211 Management of the Brain Dead Organ Donor
Transplantation is an increasingly utilized treatment option for patients with organ failure. In 2009, 28,465 organs were transplanted in the United States, with over 100,000 patients on the waiting list.1 Despite advances in immunosuppression and postoperative management, utility of transplantation is dependent on the number of available organs. Expanding indications for transplantation have further widened the gap between supply and demand.
To address this problem, the U.S. Department of Health and Human Services launched the Organ Donation Breakthrough Collaboratives in 2003 with the intent to increase the number of donors, as well as number of organs transplanted per donor. Events in the pathway of organ donation are illustrated in Figure 211-1.2 Maintenance of allocated organs was identified as a major area for improvement. The majority of donor organs are cadaveric, of which 90% are from brain dead (BD) donors. An estimated 20% to 30% of organs are lost prior to procurement despite aggressive measures in BD donors.1 This number attests to the profound physiologic variations that occur at the time of brain death but can also be attributed to what can often be suboptimal unstandardized care.3 There is a large disparity between the intensive team-based management of the trauma or stroke victim and the singularity of the organ procurement organization (OPO) coordinator left at the bedside once brain death is declared. The intensivist can therefore have a profound impact on number and quality of organs salvaged.
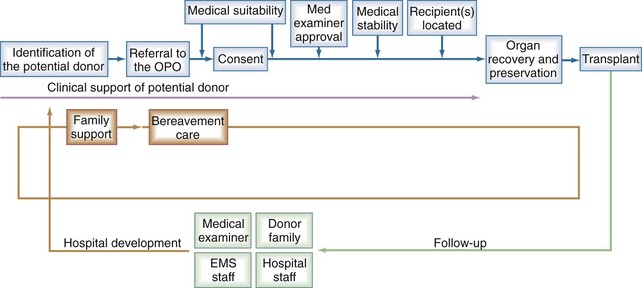
Figure 211-1 Events in the organ donation and consent process.
(From Organ Donation Breakthrough Collaborative best practices final report, September 2003. The Organ Donation Breakthrough Collaborative. Best practices final report; U.S. Department of Health and Human Services Health Resources and Services Administration; Office of Special Programs, Division of Transplantation Contract: 240-94-0037 Task Order No. 12, September 2003.)
 Declaration of Brain Death
Declaration of Brain Death
The initial process for organ donation requires heightened awareness on the part of the intensive care unit (ICU) team. Often, potential donors are excluded by the caregiver based on notions of donor criteria or concerns regarding conflict of care. Members of the local OPO are trained specifically to interact with families regarding donation issues in such a manner that the caregiver and OPO are not seen in mutual opposition. With the permission of the family, blood sampling to determine the suitability may be performed before brain death occurs.4 Once brain death is confirmed by standard criteria (see Chapter 219), the team needs to act quickly to stabilize the physiology of the donor and shorten time to transplantation.
 Physiology of Brain Death
Physiology of Brain Death
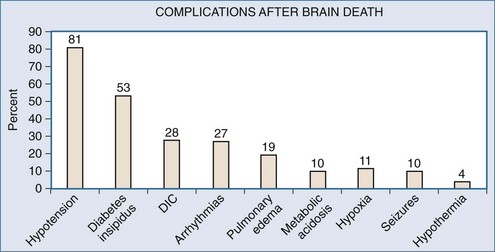
Brain injury resulting in herniation will follow a rostrocaudal progression of ischemia. Events leading up to brain death include hypertension with bradycardia (Cushing response) as the pons becomes ischemic. Further involvement of the medulla creates unopposed sympathetic stimuli, initiating a catecholamine “storm.” This surge of catecholamines damages end organs both from severe vasoconstriction and from the proinflammatory response elicited. Finally, spinal cord ischemia and loss of sympathetic denervation results in severe hypotension. Simultaneous ischemia to the pituitary and hypothalamus exacerbate this with loss of homeostatic control. These events occur in varying magnitude or velocity, making management even more difficult. The resulting physiology is characterized by hemodynamic instability with a host of secondary complications listed in Figure 211-2.
 Initial Donor Resuscitation
Initial Donor Resuscitation
Care of the BD donor requires multitasking and frequent reassessment. Donors often have associated traumatic injury and chronic health problems. To complicate matters, treatment strategies prior to brain death are directed toward maintaining cerebral perfusion, often to the detriment of other organs. Post-declaration management focuses on reversing this state and preventing further organ damage. Expeditious stabilization is paramount, as graft loss rapidly increases after 48 hours.5
Various organizations provide algorithms for standard management of the BD donor. Protocols may be organ specific, or target the donor as a whole.6–10 The United Network for Organ Sharing (UNOS) provides a sample standard pathway that includes initial workup as well as therapy (Figure 211-3). These algorithms help focus ongoing resuscitation, ensure provision of evidence-based therapy, and provide a platform for future research in the field.
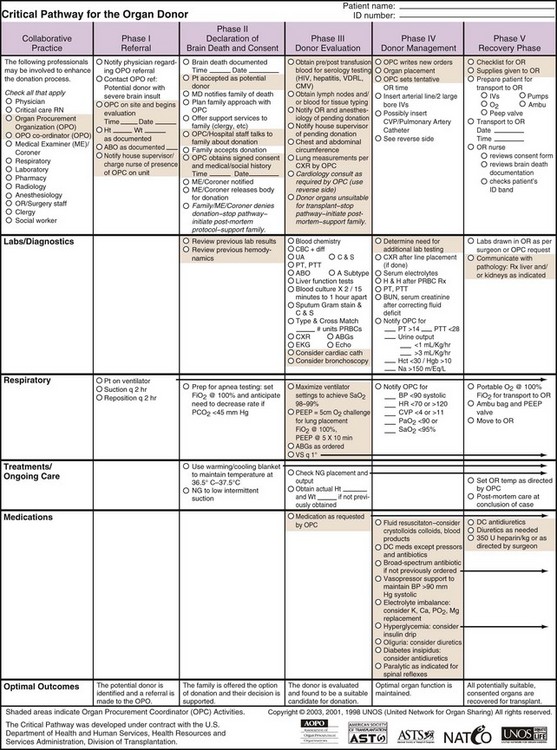
Figure 211-3 Critical pathway for organ donor.
(Reprinted with permission of UNOS, Richmond, Virginia. Access at http://www.unos.org/docs/cntical_Pathway.pdf.)
Immediate goals are establishing baseline organ function and stabilizing physiology. If not already in place, a central venous catheter and arterial catheter are inserted. Blood, urine, and bronchial cultures are obtained and baseline chemistries performed. Evaluation of the lung and heart begin with basic chest x-ray, echocardiogram, bronchoscopy, and coronary angiogram as indicated.7 Blood type and crossmatch are performed, and initial graft allocation efforts begun by the OPO coordinator.
Initial resuscitation includes crystalloid administration guided by central venous pressure or pulmonary artery pressure, although pulse pressure variation (PPV) may actually predict preload responsiveness more accurately.11 After adequate volume loading, vasopressors are often required to maintain perfusion pressure. Monitoring end organ perfusion may be achieved by measuring oxygen delivery or central venous oxygen saturation.12 Other endpoints of resuscitation are listed in Box 211-1.
Standard ICU protocols should be employed to prevent further complications. Gastrointestinal and deep vein thrombosis (DVT) prophylaxis should be continued appropriately, blood products administered for anemia or coagulopathy, aspiration precautions upheld, and electrolytes and acidosis corrected to avoid arrhythmias. Insulin therapy should be given, as it has antiinflammatory properties that may be particularly beneficial in the BD donor.13,14 A multidisciplinary approach greatly helps coordinate care.
 Specific Considerations and Controversies
Specific Considerations and Controversies
Cardiovascular
Cardiovascular management after brain death is paramount to maintaining perfusion and preserving the heart for donation. Hemodynamic collapse occurs more from loss of afterload than primary nonfunction of the heart.15 Nevertheless, the catecholamine surge during herniation can incite considerable myocardial damage.16 Right ventricle strain is common secondary to increased pulmonary capillary perfusion and pulmonary overflow injury from increased vascular resistance.17 Contractility must be frequently reassessed and quantified with echocardiography, as regional wall abnormalities will often resolve.18 Most important to cardiac function is coronary perfusion pressure, which can be affected by loss of autoregulatory reserve after brain death.19–21
Protocols based on traditional volume and vasopressor management have increased the number of donor hearts.8,22 These protocols included moderate crystalloid resuscitation followed by catecholamine use for hypotension and hormone treatment when cardiac dysfunction was diagnosed (Figure 211-4).23 Since their development, certain details have been debated, primarily hormone treatment (discussed later) and choice of vasopressor.
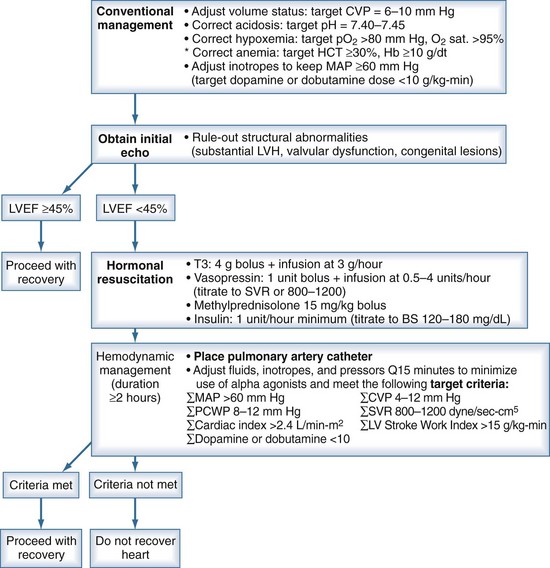
Figure 211-4 Recommendations for cardiac donor management.
(Adapted from Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant 2002;2:701-11.)
Types of vasopressors advocated include dopamine, epinephrine, and norepinephrine as well as vasopressin.7,24–27 Immunomodulatory function of the catecholamines makes them attractive in the context of the donor’s proinflammatory condition.28–30 Dopamine-stimulated induction of heme-oxygenase-1 makes kidneys more resistant to ischemic-reperfusion injury in donor models.31,32 Dopamine may suppress anterior pituitary hormones, however, and is likewise notorious for inducing tachyarrhythmias.33,34 Norepinephrine and epinephrine have been related to cardiac and kidney graft nonfunction.35–37 Vasopressin makes sense in the face of posterior pituitary ischemia and can reduce the dose of catecholamines administered.38,39
Pulmonary
Like the heart, lung function in the donor can be affected by physiologic changes with brain death, in addition to underlying pulmonary disease. Pulmonary edema after brain death results from elevated afterload from the catecholamine surge combined with increased venous return and decreased left ventricular function.40 The sympathetic discharge also up-regulates inflammation in the lung parenchyma and capillaries, leading to further edema and failure.41 These effects are significant because pulmonary edema and inflammation reduce lung donation rates to less than 20%.42
Standard criteria for lung donation include a clear chest x-ray and PaO2/FIO2 above 300, although with expanded donor criteria, these parameters are viewed as too strict.43,44 Findings such as edema and atelectasis can be reversed with adequate diuresis and recruitment maneuvers.45,46 Global oxygenation does not represent unilateral oxygenation, and the single-lung donor pool can therefore be expanded by obtaining unilateral pulmonary vein gases instead of relying on PaO2.47,48
Once a suitable donor is identified, aggressive management with a lung-specific focus is pursued.6 Most protocols use frequent chest physiotherapy and bronchoscopy, diuretics, strict aspiration precautions, empirical antibiotics, and steroid administration.49–51 Lung recruitment maneuvers and frequent bronchoscopy increase oxygenation and lung utilization.52 Diuretics are given to decrease central venous pressure (CVP) in an effort to decrease alveolar-arterial oxygen gradient, although restricting CVP does not necessarily increase lung utilization.53–55
Empirical antibiotics are generally administered based on chest x-ray findings. Although culture obtained from bronchoalveolar lavage (BAL) is the gold standard, results often are not rapid enough for specific antibiotic tapering.56 Even when bronchial culture is obtained, there is poor correlation between culture data and posttransplant pneumonia development, with an 8% transmission rate despite appropriate antibiotics.57 Steroids are also widely employed in lung donors in an effort to decrease lung water accumulation and enhance alveolar fluid clearance.58,59
Whereas there are few studies about ventilator mode, pressure-cycled modes are being used more frequently for donors.60 Lung protective strategies using low tidal volumes and moderate positive end-expiratory pressure (PEEP) can prevent further barotrauma.61,62 For the donor population specifically, acute lung injury is more common in those treated with increased tidal volumes, and sustained recruitment maneuvers should be used with caution.63 Excessive oxygen administration should likewise be avoided, as this can induce the inflammatory cascade and apoptosis.64
Renal
BD donors are typically volume depleted secondary to aggressive mannitol use and diabetes insipidus. Strategies for preventing further renal injury include avoidance of nephrotoxic agents and maintaining hydration. Larger amounts of volume administration can improve kidney and liver graft function by correction of hypernatremia.36,65,66 By contrast, hypervolemia is also deleterious, inducing right heart strain and lung dysfunction.45 Restricting CVP to improve lung function, however, did not adversely affect kidney graft function in a recent well-designed study.55
Crystalloids are primarily used for initial resuscitation. Some societies advocate colloids to avoid lung water accumulation, but data to support this are limited.53 Hydroxyethylstarch (HES) can generate nephrosis-like lesions and impair graft function in kidneys, although newer, less osmotic formulations do not demonstrate the same detrimental effects.67–69 Hypertonic saline may modulate inflammation and shows promise for donor resuscitation, although sodium levels should be monitored closely.70,71
Endocrine
One of the more debated aspects of donor management is the use of hormonal therapy. The donor suffers from a variable panhypopituitary state secondary to ischemia.72 Dysfunction of the posterior pituitary is common (90%), with resultant low to nil vasopressin levels.73 Administering desmopressin treats the subsequent diabetes insipidus that can further complicate fluid management. Dysfunction of the anterior pituitary is less consistent, with variable effects of hormones given to counteract the loss of corticotropin (ACTH) and thyroid-stimulating hormone (TSH).74 In animal models, levels of triiodothyronine (T3), cortisol, and insulin are all markedly decreased.75 Humans, however, exhibit near-normal levels of cortisol and insulin, with nonuniform decreases in T3.73 Hypophyseal blood flow may be maintained by branches off of the external carotid and could explain some of these variable hormone alterations.
Initial enthusiasm for hormone replacement therapy (HRT) was based on non-randomized data showing increased organ yield when a cocktail of T3, steroids, insulin, and vasopressin was administered.9,22,76,77 Hormone cocktails therefore became part of the UNOS protocol for cardiac donor management. Using this protocol, animal models demonstrated beneficial reduction in vasopressors when given HRT.78 With more rigorous examination, however, combination hormone therapy has not been supported. A recent randomized control trial using HRT in a protocol to increase lung donors was unable to demonstrate increased organ yield in those receiving the cocktail.79 Criticism of HRT focuses primarily on the thyroid and steroid components of therapy, and thus will be examined more closely.
Thyroid
Thyroid hormone replacement for donors originated with baboon studies in the late 1980s. Novitsky et al. observed reversal of cardiac dysfunction after administration of T3.72,80 Some societies therefore advocated T3 if donor heart dysfunction was encountered.7,9,76,78 However, further animal studies could not demonstrate benefit to T3.81,82 Numerous human studies were likewise unable to show correlation between T3, cardiac function, inotropic support, or improved organ yield.83–91
Thyroid hormone replacement with thyroxine has also yielded conflicting results.92,93 Revised UNOS recommendations concede that DDAVP (1-deamino-8-D-arginine-vasopressin), diuretics, and steroids rather than thyroid hormone administration increase organ yield.94 Reasons for the conflicting data may be the pattern of thyroid dysfunction in brain death. Characterized by normal thyroxine levels, elevated reverse T3, and low TSH, this matches the “sick euthyroid” state which has likewise failed to demonstrate benefit from hormone replacement.95–97 In cases of prolonged donor management or excess catecholamine administration, thyroid hormones may have some role; however, they also may be harmful and therefore cannot be advocated in all donors.88,98
Inflammation
Prominence of inflammatory mediators in BD donors plays a significant role in management. The ischemic brain elaborates core inflammatory mediators that then cross the blood-brain barrier.99 Elevated levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 are found in both serum and tissue.100,101 This can be cerebral in origin or secondary to local tissue ischemia-reperfusion (IR) injury as a result of the initial catecholamine surge.102,103 Free radicals elaborated by IR injury increase local expression of adhesion molecules and signal an influx of leukocytes, referred to as passenger leukocytes, in the transplanted organ.104 These primed leukocytes can then go on to influence graft rejection in the posttransplant period.
Inflammatory markers are more prominently expressed in all solid-organ grafts after BD versus living-related transplant.105 Several studies have demonstrated increased rejection of kidneys from BD donors compared to living-related, unrelated, or donation after cardiac death (DCD) donors.106–108 Increased levels of IL-6, TNF-α, and procalcitonin have also been associated with poor cardiac graft function.109,110 A recent case-control study confirmed marked elevation of plasma endotoxin and cytokines in BD donors, although this did not correlate with lower graft survival.111 Of interest, tissue from BD donors exhibited higher levels of proapoptotic gene mRNA, which may further explain graft loss beyond just having higher cytokine levels.
Steroids are used in the donor in an attempt to attenuate the inflammatory response, with the goal of reducing rejection and increasing organ yield.112,113 Methylprednisolone is the steroid of choice and is given either as a single bolus or as a drip. Some studies demonstrate reduction of cytokines and subsequent rejection with early steroid administration.114,115 A follow-up randomized control trial confirmed that while BD hearts have increased inflammatory markers and poorer graft function, these results could not be prevented with steroid administration.116 Barring an alternative antiinflammatory agent and given their low risk profile, however, steroids are currently recommended in donor resuscitation.
 Other Treatment
Other Treatment
A large number of emerging treatment modalities focus on reversing IR injury and modulating inflammation. Erythropoietin and carbamylated erythropoietin are gaining use as renal protectants.117 Benefit in the donor comes not from hematopoietic effects, but via immunomodulation.118 Naloxone is also potentially protective. Early animal studies have shown better renal function and survival in treatment groups with its use,119 as well as improvement in oxygenation and lung function.120
Smith M. Physiologic changes during brain stem death—lessons for management of the organ donor. J Heart Lung Transplant. 2004;23:S217-S222.
Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D’Alessandro A, Dec GW, et al. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations. March 28-29, 2001, Crystal City, Va. Circulation. 2002;106:836-841.
Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, et al. Aggressive pharmacologic donor management results in more transplanted organs. Transplantation. 2003;75:482-487.
Shemie SD, Ross H, Pagliarello J, Baker AJ, Greig PD, Brand T, et al. Organ donor management in Canada: recommendations of the forum on Medical Management to Optimize Donor Organ Potential. CMAJ. 2006;174:S13-S30.
Selck FW, Deb P, Grossman EB. Deceased organ donor characteristics and clinical interventions associated with organ yield. Am J Transplant. 2008;8:965-974.
Chamorro C, Falcón JA, Michelena JC. Controversial points in organ donor management. Transplant Proc. 2009;41:3473-3475.
, United Network for Organ Sharing website http://www.unos.org/
1 United Network for Organ Sharing. Organ Donation and Transplantation [Internet]. [cited 2010 Apr 14];Available from. http://www.unos.org/resources/donorManagement.asp?index=2.
2 HRSA Knowledge Gateway [Internet]. [cited 2010 Apr 14];Available from http://www.healthdisparities.net/hdc/html/collaboratives.topics.TGMC.aspx
3 Smith M. Physiologic changes during brain stem death—lessons for management of the organ donor. J Heart Lung Transplant. 2004 Sep;23(Suppl. 9):S217-S222.
4 OPTN. Organ Procurement and Transplantation Network [Internet]. [cited 2010 Apr 14]; Available from. http://optn.transplant.hrsa.gov/data/.
5 Cantin B, Kwok BWK, Chan MCY, Valantine HA, Oyer PE, Robbins RC, et al. The impact of brain death on survival after heart transplantation: time is of the essence. Transplantation. 2003 Nov 15;76(9):1275-1279.
6 Angel LF, Levine DJ, Restrepo MI, Johnson S, Sako E, Carpenter A, et al. Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 2006 Sep 15;174(6):710-716.
7 Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D’Alessandro A, Dec GW, et al. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28-29, 2001, Crystal City, Va. Circulation. 2002 Aug 13;106(7):836-841.
8 Wood KE, Becker BN, McCartney JG, D’Alessandro AM, Coursin DB. Care of the potential organ donor. N Engl J Med. 2004 Dec 23;351(26):2730-2739.
9 Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, et al. Aggressive pharmacologic donor management results in more transplanted organs. Transplantation. 2003 Feb 27;75(4):482-487.
10 Shemie SD, Ross H, Pagliarello J, Baker AJ, Greig PD, Brand T, et al. Organ donor management in Canada: recommendations of the forum on Medical Management to Optimize Donor Organ Potential. CMAJ. 2006 Mar 14;174(6):S13-S30.
11 Michard F, Teboul JL. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care. 2000;4(5):282-289.
12 Rivers E. Mixed vs central venous oxygen saturation may be not numerically equal, but both are still clinically useful. Chest. 2006 Mar;129(3):507-508.
13 Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003 Mar;88(3):1082-1088.
14 Barklin A, Larsson A, Vestergaard C, Kjaergaard A, Wogensen L, Schmitz O, et al. Insulin alters cytokine content in two pivotal organs after brain death: a porcine model. Acta Anaesthesiol Scand. 2008 May;52(5):628-634.
15 Szabó G, Hackert T, Sebening C, Vahl CF, Hagl S. Modulation of coronary perfusion pressure can reverse cardiac dysfunction after brain death. Ann Thorac Surg. 1999 Jan;67(1):18-25.
16 Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985 Apr;17(4):291-306.
17 Bittner HB, Chen EP, Biswas SS, Van Trigt P, Davis RD. Right ventricular dysfunction after cardiac transplantation: primarily related to status of donor heart. Ann Thorac Surg. 1999 Nov;68(5):1605-1611.
18 Banki NM, Zaroff JG. Neurogenic Cardiac Injury. Curr Treat Options Cardiovasc Med. 2003 Dec;5(6):451-458.
19 Szabó G, Hackert T, Buhmann V, Graf A, Sebening C, Vahl CF, et al. Downregulation of myocardial contractility via intact ventriculo-arterial coupling in the brain dead organ donor. Eur J Cardiothorac Surg. 2001 Jul;20(1):170-176.
20 Szabo G, Buhmann V, Bahrle S, Vahl CF, Hagl S. Brain death impairs coronary endothelial function. Transplantation. 2002 Jun 15;73(11):1846-1848.
21 Chamorro C, Silva JA, Segovia J, Romera MA. Use of catecholamines in cardiac donors: what is the real limit? J Heart Lung Transplant. 2004 Jul;23(7):916-917.
22 Wheeldon DR, Potter CD, Oduro A, Wallwork J, Large SR. Transforming the “unacceptable” donor: outcomes from the adoption of a standardized donor management technique. J Heart Lung Transplant. 1995 Aug;14(4):734-742.
23 Frontera J, Kalb T. How I Manage the Adult Potential Organ Donor: Donation After Neurological Death (Part 1). Neurocrit Care. 2010 Feb 1;12(1):103-110.
24 Care of the Potential Organ Donor [Internet]. 2004 Dec 23 [cited 2010 Apr 12];Available from http://content.nejm.org/cgi/content/extract/351/26/2730
25 Chamorro C, Silva JA, Romera MA. Cardiac donor management: another point of view. Transplant Proc. 2003 Aug;35(5):1935-1937.
26 Chamorro C, Silva JA, Segovia J, Romera MA. Use of catecholamines in cardiac donors: what is the real limit? J Heart Lung Transplant. 2004 Jul;23(7):916-917.
27 Cardiovascular management of a potential heart donor: A statement from the Transplantation Committee of the American College of Cardiology. Crit Care Med. 1996;24(9):1599-1601.
28 Cuende N, Miranda B, Cañón JF, Garrido G, Matesanz R. Donor characteristics associated with liver graft survival. Transplantation. 2005 May 27;79(10):1445-1452.
29 Schaub M, Ploetz CJ, Gerbaulet D, Fang L, Kranich P, Stadlbauer THW, et al. Effect of dopamine on inflammatory status in kidneys of brain-dead rats. Transplantation. 2004 May 15;77(9):1333-1340.
30 Schnuelle P, Yard BA, Braun C, Dominguez-Fernandez E, Schaub M, Birck R, et al. Impact of donor dopamine on immediate graft function after kidney transplantation. Am J Transplant. 2004;4(3):419-426.
31 Hoeger S, Gottmann U, Liu Z, Schnuelle P, Birck R, Braun C, et al. Dopamine treatment in brain-dead rats mediates anti-inflammatory effects: the role of hemodynamic stabilization and D-receptor stimulation. Transplant Int. 2007;20(9):790-799.
32 van der Woude FJ, Schnuelle P, Yard BA. Preconditioning strategies to limit graft immunogenicity and cold ischemic organ injury. J Investig Med. 2004 Jul;52(5):323-329.
33 De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010 Mar 4;362(9):779-789.
34 Debaveye YA, Van den Berghe GH. Is there still a place for dopamine in the modern intensive care unit? Anesth Analg. 2004 Feb;98(2):461-468.
35 Schnuelle P, Berger S, de Boer J, Persijn G, van der Woude FJ. Effects of catecholamine application to brain-dead donors on graft survival in solid organ transplantation. Transplantation. 2001 Aug 15;72(3):455-463.
36 Giral M, Bertola JP, Foucher Y, Villers D, Bironneau E, Blanloeil Y, et al. Effect of brain-dead donor resuscitation on delayed graft function: results of a monocentric analysis. Transplantation. 2007 5;83(9):1174-1181.
37 Blasco V, Leone M, Bouvenot J, Geissler A, Albanèse J, Martin C. Impact of intensive care on renal function before graft harvest: results of a monocentric study. Crit Care. 11(5), 2007. R103-R103
38 Pennefather SH, Bullock RE, Mantle D, Dark JH. Use of low dose arginine vasopressin to support brain-dead organ donors. Transplantation. 1995 Jan 15;59(1):58-62.
39 Rostron AJ, Avlonitis VS, Cork DMW, Grenade DS, Kirby JA, Dark JH. Hemodynamic resuscitation with arginine vasopressin reduces lung injury after brain death in the transplant donor. Transplantation. 2008 Feb 27;85(4):597-606.
40 Bittner HB, Kendall SW, Chen EP, Craig D, Van Trigt P. The effects of brain death on cardiopulmonary hemodynamics and pulmonary blood flow characteristics. Chest. 1995 Nov;108(5):1358-1363.
41 Kaneda H, Waddell TK, Perrot MD, Bai X, Gutierrez C, Arenovich T, et al. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6(3):544-551.
42 Avlonitis VS, Fisher AJ, Kirby JA, Dark JH. Pulmonary transplantation: the role of brain death in donor lung injury. Transplantation. 2003 Jun 27;75(12):1928-1933.
43 Fisher AJ, Donnelly SC, Pritchard G, Dark JH, Corris PA. Objective assessment of criteria for selection of donor lungs suitable for transplantation. Thorax. 2004 May;59(5):434-437.
44 Lardinois D, Banysch M, Korom S, Hillinger S, Rousson V, Boehler A, et al. Extended donor lungs: eleven years experience in a consecutive series. Eur J Cardiothorac Surg. 2005 May;27(5):762-767.
45 Straznicka M, Follette DM, Eisner MD, Roberts PF, Menza RL, Babcock WD. Aggressive management of lung donors classified as unacceptable: Excellent recipient survival one year after transplantation. J Thorac Cardiovasc Surg. 2002 Aug;124(2):250-258.
46 Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly J, et al. Influence of donor characteristics on outcome after lung transplantation: a multicenter study. J Heart Lung Transplant. 2005 Sep;24(9):1347-1353.
47 McGiffin DC, Zorn J, Young J, Kirklin JK, Leon KJ, Wille KM, et al. The intensive care unit oxygen challenge should not be used for donor lung function decision-making. J Heart Lung Transplant. 2005 Nov;24(11):1902-1905.
48 Aziz TM, El-Gamel A, Saad RAG, Migliore M, Campbell CS, Yonan NA. Pulmonary vein gas analysis for assessing donor lung function. Ann Thorac Surg. 2002 May;73(5):1599-1604. discussion 1604-5
49 Follette DM, Rudich SM, Babcock WD. Improved oxygenation and increased lung donor recovery with high-dose steroid administration after brain death. J Heart Lung Transplant. 1998 Apr;17(4):423-429.
50 McElhinney DB, Khan JH, Babcock WD, Hall TS. Thoracic organ donor characteristics associated with successful lung procurement. Clin Transplant. 2001;15(1):68-71.
51 Gabbay E, Williams TJ, Griffiths AP, Macfarlane LM, Kotsimbos TC, Esmore DS, et al. Maximizing the utilization of donor organs offered for lung transplantation. Am J Respir Crit Care Med. 1999 Jul;160(1):265-271.
52 Noiseux N, Nguyen B, Marsolais P, Dupont J, Simard L, Houde I, et al. Pulmonary recruitment protocol for organ donors: a new strategy to improve the rate of lung utilization. Transplant Proc. 2009 Oct;41(8):3284-3289.
53 Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2(8):701-711.
54 Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D’Alessandro A, Dec GW, et al. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations March 28-29, 2001, Crystal City, Va. Circulation. 2002;106(7):836-841.
55 Minambres E, Rodrigo E, Ballesteros MA, Llorca J, Ruiz JC, Fernandez-Fresnedo G, et al. Impact of restrictive fluid balance focused to increase lung procurement on renal function after kidney transplantation. Nephrol Dial Transplant. 2010 Feb 14. gfq054
56 Avlonitis VS, Krause A, Luzzi L, Powell H, Phillips JA, Corris PA, et al. Bacterial colonization of the donor lower airways is a predictor of poor outcome in lung transplantation. Eur J Cardiothorac Surg. 2003 Oct;24(4):601-607.
57 Bonde PN, Patel ND, Borja MC, Allan SH, Barreiro CJ, Williams JA, et al. Impact of donor lung organisms on post-lung transplant pneumonia. J Heart Lung Transplant. 2006 Jan;25(1):99-105.
58 Venkateswaran RV, Patchell VB, Wilson IC, Mascaro JG, Thompson RD, Quinn DW, et al. Early donor management increases the retrieval rate of lungs for transplantation. Ann Thorac Surg. 2008 Jan;85(1):278-286. discussion 286
59 Folkesson HG, Norlin A, Wang Y, Abedinpour P, Matthay MA. Dexamethasone and thyroid hormone pretreatment upregulate alveolar epithelial fluid clearance in adult rats. J Appl Physiol. 2000 Feb;88(2):416-424.
60 Jr GBM, Schecter MG, Elidemir O. Management of the pediatric organ donor to optimize lung donation. Pediatr Pulmonol. 2009;44(6):536-546.
61 The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-1308.
62 Botha P, Rostron AJ, Fisher AJ, Dark JH. Current strategies in donor selection and management. Semin Thorac Cardiovasc Surg. 2008;20(2):143-151.
63 Mascia L, Zavala E, Bosma K, Pasero D, Decaroli D, Andrews P, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35(8):1815-1820.
64 Bhandari V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008;13:6653-6661.
65 Totsuka E, Fung JJ, Ishii T, Urakami A, Moras NP, Hakamada K, et al. Influence of donor condition on postoperative graft survival and function in human liver transplantation. Transplant Proc. 2000 Mar;32(2):322-326.
66 Markmann JF, Markmann JW, Markmann DA, Bacquerizo A, Singer J, Holt CD, et al. Preoperative factors associated with outcome and their impact on resource use in 1148 consecutive primary liver transplants. Transplantation. 2001 Sep 27;72(6):1113-1122.
67 Legendre C, Thervet E, Page B, Percheron A, Noël LH, Kreis H. Hydroxyethylstarch and osmotic-nephrosis-like lesions in kidney transplantation. Lancet. 1993;342(8865):248-249.
68 Cittanova M, Leblanc I, Legendre C, Mouquet C, Riou B, Coriat P. Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet. 1996;348(9042):1620-1622.
69 Blasco V, Leone M, Antonini F, Geissler A, Albanese J, Martin C. Comparison of the novel hydroxyethylstarch 130/0.4 and hydroxyethylstarch 200/0.6 in brain-dead donor resuscitation on renal function after transplantation. Br J Anaesth. 2008 Apr 1;100(4):504-508.
70 Badiwala MV, Ramzy D, Tumiati LC, Tepperman ED, Sheshgiri R, Prodger JL, et al. Donor pretreatment with hypertonic saline attenuates primary allograft dysfunction: a pilot study in a porcine model. Circulation. 2009 Sep 15;120(Suppl. 11):S206-S214.
71 Tyagi R, Donaldson K, Loftus CM, Jallo J. Hypertonic saline: a clinical review. Neurosurg Rev. 2007 Oct;30(4):277-289. discussion 289-290
72 Novitzky D, Cooper DKC, Rosendale JD, Kauffman HM. Hormonal therapy of the brain-dead organ donor: experimental and clinical studies. Transplantation. 2006 Dec 15;82(11):1396-1401.
73 Gramm HJ, Meinhold H, Bickel U, Zimmermann J, von Hammerstein B, Keller F, et al. Acute endocrine failure after brain death? Transplantation. 1992 Nov;54(5):851-857.
74 Novitzky D, Cooper DK, Reichart B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation. 1987 Jun;43(6):852-854.
75 Novitzky D, Cooper DK, Morrell D, Isaacs S. Brain death, triiodothyronine depletion, and inhibition of oxidative phosphorylation: relevance to management of organ donors. Transplant Proc. 1987 Oct;;19(5):4110-4111.
76 Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, et al. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 2003 Apr 27;75(8):1336-1341.
77 Abdelnour T, Rieke S. Relationship of hormonal resuscitation therapy and central venous pressure on increasing organs for transplant. J Heart Lung Transplant. 2009 May;28(5):480-485.
78 Hing AJ, Hicks M, Garlick SR, Gao L, Kesteven SH, Faddy SC, et al. the effects of hormone resuscitation on cardiac function and hemodynamics in a porcine brain-dead organ donor model. Am J Transplant. 2007;7(4):809-817.
79 Venkateswaran RV, Patchell VB, Wilson IC, Mascaro JG, Thompson RD, Quinn DW, et al. Early donor management increases the retrieval rate of lungs for transplantation. Ann Thorac Surg. 2008 Jan;85(1):278-286.
80 Novitzky D, Cooper DK, Morrell D, Isaacs S. Change from aerobic to anaerobic metabolism after brain death, and reversal following triiodothyronine therapy. Transplantation. 1988 Jan;45(1):32-36.
81 Meyers CH, D’Amico TA, Peterseim DS, Jayawant AM, Steenbergen C, Sabiston DC, et al. Effects of triiodothyronine and vasopressin on cardiac function and myocardial blood flow after brain death. J Heart Lung Transplant. 1993 Feb;12(1 Pt 1):68-79. discussion 79-80
82 Watson A, Gao L, Sun L, Faddy S, Hicks M, Jansz P, et al. 313: Exogenous T3 causes acute pulmonary hypertension in a porcine model of brain death. J Heart Lung Transplant. 2010 Feb;29(2 Suppl. 1):S105-S106.
83 Randell TT, Höckerstedt KA. Triiodothyronine treatment is not indicated in brain-dead multiorgan donors: a controlled study. Transplant Proc. 1993 Feb;25(1 Pt 2):1552-1553.
84 Goarin JP, Cohen S, Riou B, Jacquens Y, Guesde R, Le Bret F, et al. The effects of triiodothyronine on hemodynamic status and cardiac function in potential heart donors. Anesth Analg. 1996 Jul;83(1):41-47.
85 Macoviak JA, McDougall IR, Bayer MF, Brown M, Tazelaar H, Stinson EB. Significance of thyroid dysfunction in human cardiac allograft procurement. Transplantation. 1987 Jun;43(6):824-826.
86 Randell TT, Höckerstedt KA. Triiodothyronine treatment in brain-dead multiorgan donors—a controlled study. Transplantation. 1992 Oct;54(4):736-738.
87 Jeevanandam V. Triiodothyronine: spectrum of use in heart transplantation. Thyroid. 1997 Feb;7(1):139-145.
88 Powner DJ, Hernandez M. A review of thyroid hormone administration during adult donor care. Prog Transplant. 2005 Sep;15(3):202-207.
89 Pérez-Blanco A, Caturla-Such J, Cánovas-Robles J, Sanchez-Payá J. Efficiency of triiodothyronine treatment on organ donor hemodynamic management and adenine nucleotide concentration. Intensive Care Med. 2005 Jul;31(7):943-948.
90 James SR, Ranasinghe AM, Venkateswaran R, McCabe CJ, Franklyn JA, Bonser RS. The effects of acute triiodothyronine therapy on myocardial gene expression in brainstem dead cardiac donors. J Clin Endocrinol Metab. 2010 Mar 1;95(3):1338-1343.
91 Venkateswaran RV, Steeds RP, Quinn DW, Nightingale P, Wilson IC, Mascaro JG, et al. The haemodynamic effects of adjunctive hormone therapy in potential heart donors: a prospective randomized double-blind factorially designed controlled trial. Eur Heart J. 2009 Jul;30(14):1771-1780.
92 Salim A, Martin M, Brown C, Inaba K, Roth B, Hadjizacharia P, et al. Using thyroid hormone in brain-dead donors to maximize the number of organs available for transplantation. Clin Transplant. 2007 Jun;21(3):405-409.
93 Mariot J, Sadoune LO, Jacob F, Dousset B, Perrier JF, Jacob C, et al. Hormone levels, hemodynamics, and metabolism in brain dead organ donors. Transplant Proc. 1995 Feb;27(1):793-794.
94 Selck FW, Deb P, Grossman EB. Deceased organ donor characteristics and clinical interventions associated with organ yield. Am J Transplant. 2008;8(5):965-974.
95 Masson F, Thicoïpe M, Latapie MJ, Maurette P. Thyroid function in brain-dead donors. Transpl Int. 1990 Dec;3(4):226-233.
96 Novitzky D. Triiodothyronine replacement, the euthyroid sick syndrome, and organ transplantation. Transplant Proc. 1991 Oct;23(5):2460-2462.
97 Bennett-Guerrero E, Jimenez JL, White WD, D’Amico EB, Baldwin BI, Schwinn DA. Cardiovascular effects of intravenous triiodothyronine in patients undergoing coronary artery bypass graft surgery. A randomized, double-blind, placebo-controlled trial. Duke T3 study group. JAMA. 1996 Mar 6;275(9):687-692.
98 Chamorro C, Falcón JA, Michelena JC. Controversial points in organ donor management. Transplant Proc. 2009 Oct;41(8):3473-3475.
99 McKeating EG, Andrews PJ, Signorini DF, Mascia L. Transcranial cytokine gradients in patients requiring intensive care after acute brain injury. Br J Anaesth. 1997 May;78(5):520-523.
100 Skrabal CA, Thompson LO, Potapov EV, Southard RE, Joyce DL, Youker KA, et al. Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res. 2005 Jan;123(1):118-125.
101 Kusaka M, Pratschke J, Wilhelm MJ, Ziai F, Zandi-Nejad K, Mackenzie HS, et al. Early and late inflammatory changes occurring in rat renal isografts from brain dead donors. Transplant Proc. 2001 Mar;33(1-2):867-868.
102 van Der Hoeven JA, Ter Horst GJ, Molema G, de Vos P, Girbes AR, Postema F, et al. Effects of brain death and hemodynamic status on function and immunologic activation of the potential donor liver in the rat. Ann Surg. 2000 Dec;232(6):804-813.
103 Kusaka M, Pratschke J, Wilhelm MJ, Ziai F, Zandi-Nejad K, Mackenzie HS, et al. Activation of inflammatory mediators in rat renal isografts by donor brain death. Transplantation. 2000 Feb 15;69(3):405-410.
104 Hevesi ZG, Lopukhin SY, Angelini G, Coursin DB. Supportive care after brain death for the donor candidate. Int Anesthesiol Clin. 2006;44(3):21-34.
105 Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009 Apr;53(4):425-435.
106 Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000 Mar 2;342(9):605-612.
107 Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995 Aug 10;333(6):333-336.
108 Stangl M, Zerkaulen T, Theodorakis J, Illner W, Schneeberger H, Land W, et al. Influence of brain death on cytokine release in organ donors and renal transplants. Transplant Proc. 2001;33(1-2):1284-1285.
109 Birks EJ, Owen VJ, Burton PBJ, Bishop AE, Banner NR, Khaghani A, et al. Tumor necrosis factor-α is expressed in donor heart and predicts right ventricular failure after human heart transplantation. Circulation. 2000 Jul 18;102(3):326-331.
110 Wagner FD, Jonitz B, Potapov EV, Qedra N, Wegscheider K, Abraham K, et al. Procalcitonin, a donor-specific predictor of early graft failure-related mortality after heart transplantation. Circulation. 2001 Sep 18;104(12 Suppl. 1):I192-I196.
111 Adrie C, Monchi M, Fulgencio J, Cottias P, Haouache H, Alvarez-Gonzalvez A, et al. Immune status and apoptosis activation during brain death. Shock. 2010 4;33(4):353-362.
112 Pratschke J, Wilhelm MJ, Kusaka M, Beato F, Milford EL, Hancock WW, et al. Accelerated rejection of renal allografts from brain-dead donors. Ann Surg. 2000 Aug;232(2):263-271.
113 McElhinney DB, Khan JH, Babcock WD, Hall TS. Thoracic organ donor characteristics associated with successful lung procurement. Clin Transplant. 2001 Feb;15(1):68-71.
114 Kuecuek O, Mantouvalou L, Klemz R, Kotsch K, Volk HD, Jonas S, et al. Significant reduction of proinflammatory cytokines by treatment of the brain-dead donor. Transplant Proc. 2005 Feb;37(1):387-388.
115 Miki C, Gunson BK, Buckels JAC, Uchida K, Mohri Y, Kusunoki M. Methylprednisolone therapy in deceased donors reduces inflammation in the donor liver and improves outcome after liver transplantation. Ann Surg. 2009 Sep;250(3):502-503. author reply 503-504
116 Venkateswaran RV, Dronavalli V, Lambert PA, Steeds RP, Wilson IC, Thompson RD, et al. The proinflammatory environment in potential heart and lung donors: prevalence and impact of donor management and hormonal therapy. Transplantation. 2009 Aug 27;88(4):582-588.
117 Nijboer WN, Ottens PJ, van Dijk A, van Goor H, Ploeg RJ, Leuvenink HGD. Donor pretreatment with carbamylated erythropoietin in a brain death model reduces inflammation more effectively than erythropoietin while preserving renal function. Crit Care Med. 2010 Apr;38(4):1155-1161.
118 Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004 Jul 9;305(5681):239-242.
119 Toledo-Pereyra LH, Frantzis P, Prough D, Alvarez H, Hilchenbach G, Cramer T, et al. Better renal function with naloxone treatment following hemorrhage and brain death. Transplant Proc. 1990 Apr;22(2):462-463.
120 Eagan C, Keller CA, Baz MA, Thibault M. Effects of administration of intravenous naloxone on gas exchange in brain-dead lung donors. Prog Transplant. 2009 Sep;19(3):267-271.