Chapter 143 Management of Suppurative Intracranial Infections
Pathogenesis
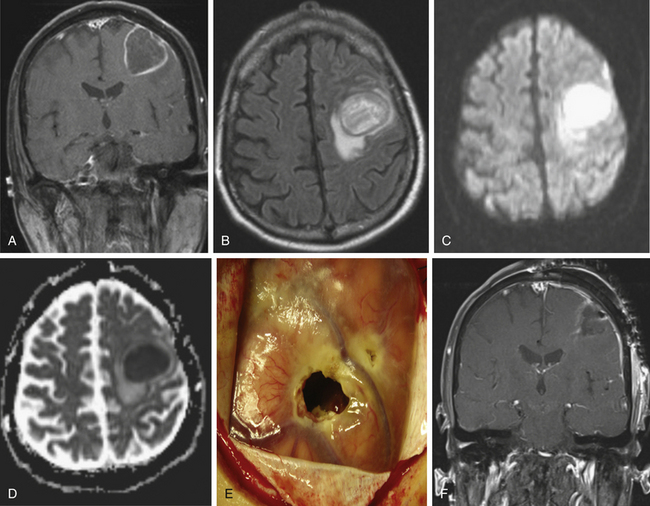
Pyogenic brain abscess is a focal collection of pus within the brain. This condition has been the subject of discussion and of surgical treatment for hundreds of years.1 The incidence of pyogenic brain abscesses is 8% of intracranial masses in the developing countries, whereas in the West the incidence is 1% to 2%, with male predominance.1,2 This condition is rare largely because of the brain natural resistance to infection, a property mediated by its abundant vascular supply, the relative impermeability of the blood–brain barrier,3 and improvement in the treatment of ear, sinus, and orofacial infections over the last decades. Peak ages vary, however, depending on predisposing influences; pediatric cases peak between the ages of 4 and 7, and a substantial minority of these children have congenital heart disease.2 The overall occurrence of brain abscess does not appear to have changed significantly in the antibiotic era, and an active neurosurgical service in a hospital in a developed country can expect to see 4 to 10 cases annually.2,4 Pyogenic brain abscess formation is initiated when bacteria gain entry into cerebral tissues with trauma, contiguous spread from a suppurative focus, or hematogenous dissemination from a distant infection. Most cases (40%-60%) are the result of contiguous spread of infection from the middle ear, oropharynx, and paranasal sinuses (Fig. 143-1).5–7 These lesions are usually solitary, and their distribution in the brain reflects the predisposing lesion (Table 143-1). Seeding of the brain probably occurs via the valveless emissary veins draining the contiguous areas that allow microorganisms to flow into the venous system of the brain from adjacent sites.7 Middle ear infections, chronic otitis media, or chronic mastoiditis can lead to temporal lobe or cerebellar abscesses by direct spread via the tegmen tympani or by translabyrinthine spread. Paranasal sinusitis can lead to frontal or temporal lobe abscesses by retrograde thrombophlebitis of the diploic veins. Frontal sinus infection can also lead to frontal lobe brain abscess when complicated by osteomyelitis of the frontal bone of the skull with dehiscence of the posterior table.
TABLE 143-1 Predisposing Lesions, Intracranial Location, and Bacteriology of a Pyogenic Brain Abscess
| Predisposing Lesion | Intracranial Location | Predicted Bacteriology |
|---|---|---|
| Paranasal sinusitis | Frontal lobe | Microaerophilic (Streptococcus intermedius group) and anaerobic strep, Haemophilus, Bacteroides, Fusobacterium, and Prevotella species |
| Otitis media, mastoiditis | Temporal lobe or cerebellum | Aerobic and anaerobic streptococci, Enterobacteriaceae, P. aeruginosa, Prevotella species, Bacteroides fragilis |
| Dental sepsis | Frontal lobe | S. viridans and anaerobic streptococci, Bacteroides, Fusobacterium, Prevotella, and Actinomyces species |
| Penetrating trauma | Related to site of wound | S. aureus, aerobic streptococci, Clostridium species, Enterobacteriaceae |
| Postoperative trauma | Related to site of surgery | Staphylococcus epidermidis, S. aureus, Enterobacteriaceae, P. aeruginosa |
| Congenital heart disease | Multiple abscesses, most commonly in distribution of middle cerebral artery | Microaerophilic and aerobic strep |
| Infective endocarditis | Same as in congenital heart disease | S. aureus, S. viridans, Enterococcus species |
| Pulmonary infection (lung abscess, empyema) | Same as in congenital heart disease | Microaerophilic and anaerobic streptococci, Actinomyces, Fusobacterium, Nocardia, and Prevotella species |
| Intra-abdominal infectionCompromised host (AIDS, cancer chemotherapy, chronic steroids, lymphoma) | Same as in congenital heart disease | Streptococcus species, B. fragilis, Enterobacteriaceae toxoplasmosis, Nocardia species, EBV lymphoma, TB, fungi |
EBV, Epstein-Barr virus; TB, tubercle bacillus.
Penetrating cranial trauma such as open cranial fracture with dural tear is a well-described, though relatively infrequent, cause of pyogenic brain abscess, accounting for less than 10% of these infections.5,6,8 The incidence of brain abscess was 3% in one large series of combat-acquired injuries from the Vietnam War, with most occurring in the setting of gunshot wounds to the head and retained bone fragments.9 The interval from the time of injury to diagnosis may be considerably delayed, averaging nearly 4 months in one study.8 A distinctive form of post-traumatic brain abscess that occurs largely in young children results from penetrating injuries secondary to a foreign body injury, such as to the orbital region and, less commonly, other areas of the skull, from pencil tips, wooden sticks, wooden toys, and lawn darts.10 The interval from injury to clinical presentation may extend from days to years. Treatment, as in other penetrating cranial injuries, involves early surgical debridement.11
Brain abscesses are infrequent sequelae of neurosurgery, complicating approximately 0.1% of clean neurosurgical procedures.4 Microorganisms, introduced at the time of surgery, infect the wound or bone flap and form an intracranial focus of suppuration by contiguous spread. Hematogenous dissemination or metastatic seeding from a distant primary site of infection accounts for approximately 25% of brain abscesses.4–6 These lesions are usually located in the distribution of the middle cerebral artery or parietal–occipital junction, tend to occur at the corticomedullary junction, where capillary flow is slowest, and are frequently multiple and multiloculated.4 Recognized sources of metastatic seeding include pulmonary lesions, such as arteriovenous fistulas,12 often occurring with hereditary hemorrhagic telangiectasia13; infective endocarditis, rarely complicated by macroscopic brain abscess (less than 1%) but with microabscesses found at autopsy in 4%14; and deep-seated infections, such as osteomyelitis, pulmonary empyema, pelvic infections, and intra-abdominal infections.
An increasingly important problem occurs in intravenous drug users with infected valvular vegetations supplying emboli, resulting in cerebral infarction, cerebral hemorrhage, brain abscess formation, and spinal epidural abscesses. This population is prone to toxin-mediated diseases (tetanus and botulism) with inoculation of these agents at injection sites.15 In general, however, transient bacteremia is unlikely to result in brain abscess in the absence of breaches of the blood–brain barrier or predisposing cerebral lesions, such as previous stroke or primary or metastatic neoplasms.7,16
Cerebral abscess complicates cyanotic congenital heart disease in 2% to 6% of cases,4 and cyanotic congenital heart disease is a leading underlying cause of pediatric brain abscess, accounting for 6% to 50% of cases.7 Tetralogy of Fallot and transposition of the great vessels underlie most cases, although any cardiac defect that results in significant right-to-left shunting appears to increase the risk. The pathogenesis probably involves increased blood viscosity as a result of chronic hypoxemia (due to right-to-left shunting), leading to areas of microinfarction within the brain that act as nidi for infection. The mortality from pyogenic brain abscess in this setting is high. Intrasellar, brain stem, basal ganglia, and thalamic abscesses are rare.4
Intrasellar abscesses generally occur in the setting of preexisting pituitary or sellar lesions, such as adenomas, craniopharyngiomas, or Rathke’s cleft cysts, or as a complication of trans-sphenoidal surgery.17,18 These lesions may also occur as a result of intrasellar extension from sphenoid sinusitis. Abscesses of the brain stem are generally hematogenous in origin and may extend longitudinally over several levels of the brain stem.7 The incidence of these lesions appears to have decreased over the past decades, probably as a result of improvements in the management of pediatric ear infections. Of the brain abscesses, 20% are cryptogenic. In such cases, broad antimicrobial therapy is indicated. Many of these cases are the result of nondiagnosed dental infections.
Bacteriology
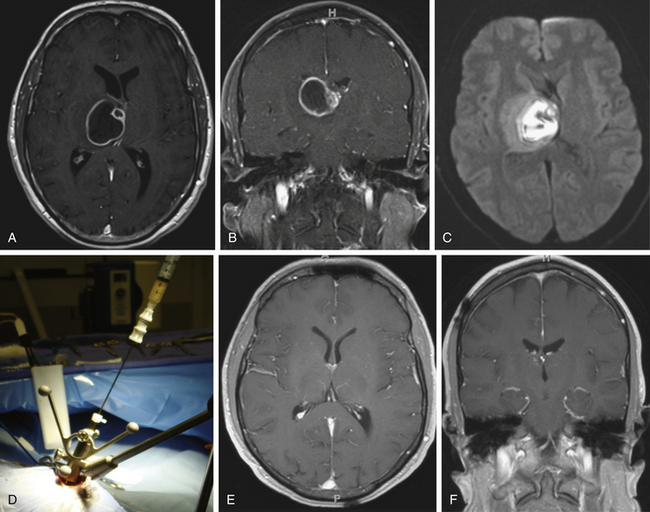
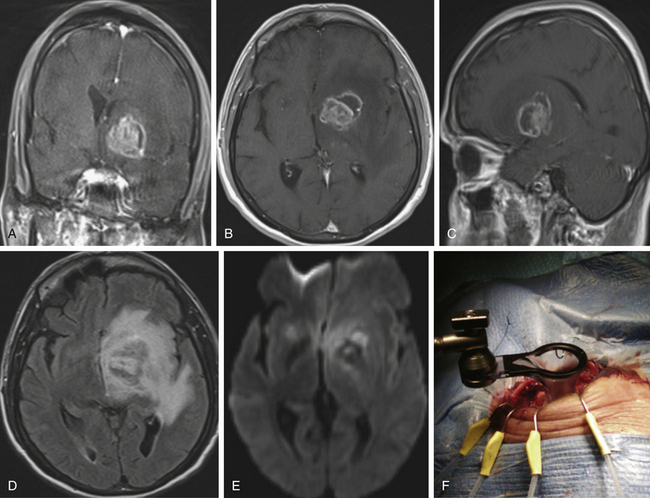
The bacteriology of brain abscesses is determined by the initial site of infection (Table 143-1). Streptococci (Streptococcus milleri and Streptococcus viridans) are the most common cause of pyogenic brain abscesses, involved in nearly two thirds of cases, because of their extension from the nasopharynx and oropharynx, as well as from endocarditis (S. viridans).4,19 Staphylococcus aureus accounts for 10% to 21% of cases, generally in the setting of trauma, postoperatively, but is also seen among patients with brain abscesses resulting from endocarditis.6,19–21 Methicillin-resistant S. aureus (MRSA) should be considered in hospitalized patients or among those who were known as MRSA colonized. Anaerobic bacteria (Bacteroides, Prevotella, Peptostreptococcus, Fusobacterium, and Actinomyces species) are also major causes of brain abscesses, usually part of polymicrobial infection.20 Brain abscesses due to Actinomyces species are commonly associated with pulmonary and odontogenic infections. Gram-negative bacilli such as Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Serratia species, and Proteus species are associated with genitourinary or intra-abdominal infections, as well as commonly isolated from brain abscesses following head trauma and postoperative infection.7,22,23 Pseudomonas species can cause brain abscess that results from otitis media or otitis externa.7,20 Other bacterial species may be cultivated from brain abscesses in various clinical settings: Clostridium species, in association with an underlying malignancy or hemolytic–uremic syndrome24; Propionibacterium acnes in the postneurosurgical patient25; Bacillus species; Listeria monocytogenes, commonly a cause of meningitis or meningoencephalitis but rarely a cause of brain abscess; and Salmonella species, associated with intracerebral hematoma.4 Nocardia species such as N. asteroides and N. farcinica comprise 1% to 2% of all cerebral abscesses, with a mortality rate of 31%.26 They are frequently seen in immunocompromised patients but also occur in immunocompetent patients.26 Nocardia brain abscess (Fig. 143-2) may be in isolated cranial pathology or may result from dissemination of cutaneous or pulmonary infection.20 Recently, the number of case reports describing Nocardia brain abscess have been increasing; this may be related to better diagnostic techniques but most likely results from an increase in their incidence.19 Fungal brain abscesses caused by yeast such as Candida or Cryptococcus species; dimorphic fungi such as Histoplasma, Coccidioides, or Blastomyces species; and molds such as Aspergillus or Rhizopus species are associated with immunocompromised states.20 Protozoa and helminths can cause parasitic brain abscesses and may be relevant in certain cases, such as cat exposure or endemic areas. Toxoplasma gondii can cause central nervous system (CNS) toxoplasmosis and brain abscess (Fig. 143-3), and Taenia solium can cause neurocystcercosis.20 Brain abscess in the neonatal period has a distinctive bacteriologic profile, with most of these lesions caused by Proteus species and Citrobacter diversus.27 In contrast to meningitis caused by other pathogens, neonatal meningitis caused by these organisms is complicated by brain abscess in 40% to 75% of cases, so early computed tomography (CT) evaluation of neonates with meningitis or bacteremia caused by Proteus or Citrobacter species is recommended.7 Interestingly, the most common bacterial causes of acute pyogenic meningitis (Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis) are rarely associated with brain abscess.4,28 The neuropathologic events that underlie brain abscess formation have been studied using α-streptococci in a canine model and found to correlate with CT scan findings.29,30 A series of histopathologic stages has been described that appear to parallel the evolution of CT findings in human brain abscesses.29,30 Early cerebritis (days 1-3) is a poorly circumscribed lesion characterized by acute inflammation and cerebral edema associated with bacterial invasion. Later (days 4-9), the zone of cerebritis expands, and necrosis develops, with pus forming at the center of the lesion. CT scanning reveals some ring enhancement with diffusion of contrast material into the necrotic center. The early capsule stage (days 10-13) demonstrates the establishment and maturation of a well-formed collagenous capsule associated with a reduction in the degree of cerebritis and some regression in the local edema. At the late capsule stage (day 14 and beyond), there is continued maturation of a thick capsule with extracapsular gliosis and dense ring enhancement with little contrast diffusion on CT scan.
Capsule formation and ring enhancement on imaging studies are generally thinner and less complete on the ventricular side of the abscess.29 This situation is probably related to the relatively poor vascularity of the deep white matter and reduced migration of fibroblasts into the area. This thinner area of capsule predisposes to ventricular rupture of the abscess.
The nature of the infecting organism influences encapsulation. Models using Bacteroides species show delayed capsule formation with multiple daughter abscesses, suggesting incomplete containment of the infection,31 whereas S. aureus experimental abscesses were larger, demonstrated delayed healing, and were associated with marked extracapsular abnormalities.32 The route of infection also appears to affect capsule formation: abscesses resulting from hematogenous spread tend to have less extensive encapsulation than those arising from a contiguous focus of infection.4 This situation is probably the result of microinfarcted areas of the brain arising from metastatic emboli, leading to tissue hypoxia, impaired angiogenesis, and impeded fibroblast migration. Host variables also contribute to encapsulation. For example, in a canine model of brain abscess, immunosuppression with prednisone and azathioprine before bacterial inoculation leads to delayed histopathologic evolution with incomplete encapsulation as assessed by the diffusion of contrast media into the necrotic center of the lesion on CT imaging.33
Clinical Presentation
Most patients with pyogenic brain abscess have symptoms for less than 2 weeks, although the disease can present indolently.2 The presenting features of brain abscess depend on the size and intracranial location of the lesions, the virulence of the infecting agents, the immunologic status of the host, and the cerebral edema caused by the expanding intracranial mass lesion. The classic triad of fever, headache, and focal neurologic deficit is present in less than 50% of cases.4 Headache, usually dull and poorly localized, is present in 50% to 70% of cases and is so nonspecific as to be a potential cause of diagnostic delays.5–7,19,20,22,34,35 Sudden worsening of a preexisting headache in a patient with a brain abscess, especially if accompanied by the acute onset of meningeal signs, suggests either herniation or intraventricular rupture of the abscess.7
Fever occurs in 25% to 50% of adults5-7,19,20,22,34,35 and is more common in children.4 Symptoms and signs related to any underlying disease (e.g., paranasal sinusitis or otitis media), if present, may aid in the diagnosis. Altered levels of consciousness are often present.5 Focal neurologic signs depend on the location of the lesions within the brain and the extent of cerebral edema2: frontal and parietal lobe abscesses are commonly associated with hemiparesis and aphasia, temporal lobe presentations may include aphasia or visual field disturbances, intrasellar lesions tend to mimic pituitary tumors, and cerebellar abscesses often present with ataxia and nystagmus.36 Seizures occur in 25% to 35% of cases.4 Patients present with multiple brain abscesses in approximately 10% of cases.35
Diagnosis
Laboratory Findings
A moderate peripheral leukocytosis are found in most patients with brain abscesses; however, blood cultures are rarely (~10%) positive.5 Despite this, it is advisable to perform blood cultures on presentation (and before antimicrobial therapy). The erythrocyte sedimentation rate and the level of C-reactive protein are elevated in the majority of the patients. Although they are nonspecific indicators of inflammation, they help in monitoring patient response. Lumbar puncture in the setting of brain abscess with mass effect is strongly contraindicated and rarely provides useful clinical information. The best opportunity to obtain a specific microbiologic diagnosis is at the time of surgery. Consultation and coordination of efforts between neurosurgeons and infectious disease specialists are crucial to ensure that the appropriate specimens are obtained and that cultures of abscess material are optimally handled and processed to enhance the chances of identifying the pathogen or pathogens. The broad-range bacterial ribosomal deoxyribonucleic acid (DNA) polymerase chain reaction (PCR) method, combined with DNA sequencing, has been used to examine pus and tissue from neurosurgical patients with suspected meningitis, brain abscess, spondylitis, or spinal epidural abscess. In one study, bacterial 23S ribosomal DNA was positive in 9 of 14 pus samples from patients with brain abscess or subdural empyemas; 8 of 14 were also positive on bacterial culture. In 6 patients with brain abscesses, bacteria were detected both by PCR and on bacterial culture. In one brain abscess, the sequencing identified several bacterial species. In 3 patients with intracranial infections, the specimens were positive by PCR but negative by culture. In 8 patients, specimens were taken while the patients were on antibiotic therapy for a mean duration of 5.3 days; in 3 of these patients, the causative bacteria could still be identified by PCR alone, even after intensive parenteral antibiotic therapy. In one case of Mycoplasma hominis, the organism was promptly identified by PCR alone, whereas standard methods require prolonged culturing of the specimen.37
Imaging
Computed Tomography
CT scan is excellent for the diagnosis of brain abscess, anatomic localization of the lesion, and evaluation of cerebral edema. Its value in the identification of cerebritis is improved by a delayed contrast-enhanced scanning technique.7 The contrast-enhanced CT appearance of brain abscess is a hypodense lesion surrounded by ring enhancement with a variable peripheral zone of cerebral edema; dense ring enhancement is not a constant feature of abscesses but depends on the maturity of the lesion. Although the sensitivity of CT scanning for brain abscess is 95% to 99%, the specificity is compromised by the inability of this modality to reliably distinguish brain abscess from metastatic tumor or some vascular lesions.4,38 Indium-111 (111In)–labeled leukocyte scanning may be used to complement CT scanning. Radiolabeled leukocytes accumulate in foci of active inflammation, enhancing the chances of distinguishing abscess from metastasis in inconclusive cases. In several small series, this technique has shown a high degree of diagnostic accuracy.39,40
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) provides imaging detail and resolution superior to CT scanning. MRI appears to be more sensitive than CT in detecting early cerebritis. Contrast-enhanced MRI has some distinct advantages over contrast-enhanced CT scanning: it is more accurate in delineating the extent of central liquefaction necrosis of the abscess, it has better sensitivity for early satellite lesions, it can detect extraparenchymal extension of the abscess (such as subdural empyema) earlier because the purulent material is hyperintense relative to cerebrospinal fluid (CSF) (as opposed to an isodense appearance on CT scan), and it lacks bone artifact.4,41 However, because the contrast-enhanced MRI reveals ring enhancement of a brain abscess that is similar to the enhancement seen in cystic or necrotic high-grade glioma or metastasis, it may be impossible to differentiate among these lesions.
Fluid-attenuated inversion recovery (FLAIR) uses heavy T2 weighting and nulling of the free water signal.42 Increased FLAIR signal intensity is caused by increased protein content, reported among patients with brain tumors, brain abscesses, and cerebrovascular insults.43 Thus, the ability of FLAIR imaging to diagnose depends on protein content and is not pathology specific44 (see Figs. 143-1B and 143-3D).
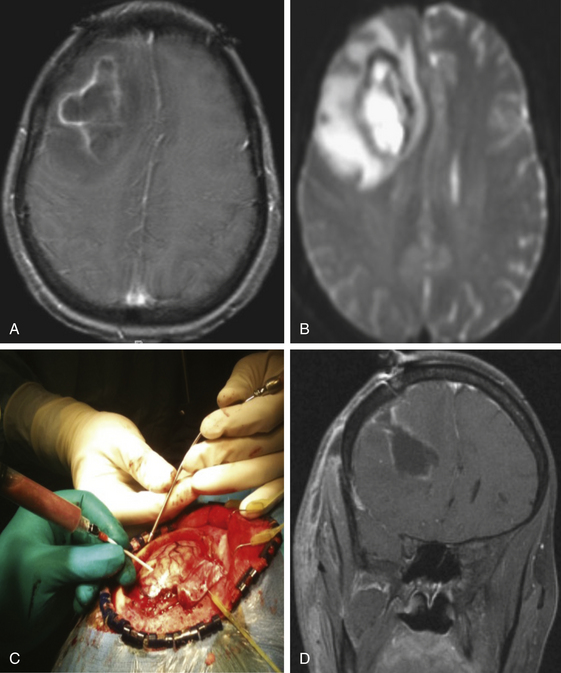
Diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) calculations can help identify brain abscesses. DWI is based on the motion of water molecules. To measure the degree of water movement, ADC maps are needed.45 Cerebral abscess contains proteins, pus, and bacteria; thus, water motion is restricted, and DWI has increased signal intensity with low ADC values (see Figs. 143-1C and D, 143-2C, 143-3E, and 143-4B). However, most necrotic tumors have low–intermediate intensity on DWI, high ADC values due to serous fluid, and fewer inflammatory cells compared to brain abscess.46 Fertikh et al.47 described the ADC ratio, calculated by dividing the ADC values from the nonenhancing cystic portion of the mass by the ADC values from the contralateral normal-appearing white matter. The mean ADC ratios were significantly higher in neoplasms than in abscesses.47
Magnetic Resonance Spectroscopy
Proton magnetic resonance (MR) spectroscopy provides a noninvasive imaging modality that can help differentiate between cystic tumors and brain abscesses. This technique detects the metabolic profile of the brain. Cytosolic amino acids (leucine, isoleucine, and valine) are usually detected in cerebral abscesses. Because these metabolites are absence in neoplasms, their detection is strongly indicative of cerebral abscess.48 Their absence, however, does not rule out a pyogenic abscess. The presence of acetate with or without succinate supports an anaerobic bacterial abscess.49 However, it does not apply to cases of parasitic abscesses (hydatid cyst), toxoplasmic abscesses, and cryptococcomas. In fungal abscesses, the amino acids are at low concentrations. At present, it is thus impossible to differentiate between toxoplasmosis and lymphoma by MR spectroscopy in patients with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS).50 It has also been suggested that response to treatment may be followed by demonstrating serial changes in spectral metabolite patterns.51 In an analysis of 24 such patients with proton MR spectroscopy, Dev et al.51 found that lactate and amino acids were noted in the spectra of all patients regardless of the timing of spectroscopy relative to combined medical and surgical therapy. Acetate and pyruvate consistently disappeared after 1 week of combined therapy, suggesting that the proton MR spectroscopy spectral patterns are specific for pyogenic brain abscess and the change in these patterns is potentially useful in monitoring response to treatment. Lai et al.48 evaluated by MRI, proton MR spectroscopy, and DWI 50 patients with intracranial cystic lesions (21 pyogenic abscesses, 23 tumor cysts, 3 epidermoid cysts, and 3 arachnoid cysts). The diagnostic accuracy, sensitivity, specificity, and positive and negative predictive values of MRI were 61.4%, 61.9%, 60.9%, 59.1%, and 63.3%, respectively, while the respective values for MR spectroscopy were 93.2%, 85.1% 100%, 100%, and 88.5%. The addition of DWI increased these values to 97.7%, 95.2%, 100%, 100%, and 95.8%, respectively.
Positron Emission Tomography
Positron emission tomography (PET) has been used preoperatively to evaluate CNS mass lesions. In a recent study of the uptake of fludeoxyglucose F 18 (FDG) and [11C]methionine (11C-Met) tracers were used in brain abscesses before treatment. The area showing increased uptake of 11C-Met corresponded to the enhanced area on CT and MRI. After treatment, the area of lesions was smaller on CT and MRI, and the PET studies showed decreased uptake. The mechanism of 11C-Met uptake in the inflammatory area is thought to be not only higher metabolic rate and active transport of amino acids but also disruption of the blood–brain barrier. The mechanism of FDG uptake is also related to the degree of the inflammatory response and the increased density of inflammatory cells in the brain abscess. PET is useful both in detecting the inflammatory lesion and in assessing the response to antibiotic therapy.52
Treatment
Medical Management of Brain Abscess
Empiric antibiotic therapy should be started as early as possible; however, every effort should be made to obtain samples from the abscess before starting the antibiotic therapy.53 Empiric intravenous antibiotic therapy should cover gram-positive, gram-negative, and anaerobic microorganisms, because brain abscesses are often polymicrobial. For example, third- or fourth-generation cephalosporin, metronidazole, and vancomycin can be used. After the pathogen is identified on culture of the aspirate, the empiric antibiotic should be modified. The options for treatment of brain abscess caused by MRSA are limited and include vancomycin and clindamycin, in combination with other agents if the patient is susceptible in vitro.21
The anti-infective agents needed to treat brain abscesses must be active against the pathogens and be capable of penetrating into the abscess cavity and achieving high levels in the abscess pus. Several studies have addressed the issue of antimicrobial penetration into abscess pus and found that penicillin G at high doses, metronidazole, trimethoprim–sulfamethoxazole, and chloramphenicol achieve therapeutic concentrations within abscess fluid.54,55 Metronidazole attains such high levels in abscess fluid (34-42 μg/ml) that it is considered an important component of most regimens when anaerobes are potentially involved.54 It must be used in combination with an agent active against microaerophilic streptococci (e.g., penicillin), because these organisms are frequent contributors to polymicrobial infection (Table 143-1) and are resistant to metronidazole. Clindamycin, aminoglycosides, and first-generation cephalosporins penetrate abscess fluid poorly. Limited data are available on vancomycin and nafcillin (Table 143-2).41 Experimental evidence supports the potential utility of ceftriaxone, ceftazidime, and other third- and fourth-generation cephalosporins, as well as quinolones (e.g., ciprofloxacin), monobactams (e.g., aztreonam), and carbapenems (e.g., imipenem), in the management of these infections,7 although imipenem and quinolones have a propensity to lower seizure thresholds.
TABLE 143-2 Empirical Antimicrobial Therapy of Suppurative Intracranial Infection Based on the Predisposing Lesion
| Predisposing Lesion | Antimicrobial Therapy∗ |
|---|---|
| Sinusitis | Cefotaxime 2 g q4-6h or ceftriaxone 2 g IV q12h + metronidazole 500 mg q6h |
| Otitis media, mastoiditis | Cefotaxime 2 g q4-6h, ceftriaxone 2 g q12h, or cefepime 2 g q8h + metronidazole 500 mg q6h |
| Dental sepsis | Penicillin G 4 mU q4h + metronidazole 500 mg q6h |
| Trauma (postneurosurgical) | Vancomycin 1 g q8-12h + ceftazidime 2 g q8h or cefepime 2 g q8h |
| Congenital heart disease | Same as in frontoethmoidal disease |
| Infective endocarditis | Vancomycin 15 mg/kg q8-12h or nafcillin/oxacillin 2 g q4h + gentamicin 1 mg/kg q8h |
| Pulmonary infection (lung abscess, empyema) | Penicillin G 4 mU q4h + metronidazole 500 mg q6h |
| Cryptogenic source | Cefotaxime 2 g q4-6h or ceftriaxone 2 g q12h + metronidazole 500 mg q6h |
| Nocardia brain abscess suspected | Add trimethoprim–sulfamethoxazole 5-6 mg/kg q6-8h |
∗ Suggested initial empirical therapy may require modification once specific microbiologic data become available; dosing may require adjustment in patients with underlying renal or hepatic disease. All agents are to be given intravenously. An infectious disease consultation is advisable.
The duration of antimicrobial therapy for brain abscess largely depends on a causative pathogen and the adequacy of surgical drainage. In most cases, a 6- to 8-week course of antimicrobial therapy is recommended, with abscess resolution monitored by serial imaging studies. However, no prospective control studies support the optimal duration of therapy; therefore, recommendations for antibiotic therapy are based on the pathogen, the antibiotic penetration into the abscess, and the patient’s response. The Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy group recommended starting with intravenous therapy for 1 to 2 weeks; then, depending on clinical response, a change to an appropriate oral regimen should be considered.19 Complete resolution of the abscess and abnormal contrast enhancement may take 3 to 4 months. A residual area of contrast enhancement may persist for more than 6 months, and in a small percentage of these patients, the abscess recurs.
The role of corticosteroids remains controversial. Several experimental models have demonstrated that these agents diminish antimicrobial entry into the CNS, reduce the elimination of viable organisms from the abscess cavity, and inhibit an effective, ring-enhancing, host inflammatory response.4,41,56 In a rat model of brain abscess, a delay in encapsulation of the lesion occurs, but no differences in outcome occur with the use of steroids.53 Limited retrospective clinical series yield conflicting data; some fail to reveal significant differences in outcome with the use of steroids,5,57 whereas others have shown a worse outcome with their use.58 The preponderance of evidence appears to weigh against the routine use of these agents as adjunctive therapy for brain abscess except when signs of increased intracranial pressure secondary to marked cerebral edema are present. In these cases, emergency drainage of the abscess is crucial, but treatment of an associated severe degree of cerebral edema mandates the use of steroids in high doses (e.g., dexamethasone at 10 mg every 6 hours), tapered as the clinical condition and serial imaging improve.7
Seizures occur in 13% to 25% of the patients with brain abscess; thus, anticonvulsants are used for the treatment and prophylaxis of seizures in these patients.59,60 We generally recommend perioperative use of these agents and their continuation after surgery, particularly in patients with seizures during their illness. The decision for long-term use depends on neurologic evaluation after the abscess has resolved.7
The nonoperative management of pyogenic brain abscess occurs in the setting of the neurologically stable patient with cerebritis, or with small lesions (less than 1.5 cm); severe concomitant medical conditions that greatly increase the surgical risk, such as severe bleeding diathesis; the presence of multiple abscesses in a surgically inaccessible, dominant, or disparate location; or the presence of multiple small abscesses.3,4,41,61–63 Although this approach may be useful in these situations, the lack of diagnostic specimens requires empirical antimicrobial therapy that necessarily involves several agents, with their combined attendant toxicities, for an extended period without the potential for narrowing the regimen. In addition, the time to resolution of lesions may be prolonged, with the possibility of serious sequelae, because the lesion may continue to expand and may rupture into the ventricle. The decision to treat such lesions empirically with antimicrobials alone probably necessitates longer parenteral therapy (e.g., 12 weeks) and frequent imaging until resolution of radiographic abnormalities is achieved.
Surgical Management of Brain Abscess
Aspiration of Brain Abscess Versus Craniotomy for Excision of Brain Abscess
Surgical treatment can be either aspiration of brain abscess content or surgical excision of abscess capsule. There is controversy regarding the appropriate surgical procedure.64 Both methods enable the surgeon to identify the causative pathogen and to tailor the antibacterial treatment.3 However, although aspiration of brain abscess has a low surgery-related morbidity and mortality rates, postaspiration relapse rates are up to 32% and such a relapse necessitates reaspiration.65 CT-guided stereotactic aspiration is a modality accurate to within a few millimeters with a diagnostic yield of 95%, is associated with transient morbidity only in 5% of patients, and is highly effective in the definitive drainage of abscesses.41,66 It has an advantage in the treatment of deep-seated brain abscesses, especially in eloquent areas such as the brain stem, thalamus, and basal ganglia. Stereotactic drainage of several abscesses can usually be accomplished; a combination of several concurrent drainage procedures and prolonged medical therapy is effective.67 The current best technique to localize and drain brain abscess involves frameless neuronavigation (see Fig. 143-2). Another reliable method to drain a brain abscess is real-time ultrasound guidance, a minimally invasive and accurate method, with fewer risks associated with CT-guided stereotactic aspiration.68 Other surgical methods are stereotactic endoscopic aspiration and irrigation. Numerous reports support these approaches, which share the advantages of precise localization, minimal craniotomy, and application to treating multiple lesions.69–73 Some have suggested a trend toward a lower incidence of seizures and other sequelae in those treated by aspiration as opposed to excision.41
Image-guided craniotomy for excision of brain abscess with its capsule has a lower recurrence rate compared to aspiration methods.74 Excision of brain abscesses is useful in large (more than 2.5 cm) superficial abscesses (see Figs. 143-1 and 143-4), in the case of multiloculated abscesses; failure of resolution after several aspirations; some posterior fossa lesions; some fungal abscesses; post-traumatic abscesses with retained bone fragments or foreign bodies9; and gas-containing abscesses, usually signifying the presence of an associated CSF fistula.75
For surgical excision of a brain abscess that has failed to respond to aspiration and antimicrobial therapy or is in a particularly dangerous location, such as a posterior fossa abscess associated with edema, mass effect, and impending or actual obstructive hydrocephalus, image-guided craniotomy is favored to excise the lesion, relieve the mass effect on the brain stem, and reduce the chances of recurrence. An image-guided keyhole approach uses a small skin incision, limited craniotomy, and brain retraction, minimizing the intraoperative trauma to the brain parenchyma with better cosmetic results76 (see Fig. 143-3).
Excision is not the procedure of choice in the cerebritis stage or in deep-seated brain abscesses, especially in eloquent areas. During the stage of cerebritis, antimicrobials are used with serial neurologic examinations and imaging studies to guide therapy. In most other settings, however, surgical intervention is undertaken. In the obtunded patient with a severe neurologic deficit and an encapsulated lesion, surgery for diagnosis and decompression is carried out emergently.
The intraventricular rupture of a brain abscess occurs with progressive growth of the lesion. As the pus increases, the abscess expands toward the ventricle and may rupture, resulting in the sudden, catastrophic deterioration of the patient. The diagnosis is confirmed by the presence of hydrocephalus and enhancement of the ventricular walls. Immediate ventricular drainage, intraventricular instillation of antibiotics, evacuation of the remaining abscess, and systemic antibiotic therapy are still associated with a management mortality rate of greater than 80%.77,78
If multiple lesions are discovered, those greater than 2.5 cm in diameter should be aspirated. If all lesions are less than 2.5 cm and do not exert mass effect, the largest or most accessible one should be aspirated for culture.79
Osteomyelitis of the Skull
Osteomyelitis of the skull usually results from the contiguous spread of infection from a paranasal sinusitis, otogenic infection, or odontogenic infection or after penetrating cranial trauma or craniotomy. Osteomyelitis of the frontal bone, subperiosteal abscess, and acute frontal sinusitis (Pott’s puffy tumor) can spread via scalp veins and thrombophlebitis of the dural sinuses and veins.80 Odontogenic infections can lead to osteomyelitis of the alveolar ridge of the mandible. Malignant otitis externa is a necrotizing osteomyelitis of the skull base caused by P. aeruginosa and occurring largely in diabetics. The predisposing lesions generally predict the site of bone infection and the expected bacteriology of the infecting organisms (see Table 143-1).
An elevated erythrocyte sedimentation rate is diagnostically helpful and provides a useful marker to monitor the efficacy of therapy using serial determinations. Blood cultures are obtained before initiating therapy. Aspiration of the incision and bone biopsy provide the definitive diagnostic tests; however, radiographic techniques are crucial to establishing the extent of the process and providing a baseline for follow-up during treatment. MRI is extremely sensitive to signal changes in the marrow cavity and provides excellent detail of the skull base. It is the procedure of choice in investigating malignant otitis externa. Gallium-67–labeled and 111In-labeled white blood cell scans are fairly sensitive, albeit nonspecific, tests whose usage appears to occur during the follow-up period. Single photon emission computed tomography technology enhances the monitoring of therapy.81 The treatment of cranial osteomyelitis is often surgical debridement, removal of infected bone flaps, and 4 to 6 weeks of parenteral antibiotic therapy.82 In addition, in cases arising from the sinuses or auditory canal, surgical debridement needs to be carried out by the otologist. Hyperbaric oxygen has been described as an adjuvant therapy in refractory cases of skull base osteomyelitis. Increasing the oxygen partial pressure and tension enhances the polymorphonuclear ability to kill aerobic pathogens.
Cranial Epidural Abscess
Cranial epidural abscess arises between the skull and the dura, usually in the frontal region, as a result of contiguous spread of infections from adjacent structures, such as the paranasal sinuses or the mastoid cells, into the epidural space.83,84 It is associated with paranasal sinusitis, cranial osteomyelitis, or head trauma or occurs postsurgery. Risk factors for intracranial epidural abscess include prior craniotomy, head injury, sinusitis, otitis media, and mastoiditis.85 The bacteriology of these lesions is analogous to that of brain abscess (see Table 143-1). Generally, the epidural abscess is an indolent lesion when compared to subdural empyema. Neurologic symptoms and complications are rare, because the dura mater protects the brain parenchyma, and the tight adherence of the dura to the skull limits the spread. The most common presenting symptoms are fever, headache, and neurologic signs.83 Untreated, this parameningeal focus can extend intracranially and involve the dural venous sinuses, resulting in septic thrombophlebitis. Gradenigo’s syndrome is a specific presentation of temporal petrous apex infection characterized by ipsilateral facial pain and abducens palsy. CT and MRI demonstrate a hypodense, enhancing lesion. MRI with gadolinium shows a thickened dural surface, which differentiates cranial epidural abscess from sterile collection. The treatment consists of antibiotic therapy, usually in combination with surgical drainage to prevent progression to subdural empyema.86 Drainage of the epidural abscess can be done either by a minimally invasive approach or by a craniotomy with removal of infected bone. A higher rate of recurrence was reported after burr hole drainage.87 In preceding sinusitis, a combination of neurosurgical and ear, nose, and throat specialists is needed, along with instillation of antibiotics combined with systemic antibiotic therapy.
Subdural Empyema
Subdural empyema is a purulent infection located between the dura and the arachnoid, mostly involves the frontal lobe, and is more common among males. It is a neurosurgical emergency, with a mortality rate of 10% to 20%, and more than half of surviving patients have neurologic residua despite intensive neurosurgical care.20,85,88–90 The subdural space is ineffective in containing the extension of the infection because it lacks a fibrin capsule and anatomic barriers; thus, the infection tends to spread rapidly through the subdural space. With its progression, subdural empyema has a tendency to behave like an expanding mass lesion, with associated increased intracranial pressure and cerebral intraparenchymal penetration. Cerebral edema and hydrocephalus also may be present secondary to disruption of blood flow or CSF flow caused by the increased intracranial pressure. Cerebral infarction may be present from thrombosis of the cortical veins or cavernous sinuses or from septic venous thrombosis of contiguous veins in the area of the subdural empyema.
The pathogens are also similar to those of brain abscess (see Table 143-1). In contrast to the epidural infection, the clinical presentation of a subdural empyema is often fulminant, with an acute febrile illness accompanied by headache and followed by rapid neurologic deterioration,88 as well as recent history of sinusitis, otitis media, mastoiditis, meningitis, cranial surgery or trauma, sinus surgery, or pulmonary infection. The median time from the onset of symptoms to diagnosis is 4 days.
CT and MRI with contrast demonstrate a crescent-shaped, hypodense mass lesion on the surface of the brain with medial membrane enhancement, along the falx, or along the tentorium. The appearance is analogous to the subdural hematoma, and occasionally a preexisting subdural hematoma may become infected, blurring these diagnoses.
Treatment consists of emergency surgical drainage of the empyema and any underlying sinusitis, debridement of necrotic tissues, and high doses of systemic antibiotics (see Table 143-2).
Prognosis
The mortality from brain abscess subdural empyema, cranial epidural abscess, and osteomyelitis of the skull has declined markedly over the past 30 years. Alderson et al.91 noted a decrease in overall mortality from 42% in the 5-year period from 1964 through 1968 to 10% during the 5-year period from 1974 through 1978 among patients with brain abscess. Others have observed similar trends with even further reductions in mortality (4% in 47 cases) during the period from 1981 through 1986.58 The most significant influence in the improved outcome of these diseases has been CT or MRI, allowing rapid diagnosis, surgical localization, and detection of postoperative complications.38 In addition, microbiologic advancements have led to enhanced isolation and identification of anaerobes as causal agents, allowing for targeted therapy. More effective antimicrobials against gram-negative and anaerobic organisms and image-guided stereotactic surgery have also contributed considerably to the management outcomes. Despite these advances, the most important predictor of outcome is the patient’s neurologic status at the time of presentation; mortality is highest in those with an altered consciousness3,41,58,83 and rapid progression.57
The clinical course of a patient with a pyogenic brain abscess is unpredictable; in 50% of patients with metastatic abscesses, death occurs within 5 to 14 days of symptoms. The management of these lesions requires an emergent response. When patients with several abscesses are properly managed, their survival rates are comparable to those with solitary lesions.3 Long-term neurologic sequelae are common in survivors, occurring in 30% to 50% of cases.41,57 Seizures and hydrocephalus are common residua. Seizures occur in about 10% of cases during the acute illness, and the subsequent incidence of seizures ranges from 35% to 75%, often starting years after the diagnosis.92
Black P., Graybill J.R., Charache P. Penetration of brain abscess by systemically administered antibiotics. J Neurosurg. 1973;38:705-709.
Carpenter J., Stapleton S., Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1-11.
Cunha B.A. Central nervous system infections in the compromised host: a diagnostic approach. Infect Dis Clin North Am. 2001;15:567-590.
Fertikh D., Krejza J., Cunqueiro A., et al. Discrimination of capsular stage brain abscesses from necrotic or cystic neoplasms using diffusion-weighted magnetic resonance imaging. J Neurosurg. 2007;106:76-81.
Honda H., Warren D.K. Central nervous system infections: meningitis and brain abscess. Infect Dis Clin North Am. 2009;23:609-623.
Kao P.T., Tseng H.K., Liu C.P., et al. Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect. 2003;36:129-136.
Keet P.C. Cranial intradural abscess management of 641 patients during the 35 years from 1952 to 1986. Br J Neurosurg. 1990;4:273-278.
Kupila L., Rantakokko-Jalava K., Jalava J., et al. Aetiological diagnosis of brain abscesses and spinal infections: application of broad range bacterial polymerase chain reaction analysis. J Neurol Neurosurg Psychiatry. 2003;74:728-733.
Lu C.H., Chang W.N., Lin Y.C., et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. QJM. 2002;95:501-509.
Lu C.H., Chang W.N., Lui C.C. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979-985.
Malone D.G., O’Boynick P.L., Ziegler D.K., et al. Osteomyelitis of the skull base. Neurosurgery. 1992;30:426-431.
Mamelak A.N., Mampalam T.J., Obana W.G., Rosenblum M.L. Improved management of multiple brain abscesses: a combined surgical and medical approach. Neurosurgery. 1995;36:76-85. discussion 85-76
Mishra A.M., Reddy S.J., Husain M., et al. Comparison of the magnetization transfer ratio and fluid-attenuated inversion recovery imaging signal intensity in differentiation of various cystic intracranial mass lesions and its correlation with biological parameters. J Magn Reson Imaging. 2006;24:52-56.
Moorthy R.K., Rajshekhar V. Management of brain abscess: an overview. Neurosurg Focus. 2008;24:E3.
Nathoo N., Nadvi S.S., van Dellen J.R., Gouws E. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44:529-535. discussion 535-526
Osenbach R.K., Loftus C.M. Diagnosis and management of brain abscess. Neurosurg Clin N Am. 1992;3:403-420.
Pal D., Bhattacharyya A., Husain M., et al. In vivo proton MR spectroscopy evaluation of pyogenic brain abscesses: a report of 194 cases. AJNR Am J Neuroradiol. 2010;31(2):360-366.
Pradilla G., Ardila G.P., Hsu W., Rigamonti D. Epidural abscesses of the CNS. Lancet Neurol. 2009;8:292-300.
Reisch R., Perneczky A., Filippi R. Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol. 2003;59:223-227.
Roche M., Humphreys H., Smyth E., et al. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Microbiol Infect. 2003;9:803-809.
Rumboldt Z., Marotti M. Magnetization transfer, HASTE, and FLAIR imaging. Magn Reson Imaging Clin N Am. 2003;11:471-492.
Sharma R., Mohandas K., Cooke R.P. Intracranial abscesses: changes in epidemiology and management over five decades in Merseyside. Infection. 2009;37:39-43.
Tseng J.H., Tseng M.Y. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65:557-562. discussion 562
Yilmaz N., Kiymaz N., Yilmaz C., et al. Surgical treatment outcome of subdural empyema: a clinical study. Pediatr Neurosurg. 2006;42:293-298.
Ziai W.C., Lewin J.J.3rd. Update in the diagnosis and management of central nervous system infections. Neurol Clin. 2008;26:427-468. viii
1. Moorthy R.K., Rajshekhar V. Management of brain abscess: an overview. Neurosurg Focus. 2008;24:E3.
2. Kaplan K. Brain abscess. Med Clin North Am. 1985;69:345-360.
3. Mamelak A.N., Mampalam T.J., Obana W.G., Rosenblum M.L. Improved management of multiple brain abscesses: a combined surgical and medical approach. Neurosurgery. 1995;36:76-85. discussion 85-76
4. Wispelwey B., DRJ, Scheld W.M. Brain Abscess. In: Scheld W., Whitley R.J., Durack D.T. Infection of the Central Nervous System. New York: Raven Press; 1991:457-486.
5. Chun C.H., Johnson J.D., Hofstetter M., Raff M.J. Brain abscess: a study of 45 consecutive cases. Medicine (Baltimore). 1986;65:415-431.
6. Yang S.Y. Brain abscess: a review of 400 cases. J Neurosurg. 1981;55:794-799.
7. Mathisen G.E., Johnson J.P. Brain abscess. Clin Infect Dis. 1997;25:763-779. quiz 780-761
8. Patir R., Sood S., Bhatia R. Post-traumatic brain abscess: experience of 36 patients. Br J Neurosurg. 1995;9:29-35.
9. Rish B.L., Caveness W.F., Dillon J.D., et al. Analysis of brain abscess after penetrating craniocerebral injuries in Vietnam. Neurosurgery. 1981;9:535-541.
10. Miller C.F., Brodkey J.S., Colombi B.J. The danger of intracranial wood. Surg Neurol. 1977;7:95-103.
11. Shih T.Y., Kuo Y.L. Development of intracranial complications following transoral stab wounds in children: report of two cases. Pediatr Neurosurg. 2002;37:35-37.
12. Finkelstein R., Engel A., Simri W., Hemli J.A. Brain abscesses: the lung connection. J Intern Med. 1996;240:33-36.
13. Press O.W., Ramsey P.G. Central nervous system infections associated with hereditary hemorrhagic telangiectasia. Am J Med. 1984;77:86-92.
14. Pruitt A.A., Rubin R.H., Karchmer A.W., Duncan G.W. Neurologic complications of bacterial endocarditis. Medicine (Baltimore). 1978;57:329-343.
15. Tunkel A.R., Pradhan S.K. Central nervous system infections in injection drug users. Infect Dis Clin North Am. 2002;16:589-605.
16. Chen S.T., Tang L.M., Ro L.S. Brain abscess as a complication of stroke. Stroke. 1995;26:696-698.
17. Wolansky L.J., Gallagher J.D., Heary R.F., et al. MRI of pituitary abscess: two cases and review of the literature. Neuroradiology. 1997;39:499-503.
18. Henegar M.M., Koby M.B., Silbergeld D.L., et al. Intrasellar abscess following transsphenoidal surgery. Surg Neurol. 1996;45:183-188.
19. Carpenter J., Stapleton S., Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1-11.
20. Honda H., Warren D.K. Central nervous system infections: meningitis and brain abscess. Infect Dis Clin North Am. 2009;23:609-623.
21. Roche M., Humphreys H., Smyth E., et al. A twelve-year review of central nervous system bacterial abscesses: presentation and aetiology. Clin Microbiol Infect. 2003;9:803-809.
22. Kao P.T., Tseng H.K., Liu C.P., et al. Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect. 2003;36:129-136.
23. de Louvois J., Gortavai P., Hurley R. Bacteriology of abscesses of the central nervous system: a multicentre prospective study. Br Med J. 1977;2:981-984.
24. Cheng Y.T., Huang C.T., Leu H.S., et al. Central nervous system infection due to Clostridium septicum: a case report and review of the literature. Infection. 1997;25:171-174.
25. Berenson C.S., Bia F.J. Propionibacterium acnes causes postoperative brain abscesses unassociated with foreign bodies: case reports. Neurosurgery. 1989;25:130-134.
26. Lin YJ, Yang KY, Ho JT, et al. Nocardial brain abscess. J Clin Neurosci 17, 250-253.
27. Renier D., Flandin C., Hirsch E., Hirsch J.F. Brain abscesses in neonates: a study of 30 cases. J Neurosurg. 1988;69:877-882.
28. Grigoriadis E., Gold W.L. Pyogenic brain abscess caused by Streptococcus pneumoniae: case report and review. Clin Infect Dis. 1997;25:1108-1112.
29. Britt R.H., Enzmann D.R. Clinical stages of human brain abscesses on serial CT scans after contrast infusion: computerized tomographic, neuropathological, and clinical correlations. J Neurosurg. 1983;59:972-989.
30. Britt R.H., Enzmann D.R., Yeager A.S. Neuropathological and computerized tomographic findings in experimental brain abscess. J Neurosurg. 1981;55:590-603.
31. Osenbach R.K., Loftus C.M. Diagnosis and management of brain abscess. Neurosurg Clin N Am. 1992;3:403-420.
32. Enzmann D.R., Britt R.R., Obana W.G., et al. Experimental Staphylococcus aureus brain abscess. AJNR Am J Neuroradiol. 1986;7:395-402.
33. Obana W.G., Britt R.H., Placone R.C., et al. Experimental brain abscess development in the chronically immunosuppressed host: computerized tomographic and neuropathological correlations. J Neurosurg. 1986;65:382-391.
34. Lu C.H., Chang W.N., Lin Y.C., et al. Bacterial brain abscess: microbiological features, epidemiological trends and therapeutic outcomes. QJM. 2002;95:501-509.
35. Sharma R., Mohandas K., Cooke R.P. Intracranial abscesses: changes in epidemiology and management over five decades in Merseyside. Infection. 2009;37:39-43.
36. Shaw M.D., Russell J.A. Cerebellar abscess: a review of 47 cases. J Neurol Neurosurg Psychiatry. 1975;38:429-435.
37. Kupila L., Rantakokko-Jalava K., Jalava J., et al. Aetiological diagnosis of brain abscesses and spinal infections: application of broad range bacterial polymerase chain reaction analysis. J Neurol Neurosurg Psychiatry. 2003;74:728-733.
38. Miller E.S., Dias P.S., Uttley D. CT scanning in the management of intracranial abscess: a review of 100 cases. Br J Neurosurg. 1988;2:439-446.
39. Bellotti C., Aragno M.G., Medina M., et al. Differential diagnosis of CT-hypodense cranial lesions with indium-111-oxine–labeled leukocytes. J Neurosurg. 1986;64:750-753.
40. Palestro C.J., Swyer A.J., Kim C.K., et al. Role of in-111 labeled leukocyte scintigraphy in the diagnosis of intracerebral lesions. Clin Nucl Med. 1991;16:305-308.
41. Wispelwey B., S.W. Brain abscess. In: Mandell GL B.J., Dolin R. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 1995:887-900.
42. Rumboldt Z., Marotti M. Magnetization transfer, HASTE, and FLAIR imaging. Magn Reson Imaging Clin N Am. 2003;11:471-492.
43. Taoka T., Yuh W.T., White M.L., et al. Sulcal hyperintensity on fluid-attenuated inversion recovery MR images in patients without apparent cerebrospinal fluid abnormality. AJR Am J Roentgenol. 2001;176:519-524.
44. Mishra A.M., Reddy S.J., Husain M., et al. Comparison of the magnetization transfer ratio and fluid-attenuated inversion recovery imaging signal intensity in differentiation of various cystic intracranial mass lesions and its correlation with biological parameters. J Magn Reson Imaging. 2006;24:52-56.
45. Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q. 1991;7:1-30.
46. Castillo M., Mukherji S.K. Diffusion-weighted imaging in the evaluation of intracranial lesions. Semin Ultrasound CT MR. 2000;21:405-416.
47. Fertikh D., Krejza J., Cunqueiro A., et al. Discrimination of capsular stage brain abscesses from necrotic or cystic neoplasms using diffusion-weighted magnetic resonance imaging. J Neurosurg. 2007;106:76-81.
48. Lai P.H., Li K.T., Hsu S.S., et al. Pyogenic brain abscess: findings from in vivo 1.5-T and 11.7-T in vitro proton MR spectroscopy. AJNR Am J Neuroradiol. 2005;26:279-288.
49. Pal D., Bhattacharyya A., Husain M., et al. In vivo proton MR spectroscopy evaluation of pyogenic brain abscesses: a report of 194 cases. AJNR Am J Neuroradiol. 2010;31(2):360-366.
50. Grand S., Passaro G., Ziegler A., et al. Necrotic tumor versus brain abscess: importance of amino acids detected at 1H MR spectroscopy—initial results. Radiology. 1999;213:785-793.
51. Dev R., Gupta R.K., Poptani H., et al. Role of in vivo proton magnetic resonance spectroscopy in the diagnosis and management of brain abscesses. Neurosurgery. 1998;42:37-42. discussion 42-33
52. Bowen B.C. Proton MR spectroscopy and the ring-enhancing lesion. AJNR Am J Neuroradiol. 1998;19:589-590.
53. Cunha B.A. Central nervous system infections in the compromised host: a diagnostic approach. Infect Dis Clin North Am. 2001;15:567-590.
54. Black P., Graybill J.R., Charache P. Penetration of brain abscess by systemically administered antibiotics. J Neurosurg. 1973;38:705-709.
55. Everett E.D., Strausbaugh L.J. Antimicrobial agents and the central nervous system. Neurosurgery. 1980;6:691-714.
56. Quartey G.R., Johnston J.A., Rozdilsky B. Decadron in the treatment of cerebral abscess: an experimental study. J Neurosurg. 1976;45:301-310.
57. Seydoux C., Francioli P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394-401.
58. Mampalam T.J., Rosenblum M.L. Trends in the management of bacterial brain abscesses: a review of 102 cases over 17 years. Neurosurgery. 1988;23:451-458.
59. Heilpern K.L., Lorber B. Focal intracranial infections. Infect Dis Clin North Am. 1996;10:879-898.
60. Tseng J.H., Tseng M.Y. Brain abscess in 142 patients: factors influencing outcome and mortality. Surg Neurol. 2006;65:557-562. discussion 562
61. Boom W.H., Tuazon C.U. Successful treatment of multiple brain abscesses with antibiotics alone. Rev Infect Dis. 1985;7:189-199.
62. Rousseaux M., Lesoin F., Destee A., et al. Developments in the treatment and prognosis of multiple cerebral abscesses. Neurosurgery. 1985;16:304-308.
63. Adachi J., Uki J., Kazumoto K., Takeda F. Diagnosis of brainstem abscess in the cerebritis stage by magnetic resonance imaging: case report. Neurol Med Chir (Tokyo). 1995;35:467-470.
64. Lu C.H., Chang W.N., Lui C.C. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979-985.
65. Sharma B.S., Khosla V.K., Kak V.K., et al. Multiple pyogenic brain abscesses. Acta Neurochir (Wien). 1995;133:36-43.
66. Shahzadi S., Lozano A.M., Bernstein M., et al. Stereotactic management of bacterial brain abscesses. Can J Neurol Sci. 1996;23:34-39.
67. Dyste G.N., Hitchon P.W., Menezes A.H., et al. Stereotaxic surgery in the treatment of multiple brain abscesses. J Neurosurg. 1988;69:188-194.
68. Strowitzki M., Eymann R., Schleifer J., Steudel W.I. Vertex epidural hematoma with communicating bifrontal subgaleal hematomas treated by percutaneous needle aspiration. Pediatr Neurosurg. 2001;35:1-4.
69. Chacko A.G., Chandy M.J. Diagnostic and staged stereotactic aspiration of multiple bihemispheric pyogenic brain abscesses. Surg Neurol. 1997;48:278-282. discussion 282–273
70. Skrap M., Melatini A., Vassallo A., Sidoti C. Stereotactic aspiration and drainage of brain abscesses: experience with 9 cases. Minim Invasive Neurosurg. 1996;39:108-112.
71. Bavetta S., Paterakis K., Srivatsa S.R., Garvan N. Brainstem abscess: preoperative MRI appearance and survival following stereotactic aspiration. J Neurosurg Sci. 1996;40:139-143.
72. Hellwig D., Bauer B.L., Dauch W.A. Endoscopic stereotactic treatment of brain abscesses. Acta Neurochir Suppl. 1994;61:102-105.
73. Fritsch M., Manwaring K.H. Endoscopic treatment of brain abscess in children. Minim Invasive Neurosurg. 1997;40:103-106.
74. Bidzinski J., Koszewski W. The value of different methods of treatment of brain abscess in the CT era. Acta Neurochir (Wien). 1990;105:117-120.
75. Young R.F., Frazee J. Gas within intracranial abscess cavities: an indication for surgical excision. Ann Neurol. 1984;16:35-39.
76. Reisch R., Perneczky A., Filippi R. Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol. 2003;59:223-227.
77. Zeidman S.M., Geisler F.H., Olivi A. Intraventricular rupture of a purulent brain abscess: case report. Neurosurgery. 1995;36:189-193. discussion 193
78. Isono M., Wakabayashi Y., Nakano T., et al. Treatment of brain abscess associated with ventricular rupture: three case reports. Neurol Med Chir (Tokyo). 1997;37:630-636.
79. Rosenblum M.L., Mampalam T.J., Pons V.G. Controversies in the management of brain abscesses. Clin Neurosurg. 1986;33:603-632.
80. Malone D.G., O’Boynick P.L., Ziegler D.K., et al. Osteomyelitis of the skull base. Neurosurgery. 1992;30:426-431.
81. Seabold J.E., Simonson T.M., Weber P.C., et al. Cranial osteomyelitis: diagnosis and follow-up with In-111 white blood cell and Tc-99m methylene diphosphonate bone SPECT, CT, and MR imaging. Radiology. 1995;196:779-788.
82. Blomstedt G.C. Craniotomy infections. Neurosurg Clin N Am. 1992;3:375-385.
83. Pradilla G., Ardila G.P., Hsu W., Rigamonti D. Epidural abscesses of the CNS. Lancet Neurol. 2009;8:292-300.
84. Kaptan H., Cakiroglu K., Kasimcan O., Kilic C. Bilateral frontal epidural abscess. Neurocirugia (Astur). 2008;19:55-57.
85. Hlavin M.L., Kaminski H.J., Fenstermaker R.A., White R.J. Intracranial suppuration: a modern decade of postoperative subdural empyema and epidural abscess. Neurosurgery. 1994;34:974-980. discussion 980–971
86. Ziai W.C., Lewin J.J.3rd. Update in the diagnosis and management of central nervous system infections. Neurol Clin. 2008;26:427-468. viii
87. Yilmaz N., Kiymaz N., Yilmaz C., et al. Surgical treatment outcome of subdural empyema: a clinical study. Pediatr Neurosurg. 2006;42:293-298.
88. Dill S.R., Cobbs C.G., McDonald C.K. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20:372-386.
89. Nathoo N., Nadvi S.S., van Dellen J.R., Gouws E. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44:529-535. discussion 535–526
90. Keet P.C. Cranial intradural abscess management of 641 patients during the 35 years from 1952 to 1986. Br J Neurosurg. 1990;4:273-278.
91. Alderson D., Strong A.J., Ingham H.R., Selkon J.B. Fifteen-year review of the mortality of brain abscess. Neurosurgery. 1981;8:1-6.
92. Legg N.J., Gupta P.C., Scott D.F. Epilepsy following cerebral abscess: a clinical and EEG study of 70 patients. Brain. 1973;96:259-268.