Chapter 13 Management of the Patient with Atopic Skin Disease
Introduction
Atopic dermatitis, also called atopic eczema or simply eczema, has been described since the days of Hippocrates. Eczema literally means to “boil over.” This is an apt description of the smoldering itch and inflammation that characterize this skin disease. Since the advent of formal diagnostic criteria in 1980, it has become clear that atopic dermatitis is an extremely common, multifactorial disease.1 The cause remains unknown but both genetic and environmental factors contribute to the clinical phenotype. The incidence of atopic dermatitis (AD) is rising globally, and the medical, psychosocial, and financial burden imposed is considerable. While the persistent inflammation and itch often resolve during the childhood years, cutaneous hyperirritability and susceptibility to allergy and infection persist in many. Eighty percent of all patients with occupational skin disease are atopic.2 Longitudinal studies have shown that symptoms of AD recur or persist in up to 60%, implying that we do our patients no favors by asserting that they will “grow out of it.”3
Epidemiology
Atopic dermatitis, along with asthma and hayfever, comprises one-third of the “atopic triad.” Though deemed “atopic” 20–60% of patients do not have IgE-mediated sensitivity to allergens or a positive family history of atopy.4 This led to recent nomenclature revision by the Nomenclature Review Committee of the World Allergy Organization.5 Up to 30% of patients with AD continue the “atopic march” to develop asthma, and 35% will eventually develop hayfever.6 AD is a pediatric disease. Sixty percent are diagnosed by 1 year of age and 90% by 5 years of age. There is no race or gender predilection. The prevalence varies widely from less than 2% in China to greater than 20% in Japan, Australia, and England.7,8 In a study in 2000 of Oregon schoolchildren, Laughter et al found a 17.2% incidence of AD.9 This contrasts with prevalence rates around 5% in the USA in the 1950s. Many candidate genes have been investigated though none explain all cases. Schultz Larsen demonstrated 0.72 concordance in monozygotic twins and 0.23 in dizygotes.10 Children with two affected parents have a 60–80% chance of developing AD. This risk drops with one or neither parent having AD, asthma, or hayfever, and markedly so if there is no atopic family history. In a longitudinal study Ben-Gashir et al noted that children living in an urban area with onset of AD accompanied by asthma, allergic rhinitis, or both during the first year of life, had more severe disease independent of other risk factors.11
Birth order studies have found a higher incidence of AD in first born children. AD rates have also been found to be lower in households with two or more farm animals, and early childhood exposure to typical infections.12 These results have suggested that some degree of exposure to antigen at an early age may induce immunologic tolerance and aid the discriminatory capability of the developing immune system. The “hygiene hypothesis,” which posits that excessively clean environments prevent exposure to such early priming antigens, has been used to explain some of these findings.13,14 These data point to significant environmental mediation of disease expression.
The impact of AD on families is often underestimated. Validated quality of life indices can provide a more accurate assessment of the impact of disease on patients and families.15 An Australian study comparing the impact of chronic diseases on children and families found that caring for a child with moderate to severe AD was significantly more stressful than caring for a child with insulin-dependent diabetes.16 Sleep loss due to itch plays a large part in this daily burden and can have wide repercussions on school performance, interpersonal confidence, social relationships both inside and outside of the family unit, and personal health. Polysomnographic studies have documented frequent nighttime awakening, reduced non-REM sleep, and poor sleep efficiency in patients with AD.17 The out of pocket costs of this disease are comparable to those of asthma.18,19
Classification
Atopic dermatitis has been classified in various ways though the most useful are by severity. Severity can be mild, moderate, or severe based upon body surface area involved, degree of itch, and signs of disease including erythema, papulation, lichenification, and excoriation. Validated scoring instruments such as the EASI (Eczema Area and Severity Index) and SCORAD (SCORing of Atopic Dermatitis) are available but are rarely used outside of clinical trials.20 In a UK survey of 1760 children from 1 to 5 years, 84% were categorized as mild, 14% moderate, and 2% severe.21
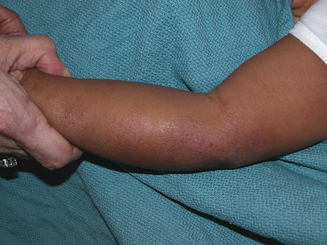
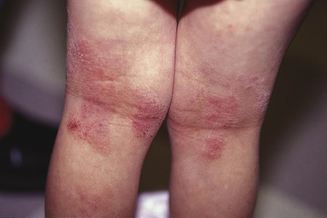
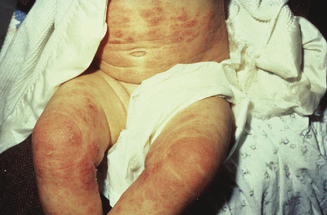
Atopic dermatitis can be categorized based upon age of presentation. Atopic dermatitis can present differently in infants and small children. Infantile eczema, though histologically indistinguishable from childhood and adult eczema, often affects a more extensor and facial distribution (Figure 13.1). Skin folds including the axilla and groin are typically spared in AD, however in infants there is frequent overlap with seborrheic dermatitis which has intertrigenous involvement. As children age the more classic flexural presentation predominates (Figure 13.2). Adult-onset eczema is uncommon and may have more follicular and nummular features.22,23 Other eczema “types” such as “nummular,” “papular,” “follicular,” or “palmoplantar” refer to the prevailing morphology or distribution in a given patient. These subtypes have similar histology and putative triggers.
An older classification scheme, utilized primarily in Europe, describes AD as either extrinsic or intrinsic. Extrinsic AD represents the 60–80% of patients who have an atopic family history, elevated IgE, and positive allergy tests. These patients would qualify as having “atopic dermatitis” under the revised nomenclature described above. The remaining 20–40% do not have these features, though clinically their disease is identical. This population is labeled “intrinsic AD.” These distinctions highlight the immunologically heterogeneous nature of atopic dermatitis.
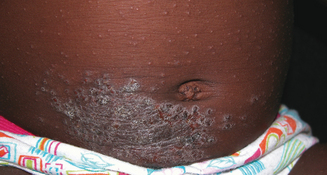
Allergic contact dermatitis, irritant contact dermatitis, and contact urticaria are three related skin conditions that are frequently confused with AD. All three occur with greater frequency in patients with AD, but do not necessarily have the same triggers or natural history. Allergic contact dermatitis (ACD) is a delayed-type hypersensitivity reaction to an external substance. Common causes of ACD in atopic patients include fragrance, nickel (Figure 13.3), latex, lanolin, Neosporin, rubber accelerators, preservatives such as formaldehyde or formaldehyde releasers, parabens, and, rarely, steroid molecules themselves (e.g., budesonide). Contact urticaria is an immediate-type hypersensitivity reaction that presents within minutes to hours after exposure (e.g., fish) and resolves completely within 24–48 hours. Irritant contact dermatitis, a nonimmunologic reaction, is due to either frequent wetting and drying (e.g., perioral rash in drooling infants) without sufficient emollient use, or the harsh effects of certain foods on the skin (e.g., citrus). Each of these conditions present with an itchy rash possibly in response to a food or environmental stimulus; however, treatment and natural history may vary so it is useful to distinguish these different cutaneous reaction patterns from typical eczema.
Anatomy/Physiology
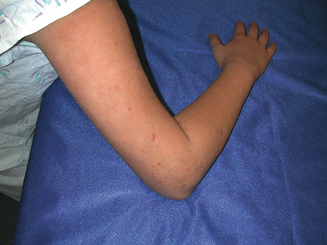
The skin is divided into three layers: epidermis, dermis, and subcutis. Atopic dermatitis is a papulosquamous skin disease and as such affects primarily the epidermis and papillary (superficial) dermis. Related conditions can present with additional findings affecting more focal cutaneous structures. Keratosis pilaris (Figure 13.4) occurs quite commonly in patients with atopic dermatitis and presents with scaly hyperkeratotic papules typically affecting the hair follicles of the upper extensor arms. Keratosis pilaris specifically affects the follicular ostia and adnexal unit, sparing the remainder of the epidermis. Ichthyosis vulgaris affects the stratum corneum or outer layer of the epidermis and the plate-like scale commonly found on the shins reflects this retained, xerotic outer skin layer. Pityriasis alba reflects diminished pigmentation within melanosomes distributed throughout the epidermis, presumably in response to the cutaneous inflammation associated with AD. Less commonly patients with AD develop an ocular deformity called keratoconus. Keratoconus is found in some patients with chronic periocular dermatitis and conjunctivitis. Asthma and rhinoconjunctivitis are also common in patients with AD and are detailed elsewhere.
Pathophysiology
The cause of AD is unknown. In the prevailing model, genetically predisposed individuals develop epidermal inflammation in response to environmental and/or emotional stimuli, presumably leading to the characteristic itch and rash of AD. The final common pathway of all types of eczema histologically is epidermal spongiosis with a mixed lymphohistocytic papillary dermal infiltrate. Cutaneous inflammation in AD is T cell mediated and there are demonstrable barrier and immunologic abnormalities. Patients have increased transepidermal water loss that manifests as xerotic, scaly skin. Biochemical analysis has shown deficiencies in certain components of the epidermal envelope including ceramide. The so-called “acid mantle,” a zone of lower pH found in normal skin that may alter bacterial attachment, is disrupted. Clinically it has long been noted that patients with AD are prone to staphylococcal skin infections whereas patients with psoriasis, another skin disorder notable for an abnormal barrier, are not. It has recently been shown that skin biopsies from lesional AD skin have decreased levels of the innate antimicrobial peptide beta-defensin, in contrast to normal or elevated levels found in lesional psoriatic skin.24 This deficiency could explain the relatively heightened skin infection risk in AD.
In addition to these barrier abnormalities, AD patients have well-described immunologic alterations including elevated IgE and eosinophil counts. AD patients have an abnormal T-helper 2 (Th2) mediated immune response as reflected by increased numbers of specific T cells producing interleukin (IL) 4, IL-5, and IL-13, and diminished interferon gamma (IFN-γ). IL-4 is involved in IgE isotype switching and IL-5 promotes eosinophilia which could explain these clinical features. This Th2 predominance may be due to preferential apoptosis of circulating Th1 cells.25 Skin biopsies have demonstrated elevated IL-4, IL-5, and IL-13 in both acutely affected and unaffected skin.26 Conversely biopsies taken from chronic lesions show increased amounts of IFN-γ, IL-5, IL-12, and GM-CSF (granulocyte macrophage colony stimulating factor).27 The factors governing the evolving inflammatory response have not been elucidated, but the chemokine MIP4/CCL18 may play a regulatory role.28 The clinical significance of this “biphasic” response is unclear but the effector cells appear to be FcRI expressing IgE epidermal dendritic cells including Langerhans cells (LC) and inflammatory dendritic epidermal cells (IDEC), both of which have been demonstrated in lesional AD skin.29
Diagnosis
▪ History
Atopic dermatitis does not usually present a diagnostic challenge. Affected patients typically present in the first year of life with focal or widespread itch and rash. Parents often suspect but cannot pinpoint an association with either a food or environmental trigger. Occasionally there is an obvious association with certain foods such as dairy products or eggs. Parents may report associated sleep loss and irritability. Gastrointestinal symptoms are less common but may present with cramping, nausea/vomiting, diarrhea, or blood in the stool. Particularly in cases of infantile eczema, the possibility of an underlying immunodeficiency must be considered. Several immunodeficiency states including hyper IgE syndrome and Wiskott–Aldrich syndrome may present with intractable eczema. The timing of presentation is similar to typical eczema which may delay diagnosis. Failure to thrive, hair loss, petechiae, or unusual type or number of extracutaneous infections in an infant with severe eczema should trigger a complete immunologic work up. Other differential considerations in this setting include severe combined immunodeficiency (SCID), Bruton’s agammaglobulinemia, and Netherton’s syndrome.30 Box 13.1 outlines a more complete differential diagnosis for AD.
BOX 13.1 Differential diagnosis of atopic dermatitis
Cutaneous T-cell lymphoma (CTCL)
When the diagnosis of AD is not clear cut, diagnostic criteria should be considered (Box 13.2). Hanifin described four major criteria including itch, family history of eczema, asthma, or hayfever, typical distribution of either extensor/facial dermatitis in an infant or flexural dermatitis in a child or adult, and a waxing and waning course.1 Three of these four major criteria plus three or more minor criteria need to be present to make a diagnosis of AD. The minor criteria include the related conditions mentioned above (keratosis pilaris, pityriasis alba), physical findings such as lip cheilitis, nipple eczema, xerosis, centrofacial pallor, a distinctive fold beneath the lower eyelids (Dennie–Morgan folds), keratoconus, and white dermographism, as well as serologic abnormalities, elevated IgE, and elevated eosinophil counts that may help when the diagnosis is uncertain. Several groups including a working party in the UK have attempted to modernize the original criteria.31 In addition to establishing a concrete diagnosis, every effort should be made to identify relevant allergic and nonallergic triggers. Foods that are most commonly associated include eggs, cows’ milk, peanuts, soy, wheat, seafood and shellfish. A recent meta-analysis of food sensitization and atopic diseases found few good studies upon which to guide our recommendations from the over 2000 that have been performed. Tarini et al identified nine such studies, five of which had a positive association between AD and early solid food exposure. The other four studies did not identify such an association. The authors concluded that there may be a slightly increased risk of developing AD but not other atopic conditions with early introduction of solid food.32 Zutavern found no increased risk if solids were delayed for 6 months, but could not rule out an increased risk if solid food is introduced prior to 4 months of age.33 Patient history should include family members with atopy, duration of breastfeeding, age of onset of skin disease, maternal and infant diet history, nature of skin reactions suspected in relationship to diet, and response to interventions or withdrawals. All medications should be reviewed. There have been reports of an increased incidence of atopic disease in association with early medication ingestion.34
▪ Physical Examination
A thorough skin exam alone may be sufficient to confirm a diagnosis of AD. The classic signs on skin exam are erythema, papulation, excoriation, and lichenification. In very young infants lack of coordination may minimize excoriation and lichenification. In this age group the provider should assess general appearance and development to rule out any concomitant failure to thrive that may herald an underlying systemic disorder or iatrogenic nutritional deficiency from restriction dieting. Sites of frequent wetting and drying such as the mouth, chin, cheeks, and chest should be evaluated. There may be more widespread involvement on the trunk and extensor extremities. The scalp can be quite scaly. Fissures may be seen at the inferior earlobes and these frequently culture Staphylococcus aureus. The folds of skin around the neck, axilla, and groin may be spared even in children with extensive dermatitis presumably from local hydration (Figure 13.5). Palms and soles are typically uninvolved in young infants and children. Older children and adults may have similar areas of involvement, but in general the disease is more flexural. The antecubital and popliteal fossae, posterior thighs, wrists, and ankles are often affected (Figure 13.2). Adult onset AD may have more follicular or nummular features. The eyes, nose, and throat should be examined for signs of rhinoconjunctivitis, and the lungs should be auscultated for wheezing or evidence of asthma.
▪ Laboratory Tests
Diagnosis of AD is typically clinical. A skin biopsy is rarely helpful and laboratory tests are not routinely ordered. In mild to moderate uncomplicated cases no serologies are indicated. If involvement is more severe, the diagnosis unclear, response to therapy unsatisfactory, or there is a question of allergic causation lab tests may be indicated. Serum IgE and eosiniphil counts are frequently elevated. The list of potential allergic triggers is extensive and testing exhaustively in an unselected fashion impractical. CAP-RAST testing, and/or prick testing to eggs, cows’ milk, peanut, soy, wheat, shellfish, and dust mites (Dermatophagoides pteronyssinus, D. farinae) can be a useful initial screen in the absence of suspected foods. This battery comprises the vast majority of relevant allergens. Skin prick and RAST tests have a negative predictive value approaching 95%. This can be very useful information for patients and parents struggling to identify the allergic contribution to chronic dermatitis. The positive predictive value is much lower (40%).35 If clinical relevance is unclear or there is a history of anaphylactic reactions, further evaluation may be necessary in a controlled setting. This may include observed or double blind placebo controlled food challenge. In light of the frequent disconnection between positive allergy tests and clinical improvement upon elimination of identified foods, providers must counsel cautious interpretation to parents as investigations continue.36,37 If allergic contact dermatitis is considered, patch testing may be obtained. A limited accessible alternative has been commercially available (TRUE test) that contains the 20 most common contact allergens, however interpretation can be challenging. These tests are reviewed elsewhere in this text.
Treatment
▪ Pharmacologic
Topical steroids are first line pharmacologic therapy. In deciding on a topical steroid regimen several factors must be taken into consideration including: (1) age of patient, (2) body site to be treated, (3) steroid vehicle (e.g., lotion, cream, ointment), and (4) risk factors for topical steroid side effects.37 Infants have a greater body surface area to mass ratio and thinner skin, increasing the risks of systemic absorption and cutaneous atrophy respectively. An over-the-counter (OTC) 1% hydrocortisone ointment or cream may be the best initial therapy in this age group. Topical steroids are generally prescribed twice daily to affected areas but there is no evidence that this provides additional benefit over once daily use.37,38 As dermatitis improves topical steroids should be gradually weaned in favor of emollients. In infants unresponsive to OTC products, a class VI agent such as aclometasone 0.05% or hydrocortisone 2.5% cream or ointment is an appropriate step up and only in unusual circumstances should anything more potent be used. In children and adults a similar strategy may be employed utilizing more potent agents as necessary. For facial dermatitis (Figure 13.1) even in adults, every effort should be made to utilize a class VI or weaker agent; however, trunk and extremity dermatitis may require a class IV or V agent such as triamcinolone 0.1% ointment. Certain regional variations such as the thick palms and soles or lichenified plaques in nickel allergic contact dermatitis (Figure 13.3) may require more potent class I, II, or III agents to achieve benefit.
Special precautions must be taken when treating dermatitis around the eyes or mouth. Periocular use incurs a small risk of glaucoma and cataracts, and hand or perioral use in infants may imply some modest oral ingestion particularly in infants. Reasonable limits in both instances should be imposed. Side effects from topical steroids include skin atrophy, telangiectasia, striae, hypopigmentation, acneiform change, hypertrichosis, HPA axis suppression, glaucoma, and cataracts. Follow-up appointments and limits on refills can help minimize these risks. Parental fear, particularly of skin atrophy, is frequently disproportionate to the actual risk. Several recent randomized trials have shown no significant skin thinning over a 16-week study period, and a 1-year study of unrestricted use of a potent topical steroid showed striae development in less than 1% of an adult population.39,40
The topical calcineurin inhibitors (TCI) tacrolimus and pimecrolimus represent an alternative to topical steroid treatment. TCIs are indicated as second line therapy in children 2 years of age and older who have failed or are intolerant of topical steroids. Although the precise anti-inflammatory mechanism for TCIs has not been elucidated, tacrolimus decreases inflammation through: (1) inhibition of translocation of cytokine transcription factor, (2) downregulation of costimulatory molecules, and (3) downregulation of FcRI expression. Tacrolimus 0.03% ointment is indicated for children aged 2–15 with moderate to severe AD, and 0.1% ointment is indicated for patients 16 and older. Pimecrolimus 1% cream is indicated in patients 2 years and older with mild to moderate AD. These products should be applied twice daily to affected areas only as needed and should be discontinued in favor of emollients when dermatitis resolves. TCIs are nonsteroidal and therefore do not affect collagen synthesis or risk cutaneous atrophy. The most common adverse effect is stinging, burning, or redness at the application site in 10–30% of patients. Application site reactions are typically transient and worse in patients with extensive excoriation.41 Pretreating excoriated areas for several days with topical steroids may help minimize this problem and improve adherence particularly in children. Paller et al found tacrolimus more effective than pimecrolimus with a similar side effect profile.42 A postmarket black box warning label has recently been assigned to this medication class due to unclear long-term risk of immunosuppression and malignancy. This FDA warning occurred in response to animal data as well as some postmarketing reports of lymphoproliferative disease and skin cancers in some patients using TCIs, though not in rates above those seen in the general population. Several allied administrative bodies including the American Academy of Allergy, Asthma and Immunology, and the American Academy of Dermatology backed by the assessment of independent oncologists have established a unified position that TCIs appear to be safe and effective when used as labeled. These groups did not feel that the data warranted a black box warning.43,44
Patients with AD frequently develop staphylococcal superinfection as evidenced by honey-colored crusting, weeping, fissuring, or pustules. Localized involvement may respond to topical antibiotics such as mupirocin 2% ointment or cream twice daily for 5–7 days. More widespread impetiginized dermatitis requires a course of systemic antibiotics (e.g., cephelexin 25–50 mg/kg/day) for 7–10 days. Nasal staphylococcal carriage and resistant (MRSA) species must also be considered in patients with recurrent skin infection.
Systemic antihistamines are frequently prescribed though their role in disease modulation is unclear. In a meta-analysis Klein et al found that while antihistamines may effectively treat concurrent rhinoconjunctivitis, little antipruritic effect has been demonstrated.45 If patients are losing sleep due to chronic itching, a sedating agent such as hydroxyzine 1 mg/kg at bedtime may be beneficial, though some concern exists that these agents interfere with normal sleep architecture.46 Some patients find antihistamines quite helpful, and less sedating agents such as loratadine or ceterizine should be made available to them for daytime use.
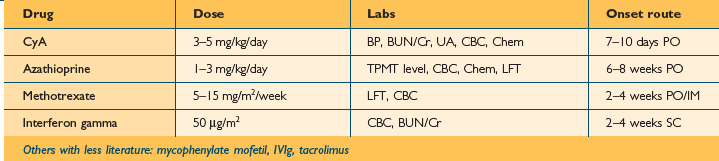
Systemic immunosuppressive therapy should be reserved for only the most severe, refractory patients. Prednisone should be avoided except in extraordinary circumstances. Prednisone is remarkably effective, however its beneficial effect is just as reliably transient. Patients often have rebound flares after cessation of prednisone. If patients are flaring uncontrollably and cannot tolerate or have failed conventional measures, a 1- to 2-week course of prednisone 1–2 mg/kg can be considered infrequently. A topical regimen must be reinitiated gradually as prednisone is weaned to avoid a rebound flare. If patients require repeated prednisone courses consideration should be given to nonsteroidal immunosuppressive therapy. Cyclosporin A, azathioprine, and interferon gamma have all been found effective in controlled trials, but each has well-documented side effects. Table 13.1 compares onset of action, route of administration, dosing, and recommended follow up. Long-term remission cannot be expected following cessation, but these agents can be effective, safe short-term weapons in the care of the most severe AD patients.47,48
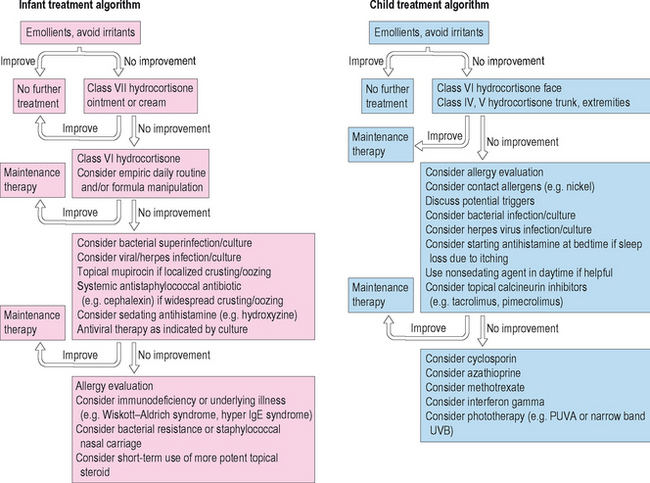
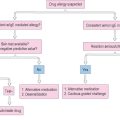
In older children and adults with severe unremitting AD, phototherapy is a viable option. Phototherapy can be utilized in the acute setting to induce a remission, or chronically to maintain control of skin disease. Several modalities have proven effective including narrow band UVB, UVA/UVB, and PUVA (psoralen ultraviolet A).49,50 The primary benefit of light therapy is ease of delivery to a large body surface area without the need for cumbersome, messy topical products. Phototherapy is not utilized widely for AD particularly in children due to the inconvenience of several times weekly office visits and the cumulative risks of light exposure including cutaneous malignancy and photo-aging. Therapeutic and diagnostic algorithms are outlined in Figure 13.6.
▪ Nonpharmacologic
Atopic dermatitis is notorious for flares triggered by a variety of environmental stimuli independent of allergy. These triggers are not universal and an in-depth discussion with each patient should attempt to identify and limit exposure to these factors. Winter months are usually more difficult due to decreased ambient humidity from indoor heaters. Simple measures such as lowering the thermostat and a small, frequently cleaned humidifier can help minimize this problem.
Stress exacerbates dermatitis in many patients. The itchy, visible nature of AD is constantly on display for peers and public. Chronic sleep loss and the burden of daily application of messy medicines and moisturizers combine to place a terrible strain on patients with severe disease. Associated stress creates a pathologic vicious cycle. Validated quality of life indices can help the provider capture the extent to which AD affects an individual patient, and psychological consultation aimed at stress reduction should be sought where appropriate.18,51,52
Certain regional considerations can play significant roles in AD care. Infants frequently develop markedly red, scaly patches around their mouths, chin, neck, and chest (Figure 13.1). This scenario can have myriad irritant and allergic causes, but the chronic wetting and drying in this area, particularly in the teething infant, only worsens the problem. Application of a thick barrier product such as Vaseline prior to and immediately following meals will help.
Avoidance of known allergens and attention to detergents and certain fabrics (e.g., wool) is prudent. Often parents and patients have suspicions that may require orderly, systematic avoidance and diary keeping, if not formal testing to identify true disease triggers. There is little evidence to support dietary exclusion in unselected cases, though some benefit has been demonstrated by egg restriction in children with demonstrated IgE antibodies to egg protein.53
Numerous studies have examined the role of dietary modification during and after pregnancy in breastfeeding mothers. The results have been conflicting. At present no definitive global recommendations regarding maternal dietary interventions and aeroallergen avoidance have been conclusively shown to alter the risk of developing AD in an infant or child.54 Probiotic use during and after pregnancy has been advocated, and a burgeoning literature suggests it may delay onset of AD though further study is required.55,56
The role of education in AD patients and their parents cannot be overstated. Caregivers may demonstrate application of topical products, thoroughly review potential triggers, and assess impact on quality of life. A recent study documented the benefit of age-related structured educational programs on long-term AD care.57
▪ Complementary/Alternative
For a variety of reasons complementary and alternative (CAM) remedies are frequently employed in the treatment of dermatologic disease and AD in particular.58,59 The cause of the disease is unknown, conventional therapy is imperfect, and there are significant allergic and environmental aspects to consider. There is some evidence to support CAM use in AD, but the vast majority of modalities are of unproven benefit. Traditional Chinese medicine (TCM) has been shown in poorly reported placebo controlled trials to benefit both pediatric and adult patients, though these results have not been duplicated and TCM carries an unclear risk of hepatotoxicity.60 Evening primrose oil has also been studied in controlled trials with mixed results but no benefit compared to placebo.61 Other modalities employed include St John’s wort, aloe vera gel, borage oil, flaxseed oil, tea tree oil, zinc, pyridoxine, vitamin E, habit reversal, autogenic training, hypnotherapy, biofeedback, and massage therapy. Naturopathic providers will typically attempt elimination dieting as part of initial therapy. Care must be taken not to spuriously link food ingestion with the naturally waxing and waning course of the disease, nor haphazardly restrict food ingestion. Numerous reports have described the potential for harm if close attention is not paid to nutritional intake.62 Katz recently reported a child with kwashiorkor from exclusive intake of Rice Dream beverage due to fears of food allergy.63
Future Directions
The twin holy grails for AD investigators are: (1) causation and (2) safe, uniformly effective systemic therapy. While immense strides have been made in the past 10 years in our collective understanding of this common, vexing skin disease, on neither of these two fronts are we particularly close to an answer. A number of candidate genes have been identified, but AD is both polygenic and polyfactorial.64–66 Ongoing outcome studies will tell us whether early aggressive treatment alters the natural history. Epidemiologic studies continue to return seemingly counterintuitive ideas regarding the role of infections, antibiotics, and pet exposure, and these “hygiene hypotheses” will continue to be tested.14 The fantastic commercial and clinical success of the TCIs tacrolimus and pimecrolimus, prior to the black box warning label, underscored the tremendous need for a safe, nonsteroidal therapy. In the immediate term much energy will be expended towards follow up of patients using these products to better ascertain their safety. Similarly, new nonsteroidal anti-inflammatory products will be marketed to fill the void created by the uncertainty over TCIs. Improved understanding of the biomechanics of the epidermal barrier will lead to “intelligent” emollients that not only protect but also replenish compounds demonstrated to be deficient in atopic skin.67,68 These advances will all occur against a backdrop of steadily increasing prevalence, rendering each step forward more critical than the last.
1 Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm (Stockh) Suppl. 1980;92:44-47.
2 Nassif A, Chan SC, Storrs FJ, et al. Abnormal skin irritancy in atopic dermatitis and in atopy without dermatitis. Arch Dermatol. 1994;130:1402-1407.
3 Musgrove K, Morgan JK. Infantile eczema. Br J Dermatol. 1976;95:365-372.
4 Flohr C, Johansson SGO, Wahlgren CF, et al. How atopic is atopic dermatitis? J Allergy Clin Immunol. 2004;114:150-158.
5 Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization. October 2003. J Allergy Clin Immunol. 2004;113:832-836.
6 Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of 5 years: a prospective study of 543 newborns. Allergy. 1983;38:339-346.
7 The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998;351:1225-1232.
8 Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med. 2005;352:2314-2324.
9 Laughter D, Istvan JA, Tofte S, Hanfin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
10 Schultz Larsen FV, Holm NV. Atopic dermatitis in a population based twin series. Concordance rates and heritability estimation. Acta Derm Venereol Suppl (Stockh). 1985;114:159.
11 Ben-Gashir MA, Seed PT, Hay RJ. Predictors of atopic dermatitis severity over time. J Am Acad Dermatol. 2004;50:349-356.
12 Benn CS, Melbye M, Wohlfahrt J, et al. Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. Br Med J. 2004;328(7450):1223.
13 Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the “hygiene hypothesis”: too clean to be true? Br J Dermatol. 2005;152:202-216.
14 Zutavern A, Hirsch T, Leupold W, et al. Atopic dermatitis, extrinsic atopic dermatitis and the hygiene hypothesis: results from a cross-sectional study. Clin Exp Allergy. 2005;35:1301-1308.
15 Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274-283.
16 Su JC, Kemp AS, Varigos GA, Nolan TM. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
17 Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics. 2004;114:607-611.
18 Kemp AS. Atopic eczema: its social and financial costs. J Paediatr Child Health. 1999;35:229-231.
19 Verboom P, Hakkaart-Van L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
20 Housman TS, Patel MJ, Camacho F, et al. Use of the Self-Administered Eczema Area and Severity Index by parent caregivers: results of a validation study. Br J Dermatol. 2002;147:1192-1198.
21 Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol. 1998;139:73-76.
22 Bannister MJ, Freeman S. Adult onset atopic dermatitis. Aust J Dermatol. 2000;41:225-228.
23 Ozkaya E. Adult-onset atopic dermatitis. J Am Acad Dermatol. 2005;52:579-582.
24 Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151-1160.
25 Akdis M, Trautmann A, Klunker S, et al. T helper (TH2) predominance in atopic diseases is due to preferential apoptosis of circulating memory/effector TH1 cells. FASEB J. 2003;17:1026-1035.
26 Hamid Q, Naseer T, Minshall EM, et al. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225-231.
27 Hamid Q, Boguneiwicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870-876.
28 Pivarcsi A, Gombert M, eu-Nosjean MC, et al. CC chemokine ligand 18, an atopic dermatitis-associated and dendritic cell-derived chemokine, is regulated by staphylococcal products and allergen exposure. J Immunol. 2004;173:5810-5817.
29 Novak N, Bohle B, Laffer J, et al. Unraveling the mission of FcERI on antigen-presenting cells. J Allergy Clin Immunol. 2003;111:38-44.
30 Krol A, Krafchik B. The differential diagnosis of atopic dermatitis in childhood. Dermatol Ther. 2006;19:73-82.
31 Williams HC, Burney PG, Hay RJ, et al. The UK Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. 1994;131:383-396.
32 Tarini BA, Carroll AE, Sox CM, et al. Systematic review of the relationship between early introduction of solid foods to infants and the development of allergic disease. Arch Pediatr Adolesc Med. 2006;160:502-507.
33 Zutavern A, Brockow I, Schaaf B, et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics. 2006;117:401-411.
34 Floistrup H, Swartz J, Bergstrom A, et al. Allergic disease and sensitization in Steiner school children. J Allergy Clin Immunol. 2006;117:59-66. Epub 2005 Nov 28.
35 Sampson HA, Albergo R. Comparison of results of skin tests, RAST, and double blind placebo controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 1984;74:26-33S.
36 Rowlands D, Tofte SJ, Hanifin JM. Does food allergy cause atopic dermatitis? Food challenge testing to dissociate eczematous from immediate reactions. Dermatol Ther. 2006;19:97-103.
37 Halbert A, Weston WL, Morelli JG. Atopic dermatitis: is it an allergic disease? J Am Acad Dermatol. 1995;33:1008-1018.
38 Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for the use of topical glucocorticosteroids. American Academy of Dermatology. J Am Acad Dermatol. 1996;35:615-619.
39 Luger TA, Lahfa M, Folster-Holst R, et al. Long term safety and tolerability of pimecrolimus cream 1% and topical corticosteroids in adults with moderate to severe atopic dermatitis. J Dermatol Treat. 2004;15:169-178.
40 Hanifin JM, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone proprionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147:528-537.
41 Reitamo S, Ortonne JP, Sand C, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2005;152:1282-1289.
42 Paller AS, Lebwohl M, Fleischer AB, et al. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in the treatment of atopic dermatitis: result from 3 randomized comparative studies. J Am Acad Dermatol. 2005;52:810-822.
43 Fonacier L, Spergel J, Charlesworth EN, et al. Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2005;115:1249-1253.
44 Qureshi AA, Fischer MA. Topical calcineurin inhibitors for atopic dermatitis: balancing clinical benefit and possible risks. Arch Dermatol. 2006;142:633-637.
45 Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525.
46 Casale TB, Blaiss MS, Gelfand E, et al. First do no harm: managing antihistamine impairment in patients with allergic rhinitis. J Allergy Clin Immunol. 2003;111:S835-S842.
47 Sidbury R, Hanifin JM. Systemic therapy of atopic dermatitis. Clin Exp Dermatol. 2000;25:559-566.
48 Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367(9513):839-846.
49 Berneburg M, Rocken M, Benedix F. Phototherapy with narrowband vs broadband UVB. Acta Derm Venereol. 2005;85:98-108.
50 Reynolds NJ, Franklin V, Gray JC, et al. Narrow-band UVB and broadband UVA phototherapy in adult atopic eczema: a randomised controlled trial. Lancet. 2001;357(9273):2012-2016.
51 Cotterill JA. Psychophysiological aspects of eczema. Semin Dermatol. 1990;9:216-219.
52 Crossen JR. Psychological assessment and treatment of patients with atopic dermatitis. Dermatol Ther. 1996;1:94-103.
53 Sampson HA. The evaluation and management of food allergy in atopic dermatitis. Clin Dermatol. 2003;21:183-192.
54 Hanifin JM, Cooper KD, Ho V, et al. Guidelines of care for atopic dermatitis. J Am Acad Dermatol. 2004;50:391-404.
55 Rautava S, Kalliomaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119-121.
56 Isolauri E. Dietary modification of atopic disease: use of probiotics in the prevention of atopic dermatitis. Curr Allergy Asthma Rep. 2004;4:270-275.
57 Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. Br Med J. 2006;332(7547):933-938.
58 Ernst E, Pittler MH, Stevinson C. Complementary/alternative medicine in dermatology: evidence assessed efficacy of two diseases and two treatments. Am J Clin Dermatol. 2002;3:341-348.
59 Artik S, Ruzicka T. Complementary therapy for atopic eczema and other allergic skin diseases. Dermatol Ther. 2003;16:150-163.
60 Sheehan MP, Atherton DJ. A controlled trial of traditional Chinese medicinal plants in widespread non-exudative atopic eczema. Br J Dermatol. 1992;126:179-184.
61 Hederos CA, Berg A. Epogam evening primrose oil treatment in atopic dermatitis and asthma. Arch Dis Child. 1996;75:494-497.
62 David TJ, Waddington E, Stanton RH. Nutritional hazards of elimination diets in children with atopic eczema. Arch Dis Child. 1984;59:323-325.
63 Katz KA, Mahlberg MJ, Honig PJ, et al. Rice nightmare: Kwashiorkor in two Philadelphia-area infants fed Rice Dream beverage. J Am Acad Dermatol. 2005;52(suppl 1):S69-S72.
64 Bu LM, Bradley M, Soderhall C, et al. Susceptibility loci for atopic dermatitis on chromosome 21 in a Swedish population. Allergy. 2006;61:617-621.
65 Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441-446. Epub 2006 Mar 19.
66 Hoffjan S, Epplen JT. The genetics of atopic dermatitis: recent findings and future options. J Mol Med. 2005;83:682-692. Epub 2005 May 18.
67 Chamlin SL, Frieden IJ, Fowler A, et al. Ceramide-dominant barrier repair lipids improve childhood atopic dermatitis. Arch Dermatol. 2001;137:1110-1112.
68 Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.