Chapter 63 Management of sleep-related breathing disorders in children
1 INTRODUCTION
Sleep-related breathing disorders (SRBD) are a common occurrence in children. While such events may be central in etiology, most affected children experience obstructive sleep disorders, caused by anatomic variations and complicated by the diminished pharyngeal muscle tone and neurophysiologic changes that typically accompany sleep. Hyperplasia of the tonsils and adenoid is the most common cause of such obstruction, but infants and toddlers may be affected at a variety of sites in the upper respiratory tract. Causes of SRBD in children may be grouped on the basis of age, simplifying the differential diagnosis for a given patient (Table 63.1). In small children, caliber of the airway may be reduced due to nasal obstruction, compromised skeletal anatomy, hyperplastic pharyngeal or laryngeal soft tissues or neuromuscular compromise. In addition, in older children, obstruction due to obesity has been encountered with increasing frequency over the last two decades.1,2
Table 63.1 Causes of sleep-related breathing disorders in children
| Neonates and infants |
| Nasal aplasia, stenosis, or atresia |
| Nasal or nasopharyngeal masses |
| Craniofacial anomalies: |
| Hypoplastic mandible (Pierre Robin, Nager’s, Treacher Collins) |
| Hypoplastic maxilla (Apert’s, Crouzon’s) |
| Macroglossia (Beckwith–Wiedemann) |
| Vascular malformations of tongue and pharynx |
| Congenital cysts of the vallecula and tongue |
| Neuromuscular disorders |
| Toddlers and older children |
| Rhinitis, nasal polyposis, septal deviation |
| Syndromic narrowing of nasopharynx (Hunter’s, Hurler’s, Down’s, achondroplasia) |
| Adenotonsillar hyperplasia |
| Obesity |
| Macroglossia (Down’s) |
| Vascular malformations of tongue and pharynx |
| Neuromuscular disorders |
| Iatrogenic |
| Nasopharyngeal stenosis |
2 DIAGNOSIS OF SRBD IN CHILDREN
In most children with SRBD, the presenting symptom is stertor, a term used to describe sonorous breathing in the upper airways, including snoring. The prevalence of snoring during sleep has been reported as 3–12% in children,3 although some studies suggest the rate may be as high as 27%.4 Primary snoring is generally not considered a form of SRBD, although some studies do suggest the possibility of associated morbidity such as elevated blood pressure and reduced arterial distensibility.5
In its mildest form, SRBD presents as upper airway resistance syndrome (UARS). Affected children demonstrate episodic arousals resulting from partial obstruction of the upper airway, associated with symptoms of heroic snoring, mouth-breathing, sleep pauses or breath-holding, gasping, perspiration, and enuresis. Daytime manifestations of sleep disturbance include morning headache, dry mouth, halitosis, and most significantly behavioral and neurocognitive disorders.6 Hypersomnolence may occur in older children and adolescents. Other signs and symptoms include audible breathing with open mouth posture, hyponasal speech, and chronic nasal obstruction with or without rhinorrhea. Approximately 40% of children who snore demonstrate more significant degrees of obstruction characteristic of obstructive hypopnea syndrome (OHS) or obstructive sleep apnea syndrome (OSAS) as defined below.6 The most severely affected patients may develop cor pulmonale, right ventricular hypertrophy, congestive heart failure, alveolar hypoventilation, pulmonary hypertension, pulmonary edema, or failure to thrive, and are at risk for permanent neurological damage and even death.
When a history of severe symptoms of sleep disturbance correlates with obvious physical findings of airway obstruction, additional studies to establish a patient’s candidacy for surgical intervention may be superfluous. However, studies suggest that in most cases accurate diagnosis of SRBD cannot be established solely on the basis of a history and physical examination.7 SRBD occurs primarily during REM sleep when children are less likely to be observed by their parents,8 and in many cases of UARS and OHS parents may misinterpret the symptoms only as snoring in the absence of obstruction. In addition, airway dynamics during sleep cannot be determined by static examination in the office setting, and fiberoptic assessment of the airway is often deceptive due to the distorted wide-angle view. Similarly, radiographic assessment of the adenoid tissue and tongue base may be difficult to interpret. In such cases, polysomnography (PSG) remains the gold standard for objective correlation of ventilatory abnormalities with sleep disordered breathing.7 However, the severity of obstruction for which intervention is recommended remains controversial, with most authors advocating aggressive management of children whose Respiratory Distress Index (RDI; obstructive apneas+hypopneas per hour) is between 1 and 5. Furthermore, the expense and scheduling difficulties associated with PSG make this a cumbersome method of assessment in many otolaryngology practices. Other techniques of assessment including audiotaping, videotaping, and home and abbreviated PSG have demonstrated favorable results, but require further study.
3 MANAGEMENT OF SRBD IN CHILDREN
3.1 NON-SURGICAL MANAGEMENT
Treatment of SRBD in children is tailored to the etiology of the airway obstruction. Medical management, such as thioxanthines and methylphenidate, may be useful in cases of central apnea. Pharmacotherapy may also be considered in less severe cases of obstructive apnea, or when surgical intervention does not address the pathology. Examples of such disorders include neonatal rhinitis, allergic rhinitis, and acute tonsillitis. Systemic steroids are effective in reducing lymphoid hyperplasia, but the results are often transient. In cases of chronic upper airway obstruction, positive airway pressure has demonstrated high efficacy in treating SRBD in children; however, the dropout rate is high and the rate and duration of nightly use are relatively low even among compliant children.9 Other alternatives, such as mechanical correction by prostheses, orthodontia, or weight loss, may be worth consideration as well, although these interventions are rarely tolerated in children and are often ineffective. As a result, even in cases of SRBD due to obesity and neuromuscular impairment, surgical management is generally considered as first-line therapy and other modalities are entertained postoperatively to address residual obstruction.
3.2 SURGICAL MANAGEMENT
3.2.1 PREOPERATIVE PLANNING
Preoperative planning is an essential component of the surgical management of patients with SRBD. Such individuals are prone to airway embarrassment during induction of anesthesia, and the surgeon and anesthetist should discuss emergency intervention for the airway should the patient become acutely obstructed. In most cases, dynamic airway collapse may be overcome by bag/mask ventilation used with an oral airway. In patients with severe micrognathia, macroglossia, or lingual tonsil hyperplasia, visualization of the larynx may be compromised. In such cases, the operating team should preoperatively assemble those items necessary for intubation with a Bullard laryngoscope, flexible fiberoptic bronchoscope, or fiberoptic intubation wand, and patients should be intubated under conditions of maximal topical and minimal systemic anesthesia, preferably in the sitting position.
Postoperative respiratory distress is common after surgery for SRBD due to effects of anesthesia, bleeding, edema, and residual airway compromise. Patients at greatest risk include those with severe OSAS, diminished neuromuscular tone (i.e. cerebral palsy), morbid obesity, skeletal and craniofacial abnormalities such as hypoplasia of the midface and/or mandible or nasopharyngeal vault, and very young children (under age 2–3).10,11 As a result, high-risk individuals who are undergoing even routine procedures such as adenotonsillectomy should be admitted to a high-visibility bed in the hospital with continuous cardiac and oxygen saturation monitoring. Intraoperative use of steroids and postoperative placement of nasopharyngeal airways and tongue sutures for anterior traction may reduce the risk of airway compromise after surgery. Narcotics and sedatives should be used sparingly in severely obstructed children. Obese patients and those with reduced neuromuscular tone may benefit from airway support with positive airway pressure. In the most extreme cases, overnight endotracheal intubation may be desirable. If it is suspected that the airway may be difficult to reintubate should obstruction occur after extubation, the tube should be remove in the operating room setting.
3.2.2 NASAL AND NASOPHARYNGEAL OBSTRUCTION
While choanal atresia may be approached by either the transpalatal or the transnasal route, improvements in endoscopic and powered instrumentation have made the transnasal approach the first choice for most otolaryngologists. Our preferred approach is as follows.
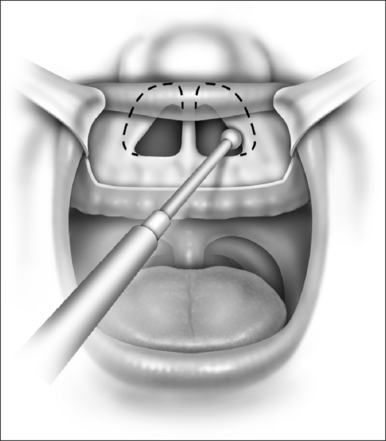
In cases of piriform aperture stenosis, the offending bone may be approached through a sublabial approach (Fig. 63.2), and the aperture is widened using the 2.9 mm round burr at high speed. Stents may be placed as in choanal atresia repair if desired.
Restenosis is the most common complication of these procedures. Success rates (permanent patency) in recent studies are quoted as 78% to 95% with 1–3 year follow-up; number of required revisions is very variable.12,13
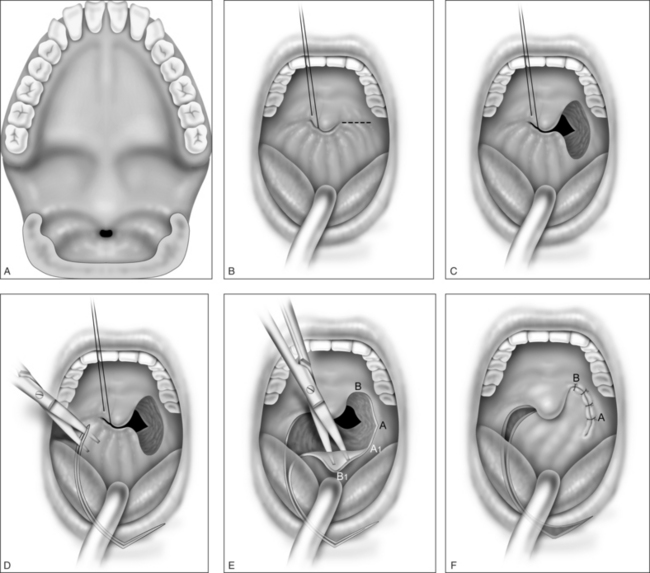
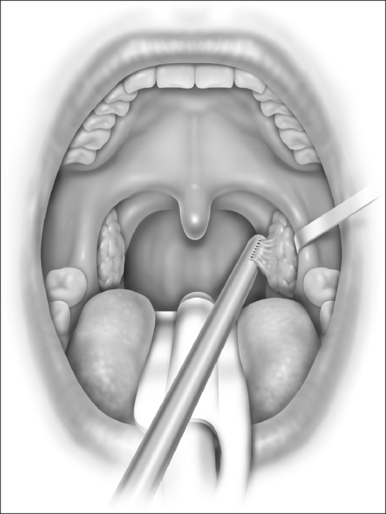
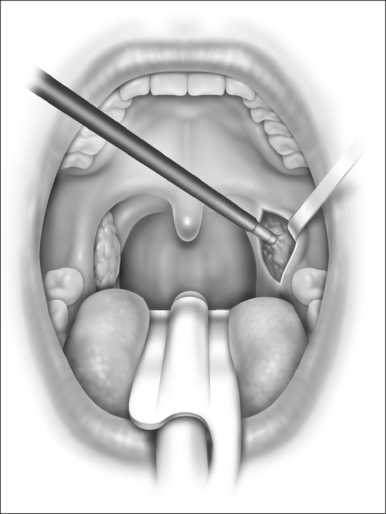
In mild cases, lysis of the scar with some combination of steroid injection, topical application of mitomycin C, and nasopharyngeal stenting may be sufficient to re-establish adequacy of the nasopharyngeal airway. However, frequently simple release of the scarred area results in recurrence, and treatment must therefore include the movement of fresh, well-vascularized tissue to cover the denuded bed. A variety of techniques has been recommended, including Z-plasty, laterally based pharyngeal flaps, other advancement and rotation flaps, and radial forearm and jejunal free flaps. In most cases, the unilateral laterally based pharyngeal flap described by Cotton14 results in an adequate nasopharyngeal airway without restenosis. The procedure is as follows (Fig. 63.3).
3.2.3 ADENOTONSILLAR HYPERPLASIA AND OROPHARYNGEAL OBSTRUCTION
Adenotonsillectomy is generally the first intervention considered for most patients with SRBD, providing they have at least mild adenotonsillar hyperplasia. Improvements in snoring and polysomnography may be anticipated postoperatively in such patients, including those in whom obesity exacerbates their airway obstruction.15–18 Improvement following adenotonsillar surgery has also been demonstrated in children with preoperative enuresis, orthodontic abnormalities, and behavioral issues prior to surgery. Validated surveys suggest an overall improvement in quality of life after adenotonsillectomy.19,20
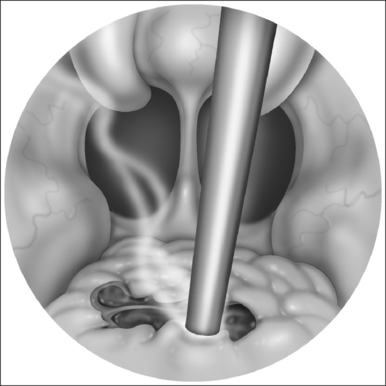
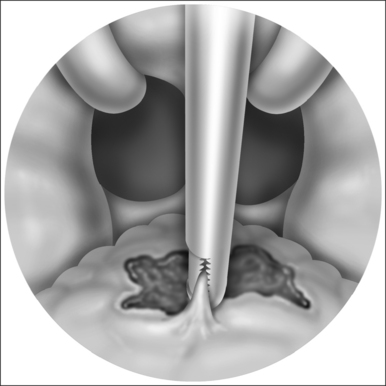
With the report by Krespi and Ling of serial tonsillectomy with CO2 laser in the outpatient setting came the notion that partial tonsillectomy was safe and less painful than traditional tonsillectomy for patients with tonsil hyperplasia.21 It is theorized that the exposure of muscle resulting from removal of the tonsil capsule is the cause of pain associated with tonsillectomy, and that leaving some small portion of the tonsil behind may vastly diminish this sequela.22 Proposed many years ago, the technique had been abandoned due to the risk of tonsil regrowth at a time when most such procedures were performed for recurring infection. Initially studied using the CO2 laser, intracapsular tonsillectomy is now more commonly performed using the microdebrider given its greater efficiency and lower cost (Fig. 63.4). Randomized studies comparing microdebrider intracapsular tonsillectomy to complete electrocautery tonsillectomy generally suggest a modest advantage in pain reduction, particularly otalgia, and return to normal activity; other outcomes yielded less consistent results.23–26 The procedure involves a slight increase in duration and blood loss. Devices for traditional tonsillectomy developed over the last 20 years, such as the harmonic scalpel (Ethicon Endo-Surgery; Cincinnati, OH), radiofrequency devices (Somnoplasty, Somnus Medical Technologies, Sunnyvale, CA), and Coblation® (Fig. 63.5; ArthroCare Corp., Sunnyvale, CA) have been studied in small trials that suggest modest reduction in pain compared with electrocautery, with further decrease in postoperative pain when the tonsil is reduced rather than excised.
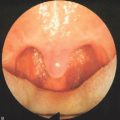
Techniques of adenoidectomy include curettage, suction electrocautery ablation (Fig. 63.6), Coblation® (Fig. 63.7) and removal by power-assisted devices (Fig. 63.8). Traditional curettage is inexpensive but is the least precise of these techniques and is associated with hemorrhage that must be controlled before leaving the operating room. Electrocautery dissection, by definition, is associated with less bleeding and is also a precise and inexpensive device. However, high settings are required on the cautery device, with the potential for thermal injury to deep structures, and surgical times have been variable. Studies of power-assisted (microdebrider) techniques have demonstrated excellent precision with rapid removal of tissue and minimal additional time for cautery, but the disposable blades add significant expense.
Uvulopalatopharyngoplasty is not commonly performed in children, perhaps since most children with sleep apnea do not demonstrate the redundant tissue found in adults with similar symptoms. Several studies have demonstrated that the procedure is efficacious in the most difficult-to-treat patients with SRBD, particularly those with obesity, neurological impairment, or Down syndrome. However, these reports are retrospective, and it remains unclear whether resection of the palate and uvula adds significantly to tonsillectomy with plication of the tonsil pillars. In addition, nasopharyngeal stenosis remains a significant risk when the procedure is performed at the same time as adenoidectomy.
3.2.4 MACROGLOSSIA AND THE PTOTIC TONGUE
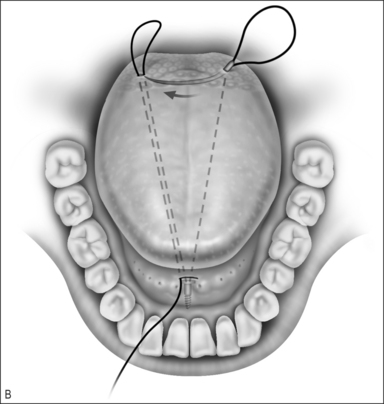
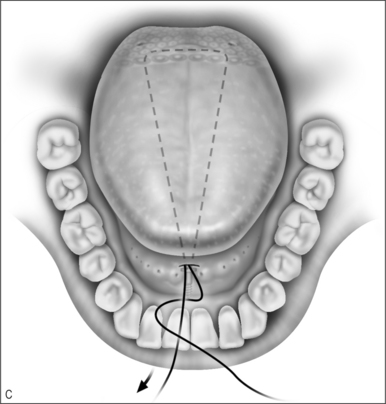
Other methods of managing macroglossia include suture suspension of the tongue, genioglossus advancement, and radiofrequency ablation. The tongue suspension suture technique (Repose®, InfluENT, Concord, NH) in children has been reported only in individual cases, and recently in a series of 16 patients refractory to treatment with adenotonsillectomy.27 The procedure is performed as follows (Fig. 63.9).
Overall success of tongue suspension was reported as 63%, but only 50% among children with Down syndrome.27
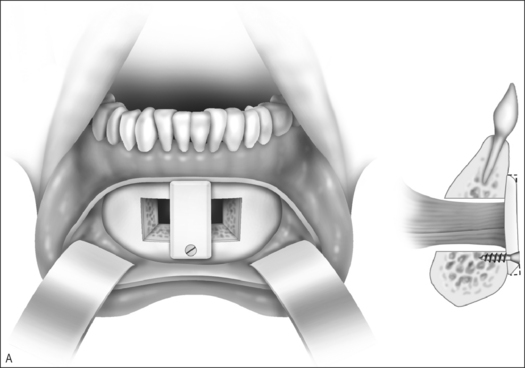
Genioglossus advancement through advancement of the genial tubercle is rarely performed in young children due to the risk of injury to the developing mandibular incisors. However, the procedure may be considered for older children whose mandibles are well developed (Fig. 63.10).
Patients may generally be discharged within 24 hours after surgery. Complications of genioglossus advancement are unusual, but include dental injury, hematoma, and hypesthesia of the teeth and chin. The procedure has not been reported in any large series of children; however, adults usually undergo simultaneous procedures such as uvulopalatopharyngoplasty and hyoid advancement since genioglossus advancement alone will rarely correct severe sleep apnea. When performed with adjunctive procedures, studies demonstrate a widened retrolingual airway, improvement in oxygen saturation, and a 70% reduction in Respiratory Distress Index.28
Radiofrequency ablation of the tongue has not been reported in children except for those with lymphatic malformations.29 In adults with sleep apnea, only modest results have been obtained, and the improvement has been shown to deteriorate over time.30 The device (Somnoplasty; Somnus Medical Technologies, Sunnyvale, CA) uses radio-frequency to create a submucosal coagulum that results in tissue contracture and reduction in tissue volume. The tongue handpiece consists of two prongs with retractable needles. After infiltration of local anesthesia, the handpiece is placed in contact with the mucosa and the needles are then advanced into the muscle. The needles are placed no further than 2 cm from the midline to avoid injury to the neurovascular pedicles. Complications include postoperative edema, abscess formation and tissue necrosis.
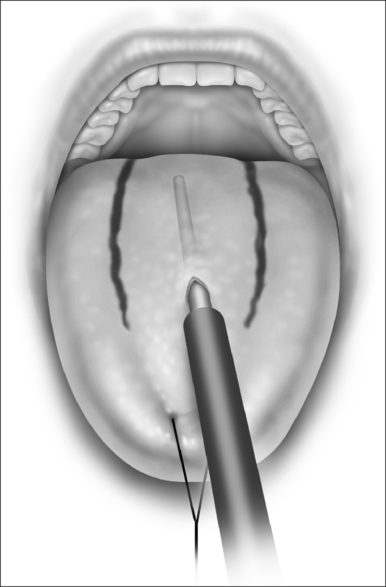
Submucosal minimally invasive lingual excision (SMILE) has recently been described as a novel method of reducing tissue of both the anterior tongue and the tongue base.31 The procedure uses ultrasound and endoscopically guided Coblation® reduction of lingual tissue through a small incision in the anterior tongue (Fig. 63.11).
Vascular malformations of the tongue are generally of lymphatic or venous origin. Microcystic lymphatic malformation causing the tongue to be both wide and thick is extremely difficult to treat. Surgical resection may be performed transorally or transcervically and is tailored to sites of obstruction and dentofacial deformity. Regardless of approach or technique, recurrence is exceedingly common. Limited success has been reported using radiofrequency and Coblation® technology. Many such patients retain a tracheotomy indefinitely. Conversely, lymphatic malformations that are macrocystic may be virtually eliminated by sclerotherapy with OK-432, alcohol, bleomycin, or tetracycline.32 All of these sclerosants are known to cause significant inflammation; hence all patients with involvement of the neck, pharynx, or larynx who are not already tracheotomized should be monitored for postoperative airway obstruction. Venous malformations of the tongue may be reduced considerably using a combination of superficial and intralesional Nd:YAG laser therapy, alcohol sclerosis, and/or excision.33
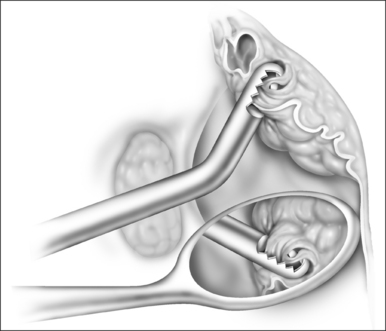
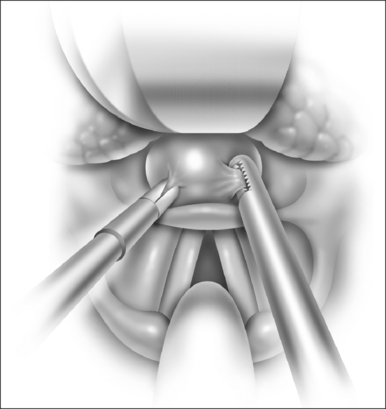
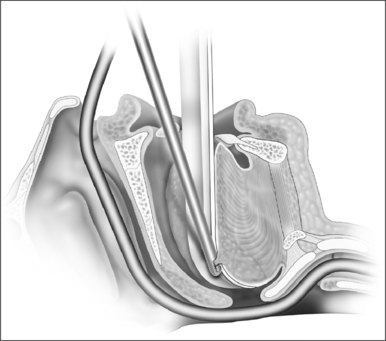
Ductal cysts of the vallecula may present with sleep disordered breathing in neonates. Occasionally, the presentation may also be consistent with laryngomalacia. These lesions are thought to result from mucous gland obstruction, but the etiology has not been definitively elucidated. The diagnosis may be difficult to make endoscopically due to the position of the mass, and lateral radiograph of the upper airway may be useful when the diagnosis is suspected. The lesion is managed surgically as follows (Fig. 63.12).
Patients are monitored overnight on the floor or in the intensive care unit depending on their preoperative degree of obstruction. Two to three doses of steroid are given postoperatively and pain is managed appropriately. A regular diet may be taken. Complications include taste disturbance, scarring or injury to the neurovascular bundle if the dissection is excessive, and bleeding. Patients should be monitored for at least a year for evidence of tissue regrowth. There are no large-scale studies of the efficacy of the procedure in children or adults.
3.2.5 HYPOPLASIA OF THE MIDFACE AND MANDIBLE
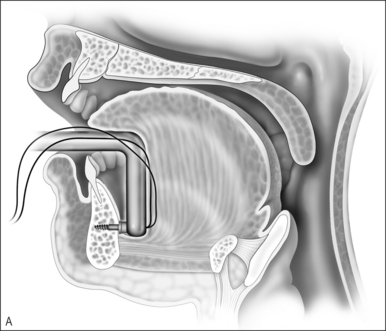
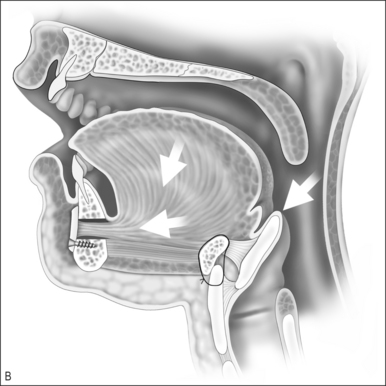
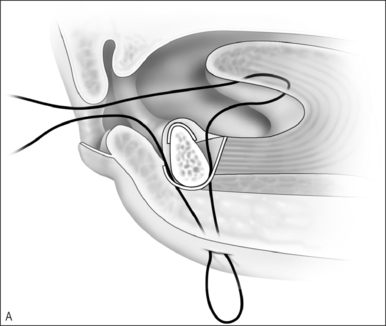
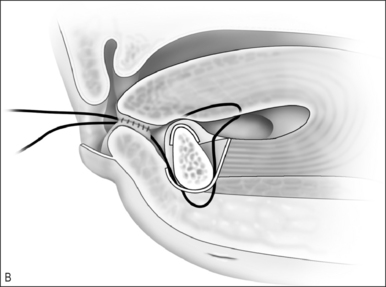
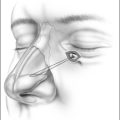
Upper airway obstruction due to hypoplasia of the midface and mandible is usually associated with craniofacial syndromes. Micrognathia due to Pierre Robin sequence often improves within the first 2 years of life without surgical intervention for the mandible. In cases of mild airway obstruction, children with competent caretakers may be managed by prone positioning and nasopharyngeal stenting via nasal trumpet or similar device. When symptoms are more severe, airway obstruction may be relieved by temporary repositioning of the ptotic tongue through labioglossopexy. The surgical technique is as follows (Fig. 63.14).
Postoperative management includes nasogastric feeding to prevent aggressive sucking. The nasopharyngeal airway can be discontinued several days postoperatively and oral feedings are usually attempted in about 1 week. Oral feeding is usually unaffected by the procedure. The child can be discharged home once the airway is deemed adequate and the patient is able to obtain adequate nutrition by mouth. Dehiscence of the adhesion is the most common complication of labioglossopexy, but fewer than 5% require a return to the operating room for revision. Other reported complications include tongue lacerations, Wharton duct injury, wound infection, and scarring. Some authors feel that syndromic patients tend to be more difficult to manage due to numerous anomalies or conditions contributing to airway obstruction and impaired feeding. Kirschner et al.34 had an 83% rate of conversion to tracheostomy in syndromic children.
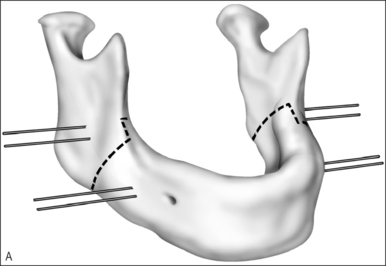
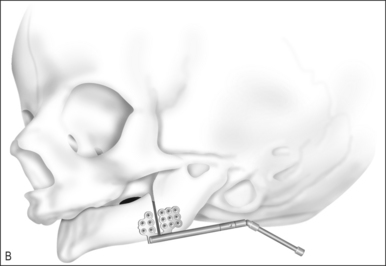
First described for the treatment of limb length discrepancies, osteotomy with distraction of bone is now widely accepted as the procedure of choice in the early management of airway obstruction due to craniofacial disproportion.35–38 The procedure takes advantage of the rapid healing and capacity for growth in the pediatric skeleton. Distraction osteogenesis has been used for over a decade to advance the mandible in cases of retrognathia and micrognathia, but indications have expanded to include neuromuscular disorders. With modifications to the expansion devices, distraction of the midface is also beginning to replace Le Fort osteotomies with bone grafting.37,38
The operative technique is as follows.
Avoidance of or decannulation from tracheotomy in appropriately selected patients undergoing distraction of the mandible is >80%.35–38 Complications include pin site infections, injury to the inferior alveolar and marginal nerves, damage to tooth buds, resorption and ankylosis of the temporomandibular joint, and failure to maintain distraction. Complication rates are difficult to quantify since most series are small and follow-up is limited.
1. O’Brien LM, Sitha S, Baur LA, Waters KA. Obesity increases the risk for persisting obstructive sleep apnea after treatment in children. Int J Pediatr Otorhinolaryngol. 2006;70:1555-1560.
2. Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatr Respir Rev. 2006;7:247-259.
3. Schechter MS. Section on Pediatric Pulmonology. Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69.
4. Owen GO, Canter RJ, Robinson A. Snoring, apnoea, and ENT symptoms in the paediatric community. Clin Otolaryngol. 1996;21:130-134.
5. Kwok KL, Ng DK, Cheung YF. BP and arterial distensibility in children with primary snoring. Chest. 2003;123:1561-1566.
6. Gozal D. Sleep disordered breathing and school performance in children. Pediatrics. 1998;102:616-620.
7. Section on Pediatric Pulmonology. Subcommittee on Obstructive Sleep Apnea Syndrome. Clinical Practice Guideline: Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics. 2002;109:704-712.
8. Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:682-686.
9. Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442-e451.
10. Biavati MJ, Manning SC, Phillips DL. Predictive factors for respiratory complications after tonsillectomy and adenoidectomy in children. Arch Otolaryngol Head Neck Surg. 1997;123:517-521.
11. Rosen GM, Muckle RP, Mahowald MW, et al. Postoperative respiratory compromise in children with obstructive sleep apnea syndrome: can it be anticipated? Pediatrics. 1994;93:784-788.
12. Van Den Abbeele T, Francois M, Narcy P. Transnasal endoscopic treatment of choanal atresia without prolonged stenting. Arch Otolaryngol Head Neck Surg. 2002;128:936-940.
13. Kubba H, Bennett A, Bailey CM. An update on choanal atresia surgery at Great Ormond Street Hospital for Children: preliminary results with Mitomycin C and the KTP laser. Int J Pediatr Otorhinolaryngol. 2004;68:939-945.
14. Cotton RT. Nasopharyngeal stenosis. Arch Otolaryngol. 1995;111:146-148.
15. Suen JS, Arnold JE, Brooks LJ. Adenotonsillectomy for treatment of obstructive sleep apnea in children. Arch Otolaryngol Head Neck Surg. 1995;121:525-530.
16. Mora R, Salami A, Passali FM, et al. OSAS in children. Int J Pediatr Otorhinolaryngol. 2003;67(Suppl 1):S229-S231.
17. Mitchell RB, Kelly J. Outcome of adenotonsillectomy for severe obstructive sleep apnea in children. Int J Pediatr Otorhinolaryngol. 2004;68:1375-1379.
18. Mitchell RB, Kelly J. Adenotonsillectomy for obstructive sleep apnea in obese children. Otolaryngol Head Neck Surg. 2004;131:104-108.
19. Stewart MG, Glaze DG, Friedman EM, et al. Quality of life and sleep study findings after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2005;131:308-334.
20. De Serres LM, Derkay C, Sie K, et al. Impact of adenotonsillectomy on quality of life in children with obstructive sleep disorders. Arch Otolaryngol Head Neck Surg. 2002;128:489-496.
21. Krespi YP, Ling EH. Laser-assisted serial tonsillectomy. J Otolaryngol. 1994;23:325-327.
22. Koltai PJ, Solares CA, Mascha EJ. Xu M. Intracapsular ‘partial’ tonsillectomy for pediatric tonsillar hypertrophy. Laryngoscope. 2002;112(8 Pt 2):17-19.
23. Derkay CS, Darrow DH, Welch C, Sinacori JT. Post-tonsillectomy morbidity and quality of life in pediatric patients with obstructive tonsils and adenoid: microdebrider vs. electrocautery. Otolaryngol Head Neck Surg. 2006;134:114-120.
24. Lister MT, Cunningham MJ, Benjamin B, et al. Microdebrider tonsillotomy vs. electrosurgical tonsillectomy: a randomized, double-blind, paired control study of postoperative pain. Arch Otolaryngol Head Neck Surg. 2006;132:599-604.
25. Mixson CM, Weinberger PM, Austin MB. Comparison of microdebrider subcapsular tonsillectomy to harmonic scalpel and electrocautery total tonsillectomy. Am J Otolaryngol. 2007;28:13-17.
26. Sobol SE, Wetmore RF, Marsh RR, et al. Postoperative recovery after microdebrider intracapsular or monopolar electrocautery tonsillectomy: a prospective, randomized, single-blinded study. Arch Otolaryngol Head Neck Surg. 2006;132:270-274.
27. Shott SR. Suspension or Repose® genioglossus advancement – surgical treatment of OSA beyond T&A. Presented at the Annual Meeting of the American Society of Pediatric Otolaryngology, San Diego, CA, April 2007.
28. Miller FR, Watson D, Boseley M. The role of the Genial Bone Advancement Trephine system in conjunction with uvulopalatopharyngoplasty in the multilevel management of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2004;130:73-79.
29. Grimmer JF, Mulliken JB, Burrows PE, Rahbar R. Radiofrequency ablation of microcystic lymphatic malformation in the oral cavity. Arch Otolaryngol Head Neck Surg. 2006;132:1251-1256.
30. Li KK, Powell NB, Riley RW, Guilleminault C. Temperature-controlled radiofrequency tongue base reduction for sleep disordered breathing: long-term outcomes. Otolaryngol Head Neck Surg. 2002;127:230-234.
31. Maturo SC, Mair EA. Submucosal minimally invasive lingual excision: an effective, novel surgery for pediatric tongue base reduction. Ann Otol Rhinol Laryngol. 2006;115:624-630.
32. Alomari AI, Karian VE, Lord DJ, et al. Percutaneous sclerotherapy for lymphatic malformations: a retrospective analysis of patient-evaluated improvement. J Vasc Interv Radiol. 2006;17:1639-1648.
33. Waner M, Suen JY. Treatment options for the management of vascular malformations. In: Waner M, Suen JY, editors. Hemangiomas and Vascular Malformations of the Head and Neck. New York: Wiley-Liss; 1998:315-347.
34. Kirschner RE, Low DW, Randall P, et al. Surgical airway management in Pierre Robin sequence: is there a role for tongue–lip adhesion? Cleft Palate Craniofac J. 2003;40:13-18.
35. Sidman JD, Sampson D, Templeton B. Distraction osteogenesis of the mandible for airway obstruction in children. Laryngoscope. 2001;111:1137-1146.
36. Imola MJ, Hamlar DD, Thatcher G, Chowdury K. The versatility of distraction osteogenesis in craniofacial surgery. Arch Facial Plast Surg. 2002;4:8-19.
37. Imola MJ, Tatum SA. Craniofacial distraction osteogenesis. Facial Plast Surg Clin North Am. 2002;10:287-301.
38. Mandell DL, Yellon RF, Bradley JP, et al. Mandibular distraction for micrognathia and severe upper airway obstruction. Arch Otolaryngol Head Neck Surg. 2004;130:344-348.