Management of the Difficult Airway
 supraglottic airway device (SAD) placement and ventilation
supraglottic airway device (SAD) placement and ventilation
 emergency surgical airway (including cannula cricothyroidotomy and surgical techniques).
emergency surgical airway (including cannula cricothyroidotomy and surgical techniques).
CICV is an abbreviation for ‘Can’t intubate, can’t ventilate’.
BEFORE MANAGING THE DIFFICULT AIRWAY
The key to safe management of the difficult airway is preparedness.
Organizational Preparedness
Guidelines: Organizational preparedness requires that those events which might reasonably be anticipated to occur can be managed appropriately in the organization. This in turn requires guidelines (or policies) and equipment. As a minimum, the guidelines should cover the following:
Equipment: Logic dictates that the equipment needed to satisfy institutional preparedness is that which is needed for all the guidelines to be carried out in their entirety. This equipment should be procured, stored, maintained and checked appropriately to ensure that it is readily available whenever and wherever it is required. Difficult airway equipment (perhaps better described as advanced airway equipment) is usually maintained in an airway trolley (Fig. 22.1). It is advisable for all airway trolleys in an organization to have the same content and layout; this includes areas such as ICU and the emergency department. Organizing the airway trolley so that the layout of the equipment matches the flow of the airway guideline may improve compliance with the guideline and patient care; an example based on the DAS guideline is shown in Figure 22.2.
Communication and Training: The final aspect of institutional preparedness is communication and training. Guidelines are of limited value if they are not understood, accepted and practised by the relevant staff. Many hospitals have access to training in advanced airway management. Guidelines should be distributed widely. Training should involve the use of local guidelines and locally available equipment to ensure relevance. Where possible, those individuals who work together in teams should be trained together so that the chances of the team working well in an emergency are enhanced. The ‘team’ need not be limited to the anaesthetist and anaesthetic assistant, and some training (rather like a trauma team) allocates specific roles to surgeons, scrub nurses and other anaesthetists who attend to help in a crisis. While there is no evidence that such an approach improves outcome in real airway emergencies, it probably enables an organized, systematic approach and has the value of enabling a ‘team leader’ to oversee the management of the crisis, perhaps avoiding ‘task fixation’ and promoting ‘situation awareness’.
Personal Preparedness
Assessment and Planning a Strategy: While it is accepted that not all cases of airway difficulty can be anticipated (perhaps 50% are unanticipated), many can be. Airway assessment is discussed in detail in Chapter 21. Only principles are discussed here.
MANAGEMENT OF THE DIFFICULT AIRWAY
Before Approaching the Difficult Airway
 Will delivery of oxygen be difficult?
Will delivery of oxygen be difficult?
 Will face-mask ventilation be difficult?
Will face-mask ventilation be difficult?
 Will SAD placement be difficult?
Will SAD placement be difficult?
 Will tracheal intubation be difficult?
Will tracheal intubation be difficult?
 Will direct access to the trachea be difficult?
Will direct access to the trachea be difficult?
 Will there be problems with patient consent or co-operation?
Will there be problems with patient consent or co-operation?
Consider the relative merits of:
 securing the airway with the patient awake or anaesthetized
securing the airway with the patient awake or anaesthetized
 making the initial approach to tracheal intubation direct or indirect
making the initial approach to tracheal intubation direct or indirect
 maintaining spontaneous ventilation or ablating it during airway management.
maintaining spontaneous ventilation or ablating it during airway management.
Securing the Airway Awake
Administration of Muscle Relaxants in the Patient with a Difficult Airway
The subject remains controversial but can be summarized as follows:
 neuromuscular blockade often makes difficult mask ventilation easier
neuromuscular blockade often makes difficult mask ventilation easier
 this is not universal and is uncertain in anatomically abnormal patients
this is not universal and is uncertain in anatomically abnormal patients
 administration of a neuromuscular blocker removes the possibility of waking the patient
administration of a neuromuscular blocker removes the possibility of waking the patient
 if neuromuscular blockade is used to manage difficult mask ventilation, the anaesthetist must be adequately prepared to secure the airway, including managing a CICV situation
if neuromuscular blockade is used to manage difficult mask ventilation, the anaesthetist must be adequately prepared to secure the airway, including managing a CICV situation
 even if it was not part of the initial airway management strategy, if CICV occurs and waking the patient is not an option, a muscle relaxant should be given before determining the need to proceed to a surgical airway.
even if it was not part of the initial airway management strategy, if CICV occurs and waking the patient is not an option, a muscle relaxant should be given before determining the need to proceed to a surgical airway.
Selecting an Appropriate Size of Tracheal Tube
 passes though some narrow lumens which a larger tracheal tube will not passlarger tracheal tube will not pass
passes though some narrow lumens which a larger tracheal tube will not passlarger tracheal tube will not pass
 passes through a narrow lumen with greater ease and with less risk of trauma to the airway
passes through a narrow lumen with greater ease and with less risk of trauma to the airway
 is easier to ‘railroad’ over a bougie or exchange catheter with less risk of ‘hold-up’ during insertion.
is easier to ‘railroad’ over a bougie or exchange catheter with less risk of ‘hold-up’ during insertion.
DIFFICULT AIRWAYS AND THEIR MANAGEMENT
The first guidelines were published in 1993 by the ASA. The introduction of guidelines in the USA has been demonstrated to have led to a reduction in death and brain damage claims (and therefore probably critical incidents) related to airway management, most notably at the time of induction of anaesthesia. Many European countries developed their own guidelines in subsequent years. While all these claim to be evidence-based, the paucity of robust evidence means that most guidelines differ significantly from each other, often reflecting local preferences. The UK guidelines were published by the Difficult Airway Society in 2004. The major differences between the guidelines published by the ASA and DAS are summarized in Table 22.1. Both emphasize the most important principles in airway management:
TABLE 22.1
Comparison between the American Society of Anesthesiologists’ and Difficult Airway Society’s Guidelines Dealing with Management of the Difficult Airway
| ASA Guidelines 2012 | DAS Guidelines 2004 | |
| Breadth of ‘difficult airway’ management covered | A clinical situation in which a conventionally trained anesthesiologist experiences difficulty with face mask ventilation of the upper airway, difficulty with tracheal intubation or both | A Cormack and Lehane grade 3 or 4 view despite optimal direct laryngoscopy, using an alternative laryngoscope and external laryngeal manipulation |
| Evaluation of the airway | 11 non-reassuring findings | Not covered |
| Number of attempts at laryngoscopy allowed before moving to a different technique | > 3, multiple attempts | Up to 4 during routine intubation and 3 during RSI. A further single attempt if a more experienced anaesthetist arrives. |
| Techniques recommended for difficult intubation | Multiple including AFOI, blind, retrograde, LMA used as conduit and invasive airway access | Optimal laryngoscopy with gum elastic bougie (Plan A), then LMA/ILMA as conduit using fibreoptic control (Plan B)* then invasive airway access (Plan D) |
| Order of techniques | None given | Clear flow chart |
| Recommendations for extubation | Yes | Published separately (2012) |
| Recommendations on training | None given | Should form part of all anaesthetist training |
 to have devised a plan for airway management in the eventuality of it proving difficult, and a backup plan(s) which has been prepared for and practised
to have devised a plan for airway management in the eventuality of it proving difficult, and a backup plan(s) which has been prepared for and practised
 priority must be given to ensuring oxygenation and preventing iatrogenic trauma to the airway at all times.
priority must be given to ensuring oxygenation and preventing iatrogenic trauma to the airway at all times.
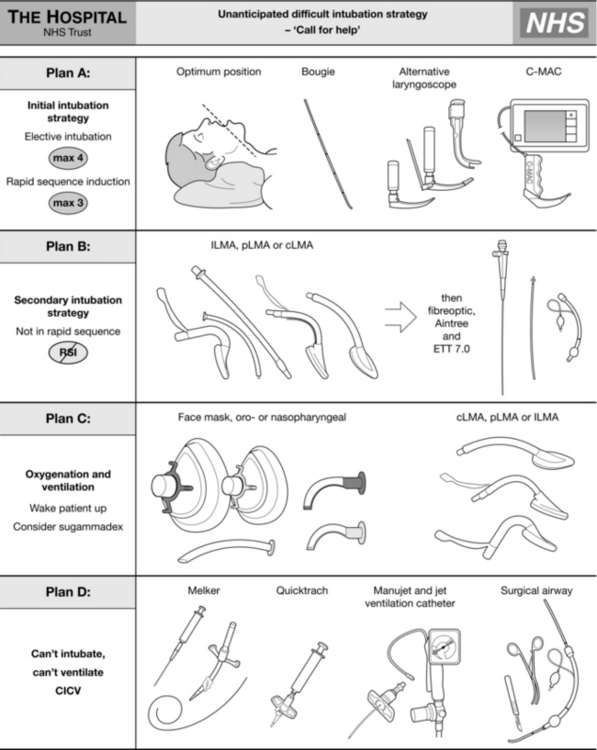
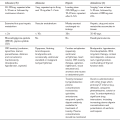
The ASA guidelines cover airway assessment and a number of different difficult airway situations. They offer the user a wide choice of options at each point of airway difficulty. They recommend multiple different techniques and it is likely that not all will be within the competence of all anaesthetists. They offer ‘choices’. In contrast, the DAS guidelines are didactic and present a single recommended pathway arranged in plans A to D and differentiating between the patient undergoing routine intubation or rapid sequence induction. They do not prescribe any advanced techniques but recommend simple procedures using equipment that should be familiar to all anaesthetists in training. They also strongly emphasize the need for regular practice of the recommended techniques using simulators and manikins where appropriate. The DAS guidelines are shown in Figures 22.3–22.5.
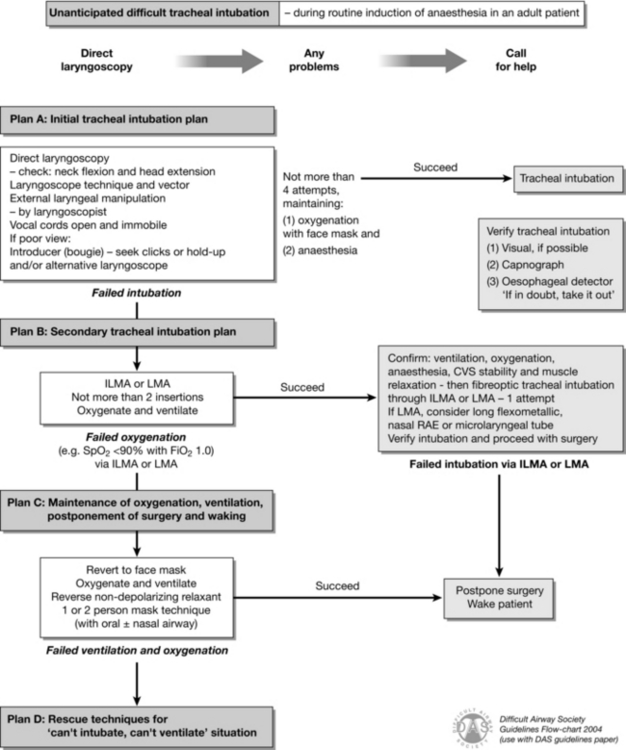
FIGURE 22.3 Difficult Airway Society guideline for unanticipated difficult tracheal intubation during routine induction of anaesthesia in an adult patient. (Adapted from Henderson JJ, Popat MT, Latto IP, Pearce AC. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004; 59:675–964, with permission from Blackwell Publishing Ltd. LMA, laryngeal mask airway; ILMA, intubating laryngeal mask airway.)
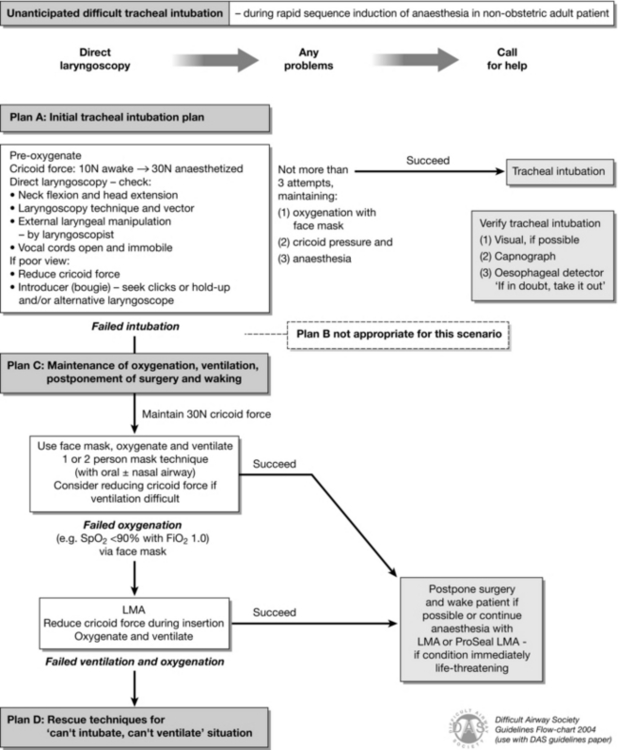
FIGURE 22.4 Difficult Airway Society guideline for unanticipated difficult tracheal intubation during rapid sequence induction of anaesthesia in a non-obstetric adult patient. (Adapted from Henderson JJ, Popat MT, Latto IP, Pearce AC. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004; 59:675–694, with permission from Blackwell Publishing Ltd.)
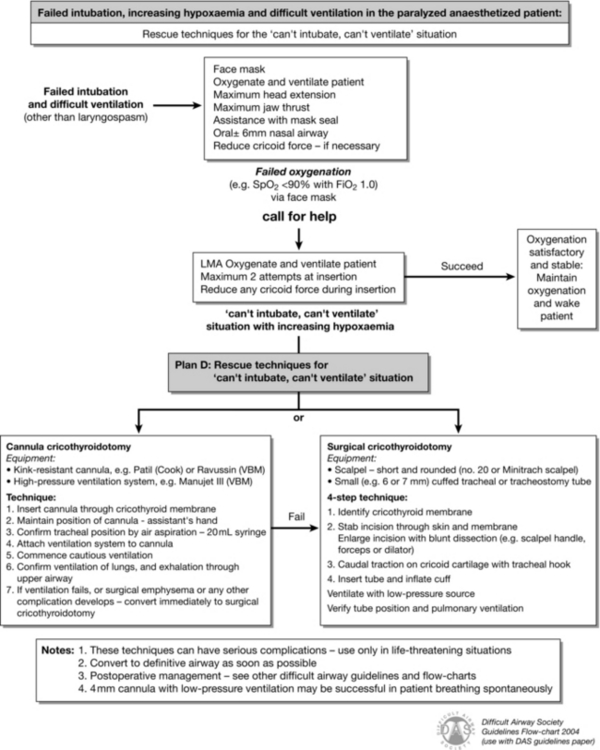
FIGURE 22.5 Difficult Airway Society guideline for management of failed intubation, increasing hypoxaemia and difficult ventilation in the paralysed, anaesthetized patient, with rescue techniques for the ‘can’t intubate, can’t ventilate’ situation. (Adapted from Henderson JJ, Popat MT, Latto IP, Pearce AC. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004; 59:675–694, with permission from Blackwell Publishing Ltd.)
Difficult Mask Ventilation
The first problem encountered in any difficult airway situation is often difficulty with face-mask ventilation. This is a vital step because it represents the basic and least invasive way of ensuring oxygenation of the patient. For mask ventilation to occur, a clear, sealed and patent airway from face mask to the lower airway is required. Difficulty can be diagnosed when there is inadequate chest movement despite high airway pressures (in the case of obstruction) or very low airway pressures (due to a leak during inspiration). Capnography and spirometry, both of which are available on most modern anaesthetic machines, can also identify poor ventilation before hypoxaemia occurs (Fig. 22.6).
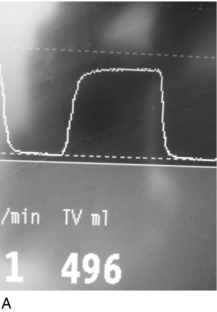
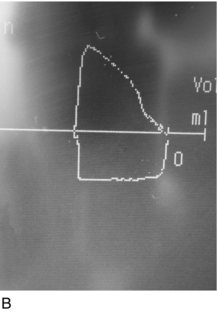
FIGURE 22.6 (A) A normal capnograph trace. (B) A flow-volume loop from a patient with an unobstructed airway.
Difficulties with Mask Ventilation can be due to:
 failure to maintain a patent upper airway (by far the most common problem)
failure to maintain a patent upper airway (by far the most common problem)
 laryngeal obstruction (either spasm or pathology)
laryngeal obstruction (either spasm or pathology)
 obstruction below the larynx, in the trachea, bronchi or in patients with reduced pulmonary compliance.
obstruction below the larynx, in the trachea, bronchi or in patients with reduced pulmonary compliance.
Face-mask ventilation requires the combination of: establishing a seal between the mask and the face; maintaining a clear upper airway; and ventilation of the lungs. Flexion of the lower cervical spine, extension of the upper cervical spine and mandibular protrusion are required, ideally with good quality facial soft tissues to enable an adequate seal with the mask. Using a ‘C-grip’, the thumb and first finger are used to hold the mask pushing downwards while the remaining three fingers pull the chin, jaw and soft tissues into the mask while also maintaining head and neck positions (Fig 22.7). Manual ventilation is performed with the anaesthetist’s other hand. Problems with the upper airway can often be predicted by prior airway assessment. Patients for whom obtaining an adequate mask seal is often problematic include the edentulous elderly, bearded patients and those who require high ventilation pressures, such as the morbidly obese.
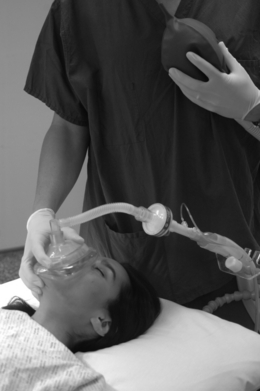
FIGURE 22.7 Mask ventilation with one person. Note the C-grip of the fingers over the mask and the three fingers supporting the airway.
MANAGEMENT OF UNPREDICTED DIFFICULT INTUBATION
Every anaesthetist should have a strategy prepared for dealing with problems with intubation, including plans for the more serious situation of CICV. The DAS algorithm is a suitable approach and is shown in Figures 22.3–22.5. This, or another equally valid strategy, should be familiar to all anaesthetists who are practising without direct supervision.
Plan A: Primary Intubation Attempt
There is no necessity to use the same laryngoscope blade for all attempts at intubation. Alternative blades include a long (size 4) Macintosh blade, the McCoy (levering laryngoscope) blade and a number of straight-bladed laryngoscopes (e.g. Miller, Henderson). Arguably, at least one attempt should be with a McCoy blade (Fig. 22.10) because it is recognized to move the fulcrum of the force applied to the airway distally and to improve the view when laryngoscopy is awkward. Use of a straight blade may offer the benefit of using an alternative approach to the laryngoscopy, such as a retromolar approach (Fig. 22.11), and this may be helpful, particularly if there is a small mandibular space.
Plan B: Secondary Intubation Attempt
Intubation Via a SAD: The cLMA is recommended because it is a device with which all anaesthetists are familiar. The cLMA does have several important limitations for such a use.
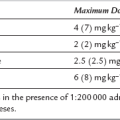
 The tube lumen is relatively narrow. To intubate through a size 3 cLMA requires a tracheal tube of maximum internal diameter of 6 mm (size 4, 6.5 mm; size 5, 7.0 mm).
The tube lumen is relatively narrow. To intubate through a size 3 cLMA requires a tracheal tube of maximum internal diameter of 6 mm (size 4, 6.5 mm; size 5, 7.0 mm).
 The LMA aperture bars can obstruct the passage of the tube.
The LMA aperture bars can obstruct the passage of the tube.
 The length of the cLMA tube requires a long tracheal tube to ensure that the trachea is entered.
The length of the cLMA tube requires a long tracheal tube to ensure that the trachea is entered.
 Removal of the LMA may be awkward once the tracheal tube has been successfully inserted and may risk displacing the tracheal tube.
Removal of the LMA may be awkward once the tracheal tube has been successfully inserted and may risk displacing the tracheal tube.
In view of these limitations, a microlaryngeal or long flexometallic tube is recommended.
An important modification of this technique, which is not included in the DAS guidelines, is to use an Aintree Intubation Catheter (AIC). The AIC (internal diameter 4.6 mm) is slid over the fibrescope and fibreoptic intubation is then performed via the SAD (Fig. 22.12). The AIC is left in the trachea (close to the carina) while first the fibrescope and then the SAD are removed. If oxygenation is required, a connector is available to enable ventilation via a standard anaesthetic breathing system. A lubricated tracheal tube is then railroaded over the AIC into the trachea and the AIC removed. The AIC has an external diameter of approximately 7.0 mm, requiring a standard tracheal tube of at least this size internal diameter to be inserted, although a 6.5 mm internal diameter ILMA tracheal tube is also accommodated. These techniques require practice and it is strongly recommended that, if such techniques are part of departmental guidance, then an appropriate regular training programme should be in place.
Intubation Via an ILMA: The ILMA is also an option for management of plan B. Unlike the other SADs, it is specifically designed for facilitating tracheal intubation. The ILMA has a mask end which is similar to a cLMA but the grille is replaced by an ‘epiglottic elevating bar’ which is a firm piece of silicone anchored at one end only. The stem of the ILMA is both shorter and wider than other LMAs and is rigid, with a handle attached on the concave side (Fig. 22.13); the stem passes through an angle of approximately 110o. The ILMA is supplied with a specifically designed ILMA tracheal tube (ILMA TT) which is straight, reinforced and has a soft bullet-shaped tip. The ILMA is supplied in three sizes (3–5, for patients of 30–100 kg) and each accommodates all sizes of ILMA TT from 6.0–8.0 mm internal diameter (Fig. 22.13).
The position of the ILMA over the airway must then be optimized using several steps.
 The anaesthetic circuit is attached and the patient’s lungs are ventilated manually.
The anaesthetic circuit is attached and the patient’s lungs are ventilated manually.
 The ILMA is advanced or withdrawn partially or lifted anteriorly until the optimal ventilation position is found.
The ILMA is advanced or withdrawn partially or lifted anteriorly until the optimal ventilation position is found.
 If ventilation is poor, the ILMA can be removed 6 cm and re-inserted to resolve epiglottic down-folding.
If ventilation is poor, the ILMA can be removed 6 cm and re-inserted to resolve epiglottic down-folding.
The ILMA TT can then be advanced into the trachea (Fig. 22.14). It should enter the trachea in no more than a further 6–8 cm.
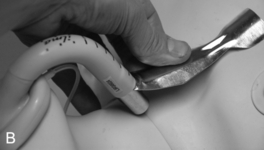


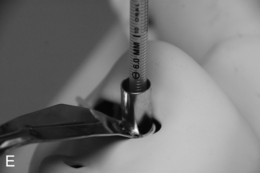

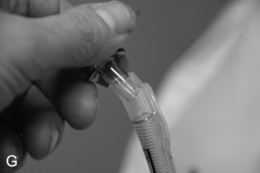
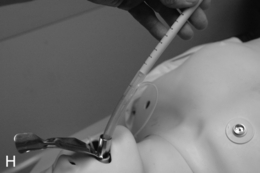
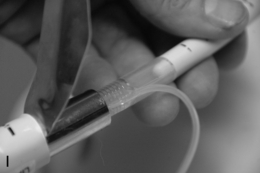
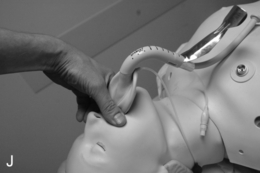

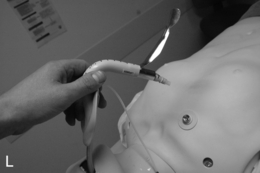
FIGURE 22.14 The use of the intubating laryngeal mask airway (ILMA). (A) ILMA TT is checked; (B) ILMA is inserted; (C) ventilation is optimized; (D) ILMA TT is passed through ILMA; (E) ILMA TT inside ILMA and position confirmed; (F) cuff of ILMA is deflated while TT remains inflated; (G) TT connector is removed: (H) tube stabilizing rod inserted in end of TT; (I) position of stabilizing rod maintained in same position as ILMA withdrawn over TT and stabilizer; (J) TT stabilized in mouth before ILMA removed completely; (K) stabilizing rod removed to prevent cuff trapping of TT cuff between rod and ILMA and cuff avulsion; (L) ILMA removed completely.
When the trachea has been intubated, the ILMA should usually be removed. If left in place, it may cause minor morbidity because it exerts a moderate pressure against the pharyngeal wall. A ‘tube stabilizer’ is provided to facilitate this. The 15-mm connector of the ILMA TT is removed (ideally having been loosened before intubation) and the stabilizing rod is placed into the proximal end of the tube. The ILMA TT cuff is confirmed as inflated and the ILMA cuff is deflated (Fig. 22.14). The stabilizing rod is then used to maintain the ILMA TT position while the ILMA is withdrawn; at the point at which the ILMA is almost out of the mouth, the stabilizing rod is removed and the anaesthetist’s gloved hand is inserted into the mouth to hold the tube in position. There are two important points: first, the stabilizing rod is not a ‘pusher’ and the ILMA TT should not be advanced during this procedure; second, if the stabilizing rod is not removed before the ILMA is withdrawn completely, the pilot cuff of the ILMA TT is avulsed and the cuff deflates (this is problematic, but can be rescued by inserting an i.v. cannula into the cut end of the pilot tube to enable re-inflation, followed by clamping) (Fig. 22.14).
Plan C: Failed Intubation, Oxygenation and Waking
Management of Unanticipated Difficult Intubation During RSI: The principal differences in the guidelines for unanticipated difficult intubation during RSI are as follows:
 The patient is always fully pre-oxygenated, which creates more time before oxygen desaturation in the event of difficulties with intubation.
The patient is always fully pre-oxygenated, which creates more time before oxygen desaturation in the event of difficulties with intubation.
 Cricoid pressure is used to prevent pulmonary aspiration of any regurgitated gastric contents.
Cricoid pressure is used to prevent pulmonary aspiration of any regurgitated gastric contents.
 Cricoid pressure should be reduced or briefly removed if it is impeding laryngoscopy. This should be done with ‘sucker in hand’ so that regurgitated material can be removed promptly.
Cricoid pressure should be reduced or briefly removed if it is impeding laryngoscopy. This should be done with ‘sucker in hand’ so that regurgitated material can be removed promptly.
 There is no plan B for secondary attempts at tracheal intubation because the patient is at risk of aspiration, and succinylcholine has a relatively short duration of action. Consequently, in all but life-threatening situations, the safest course of action is to postpone the surgery and allow the patient to wake. If rocuronium is used as part of a modified RSI technique, sugammadex may be used to reverse it, although this can only be achieved in a timely fashion if the drug is immediately available before induction commences.
There is no plan B for secondary attempts at tracheal intubation because the patient is at risk of aspiration, and succinylcholine has a relatively short duration of action. Consequently, in all but life-threatening situations, the safest course of action is to postpone the surgery and allow the patient to wake. If rocuronium is used as part of a modified RSI technique, sugammadex may be used to reverse it, although this can only be achieved in a timely fashion if the drug is immediately available before induction commences.
 If (as is often the case) active airway management is required to maintain oxygenation while the patient wakes, the ProSeal LMA is also advocated as a rescue device.
If (as is often the case) active airway management is required to maintain oxygenation while the patient wakes, the ProSeal LMA is also advocated as a rescue device.
Plan D: Management of the CICV Situation
All organizations should have a guideline (and individuals a plan) for the management of CICV. The DAS guideline is one option (Figs 22.3–22.5).
The principles behind management of CICV are:
1. Give 100% oxygen and call for help
2. Perform optimal face-mask ventilation
4. Wake the patient if this is feasible
5. If waking is not feasible and CICV persists, administer a neuromuscular blocking drug if not already administered
6. If the situation is not resolved, secure the airway with direct tracheal access.
Give 100% Oxygen and Call for Help: CICV is a life-threatening emergency. Oxygen should be delivered at high flow, maximum concentration. Even experienced anaesthetists require assistance in these circumstances. Call for the emergency airway trolley. However the main assistant should not be sent away – rather call on others to help and ensure all around understand clearly that the situation is a critical emergency.
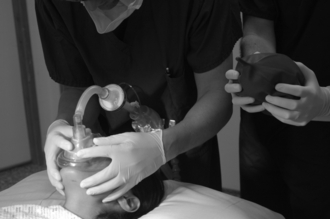
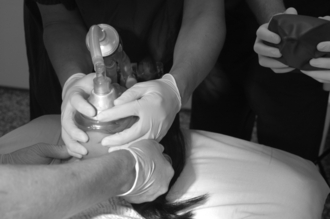
Perform Optimal Face Mask Ventilation: This involves a tight-fitting appropriately sized mask. Continuous positive airways pressure (CPAP) and an anti-Trendelenburg position may be helpful. An oropharyngeal (and perhaps nasopharyngeal) airway should be inserted. Two-handed (one person) mask ventilation should be changed to four- or six-handed ventilation (Figs 22.8–22.9).
Insert an Appropriate SAD: The ideal SAD for rescuing the airway during CICV has several properties:
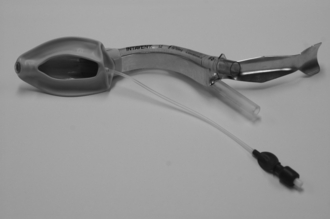
Based on risk factors and epidemiology, CICV might typically occur in an obese, paralysed patient at risk of aspiration and undergoing emergency surgery. The evidence base supports the use of a second generation SAD with a high seal pressure. The ProSeal LMA, inserted over a gum-elastic bougie placed in the oesophagus, is logically the best choice as this combines high first-time success with the best airway seal of all SADs and increased protection against aspiration (Fig. 22.15). Alternatives include the i-gel, LMA Supreme or laryngeal tube suction II.
Wake the Patient if this is Feasible: If the airway cannot be rescued, the default option should be to seek a ‘place of safety’ which in most cases involves waking the patient up. As a rule of thumb, this should be considered actively in all cases of airway difficulty and, if it is feasible, it is the safest option. In cases where difficulty may be anticipated it is particularly useful to adopt a ‘wake up trigger’ as part of the airway strategy and communicate it to all around before commencing anaesthesia. The airway can subsequently be secured awake. This option is equally valid when the airway has been secured with a SAD.
If Waking is not Feasible and CICV Persists, Administer a Neuromuscular Blocking Drug if not Already Administered: This may be considered by some to be controversial but we believe it is not; rather, it is entirely logical. It is well recognized that neuromuscular blockade may assist ventilation. The only strong argument for not administering a neuromuscular blocker when ventilation is difficult is the concern about how to manage the airway if ventilation is not improved. In the circumstance of CICV, where waking is not feasible, it is not possible for the situation to get worse and the realistic possibility that neuromuscular blockade may improve the airway means that it is entirely logical because it may prevent the need to provide an emergency surgical airway. Even if it does not enable ventilation, it is likely that performance of an emergency surgical airway will be easier after neuromuscular blockade.
If the Situation is not Resolved, Secure the Airway with Direct Tracheal Access: When CICV is not resolved by optimal face-mask ventilation, attempted SAD insertion and neuromuscular blockade, profound hypoxia and cardiac arrest occur rapidly (usually within 3–5 min) unless an airway is established and the blood is re-oxygenated. There are three options:
Decision making in CICV: As, or arguably more important than, choosing the correct device to rescue the airway is making the decision that a rescue procedure is needed. There have been numerous studies which illustrate that many emergency surgical airways are performed too late to prevent hypoxic brain injury or death. When CICV is unresolved and hypoxia is progressing, the decision should be made and communicated to those around, and prompt actions should be taken. Even in ideal circumstances, it is unlikely the airway will be secured after this decision is made in less than 3 min. An appropriate early decision may save a life.
EMERGENCY SURGICAL AIRWAY TECHNIQUES
Devices
Narrow-Bore Cannula with High Pressure Source Ventilation: A narrow-bore cannula (most are approximately 2 mm internal diameter) is usually inserted as a cannula-over-needle technique through the cricothyroid membrane. Once inserted, ventilation is based on two important principles: first, a high driving pressure is required to inflate the lungs due to the enormous resistance to flow through the cannula; second, expiration must take place though the patient’s upper airway. It is appropriate to use specifically designed devices (e.g. Ravussin cannula; Fig. 22.16A) which sit against the neck correctly (Fig. 22.16B) and are less likely to kink. Similarly, a specifically designed ventilating device (e.g. Manujet injector; Fig. 22.17) is strongly recommended. Use of intravenous cannulae (which cannot be fixed securely and tend to kink) and ‘Heath-Robinson’ assemblies for ventilation are difficult to justify in settings such as operating theatres where it can be predicted that such a technique will be required from time to time: avoiding the need for such ad hoc devices is part of institutional and personal preparedness.
Wide-Bore Cannula (≥ 4 mm): Wide-bore cannulae may be cannula-over-needle devices (e.g. QuickTrach) or those requiring a Seldinger insertion technique (e.g. Melker cricothyroidotomy devices; Fig. 22.18). Cannula-over-needle devices require a large sharp needle which risks significant tissue trauma if misplaced and such devices may be too short to reach the trachea in patients with an obese neck. Seldinger-type devices take somewhat longer to insert but the technique is familiar (and generally favoured) by anaesthetists.
Surgical Airway: A surgical airway technique comprises four steps.
1. The cricothyroid membrane is identified.
2. An incision is made in the neck over the cricothyroid membrane (a round-ended No. 20 blade is recommended) extending deep into the membrane and the scalpel is kept in place.
3. A ‘cricoid hook’ is inserted and the cricoid cartilage pulled forward.
4. A 6.0 mm internal diameter tracheal tube (or tracheostomy tube) is inserted.
Ventilation and Expiration Via Cricothyroidotomy Devices
Narrow-Bore Cannula: When ventilating through a narrow-bore cannula, a high pressure source is needed to overcome the resistance of the cannula. However, the pressure changes in the trachea during this type of ventilation are similar to those during conventional ventilation. The technique should not be confused with ‘jet ventilation’ or ‘oscillation’; what is achieved is ‘high pressure source conventional ventilation’. Appropriate sources of the high pressure for such ventilation are either wall oxygen or an oxygen cylinder, both approximately 400 kPa (4 bar, 4000 cmH2O, 58 psi). If wall oxygen is used, the flow rate should be set at greater than 15 L min −1. When using an anaesthetic machine, pressure regulators and ‘blow-off’ valves reduce the pressure available; an attached anaesthetic breathing system with a conventional anaesthetic reservoir bag limits pressure to 6 kPa (60 cmH2O), which is totally inadequate for ventilating through a narrow cannula. If the anaesthetic flush is deployed continuously, a pressure of 30–60 kPa (300–600 cmH2O) can be achieved and this may be just sufficient to ventilate the lungs. Maximum flow rates from an anaesthetic machine are 15 L min −1 via a flowmeter and 30–60 L min −1 when the oxygen flush button is depressed. An anaesthetic machine cannot provide a reliably high pressure source unless a connection is made to a high pressure source outlet at the back of the machine, usually a ‘mini-Schrader’ connector which can then be used to drive an injector or similar device (Fig. 22.19). An oxygen ‘injector’ such as a Sanders or Manujet injector can deliver a high pressure source ranging from 0.5–4 bar (500–4000 kPa) at a flow rate of up to 1000 mL s −1. Wall oxygen or the use of an injector may inflate the chest by 500 mL within 0.5 s. Using a Manujet, a driving pressure of 1 bar may be adequate to ventilate most slim patients, and reduces the risk of barotrauma. Neuromuscular blockade, and inserting a SAD to maximize the expiratory route, also increase the success of the technique.
Wide-Bore Cannula and Surgical Techniques: Wide-bore cannulae are defined as those with an internal diameter of at least 4 mm because this is the minimum calibre through which an adult can exhale with adequate speed to maintain a normal minute volume. This applies whether inspiration is mechanical or spontaneous, because expiration is a passive process.
 Small cannula (approx. 2 mm) techniques (usually inserted as cannula over needle) require a high pressure gas source to overcome device resistance and rely on a patent upper airway for exhalation. Entrainment may augment inspiratory flow.
Small cannula (approx. 2 mm) techniques (usually inserted as cannula over needle) require a high pressure gas source to overcome device resistance and rely on a patent upper airway for exhalation. Entrainment may augment inspiratory flow.
 Large cannula (≥ 4 mm) techniques enable ventilation with lower pressures but require either a tracheal cuff, or the upper airway to be obstructed, to prevent loss of driving gas through the upper airway. Entrainment is minimal or non-existent.
Large cannula (≥ 4 mm) techniques enable ventilation with lower pressures but require either a tracheal cuff, or the upper airway to be obstructed, to prevent loss of driving gas through the upper airway. Entrainment is minimal or non-existent.
 Surgical airway techniques allow insertion of a large tube into the trachea and conventional (low pressure) ventilation, as for large cannulae.
Surgical airway techniques allow insertion of a large tube into the trachea and conventional (low pressure) ventilation, as for large cannulae.
MANAGEMENT OF THE PREDICTED DIFFICULT AIRWAY
When airway management is predicted to be difficult, the strategy can be based around achieving:
MANAGEMENT OF THE OBSTRUCTED AIRWAY
 What is the level of the obstruction?
What is the level of the obstruction?
 What is the degree of obstruction?
What is the degree of obstruction?
 What is its cause (e.g. tumour, haematoma, infection)?
What is its cause (e.g. tumour, haematoma, infection)?
 Is it friable or likely to bleed?
Is it friable or likely to bleed?
 Has there been previous airway or neck surgery, or radiotherapy? All place the patient in a higher risk category.
Has there been previous airway or neck surgery, or radiotherapy? All place the patient in a higher risk category.
 Can the patient’s airway be improved? If time allows, nebulized adrenaline (epinephrine) or steroids may improve the airway for short periods of time. Heliox (a mixture of helium and oxygen) may be beneficial before anaesthesia in critical cases. Radiotherapy may improve the airway, although often this is impractical.
Can the patient’s airway be improved? If time allows, nebulized adrenaline (epinephrine) or steroids may improve the airway for short periods of time. Heliox (a mixture of helium and oxygen) may be beneficial before anaesthesia in critical cases. Radiotherapy may improve the airway, although often this is impractical.
 Is there any important co-morbidity?
Is there any important co-morbidity?
 What is the surgical plan and preferred route for anaesthetic access? These patients illustrate the complexity of a ‘shared airway’ and there is no point in planning an anaesthetic approach which is not compatible with an agreed surgical plan.
What is the surgical plan and preferred route for anaesthetic access? These patients illustrate the complexity of a ‘shared airway’ and there is no point in planning an anaesthetic approach which is not compatible with an agreed surgical plan.
 What is the urgency of the intervention? It is important to differentiate patients in whom anaesthesia is planned to achieve surgery to improve the airway from those in whom anaesthesia is necessary to secure the airway in order to preserve life.
What is the urgency of the intervention? It is important to differentiate patients in whom anaesthesia is planned to achieve surgery to improve the airway from those in whom anaesthesia is necessary to secure the airway in order to preserve life.
Patients with an obstructed airway can be considered according to the level of obstruction.
Mid-Tracheal Obstruction
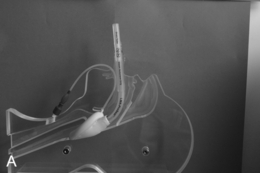
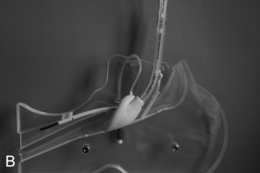
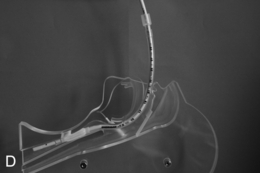
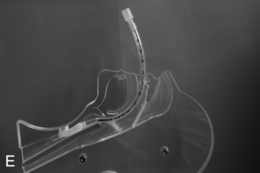
This presents an entirely different problem and is often caused by a retrosternal thyroid mass, although malignancies and infections may be the cause (Fig. 22.20). In the presence of a thyroid mass, the onset of airway compromise is usually slow and further radiological assessment is possible. A CT scan is vital to show the level and extent of the obstruction. Lesions below the larynx do not interfere with laryngoscopy (although the larynx and trachea may be displaced; Fig. 22.21) or the ability to use a face mask or SAD but they may interfere with ventilation after induction of anaesthesia and the ability to insert a tracheal tube.
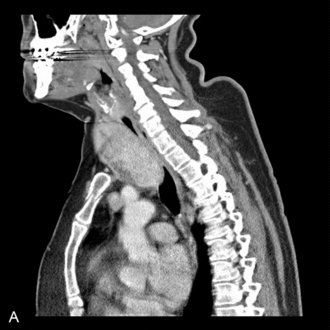
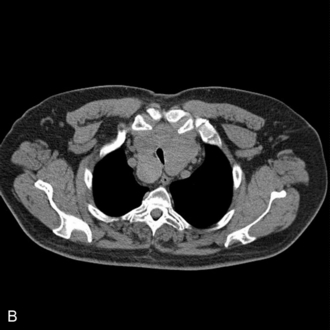
FIGURE 22.20 Sagittal (A) and axial (B) images of a thyroid mass partly obstructing the mid-trachea.
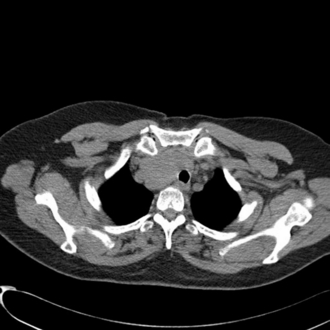
FIGURE 22.21 Axial image of a large thyroid mass which has displaced but not compressed the trachea.
There are several key issues which must be clarified before anaesthesia.
 How wide is the tracheal lumen and what size of tracheal tube may be used?
How wide is the tracheal lumen and what size of tracheal tube may be used?
 Is there space below the obstruction and above the carina to accommodate the end of the tracheal tube and the cuff?
Is there space below the obstruction and above the carina to accommodate the end of the tracheal tube and the cuff?
 Is the tracheal wall itself invaded? If so, there is a risk of collapse after any external mass has been removed.
Is the tracheal wall itself invaded? If so, there is a risk of collapse after any external mass has been removed.
 Would an emergency tracheostomy be possible and would a standard tracheostomy tube be long enough to bypass the obstruction?
Would an emergency tracheostomy be possible and would a standard tracheostomy tube be long enough to bypass the obstruction?
SPECIFIC TECHNIQUES
Supraglottic Airway Devices for Airway Rescue
 reliable ventilation (even if lung compliance is low)
reliable ventilation (even if lung compliance is low)
 early detection of regurgitation and increased protection against aspiration
early detection of regurgitation and increased protection against aspiration
 suitable as a conduit for subsequent fibreoptic guided tracheal intubation.
suitable as a conduit for subsequent fibreoptic guided tracheal intubation.
‘Videolaryngoscopes’, Rigid Indirect Laryngoscopes, Optical Stilettes and Advanced Intubation Aids: (see also Chapter 15)
Conduited intubation guides include several rigid fibreoptic laryngoscopes (Pentax AWscope, AP Advance laryngoscope) and the Airtraq (Fig. 22.22), which uses optical prisms rather than fibreoptics to illuminate the object and transmit an image.

FIGURE 22.22 An Airtraq conduited rigid fibreoptic laryngoscope with a tracheal tube mounted in the conduit.
Optical stilettes are metal rods with an internal fibreoptic or video system which enables the user to view the image from the distal end directly from the viewing port or on a remote screen. Most stilettes are rigid with a fixed angle (e.g. Bonfils, Levitan). The Shikani stilette (Fig. 22.23) is semi-malleable and the Sensascope has a flexible fibreoptic tip which allows some manipulation. Optical stilettes are placed within the lumen of the tracheal tube and then directed into the larynx before the tracheal tube is advanced into the airway. The main advantage of the stilettes is that they require minimal mouth opening (as little as 1 cm) and can be advanced with negligible tissue disruption. Their main disadvantage is the inability to manipulate and displace airway structures in the manner that a bladed instrument can.
The potential advantages of these devices include:
 ability to ‘see round corners’
ability to ‘see round corners’
 reduced head and neck movement to achieve a view of the larynx
reduced head and neck movement to achieve a view of the larynx
 reduced tissue distortion and haemodynamic responses to laryngoscopy
reduced tissue distortion and haemodynamic responses to laryngoscopy
 ability to display the image on a remote screen, which permits:
ability to display the image on a remote screen, which permits:
 improved supervision by trainers of intubation performed by trainees
improved supervision by trainers of intubation performed by trainees
 an assistant to observe what the intubator sees (e.g. guiding cricoid pressure)
an assistant to observe what the intubator sees (e.g. guiding cricoid pressure)
 recording for medical notes and medicolegal reasons
recording for medical notes and medicolegal reasons
 recording of an objective image or video for presentation, teaching or research purposes.
recording of an objective image or video for presentation, teaching or research purposes.
EXTUBATION AND RECOVERY
 patients who had problems at intubation or induction
patients who had problems at intubation or induction
 blood in the airway from surgical or anaesthetic causes
blood in the airway from surgical or anaesthetic causes
 patients who may have airway oedema from anaesthetic or surgical interventions
patients who may have airway oedema from anaesthetic or surgical interventions
 obese patients, particularly those with obstructive sleep apnoea
obese patients, particularly those with obstructive sleep apnoea
 intraoral or airway surgery (risks both oedema and aspiration of blood).
intraoral or airway surgery (risks both oedema and aspiration of blood).
Guidelines on Management of Extubation
There has been a steady increase in interest in the topic of extubation. In 2012, DAS published the first national guidance on extubation of adult patients (Fig. 22.24). This guidance divides extubation into four phases: plan, prepare, perform and post-extubation care. It recommends that an early assessment is made to determine whether extubation is low (fasted, uncomplicated airway, no other risk factors) or high risk (others). After taking the precautions described above, low risk extubation can usually be managed awake or ‘deep’ according to the anaesthetist’s preference and judgement, though making clear that the default method is awake. For patients requiring high risk extubation a number of options are offered: awake extubation, advanced techniques, delayed extubation and tracheostomy. Advanced techniques are conversion to a laryngeal mask with the tracheal tube in situ before extubation, a ‘remifentanil extubation’ or use of an AEC. Deep extubation in the high risk setting is not advocated. All the advanced techniques are described in detail but require expertise and practice before use in an acute situation.
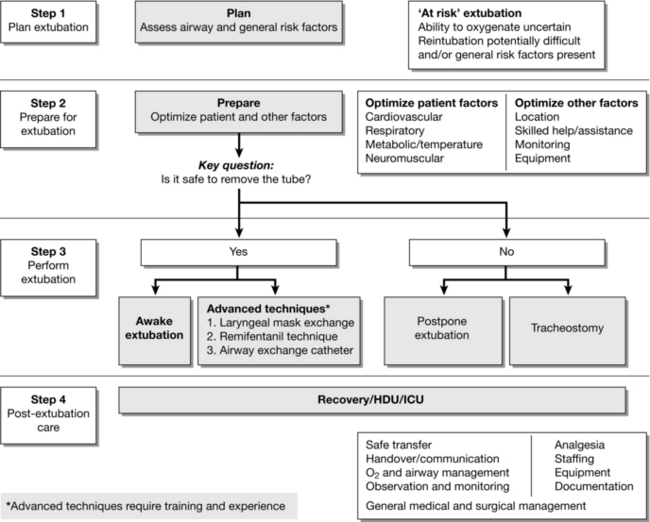
FIGURE 22.24 Algorithm for management of the high risk extubation. (Adapted from Popat M, Mitchell V, Dravid R, Patel A, Swampillai C, Higgs A. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012; 67:318–340, with permission from the Association of Anaesthetists of Great Britain & Ireland/Blackwell Publishing Ltd.)
THE DIFFICULT AIRWAY IN OTHER LOCATIONS
 Hazardous environment: an environment not designed for airway management and where this is sometimes not considered a main priority.
Hazardous environment: an environment not designed for airway management and where this is sometimes not considered a main priority.
 Failure to recognize and plan for patients with a known or predictably difficult airway.
Failure to recognize and plan for patients with a known or predictably difficult airway.
 A more limited range of equipment for management of the difficult airway than in theatres.
A more limited range of equipment for management of the difficult airway than in theatres.
 Less skilled intubators if not from an anaesthetic background.
Less skilled intubators if not from an anaesthetic background.
 Lack of experienced and skilled assistance.
Lack of experienced and skilled assistance.
 Absence of guidelines for management of routine airway problems (tracheostomy or tracheal tube displacement or failed RSI).
Absence of guidelines for management of routine airway problems (tracheostomy or tracheal tube displacement or failed RSI).
MANAGEMENT OF THE DIFFICULT AIRWAY IN CHILDREN
These were published in 2013 and are available on http://www.das.uk.com/content/paediatric-difficult-airway-guidelines and http://www.apagbi.org.uk/publications/apa-guidelines. The basic principles of difficult airway management described above apply equally in children but the airway management options are more limited.
 Awake techniques are generally impractical because of issues of co-operation.
Awake techniques are generally impractical because of issues of co-operation.
 Anatomical differences mean that some techniques (e.g. cricothyroidotomy) are less suitable in children and infants.
Anatomical differences mean that some techniques (e.g. cricothyroidotomy) are less suitable in children and infants.
 Newly developed equipment is often available only in adult sizes in the first instance. It can be many years before paediatric versions become available (e.g. videolaryngoscopes, second generation SADs) and some are not available at all (e.g. ILMA tracheal tubes, Aintree Intubation Catheter, several videolaryngoscopes).
Newly developed equipment is often available only in adult sizes in the first instance. It can be many years before paediatric versions become available (e.g. videolaryngoscopes, second generation SADs) and some are not available at all (e.g. ILMA tracheal tubes, Aintree Intubation Catheter, several videolaryngoscopes).
Alternative devices available to facilitate intubation in children include:
 bladed videolaryngoscopes (Glidescope)
bladed videolaryngoscopes (Glidescope)
 conduited videolaryngoscopes (Airtraq)
conduited videolaryngoscopes (Airtraq)
 optical stilettes (several adult versions may be used for children)
optical stilettes (several adult versions may be used for children)
AFTER DIFFICULT AIRWAY MANAGEMENT
Long-Term Management
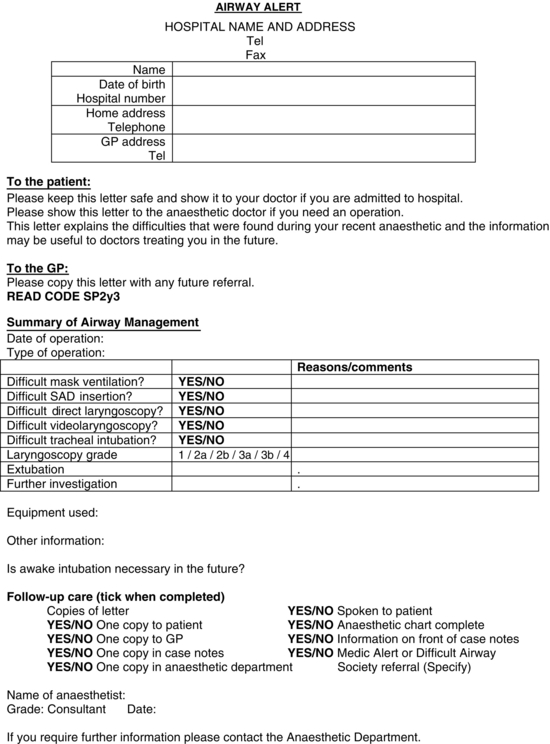
When the patient has recovered fully and before discharge from hospital, the senior anaesthetist involved should inform the patient of the relevant facts and the ways in which the difficulties experienced may affect airway management in future anaesthetics. It is probably appropriate that any patient whose airway is likely to prove difficult to manage during RSI by a junior anaesthetist is given written information to that effect. The anaesthetic record should contain a clear record of the problem, what was done, what did and did not work, and a judgement as to the likely problems and solutions in the future. This information should be given to the patient, sent to the patient’s general practitioner and filed in the hospital records. An example of a proforma is shown in Figure 22.25. The general practitioner should be asked to include the information in any future referrals. The Read code for difficult intubation can usefully be included in such letters and is SP2y3. It may be appropriate for the patient to wear a medical alert bracelet.
Cook, T.M., Nolan, J.P., Cranshaw, J., Magee, P. Needle cricothyroidotomy. Anaesthesia. 2007;62:289–290.
Cook, T.M., Woodall, N., Frerk, C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1 Anaesthesia. Br. J. Anaesth. 2011;106:617–631.
Cook, T.M., Woodall, N., Harper, J., Benger, J. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2 Intensive Care and Emergency Department. Br. J. Anaesth. 2011;106:632–642.
Cook, T.M., McDougall-Davis, S.R. Complications and failure of airway management. Br. J. Anaesth. 2012;109:i68–i85. (Suppl 1),
Harmer, M. Independent Review on the care given to Mrs Elaine Bromiley on 29 March 2005. Clinical Human Factors Group. http://www.chfg.org/resources/07_qrt04/Anonymous_Report_Verdict_and_Corrected_Timeline_Oct_07.pdf, 2005.
Hawthorne, L., Wilson, R., Lyons, G., Dresner, M. Failed intubation revisited: 17-yr experience in a teaching maternity unit. Br. J. Anaesth. 1996;76:680–684.
Mihai, R., Blair, E., Kay, H., Cook, T.M. A quantitative review and meta-analysis of performance of non standard laryngoscopes and rigid fibreoptic intubation aids. Anaesthesia. 2008;63:745–760.
Peterson, G.N., Domino, K.B., Caplan, R.A., et al. Management of the difficult airway: a closed claims analysis. Anesthesiology. 2005;103:33–39.
Rose, D.K., Cohen, M.M. The incidence of airway problems depends on the definition used. Can. J. Anaesth. 1996;43:30–34.
Samsoon, G.L.T., Young, J.R.B. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487–490.
Sheriffdom of Glasgow and Strathkelvin, 2010. Determination of sheriff Linda Margaret Ruxton in fatal accident inquiry into the death of Gordon Ewing. http://www.scotcourts.gov.uk/opinions/2010FAI15.html