same as that for allergic and anaphylactic reactions (see below). In the case of opiates, especially when clinically necessary as in palliative care, the physician often may treat through the reaction with H1 and H2 blockers or steroids. More severe reactions are rarely seen, and tolerance seems to develop just as it does to other side effects and therapeutic effects of the narcotic. Not all narcotics or synthetic narcotics have a similar risk of inducing pseudoallergic reactions. Morphine and codeine are frequently involved while fentanyl is not.
BOX 11.1 Causes of pseudoallergic drug reactions
Medications associated with pseudoallergic reactions
Radiocontrast media (higher osmolar especially)
There is a 1 in 50 incidence of having a pseudoallergic reaction to radiocontrast media. Even though the reactions to radiocontrast media are pseudoallergic reactions, they should be taken seriously as they may be severe and are fatal in up to 1 in 50 000 events. Patients that react to radiocontrast media have a high likelihood, up to 44%, of having a reaction again on a second exposure. Premedication with antihistamines such as diphenhydramine and corticosteroids starting 12–24 hours before the procedure combined with the use of newer, lower osmolar contrast agents may minimize the chance of subsequent reaction to 1%.9,10 This risk is not zero, however, as evidenced by a report of anaphylaxis despite premedication, thus patients should be apprised of the risks with informed consent prior to procedures.11 While more controversial, oral ephedrine and oral albuterol may also be considered as prophylaxis in these cases of reaction to radiocontrast media. The physician must recall that radiocontrast media is also used in myelograms, hystosalpingograms, and retrograde pyelograms. Atopic individuals, those with asthma, chronic obstructive pulmonary disease, unstable heart disease using beta blockers, and those with previous adverse events to contrast in the past, are considered at risk of adverse events when receiving radiocontrast.12,13 In contrast to a popular myth, radiocontrast does not cross-react with seafood or iodine.
Administration of vancomycin may be accompanied by erythema, flushing, and allergic-like symptoms. Known as red man’s syndrome, this type of reaction does not reflect true allergy and can usually be managed by slowing the infusion rate and using premedication such as antihistamines.14 However, although very rare, IgE-mediated anaphylaxis to vancomycin may occur. Pseudoallergic reaction to iron dextran has also been reported, and for patients who require this medication, a successful graded challenge protocol has been described.15
▪ Idiosyncratic Reactions
Most reactions to aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) fit into this category, although true IgE-mediated reactions to isolated agents do occur rarely.16 By non-IgE-mediated reactions, aspirin and NSAID reactions may manifest as urticaria, angioedema, wheezing, nasal congestion, or may trigger recurrence of urticaria and angioedema in a person with chronic idiopathic urticaria. The mechanism to explain why patients with aspirin allergy, nasal polyps often leading to sinusitis, and asthma, which make up Samter’s triad, is also unknown.17 In those patients with Samter’s triad, a decrease in prostaglandin E2 (PGE2) and an increase in leukotrienes may be found, implicating a mechanism of action based on the cyclooxygenase and lipoxygenase pathways following breakdown of arachidonic acid.18 Involvement of this pathway also explains cross-reactive drug reaction with high dose acetaminophen in a third of these individuals. There is no value in skin testing these patients, since the mechanism is not IgE-mediated. These patients may respond to antileukotriene medications such as montelukast and zafirlukast as well as the 5-lipoxygenase inhibitor, zileuton. These patients with aspirin sensitivity may respond to graded challenge to aspirin as described below. Graded challenge leads to improved quality of life, improved asthma, less need for polypectomy and sinus surgery, reduced symptom of congestion and decreased emergency room visits and hospitalizations. Graded challenge protocols or desensitizations are usually not effective in patients with aspirin-induced urticaria or angioedema.19
Another idiosyncratic reaction is angioedema to ACE inhibitors. Up to a quarter of patients on ACE inhibitors may develop a persistent cough that may last for weeks after the drug is discontinued. This is more common in females and more likely to occur early during therapy.20 Angiotensin receptor blockers have not been associated with cough. Angioedema secondary to ACE inhibitors is much less common, but can be serious, with one-third of patients hospitalized as a result and one tenth requiring intensive care21 (Figure 11.1). Physicians must have a high degree of suspicion to diagnose this condition, as it may occur after a patient has been asymptomatically on the medication for months to years. Unlike in the case with cough, angioedema to angiotensin receptor blockers has been described.22
▪ Immune Mediated
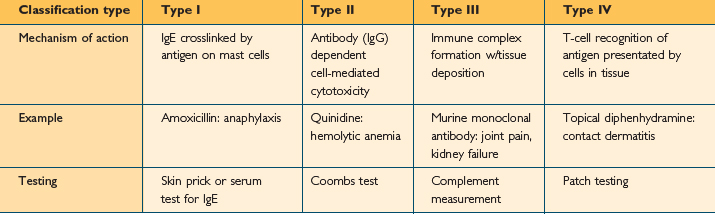
Many, although not all, type B reactions with an immune basis can be classified using the Gell and Coombs classification23 (Table 11.2). Under this classification, reaction types are numbered I through IV based on the immune system components thought to be responsible. This may be confusing, as a single drug can lead to different reactions in different patients, or the same patient, each of which may fit a different Gell and Coombs type. Also, this classification is for discussion and the reactions rarely occur in isolation. For instance, penicillin has been associated with reactions in each classification. Furthermore, the mechanism for many drug reactions is unknown, and other reaction types such as “typical maculopapular drug rash”, do not fit neatly into the classification scheme. However, the Gell and Coombs classification is frequently utilized in the literature to categorize drug reactions on an immunologic basis. Therefore physicians should have an understanding of its classes as well as examples of each type.
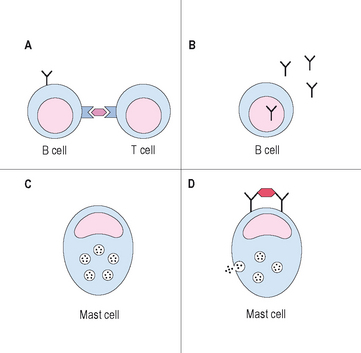
Type I reactions are mediated by IgE and are the reactions that physicians usually consider to be true drug “allergies.” Prior exposure and sensitization is necessary in order to develop IgE. It is thought that either the medication or one of its metabolites is presented by MHC class II complexes on antigen presenting cells. As many drugs have low molecular weights, they need to covalently bond to self proteins to be immunogenic, a process termed haptenizing.24 If the antigen presenting cell containing the drug/hapten complex is a B cell, and is recognized by a MHC/antigen-specific T cell in the presence of the correct cytokine milieu, usually IL-4 and IL-13, the B cell will be activated, leading to IgE production. On second exposure to the drug, drug-specific IgE molecules on mast cells may become cross-linked, leading to degranulation of mast cells and then the typical symptoms consistent with an allergic reaction (Figure 11.2). An example of a type 1 reaction would be the development of urticaria and anaphylaxis immediately following infusion of IV ampicillin, in a patient that had received the medication on a previous hospitalization without difficulty. In addition to the early phase of type 1 reactions, which are mainly secondary to histamine release from mast cells and basophils, a late phase may occur 3–6 hours later. This late phase is secondary to the influx and activation of inflammatory cells, such as the eosinophil, and may cause worsening symptoms hours after the initial event. Type 1 reactions may be evaluated by skin testing under the guidance of an allergist and often can be treated by desensitization protocols if necessaary.
Type 2 reactions are caused by antibody-dependent, cell-mediated cytotoxicity. The antigens, in this case the drug or its metabolite, becomes associated with cells or extracellular matrix proteins. IgG antibodies form against the antigen, in a mechanism similar to, although not identical to, the mechanism described above for IgE antibody formation. These IgG antibodies bind the drug/cell or drug/matrix complex. Complement fixation occurs and effector cells such as phagocytes and natural killer recognize the reaction by binding the Fc portion of the IgG, and lead to cytotoxic events that destroy surrounding tissue. Symptoms depend on the tissue or cells involved. An example of this type of reaction would be immune-mediated, hemolytic anemia causing destruction of red blood cells after taking penicillin or a related compound. Quinidine and alpha-methyldopa have also been implicated in this type of reaction.25,26 Thrombocytopenia is frequently caused by this mechanism as well.
Type 3 reactions are usually due to the formation of immune complexes, containing IgM or IgG plus complement and a soluble antigen. This reaction is most likely to occur in cases of slight antigen excess, often a few weeks after the last dose of the medication. These complexes deposit in specific bodily tissues and when complement fixation or effector cell mediator release occurs, the organs or tissues into which the complexes have lodged become damaged, as innocent bystanders. Examples of this type of reaction include the Arthus’ reaction and serum sickness. This class of drug reaction may become more clinically relevant as more monoclonal antibodies containing mouse and other species’ proteins are marketed.
▪ Others
Still, many other reactions are not well described by the Gell and Coombs classification table. Following multiple drugs, especially antibiotics, patients develop a morbilliform rash (Figures 11.3, 11.4) or bullous lesions, days after the medication has been started. Organ damage through fibrosis and inflammation occurs with broad medication classes and risk cannot be predicted for an individual patient. The lungs are at particular risk for drug reactions, and depending on the offending medication, almost every pathologic type of lung disease may occur. Sulfonamides, NSAIDs, and others may cause an acute pneumonitis picture. Narcotic drug abusers may develop a noncardiac pulmonary edema. Churg–Strauss vasculitis has been associated with the withdrawal of leukotriene receptor antagonists secondary to the ability to withdraw corticosteroids in asthmatic patients on these medications.27 Methicillin and sulfonamides are classic as causes of interstitial nephritis. Urine eosinophils and increased IgE may be found and may aid in diagnosis in these cases.28 Kidney biopsy is likely not necessary and function often returns to normal after drug discontinuation. Sun-induced rash is described with various antibiotics and some diuretics, among other medications. The frequently used medication for Parkinson’s disease, carbidopa/levidopa, is associated with a hypersensitivity pneumonitis. This may be an important association to keep in mind for those in geriatric medicine or who care for elderly patients. A spectrum of diseases, from erythema multiforme to Stevens–Johnson syndrome to toxic epidermal necrolysis (TEN), occurs most commonly following sulfonamides but also with other drugs. Stevens–Johnson syndrome has less than 10% involvement of the mucous membranes, while TEN has >30% mucosal involvement.
Pathophysiology
On a cellular level, an understanding of antigen presentation and involved cells and cytokines in relation to drug allergy is still being determined. Processing of drugs in a certain way by keratinocytes in the skin may have a role, especially as cutaneous manifestation of drug allergy is the most common symptom. T-cell response is critical.29 In many cases, the reaction is specific, as can be demonstrated by drug-specific T-cell response to sulfonamide antibiotics. Depending on the way in which a drug is processed, T-cell response to that particular agent may be secondary to CD4 or CD8 pathways, or to both pathways. The familiar maculopapular morbilliform rash seen in reaction to beta lactam antibiotics is associated with CD8+ T cells with interleukin (IL) 2 and interferon gamma production.30 In an immediate reaction to penicillin, CD4 cells are participating and IL-4 is elevated. As there are alternative methods of inducing immmunogenicity, neither pathway could be involved.
Beta lactam containing antibiotics, such as penicillin, are the most common culprits in IgE-mediated drug reaction in the USA. Up to 10% of hospitalized patients report a penicillin allergy. Each dose of penicillin is associated with a 0.01–0.05% chance of reaction.31 Beta lactam antibiotics are also responsible for the majority of drug-related deaths, causing 75% of fatal anaphylaxis events in the USA each year.32 A person’s IgE to penicillin declines over time.33 It is absent in 70% of adults with documented penicillin allergy upon retesting 10 years later. Perhaps this explains why greater than 80% of patients with a historical penicillin allergy are negative on evaluation.34–36 Patients more likely to retain skin test positivity to penicillin include those with a history of anaphylaxis, 46% positive on skin testing, versus those with hives and swelling, 15% of whom remain positive.37
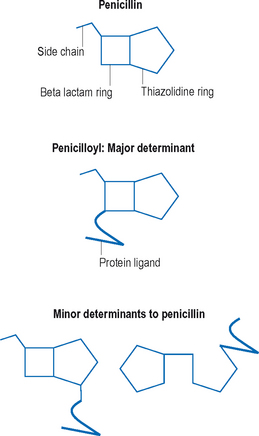
As the name suggests, beta lactam antibiotics are composed of a beta lactam and a thiazolodine ring. The beta lactam ring is unstable and opens easily. Unlike many other medications, metabolism of penicillin is not necessary for haptenization, the parent compounds are capable of binding serum proteins on their own. Amide linkages are formed with the lysine structures on self-proteins, leading to the major determinant, penicilloyl.38 Minor determinants form through haptenization with carboxyl and thiol groups39 (Figure 11.5). Specific IgE-binding sites have been identified on penicillin.40 Between the major and minor determinants, the majority of IgE-mediated reactions can be explained. Other reactions may be due to side chains, and this may be an important source of cross-reactions with some of the cephalosporin group.40
Drug reactions to other classes of antibiotics are less common than those to the beta lactams, and compromise approximately 3% of drug reactions. Trimethaprim-sulfamethoxazole (TMP/SMX) is a common cause of hypersensitivity reaction and occurs in up to 4% of healthy individuals and half of all individuals with HIV infection. Drug reaction to TMP/SMX usually involves the aromatic amine at the N4 ring position. There is also an N1-substituted ring. Sulfonamides are usually metabolized via N-acetylation to form relatively inactive metabolites. They may also undergo N-oxidation to reactive metabolites, some of which may be reduced by glutathione reductase.41 Problems with cell glutathione levels may explain the tendency for some individuals, such as HIV-infected patients, to react not only to sulfonamides but also to other medications that utilize these pathways.42 As all active metabolites for drug reaction are not know, skin testing is not predictive of future response in patients with TMP/SMX reaction. As nonantibiotics, sulfa-containing medications do not have this structure, and the chance of cross-reaction between them is very low and has not been convincingly described.
Presentations may vary, and this is another medication that through varied immune processing and presentation can cause various cutaneous and extracutaneous features. Reaction to TMP/SMX can include a morbilliform rash, which often develops between 1 and 1.5 half weeks after starting the medication. TMP/SMX is a common cause of Stevens–Johnson syndrome and mucocutaneous lesions are an absolute contraindication to repeated use of the drug. The metabolic machinery of a particular patient may put them at risk, as slow acetylators of TMP/SMX may be more likely to have mucocutaneous complications. A significant portion of HIV-positive patients develops a drug reaction to sulfonamides.43 Even many HIV patients without clinical symptoms of drug reaction have measurable IgG and IgM antibodies toTMP/SMX. The utility and predictive value of these antibodies is unknown.
Drug allergy to other antibiotic classes such as aminoglycosides and macrolides is less frequent. Telithromycin, although a new class of medications, may have cross-reactivity with those with drug reactions to related macrolides. Drug reaction to quinolones, topoisomerase inhibitors, are associated with cutaneous lesions in 2% of people on the first dose, responding to an unknown mechanism.44 There is usually cross-reactivity within the quinolone class of antibiotics, with allergy to one medication in the class eliminating other members for use.45 Skin testing to these medications is not standardized.
IgE-mediated reactions have been described to several anticancer therapeutics, such as L-asparaginase, doxorubicin, and cisplatin and carboplatin. Skin testing to the platins has a 91% negative predictive value.46 Desensitizations to these medications have been performed, but success is variable.47 Prior to the use of L-asparaginase, some chemotherapy protocols call for an intradermal skin test placement, as the risk of reaction to this drug may be greater than 10%. Other frequently used products, like Cremophor El, the solvent for paclitaxel, have been implicated in anaphylactoid reactions. Bleomycin, busulfan and cyclophosphamide are implicated in cytotoxic pulmonary fibrosis, but are unlikely culprits in IgE-mediated disease.48 Methotrexate may be associated with an acute presentation of fever, cough, and shortness of breath months after the medication was started. Again, this reaction is unlikely to be IgE mediated. As long as the diagnosis is made and the medication discontinued in time, diffuse infiltrates usually clear from chest X-ray.49
Perioperative drug reactions are also common. Muscle relaxants are the most common agent involved in an IgE-mediated reaction during anesthesia. They are responsible for one reaction in 4500 cases. Some muscle relaxants may be potent histamine releasers, but IgE has been demonstrated as well to muscle relaxants.50 Induction agents, like thiopental, should also be considered. During investigation of perioperative anaphylaxis, not only anesthetic agents should be evaluated, but also latex and prophylactic antibiotics should be considered in the differential. “Allergic” reactions to local anesthetics, although a frequent cause of referral to the allergist’s office, are not likely to be secondary to IgE mediation, but rather to vasovagal reactions or toxic reactions following accidental injection into the vascular system.51 Local anesthetics belong to one of two classes, the amides and the esters. Despite past belief, there may be cross-reaction both within and between classes. Testing is usually conducted by a graded challenge, if prick testing to full strength material is negative. Intracutaneous doses of 0.1 ml of dilutions starting at 1 : 1000 or 1 : 100 of multiple local anesthetic agents should be tested.
Reaction to blood products during transfusion may be secondary to the presence of anti-IgA antibodies in IgA-deficient patients reacting to IgA in the product. Patients with diabetes, on neutral protamine hagedorn (NPH) insulin, who undergo coronary artery bypass surgery, are at risk for drug reaction to the protamine given to reverse the effects of heparin at the end of the procedure. This risk is elevated 50 times in these patients as a significant portion of them have become sensitized to protamine through their NPH insulin product.52 Diverse reactions to heparin have been described, from urticaria, increased eosinophils and anaphylaxis to thrombocytopenia.53 The latter reaction may be secondary to IgG antibody formation to platelet factor 4. Cross-reactivity with low molecular weight heparin exists, but cross-reaction to synthetic products are minimal.
Diagnosis
▪ History and Physical
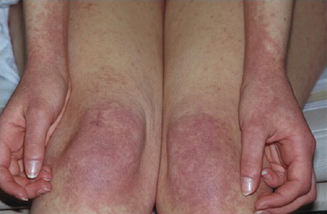
The importance of a thorough history and clinical evaluation cannot be underestimated in the evaluation and treatment of these patients. Diagnosis can be difficult as signs and symptoms are often varied and nonspecific. Cutaneous manifestations are the most common presentation of drug allergy. Patients may present with any of a number of rashes that may include, but are not limited to, wheal and flare lesions (Figures 11.6–11.8), macules, papules, bullous lesions, mucosal erosion, photosensitivity reactions, generalized erythema, target lesions, lichenification and morbilliform eruptions, purpura, or pruritis alone. Often, drug-induced exanthemas begin in dependent areas and then generalize as they progress. For the majority, symptoms occur 7–10 days following initiation of the medication, although may occur sooner when it is the second exposure to a drug.
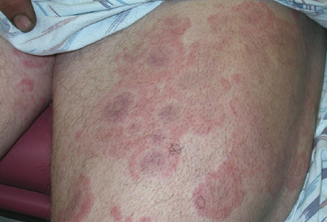
Figure 11.8 Urticaria 3. A close up of the urticaria from the patient in Figure 11.6. Some of the lesions almost appear to have a bruise, which may associate with accompanying drug-induced vasculitis.
Fixed drug eruptions are another common presentation, especially with sulfonamides and tetracycline antibiotics, NSAIDs, and salicylates. These reactions are so named because postinflammatory pigment occurs, and a recurrence of a reaction on reexposure is in an identical location to the first reaction (Figure 11.9). Face and genitals are frequently affected. Lichenoid drug eruptions, resembling lichen planus with extreme pruritis and characteristic violaceous papules, is associated with multiple antihypertensive agents such as beta-blockers, ACE inhibitors, and methyldopa as well as quinidine and NSAIDs (Figure 11.10). Mucosal involvement is frequent in this disorder.54
History should include the symptom of worsening on exposure to sun, as the combination of the drug with sunlight can lead to phototoxic and photoallergic reactions. In the phototoxic reaction, the drug absorbs certain bands of ultraviolet light, which is then released into tissues with cytotoxic effects, manifesting as sunburn. NSAIDs and tetracycline are classic examples. In photoallergic reactions, light biologically alters the drug or its metabolite to a more immunogenic compound which can then stimulate a lymphocytic response. Symptoms may be similar to eczema. Topical agents, their preservatives, and fragrances are often responsible.55
Factors that should be included in the history, besides presence or absence of the symptoms mentioned above, include concomitant medications, timing of the reaction in regards to medication start date as well as time elapsed between administration of last dose and development of symptoms. IgE-mediated reactions tend to occur immediately to within an hour of administration. A history of past reactions to medications is also of importance to determine prior reactions to cross-reactive drugs, as in the case of penicillin and cephalosporin allergy, and to determine if the person had the medication in the past and could therefore have become sensitized. Sometimes, even in the case of a clearly IgE-mediated reaction, no history of prior administration can be found. Such an individual may have had the medication as a small child or had a cross-reacting medication. Still, some allergic drug reactions have been described even with first administration. Reaction to ciprofloxacin on first administration is an example.44
There may also be a family history of specific drug reaction that is heritable and clinically relevant in your patient. The enzyme epoxide hydrolase, responsible for the metabolism of arene oxide intermediates, may predispose patients on certain anticonvulsant medications to drug adverse events. Patients taking phenytoin, phenobarbital, or carbamazapine, among other drugs, and who lack this enzyme or are slow metabolizers, may present with a syndrome including fever, rash, lymphadenopathy, hepatosplenomegaly, and end organ damage.56 With phenytoin, this occurs in 1 : 1000 to 1 : 10 000 patients, usually 2–6 weeks after starting the medication. These patients should not receive the offending anticonvulsant or related anticonvulsants given the risk of morbidity and mortality with reexposure. On reexposure, the reaction may be more severe and rapid in onset. As the trait is passed down generation to generation in an autosomal dominant, variably expressed pattern, family members of a patient who has experienced a reaction should also avoid the medication and related compounds. Similarly, there is a hereditary basis secondary to altered drug metabolism of the cephalosporins cefaclor and cefprozil. Affected individuals may develop a serum sickness-like reaction when taking one of these two medications. There does not seem to be an antibody basis for this reaction.57 While little evidence exists for the so-called “multidrug allergy syndrome,” certain patients may have a higher likelihood of having a reaction to a second medication versus the general population.58 However, others have presented contradictory evidence refuting this claim.59
▪ Testing
Skin testing for IgE-mediated reaction to penicillin and to insulin have well standardized testing protocols for evaluation of drug allergy. Skin testing to local anesthetics also has a very good negative predictive value, and can be used to exclude IgE-mediated reactions. Skin testing is only helpful in evaluating IgE-mediated reactions. In the case of penicillin, patients should be tested to major and minor determinants. The major determinant, penicilloyl, conjugated with polylysine and known as PrePen, is not currently available due to a manufacturing issue in the USA, although through the efforts of lobbying groups this will hopefully be remedied in the future. Testing with only the major determinant will miss up to 10–20% of penicillin allergy. Minor determinants of penicillin, unlike most other drugs, have been classified and include benzyl penicilloate, benzyl penilloate, and benzyline propylamine. Minor determinant mixtures are available for testing in some specialized laboratories, but generally penicillin G is used for testing. However, there is a minute chance that a few patients with true allergy to penicillin may be missed with penicillin G. To perform skin testing, an epicutaneous test to the full strength product is placed along with a positive and negative control, followed by intracutaneous testing of the diluted products.60
Chances of a positive skin test to penicillin vary by history of particular reaction to the drug. A positive skin test was found in up to 46% of patients with a history of anaphylaxis, 12% with a history of urticaria, 4% with a history of exanthem, and 1.7% of the population with no penicillin allergy by history.61 Other studies suggest that there was no higher incidence of positive skin testing in someone with history of only a maculopapular rash versus the population in general.62 A positive skin test for penicillin is associated with a 50% chance of having a clinical reaction upon administration of the drug. Therefore, in the case of positive skin test to penicillin, an alternative choice should be made, or in the case of no alternative, desensitization to penicillin should be performed (see below). The negative predictive value of penicillin skin testing with PrePen, minor determinants, and penicillin G approaches 99%.60 The risk for these patients of reacting to penicillin administration is no greater than that of the population at large. More importantly, no anaphylaxis has been reported in patients who have tested negative to appropriate penicillin skin testing.63 Furthermore, meta-analysis has also suggested a high negative predictive value for cephalosporin reactivity following negative skin testing to penicillin. Among patients with a history of penicillin allergy by this meta-analysis, 4.4% of penicillin skin test positive patients reacted to a cephalosporin on challenge versus only 1.3% positivity to cephalosporin when penicillin skin testing was negative.64 The skin testing procedure for patients with suspected penicillin allergy is considered safe. Poor outcome, such as anaphylaxis or death from the testing, is usually associated with failing to do prick tests first or starting with intracutaneous placement of more potent product. Despite concerns by some that testing with penicillin would sensitize a patient, theoretically placing them at risk for allergic reaction when exposed to the medication with a future therapeutic course, these fears have not been realized. Resensitization after negative testing and successful drug administration is also unknown, but theoretically possible. If skin test is negative, but suspicion is high, skin testing should be repeated before initiation of a second course of therapy. If a patient has a history of a severe reaction by history, a positive skin test, but has successfully received a course of penicillin after desensitization and needs a second, desensitization should be performed again.
Skin prick testing to other antibiotics besides penicillin, including skin testing to cephalosporins, is not standardized. False positive reactions can occur, especially at the higher concentrations, secondary to an irritant response. False negatives may also occur, as all the potentially active metabolites of drugs other than penicillin have not been characterized. This is especially the case with ciprofloxacin and vancomycin, the latter of which is associated with false positive irritant responses at concentrations of 100 μg. For skin prick testing to antibiotics such as cephalosporins, if this approach is to be used, 3 mg/ml is usually a nonirritating dose for the prick test, followed by intracutaneous testing to dilutions. There can be no guarantee that a patient will not have a clinically significant reaction even with a negative response to these medications with skin testing. However, Romano et al studied 128 adults with penicillin reaction and at least one positive on skin prick testing to penicillins. He then performed skin prick testing at 2 mg/ml to various cephalosporins: cefuroxime, ceftriaxone, ceftazidime, and cefotaxime. Patients with no positives on the cephalosporin skin testing were challenged with ceftriaxone and cefuroxime.65 Of 101 patients that met criteria and underwent the challenge, all tolerated these two cephalosporins, suggesting that those with negative penicillin and cephalosporin skin testing may be given cephalosporins with minimal risk. Several smaller studies have corroborated these findings.
The mechanism of action for insulin allergy is usually IgE. IgE may be responsible for local reactions as well as systemic symptoms of anaphylaxis.66,67 Therefore, skin prick testing to insulin preparations, in order to find the least allergenic for a particular individual, is very practical in the case of insulin allergy. Local reactions at injection sites are usually easily managed, just as one would premedicate for local reactions with allergy immunotherapy with antihistamines or steroids if necessary.66 Desensitization has been successful in treating anaphylactic reactions.67 The medication should not be discontinued. If a patient has systemic reaction to insulin, one-third to one-half of the dose should be given at the next scheduled dosing time, with subsequent desensitization to the full, desired dose.
If a reaction may be allergic, but a question still persists, then tryptase serum levels done at the time of the reaction may help in the differential. An elevated total serum tryptase level may also be measured, indicating the reaction was likely mediated by mast cell releasing mechanism.68 Unlike serum histamine, which is elevated only briefly, serum tryptase peaks in 1–2 hours and is often elevated for several hours, allowing an adequate window for clinical capture. It should be transported for evaluation on ice, immediately after blood draw, to obtain the most accurate result. N-methyl histamine excreted in the urine is less practical to collect.
Patch testing may aid in evaluation of contact dermatitis to medications such as topical antibiotics, topical steroids, or eye drops. Patch testing involves placement of titrated amounts of suspected substances or chemicals as well as their components on the skin with a dressing for 48 hours. After removal of the dressing, response is graded based on degree of erythema, papules, or vesicles to aid in identifying the responsible agent and making a diagnosis. The predictive value of patch testing, especially for the evaluation of noncontact dermatitis related drug reactions, is not known. Some work has been done on amoxillin and ampicillin patch testing in the evaluation of more delayed drug reaction to these medications. In one study, patch test negative patients tolerated an oral challenge.69
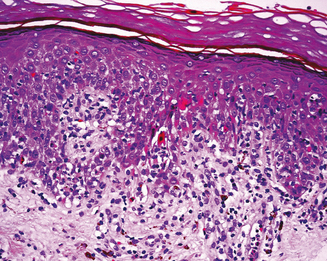
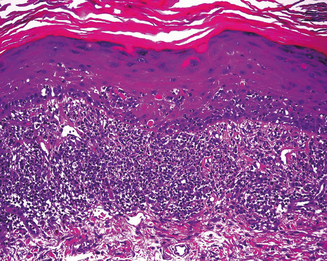
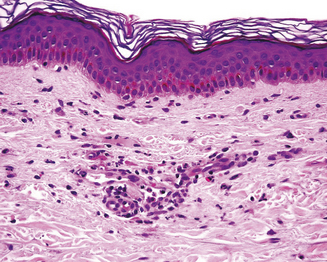
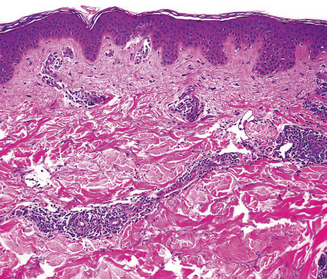
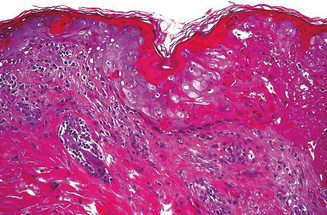
Skin biopsy may be indicated in some situations. Urticarial lesions may show a typical infiltrate as demonstrated by (Figure 11.11). CD8+ T cells and cytokines that fit the inflammatory T-helper 1 profile are found in skin biopsies of many delayed morbilliform rashes (Figure 11.12) and in bullous or blistering lesions, suggesting a causal role for these cells in those disease processes.70 Stevens–Johnson syndrome and TEN are associated with full thickness necrosis of the epidermis (Figure 11.13). During hypersensitivity vasculitis, biopsy may show neutrophils around arterioles or venules. Biopsy of lymphadenopathy associated with drug reaction to anticonvulsant drugs often resembles malignancy, a pseudolymphoma architecturally.
Treatment
Treatment of drug reactions is based on the type of reaction and symptoms manifested. The offending agent, or any potential offending agents in the case of severe reactions, should be discontinued immediately. Anaphylactic and anaphylactoid reactions should be treated as recommended in the Practice Parameter for Anaphylaxis published by the American Academy of Allergy, Asthma and Immunology (AAAAI) and the American College of Allergy, Asthma and Immunology (ACAAI).71 ABCs are followed as per adult and pediatric resuscitation protocols. The airway should be maintained and oxygenation ensured. The treatment of anaphylaxis is epinephrine (adrenaline), usually given at a dose of 0.3–0.5 mg intramuscularly in adults, and 0.01 mg/kg in children. Antihistamines, corticosteroids, and inhaled bronchodilator treatments are supplementary treatment, not primary treatment, in the management of anaphylaxis. The addition of an H2 blocker such as ranitidine to the antihistamine when managing anaphylaxis may confer some additional benefit. Corticosteroids may be helpful in the treatment of drug reactions secondary to other, non-IgE-induced mechanisms.
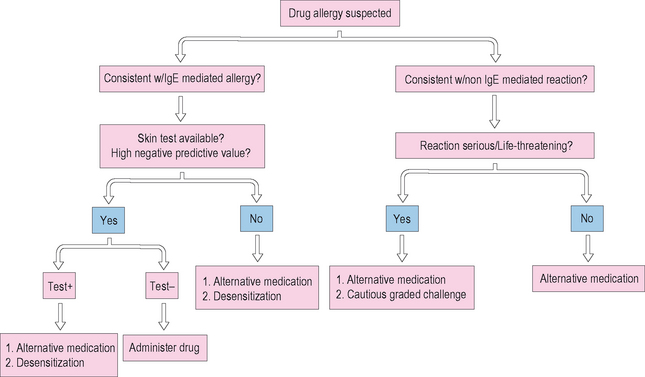
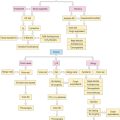
The management of patients with drug allergy is summarized in Figure 11.14. Management often includes simply avoiding the offending medication. In many cases, in regard to antibiotic use especially, medication may not be clinically warranted and is often overprescribed. If professional guidelines are followed and a medication is still indicated, an alternative should be chosen if available. It is important to avoid clinically significant cross-reacting medications. An example is the carbapenams, such as imipenem, which may cross-react with penicillin.72 The monobactam, aztreonam, is structurally similar to penicillin. However, monobactams have a monocyclic ring structure and cross-reaction between aztreonam and penicillin is very rare. Adkinson et al demonstrated that in 41 patients with skin test positivity to penicillin, 37 of the 41 were unequivocally negative to aztreonam. The side chains of aztreonam are similar and cross-react with ceftazadime.73
Anaphylaxis to cephalosporins occurs in up to 0.1%. Cross-reactions with cephalosporins in penicillin-allergic individuals occur in a small number, up to 3%. First generation cephalosporins are more likely to be cross-reactive versus second generation which are more likely than third generation cephalosporins. The steric hindrance of the side chains of the more recent generations of cephalosporins prevent reaction with the beta lactam ring. If a patient has previously demonstrated tolerability to an individual cephalosporin, and the patient is going to receive the same cephalosporin again, then testing is not needed, even with a past history of penicillin allergy, as tolerance has been demonstrated. If a patient has a suspected cephalosporin allergy, side chains may be an issue, and then options include cautious graded challenge or skin test. Romano et al studied a small group of 30 patients with either cephalosporin allergy by in vitro or skin testing or penicillin allergy by skin testing.74 He identified side chains as a predominant risk for cross-reactivity between these groups. These results were also mirrored by Antunez et al who noted cross-reactivity in the cephalosporin group was also predictable by R1 side chains, such as the second generation cephalosporins cefotaxime and ceftriaxone.75 These data suggest that chance of reaction can be minimized by avoiding agents with cross-reactive side chains. The current practice parameters for evaluation and management of drug allergy as published by the AAAAI and ACAAI would recommend caution. Testing for cephalosporin allergies, although performed by some, is not standardized and there is no guarantee that a patient who tests negative will not have an adverse event. Although some have advocated the use of cephalosporins in penicillin-allergic patients, given the low risk of cross-reaction, a significant proportion of the albeit small number of reactions have resulted in death on first administration of a cephalosporin in penicillin-allergic patients. Therefore, a positive skin test to penicillin should preclude administration of a cephalosporin without desensitization. If during the evaluation of allergy for penicillin, skin testing cannot be completed successfully, rapid desensitization should be performed if there is no alternative medication.
Under no circumstances should a drug be reintroduced or a rapid desensitization or graded challenge done if the reaction was consistent with blistering or bullous lesions such as Stevens–Johnson syndrome or TEN. Reintroduction of the medication in such a case can lead to TEN and resulting severe morbidity and mortality. Mortality in TEN is at least 50%. Corticosteroids are contraindicated in TEN as they may predispose to infection and worsen the condition. There may be a role for intravenous immunoglobulin (IVIG) in these patients.76 In the case of the well-described reaction to the anticonvulsants phenytoin, phenobarbital, and carbamazipine among others, medication should also never be reintroduced. Even in IgE-mediated reactions, giving a test dose of a medication without prior evaluation and testing may be dangerous. The immunologic mechanisms responsible for IgE-mediated anaphylaxis, especially, require only minute amounts of the antigen to cause a life-threatening reaction.
Individuals with HIV are also at risk for development of drug allergy to multiple drugs, and their physicians should be aware of special management issues and options. Their increased susceptibility may be secondary to the underlying immunodeficiency.77 However, drug reactions to sulfonamides may decrease as HIV disease progresses. Multiple theories have been postulated to describe this phenomenon from altered metabolism of the medication, to coexistent viral infection or glutathione deficiency. Most of the reactions are not likely due to an IgE or Gell and Coombs type 1 reaction. Morbilliform rash after 10 days is the most common presentation and may not be an indication to discontinue therapy, unless more concerning systemic symptoms develop, or any sign of blistering disease. Successful desensitization procedures, described below, have been published not only to TMP/SMX, to which a significant number of HIV-infected individuals develop hypersensitivity, but also to anti-tuberculin medications and to therapeutic agents such as AZT.78 Patients experiencing a hypersensitivity-like reaction to the nucleoside reverse transcriptase inhibitor, abacavir, should never be rechallenged with the agent, as the risk for life-threatening drug reaction with rechallenge is high.79
If a drug to which a patient has had a reaction needs to be used, several options are available. Of course, an alternative medication should be used if at all possible. If evaluation and testing was consistent with an IgE-mediated process, a rapid desensitization can be done. An example of a successful desensitization protocol to a beta lactam antibiotic at our institution is given in Box 11.2. The patient should be admitted to a monitored unit with the capacity to respond to anaphylaxis quickly. Informed consent should be obtained, as the patient is at risk for recurrence of anaphylaxis. The medication is administered in increasing doses at 15- to 30-minute intervals, starting with at least a 1 : 1000 dilution of the drug. Vital signs and peak flows, especially in the case of patients with asthma or those with respiratory symptoms as a manifestation of their reaction, should be obtained prior to each dose. Mild symptoms can be treated with medications such as antihistamines and by backing up to a previously tolerated dose in the schedule and proceeding again slowly if the medication is tolerated. When the patient reaches the desired dose of the medication, it may be continued at the regular dosage interval. However, patients and referring physicians need to be aware that the desensitization is valid only as long as the medication is continued regularly. If more than a few doses are missed, or if the patient needs a second course after an interrupted treatment interval, desensitization needs to be repeated, as the protection may be lost, putting the patient at risk for anaphylaxis.
BOX 11.2 Desensitization to ampicillin-sulbactam (Unasyn): allergy and immunology Hershey Medical Center
Vital signs should be obtained at baseline and prior to each dose
If the necessary medication is thought to be secondary to a non-IgE-mediated mechanism, and the medication is essential, a graded challenge may be performed. This is often the case with aspirin or with TMP/SMX. Many well-publicized protocols exist for both medications, with desensitization over hours to days. Several options for TMP/SMX graded challenge are summarized well by Gruchalla.80 The process is similar to the procedure described above for rapid desensitization, with the exceptions that the starting dose of the medication is usually higher, and the interval between doses is often longer.
Future Directions
The future of the evaluation and treatment of drug allergy is very broad. There needs to be more work in the development of new testing procedures to evaluate for both IgE and non-IgE-based drug reactions, as well as to define latex allergens for testing. By identifying active drug metabolites, this may be more feasible. Available diagnostic tools, such as PrePen for penicillin testing and minor determinant mixtures, need to be available for physicians. With the advent of newer biologic agents, such as monoclonal antibodies used in treatments from autoimmune diseases, to asthma and allergies, to cancer, more drug reactions may be expected. These monoclonal antibodies, although often humanized, may contain portions of immunogenic foreign species protein. They are also complex structures of high molecular weight, characteristics that may make them particularly immunogenic. Etanercept, a genetically designed, soluble tumor necrosis factor (TNF) alpha receptor used in the treatment of rheumatoid arthritis, is associated with injection site reactions in up to 20% of patients on this therapy within the first few months of therapy.81 Our group has described a case of refractory urticaria with etanercept use which improved following a desensitization protocol which we developed to the medication.82
Despite published guidelines by national organizations on the management of drug allergy, there is a need for improvement. In a survey of physicians on the management of penicillin allergy, only one chose the option of requesting penicillin testing. Only two would consult an allergist. Many physicians in this survey picked a cephalosporin as an acceptable alternative.83 The use of electronic records is, fortunately, increasing. Electronic systems with checks and balances are able to not only keep track of patient drug allergies but also to identify possible cross-reacting substances if they are erroneously ordered by physicians. Overall, these improvements will reduce patient morbidity and mortality from drug reactions while still allowing physicians to actively treat disease.
Antunez C, Blanca-Lopez N, Torres MJ, et al. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J Allergy Clin Immunol. 2006;117:404-410.
Aster RH. Drug-induced immune cytopenias. Toxicology. 2005;209:149-153.
The Diagnosis and Management of Anaphylaxis. J Allergy Clin Immunol. 1998;101:S465-S528.
Drug Hypersensitivity. Ann Allergy Asthma Immunol. 1999;83:665-700.
Gerber BO, Pichler WJ. Cellular mechanisms of T cell mediated drug hypersensitivity. Curr Opin Immunol. 2004;16:732-737.
Gollapudi RR, Teirstein PS, Stevenson DD, Simon R. Aspirin sensitivity: implications for patients with coronary artery disease. JAMA. 2004;292:3017-3023.
Idee JM, Pines E, Prigent P, Corot C. Allergy-like reactions to iodinated contrast agents. Fund Clin Pharmacology. 2005;19:263-281.
Johnson KK, Green DL, Rife JP, Limon L. Sulfonamide cross-reactivity: fact or fiction? Ann Pharmacother. 2005;39:290-301.
Kelkar PS, Li JT. Cephalosprorin allergy. N Engl J Med. 2001;345:804-809.
Pichichero ME. A review of the evidence supporting the American Academy of Pediatrics recommendations for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005;115:1048-1057.
Pichler W, Yawalkar N, Schmid S, Helbling A. Pathogenesis of drug-induced exanthems. Allergy. 2002;57:493-884.
Riedl M, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician. 2003;68:1781-1790.
Task Force Report. Future research needs for the prevention and management of immune-mediated drug hypersensitivity reactions. J Allergy Clin Immunol. 2002;109:S461-S478.
Torres MJ, Blanca M, Fernandez J, et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003;58:961-972.
Vandebriel RJ. Gene polymorphisms within the immune system that may underlie drug allergy. Arch Pharmacol. 2004;369:125-132.
1 Nelson KM, Talbert RL. Drug-related hospital admissions. Pharmacotherapy. 1996;16:701-707.
2 Winterstein AG, Sauer BC, Hepler CD, Poole C. Preventable drug-related hospital admissions. Ann Pharmacother. 2002;36:1238-1248.
3 Suh DC, Woodhall BS, Shin SK, Hermes-De Santis ER. Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother. 2000;34:1373-1379.
4 Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200-1205.
5 Gholami K, Parsa S, Shalviri G, et al. Anti-infectives-induced adverse drug reactions in hospitalized patients. Pharmacoepidemiol Drug Safety. 2005;14:501-506.
6 Adkinson NF. Risk factors for drug allergy. J Allergy Clin Immunol. 1984;74:1254-1256.
7 Kerns DL, Shira JE, Go S, et al. Ampicillin rash in children: relationship to penicillin allergy and infectious mononucleosis. Am J Dis Child. 1973;125:187-190.
8 Banks JR, Kagey-Sobotka A, Lichtenstein LM, Eggleston PA. Spontaneous histamine release after exposure to hyperosmolar solutions. J Allergy Clin Immunol 78:51–57.
9 Siegle RL, Halvorsen RA, Dillion J, et al. The use of iohexol in patients with previous reactions to ionic contrast material: a multicenter clinical trial. Invest Radiol. 1991;26:411-416.
10 Greenberger PA, Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol. 1991;87:867-872.
11 Worthley DL, Gillis D, Kette F, Smith W. Radiocontrast anaphylaxis with failure of premedication. Intern Med J. 2005;35:58-60.
12 Enricht T, Chau-Lim A, Duda E, Lim DT. The role of a documented allergic profile as a risk factor for radiocontrast media reaction. Ann Allergy. 1989;62:302-305.
13 Lang DM, Alpern MB, Visintainer PF, Smith ST. Increased risk for anaphylactoid reaction from contrast media in patients on beta-adrenergic blockers or with asthma. Ann Intern Med. 1991;115:270-276.
14 Sehai J, Healy DP, Garris R, et al. Influence of antihistamine pretreatment on vancomycin-induced red man syndrome. J Infect Dis. 1989;160:876-881.
15 Monaghan MS, Glasco G, St John G, et al. Safe administration of iron dextran in a patient who reacted to the test dose. S Med J. 1994;87:1010-1012.
16 Amos HE, Wilson DV, Taussig MJ, Carlton SJ. Hypersensitivity reactions to acetylsalicylic acid. Clin Exp Immunol. 1971;8:562-573.
17 Samter M, Beers RF. Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975.
18 Szezeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis and management. J Allergy Clin Immunol. 1999;104:5-13.
19 Mathison DA, Lumry WR, Stevenson DD, et al. Aspirin in chronic urticaria and/or angioedema: studies of sensitivity and desensitization. J Allergy Clin Immunol. 1982;69:134.
20 Coulter DM, Edwards IR. Cough associated with captopril and enalapril. Br Med J. 1987;296:1521-1523.
21 Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor-associated angioedema. JAMA. 1997;278:232-233.
22 Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor associated angioedema. Pharmacotherapy. 2002;22:1173-1175.
23 Janeway CA, et al, editors. Immunobiology, 6th edn., New York: Garland Science, 2005.
24 Naisbitt D, Williams D, Pirmohamed M, et al. Reactive metabolites and their role in drug reactions. Curr Opin Allergy Immunol. 2001;1:317-325.
25 Shulman NR. Immunoreactions involving platelets. I. A steric and kinetic model for formation of a complex from a human antibody, quinidine as a haptene, and platelets; and for fixation of complement by the complex. J Exp Med. 1958;107:665.
26 Worlledge SM, Carstairs KC, Dacie JV. Autoimmune hemolytic anemia associated with methyldopa therapy. Lancet. 1966;2:135.
27 Churg A, Brallas M, Cronin SR, et al. Formes frustes of Churg–Strauss syndrome. Chest. 1981;108:320-323.
28 Ooi BS, Pesce AJ, First MR, et al. IgE levels in interstitial nephritis. Lancet. 1974;1:1254-1256.
29 Pichler W, Schnyder B, Zanni M, et al. Role of T cells in drug allergies. Allergy. 1998;53:225-232.
30 Hertl M, Geisel J, Boecker C, Merk H. Selective generation of CD8+ T cell clones from the peripheral blood of patients with cutaneous reactions to beta-lactam antibiotics. Br J Dermatol. 1993;128:619-626.
31 Idsoe O, Guthe T, Wilcox RR, DeWeck AL. Nature and extent of penicillin side-reactions, with particular references to fatalities from anaphylactic shock. WHO. 1968;38:159-188.
32 Neugut A, Ghatak A, Miller R. Anaphylaxis in the United States: an investigation into its epidemiology. Arch Intern Med. 2001;161:15-21.
33 Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol 103:918–924.
34 Shepherd G, Mendelson L. The role of skin test positivity for penicillin allergy. Arch Intern Med. 1992;152:2505.
35 Shepherd GM. Clinical experience using only Pre-Pen and penicillin G to detect penicillin allergy in hospitalized adults. J Allergy Clin Immunol. 1997;99:S134.
36 Mendelson LM, Ressler C, Rosen JP, Selcow JE. Routine elective penicillin allergy skin testing in children and adolescents: study of sensitization. J Allergy Clin Immunol. 1984;73:76-81.
37 Green GR, Rosenbloom AH, Sweet LG. Evaluation of penicillin hypersensitivity: values of clinical history and skin testing with penicillin-polylysine and penicillin G. J Allergy Clin Immunol. 1977;60:339-345.
38 Batchelor F, Dewdney J, Gazzard D. Penicillin allergy: the formation of the penicilloyl determinant. Nature. 1965;206:362-364.
39 Levine B. Immunologic mechanisms of penicillin allergy. N Engl J Med. 1966;275:1115-1125.
40 Baldo B. Penicillins and cephalosporins as allergens: structural aspects of recognition and cross-reactions. Clin Exp Allergy. 1999;29:744-749.
41 Crib A, Spielberg S. Hepatic microsomal metabolism of sulfamethoxazole to the hydroxylamine. Drug Metabol Disp. 1990;18:784-787.
42 Reilly T, Lash L, Doll M, et al. A role for bioactivation and covalent binding within epidermal keratinocytes in sulfonamide-induced cutaneous drug reactions. J Invest Dermatol. 2000;114:1164-1173.
43 Carr A, Cooper DA, Penny R. Allergic manifestations of human immunodeficiency virus (HIV) infection. J Clin Immunol. 1991;11:55-64.
44 Deamer RL, Prichare JG, Loman GJ. Hypersensitivity and anaphylactoid reactions to ciprofloxacin. Ann Pharmacother. 1992;26:1081-1084.
45 Heine H. Cutaneous adverse reaction to ciprofloxacin: demonstration of specific lymphocyte proliferation and cross-reactivity to ofloxacin in vitro. Acta Dermato-Venereol. 1997;77:285-286.
46 Zanotti K, Rybicki L, Kennedy A, et al. Carboplatin skin testing: a skin-testing protocol for predicting hypersensitivity to carboplatin chemotherapy. J Clin Oncology. 2001;19:3126-3129.
47 Broome C, Schiff R, Friedman H. Successful desensitization to carboplatin in patients with ovarian and peritoneal carcinoma. Int J Gynecol Cancer. 1998;8:365-366.
48 Cooper J, White DA, Matthay RA. Drug-induced pulmonary disease. Part 1. Cytotoxic drugs. Am Rev Resp Dis. 1986;133:321-340.
49 Alarcon GA, Kremer JM, Macaluso M, et al. Risk factors for methotrexate-induced lung injury in patients with rheumatoid arthritis. Ann Intern Med. 1997;127:356-364.
50 Baldo BA, Fisher MM. Detection of serum IgE antibodies that react with alcuronium and tubocurarine after life-threatening reactions to muscle relaxant drugs. Anesthes Intensive Care. 1983;11:194-197.
51 Gall H, Kaufmann R, Kalveram CM. Adverse reactions to local anesthetics: analysis of 197 cases. J Allergy Clin Immunol. 1996;97:933-937.
52 Levy JH, Schwieger IM, Zaidan JR, et al. Evaluation of patients at risk for protamine reactions. J Thor Cardiovasc Surg. 1989;98:200-204.
53 Maclean JA, Moscicki R, Block KJ. Adverse reactions to heparin. Ann Allergy. 1990;65:254-259.
54 Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561.
55 Gonzalez E, Gonzalez S. Drug photosensitivity, idiopathic photodermatoses, and sunscreens. J Am Acad Dermatol. 1996;35:871.
56 Conger LAJr, Grabski WJ. Dilantin hypersensitivity reaction. Cutis. 1996;57:223.
57 Herbert AA, Syman ES, Levy ML. Serum sickness-like reactions from cefaclor in children. J Am Acad Dermatol. 1991;25:805-808.
58 Sullivan TJ, Ong RC, Gilliam LK. Studies of the multiple drug allergy syndrome. J Allergy Clin Immunol. 1989;83:270.
59 Khoury L, Warrington R. The multiple drug allergy syndrome: a matched-control retrospective study in patients allergic to penicillin. J Allergy Clin Immunol. 1996;98:462-464.
60 Sogn DC, Evans R, Shepherd GM, et al. Results of the National Institute of Allergy and Infectious Diseases collaborative clinical trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med. 1992;152:1025-1032.
61 Gadde J, Spence M, Wheeler R, et al. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. 1993;270:S134.
62 Green GR, Rosenbloom AH, Sweet LG. Evaluation of penicillin hypersensitivity: values of clinical history and skin testing with penicillin-polylysine and penicillin G. J Allergy Clin Immunol. 1977;60:339-345.
63 Weiss M, Adkinson NF. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy. 1988;18:515-540.
64 Solley GO, Gleich GJ, Van Dellen RG. Penicillin allergy: clinical experiences with a battery of skin test reagents. J Allergy Clin Immunol. 1982;69:238-244.
65 Romano A. Gueant-Rodriguez RM, Viola M, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med. 2004;141:16-22.
66 Pratt E, Miles P. Localized insulin allergy treated with continuous subcutaneous insulin. Diabetic Med. 2001;18:515-516.
67 Wessbecker R, Kiehn M, Stoffel E, Moll I. Management of insulin allergy. Allergy. 2001;56:919-920.
68 Schwartz LB, Yunginger JW, Miller J, et al. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83:1551-1555.
69 Romano A, Quarantino D, DiFonso M, et al. A diagnostic protocol for evaluating nonimmediate reactions to aminopenicillins. J Allergy Clin Immunol. 1999;103:1186-1190.
70 Hertl M, Mark HF. Lymphocyte activation in cutaneous drug reactions. J Invest Dermatol. 1995;105:955-985.
71 The diagnosis and management of anaphylaxis. J Allergy Clin Immunol. 1998;101:S465-S528.
72 Saxon A, Adelman DC, Patel A, et al. Imipenem cross reactivity with penicillin in humans. J Allergy Clin Immunol. 1988;82:213-217.
73 Adkinson NFJr. Immunogenicity and cross-allergenicity of aztreonam. Am J Med. 1990;88:125-155. 385–425.
74 Romano A, Matorga C, Torres MJ. Immediate allergic reactions to cephalosporins: cross reactivity and selective responses. J Allergy Clin Immunol. 2000;106:1177-1183.
75 Antunez C, Blanca-Lopez N, Torres MJ, et al. Immediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J Allergy Clin Immunol. 2006;117:404-410.
76 Viard I, Wehrti P, Bullani R, et al. Inhibition of toxic epidermal necrolysis by blockage of CD95 with human intravenous immunoglobulin. Science. 1998;282:490-493.
77 Bayard PJ, Berger TG, Jacobson MA. Drug hypersensitivity reactions and human immunodeficiency virus disease. J AIDS. 1992;5:1237-1257.
78 Carr A, Penny R, Cooper DA. Allergy and desensitization to zidovudine in patients with acquired immunodeficiency syndrome. J Allergy Clin Immunol. 1993;91:683-685.
79 Mallal S, Nolan D, Witt C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor Abacavir. Lancet. 2002;359:727.
80 Gruchalla RS. Drug allergy. J Allergy Clin Immunol. 2003;111:S554.
81 Zeltser R, Valle L, Tanck C, et al. Clinical, histological, and immunophenotypic characteristics of injection site reactions associated with etanercept. Arch Dermatol. 2001;137:893-899.
82 Fisher LH, Craig TJ. Successful desensitization to etanercept following urticaria associated with administration. (Publication pending).
83 Solensky R, Earl H, Gruchalla R. Clinical approach to penicillin allergic patients: a survey. J Allergy Clin Immunol. 1999;103:S33.