Chapter 15 Management of the Patient with Anaphylaxis
Introduction
The first documented case on possible anaphylaxis in humans occurred in 2640 Bc. Hieroglyphics suggest that the Egyptian pharaoh Menes may have died from a wasp sting.1 Anaphylaxis is a term first used by Richet and Portier in 1902. During attempts to immunize dogs to sea anemone venom, fatal reactions occurred using doses that previously were tolerated. The Greek term “phylaxis” means protection and “ana” translates into backwards. Since the fatal reactions were the opposite of the “phylaxis” they were trying to achieve, they used the term anaphylaxis to describe the opposite of protection.2 Unfortunately, a universally accepted definition of anaphylaxis still does not exist, and the point at which a severe “allergic reaction” becomes anaphylaxis remains unclear. This lack of a clear entity can cause confusion when examining medical literature or attempting to learn more about anaphylaxis. In the allergy treatment setting, the term “systemic reaction” is commonly used in place of “anaphylaxis.” This lack of a universal classification system limits our knowledge concerning the prevalence of this problem, contributes to its underrecognition by health care professionals, and probably results in the undertreatment of anaphylactic episodes.
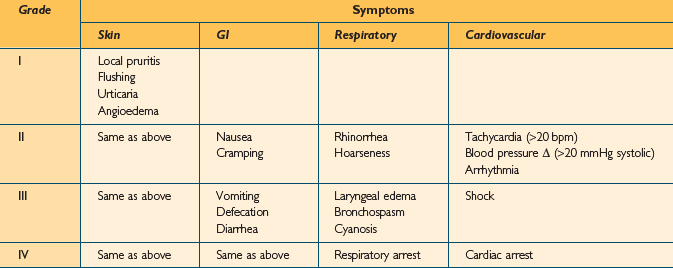
In an attempt to improve communication concerning the understanding of anaphylaxis, several authors have developed severity grading scales. Unfortunately, no single system has proven more useful than another, and none is universally accepted.3 Grading scales may be useful, however, especially when communicating among doctors, reporting results, or analyzing treatment options. One such grading scale is shown in Table 15.1. In Ring and Messner’s classification, grade I anaphylaxis is characterized by cutaneous manifestations and angioedema. In grade II anaphylaxis, milder respiratory and cardiovascular effects are noted. With grade III anaphylaxis, there is severe multisystem involvement, and finally, in grade IV, anaphylactic death is imminent. A similar, but simpler grading system was developed by Brown. In this system, anaphylaxis is divided into mild (generalized erythema, urticaria, periorbital edema, or angioedema), moderate (dyspnea, stridor, wheeze, nausea, vomiting, dizziness, diaphoresis, chest or throat tightness, or abdominal pain), and severe (hypoxia, hypotension, neurologic compromise, cyanosis, confusion, collapse, loss of consciousness, incontinence).4
Etiology
A large variety of agents are capable of eliciting anaphylactic reactions. By definition, IgE-mediated anaphylaxis requires prior exposure and sensitization. Non-IgE-mediated (anaphylactoid) reactions do not require antigen processing or prior exposure. Common causes of anaphylaxis are foods, drugs, insect venoms, and immunotherapy (IT) (Box 15.1), with foods appearing to be the most common cause.5 Of foods, the usual culprits are tree nuts, peanuts, milk, eggs, soy, wheat, shellfish, and fish. As for drugs, the most common instigators for anaphylaxis include beta lactam antibiotics, anesthetic drugs, opioid analgesics, radiocontrast media, NSAIDs, and aspirin. Drug reactions in the operating room are also possible. In the perioperative period and during medical procedures, the risk of anaphylaxis is somewhere between 1 in 4500 and 1 in 25 000, with a fatality rate between 3% and 6%. The most common agents implicated in surgical procedures are muscle relaxants and latex.6 Exercise can also cause anaphylaxis.7 Finally, a likely cause for anaphylaxis cannot be found for many episodes, and these cases are considered idiopathic.
Epidemiology
The epidemiology of anaphylaxis is incompletely understood due to the variability in case definition and differences in methodology. Additionally, many cases of anaphylaxis are probably never diagnosed. With these caveats, a fairly broad description of the incidence and etiologies of anaphylactic and anaphylactoid reactions is possible. Yocum et al studied the incidence of anaphylaxis in a defined population in Olmstead County, Minnesota over a 5-year period and found an average annual incidence of 21 cases per 100 000 persons per year. The case fatality rate was 0.65%.8 Analysis of data from multiple countries yielded an incidence rate of 1–3 cases of severe anaphylaxis per 10 000 people and a fatal anaphylaxis rate of 1–3 per million people. These rates included anaphylaxis from all causes.9 It is estimated that 1.24–16.8% of the US population may suffer an anaphylactic reaction, and 0.002% may die. It is has been reported that there are up to 1500 deaths per year in the USA from anaphylaxis.10
Neugut et al published a review of anaphylaxis in the USA.10 Several points of interest can be found in that text about the various etiologies of anaphylaxis. Approximately 1% of the US population suffers from food allergies, and approximately 0.0004% of the population has a severe allergic or anaphylactic reaction each year, with about 100 deaths per year. Penicillin is the most common drug that causes anaphylaxis, and one report suggests that penicillin causes approximately 75% of all fatal anaphylactic cases in the USA each year. Penicillin causes fatal anaphylaxis at a rate of 0.002% among the general population (about 500–1000 deaths per year), and nonfatal penicillin-induced anaphylaxis may occur in 0.7–10% of the population taking penicillin. Radiocontrast media causes anaphylactoid reactions in 0.22–1% of exposures. Newer nonionic contrast media have lower rates however. Latex allergy has become more common over the last few decades, mostly due to the increasing use of latex gloves for universal precautions in the health care setting. The annual incidence of latex-induced anaphylactic reactions is estimated to be 220 per year. Stinging insects, especially those in the hymenoptera, are common causes of anaphylaxis. These insects include bees, wasps, hornets, and ants. Anaphylaxis from hymenoptera stings may occur in 0.5%–5% of stings, resulting in approximately 40–100 deaths per year in the USA. On subsequent stings, individuals who have had an anaphylactic reaction have a 30–60% chance of another anaphylactic reaction. In patients who receive venom immunotherapy this risk decreases to 3%.
One of the greatest risk factors for anaphylaxis appears to be atopy.8,11 Atopic patients are also at greater risk for anaphylactoid reactions (such as those from radiocontrast media, for example), even though these reactions are not necessarily IgE mediated. It is postulated that increased production of interleukin (IL) 4, IL-5, and IL-13 may make the atopic patient prone to mast cell mediator release, as these cytokines have effects on mast cell degranulation.11 Although anaphylaxis seems most common in adults, incidence varies in different age groups based on the various triggers. Gender may play a role as well, as anaphylaxis is more common in males under 16 and females over 30.11 Asthma is also a significant risk factor for anaphylaxis, and asthmatics may be more prone to respiratory involvement in anaphylactic episodes.
Probably the greatest concern of the clinical allergist, with regard to anaphylaxis, is the occurrence of anaphylactic responses to antigen immunotherapy. Unfortunately, as mentioned above, the allergy patient is at higher risk for an anaphylactic response to injected antigen immunotherapy. In the allergy treatment setting, anaphylactic reactions are often referred to as “systemic reactions.” Subcutaneous antigen immunotherapy can be fatal, and proper practices are required to minimize risk to the patient. Information about the incidence of fatal reactions to immunotherapy injections is relatively sparse. In a study including 36 359 injections of specific antigen immunotherapy with aluminum-adsorbed vaccine, there was an incidence of serious systemic side effects in approximately 0.1% of all injections.12 All severe reactions occurred within 30 minutes of injection. Among these allergy patients, asthmatic subjects proved at higher risk for systemic reaction, with most episodes occurring during dose escalation. However, there was no risk associated with age, gender, degree of skin test reactivity, or allergen injected. Survey studies conducted by the American Academy of Allergy and Immunology (AAAI) revealed a total of 40 deaths after skin testing or immunotherapy in the period from 1945 to 1983, 17 immunotherapy-associated deaths from 1985 to 1989, 10 deaths from 1990 to 1991, and six deaths from 1992 to 1996. The risk of death in American patients receiving immunotherapy from 1985 to 1989 was estimated to be 1 for every 2 million injections. In 1997, the American College of Allergy, Asthma and Immunology (ACAAI) estimated that deaths from immunotherapy occurred at a rate of 0.67 per 1 million injections.13 Other studies from England and Germany in the 1980s seemed to show a spike in immunotherapy deaths attributable to new, higher potency, standardized extracts.13 In the US survey covering 1985 to 1989, 77% of fatalities occurred in patients with asthma, even though the vast majority of patients were being treated for rhinitis.13 In all of the survey studies, there was a high proportion of fatalities from those who suffered from asthma or whose asthma was steroid dependent or poorly controlled. This occurrence makes it clear that symptomatic or severe asthma is a risk factor for severe systemic reactions to immunotherapy. Another important point is that most fatal immunotherapy reactions occur during the dose escalation phase, rather than the maintenance phase, of immunotherapy. Similarly, there is an increased frequency of nonfatal systemic reactions during dose escalation. There is little information about the incidence of anaphylaxis using different dose escalation protocols. However, rushed protocols appear to be associated with an increased rate of systemic reactions.13
High levels of sensitivity to the injected allergen have been found in a large portion of fatal cases, but as mentioned previously, skin sensitivity does not necessarily predict systemic reactions. Fatal cases appear more likely during a patient’s allergy season. The European Academy of Allergology and Clinical Immunology (EAACI) and the American Academy of Allergy, Asthma and Immunology (AAAAI) practice parameters both recommend reducing the seasonal pollen doses administered during a patient’s pollen season. Beginning a new vial during immunotherapy is also a risk factor for systemic reactions and is associated with fatalities, probably because of variability in extract potency associated with lot-to-lot variation or mixing errors. In some studies, pollen and dust mite antigens are more likely to provoke a severe or fatal systemic reaction than hymenoptera venom immunotherapy,13 but whether particular antigens are more likely to provoke a systemic reaction is controversial. Venom immunotherapy, in which subjects have already suffered an anaphylactic reaction prompting their need for immunotherapy, is a special case. Rush protocols are often employed to yield protection as soon as possible. Perhaps because of the frequent use of rush protocols, the incidence of systemic reactions during hymenoptera venom immunotherapy may be as high as 35%.14 Depot allergens may be less likely to provoke a systemic reaction than aqueous extracts, but these allergens are not available in the USA.12 One important risk factor for a systemic reaction to an immunotherapy injection is a previous reaction to an immunotherapy injection.13 Patients who have had systemic reactions should have their dosage reduced on subsequent administration. Most severe systemic reactions after immunotherapy injections occur within 30 minutes, and patients who had systemic reactions should be observed for this length of time so that medical intervention can be promptly initiated, if necessary. Above all, it must be noted that human error is one of the greatest risk factors for fatal reactions to immunotherapy.13
The largest study concerned with the safety of allergy immunotherapy was conducted by a group of physicians in the American Academy of Otolaryngic Allergy.15 The study evaluated the overall safety of immunotherapy given in the office environment and at home. This study included 1144 000 injections given in the prescribing physician’s office, at home or in other physician’s offices. The overall minor reaction rate was 0.009%, while the rate of major reactions was 0.005%. There were no hospitalizations and no deaths. Due to proper selection of patients eligible for home injections, the rate of reactions was less in the home injection group compared to the office injection group. The study also identified risk factors for major reactions during immunotherapy. Table 15.2 lists the percentages of patients experiencing a major reaction who exhibited each risk factor in this study.
TABLE 15.2 Major risk factors for immunotherapy-related anaphylaxis
| Risk factor | % of major reactions associated with risk factor |
|---|---|
| Build up immunotherapy | 90 |
| Active asthma | 46 |
| New vial, first injection | 10 |
| Prior systemic reaction | 7 |
| Vial prepared in another office | 6 |
| Beta-blocker treatment | 4 |
| Error (wrong patient’s vial) | 3 |
Immunology and Pathophysiology
Systemic anaphylaxis results from the release of mast cell and basophil mediators in sufficient quantity to evoke a systemic response involving multiple end organs. Anaphylaxis begins when antigen cross-linking of receptor-bound IgE causes mast cell mediator release. The exact mechanisms and thresholds required to initiate anaphylaxis after sensitizing antigen exposure are uncertain. This IgE-mediated mechanism of anaphylaxis requires systemic distribution of the offending agent, hence parenteral or enteral exposures are common routes for anaphylactic reactions. The signs and symptoms elicited by mast cell mediator release depend on the organ system in which those mast cells reside: skin, gastrointestinal tract, respiratory tract, and cardiovascular system. Mast cells in perivascular locations can have a significant effect on hemodynamics. Other cell types, including basophils, monocytes, eosinophils, antigen presenting cells, and epithelial cells, may participate in this process and affect the duration and intensity of the reaction with their interactions and secreted products.
One of the most important mediators is histamine, which causes vasodilation, increased vascular permeability, mucous hypersecretion, smooth muscle spasm, and eosinophil chemotaxis and activation. Serum histamine levels correlate with the severity of cardiopulmonary manifestations and GI manifestations in anaphylaxis episodes.16 Histamine mediates its effects through H1 and H2 receptors. Histamine causes extravasation of blood volume and decreased peripheral vascular resistance. Tryptase, chymase, heparin, and other chemokines and chemotactic factors are also involved. These above mediators activate other inflammatory systems, but may also have an attenuating role in the chain reaction of inflammatory events in an anaphylactic episode.11
Mast cell degranulation products can activate other important biochemical pathways that contribute to an anaphylactic episode, like the kininogen–kallikrein system, coagulation cascade, and the complement cascade through the actions of tryptase.11 Tryptase, which is stored in mast cell secretory granules, can activate the kinin system, clotting cascade, and complement cascade.17 Levels of C4 and C3 decrease in anaphylaxis, and increases in C3a have been measured, suggesting that complement activation plays a role in the process. Factors V, XIII, and fibrinogen decrease with anaphylaxis, suggesting involvement of these systems in anaphylactic episodes.16 Nitric oxide production is dramatically increased in anaphylactic episodes. The net effect of nitric oxide production is vasodilation and increased vascular permeability.11 In addition to preformed mediator release from mast cells, newly generated lipid mediators, including leukotrienes (LT) B4, C4, D4, E4, platelet activating factor, prostaglandin D2, and others, are involved. LT B4 is chemotactic and may play a role in the late manifestations of anaphylaxis. Recurrent or biphasic anaphylaxis may be secondary to inflammatory cell activation and recruitment (like eosinophils) and may occur 12 hours after the initial attack.16
Anaphylaxis is primarily an IgE-mediated phenomenon, but can involve IgG or IgM antibodies.3 In mouse models of anaphylaxis, there is an IgG-dependent pathway that depends on the involvement of macrophages, which secrete platelet activating factor.18 Antigen–antibody complexes may activate complement and trigger “immune aggregate anaphylaxis,” and this mechanism is implicated in anaphylactoid reactions to protamine, dextran, and albumin.17
Some anaphylactoid reactions due to drugs, exercise, or physical factors may be due to direct release of mediators from mast cells. Aspirin- and NSAID-induced anaphylactoid reactions are due to altered arachidonic acid metabolism. It is thought that inhibited production of prostaglandin E (which prevents mast cell degranulation) and excess production of leukotriene C (which stimulates degranulation) is responsible for mediator release in NSAID-induced anaphylactoid reactions. Radiocontrast material may provoke anaphylactoid reactions by activating multiple systems, including the kallikrein–kinin, clotting system, and complement system.17 Non-IgE-mediated complement activation can occur from exposure to radiocontrast media, liposomal drug preparations, NSAIDs, and other drugs in a reaction now called CARPA (C activation-related pseudo-allergy). A CARPA reaction can occur on first exposure to an agent. In the case of radiocontrast media, the classical and alternative complement pathways are activated.19
Physiologic Changes in Anaphylaxis
The main cardiovascular clinical feature of anaphylaxis is hypotension. Anaphylactic hypotension is due to fluid extravasation and vasodilation, resulting in a mixed distributive-hypovolemic shock. There may be vasodilation, reversible cardiac depression, and paradoxically, bradycardia.20 In anaphylaxis, blood pressure declines and heart rate increases, but depleted intravascular volume leads to worsening cardiac output. While peripheral vascular resistance initially decreases, it may recover, perhaps due to endogenous epinephrine, angiotensin II, or endothelin.11 With anaphylaxis, massive fluid shifts occur that affect cardiovascular stability. There may be up to a 50% decrease in circulating blood volume as quickly as 10 minutes after the onset of symptoms.16
Anaphylactic shock is a severe and prolonged hypotension caused by mediators such as histamine and prostaglandins released by tissue mast cells and circulating basophils. These mediators cause vasodilation and increased capillary permeability. In addition to the hemodynamic effects mentioned, mediators of anaphylaxis directly affect the heart. H1 receptors can “mediate” coronary vasoconstriction, and H2 receptors can increase atrial and ventricular contractility, the sinoatrial node firing rate, and coronary artery dilation. Platelet activating factor decreases coronary blood flow and decreases cardiac output.16 Histamine and other mediator production are affected by beta-adrenergic neurohumoral mechanisms acting via cyclic AMP (adenosine monophosphate). Beta-blockade may increase the synthesis and release of anaphylactic mediators, increase the sensitivity of peripheral end organs, and in experimental anaphylaxis increase the risk of death.21 Beta-blockers prevent the salutary effects of epinephrine (adrenaline), when given in an anaphylactic emergency. Unopposed alpha-adrenergic stimulation from epinephrine may cause coronary constriction or exaggerate the systemic pressor effects of epinephrine. Finally, beta-blocker treatment may increase the risk of anaphylaxis up to threefold in patients receiving immunotherapy.21
In anaphylactic deaths, pathologic findings include laryngeal edema, mucous plugging and hyperinflated lungs, and myocardial damage. Also, a dilated right ventricle, eosinophilia in the pulmonary vessels and GI tract, as well as congestion of abdominal viscera are noted. These findings indicate that death from anaphylaxis is usually a result of cardiovascular collapse or upper/lower airway obstruction.17
Signs and Symptoms of Anaphylaxis
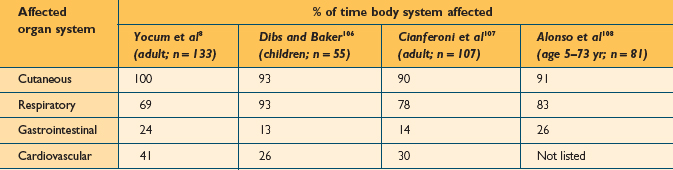
The clinical manifestations of anaphylaxis occur as a result of the systemic release of mediators from basophils and mast cells, and therefore involve some predictable signs and symptoms in organs with high concentrations of these cells.22 The upper and lower airways, skin, conjunctiva, gastrointestinal and cardiovascular systems are often affected individually or in combination. The most common signs and symptoms have been described in several publications. Kemp et al published the largest series detailing the clinical manifestations of anaphylactic reactions in 266 patients. The findings of this study are listed in Table 15.3. Several other publications have reported on the organ systems commonly affected during anaphylactic reactions in smaller series of patients (Table 15.4). The onset of symptoms of anaphylaxis is usually fairly rapid. When an antigen is introduced by injection, symptoms typically begin within 5–20 minutes,23 but more delayed initial reactions can certainly occur. It is rare for anaphylaxis to occur beyond 60 minutes after antigen injection.23,24 Ingestion of offending foods and drugs tends to result in a slower onset of anaphylaxis. It may take several hours after ingestion for anaphylaxis symptoms to begin, but in some cases, there can be rapid onset within minutes. The median time for anaphylaxis to occur after an insect sting is 15 minutes.10,25,26 It is generally considered that the more rapid the onset, the more severe the overall reaction.10,27,28
| % of patients affected (Kemp et al105; n = 266) | |
|---|---|
| Urticaria, angioedema | 90 |
| Shortness of breath, wheezing | 60 |
| Dizziness, syncope | 29 |
| Flushed skin | 28 |
| Abdominal cramps, diarrhea | 26 |
| Laryngeal or tongue edema, choking dysphagia | 24 |
| Nausea, emesis | 20 |
| Hypotension | 20 |
| Rhinitis symptoms | 16 |
| Conjunctivitis symptoms, periorbital edema | 12 |
| Substernal or esophageal discomfort | 6 |
| Headache | 5 |
| Generalized pruritis (no rash) | 4 |
| Vision change | 2 |
| Seizure | 2 |
Cutaneous involvement is by far the most common finding with anaphylaxis. Urticaria and edema are the most common cutaneous manifestations. Although these skin reactions are almost always present, the lack of skin involvement cannot completely rule out anaphylaxis. Some authors have reported series of patients who presented with severe anaphylaxis without known cutaneous symptoms.29,30
The respiratory tract is also commonly involved in an anaphylactic reaction. Exacerbation of nasal allergy symptoms often occurs early in the process. A person might experience a feeling of tightness in the throat. The voice may become hoarse as edema develops in the larynx. Lower airway involvement leads to cough, wheezing, and dyspnea as anaphylaxis progresses.31
Tachycardia is usually a reliable early sign of cardiovascular involvement due to a decrease in effective vascular volume. Skin flushing and erythema from vasodilation may occur. As circulatory collapse ensues, the skin may become cool and clammy. Hypotension can result in multiple generalized symptoms, including dizziness, confusion, nausea, emesis, and abdominal cramps. Coronary vasospasm can result in chest pain, arrhythmias, and myocardial infarction.32,33 In severe anaphylaxis, cardiovascular shock is the endpoint, with multiple organ failure.20
Anaphylaxis may occur in biphasic or protracted patterns. After recovering from an initial episode of anaphylaxis, recurrent symptoms may develop. This process is known as biphasic anaphylaxis. This is thought to be a consequence of the classic late-phase allergic reaction. The recurrent symptoms begin from 1 to 28 hours after resolution of the initial episode. The secondary reaction usually involves the same organ symptoms as the original reaction.34 The true incidence of biphasic anaphylaxis is not known. The reported frequency varies from 4 to 23% of anaphylaxis cases. The highest reported frequency (23%) occurred with food reactions. It has been reported that a biphasic reaction is more likely to occur if the initial reaction is severe. A biphasic reaction occurs in up to 30% of severe food-induced anaphylaxis cases.35 Allergen injections have a much lower reported frequency, occurring in about 4% of anaphylaxis cases.32 Due to the possibility of biphasic reactions, the clinician should consider a 12- to 24-hour hospital observation of a patient after the successful treatment of an initial episode of anaphylaxis.36,37 Protracted anaphylaxis can also occur, with prolonged symptoms lasting 5–32 hours. Protracted anaphylaxis usually involves prolonged respiratory distress or hypotension.32
Differential Diagnosis
Anaphylactoid reactions produce symptom complexes identical to anaphylaxis, but are not mediated by an IgE allergic process. Anaphylactoid reactions can be triggered by direct exposure to allergic mediators, such as in scombroid food poisoning, but most cases are thought to be triggered by exposure to substances that cause a direct, nonallergic release of allergic mediators.38 Multiple drugs, such as narcotics and vancomycin, have been reported to cause anaphylactoid reactions by stimulating release of allergic mediators.39 Radiologic contrast agents are also a relatively common cause of this type of reaction. There are multiple other substances that have been reported as possible initiators of anaphylactoid reactions. Despite the cause, anaphylactoid reactions have a clinical presentation exactly like anaphylaxis, and are treated in the same manner.40
Many conditions need to be considered in the differential diagnosis of anaphylaxis (Box 15.2). Many of these conditions are not likely to occur in the physician’s office, but are important when trying to determine the cause of a previous event. Vasovagal reactions are a fairly common occurrence. The exact mechanism of this reaction is not known, but it is often related to an emotionally disturbing or traumatic event. The increased vagal tone results in hypotension, bradycardia, vasodilation, and if severe enough, can result in loss of consciousness.41 A patient usually will report feeling “funny” or lightheaded and nauseated. Characteristic findings include slow pulse, pale skin, cold sweating, and normal recumbent blood pressure. Several features help distinguish vasovagal reactions from anaphylaxis. Patients with vasodepressor reactions typically will have normal recumbent blood pressures, instead of the hypotension commonly observed in anaphylaxis. Over 90% of patients with anaphylaxis have cutaneous manifestations, such as itching, urticaria, or flushing. Vasovagal reactions do not include these skin findings. Although bradycardia and skin pallor can occur with anaphylaxis, they are not common findings.42 Respiratory symptoms, such as cough or wheezing, commonly seen in anaphylaxis are absent in the vasovagal response.
Scombroid fish poisoning can occur after ingesting spoiled fish.43 Bacteria infesting the fish produce enzymes that result in the formation of histamine. Scombroid poisoning commonly results in skin flushing, urticaria, nausea, vomiting, and diarrhea. Other substances in foods, such as monosodium glutamate (MSG) and sulfites, can cause reactions that may be confused with anaphylaxis.
Laboratory evaluation is usually not practical, but can be used in some instances to help distinguish anaphylaxis from mimicking conditions. Histamine levels can be measured in the serum, but it has a short half-life and will only remain elevated for about 60 minutes.44 Tryptase is released from mast cells during anaphylaxis and remains elevated for several hours after the event.45 Serum IgE levels can sometimes help identify a cause of anaphylaxis. This can be particularly useful when there is exposure to a particular food within a few hours prior to development of anaphylaxis symptoms.46 Serum IgE levels should not replace clinical observations. When there is an observed relation of single food ingestion, such as plain peanuts, to immediate onset of anaphylaxis, the food should be considered the cause despite the findings of serum IgE testing.
Prevention of Anaphylaxis
Certain medications should be avoided by persons who have experienced anaphylaxis. These drugs may increase the chances of a severe reaction, and complicate treatment of anaphylaxis should it occur. Beta-blockers, tricyclic antidepressants, monoamine oxidase inhibitors, and angiotensin-converting enzyme (ACE) inhibitors are examples of medications that should be avoided if possible. Beta-blockers can amplify an allergic response by increasing the production of allergic mediators. Beta-blockers also complicate the treatment of anaphylaxis by blocking some of the beneficial effects of epinephrine, such as smooth muscle relaxation of the respiratory tract.21,47 Beta-blockade can also cause hypertensive crisis when epinephrine is administered by allowing unopposed alpha-adrenergic stimulation. Monoamine oxidase inhibitors prevent degradation of catecholamines, such as epinephrine. Severe hypertensive crisis may result when standard doses of epinephrine are administered during treatment of anaphylaxis. Tricyclic antidepressants prevent catecholamine reuptake at nerve junctions, resulting in a relative increase in the beta-adrenergic effects of epinephrine. This increases the risk of hypertensive crisis and arrhythmias. Tricyclic antidepressants may also suppress the alpha-adrenergic effects of vasopressor agents given to treat hypotension during anaphylaxis.48 ACE inhibitors have the potential to potentiate bradykinin-mediated reactions which are involved in anaphylactic reactions.40,49 ACE inhibitors have been associated with increased risk of developing angioedema, which is sometimes confused with an anaphylactic reaction.49,50
Several measures can be taken to decrease the risk of anaphylaxis related to allergy testing and immunotherapy. Physicians should begin by recognizing the factors that increase the risk of anaphylaxis (Table 15.2). Patients who have brittle medical conditions should be tested and treated with more caution.51 Allergic asthma is a common indication for immunotherapy. Uncontrolled asthma is a significant risk factor for anaphylaxis and extra watchfulness is necessary.52,53 Additional caution should be used with skin testing or giving immunotherapy whenever there is high environmental exposure to an allergen, such as during peak allergen season. It is important to minimize variations in the antigen potency in testing and treatment vials. Testing and treatment sets should be remixed at appropriate intervals to prevent loss of antigen potency due to degradation. Variations in vial antigenicity can also be reduced by the use of glycerin preservative, refrigeration, and proper dating of vials. It is wise to use the same manufacturer when purchasing replacement antigen. Consideration should be given to repeat testing if a new lot of antigen or different antigen strength is purchased before continuing with allergen injections. Since the first injection from a new vial is more likely to result in a major reaction than subsequent injections, an intradermal vial test should be considered when beginning injections from a new vial.54 It is prudent to observe a patient in the office for 20–30 minutes after an immunotherapy injection, since most anaphylactic reactions occur within that time period.
The safety study conducted by Hurst et al (previously described in this chapter) demonstrated the low incidence of severe reactions when proper precautions and techniques are utilized during allergen immunotherapy.15 The relative low risk of anaphylaxis reported in this study should not result in laxity with safety precautions when prescribing allergen immunotherapy. Instead, this study should be a confirmation that careful attention to safety measures is important to minimize the risk of these life-threatening reactions.
Treatment of Anaphylaxis
Treatment of anaphylaxis in the office begins with proper preparation in advance. As with most office procedures, it is helpful to have a plan already in place for treating allergy emergencies. The plan should give detailed instructions on the role of each office employee. Every employee who may be involved should know where the allergy emergency equipment is located in the office. One employee should be in charge of making sure the emergency equipment and medication is up to date at regular intervals, such as every 3 months. It is also a good idea to have all office personnel trained in basic cardiopulmonary resuscitation (CPR). If the office is located more than a few miles from a hospital, the physician should consider becoming certified in advanced cardiac life support (ACLS). Having personnel in the office with CPR and ACLS training may improve the outcome in the event an anaphylactic reaction occurs.36 Consideration should be given to having occasional drills to make sure everyone in the office feels comfortable with their role in the process.
If an anaphylactic reaction occurs, the primary goal of the physician should be to quickly recognize the problem and initiate the proper emergency care to stabilize the patient until the patient can be transferred to a hospital with emergency care specialists and comprehensive resuscitation equipment. To accomplish this goal, the physician needs to be aware of the most likely causes of death from an anaphylactic reaction, and be prepared to intervene appropriately. Death from anaphylaxis most frequently occurs from intractable asthma or upper airway edema, with resultant hypoxia. The remaining deaths are related to cardiovascular failure.21 The primary cardiovascular change during anaphylaxis is vasodilation and fluid extravasation resulting in hypotension. Myocardial dysfunction is another common finding in anaphylaxis which may be due to ischemia from low blood pressure.20 The physician should therefore maintain basic supplies to establish an emergency airway, aggressively treat bronchial constriction, and support cardiac and circulatory function.
In order to provide appropriate treatment of anaphylaxis, equipment and medications need to be available. Several factors help determine what equipment and medications should be maintained in a particular office. From a legal standpoint, many state medical societies have a list of minimum supplies which must be available in an office depending on the services provided in the office. On a more practical basis, the distance of the office from definitive hospital care has a large effect on the amount of advanced equipment needed. The further an office is from a hospital, the more equipment necessary to support a patient until help arrives. Several organizations have released position papers detailing what equipment should be available.55–57 Box 15.3 is a list of equipment for treatment of anaphylaxis which is based on these published position papers, as well as opinion by the authors. It is not mandatory to have every item in the list. The amount of equipment necessary in each office can be modified based on the physicians’ assessments of their particular circumstances. It is helpful to have the supplies and equipment stored in one location for easy access in the case of an emergency.
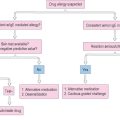
Due to the nature of anaphylaxis, it is difficult to develop controlled, randomized clinical trials to evaluate treatment algorithms. The treatment of anaphylaxis has been based on animal models and clinical experience blended with an understanding of the mechanisms involved in anaphylaxis.58 Medications are used to counteract these pathologic mechanisms. It is useful to have a written basic anaphylaxis treatment algorithm stored with the equipment for quick reference if necessary (Box 15.4). Box 15.5 lists the basic drugs commonly used in the treatment of anaphylaxis. A more extensive list of medications, including common doses, is provided in Box 15.6. As in any medical emergency, treatment should start with basic life support measures. Once the diagnosis of anaphylaxis is established, an ambulance should be immediately called for transport to a hospital setting. Next, the person’s airway and breathing should be assessed, and supported as necessary. Pulse and blood pressure measurements are used to assess circulation. The patient should be placed in a supine position with the feet elevated to increase venous return. If anaphylaxis has been caused by allergen injection, a tourniquet should be applied proximal to the injection site to reduce the rate of allergen absorption.40 Every 5 minutes the tourniquet should be loosened for several minutes. A dilute solution of epinephrine can also be injected around the allergen administration site to further decrease absorption. A local anesthetic premixed with epinephrine is commonly available and sufficient for this task. The injection of epinephrine around the allergen administration site should not delay or take place of administering a therapeutic dose of epinephrine.
BOX 15.4 Anaphylaxis treatment protocol
BOX 15.5 Basic medications needed for office management of anaphylaxis
BOX 15.6 Medications utilized in the management of anaphylaxis
The most important drug in the treatment of anaphylaxis is epinephrine.31 Epinephrine has multiple pharmacological effects that may be beneficial during an anaphylactic reaction. It increases blood pressure by several potential mechanisms. It has both inotropic and chronotropic effects on the heart. It also causes constriction of the precapillary vessels in the skin, kidney, and mucosa. Epinephrine has been shown to increase coronary blood flow. Epinephrine dilates smooth muscle, which can be beneficial in reversing bronchoconstriction during anaphylaxis. Epinephrine may also prevent mast cell and basophil degranulation and inflammatory mediator release.59
Several studies have addressed the route of administration of epinephrine.60–62 The preferred route of administration is intramuscular (IM). It has been shown that maximum plasma concentrations occur up to four times faster with intramuscular versus subcutaneous administration. Local vasoconstriction may slow absorption when epinephrine is given subcutaneously. Injection in the vastus lateralis muscle of the lateral thigh is preferred due to its rich vascularity. The typical dose for intramuscular injection in adults is 0.3–0.5 ml of a 1 : 1000 solution. For children, the IM dose is 0.01–0.03 mg/kg, which corresponds to 0.1–0.3 ml of a 1 : 1000 solution. These IM doses can be obtained from single or multi-use 1 : 1000 epinephrine vials. Some physicians stock commercially available pediatric and adult 1 : 1000 epinephrine auto-injectors in their emergency kit for quick use. Intravenous administration provides rapid systemic distribution, but has been associated with induction of cardiac arrhythmias and myocardial infarction. Intravenous injection should therefore be reserved for severe anaphylaxis and hypotension not responding to intramuscular administration.63 There is no established intravenous dose, though several regimens have been published.64–66 The following is a suggested IV protocol. To prepare a 1 : 10 000 solution, epinephrine 1 : 1000 solution can be diluted with 9 ml of normal saline. A 1 : 10 000 solution preloaded syringe is also commercially available. The 1 : 10 000 epinephrine solution can then be administered at a dose of 1–3 ml, repeated every 5–10 minutes as needed. Epinephrine is rapidly absorbed when administered near the carina through an endotracheal tube. An intravenous dose should be utilized via this route.67 Systemic availability with pressurized inhalation of epinephrine has shown conflicting results.63 A recent study reported on the use of a sublingual epinephrine tablet in an animal model.68 This study demonstrated high levels of plasma epinephrine with a rapid peak, similar to those found with intramuscular injections. This study suggests that the sublingual route may be an acceptable alternative route for epinephrine administration in the future.
It is important to administer epinephrine early during an anaphylactic reaction. Several publications site the increased risk of fatal outcomes when epinephrine is not given early during the course of anaphylaxis.36,69 It has been postulated that the delayed administration of epinephrine stems from a fear of the potential harmful effects of epinephrine. The benefits of epinephrine use have been documented to far outweigh the potential harmful side effects when used during anaphylaxis treatment.37
Oxygen is another important intervention during the treatment of anaphylaxis. Since hypoxemia may initiate or worsen cardiovascular collapse, supplemental oxygen may be helpful to decrease this effect. It has been reported that it is better to start with low flow oxygen, increasing to 10–15 liter per minute (via face mask) if impending shock develops. Ventilation support may also become necessary. Laryngeal edema and bronchospasm are common finding with anaphylaxis. Upper airway edema has been documented in 40–60% of post-mortem exams in patients who succumbed to anaphylaxis.70,71 Bronchial obstruction was found in 50% of cases. It is therefore important to aggressively treat airway and ventilation compromise during anaphylaxis. Epinephrine is effective in treating bronchospasm, as previously described. Beta agonist inhalers, such as albuterol, can be effective in overcoming bronchospasm, though multiple administrations may be required.31,72 If bronchospasm does not adequately reverse with albuterol, inhaled ipratropium may be a useful adjunctive treatment, especially in patients on beta-blockers. Ipratropium is an anticholinergic agent and is not affected by beta-blockade. Higher than normal doses (up to 15–30 puffs) may be necessary to achieve the desired response.72 Studies have also suggested a synergistic effect when beta agonists and anticholinergic agents are used in combination.73 Aminophylline has commonly been used in the treatment of bronchospasm, but multiple publications have questioned its use in emergency settings.74,75 Other adjunctive measures for resistant bronchospasm have been described and will be detailed later in a section describing alternative treatments in beta-blockaded patients.
Although not nearly as important as epinephrine, antihistamines can be useful adjunctive medications.76–78 Antihistamines are not only useful in relieving skin symptoms, such as itching and urticaria, they may also counteract some of the negative effects of histamine on myocardial function.79,80 Studies have shown superior results when combination H1 and H2 antihistamine agents are used concomitantly.76,77 Intravenous antihistamines should be administered slowly to reduce potential adverse cardiovascular effects. H2 antihistamines administered alone may be detrimental during anaphylaxis, so they should not be administered alone, and H1 agents should be administered first.
Hypotension can be severe, protracted and resistant to therapy during anaphylaxis. As previously described, hypotension can result from a combination of decreased cardiac function, loss of vascular tone, and shift of intravascular volume to extravascular spaces. Replacement of intravascular volume is the most important first step in treating hypotension. Large volumes of fluid should be rapidly administered. There is debate about whether crystalloid versus colloid fluids should be administered.81 An approach often used is to first administer crystalloid fluids, such as normal saline or lactated Ringer’s solution. In adults, roughly 1000–2000 ml should be administered first. This can be increased to 20 ml/kg if necessary. The rate should be adjusted based on overall medical conditions and blood pressure response.40 Children may require up to 30 ml/kg in the first hour, once again titrated to blood pressure effects.82 If blood pressure is not responding, then a colloid agent (such as those containing dextrose or albumin) may need to be infused.40 Periodic re-dosing of epinephrine can also be used to help support pressure. For persistent hypotension not responsive to fluid administration, additional vasopressor agents may be necessary. Dopamine is considered the vasopressor of choice. It should be administered intravenously at a rate of 2–20 μg/kg per minute. The actual rate is titrated to the blood pressure response. Any patient requiring vasopressors to maintain blood pressure should be monitored in an intensive care setting.
The use of corticosteroids in the treatment of anaphylaxis has been recommended in the past, mostly in an attempt to decrease the incidence of biphasic reactions.83 Steroids are not considered helpful in the acute phase of anaphylaxis. For patients experiencing a severe anaphylactic reaction, intravenous dosing is appropriate. Commonly used steroids include dexamethasone, hydrocortisone, and methylprednisolone (see Box 15.6 for common dosing suggestions). Intravenous steroids should be given slowly to prevent complications, such as coronary spasm.83 In less severe cases of anaphylaxis, steroids may be given by the oral route. Prednisone is a commonly used oral steroid dosed at 40–50 mg for adults, and 1 mg/kg for children.83,84 Due to the relatively low rate of significant adverse events with steroid use, they should probably be given to all patients with anaphylaxis and even severe generalized reactions. Corticosteroids should always be given to patients with severe asthma and those who chronically take systemic corticosteroids.85
As mentioned earlier, several medications can make anaphylaxis treatment more difficult. Patients on beta-blockers present a particular challenge. Although many physicians do not knowingly start immunotherapy on patients taking beta-blockers, patients are often started on new medications after immunotherapy is in progress. It is therefore important to question the patient or family member at the onset of anaphylaxis treatment to make sure no beta-blockers have been added. The mechanisms by which beta-blockers complicate treatment were discussed earlier in the chapter. Patients on beta-blockers may be resistant to routine anaphylaxis treatment, or may be subject to greater risk of treatment adverse events.86–88 To decrease the risk of hypertensive crisis due to unopposed alpha-adrenergic stimulation, some recommend decreasing the initial dose of epinephrine to 0.2 ml.40 If severe hypertension develops from unopposed alpha-adrenergic stimulation by epinephrine, phentolamine may be useful to reduce the blood pressure. Phentolamine is a pure alpha-adrenergic blocker administered in 5–10 mg doses (0.1 mg/kg in children) every 10 minutes. Atropine is sometimes useful if the patient is experiencing bradycardia from beta-blockade, or reflexive bradycardia from epinephrine-related hypertensive crisis. It is injected subcutaneously or intramuscularly. The most common adult dose is 0.3–0.5 mg. The dose may be repeated every 10 minutes to a maximum dose of 2 mg. Glucagon is another medication that has been recommended as an adjunctive treatment for patients on beta-blockers, as well as those with protracted anaphylaxis.89–92 Glucagon is a positive inotrope and chronotrope, and these properties are not dependent on catecholamine receptors.93,94 It can help reverse the adverse cardiac sequela of anaphylaxis.40 There is experimental data supporting the use of glucagon to reverse hypotension found in anaphylactic shock.95 The usual recommended dose is 1–5 mg intravenous push, followed by an infusion of 5–15 μg per minute.89 For persistent hypotension, one can also consider using the pure beta-agonist, isoproterenol. The initial dose of this medication is 0.1 μg/kg/min.
Heparin is another medication that has potential benefit in beta-blockaded patients or refractory anaphylaxis. Heparin causes the release of histaminase (a diamine oxidase) into the circulation, thus lowering histamine levels. It also absorbs and inactivates histamines.40 Studies have shown that heparin inhibits immunologically induced contraction of tracheal smooth muscle.96 An animal model has demonstrated the ability of heparin to prevent and treat anaphylaxis.97 The dose in adults is 10 000 units intravenous. Children should receive 50–75 units/kg. If success is obtained with this initial dose, a continuous infusion can be given at a rate of 1000 units/hour in adults and 25 units/kg/hour in children. Partial thromboplastin times should be monitored to prevent overdosing.40
If a patient on beta-blockers experiences protracted bronchospasm during anaphylaxis, magnesium is an alternative medication to administer.40 It has been used to control acute asthma in the emergency care setting.98,99 The initial dose is 1 g diluted in 50 ml normal saline given IV. Additional doses may be given if necessary. A total of 4 g can be given in the first 20 minutes and then 1 g per hour thereafter. A reduction in deep tendon reflexes is a sign of overdose.40,100
Vitamin C has shown significant ability to reduce acute allergic bronchospasm.101,102 Other publications have questioned the effectiveness of vitamin C in this setting.103,104 Vitamin C has very low toxicity and may be worth adding as another adjunctive measure in refractory bronchospasm during anaphylaxis. The dose of vitamin C is 2 g IV.40
Other medications have the potential to complicate anaphylaxis treatment. Tricyclic antidepressants block catecholamine reuptake at nerve junctions which can increase the chances of hypertension or arrhythmias with vasopressor use. One should consider reducing the initial dose of epinephrine or dopamine. Monoamine oxidase inhibitors block the degradation of catecholamines, increasing the risk of complications with vasopressor use. The doses of epinephrine and dopamine should be reduced by 90% to reduce the chances of severe adverse effects.40
1 Novembre E, et al. Anaphylaxis in children: clinical and allergologic features. Pediatrics. 1998;101:E8.
2 James JM. Anaphylaxis: multiple etiologies-focused therapy. J Arkansas Med Soc. 1996;93:281-287.
3 Ring J, Brockow K, Behrendt H. History and classification of anaphylaxis. Novartis Found Symp. 2004;257:6-16. discussion 16–24.
4 Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114:371-376.
5 Foucard T, Malmheden-Yman I. Food-induced anaphylaxis. Pediatr Allergy Immunol. 2001;12(Suppl 14):97-101.
6 Lieberman P. Anaphylactic reactions during surgical and medical procedures. J Allergy Clin Immunol. 2002;110(2 suppl):S64-S69.
7 Sampson HA, et al. Symposium on the definition and management of anaphylaxis: summary report [see comment]. J Allergy Clin Immunol. 2005;115:584-591.
8 Yocum MW, et al. Epidemiology of anaphylaxis in Olmsted County: a population-based study [see comment]. J Allergy Clin Immunol. 1999;104(2 Pt 1):452-456.
9 Moneret-Vautrin DA, et al. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy. 2005;60:443-451.
10 Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology [see comment]. Arch Intern Med. 2001;161:15-21.
11 Lieberman P. Anaphylaxis. Med Clin North Am. 2006;90:77-95.
12 Nettis E, et al. Safety of inhalant allergen immunotherapy with mass units – standardized extracts. Clin Exp Allergy. 2002;32:1745-1749.
13 Borchers AT, Keen CL, Gershwin ME. Fatalities following allergen immunotherapy. Clin Rev Allergy Immunol. 2004;27:147-158.
14 Bousquet J, et al. Immunotherapy with Hymenoptera venoms. Position paper of the Working Group on Immunotherapy of the European Academy of Allergy and Clinical Immunology. Allergy. 1987;42:401-413.
15 Hurst DS, et al. Safety of home-based and office allergy immunotherapy: a multicenter prospective study. Otolaryngology – Head Neck Surg. 1999;121:553-561.
16 Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol. 2002;110:341-348.
17 Lieberman P. Anaphylaxis and anaphylactoid reactions. In Middleton’s allergy principles and practice. Philadelphia: Mosby; 2003.
18 Finkelman FD, et al. Molecular mechanisms of anaphylaxis: lessons from studies with murine models [see comment]. J Allergy Clin Immunol. 2005;115:449-457. quiz 458.
19 Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216(2–3):106-121.
20 Brown SG. Cardiovascular aspects of anaphylaxis: implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol. 2005;5:359-364.
21 Toogood JH. Beta-blocker therapy and the risk of anaphylaxis. CMAJ Canad Med Assoc J. 1987;136:929-933.
22 Soto-Aguilar MC, deShazo RD, Waring NP. Anaphylaxis. Why it happens and what to do about it. Postgrad Med. 1987;82:154-160.
23 Bousquet J, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. Systemic reactions during the rush protocol in patients suffering from asthma. J Allergy Clin Immunol. 1989;83:797-802.
24 Greenberg MA, et al. Late systemic-allergic reactions to inhalant allergen immunotherapy. J Allergy Clin Immunol. 1988;82:287-290.
25 Kelly KJ, et al. The diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 1994;93:813-816.
26 Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144-1150.
27 Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111(6 Pt 3):1601-1608.
28 Dykewicz MS. Anaphylaxis and stinging insect reactions. Comp Ther. 1996;22:579-585.
29 Soreide E, Buxrud T, Harboe S. Severe anaphylactic reactions outside hospital: etiology, symptoms and treatment. Acta Anaesth Scand. 1988;32:339-342.
30 Viner NA, Rhamy RK. Anaphylaxis manifested by hypotension alone. J Urol. 1975;113:108-110.
31 Kniker W. Anaphylaxis in children and adults. In: Bierman C, Pearlmam D, editors. Allergic diseases from infancy to adult. Philadelphia: WB Saunders; 1988:667-677.
32 Golden DB. Patterns of anaphylaxis: acute and late phase features of allergic reactions. Novartis Found Symp. 2004;257:101-110. discussion 110–115.
33 Criep LH, Woehler TR. The heart in human anaphylaxis. Ann Allergy. 1971;29:399-409.
34 Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics. 2000;106:762-766.
35 Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents [see comment]. New Engl J Med. 1992;327:380-384.
36 Fisher MM, Baldo BA. Acute anaphylactic reactions. Med J Aust. 1988;149:34-38.
37 Anonymous. Anaphylaxis: statement on initial management in nonhospital settings. Laboratory Centre for Disease Control. CMAJ Canad Med Assoc J. 1996;154:1519-1522.
38 Marone G, et al. Nonspecific histamine-releasing properties of general anesthetic drugs. Clin Rev Allergy. 1991;9(3–4):269-280.
39 Naguib M, Magboul MM. Adverse effects of neuromuscular blockers and their antagonists. Drug Safety. 1998;18:99-116.
40 Gordon BR. Anaphylaxis: prevention and treatment. In: Krouse J, et al, editors. Allergy and immunology: an otolaryngic approach. Philadelphia: Lippincott Williams & Wilkins; 2002:99-113.
41 Fenton AM, et al. Vasovagal syncope. Ann Intern Med. 2000;133:714-725.
42 Anonymous. Emergency medical treatment of anaphylactic reactions. Project Team of The Resuscitation Council (UK). Resuscitation. 1999;41:93-99.
43 Lehane L. Update on histamine fish poisoning. Med J Aust. 2000;173:149-152.
44 Laroche D, et al. Biochemical markers of anaphylactoid reactions to drugs. Comparison of plasma histamine and tryptase. Anesthesiology. 1991;75:945-949.
45 Schwartz LB, et al. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83:1551-1555.
46 Yunginger JW, et al. Laboratory investigation of deaths due to anaphylaxis. J Foren Sci. 1991;36:857-865.
47 Hepner MJ, et al. Risk of systemic reactions in patients taking beta-blocker drugs receiving allergen immunotherapy injections. J Allergy Clin Immunol. 1990;86(3 Pt 1):407-411.
48 Kemp SF, Lieberman P. Inhibitors of angiotensin II: potential hazards for patients at risk for anaphylaxis? Ann Allergy Asthma Immunol. 1997;78:527-529.
49 Nikpoor B, Duan QL, Rouleau GA. Acute adverse reactions associated with angiotensin-converting enzyme inhibitors: genetic factors and therapeutic implications. Exp Opin Pharmacother. 2005;6:1851-1856.
50 Mathelier-Fusade P. Drug-induced urticarias. Clin Rev Allergy Immunol. 2006;30:19-23.
51 Greineder DK. Risk management in allergen immunotherapy. J Allergy Clin Immunol. 1996;98(6 Pt 3):S330-S334.
52 Lockey RF, et al. Fatalities from immunotherapy (IT) and skin testing (ST) [see comment]. J Allergy Clin Immunol. 1987;79:660-677.
53 Bernstein DI, et al. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990–2001 [see comment]. J Allergy Clin Immunol. 2004;113:1129-1136.
54 Hurst DS, et al. Safety of home-based and office allergy immunotherapy: a multicenter prospective study. Otolaryngol Head Neck Surg. 1999;121:553-561.
55 Joint Task Force on Practice Parameters, et al. The diagnosis and management of anaphylaxis: an updated practice parameter [see comment]. J Allergy Clin Immunol. 2005;115(3 suppl 2):S483-S523.
56 The American Academy of Pediatrics Committee on Drugs. Anaphylaxis. Pediatrics. 1973;51:136.
57 World Health Organization position paper on allergen immunotherapy, therapeutic vaccines for allergic diseases. Allergy. 1998;53(suppl):S20.
58 Bonner JR. Anaphylaxis. Part I. Etiology and pathogenesis. Alabama J Med Sci. 1988;25:283-287.
59 Lieberman P. Use of epinephrine in the treatment of anaphylaxis. Curr Opin Allergy Clin Immunol. 2003;3:313-318.
60 Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001;108:871-873.
61 Gu X, Simons FE, Simons KJ. Epinephrine absorption after different routes of administration in an animal model. Biopharm Drug Disp. 1999;20:401-405.
62 Simons FE, et al. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 1998;101(1 Pt 1):33-37.
63 McLean-Tooke AP, et al. Adrenaline in the treatment of anaphylaxis: what is the evidence? Br Med J. 2003;327(7427):1332-1335.
64 Levy JH, Levi R. Diagnosis and treatment of anaphylactic/anaphylactoid reactions. Monogr Allergy. 1992;30:130-144.
65 Barach EM, et al. Epinephrine for treatment of anaphylactic shock. JAMA. 1984;251:2118-2122.
66 Fath JJ, Cerra FB. The therapy of anaphylactic shock. Drug Intelligence Clin Pharm. 1984;18:14-21.
67 Powers RD, Donowitz LG. Endotracheal administration of emergency medications. Southern Med J. 1984;77:340-341.
68 Gu X, Simons KJ, Simons FE. Is epinephrine administration by sublingual tablet feasible for the first-aid treatment of anaphylaxis? A proof-of-concept study. Biopharm Drug Disp. 2002;23:213-216.
69 Sampson HA. Peanut anaphylaxis. J Allergy Clin Immunol. 1990;86:1-3.
70 Pumphrey RS, Roberts IS. Postmortem findings after fatal anaphylactic reactions. J Clin Pathol. 2000;53:273-276.
71 Delage C, Irey NS. Anaphylactic deaths: a clinicopathologic study of 43 cases. J Forensic Sci. 1972;17:525-540.
72 Murphy S, Kelly H. Acute asthma in children: when first-line therapy isn’t enough. J Respir Dis. 1990;11:589.
73 Beakes D. The use of anticholinergics in asthma. J Asthma. 1997;34:357-368.
74 Self TH, et al. Inhaled albuterol and oral prednisone therapy in hospitalized adult asthmatics. Does aminophylline add any benefit? Chest. 1990;98:1317-1321.
75 Littenberg B. Aminophylline treatment in severe, acute asthma. A meta-analysis. JAMA. 1988;259:1678-1684.
76 Lin RY, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000;36:462-468.
77 Runge JW, et al. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med. 1992;21:237-242.
78 Mayumi H, et al. Intravenous cimetidine as an effective treatment for systemic anaphylaxis and acute allergic skin reactions. Ann Allergy. 1987;58:447-450.
79 Chrusch C, et al. Histamine H3 receptor blockade improves cardiac function in canine anaphylaxis. Am J Resp Crit Care Med. 1999;160:1142-1149.
80 Raper RF, Fisher MM. Profound reversible myocardial depression after anaphylaxis. Lancet. 1988;1(8582):386-388.
81 Shine KI, et al. Aspects of the management of shock. Ann Intern Med. 1980;93:723-734.
82 Saryan JA, O’Loughlin JM. Anaphylaxis in children. Pediatr Ann. 1992;21:590-593.
83 Brown AF. Therapeutic controversies in the management of acute anaphylaxis. J Accident Emerg Med. 1998;15:89-95.
84 Classic Shirt-Pocket, editor. Tarascon Pocket Pharmacopoeia. Lompoc: Tarascon Publishing, 2006.
85 Jantz MA, Sahn SA. Corticosteroids in acute respiratory failure [see comment]. Am J Resp Crit Care Med. 1999;160:1079-1100.
86 Zanjanian MH. Potentiated anaphylaxis to allergenic extracts with pharmacologic beta-adrenergic blockage. J Med Soc NJ. 1983;80:359-360.
87 Jacobs RL, et al. Potentiated anaphylaxis in patients with drug-induced beta-adrenergic blockade. J Allergy Clin Immunol. 1981;68:125-127.
88 Newman BR, Schultz LK. Epinephrine-resistant anaphylaxis in a patient taking propranolol hydrochloride. Ann Allergy. 1981;47:35-37.
89 Lvoff R, Wilcken DE. Glucagon in heart failure and in cardiogenic shock. Experience in 50 patients. Circulation. 1972;45:534-542.
90 Gavalas M, Sadana A, Metcalf S. Guidelines for the management of anaphylaxis in the emergency department [see comment]. J Accident Emerg Med. 1998;15:96-98.
91 Pollack CVJr. Utility of glucagon in the emergency department. J Emerg Med. 1993;11:195-205.
92 Lee ML. Glucagon in anaphylaxis. J Allergy Clin Immunol. 1982;69:331-332.
93 Nobel-Allen N, Kirsch M, Lucchesi BR. Glucagon: its enhancement of cardiac performance in the cat with chronic heart failure. J Pharmacol Exp Ther. 1973;187:475-481.
94 Glick G, et al. Glucagon. Its enhancement of cardiac performance in the cat and dog and persistence of its inotropic action despite beta-receptor blockade with propranolol. Circulation Res. 1968;22:789-799.
95 Zaloga GP, et al. Glucagon reversal of hypotension in a case of anaphylactoid shock. Ann Intern Med. 1986;105:65-66.
96 Abraham WM, Abraham MK, Ahmed T. Protective effect of heparin on immunologically induced tracheal smooth muscle contraction in vitro. Int Arch Allergy Immunol. 1996;110:79-84.
97 Dhar HL, Mukherjee B, Sanyal RK. The effect of heparin on the heart in anaphylaxis. Am Heart J. 1967;74:489-495.
98 Monem GF, Kissoon N, DeNicola L. Use of magnesium sulfate in asthma in childhood. Pediatr Ann. 1996;25(3):136.
99 Skobeloff EM, et al. Intravenous magnesium sulfate for the treatment of acute asthma in the emergency department [see comment]. JAMA. 1989;262:1210-1213.
100 Swartjes JM, Schutte MF, Bleker OP. Management of eclampsia: cardiopulmonary arrest resulting from magnesium sulfate overdose [see comment]. Eur J Obstet Gynecol Reprod Biol. 1992;47:73-75.
101 Bucca C, et al. Effect of vitamin C on histamine bronchial responsiveness of patients with allergic rhinitis. Ann Allergy. 1990;65:311-314.
102 Fogarty A, et al. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Resp Med. 2006;100:174-179.
103 Ram FS, Rowe BH, Kaur B. Vitamin C supplementation for asthma [update of Cochrane Database Syst Rev 2001;(4):CD000993;PMID:11687089]. Cochrane Database Syst Rev. (3):2004. CD000993.
104 Boskabady MH, Ziaei T. Effect of ascorbic acid on airway responsiveness in ovalbumin sensitized guinea pigs. Respirology. 2003;8:473-478.
105 Kemp SF, et al. Anaphylaxis. A review of 266 cases [see comment]. Arch Intern Med. 1995;155:1749-1754.
106 Dibs SD, Baker MD. Anaphylaxis in children: a 5-year experience. Pediatrics. 1997;99(1):E7.
107 Cianferoni A, et al. Clinical features of acute anaphylaxis in patients admitted to a university hospital: an 11-year retrospective review (1985–1996). Ann Allergy Asthma Immunol. 2001;87(1):27-32.
108 Tejedor Alonso MA, et al. Idiopathic anaphylaxis: a descriptive study of 81 patients in Spain. Ann Allergy Asthma Immunol. 2002;88:313-318.