Chapter 8 Management of the Child with Otitis Media
Otitis media (OM) is a significant problem for infants and children. Acute otitis media (AOM) is the second most common infectious diagnosis in the first year of life, and the number one reason for the use of antibiotics in this age group. Acute otitis media and recurrent acute otitis media (RAOM) are both considerable clinical problems in infants and children. From an economic standpoint an estimated US$5 billion a year is expended combating this problem in the USA.1 Recent studies have also suggested that the overall incidence of AOM is increasing. Joki-Erkkila et al evaluated the diagnosis of AOM from 1978 through 1979 and compared it to 1994 through 1995. They demonstrated an increased incidence of 68% with a 95% confidence interval.2
As a general rule most episodes of AOM are treated with at least one course of oral antibiotics by primary care practitioners. Besides the significant potential side effects of oral antibiotics, each episode of AOM is associated with a conductive hearing loss due to residual middle ear fluid present in 50% of treated ears after 1 month of infection. As described above, a subset of children with acute otitis media develop multiple episodes of AOM known as recurrent acute otitis media (RAOM). These children with RAOM are also designated as otitis prone. Therapeutic options for children with RAOM are limited to either long-term administration of prophylactic antibiotics or placement of tympanostomy tubes. Tympanostomy tube placement is the most common suggested surgical intervention performed in children.3–5 Although this is a relatively simple procedure, it still requires a general anesthetic and reported complications include prolonged otorrhea and persistent tympanic membrane perforations.6–16
Although recurrent acute otitis media is a very common diagnosis, the pathophysiological mechanism under this disorder is multifactorial and poorly understood. There are numerous risk factors for the development of RAOM. These include male gender, sibling history of recurrent otitis media, early occurrence of infection, absence of breast-feeding, day care attendance, and second-hand smoke.17 A recent meta-analysis conducted by Uhari and colleagues analyzed risk factors associated with AOM. They found sibling history, day care attendance, parental smoking, breast-feeding less than 3 months, and pacifier use as significant risk factors for RAOM.18
Epidemiology
As described above, there are several risk factors involved in the development of acute otitis media, not the least of which is crowding in large day care centers. Indeed day care attendance is a complex risk factor for many infectious illnesses in infants and young children. Alho and colleagues looked at risk factors for RAOM and upper respiratory tract infections (URTI). They found both day care attendance and short duration of breast-feeding to be significant risk factors, increasing the risk of both RAOM and URTIs.19 Fleming and colleagues looked at two groups of children, those attending day care on a regular basis and those who did not attend day care. They also found children in day care had an increased risk of URTIs and an increased risk of ear infections. These investigators found that 66% of ear infections were linked to day care attendance.20 Collet and colleagues did an 8.5-month prospective study of children in day care. Their study demonstrated an increased risk factor of greater than or equal to five episodes of URTIs, two episodes of AOM, and two episodes of conjunctivitis.21 Other investigators have attempted to understand the basis for this increased risk. Stenstrom and Ingvarsson performed a retrospective review that looked at general illness in otitis prone (OP) versus nonotitis prone (NOP) children. They found otitis prone children accounted for between two and four times the number of episodes of rhinopharyngitis, sinusitis and tonsillitis, and significantly more episodes of bronchopulmonary infections, gastrointestinal and urinary tract infections.22 Kvaerner and colleagues evaluated exposure to upper respiratory pathogens in AOM. They found that sibling attendance in day care was the most prominent risk factor for the development of AOM, followed by the number of children in the day care center.23
It has been suggested that day care attendance may create differences in nasopharyngeal colonization. These differences in chronic nasopharyngeal colonization may play a significant role in the development of the subset of children designated as otitis prone. Indeed the incidence of bilateral myringotomy and tympanostomy intubation in children in day care is 31% versus 11% for the population not in day care.24 Brook and Gober evaluated the type of organisms found in the nasopharynx of otitis prone children versus nonotitis prone children. They found nonotitis prone children tended to have more diverse flora possibly thought to inhibit the attachment of middle ear pathogens in the nasopharynx.25 Ito and colleagues evaluated nasopharyngeal colonization of healthy children who attended day care and compared this to healthy children who did not attend day care. They demonstrated day care children were much more likely to have penicillin-resistant Streptococcus pneumoniae cultured from the nasopharynx compared to the controls not in day care.26 Other studies have shown that nasopharyngeal colonization with resistant organisms leads to an increased rate of AOM and unresolved otitis media.27 Farjo and colleagues evaluated different strains of Haemophilus influenzae in the nasopharynx of children at 16 day care centers. They demonstrated sharing of the same strains in 13 out of 15 day care centers. These authors suggested that this degree of sharing demonstrated transmission of the pathogenic organisms in the day care center.28
Relevant Anatomy and Physiology
▪ Eustachian Tube
The Eustachian tube is an important conduit between the nasopharynx and the middle ear. Composed of both bony and cartilaginous segments, it functions to equilibrate middle ear pressure, drain fluid in the middle ear, and protect the middle ear from infected nasopharyngeal secretions. Under most conditions, the Eustachian tube is closed, opening intermittently with swallowing to provide the tubal functions noted above. Numerous investigators have demonstrated that there are subsets of both children and adults who have Eustachian tube dysfunction.29–31 One major cause of Eustachian tube dysfunction involves lack of appropriate opening of the lumen, such as in patients suffering from cleft lip and cleft palate malformations. These individuals may easily develop middle ear atelectasis, and run significant risk for aspiration of nasopharyngeal pathogens. Mechanical obstruction due to inflammation or compression by a nasopharyngeal tumor or mass will also predispose patients to significant problems with middle ear atelectasis, leading to aspiration of middle ear pathogens from the nasopharynx. Alternatively, other patients have patulous Eustachian tubes that are abnormally open and provide poor protection of the middle ear from nasopharyngeal infecting agents. Age-related anatomic and functional differences have also been noted in the Eustachian tube.32 In adults the Eustachian tube is approximately 35 mm and inclined at a 45 degree angle. In infants the tube is around 18 mm, about half the size of adults, and positioned closer to a 0 degree angle. The shorter length and position of the Eustachian tube in infants and children is thought to lead to an increased likelihood of nasopharyngeal reflux and a decrease in clearance function.
▪ Middle Ear
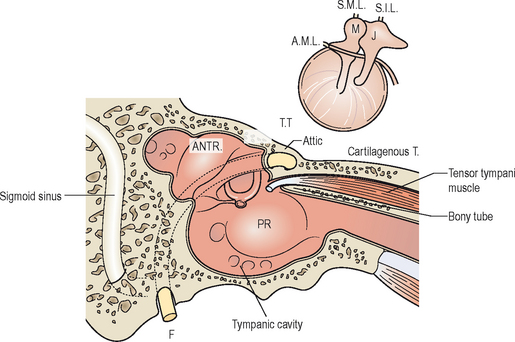
The middle ear is divided into three parts, the hypotympanum, mesotympanum, and epitympanum. The hypotympanum is defined as the area of the middle ear inferior to the tympanic membrane, while the mesotympanum covers the region of the middle ear medial to the tympanic membrane, and the epitympanum the superior aspect of the tympanic membrane. The direct open pathway of the middle ear at the level of the epitympanum to the aditus ad antrum and the mastoid air cells provides a potential route for spread of infectious processes from the middle ear to the mastoid cavity that could lead to development of acute mastoiditis, as shown in Figure 8.1.
Histology
The normal middle ear contains primarily cylindrical or layered cubic epithelium interspersed with ciliated cells. Adjacent to the Eustachian tube orifice the tympanic cavity epithelium changes to pseudo-stratified ciliated cells, as it is in the entire length of the Eustachian tube. There are different histological changes that occur during development of acute otitis media in normal ears, ears with some inflammatory change, and ears with a previous history of otitis media with effusion. For AOM in a normal ear, the goblet cell density is relatively low in the middle ear, immunologic defenses are not present, and recovery to a normal epithelium is usually fairly rapid. Development of AOM in a pathologic ear presents differently, as the epithelium is pseudo-stratified and cylindrical goblet cell glands are increased in density, resulting in a prolonged return to a normal histology. Conversely, the middle ear mucosa in an AOM-infected ear with a previous history of OME is pathologic and secretory, and filled with both mucus and secretions, with any additional episodes of AOM further deteriorating the histologic condition of the middle ear. For OME, initial histologic changes are similar to AOM, with an increase in goblet cells, basal cell hyperplasia, vessel dilation, and new gland formation. This may then go on to a secretory stage which is dominated by mucous secretions, an increase in goblet and mucous glands within the middle ear, and infiltration of lymphocyte fibroblasts and plasma cells. During the restoration phase of OME there is a period of decreased mucous production, degeneration of the mucous glands, and a pseudo-stratified epithelium that goes back to a one- or two-layered cubic epithelium, followed by a return of the middle ear to a more normal histologic condition.33
Pathophysiology
▪ Bacterial Infections
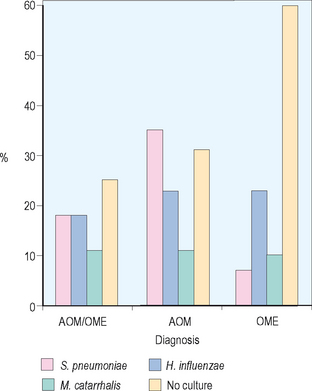
Acute otitis media has been revealed to be primarily a bacterial infection, with recent investigations even suggesting that COME is an indolent bacterial infection. Numerous investigators have demonstrated the importance of middle ear pathogens in the development of AOM. Bluestone and colleagues reviewed approximately 7400 middle ear isolates from children undergoing tympanostomy tube placement. They found Streptococcus pneumoniae and Haemophilus influenzae at 18%, Moraxella catarrhalis at 11%, mixed growth at 11%, and no growth at 25% overall in these 7400 isolates, as shown in Figure 8.2. When divided by diagnosis for children with AOM, 35% grew out S. pneumoniae, 23% H. influenzae, and 11% M. catarrhalis. For children with OME the predominant middle ear pathogen was H. influenzae at 23%, followed by M. catarrhalis 10%, and S. pneumoniae 7%. These investigators also demonstrated shifts in antibiotic resistance with beta-lactamase production from M. catarrhalis increasing from 60% to 80% in AOM and 60% to 100% in OME. Regarding H. influenzae they showed an increase of 15% to 25% for acute otitis media and 20% to 30% for OME.34
Other investigators have looked at the incidence of pneumococcal isolates to penicillin resistance and found that 61% of nasopharyngeal pneumococcal isolates from day care centers were penicillin resistant, versus a 33% rate of incidence of nasopharyngeal pneumococcal isolates that were penicillin resistant from a county health care center.35 There also may be significant clinical implications for shifts in antibiotic sensitivity. Antonelli and colleagues looked at the incidence of admission for mastoiditis at a major tertiary medical center between 1987 and 1997. Their study demonstrated a significant increase in the incidence of mastoiditis. S. pneumoniae was found to be the causative agent in the majority of these infections, with all but one strain of S. pneumoniae found to be penicillin resistant.36
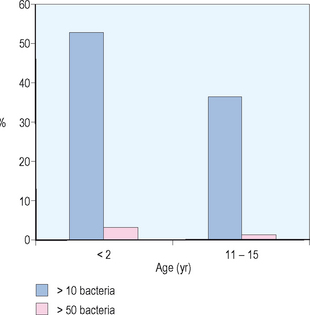
Although the normal middle ear is sterile, it has been suggested that ascending infection from the nasopharynx associated with Eustachian tube dysfunction is a critical step in the development of AOM. There is also an important relationship between nasopharyngeal colonization and otitis media. Stenfors and Raisanen evaluated attachment of bacterial cells in the nasopharynx in healthy children of different ages. They used immunofluorescence and graded the number of bacteria attached per nonciliated cell in the nasopharynx. As demonstrated in Figure 8.3, these investigators found for children less than 2 years old, 53% of nonciliated cells had greater than 10 attached bacteria and 36% of nonciliated cells had greater than 50 attached bacteria. For children 11–15 years old, 3% of nonciliated cells had greater than 10 attached bacteria while less than 1% of nonciliated cells had greater than 50 attached bacteria. These investigators also evaluated culture forming units of different middle ear pathogens. They found for children less than age 2, 100% had middle ear pathogens in the nasopharynx and for children 11–15 years of age only 44% had middle ear pathogens.37
▪ Viral Infections
The relationship between viral upper respiratory tract infections and acute otitis media is complex and not well understood. Ruskannen and colleagues recovered nasopharyngeal samples from 5092 children over a 5-year period and performed viral assays looking at the incidence of AOM. They found an increased incidence of AOM correlating in peaks from viral isolation of respiratory syncytial virus (RSV) from 1991 to 1992 and from 1993 to 1994. They also found an increased incidence of AOM in the fall of 1994 and early 1994 associated with adenovirus, parainfluenza virus, and influenza A virus. They found that 34% of patients with viral infections had AOM. The most common virus seen was RSV, followed by influenza A, parainfluenza type 3, adenovirus, and influenza B.38
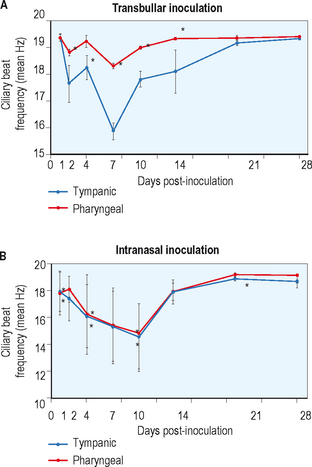
Park et al performed a study using chinchillas to try to understand the potential role of viral infections in the pathogenesis of AOM.39 These investigators evaluated ciliary activity and dye transport function in chinchillas inoculated either intranasally or transbullarly with influenza type A virus. They used light microscopy and electron microscopy and demonstrated a disorganized epithelium and the presence of cellular debris in the Eustachian tube lumen 24 hours after injection. They also noted the presence of abnormal cilia and focal ciliated cell death seen up to 7 days post injection histopathologically. In addition, these investigators demonstrated extensive inflammatory and cellular infiltrate in the epithelial space at day 14. By 28 days the histology appeared normal. The same histopathologic features were identified whether the route of injection was transnasal or intrabullar. Evaluating ciliary beat frequency and dye transport, they showed a maximum decrease between 7 and 14 days with the return of normal activity by 28 days, as displayed in Figure 8.4. There was a close correlation between histopathological changes identified on light and electron microscopy and the functional analysis of ciliary motility as seen by ciliary beat frequency and dye transport. This study demonstrates the disruptive effect that viral infections can have on Eustachian tube function, and how this may lead to intrinsic obstruction of the Eustachian tube associated with inflammatory change.
▪ Altered or Impaired Immunity
A second host factor that is important in the development of RAOM is altered or impaired immunity. It has been demonstrated that certain bacteria may liberate exotoxins, toxic cell wall components, and endotoxins (i.e., purulent sinusitis transmembrane protein P15E can cause immunosuppression). This, along with an impaired immune response, may be a major factor in a predilection for RAOM. Bernstein looked at overall antibody titers of the IgG and IgG subclasses, and demonstrated that otitis prone children have significant reduction in antibody titers as compared to nonotitis prone children for numerous bacterial pathogens.40
Diagnosis
▪ History
Like any complex clinical problem in children, a thorough history is required to arrive at an accurate diagnosis. Children presenting with AOM generally will be febrile, irritable, and may also have all of the signs and symptoms associated with viral upper respiratory tract infections. Conversely, children presenting with OME are relatively asymptomatic with only muffled hearing and possibly some mild disequilibrium. When evaluating children with otitis media, a risk factor assessment for RAOM is also valued.
As mentioned earlier, there are many documented risk factors associated with otitis media. Male gender has been shown by several studies to be a prominent risk factor, as well as sibling history of recurrent otitis media.41,42 An early occurrence of otitis media in a child can be another important early warning sign of increased susceptibility to ear infections. Other risk factors include an absence of breast feeding, presence in a day care facility, and passive exposure to cigarette smoke in a household.41–43 Although these simple diagnostic risk factors are a good indicator of predisposition to otitis media infections, this is only the first step to early and effective medical treatment.
▪ Physical Examination
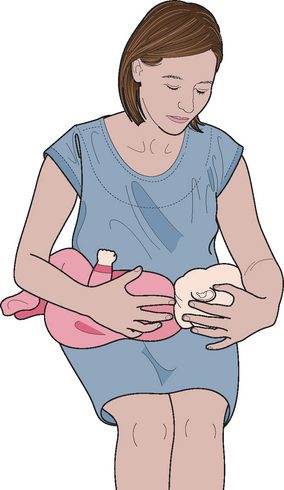
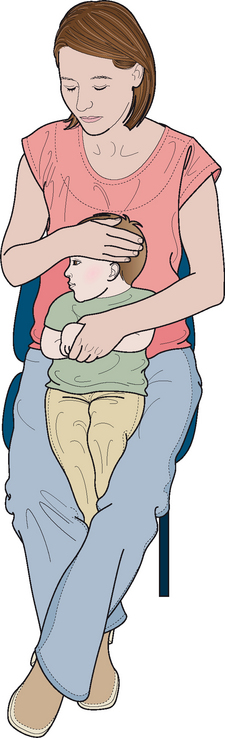
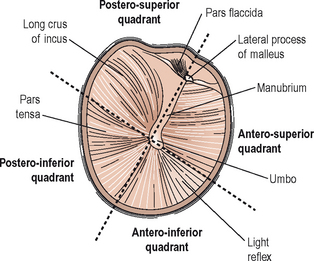
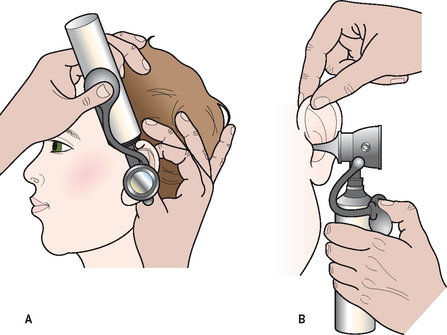
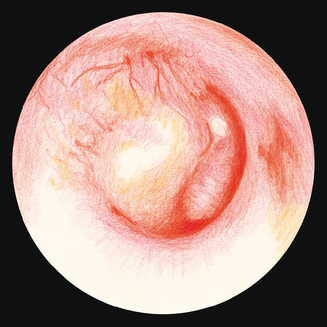
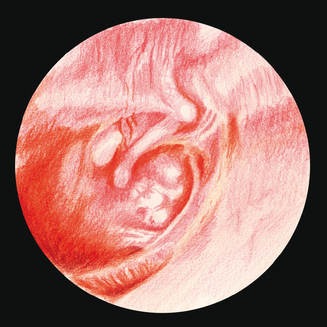
Examination of the middle ear in an infant or child requires a systematic approach. The first step involves appropriate positioning of the child with the parent. Two appropriate positions are shown in Figures 8.5 and 8.6. Next, gentle cleaning of the ear canal of any cerumen that would obstruct the physician’s view of the tympanic membrane is recommended. Due to the friable skin of the external auditory canal, a considerable amount of care should be taken not to traumatize it and cause a frustrating situation with the patient or parent. A careful inspection is then performed of the tympanic membrane, with attention directed toward visualization of the manubrium of the malleus, pars flaccida versus pars tensa. Other landmarks that may be visible would include the incudostapedial joint seen through a very thin transparent tympanic membrane in the posterior superior quadrant and a secondary light reflex from the promontory in the posterior inferior quadrant, as seen in Figure 8.7. A pneumatic otoscope with an appropriate sized speculum should always be used, as pneumatic otoscopy is a necessity to properly evaluate the condition of the middle ear, depicted by Figure 8.8. As Figure 8.9 demonstrates, four different characteristics of the tympanic membrane can be evaluated by this technique, which include mobility, position, transparency, and color of the tympanic membrane. In a normal, well-ventilated middle ear free of any pathologic changes to the tympanic membrane, the drum should be freely mobile, positioned in a neutral setting, transparent, and gray to white in color depending on whether or not the child is crying. Conversely, a patient with acute otitis media would demonstrate a non-mobile, bulging tympanic membrane around the manubrium, with obscurity of normal landmarks surrounding the tympanic membrane, as shown in Tables 8.1 and 8.2. The TM is usually thick, opaque, and red to white depending on the vascular system. Also of note for AOM, thick annular vessels are frequently identified, which may be a unique identifying feature for AOM. As Table 8.3 and Figure 8.10 demonstrate, the ear drum of a patient suffering from otitis media with effusion without active acute changes may have limited mobility or may be non-mobile, be in a neutral or retracted position, appear translucent to opaque, and have a variable color. An ear with significant middle ear atelectasis may demonstrate retraction with obvious middle ear effusion, as shown in Figure 8.11.
TABLE 8.1 The characteristics of a normal, uninfected tympanic membrane. This drum would be mobile in a neutral position, transparent, and gray to white in color
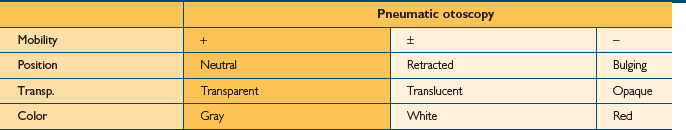
TABLE 8.2 The defining characteristics of an ear drum affected by an episode of acute otitis media. This drum would present as bulging with no mobility, appear opaque, and white to red in color

TABLE 8.3 A good look at the characteristics of an ear drum of a patient with otitis media with effusion. The drum may or may not be mobile, sits in a retracted position, and appears translucent and gray to white color
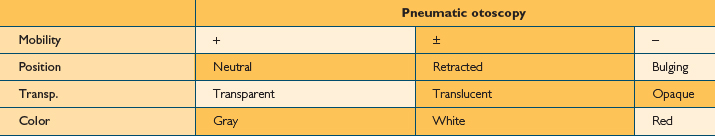
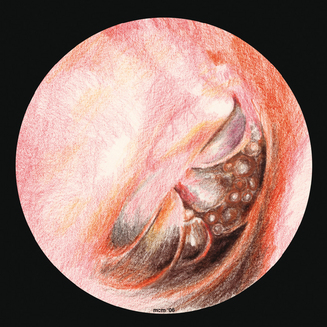
Figure 8.10 The tympanic membrane of an ear that has OME. Of note are the air–fluid levels within the middle ear.
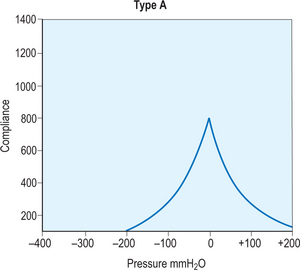
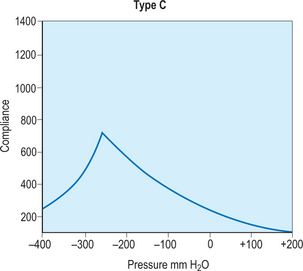
A simple assessment that provides basic functional and anatomic information about the middle and inner ear is a tympanometry test, which uses air pressure to measure the integrity of the ear. The first step in this process requires a quick otoscopy examination to locate any foreign bodies or other objects that would obscure a direct path to the tympanic membrane. Once the ear is cleared for testing, a small plastic probe-tip is fitted into the ear, and the machine alters the air pressure in the ear to produce a pure tone. The tympanometer then measures the eardrum responses to the different sound and pressure and records all of the information on a curve called a tympanogram. This can be useful in many circumstances, but in regards to otitis media, it helps to determine if there is fluid or effusion in the middle ear. This information is then interpreted by an audiologist from the different types of curves displayed by the tympanogram. A type A curve would demonstrate a normal ear, in regards to pressure and mobility of the tympanic membrane, as shown in Figure 8.12. Figures 8.13 and 8.14 demonstrate type B and C curves, which may be indicative of middle ear effusion, tympanic membrane perforation. Although helpful to locate and identify certain complications, it is not a self-exhaustive diagnostic tool, and should be coupled with other tests to substantiate its findings. Also, it cannot be used to differentiate between types of otitis media, limiting its diagnostic effectiveness.
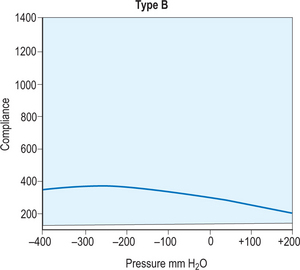
Figure 8.13 A type B tympanogram curve. This curve, classified as flat, is indicative of an abnormal or effused ear.
The best technique for evaluation of the middle ear is coupling of a tympanometry examination with pneumatic otoscopy. As described earlier, pneumatic otoscopy allows the practitioner to measure mobility, transparency, color, vascularity, and position of the tympanic membrane. The otoscopic appearance of the tympanic membrane along with tympanic membrane mobility using tympanometry data provides complementary information to understanding the middle ear condition. Jones and Kaleida showed that pneumatic otoscopy greatly improved the accuracy of diagnosis of middle ear effusion.44 Other investigators have demonstrated that evaluating characteristics of the tympanic membrane and other aspects of the external ear canal help to draw a parallel between tympanometry results and middle ear condition.45–47 It should also be noted that children under 7 months should not undergo tympanometry because of the high level of compliance of their tympanic membrane.
Treatment
There is a myriad of treatment options for care of the patient with otitis media. These range from observation with close clinical follow-up to surgical intervention such as tympanocentesis or myringotomy with tympanostomy tube placement. Observation is an acceptable option for children of 2 years or older not attending day care presenting with no suppurative complications or significant sequelae of AOM such as pain or persistent fever. If observation is elected, mandatory clinical follow-up after 48 hours is indicated to verify clinical improvement. This is appropriate because 6 out of 8 to 8 out of 9 episodes of AOM resolve spontaneously.48,49 Alternatively, if there is no documented clinical improvement in either the otoscopic appearance of the tympanic membrane, or systemic signs such as fever after 48 hours, appropriate oral antibiotics would be indicated. Although observation is one option for management of AOM, some investigators have demonstrated that this practice may lead to an increase in suppurative complications of AOM, which will be discussed later.
▪ Pharmocologic Treatment
Otitis media is the most common clinical diagnosis, with 20–25% of all antibiotic prescriptions are being filled for this condition every year in the USA.50–52 Streptococcus pneumoniae is the most frequent bacterial agent implicated in AOM episodes, characterized by standard symptoms of effusion, pain, and redness and swelling of the tympanic membrane. The first line of defense for such an infection is generally amoxicillin, provided the patient is not allergic to penicillin. If atopy is an issue, trimethoprim/sulfamethoxazole or erythromycin/sulfisoxazole are viable options. These are also effective if a single course of amoxicillin fails to clear up symptoms of the S. pneumoniae infection on its own. Another effective secondary antibiotic is an amoxicillin/clavulanate based antibiotic such as augmentin. Other good second line antibiotics are cefixime, clarithromycin, cefpodoxime proxetil, and cefprozil. Standard antibiotic therapy for all patients diagnosed with AOM is a 10–14 day treatment.
Although antibiotics are the traditional treatment modality for the majority of cases of AOM, as the prevalence of antibiotic-resistant bacteria continues to increase at an uncontrollable rate, the indication for such treatment declines. It has been well documented that antibiotics are becoming less and less effective at eliminating these pathogens.53–57 Also, as the basic understanding of bacterial agents increases, antibiotic treatment options appear more limited in their ability to fight these infections. There is a great deal of recent information documenting the ability of bacteria to create an extracellular matrix that surrounds and protects them, decreasing their susceptibility to the dangers of their outside environment, including antibiotic treatment, which will be addressed later in the chapter.
▪ Surgical Options
A set of clinical practice guidelines for treatment of OME in young children has been established by the AAFM (American Academy of Family Physicians), AAO-HNS (American Academy of Otolaryngology-Head and Neck Surgery, the AAP (American Academy of Pediatrics), and the AHRQ (Agency for Healthcare Research and Quality) for use by family practitioners and otolaryngologists. The guidelines set forth are only recommended for patients suffering from OME in the ages from 1 to 3. Children experiencing bilateral middle ear effusion should undergo a complete hearing evaluation in addition to the aforementioned audiological tests. Therapeutic interventions have also been outlined by the guidelines to give clinical practitioners recommendations for treatment modalities of different forms of OME. For a child suffering from bilateral middle ear effusion (MEE) for less than 4–6 months, with less than 20 dB of a hearing threshold in the better hearing ear, clinical observation or antibiotic therapy is recommended. For an infant or child who has experienced bilateral MEE for 3 months with greater than a 20 dB hearing threshold, tympanostomy tubes are optional. For young children experiencing bilateral MEE greater than 4–6 months, with a hearing deficit greater than 20 dB, tympanostomy tubes are recommended.
▪ Complications
There are a variety of suppurative complications associated with otitis media. These complications, although relatively rare, may lead to significant morbidity such as subdural or epidural empyema or brain abscess. These complications could result in intratemporal or extratemporal spread beyond the temporal bone. Intratemporal complications include facial paresis, labyrinthitis, acute mastoiditis, and petrositis.58 Patients experiencing AOM-induced facial paresis would present with facial neuropalsy and other common suppurative complications associated with AOM. Because of the significant morbidity factor, these patients should urgently undergo a myringotomy and tympanostomy tube insertion, and a culture should be obtained for a complete pathological work-up. The patient should subsequently be placed on broad spectrum antibiotics. High resolution temporal bone computed tomography should also be performed to rule out coalescent mastoiditis or cholesteatoma as the prevailing cause of complications. Labyrinthitis may present as disequilibrium or balance disturbances associated with AOM or OME. Patients suffering from this complication again should undergo prompt tympanocentesis or myringotomy and tympanostomy tube placement with IV antibiotics. Temporal bone imaging is again needed to rule out mastoiditis or cholesteatoma. A complete audiological evaluation is also indicated to help differentiate between serous versus suppurative labyrinthitis. Serous labyrinthitis would create a mild to moderate conductive hearing loss, whereas a patient with suppurative labyrinthitis would experience profound sensorineural hearing loss.59
A patient with acute mastoiditis will generally demonstrate all signs and symptoms of AOM, in addition to increased temperature, postauricular tenderness, and possible fullness associated with a subperiosteal skull abscess. These patients should be placed on IV antibiotics and undergo immediate computed tomography imaging. If the scan demonstrates acute mastoiditis without bone destruction or abscess, prompt myringotomy and tympanostomy tube placement with parental antibiotics is an option. If the scan demonstrates coalescent abscess or mastoiditis, a mastoidectomy is indicated. Petrositis is a very rare complication of AOM or OME and presents with a triad of symptoms including retro-orbital pain, diplopia, and ear pain. This very serious complication requires immediate surgical intervention via mastoidectomy with an extratemporal approach into the petrous air cells, followed by long term IV antibiotic therapy.
Future Directions
▪ Otitis Media as a Biofilm Disease
In the past, otitis media and COME were considered inflammatory conditions of the middle ear partially due to the lack of response of systemic antibiotics. This concept was further reinforced by the difficulty of culturing living bacteria from OME specimens. Work by Post et al demonstrated that 85% of OME specimens were PCR positive for middle ear pathogens.60 Further work by this group demonstrated, using RT-PCR, bacterial RNA for middle ear pathogens in 85% of these specimens.61 This second study strongly supports the concept of viable middle ear pathogens in OME which are difficult to culture and resistant to systemic antibiotics. The role of these formations, known as biofilms, was further supported by a chinchilla model of AOM which demonstrated biofilm production within 24 hours of the development of AOM.62 Expanding on this paradigm, other more recent investigators have examined adenoid specimens removed from children with RAOM and OME and have found dense biofilms covering the mucosal surface when compared to controls.
Even with the advanced medical treatment modalities at the disposal of physician’s today, the number of cases of OM in children has been increasing and is still on the rise.60 This is a good indicator that there are other processes of this disease not well understood that are enhancing the pervasive properties of otitis media infections. Biofilm formation by bacteria is a well-recognized phenomenon. This process occurs when individual cells coalesce and adhere to various surfaces. Outside of the human, this occurs on mineral surfaces, living and dead plant or animal tissue, polymers, ceramics, and metal alloys. The exact bimolecular mechanism of this transformation varies accordingly with each species that partakes in it. Eventually, the proximity of the cells to a surface stimulates the production of exopolysaccharides (EPS) – it is this hydrated EPS matrix that forms much of the volume of a biofilm community, allowing microchannels to form connections among the microbes and perpetual shedding of planktonic cells.
Amplifying all of this is the ability of a biofilm to render seemingly appropriate antimicrobial therapy entirely futile. The necessity to remove and replace infected indwelling devices highlights the importance of this point. This resistance seems to be a unique function of the biofilm complex, as known mechanisms of altered sensitivity such as efflux pumps, modifying enzymes, and target mutations do not play a role in the resistance imparted by biofilms. Even bacteria that are sensitive to antibiotics in vitro show reduced susceptibility in biofilms. As an example, Klebsiella pneumoniae organisms with an MIC (minimum inhibitory concentration) of 2 μg/ml ampicillin in aqueous solution demonstrated 66% survival when the same organisms in a biofilm complex were treated for 4 hours with 5000 μg/ml of ampicillin. Adding to the evidence against a genetic mechanism for this resistance is the observed susceptibility of organisms upon shedding to planktonic forms. One possible explanation of resistance is slow or incomplete penetration of antimicrobials into biofilms, although interestingly, biofilms have not been clearly shown to be generic mechanical barriers to the diffusion of solutes with comparable size to antibiotics. This incomplete penetration may in fact be due to the inactivation of antimicrobials during the diffusion process; presumably, this inactivation occurs at a more rapid rate than antimicrobial diffusion. Another explanation involves studies showing altered chemical microenvironments within biofilms.
1 Klein JO. The burden of otitis media. Vaccine. 2000;19(suppl 1):S2-8.
2 Joki-Erkkila VP, Laippala P, Pukander J. Increase in pediatric acute otitis media diagnosed by primary care in two Finnish municipalities – 1994–5 versus 1978–9. Epidemiol Infect. 1998;121:529-534.
3 Berman S. Otitis media in children. N Engl J Med. 1995;332:1560-1565.
4 Gates GA. Cost-effectiveness consideration in otitis media with effusion. In: Cumming CW, editor. Otolaryngology – head and neck surgery. 2nd edn. St Louis, MO: Mosby; 1998:461-477.
5 Stool SE, Berg AO, Berman S, et al. Otitis media with effusion in young children. AHCPR publication No. 94–0622. Clinical Practice Guidelines No. 12. Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Policy and Research, July 1994.
6 Adkins AP, Friedman EM. Surgical indications and outcomes of tympanostomy tube removal. Int J Ped Otorhinolaryngol. 2005;69:1047-1051.
7 Barfoed C, Rosborg J. Secretory otitis media. Arch Otolaryngol. 1980;106:552-556.
8 Balkany TH, Barkin RM, Suzuki BH, Watson WJ. A prospective study of infection following tympanostomy and tube insertion. Am J Otol. 1983;4:288-291.
9 Gates GA, Avery C, Prihoda TJ, Holt GR. Post-tympanostomy otorrhea. Laryngoscope. 1986;96:630-634.
10 Baker RS, Chole RA. A randomized clinical trial of topical gentamicin after tympanostomy tube placement. Arch Otolaryngol Head Neck Surg. 1988;114:755-757.
11 Giebink SG, Daly K, Buran DJ, Satz M, Ayre T. Predictors for postoperative otorrhea following tympanostomy tube placement. Arch Otolaryngol Head Neck Surg. 1992;118:491-494.
12 Salam MA, Cable HR. The use of antibiotic/steroid drops to reduce postoperative otorrhea and blockage of ventilation tubes. J Laryngol Otol. 1993;107:188-189.
13 Younis RT, Lazar RH, Long TE. Ventilation tubes and prophylactic antibiotic eardrops. Otolaryngol Head Neck Surg. 1992;106:193-195.
14 Shinkwin CA, Murty GE, Simo R, Jones NS. Pre-operative antibiotic/steroid prophylaxis of tympanostomy tube otorrhea. J Laryngol Otol. 1996;110:531-533.
15 Mangat KS, Morrison GA, Ganniwalla TM. T-tubes: A retrospective review of 1274 insertions over a 4-year period. Int J Pediatr Otorhinolaryngol. 1993;25:119-125.
16 Goode RL. Long-term middle ear ventilation with t-tubes: the perforation problem. Otolaryngol Head Neck Surg. 1996;115:500-501.
17 Baraibar R. Incidence and risk factors of acute otitis media in children. Clin Microbiol Infect. 1997;3(suppl 3):S13-S22.
18 Uhari M, Mantysaari K, Niemela M. A meta-analytic review of the risk factors for acute otitis media. Clin Infect Dis. 1996;22:1079-1083.
19 Alho OP, Koivu M, Sorri M, Rantakallio P. Risk factors for recurrent acute otitis media and respiratory infection in infancy. Int J Pediatr Otorhinolaryngol. 1990;19:151-161.
20 Fleming DW, Cochi SL, Hightower AW, Broome CV. Childhood upper respiratory tract infections: to what degree is incidence affected by day-care attendance? Pediatrics. 1987;79:55-60.
21 Collet JP, Burtin P, Gillet J, Bossard N, Ducruet T, Durr F. Risk of infectious diseases in children attending different types of day-care setting. Epicreche Research Group. Respiration. 1994;61(suppl 1):16-19.
22 Stenstrom C, Ingvarsson L. General illness and need of medical care in otitis prone children. Int J Pediatr Otorhinolaryngol. 1994;29:23-32.
23 Kvaerner KJ, Nafstad P, Hagen J, Mair IW, Jaakkola JJ. Early acute otitis media: determined by exposure to respiratory pathogens. Acta Otolaryngol Suppl. 1997;529:14-18.
24 Postma DS, Poole MD, Wu SM, Tober R. The impact of day-care on ventilation tube insertion. Int J Pediatr Otorhinolaryngol. 1997;41:253-262.
25 Brook I, Gober AE. In vitro bacteria interference in the nasopharynx of otitis-media prone and non-otitis media-prone children. Arch Otolaryngol Head Neck Surg. 2000;126:1011-1013.
26 Ito M, Ito K, Yoshizaki T, Nishimura T, Miwa T, Furukawa M. Nasopharyngeal penicillin-resistant Streptococcus pneumoniae strains among young children in Japan. Otol Neurotol. 2002;23:349-352.
27 Leiberman A, Dagan R, Leibovitz E, Yagupsky P, Fliss DM. The bacteriology of the nasopharynx in childhood. Int J Pediatr Otorhinolaryngol. 1999;49(suppl 1):S151-S153.
28 Farjo RS, Foxman B, Patel MJ, et al. Diversity and sharing of Haemophilus influenzae strains colonizing healthy children attending day-care centers. Pediatr Infect Dis J. 2004;23:41-46.
29 Silverstein H, Light JP, Jackson LE, Rosenberg SI, Thompson JHJr. Direct application of dexamethasone for the treatment of chronic eustachian tube dysfunction. ENT J. 2003;82:28-32.
30 Lazo-Saenz JG, Galvan-Aguilara AA, Martinez-Ordaz VA, et al. Eustachian tube dysfunction in allergic rhinitis. Otolaryngol Head Neck Surg. 2005;132:626-629.
31 Poe DS, Abou-Halawa A, Abdel-Razek O. Analysis of the dysfunctional Eustachian tube by video endoscopy. Otol Neurotol. 2001;22:590-595.
32 Grimmer JF, Poe DS. Update on Eustachian tube dysfunction and the patulous Eustachian tube. Curr Opin Otolaryngol Head Neck Surg. 2005;13:277-282.
33 Tos M. Anatomy and histology of the middle ear. Clin Rev Allergy. 1984;2:267-284.
34 Bluestone CD, Stephenson JS, Martin LM. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992;11:s7-s11.
35 Centers for Disease Control and Prevention (CDC). Drug-resistant Streptococcus pneumoniae. Kentucky and Tennessee, 1993. MMWR. 1994;43:23-31.
36 Antonelli PJ, Dhanani N, Giannoni C. Impact of resistant pneumococcus on rates of acute mastoiditis. Otolaryngol Head Neck Surg. 1999;121:190-194.
37 Stenfors LE, Raisanen S. Is attachment of bacteria to the epithelial cells of the nasopharynx the key to otitis media? Int J Pediatr Otorhinolaryngol. 1991;22:1-8.
38 Ruuskanen O, Arola M, Putto-Laurila A, et al. Acute otitis media and respiratory virus infection. In: Lim DJ, editor. Recent advances in otitis media: proceedings of the Fourth International Symposium, June 1–4, 1987, Bal Harbour, Florida. Toronto; Philadelphia: Decker; 1987:307-309.
39 Park JY, Bakaletz LO, Coticchia J, Lim DJ. Effect of influenza A virus infection on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann Otol Rhinol Laryngol. 1993;102:551-558.
40 Bernstein JM. New perspectives on immunologic reactivity in otitis media with effusion. Ann Otol Rhinol Laryngol. 1988;97:19-23.
41 Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in Greater Boston: a prospective cohort study. J Infect Dis. 1989;160:83-94.
42 Hardy AM, Fowler MG. Child care arrangements and repeated ear infections in young children. Am J Public Health. 1993;83:1321-1325.
43 Saarinen UM. Recurrent otitis media in breast-fed infants. Acta Paediatr Scand. 1982;71:567.
44 Jones WS, Kaleida PH. How helpful is pneumatic otoscopy in improving diagnostic accuracy? Pediatrics. 2003;112:510-513.
45 Johansen EC, Lildholdt T, Damsbo N, Eriksen EW. Tympanometry for diagnosis and treatment of otitis media in general practice. Fam Pract. 2000;17:317-322.
46 Kaleida PH, Fireman P. Diagnostic assessment of otitis media. Clin Allergy Immunol. 2000;15:247-262.
47 Green M. The ears. In: Green M, editor. Pediatric diagnosis: interpretation of symptoms and signs in children and adolescents. 6th edn. Philadelphia: Saunders; 1998:37-43.
48 Rosenfeld RM, Vertrees JE, Carr J, et al. Clinical efficacy of antimicrobial drugs for acute otitis media: meta-analysis of 5400 children from thirty-three randomized trials. J Pediatr. 1994;124:355-367.
49 Maryc M, Takata G, Shekelle P, et al. Management of acute otitis media. Evidence Report/Technology Assessment No. 15. AHRQ publication No. 01-E010. Rockville, MD: Agency for Healthcare Research and Quality, May 2001.
50 Schappert SM. Office visits for otitis media: United States, 1975–90. Advance Data From Vital and Health Statistics, No. 214: 1–18. Hyattsville, MD: National Center for Health Statistics, 1992.
51 Roche Laboratories, Inc. Acute otitis media. Total Res Utilization Conspectus. 1999;1:1-56.
52 Levy SB. The challenge of antibiotic resistance. Sci Am. 1998;278:46-53.
53 Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
54 Jacobs MR, Dagan R, Appelbaum PC, Burch DJ. Prevalence of antimicrobial-resistant pathogens in middle ear fluid: multinational study of 917 children with acute otitis media. Antimicrob Agents Chemother. 1998;42:589-595.
55 McCracken GH. Treatment of acute otitis media in an era of increasing microbial resistance. Pediatr Infect Dis J. 1998;17:576-579.
56 Thorburn CE, Knott SJ, Edwards DI. In vitro activities of oral beta-lactams at concentrations achieved in humans against penicillin-susceptible and -resistant pneumococci and potential to select resistance. Antimicrob Agents Chemother. 1998;42:1973-1979.
57 Doern GV, Pfaller MA, Kugler K, Freeman J, Jones RN. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY antimicrobial surveillance program. Clin Infect Dis. 1998;27:764-770.
58 Zernotti ME, Casarotto C, Tosello ML, Zernotti M. Incidence of complications of otitis media. Acta Otorhinolaryngol Esp. 2005;56:59-62.
59 Papp Z, Rezes S, Jókay I, Sziklai I. Sensorineural hearing loss in chronic otitis media. Otol Neurotol. 2003;24:141-144.
60 Ehrlich GD, Veeh R, Wang X, et al. Mucosal biofilm formation on the middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287:1710-1715.
61 Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA. 1998;279:296-299.
62 Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope. 2001;111:2083-2094.
63 Lanphear BP, Byrd RS, Auinger P, Hall CB. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics. 99, 1997. Electronic article. http://pediatrics.aappublications.org/cgi/content/full/99/3/e/