Chapter 5 Management of the Patient with Asthma
Definition
Asthma is defined as a chronic inflammatory disorder of the airways causing recurrent episodes of cough, wheeze, shortness of breath, and/or chest tightness.1 These episodes are associated with increased airway sensitivity to a variety of stimuli leading to variable airflow obstruction that is often either preventable or reversible with proper treatment. Clinical manifestations of asthma can be controlled with appropriate management. With asthma controlled, no more than seldom flare-ups should occur, with severe exacerbations presenting rarely, if ever.
This definition of asthma has evolved over the past three decades. Prior to the 1990s asthma was considered primarily a bronchospastic disease.2 The role of inflammation was not appreciated, and treatment paradigms therefore focused on management of the bronchospasm without consideration of addressing the underlying progressive chronic inflammation. With a better appreciation of the pathophysiology of asthma, effective treatments have been developed that treat not only the acute symptoms of asthma but also the underlying inflammatory disease at its core.
Epidemiology
Asthma affects approximately 300 million individuals worldwide. The World Health Organization (WHO) has estimated 15 million disability-adjusted life years are lost each year due to asthma, representing a staggering 1% of the total global disease burden. Asthma mortality is at 250 000 lives lost yearly irrespective of disease severity.3 There is also significant international, national, and regional variability in asthma prevalence, a phenomenon which is not well understood. The economic impact of the disease is driven by level of control and extent of emergent care, highlighting the need for a systematic approach to management.
Pathophysiology
Airway inflammation is the hallmark of disease pathophysiology in asthma. As with other allergic conditions, activated mast cells, eosinophils, and T cells (Th2 and invariant natural killer T cells) are associated with disease expression. Inherent structural cells, such as airway epithelial and smooth muscle cells, also can play a role in the disease pathology.4
Airway narrowing is the final common pathway that leads to the symptoms of asthma. In a variable and complex interplay, smooth muscle contraction, airway edema, airway thickening, and mucous hypersecretion may each play a role in airway narrowing.5
Diagnosis
The clinical diagnosis of asthma includes symptoms of breathlessness, wheezing, cough, and/or chest tightness.1 These symptoms are usually episodic and recur with allergen or irritant exposure, seasonal variability, exercise, and/or concomitant respiratory infection. A family history of asthma and atopy is helpful, but not necessary, in making the diagnosis. Clinical and/or pulmonary function response to bronchodilation assists the diagnosis confirmation. Symptoms can occur at any time of day but are more frequently worse at night due to the circadian pathophysiology described above. A useful set of questions is important for all clinicians to consider in making the diagnosis.
Cough may present as the sole symptom of asthma and in most cases should not be treated differently than other symptoms. The differential diagnosis for cough, is of course, quite broad and therefore the clinician should carefully rule out other causes if the clinical diagnostic picture is unclear. In conditions where lung function and its variability are normal, diagnoses such as chronic sinusitis, allergic rhinitis, gastroesophageal reflux, chronic rhinitis with postnasal drainage, vocal cord dysfunction, eosinophilic bronchitis, and cough associated with angiotensin-converting enzyme (ACE) inhibitors should be considered.
▪ Pulmonary Function Testing
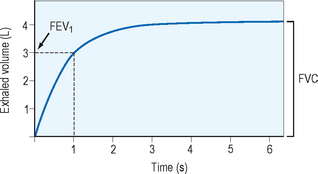
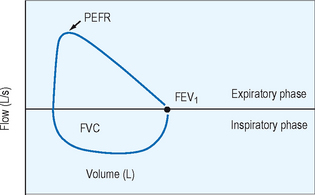
Spirometry provides the best assessment of airflow obstruction available to the clinician. Measurements of forced expiratory volume in one second (FEV1) in relation to the forced vital capacity (FVC), as well as forced expiratory flow and peak flow, are compared to population normative values to provide clinical comparison. Published recommendations for standardized testing are available. Spirometric testing quality is maximized by well and properly trained technicians and, for younger patients, interactive software. Because of anthropomorphic differences, population normative values chosen should consider ethnic variation. A normal spirometric tracing is represented in Figure 5.1. An example of a flow–volume loop is displayed in Figure 5.2.
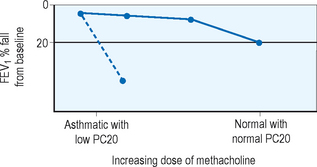
In patients who exhibit normal lung function without symptoms at the time of clinical evaluation, measurement of airway hyperresponsiveness is often helpful. Usually conducted within a specialist’s office or laboratory, the airway challenge is a sensitive tool which, if normal, helps to rule out the diagnosis of asthma. Various challenge agents are used such as methacholine, histamine, cold air, mannitol, or exercise to evaluate airway response. A 20% fall in FEV1 in relation to the quantity needed to provoke that fall gives the clinician a quantifiable value for airway sensitivity. An example of the change in FEV1 with methacholine challenge is displayed in Figure 5.3. Airway hyperresponsiveness has been described in illnesses other than asthma such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, and bronchiectasis so this tool, while sensitive, is not specific and the clinician should consider other diagnostic possibilities when faced with a positive test result.
Other Testing
Because of the strong association between asthma and allergic rhinitis, the presence of allergies, allergic diseases, and allergic rhinitis, in particular, increases the probability of a diagnosis of asthma in patients with respiratory symptoms. Moreover, the presence of allergies in asthma patients (identified by skin testing or measurement of specific IgE in serum) can help to identify risk factors that cause asthma symptoms in individual patients. Deliberate provocation of the airways with a suspected allergen or sensitizing agent may be helpful in the occupational setting, but is not routinely recommended, because it is rarely useful in establishing a diagnosis, requires considerable expertise, and can result in life-threatening bronchospasm. Skin testing (see Chapter 2) remains the primary diagnostic method to determine allergic status.
Asthma Control
Validated measures of assessing asthma clinical control (with and without the use of pulmonary function) are important clinical tools for every clinician. Examples of validated instruments are the Asthma Control Test (ACT; http://www.asthmacontrol.com), the Asthma Control Questionnaire (ACQ; http://www.qoltech.co.uk/Asthma1.htm), the Asthma Therapy Assessment Questionnaire (ATAQ; http://www.ataqinstrument.com), and the Asthma Control Scoring System.
Scoring System
The Global Initiative for Asthma (GINA) guidelines, published in 2006, suggest specific indicators of asthma control based on daytime and nighttime symptoms, limitations of activities, use of rescue medication, and lung function.6 These guidelines for the description of asthma control are displayed in Table 5.1.
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
The goals of optimal and successful asthma management, therefore, are to:
The expectations of asthma control for patient, family, and clinician should include:
Asthma Management
Current concepts in the management of the patient with asthma use therapeutic options based on the level of the patient’s asthma control, rather than directly on the severity of the disease as assessed by pulmonary function. Control is a more dynamic index of the patient’s current status, and can be more readily assessed and treated actively than through the use of a static concept of severity. The 2006 GINA guidelines therefore stress a control-based strategy for management of the asthmatic patient. In addition, the management of the patient with asthma requires an ongoing partnership between the physician, the patient, and the patient’s family that involves not only the manipulation of pharmacologic therapies, but also a dynamic strategy for bringing asthma under the best control that is practical. Components of this partnership are noted in Box 5.1.
BOX 5.1 Key components of the patient–doctor partnership (GINA 2006)6
Rights were not granted to include this data in electronic media. Please refer to the printed book.
Measures to prevent the development of asthma, acute and chronic asthma symptoms, and the progression of disease by avoiding and reducing exposure to risk factors should be a hallmark of asthma management and utilized whenever possible. The nonpharmacologic element of care is a critical component of disease control and must be discussed openly in patient–doctor communication.
Other than preventing tobacco exposure during pregnancy and postnatally, there are no widely accepted or proven interventions that prevent the development of asthma. Exacerbations of asthma are caused by a myriad of factors, or “triggers,” that include allergens, viral infections, pollutants or irritants, and medications. Reducing exposure to known triggers improves asthma control and reduces need for medication. Common indoor allergens include house dust mites, dander-laden pets, cockroaches, and fungi/mold. Evidence to reduce isolated allergen levels leading to clinical benefit is scanty (Table 5.2).7 Combined, targeted multiallergen reduction studies are yet to be conducted.
TABLE 5.2 Effectiveness of avoidance in certain indoor allergens (GINA 2006)6
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
Outdoor allergens are obviously difficult to avoid. A keen understanding of when certain tree, grass, weed, or plant allergens reach their seasonal zenith can assist the patient and family with self-management and control strategies. Outdoor air pollutants are known to aggravate asthma symptoms. Ozone, nitrogen oxides, acid aerosols, and particulate matter are all associated with symptoms and exacerbations of asthma. Community air quality policy and information can help asthma patients make critical decisions about outdoor activity and exercise that may affect asthma control.
Asthma Medications
Inhaled corticosteroids are the most effective asthma controller therapy. Their mechanism of action is via control or reduction of airway inflammation with demonstrated efficacy in reducing asthma symptoms, improving quality of life, improving lung function, decreasing airway hyperresponsiveness, reducing frequency and severity of exacerbations, and reducing asthma mortality. Despite their superiority, inhaled steroids are not a cure and up to 30% of asthma patients may have suboptimal response to therapy, and understanding controller treatment options is a critical component of the clinician’s asthma management. Inhaled steroids differ in potency and bioavailability, however lower doses are preferred given their relatively flat dose–response relationship and the desire to minimize potential medication side effect. Relative potencies of the inhaled corticosteroids are displayed in Table 5.3 for adults and in Table 5.4 for children.
TABLE 5.3 Estimated equipotent inhaled steroid dosing in adultsa (GINA 2006)6
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
TABLE 5.4 Estimated equipotent inhaled steroid dosing in childrena (GINA 2006)6
| Rights were not granted to include this data in electronic media. Please refer to the printed book. |
Mouth washing (rinsing with water, gargling, and spitting out) after inhalation may reduce oral candidiasis. The use of prodrugs that are activated in the lungs but not in the pharynx (e.g., ciclesonide), and new formulations and devices that reduce oropharyngeal deposition, may minimize such effects without the need for a spacer or mouth washing.
Inhaled glucocorticosteroids are absorbed from the lung, accounting for some degree of systemic bioavailability. The risk of systemic adverse effects from an inhaled steroid depends upon its dose and potency, the delivery system, systemic bioavailability, first-pass metabolism (conversion to inactive metabolites) in the liver, and half-life of the fraction of systemically absorbed drug (from the lung and possibly gut). Therefore, the systemic effects differ among the various inhaled steroids. Several comparative studies have demonstrated that ciclesonide, budesonide, and fluticasone propionate at equipotent doses have less systemic effect. Current evidence suggests that in adults, systemic effects of inhaled glucocorticosteroids are not a problem at doses of 400 μg or less budesonide or equivalent daily.
The regular use of β2-agonists in both short- and long-acting forms may lead to relative refractoriness to β2-agonists. Data indicating a possible increased risk of asthma-related death associated with the use of salmeterol led to advisories from the US Food and Drug Administration (FDA) and Health Canada that long-acting β2-agonists are not a substitute for first-line anti-inflammatory therapy, and should only be used in combination with an appropriate dose of inhaled steroid as determined by a physician. A study has identified that the asthma of subjects with an unusual genotype for the β-adrenergic receptor (with substitution of arginine for glycine at position B-16) may deteriorate with regular use of salmeterol whether or not administered with inhaled steroids.
An algorithm for asthma management based on control, based on principles drawn from the 2006 GINA guidelines, is shown in Figure 5.4.6
1 National Asthma Education and Prevention Program (NAEPP). Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol. 1991;88:425-534.
2 Tang E, Wiesch D, Samet J. Epidemiology of asthma and allergic disease. In: Adkinson NFJr, Yunginger JW, Busse WW, editors. Middleton’s Allergy: Principles and Practice. 6th edn. Philadelphia: Mosby; 2003:1127-1144.
3 Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination World Health Organization Committee Report. Allergy. 2004;59:469-478.
4 National Heart, Lung, and Blood Institute. Expert Panel Report 2. Guidelines for the Diagnosis and Management of Asthma, pub no. 97–4051. Bethesda, MD: National Institutes of Health, 1997.
5 Busse WW, Lemanske RFJr. Asthma. N Engl J Med. 2001;344:350-362.
6 Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2006. Online. Available: www.ginasthma.org.
7 Custovek A, Wijk RG. The effectiveness of measures to change the indoor environment in the treatment of allergic rhinitis and asthma: ARIA update. Allergy. 2005;60:1112-1115.