Management of the High-Risk Surgical Patient
Cardiothoracic anaesthesia and emergency anaesthesia are considered elsewhere (Chs 33, 34 and 37), and this chapter will concentrate on the patient undergoing scheduled major non-cardiac surgery. However, the principles of treatment, particularly fluid management, generally apply also to the patient undergoing an emergency procedure.
RISK PREDICTION SCORING SYSTEMS
Shoemaker’s Criteria
 Previous severe cardiorespiratory illness (acute MI, COPD, stroke)
Previous severe cardiorespiratory illness (acute MI, COPD, stroke)
 Extensive ablative surgery planned for carcinoma
Extensive ablative surgery planned for carcinoma
 Severe multiple trauma (more than three organs or two body cavities involved)
Severe multiple trauma (more than three organs or two body cavities involved)
 Massive acute blood loss (more than 8 units)
Massive acute blood loss (more than 8 units)
 Age over 70 years with limited physiological reserve
Age over 70 years with limited physiological reserve
 Shock (mean arterial pressure less than 60 mmHg)
Shock (mean arterial pressure less than 60 mmHg)
 Acute abdominal catastrophe with haemodynamic instability (pancreatitis, bowel infarction, perforated viscus, GI bleeding)
Acute abdominal catastrophe with haemodynamic instability (pancreatitis, bowel infarction, perforated viscus, GI bleeding)
Revised Cardiac Risk Index
POSTOPERATIVE PULMONARY COMPLICATION RISK PREDICTORS
LABORATORY INVESTIGATIONS FOR RISK ASSESSMENT
Cardiopulmonary Exercise Testing (CPET)
Anaerobic Threshold
AT is identified as the point at which there is onset of lactate production through the activation of anaerobic pathways. The lactate produced is buffered by bicarbonate to produce water and carbon dioxide. The net effect is an increase in the slope of the graph of carbon dioxide production relative to oxygen uptake (Fig. 23.1)
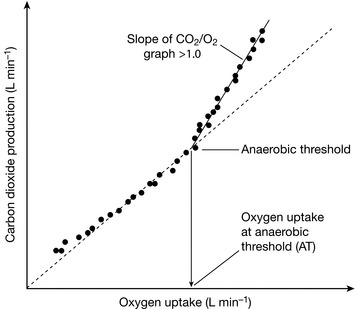
FIGURE 23.1 V-slope method for estimating Anaerobic Threshold. Oxygen uptake increases as exercise intensity increases. During the initial aerobic phase the slope of the graph of CO2 production vs. O2 uptake is less than 1. After the onset of lactate production from anaerobic pathways (the Anaerobic Threshold), extra CO2 is generated by the buffering of lactate with bicarbonate, and the slope of the CO2/O2 graph is greater than 1.
Identification of Myocardial Dysfunction
 Oxygen uptake per heart beat (VO2/HR, also known as the ‘oxygen pulse’). This should increase steadily during exercise as a reflection of increases in underlying stroke volume initially, followed by increases in oxygen extraction. Flattening of the slope of the graph during exercise may reflect underlying myocardial wall motion abnormalities.
Oxygen uptake per heart beat (VO2/HR, also known as the ‘oxygen pulse’). This should increase steadily during exercise as a reflection of increases in underlying stroke volume initially, followed by increases in oxygen extraction. Flattening of the slope of the graph during exercise may reflect underlying myocardial wall motion abnormalities.
 Oxygen uptake to work rate relationship (VO2/WR) (Fig. 23.2). For each 1 W increase in work rate, oxygen uptake should increase by 10 mL min− 1.
Oxygen uptake to work rate relationship (VO2/WR) (Fig. 23.2). For each 1 W increase in work rate, oxygen uptake should increase by 10 mL min− 1.
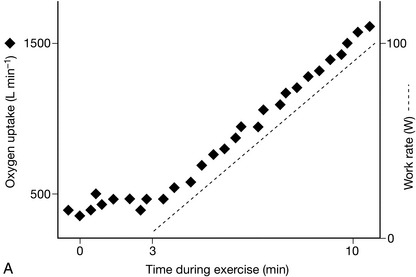
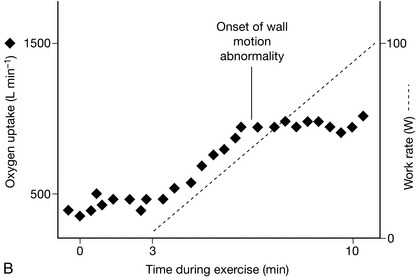
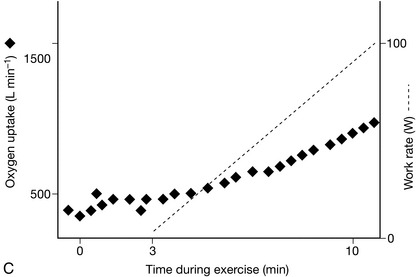
FIGURE 23.2 Relationship between oxygen uptake and work rate (VO2/WR). (A) Normal: for each 1 W increase in work rate intensity, oxygen uptake increases by 10 mL min− 1. In this example, O2 uptake is 500 mL min− 1 at the onset of loaded cycling, and 1500 mL min− 1 after 100 W, an increase of 10 mL min− 1 W− 1. (B) Ischaemic heart disease: The VO2/WR relationship starts normally, but O2 uptake abruptly stops increasing at the critical ischaemic point as wall motion abnormalities develop. (C) Chronic heart failure: VO2/WR is significantly decreased, as the myocardial pump does not respond normally to increasing exercise intensity.
REDUCING RISK BEFORE SURGERY
 Is the patient fit enough for the proposed surgery, or would a less invasive procedure, or even postponement of surgery, be more suitable?
Is the patient fit enough for the proposed surgery, or would a less invasive procedure, or even postponement of surgery, be more suitable?
 Can the patient’s medical condition be improved before surgery, with consequent risk reduction?
Can the patient’s medical condition be improved before surgery, with consequent risk reduction?
 Does the patient need to go to a high dependency care or intensive care unit after surgery, or is postoperative care in a general ward appropriate?
Does the patient need to go to a high dependency care or intensive care unit after surgery, or is postoperative care in a general ward appropriate?
PREOPERATIVE INTERVENTIONS TO REDUCE RISK
IDENTIFYING PATIENTS IN NEED OF POSTOPERATIVE CRITICAL CARE
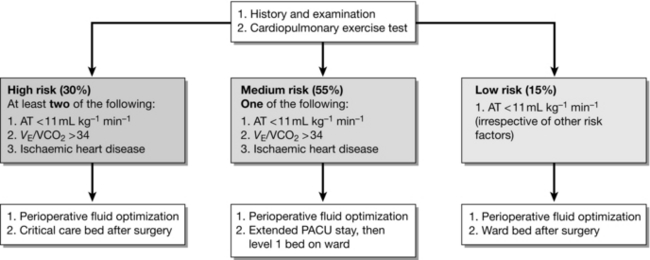
A reduced value of anaerobic threshold (AT), measured from cardiopulmonary exercise testing (CPET), has been used to define groups at higher risk of complications after surgery. A protocol for triage of patients to ICU, HDU or to ward care after surgery based on CPET results was described by Older in 1999. Mortality rates were very low among patients who were allocated to ward care on the basis of good AT values, indicating that CPET was accurate in identifying patients who did not need HDU care, irrespective of clinical history or age. This model of care is now becoming established in hospitals with access to preoperative CPET. A typical protocol is shown in Figure 23.3.
PERIOPERATIVE MANAGEMENT OF THE HIGH-RISK PATIENT
Haemodynamic Monitoring, Fluid Therapy, and Optimization of Oxygen Delivery
Oxygen delivery (DO2) is dependent upon cardiac output and the oxygen content of arterial blood.
Additionally, there is a small but clinically insignificant amount of dissolved oxygen.
Stroke Volume
 Preload decreases if the patient becomes hypovolaemic, either through blood loss, insensible losses or relative hypovolaemia through excessive vasodilatation and capacitance increase of the circulation.
Preload decreases if the patient becomes hypovolaemic, either through blood loss, insensible losses or relative hypovolaemia through excessive vasodilatation and capacitance increase of the circulation.
 Afterload is dependent on systemic vascular resistance (SVR), which can decrease through the vasodilator effects of anaesthetic agents on the peripheral circulation, or epidural-induced sympathetic blockade. SVR can increase if sympathetic tone is increased, for example by circulating catecholamine increases in response to surgical stimulation.
Afterload is dependent on systemic vascular resistance (SVR), which can decrease through the vasodilator effects of anaesthetic agents on the peripheral circulation, or epidural-induced sympathetic blockade. SVR can increase if sympathetic tone is increased, for example by circulating catecholamine increases in response to surgical stimulation.
 Contractility can decrease through direct action of anaesthetic agents or circulating inflammatory cytokines, or increase if sympathetic activity increases.
Contractility can decrease through direct action of anaesthetic agents or circulating inflammatory cytokines, or increase if sympathetic activity increases.
Figure 23.4 shows the effect of boluses of fluid on stroke volume at different points on the Frank–Starling curve. On the lower portion of the curve, when preload is low, a fluid bolus is likely to produce an increase in stroke volume of greater than 10%. As preload increases, the increases in stroke volume reduce, until a rise of less than 10% is obtained, indicating that the plateau of the Frank–Starling curve has been reached, and that further fluid boluses are not required.
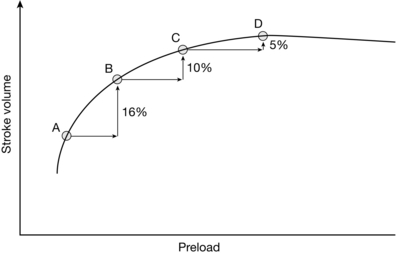
FIGURE 23.4 Optimizing stroke volume with fluid: the Frank–Starling curve. At point A on the curve, the patient is significantly hypovolaemic and responds to a fluid bolus with a 16% increase in stroke volume at point B. The second bolus (B to C) produces a smaller but still significant increase, but the third bolus (C to D) produces only a small (< 10%) increase in SV, signifying that the plateau portion of the Frank–Starling curve has been reached and that further fluid boluses will not lead to an increase in stroke volume.
Intraoperative Stroke Volume Optimization
The measured parameters of the Doppler waveform (Fig. 23.5) include:
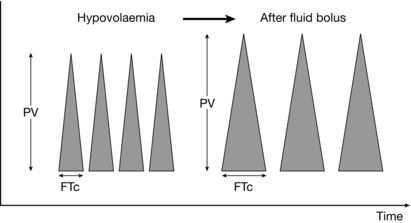
FIGURE 23.5 Oesophageal Doppler trace with response to fluid. The height of the trace represents the peak velocity (PV), a marker of contractility. The corrected Flow Time (FTc) is the systolic ejection time in milliseconds; a value less than 330 ms indicates hypovolaemia. The hypovolaemic patient responds to a fluid bolus by increasing both flow time and peak velocity.
 peak velocity (PV), considered a surrogate marker of contractility
peak velocity (PV), considered a surrogate marker of contractility
 stroke distance, the distance which a column of blood moves in the aorta during systole, from which stroke volume is calculated using a nomogram based on the patient’s height, weight and age.
stroke distance, the distance which a column of blood moves in the aorta during systole, from which stroke volume is calculated using a nomogram based on the patient’s height, weight and age.
Figure 23.6 shows a typical protocol for Doppler-guided fluid administration in which boluses of fluid are given until no further increases in stroke volume occur.
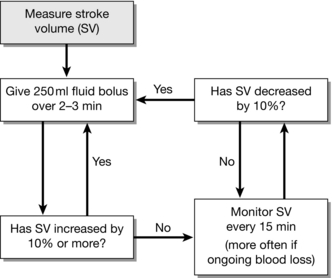
FIGURE 23.6 Protocol for administering fluid guided by oesophageal Doppler. In the anaesthetized patient, stroke volume is the optimal parameter to guide fluid therapy when using the oesophageal Doppler. The approach followed in this example aims to optimize the patient’s Frank–Starling curve (see Fig. 23.4) by sequential boluses of fluid until no further increases in stroke volume are observed.
Preload Responsiveness to Guide Fluid Therapy
The ability of these variables to determine preload responsiveness is based on observation of the cyclical changes which occur in stroke volume in response to mechanical ventilation (Fig. 23.7).
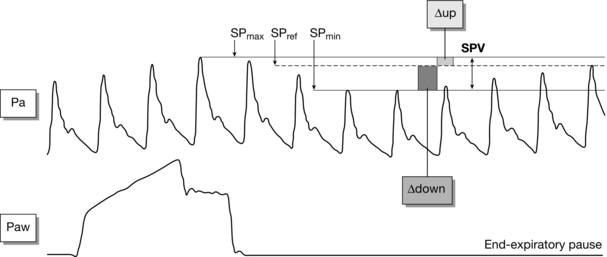
FIGURE 23.7 Variation in arterial pulse with ventilation. In the mechanically ventilated patient, the amplitude of the arterial pulse varies during the ventilatory cycle. Variations in the systolic pulse (SPV) greater than 12–14% indicate that a patient is hypovolaemic and likely to respond to fluid by increasing stroke volume. (Adapted from: Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005; 103:419–428.)
 Right ventricular stroke volume decreases due to a decrease in preload as raised intrathoracic pressure causes decreased flow in the inferior vena cava and increased afterload.
Right ventricular stroke volume decreases due to a decrease in preload as raised intrathoracic pressure causes decreased flow in the inferior vena cava and increased afterload.
 Left ventricular stroke volume increases initially as increased alveolar pressure causes an increase in flow of blood already in the low-pressure pulmonary capillary system returning to the left atrium.
Left ventricular stroke volume increases initially as increased alveolar pressure causes an increase in flow of blood already in the low-pressure pulmonary capillary system returning to the left atrium.
 Left ventricular stroke volume subsequently decreases as the decrease in right ventricular stroke volume causes a decrease in left ventricular preload.
Left ventricular stroke volume subsequently decreases as the decrease in right ventricular stroke volume causes a decrease in left ventricular preload.
POSTOPERATIVE MANAGEMENT
Monitoring on the General Ward
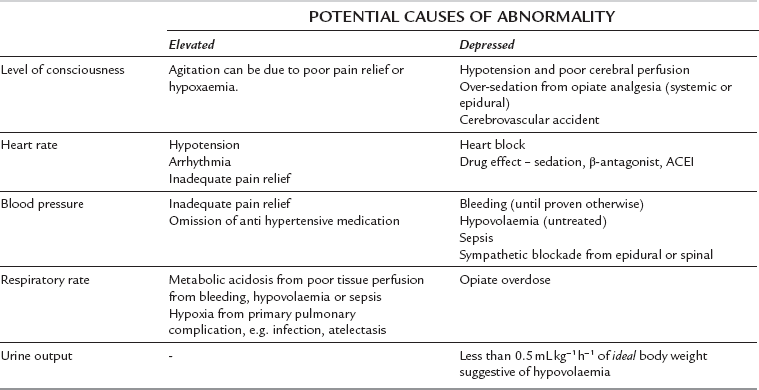
General ward monitoring is normally restricted to standard clinical observations, although some wards may be able to monitor central venous pressure (CVP) as well. The same principles of monitoring apply to both general ward patients and critical care patients in that the clinician is using the monitoring to detect organ dysfunction and decreased tissue perfusion at the earliest possible stage. The variables shown in Table 23.1 should be monitored routinely. These variables are usually combined into a scoring system known as a ‘patient at risk’ (PAR) score, or ‘early warning system’ (EWS) score. A particular PAR or EWS score value, or change in value, is pre-defined as a trigger point for nursing staff to summon medical assistance so that a potentially deteriorating patient can be identified early and appropriate treatment can be given, including transfer to critical care if indicated.
Monitoring in the Critical Care Unit
Central Venous Catheter
 Central venous pressure (CVP). This can be used to assess circulating volume status if the response to a carefully administered fluid challenge is observed.
Central venous pressure (CVP). This can be used to assess circulating volume status if the response to a carefully administered fluid challenge is observed.
 Central venous oxygen saturation (SCVO2). Oxygen saturation of blood returning to the heart of less than 70% has been associated with an increased risk of complications. Decreased SCVO2 reflects decreased oxygen delivery and a consequent increase in oxygen extraction.
Central venous oxygen saturation (SCVO2). Oxygen saturation of blood returning to the heart of less than 70% has been associated with an increased risk of complications. Decreased SCVO2 reflects decreased oxygen delivery and a consequent increase in oxygen extraction.
Arterial Cannula
 Adequacy of oxygenation and ventilatory function through measurement of PaO2 and PaCO2.
Adequacy of oxygenation and ventilatory function through measurement of PaO2 and PaCO2.
 Assessment of pH and identification of causes of abnormalities, e.g. whether an acidosis is metabolic, respiratory or hyperchloraemic in origin.
Assessment of pH and identification of causes of abnormalities, e.g. whether an acidosis is metabolic, respiratory or hyperchloraemic in origin.
 Assessment of tissue perfusion using lactate and bicarbonate, with an elevated lactate, decreased bicarbonate (‘base deficit’) and decreased pH indicating that tissue perfusion is inadequate, and that treatment with fluids and possibly inotropic drugs may be required.
Assessment of tissue perfusion using lactate and bicarbonate, with an elevated lactate, decreased bicarbonate (‘base deficit’) and decreased pH indicating that tissue perfusion is inadequate, and that treatment with fluids and possibly inotropic drugs may be required.
 Cardiac output monitoring using a pulse contour analysis device. Using this technology, stroke volume and cardiac output can be estimated in the awake patient without the need for pulmonary artery catheterization. Fluid challenges can be given to increase stroke volume, and subsequent improvements in tissue perfusion can be assessed using base deficit, lactate or central venous oxygen saturation as described above.
Cardiac output monitoring using a pulse contour analysis device. Using this technology, stroke volume and cardiac output can be estimated in the awake patient without the need for pulmonary artery catheterization. Fluid challenges can be given to increase stroke volume, and subsequent improvements in tissue perfusion can be assessed using base deficit, lactate or central venous oxygen saturation as described above.
Canet, J., Galart, L., Gomar, C., et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350.
Copeland, G.P., Jones, M.W. POSSUM: a scoring system for surgical audit. Br. J. Surg. 1991;78:355–360.
Davies, S.J., Wilson, R.J.T. Preoperative optimisation of the high-risk surgical patient. Br. J. Anaesth. 2004;93:121–128.
Davies, S.J., Wilson, R.J.T. Rationalising the use of surgical critical care: the role of cardiopulmonary exercise testing. In: Vincent J.L., ed. Yearbook of Intensive Care and Emergency Medicine. Berlin: Springer-Verlag, 2009.
Grocott, M.P.W., Mythen, M.G., Gan, T.J. Perioperative fluid management and clinical outcomes in adults. Anesth. Analg. 2005;100:1093–1106.
Lee, T.H., Marcantonio, E.R., Mangione, C.M., et al. Derivation and validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049.
Loftus I., ed. Care of the critically ill surgical patient, third ed., London: Hodder Arnold, 2010.
McConachie I., ed. Anaesthesia for the high-risk patient, second ed., Cambridge: Cambridge University Press, 2009.