Management of Secondary Jaw Deformities after Maxillofacial Trauma
Posttraumatic Temporomandibular Joint Ankylosis in the Pediatric Population
Background
The treatment of TMJ ankylosis poses challenges to the maxillofacial surgeon as a result of technical difficulties associated with access to the joint, the currently available autogenous and prosthetic TMJ replacement options, and the high incidence of ankylosis recurrence. Failure to restore adequate mandibular opening is likely to result in speech and swallowing impairment; difficulty with mastication; poor oral hygiene and dental neglect; continued facial growth disturbances; and the potential for airway compromise. The surgical management of bony ankylosis of the TMJ requires the complete excision of the involved osseous mass with intraoperative achievement of satisfactory passive mouth opening. The immediate reconstruction with a costochondral rib graft is often carried out, and this is followed by a postoperative physiotherapy regimen to maintain mouth opening. A number of authors have critically evaluated the treatment of TMJ ankylosis in adults, with less emphasis placed on addressing this problem exclusively in the pediatric population.*
In a study by Posnick and colleagues, a consecutive series of nine pediatric patients (mean age, 7.7 years) who underwent a standardized treatment protocol for 13 affected ankylosed temporomandibular joints was reviewed.88 Four patients had unilateral TMJ ankylosis, and five had bilateral ankylosis. One child required bilateral release but only unilateral reconstruction. Radiographic evidence demonstrated bony ankylosis in all 13 operated joints. Two patients had previously undergone surgical intervention of the TMJ. The cause of ankylosis within the study group was primarily traumatic or congenital. The protocol that was followed included complete excision of the involved ankylotic structures with mobilization through coronal scalp and Risdon neck incisions as well as the achievement of wide mouth opening. This was followed by immediate costochondral grafting. Fixation with mini-plates and screws allowed for early mobilization with the rapid institution of a physiotherapy program (Fig. 35-1).
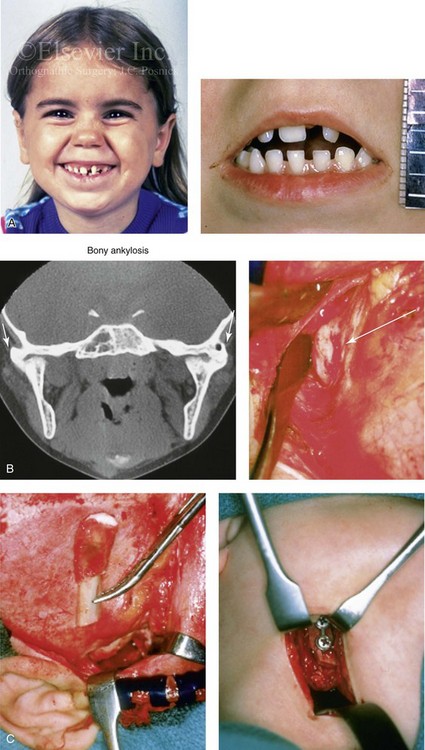
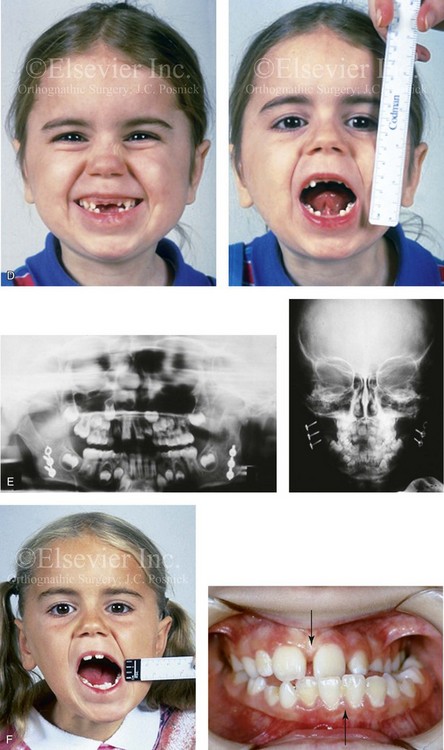
Figure 35-1 A 5-year-old girl was referred to this surgeon after intracapsular condyle fractures of the mandible resulted in bilateral temporomandibular joint ankylosis. These fractures were unrecognized and went untreated at the time of injury 1 year earlier. A, Preoperative frontal views showing maximal opening limited to 4 mm. B, Preoperative computed tomography scan that demonstrates the bony ankylosis of each condyle to its zygomatic arch. Intraoperative view demonstrates ankylosis to the zygomatic arch to the condyle as viewed through the coronal scalp incision. C, Intraoperative view of costochondral graft placement through the coronal scalp incision. Stabilization of the rib graft through Risdon neck incision with the use of a mini-plate and screws. D, Facial views 1 year after reconstruction showing good cranial nerve VII function and satisfactory vertical mouth opening being maintained. E, Postoperative Panorex and anteroposterior facial radiographs. F, Facial view with maximum mouth opening maintained 3 years after reconstruction but with asymmetrical overgrowth of the costochondral grafts. Occlusal view 3 years after reconstruction that shows an asymmetrical Angle class III malocclusion as a result of the overgrowth.
Controversies and Unresolved Issues
Knowing the cause of TMJ ankylosis helps with the understanding of its pathophysiology. In developed countries, the most common etiology is trauma, followed by infection.33,48,121,125,126 Topazian found an association with trauma in 39% and with infection in 43% of patients with TMJ ankylosis (N = 229).119 Since the late 1970s, authors have found trauma to be a more frequent cause than infection, probably because of the use of antibiotics to combat the latter. In a report of a series of patients with TMJ ankylosis, Rajgopal and colleagues concluded that 80% of cases were traumatic in origin.96 Unfortunately, these reviews lumped both pediatric and adult patients together.
Surgical attempts to release TMJ ankylosis have been described dating back to 1850.5 Since then, ankylosis release followed by reconstruction using a variety of alloplastic and autogenous materials has been repoted.9,15,34,46,50,53,62,68,72,79,81,101,114,124,129 Although Gillies first described costochondral grafting for TMJ reconstruction in 1920,30 it was Poswillo93,94 and MacIntosh and Henny53 who popularized its use during the 1970s. Poswillo attempted to demonstrate the histologic and physiologic similarities between the mandibular condyle and rib cartilage in humans.93 MacIntosh and Henny described 26 cases of costochondral grafting for mandibular condyle replacement, with 6 of them being for ankylosis.53 Their approach included graft stabilization with interosseous wires and intermaxillary fixation (IMF) for 6 to 8 weeks. They claimed subsequent growth of the grafts in their pediatric patients, but they failed to provide any objective data.
In 1973, Kennett reported on two cases of unilateral TMJ ankylosis in young patients who underwent the combination of condylectomy, coronoidectomy, and reconstruction with a wired rib graft.45 He concluded that ankylosis in children should be treated as soon as possible and that recurrence is the most frequent complication. In 1986, Munro and colleagues reviewed their series of 18 adult and pediatric patients with TMJ ankylosis and facial deformity.67 Their reconstruction included interosseous wiring of the rib graft and 8 weeks of IMF followed by physiotherapy. They demonstrated better results in unilateral ankylosis cases than in bilateral cases. The next year, Lindqvist and associates described 27 patients of varying ages with TMJ ankylosis; 25 of them underwent costochondral graft reconstruction. Fixation was also by direct interosseous wires, but IMF was reduced to 3.5 weeks to encourage early mobilization and to limit recurrence. The maximum incisor opening improved from 16 mm to 31 mm. In 1987, Politis and others reported satisfactory results in six patients (five adults) with ankylosis.81 They employed a preauricular approach that involved the use of costochondral grafts and either wire or plate and screw fixation followed by 2 or 6 weeks of IMF, respectively. However, 50% of their patients developed seventh cranial nerve palsies. In 1990, Kaban and colleagues described their experience in 14 adult and pediatric patients with TMJ ankylosis and indicated satisfactory results.41,42 The protocol reported by Posnick and colleagues was similar to that of Kaban and colleagues except that the former found that the use of a mini-plate with screw fixation improved stability (as compared with interosseous wire fixation), thereby allowing for the minimal use of IMF and the early initiation of mandibular range of motion with active physiotherapy.88
In 2009, Kaban and colleagues described an updated protocol for the management of TMJ ankylosis exclusively in children.78,79,122,123 They restated their observation that the most common cause of treatment failure was inadequate resection of the ankylotic mass followed by the failure to achieve adequate passive maximum opening in the operating room. Clearly, unless adequate passive mandibular vertical opening is achieved in the operating room, failure will inevitably occur. Unfortunately, this is no guarantee that mouth opening will be maintained. Their seven-step protocol consisted of the following:
1. Complete excision of the fibrous and/or bony ankylotic mass
2. Coronoidectomy of the affected side
3. Coronoidectomy of the contralateral side if steps 1 and 2 do not result in a maximum incisal opening (>35 mm) or opening to the point of dislocation of the unaffected TMJ
4. Lining of the TMJ with either a temporalis myofascial flap or the native disc, if it can be salvaged
5. Reconstruction of the ramus condyle unit with either proximal segment osteotomy and distraction osteogenesis or costochondral graft and plate and screw fixation
6. Early mobilization of the jaws: If distraction osteogenesis is used to reconstruct the ramus condyle unit, mobilization begins the day of operation. In patients who are undergoing costochondral graft reconstruction, mobilization begins after 10 days of IMF.
7. All patients receive rigorous physiotherapy after surgery to maintain mouth opening, generally for at least 3 to 6 months
Observations and Recommendations
Several generalizations can be made about pediatric patients with TMJ ankylosis. First, in first-world countries as in the adult population, trauma remains a prevalent cause, and this is followed closely by congenital issues. In underdeveloped countries, infection continues to be an important causative factor.92 Second, although patients with unilateral ankylosis present with a severely limited preoperative opening, they are more likely to achieve satisfactory long-term functional results. Third, children with bilateral congenital TMJ ankylosis generally attain poor long-term results, despite adequate intraoperative release. This is likely the result of associated masticatory muscle anomalies, neuromuscular discoordination, or longstanding muscular disuse atrophy. Fourth, the use of mini-plate and screw fixation of the graft diminishes the need for immobilization, thereby allowing for the early institution of physiotherapy. Unfortunately, this does not guarantee the maintenance of a satisfactory long-term opening. Fifth, when a costochondral graft is used in children, only several millimeters of cartilage should remain. This is to limit the frequent complication of overgrowth and its complex secondary deformities. In clinical practice, overgrowth after costochondral grafting remains a frequent occurrence (see Chapter 28 and Fig. 35-1). Sixth, for most patients, reconstruction to achieve long-term maxillomandibular harmony must wait until they have achieved skeletal maturity (i.e., 14 to 18 years of age).
Posttraumatic Saddle-Nose Deformity
Background
Nasal injuries are often recognized but then ignored as being unimportant.11,82,86 When a depressed nasal fracture occurs in a child, injury to the growth center leaves the nose prone to a so-called “saddle deformity” with flattening of the osseocartilaginous vault.63 In general, the overlying soft tissues, the upper and lower lateral cartilages, and the nasal lining are distorted but remain intact. Reconstruction of a saddle deformity that involves the bony and cartilaginous dorsum is generally carried out with an autogenous graft (e.g., chondral, costochondral, iliac, split- or full-thickness cranial) (Fig. 35-2).27,28,32,38,60,74,91,117,118,131 The use of an allogenic graft (e.g., porous polypropylene) is always a second choice as a result of a higher incidence of associated infection and extrusion.43 The type of graft material selected (e.g., bone, cartilage, allograft), the method of fixation, the incisions required for access, the extent of dorsum that requires reconstruction, and the timing of treatment are all important details and will vary according to the age of the patient, the extent of the deformity, and the surgeon’s personal preferences (see Chapter 38).
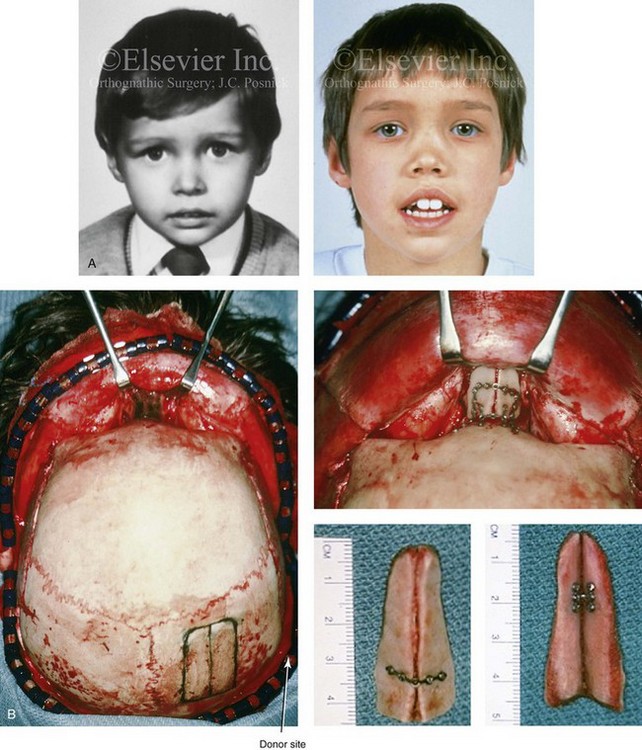
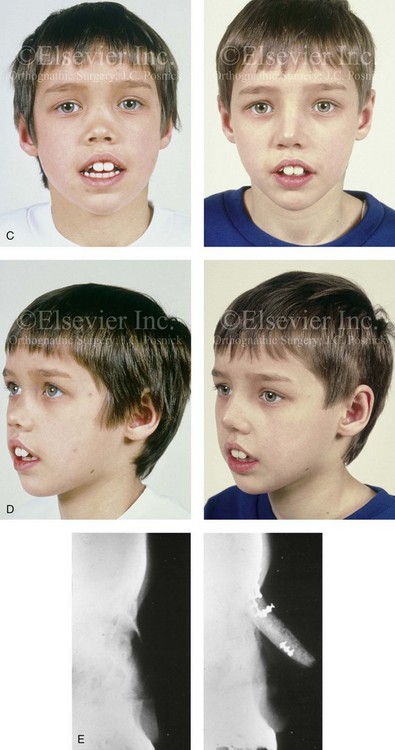
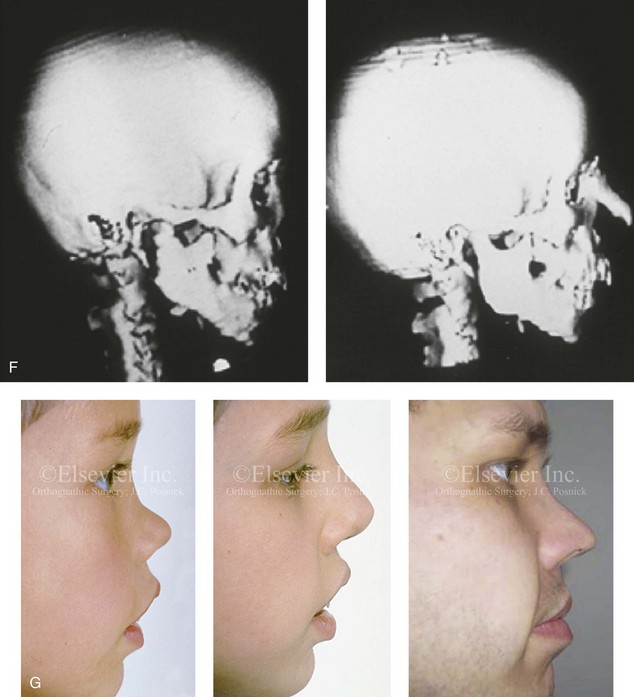
Figure 35-2 A 4-year-old boy fell and hit the bridge of his nose on a hard tabletop, which resulted in a saddle-nose deformity (i.e. osseous and cartilagenous vault). He arrived for evaluation when he was 8 years old and then underwent reconstruction through a coronal scalp incision with nasal osteotomies (in-fracture) and the placement of fixed crafted full-thickness cranial grafts. A, Frontal view at 3 years of age just before injury and at 8 years of age just before surgery. B, Intraoperative views through the coronal scalp incision that show the frontonasal region and the proposed right calvarial donor site. Crafted full-thickness cranial bone grafts are shown before placement for nasal reconstruction. Stabilization is achieved with microplates and screws. A close-up view of the frontonasal region shows the full-thickness cranial bone grafts in place and stabilized with microplates and screws. C, Frontal views before and 6 months after reconstruction. D, Oblique views before and 6 months after reconstruction. E, Lateral radiographs of the nose before and 6 months after reconstruction. F, Computed tomography scan views before and 6 months after reconstruction. G, Close-up lateral view just before surgery, 6 months after treatment, and then 16 years after reconstruction. A, B, C, E, F, from Posnick JC: The role of plate and screw fixation in the management of pediatric facial fractures. In Gruss JS, Manson PM, Yaremchuk MJ, eds: Rigid fixation of the craniomaxillofacial skeleton, Stoneham, Mass, 1992, Butterworth-Heinemann, p 1412.
Involvement of Both the Bone and the Cartilage Vault
The autogenous graft used (e.g., chondral, costochondral, rib, iliac, split- or full-thickness cranial) is contoured to provide reasonable nasal dorsum morphology. When the whole dorsum requires reconstruction (i.e., the radix to the nasal tip), the lower lateral cartilages are sutured over the top of the graft to provide a more natural tip contour and feel (see Fig. 35-2). When a bone graft extends from the radix to the tip, minor degrees of graft resorption at the tip should be anticipated. When correcting the saddle deformity with bone graft, freshening the base of the nasal bones with a rotary drill before graft placement is carried out; the onlay graft will then rest on a bleeding base. The accurately shaped graft is either dovetailed into a bony groove at the nasofrontal process or abutted to the frontal bones while resting evenly on the contoured and freshened)dorsal base to establish the correct nasofrontal angle. Stabilization of the bone graft is either with microplates and screws or transcutaneous Kirschner wires, depending on the access provided by the incisions that are used for the reconstruction. If a coronal scalp incision or a direct vertical nasal incision is used, plate and screw fixation is easily accommodated. An open approach (i.e., a columella splitting incision) should be combined when a coronal scalp incision is used to best manage the lower lateral cartilages and the nasal tip (see Chapter 38 and Fig. 35-2).
Involvement of the Cartilage Vault Only
When the saddle deformity causes collapse of the septal cartilage without injury to the nasal bones, then a cartilage graft reconstruction is preferred. A rib cartilage dorsal strut graft is crafted and placed flush with the nasal bones. The graft then extends caudal to the tip. The tip of the dorsal strut graft is joined to a second rib cartilage graft (caudal strut) that extends from the base of the maxilla to the nasal tip. The caudal strut is stabilized with a short buried Kirschner wire (no. 35 threaded) that is secured to the base of the maxilla/anterior nasal spine region and then pierced into the graft (Fig. 35-3). There is no advantage to harvesting the L-shaped rib cartilage graft as one unit. The separately crafted dorsal strut and caudal strut grafts are then joined together at the tip with non-resorbable suture. The lower lateral cartilages are sutured together and over the grafts to form the new nasal tip. This technique is efficient and results in a “soft” nasal tip that looks and feels natural (see Chapter 38).
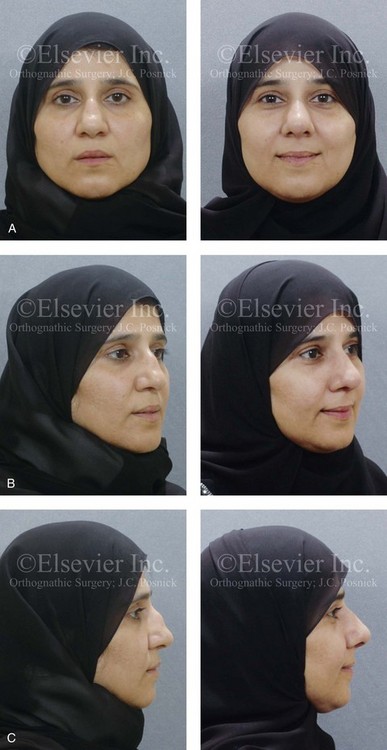
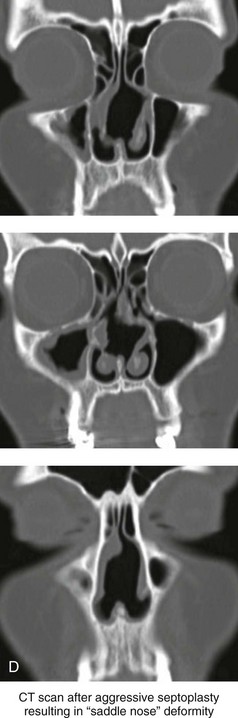
Figure 35-3 A middle-aged woman of Arabic origin with a postsurgical (iatrogenic) saddle-nose deformity. Two years earlier, she underwent an aggressive septoplasty procedure at another institution. This resulted in a large septal perforation with collapse of the cartilaginous vault. She arrived for evaluation and then underwent nasal reconstruction that included rib cartilage grafting (caudal and dorsal struts). A, Frontal views before and after reconstruction. B, Left oblique view before and after reconstruction. C, Profile view before and after reconstruction. D, Computed tomography scan views (coronal cut) of nasal septum confirming the extent of the septal deficits before reconstruction.
Posttraumatic Orthognathic Deformities
Background
Posttraumatic secondary maxillomandibular deformities may occur as a result of inadequate initial anatomic reduction or stabilization and fixation of a jaw fracture or the late resorption and remodeling of the bones resulting in malunion.23–25,35,101,105,111,115,132 The segmental loss of maxillary or mandibular dentoalveolar components may also have occurred, as discussed later in this chapter. In the child, limited growth of the bones after the initial injury (e.g., condylar fracture) or as a result of the surgical intervention carried out to reduce and stabilize the fracture may also cause secondary deformities.58,108,113
Malunion after Midface (Le Fort I) Fracture
An elongated face with an anterior open-bite malocclusion (counterclockwise rotated maxilla and clockwise rotated mandible) can occur when a Le Fort fracture is allowed to heal with inadequate reduction or stabilization at the time of initial management.30,40,87,132 The displaced maxilla often heals inferiorly with counterclockwise rotation. The mandible is forced into a clockwise-rotated position that involves a retrusive pogonion seen in profile. Another common reason for malocclusion after midface fracture is an unrecognized palatal split with secondary arch-form deformity.25,132 Reconstruction requires osteotomies to recreate the midface fractures followed by the surgical repositioning of the maxilla into the correct (preinjury) anatomic location. The uninjured mandible will then counterclockwise rotate to close the anterior open bite, to improve the horizontal projection at the pogonion, and to reduce the vertical height of the lower face.43,87 Another frequent scenario is when a midface fracture occurs during childhood and then results in growth disturbance. At the time of facial growth maturity (i.e., 14 to 18 years of age), maxillary hypoplasia with a skeletal class III malocclusion will be evident (Fig. 35-4).
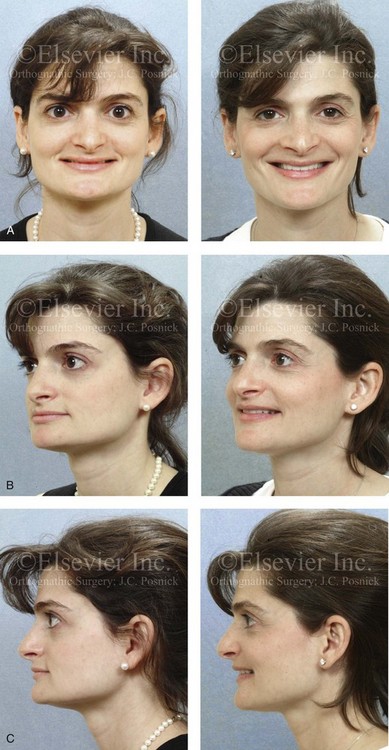
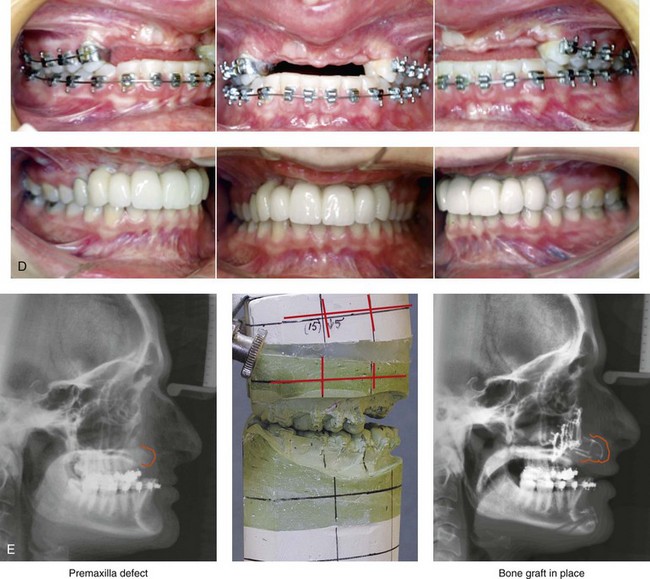
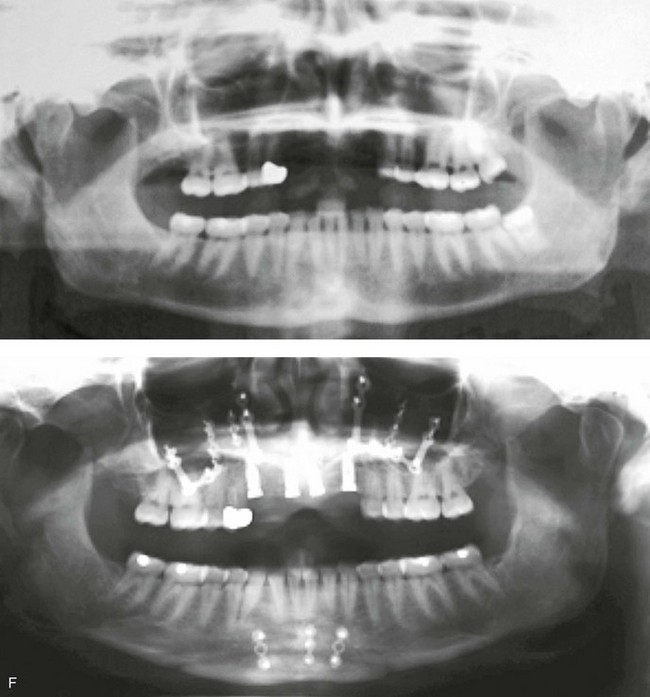
Figure 35-4 In this patient, midface trauma occurred during childhood and included the loss of the anterior teeth and the alveolar bone component. She presented during adulthood with maxillary hypoplasia, Class III malocclusion, and an anterior segmental dentoalveolar defect. Comprehensive reconstruction and dental rehabilitation involved a surgeon, a periodontist, an orthodontist, and a restorative dentist. The patient underwent Le Fort I osteotomy (horizontal advancement and vertical lengthening) with interpositional corticocancellous iliac bone grafting. A corticocancellous (iliac) bloc graft was simultaneously crafted to match the anterior maxillary defect; it was inset and secured with microplates and screws. This was followed 4 months later by the placement of four implants and then final restorations 6 months later. A, Frontal views with smile before and after reconstruction and rehabilitation. B, Oblique views before and after reconstruction and rehabilitation. C, Profile views before and after reconstruction and rehabilitation. D, Occlusal views before and after reconstruction and rehabilitation. E, Lateral cephalometric radiographs and analytic model planning before and after reconstruction. F, Panorex radiographs before and after reconstruction and rehabilitation.
Late Secondary Consequences of a Condylar Fracture
There are many published opposing treatment protocols for the primary management of mandibular condyle fractures.2,10,21,22,31,59,63–66,112,127,133 Despite much discussion, controversy remains regarding which type of condyle fracture should be treated open rather than closed and, if operated, what approach (i.e., incisions for access, extent of condylar fragment degloving, method of fixation, postoperative immobilization and remobilization protocol) is optimal for each specific fracture. In addition, when a condyle injury or fracture occurs in a child, it can easily be overlooked or misdiagnosed, especially if the individual has no other apparent injuries. The child may not even arrive for evaluation; if evaluation does occur, the patient may be seen by a general practitioner rather than a specialist. Radiographs may be limited as a result of the need for sedation or concern by the parents about ionized radiation exposure.49,83,84
Regardless of the primary treatment rendered, a frequently seen pattern of late secondary deformity results when the condylar injury occurs prior the completion of mandibular growth (Figs. 35-5 through 35-10).26,29,69,83–85,87,89,90,95,109,110 Often there is asymmetrical growth of the mandible from that point forward, with ipsilateral mandibular hypoplasia (see Chapter 4). Canting of the mandible (up on the ipsilateral side), shift of the dental midline (toward the fracture), and malocclusion (ipsilateral Class II) are frequently seen. The upper jaw is secondarily affected, growing with vertical asymmetry (canting) and with a shift of the maxillary dental midline toward the side of the fracture.
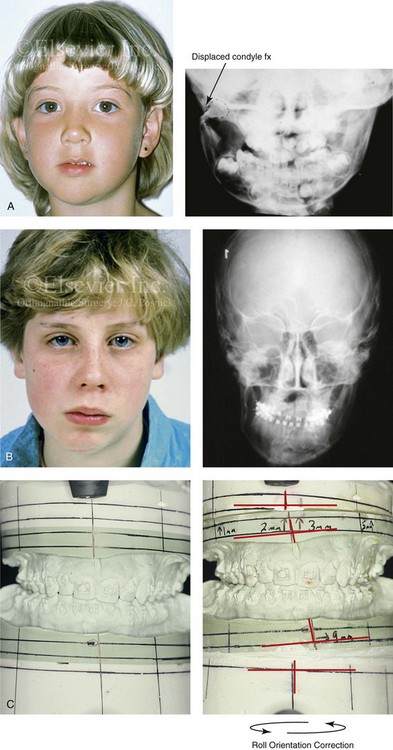
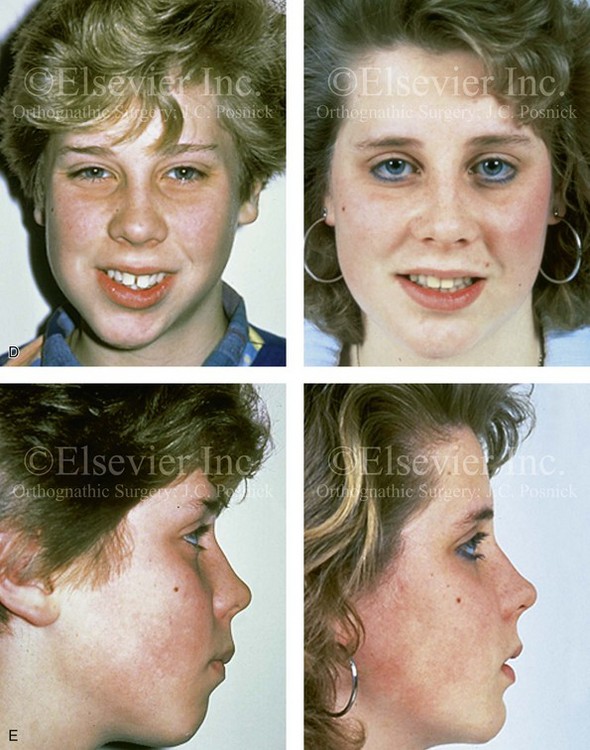
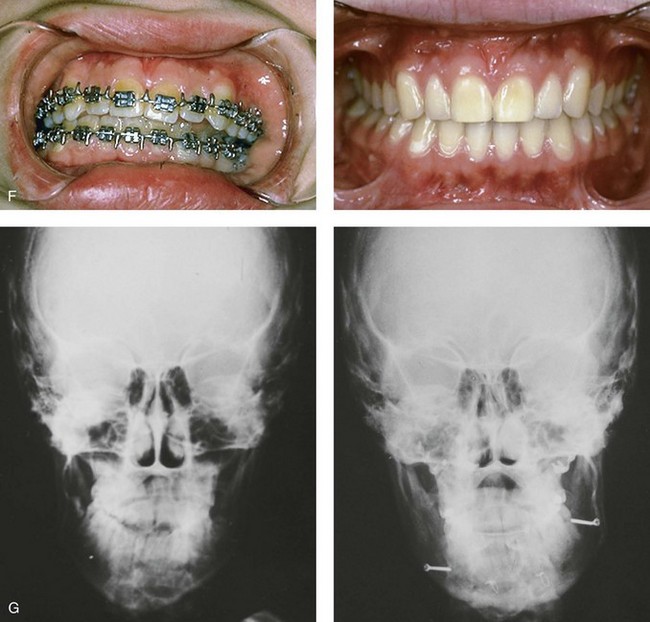
Figure 35-5 A 5-year-old girl sustained a right condyle fracture of the mandible and then presented as a teenager with resulting facial asymmetry that involved the maxilla and the mandible. She then underwent a comprehensive orthodontic and surgical approach that included a Le Fort I osteotomy; bilateral sagittal split ramus osteotomies; and an osseous genioplasty. A, Frontal view at 6 years of age, 1 year after right condyle fracture. The anteroposterior facial radiograph indicates a medially displaced right condyle fracture. B, Frontal view at 15 years of age. An anteroposterior cephalometric radiograph confirms facial asymmetry. C, Articulated dental casts that indicate analytic model planning. D, Frontal views before and 2 years after reconstruction. E, Profile views before and 2 years after reconstruction. F, Occlusal views before and 2 years after reconstruction. G, Panorex radiographs before and after reconstruction. H, Anteroposterior cephalometric radiographs before and after reconstruction.

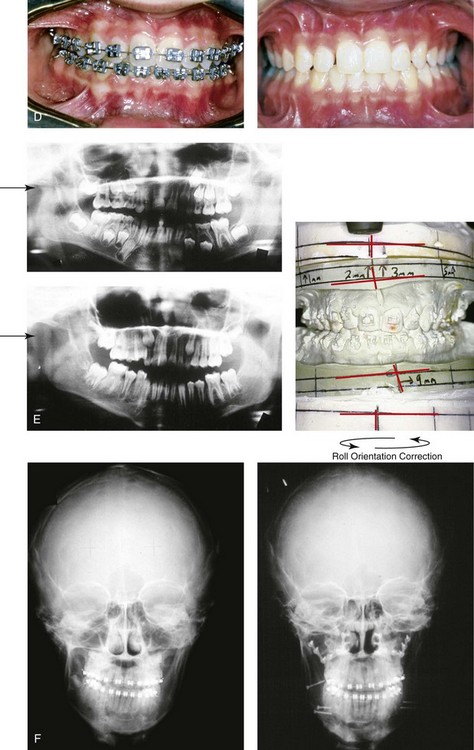
Figure 35-6 A 15-year-old boy who had sustained a fracture of the right condyle of the mandible during early childhood presented with facial asymmetry that involved the maxilla and the mandible. He underwent a comprehensive orthodontic and surgical approach that included a Le Fort I osteotomy; bilateral sagittal split ramus osteotomies; and an osseous genioplasty. A, Frontal view in repose before and 2 years after reconstruction. B, Frontal view with smile before and 2 years after reconstruction. C, Profile view before and 2 years after reconstruction. D, Occlusal view before and 3 years after reconstruction. E, Longitudinal Panorex radiographs before surgery. Articulated dental casts that indicate analytic model planning. F, Anteroposterior cephalometric radiographs before and after reconstruction.
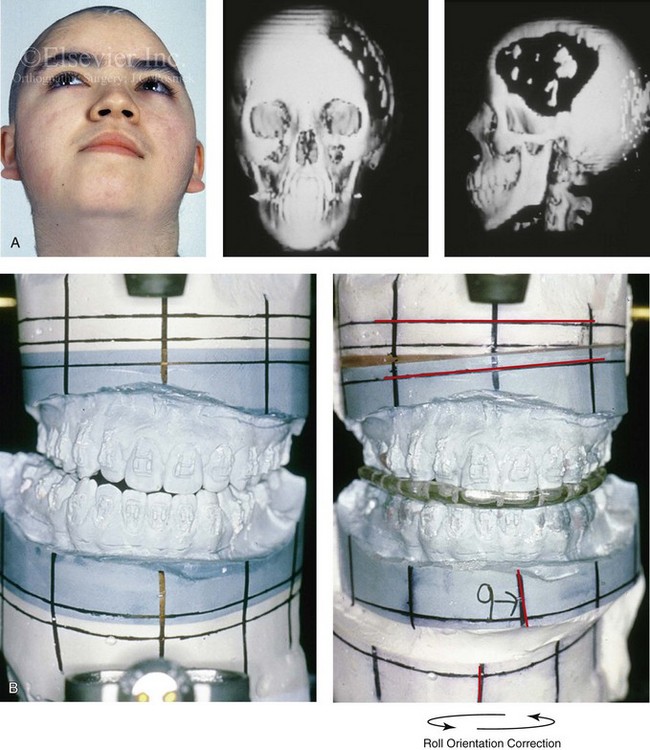
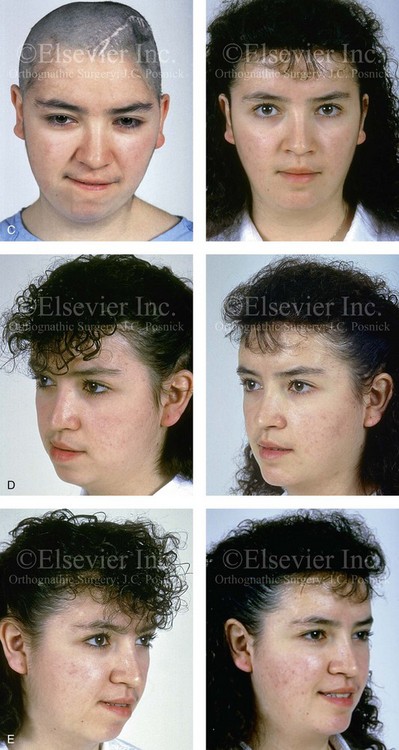
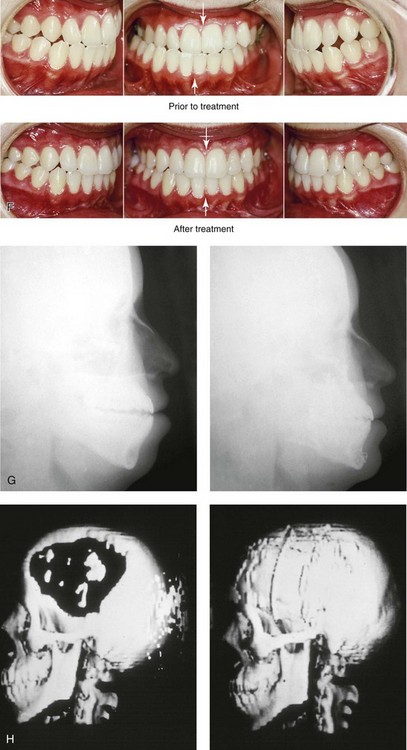
Figure 35-7 A 14-year-old girl sustained left cranial vault and left facial trauma. She required cranial debridement that resulted in a large, full-thickness frontotemporal skull defect. She developed a lower-face deformity that was characterized as an asymmetrical mandibular excess, likely as a result of the traumatic stimulation of the left condyle. A secondary maxillary deformity also occurred (i.e., canting). She was then referred for reconstruction. A, Worm’s-eye facial view before reconstruction. Three-dimensional computed tomography scan views that indicate the extent of skull defect and jaw asymmetry. B, Articulated dental casts that indicate analytic model planning. C, Frontal view before and after the reconstruction of the skull defect and the orthognathic surgery that included Le Fort I osteotomy, bilateral sagittal split ramus osteotomies, and an osseous genioplasty. D, Right oblique views before and after jaw reconstruction. E, Left oblique views before and after reconstruction. F, Occlusal views before and after reconstruction. G, Lateral cephalometric radiographs before and after reconstruction. H, Three-dimensional computed tomography scan views before and after cranial vault reconstruction. A (right), C (left), H, from Posnick JC: The role of plate and screw fixation in the management of pediatric facial fractures. In Gruss JS, Manson PM, Yaremchuk MJ, eds: Rigid fixation of the craniomaxillofacial skeleton, Stoneham, Mass, 1992, Butterworth-Heinemann, p 416.
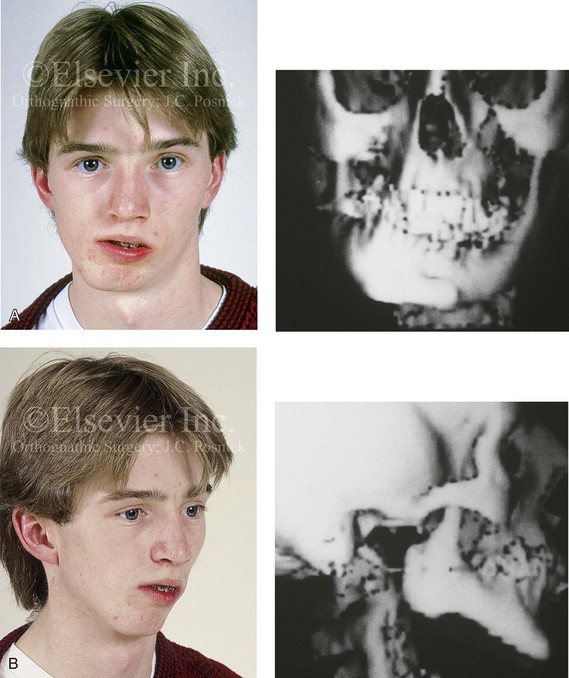
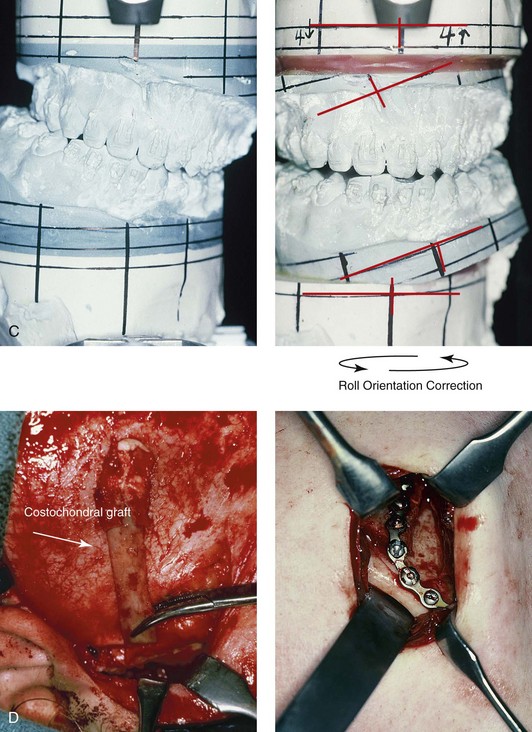

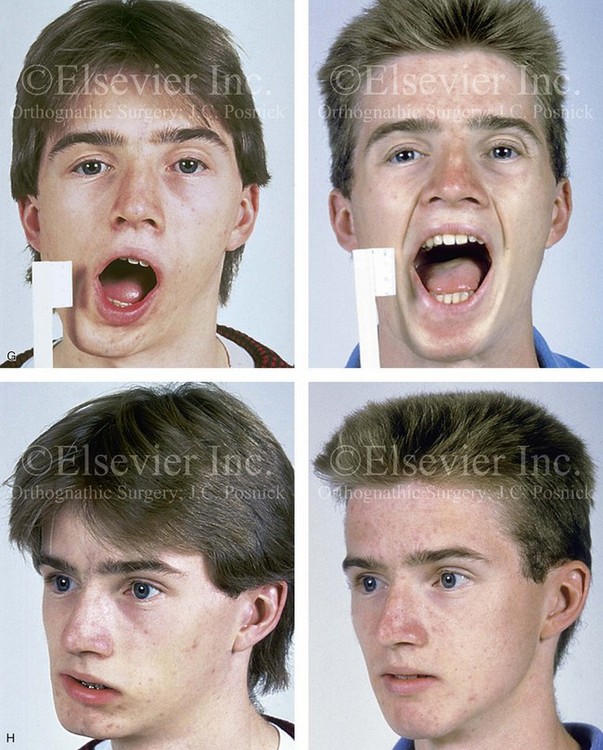
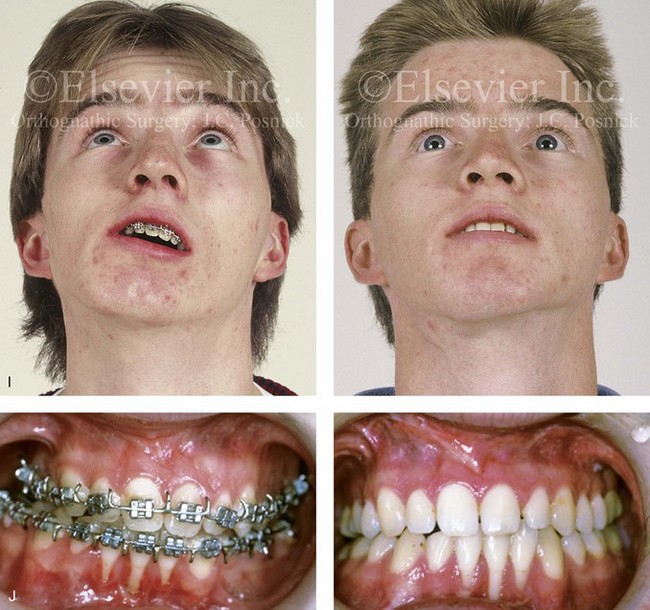
Figure 35-8 A 16-year-old boy who had sustained a fracture of the right condyle of the mandible during early childhood presented with resorption of the condylar fragment and resulting facial asymmetry. He previously underwent several years of compensating orthodontics to neutralize the occlusion, which resulted in the loss of labial bone along the anterior mandibular teeth. He then underwent a comprehensive orthodontic and orthognathic surgical approach that included Le Fort I osteotomy, left sagittal split ramus osteotomy, reconstruction of the right condyle–ascending ramus with a costochondral graft, and osseous genioplasty. A, Frontal facial view in repose before surgery. The computed tomography scan demonstrates facial asymmetry that affects the maxilla and the mandible. B, Oblique facial view before surgery. The computed tomography scan demonstrates an absent right condyle. C, Articulated dental casts that indicate analytic model planning. D, Costochondral graft is inserted through coronal scalp incision. The stabilization of the graft involves an extended mini-plate and screws placed through a Risdon neck incision. E, Frontal views in repose before and after reconstruction. F, Frontal views with smile before and after reconstruction. G, Maximum vertical mouth opening before and after reconstruction. H, Oblique views before and after reconstruction. I, Worm’s-eye views before and after reconstruction. J, Occlusal views before and after reconstruction. Note the recession along the labial aspects of the mandibular incisors both before and after surgery. Gingival grafting is indicated. A (right), B (right), C, F, G, I, from Posnick JC: Management of facial fractures in children and adolescents, Ann Plast Surg 33:442-457, 1994.


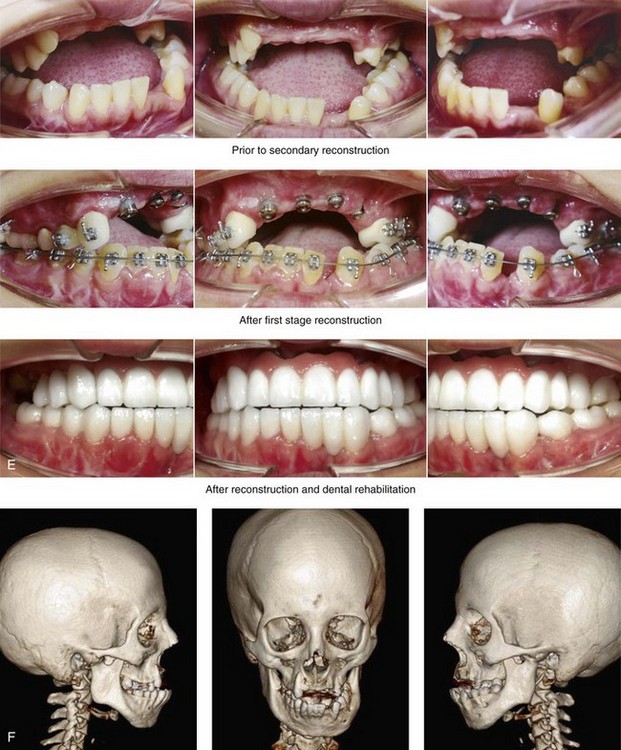
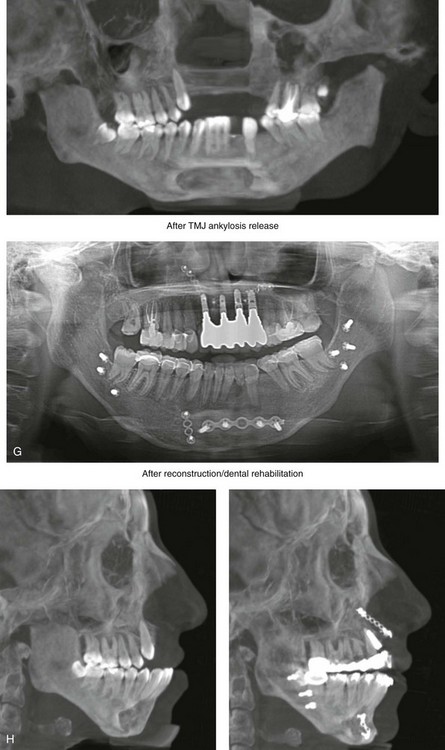
Figure 35-9 When she was 16 years of age, this patient fell from a height of 6 meters and sustained maxillofacial and lower extremity trauma. Her maxillofacial injuries included a displaced left condyle fracture, a displaced right condyle fracture, a comminuted left anterior mandibular fracture with the loss of teeth, and left anterior maxillary dentoalveolar fractures. She was treated at another institution with tracheostomy, open reduction and internal fixation of the comminuted anterior mandibular fractures, debridement of the anterior maxilla and the associated teeth, and closed reduction of the bilateral condyle fractures. The lower extremity fractures required the placement of left and right femoral rods and open reduction and internal fixation of the right ankle and foot fractures. The patient underwent several months of intermaxillary fixation. With the release of the fixation, radiographs confirmed bilateral temporomandibular joint (TMJ) ankylosis. One year later, the patient was taken to the operating room for the removal of the internal fixation of the left anterior mandible.
She was referred to this surgeon when she was 20 years old for secondary reconstruction. She was found to have bilateral TMJ ankylosis, malunion and displacement of the mandible, anterior maxillary dentoalveolar defects, the loss of multiple teeth in both arches, and impacted wisdom teeth. She underwent a comprehensive assessment that also included the evaluation of her dental rehabilitative needs. An orthodontist and a prosthodontist were consulted, and perioperative orthodontic treatment was instituted. A, Frontal views in repose before and after reconstruction and dental rehabilitation. B, Frontal views with smile before and after reconstruction and dental rehabilitation.
Staged maxillofacial reconstruction included the following:
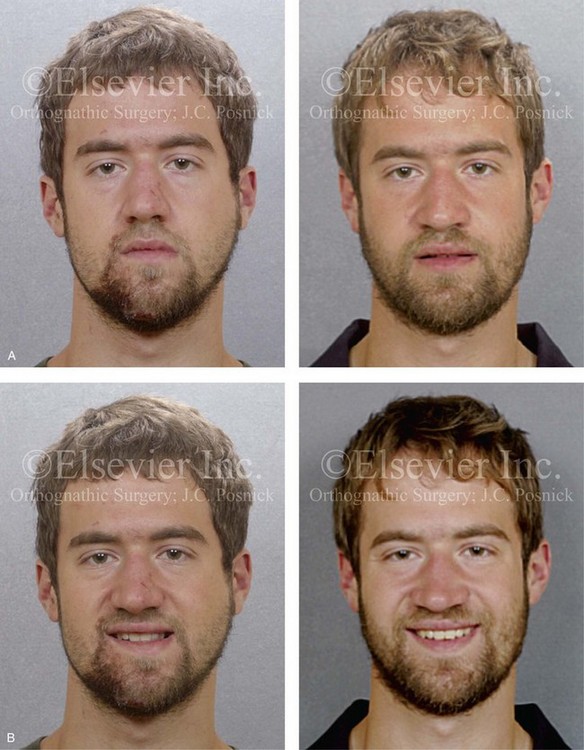
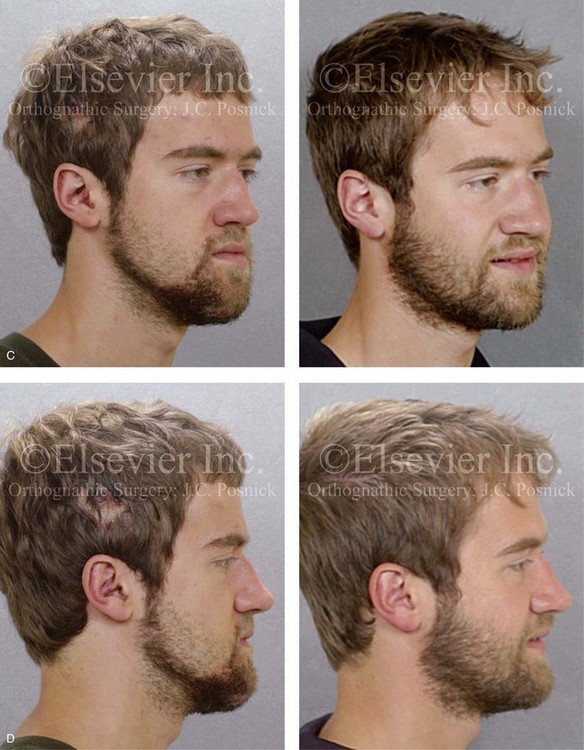
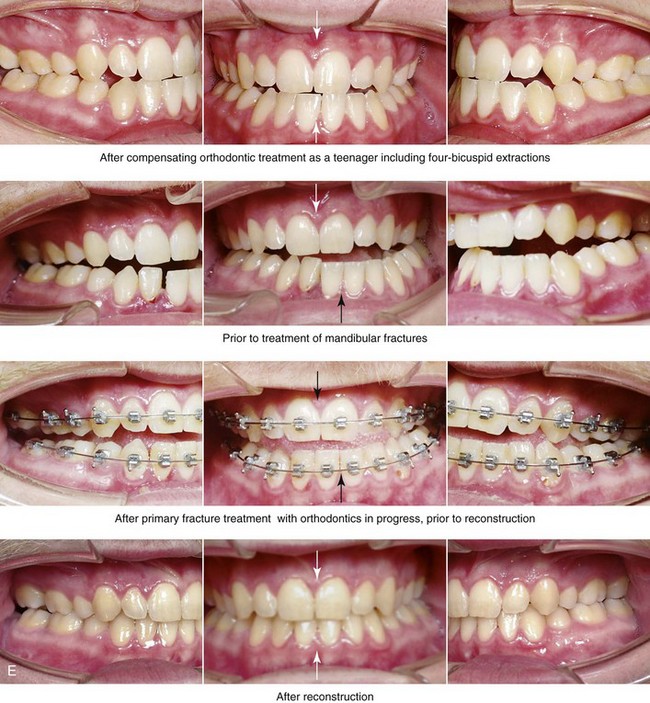
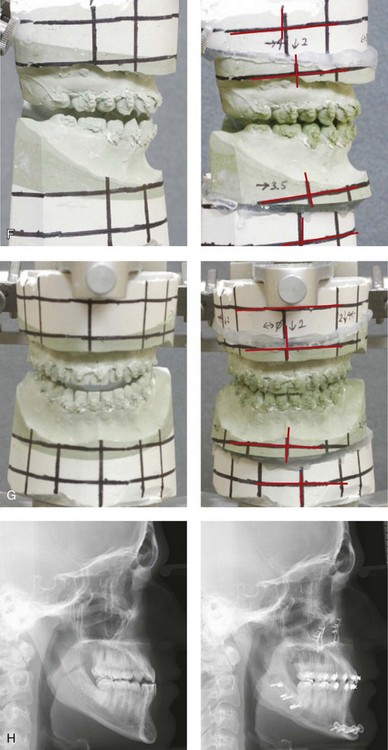
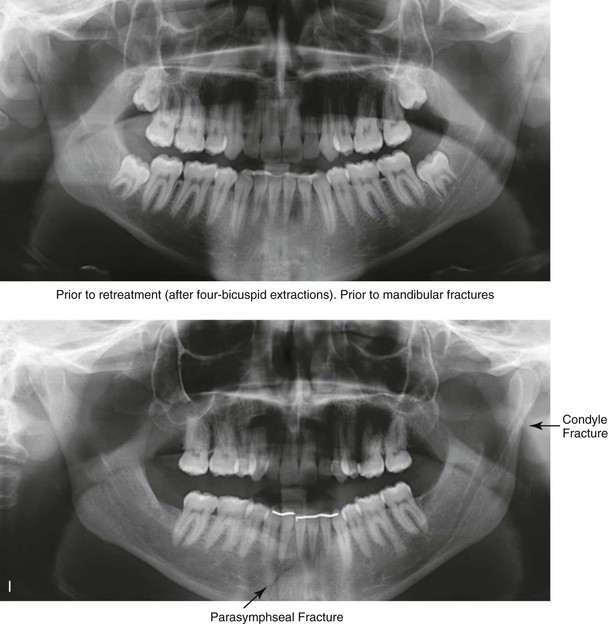
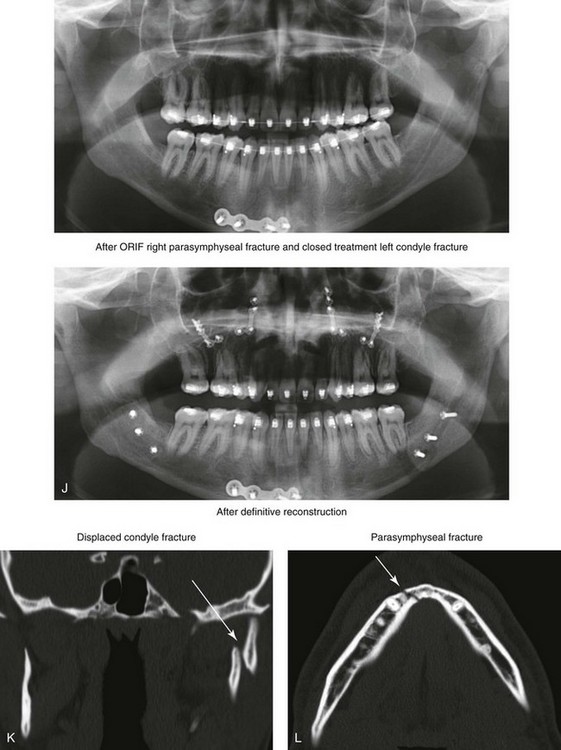
Figure 35-10 A recent college graduate was referred by his orthodontist for a surgical consult. He was known to have a developmental jaw deformity that was characterized as an asymmetric mandibular excess growth pattern in combination with maxillary deficiency. He had undergone orthodontic mechanics earlier during his life in an attempt to modify growth and then to straighten the teeth; this included four bicuspid extractions. An asymmetric Angle Class III negative overjet malocclusion remained. The patient had a lifelong history of chronic obstructive nasal breathing that was unresponsive to nasal sprays. At this time, he made a personal decision to proceed with orthognathic surgical reconstruction. Just 1 week after the surgical consult, he was assaulted while walking on a city street. Maxillofacial injuries included a displaced right parasymphyseal fracture and a displaced left condyle fracture. A, Facial views in repose before and after reconstruction. B, Facial views with smile before and after reconstruction. This further altered the occlusion and involved a shift of the mandible to the left and a step-off between the right central and lateral incisors. The fractures were treated with the placement of surgical arch wires; open reduction and internal fixation of the right parasymphyseal fracture; and closed treatment of the left condyle fracture. Four weeks later, the patient was converted to orthodontic appliances, and orthodontic treatment continued in preparation for jaw reconstruction. Six months after the maxillofacial injuries and with orthodontic preparation complete, he underwent definitive jaw and intranasal surgery to improve the airway, occlusion, and facial morphology. The patient’s procedures included maxillary Le Fort I osteotomy (vertical lengthening and horizontal advancement); bilateral sagittal split osteotomies of the mandible (lengthening of the left posterior facial height and horizontal advancement); septoplasty; and the reduction of the inferior turbinates. Orthodontic maintenance and detailing continued until the appliances were removed 6 months after surgery. C, Oblique facial views before and after reconstruction. D, Profile views before and after reconstruction. E, Occlusal views before retreatment, after the mandibular fractures, after primary fracture treatment with orthodontics in progress, and after the completion of the reconstruction. F and G, Articulated dental casts that indicate analytic model planning. H, Lateral cephalometric radiographs before and after reconstruction. I and J, Panorex radiographs taken before any surgery; after the mandibular fractures were sustained; after treatment of the mandibular fractures but before orthognathic surgery; and after orthognathic surgery. K and L, Computed tomography scans that indicate the locations of the mandibular fractures (left condyle and right parasymphysis) before treatment.
When facial growth is complete, orthodontic alignment to eliminate dental compensations in combination with orthognathic surgery (Le Fort I osteotomy, sagittal ramus osteotomies of the mandible, and osseous genioplasty) will allow three-dimensional repositioning of the jaws to improve facial symmetry, to restore Euclidian proportions, and to correct the malocclusion (see Figs. 35-5 and 35-7). This classic posttraumatic dentofacial deformity and orthognathic approach to reconstruction assumes a stable posterior stop when seating the ipsilateral condyle, adequate mouth opening, and minimal TMD.
Orthognathic surgery is not preferred for patients with significant mandibular hypomobility. Limited mouth opening makes intraoral surgery difficult; in some cases, it may worsen when the cicatricial effects of a surgical wound are added.14,20,70,77,106 If feasible, the hypomobility is resolved before the jaw deformity is definitively addressed (see Fig. 35-9).
When considering the timing of orthognathic surgery for a patient after a condyle fracture, another essential aspect is to wait until the temporomandibular articulation has stabilized. Becking and colleagues reported stable orthognathic results in their patients who had sustained displaced condylar fracture with a loss of posterior facial height.3 They postponed definitive orthognathic treatment until at least 9 months after the initial injury. In clinical practice, each of these patients should be considered individually with regard to the stability of the temporomandibular articulation. In general, I like to allow 6 months of healing before proceeding with definitive reconstruction (see Fig. 35-10). Unfortunately, no one test or radiographic study can reliably answer the question of condylar stability (see Chapter 4).
When a condylar neck fracture heals with resorption of the entire proximal condylar fragment, not only is there significant loss of ipsilateral posterior facial height but there is also an unstable posterior stop. Correction will require the construction of a neocondyle.15,37,114,124,128,129 In this case, as long as there is satisfactory mouth opening, use a costochondral graft for reconstruction is preferred (see Chapter 28).4,9,51,54,55,72,75,97,107 If the initial fracture occurred during childhood and was followed by condylar resorption, then additional secondary deformities of the maxilla and chin will have also occurred during growth. In addition to reconstruction of the ipsilateral mandible with a costochondral graft, surgical correction of the maxilla above and the chin below is often required. This includes use of Le Fort I osteotomy (primarily for cant and dental midline correction); contralateral sagittal split ramus osteotomy (asymmetry correction); and osseous genioplasty7 (see Fig. 35-8).
When a displaced condylar fracture occurs in an adult and healing involves the loss of the ipsilateral posterior facial height, a predictable shift in the occlusion is also expected (Figs. 35-10 through 35-15). Depending on the individual’s masticatory musculature and condylar (TMJ) adaptations, a significant centric relation–centric occlusion discrepancy may have occurred.52,130,134 This can result in difficulty with chewing, swallowing, speech, secondary occlusal trauma, symptomatic TMD, and significant facial asymmetry. If so—and assuming satisfactory mandibular mobility (i.e., vertical mouth opening)—standard sagittal split ramus osteotomies can generally be carried out to reestablish the following: 1) the ipsilateral posterior facial height; 2) a consistent preinjury occlusion; 3) the restoration of facial symmetry; and 4) the relief of TMD (see Chapter 4 and Figs. 35-10 through 35-15). In most cases, after the individual sustains a displaced condyle fracture, the combination of condylar remodeling and masticatory muscle adaptation will result in a manageable occlusion and an adequate range of motion without the need for secondary reconstruction (see Fig. 35-15).
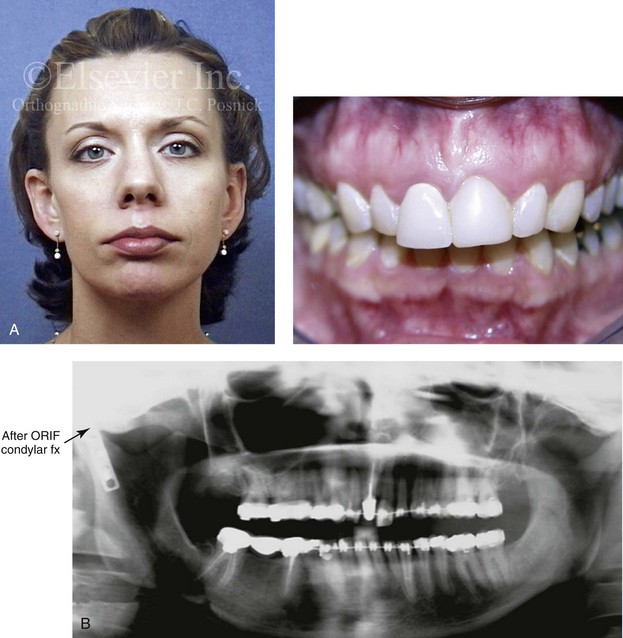
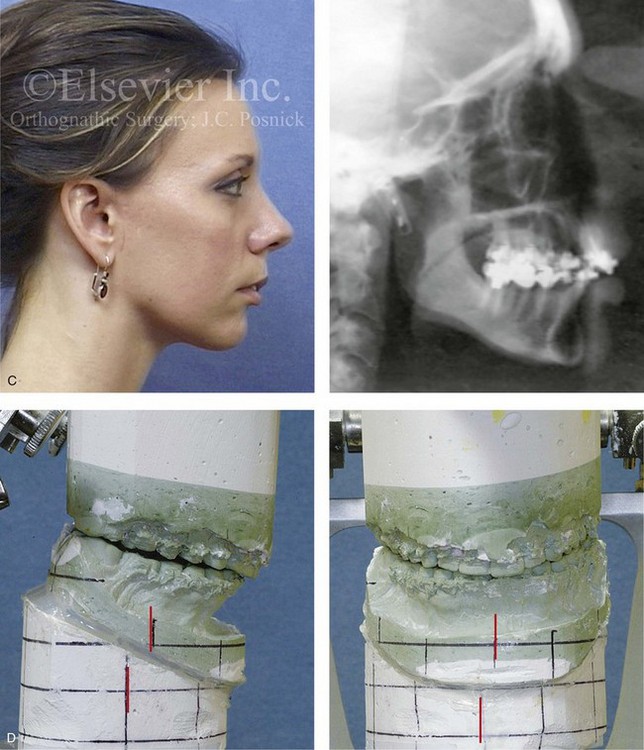
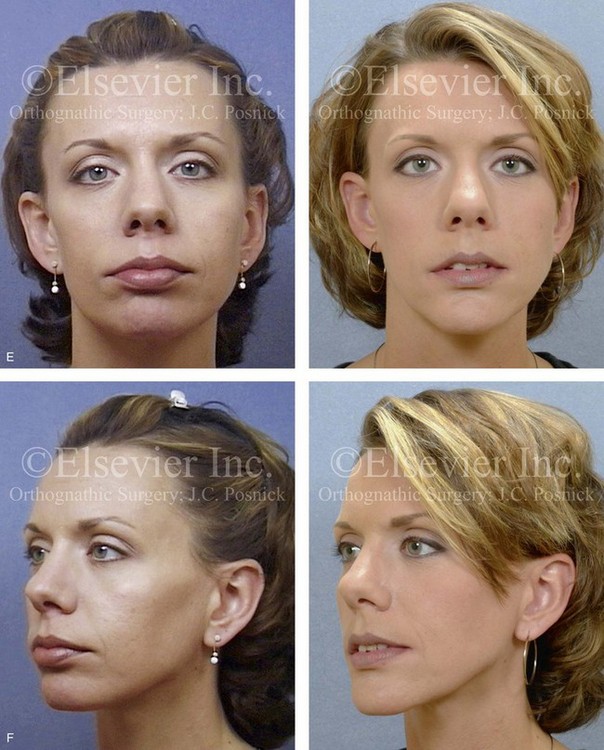
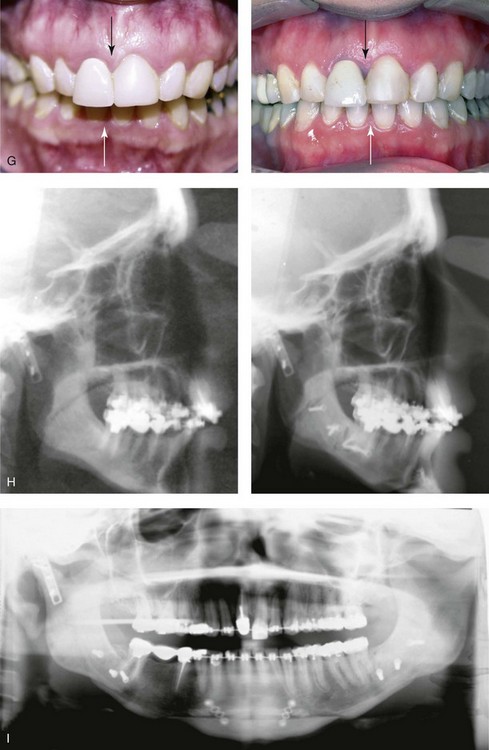
Figure 35-11 A woman in her late 20s was in a bicycle accident and sustained a displaced right condylar fracture and anterior maxillary dentoalveolar trauma. She underwent open reduction and internal fixation of the right condyle fracture with plate and screw fixation. There was partial necrosis of the proximal (condylar) segment with a loss of posterior facial height. This resulted in a shift of the mandible on the ipsilateral side. There was a significant centric relation–centric occlusion discrepancy; the patient had difficulty chewing, symptomatic temporomandibular disorder, and facial asymmetry. She was referred to this surgeon and underwent perioperative orthodontics, bilateral sagittal split ramus osteotomies, and an osseous genioplasty. A, Frontal facial and occlusal views before reconstruction. B, Panorex radiograph before reconstruction. C, Profile and cephalometric views before reconstruction. D, Articulated dental casts that indicate analytic model planning. E, Frontal views before and after reconstruction. F, Oblique views before and after reconstruction. G, Occlusal views before and after reconstruction. H, Lateral cephalometric views before and after reconstruction. I, Panorex radiograph after reconstruction.
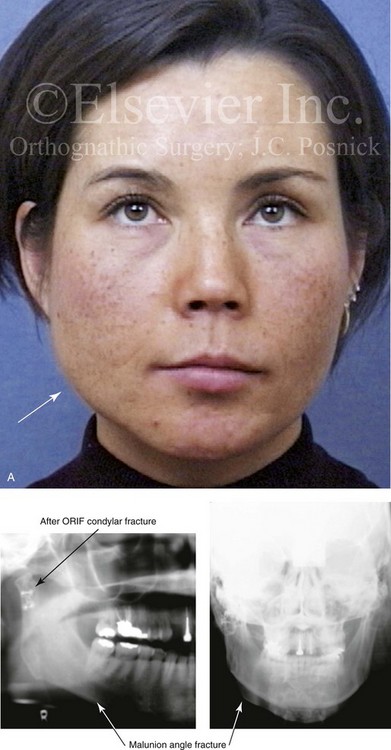
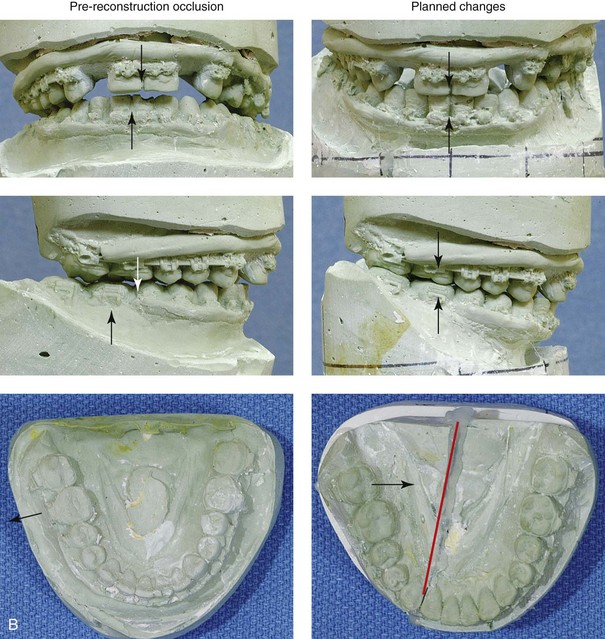
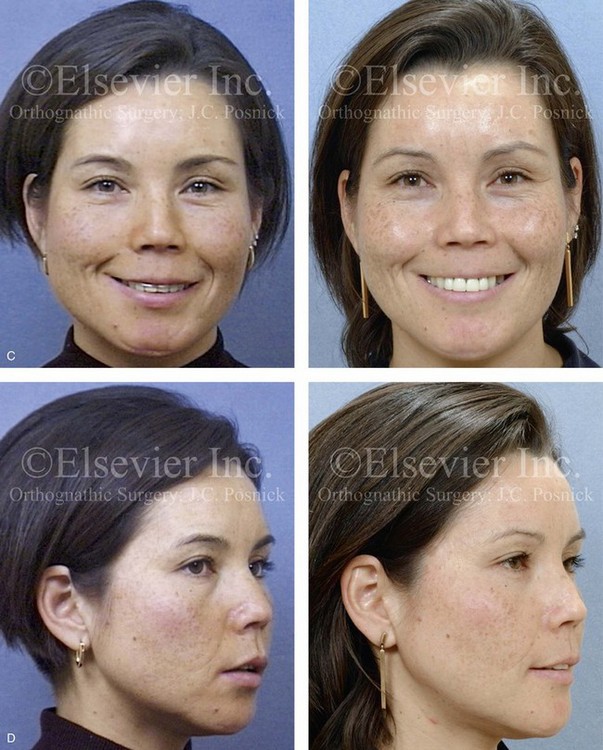
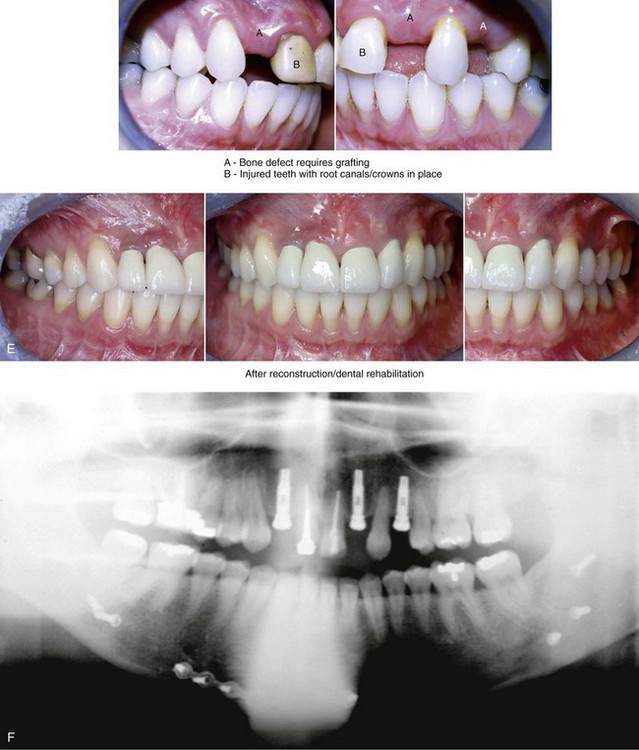
Figure 35-12 A woman in her late 20s was a pedestrian who was struck by a motor vehicle. She sustained a right condylar fracture, a right parasymphyseal fracture, and maxillary dentoalveolar trauma. Primary fracture management included open reduction and internal fixation of the right condyle fracture, closed reduction of the right parasymphyseal fracture, and closed reduction of the dentoalveolar injury. She arrived to this surgeon for the evaluation of secondary deformities that included the partial resorption of the condylar segment, malunion of the parasymphyseal fracture (widening), and the loss of maxillary dentoalveolar components. Comprehensive evaluation included additional consultations with an orthodontist, a periodontist, and a restorative dentist. Surgery included bilateral sagittal split ramus osteotomies (correction of asymmetry), right parasymphyseal osteotomy (narrowing), and bloc iliac grafting of the maxillary dentoalveolar defects. Dental implants were placed 4 months later. Restorative work was completed 6 months after implant placement. A, Frontal view before secondary reconstruction. The Panorex radiograph indicates the loss of condylar height. The anteroposterior cephalometric radiograph and facial views indicate mandibular width asymmetry. B, Dental casts before and after model surgery planning. C, Facial views before and after reconstruction and rehabilitation. D, Oblique views before and after reconstruction and rehabilitation. E, Occlusal views before and after reconstruction and rehabilitation. F, Panorex radiograph after reconstruction and rehabilitation.
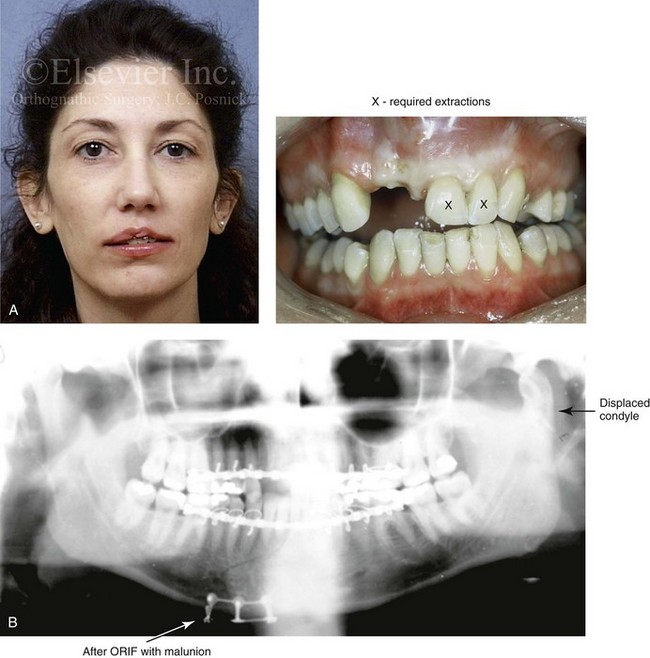
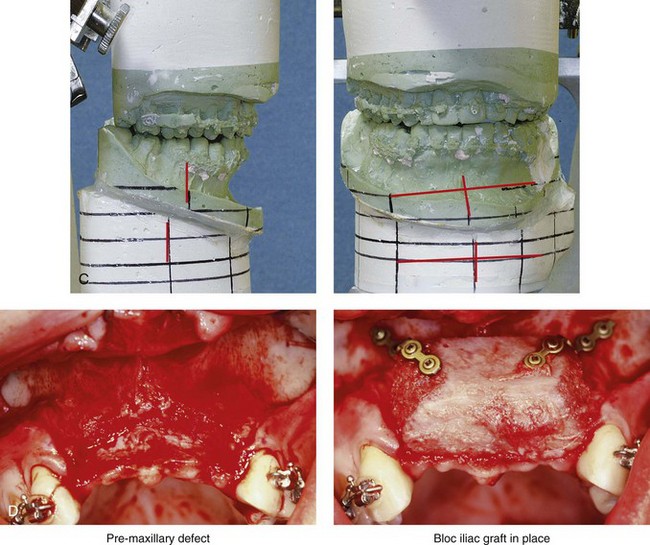
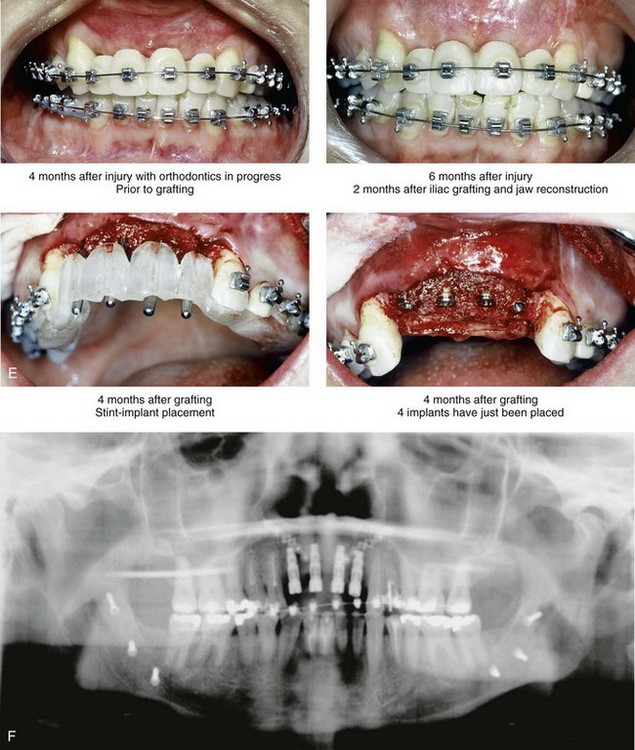
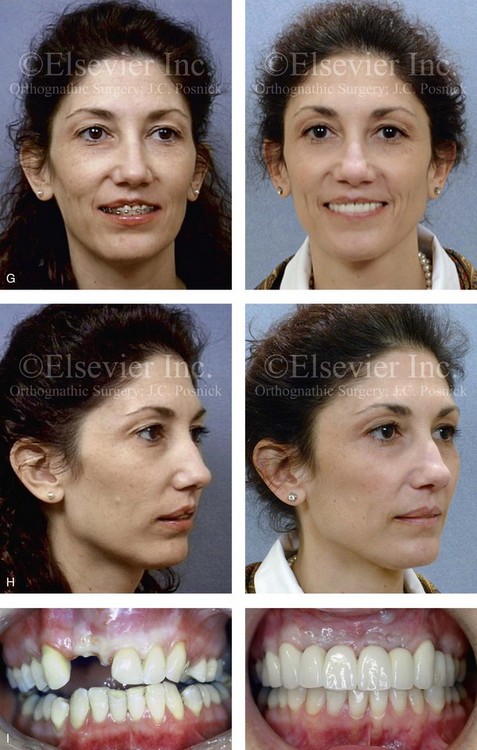
Figure 35-13 A woman in her 20s was in a moped accident and sustained maxillofacial trauma that included a displaced left condyle fracture, a right parasymphyseal fracture, maxillary dentoalveolar trauma, and the laceration of the upper lip. She was initially treated with closed reduction of the left condyle fracture, open reduction and internal fixation of the right parasymphyseal fracture, debridement of the right maxillary incisors, and reimplantation of the left maxillary incisors. By 3 months after treatment, malunion of the mandible resulted in malocclusion, difficulty chewing, symptomatic temporomandibular disorder, and facial asymmetry. The patient was referred to this surgeon for comprehensive evaluation included additional consultation with a periodontist, and an orthodontist. She underwent preoperative orthodontics and reconstruction that included bilateral sagittal split ramus osteotomies. A bloc corticocancellous graft was simultaneously crafted and secured to the anterior maxillary dentoalveolar defect. Four months later, four dental implants were placed. This was followed 4 months later by the placement of crowns. A, Frontal and occlusal views 1 week after the accident and primary fracture management. B, Panorex radiograph taken just after primary fracture management. C, Three months after primary fracture management, analytic model planning indicates details for the mandibular reconstruction. D, Intraoperative view of the maxillary dentoalveolar defect and the crafted bloc corticocancellous iliac graft fixed in place. E, Close-up views of the anterior maxilla indicate the sequence of events: 4 months after injury but before grafting; 6 months after injury and 2 months after grafting; 4 months after grafting with stent and implant placement; and then just after the placement of four implants. F, Panorex radiograph after mandibular and maxillary reconstruction and dental rehabilitation. G, Frontal views before and after reconstruction and rehabilitation. H, Oblique views before and after reconstruction and rehabilitation. I, Occlusal views before and after reconstruction and dental rehabilitation.
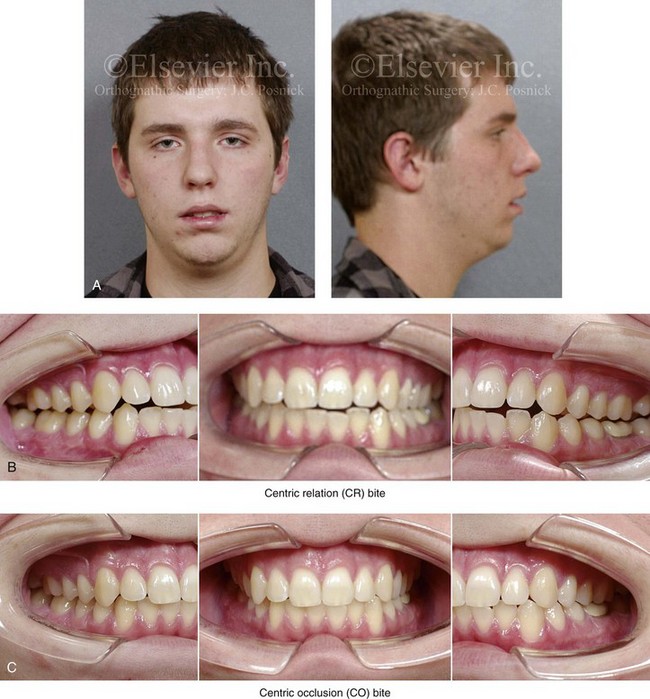
Figure 35-14 When he was between 13 and 15 years old, this patient underwent standard orthodontic treatment and achieved a normal occlusion without a centric relation–centric occlusion (CR-CO) discrepancy. When he was 16 years of age, he was in a motor vehicle accident and sustained injuries that included a displaced left condyle of the mandible fracture. The fracture was treated with closed treatment and intermaxillary fixation for 2 weeks followed by guiding elastics for an additional 4 weeks. The patient also sustained fracture of the left mandibular first and second molars, which required the placement of full crowns. With healing, there was a loss of posterior facial height and a shift of the mandible toward the fracture. There was a significant CR-CO slide with masticatory muscle discomfort and left temporomandibular joint noise. The patient underwent computed tomography scanning and magnetic resonance imaging of the temporomandibular joint, which indicated the following: left condylar head flattening and remodeling; the meniscus being in good alignment with a deformed condylar head; and, on the right side, the mandibular condyle and meniscus appear to be unremarkable. The patient maintained an adequate range of mandibular motion. He was being treated with a splint that prevented CR-CO slide, and his temporomandibular disorder has improved. Bilateral sagittal split osteotomies of the mandible were recommended to establish a consistent CR-CO without prematurities. A, Facial views 2 years after left condyle fracture with closed reduction. B, Intraoral views with the patient in CR bite. C, Intraoral views with the patient in CO bite.
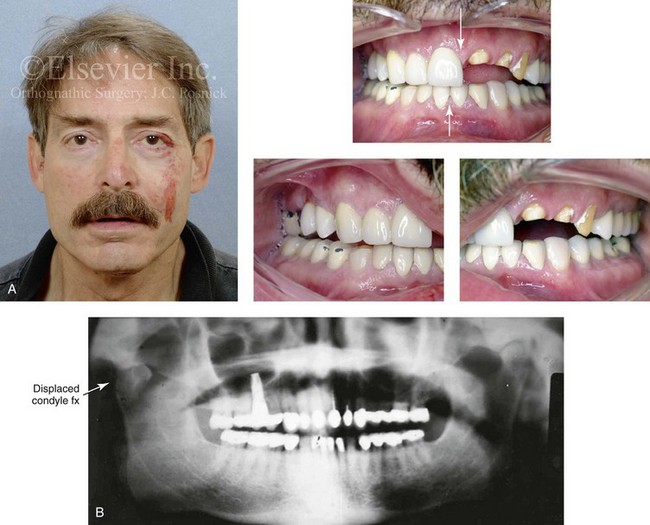
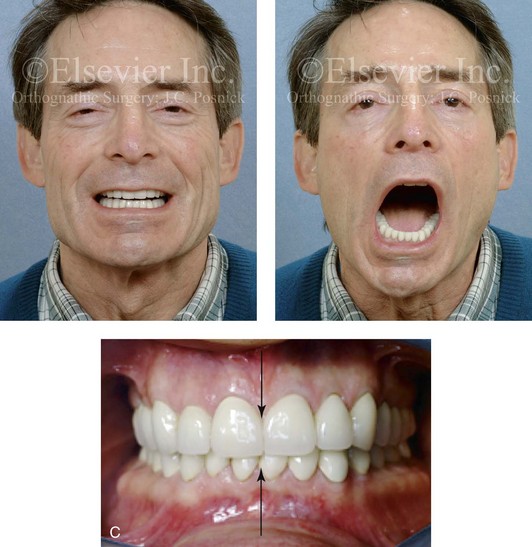
Figure 35-15 A middle-aged man had a syncopal episode and sustained a displaced right condyle fracture of the mandible and maxillary dentoalveolar trauma. This was managed with closed treatment and 2 weeks of intermaxillary fixation followed by elastics, masticatory muscle retraining, and then dental rehabilitation. A comfortable occlusion with minimal centric relation–centric occlusion discrepancy and resolution of temporomandibular disorder was possible without the need for surgical intervention. A, Facial and occlusal views 2 days after injury. B, Panorex radiograph 4 weeks after injury that indicates right subcondylar fracture and dislocation and left maxillary dentoalveolar trauma. C, Frontal view at 18 months after injury. Effective muscle retraining achieved a stable occlusion. With wide vertical opening, there is a shift of the chin to the right. The centric occlusion view is shown 18 months after injury with successful dental rehabilitation.
Posttraumatic Segmental Dentoalveolar Defects
A frequently seen pattern of posttraumatic secondary deformity occurs after the avulsion or devascularization of an anterior dentoalveolar segment (i.e., the maxilla, the mandible, or both).39 This may occur as an isolated injury or in conjunction with other jaw fractures (Figs. 35-16 and 35-17; see also Figs. 35-4, 35-9, 35-12, and 35-13). To secondarily correct this segmental dentoalveolar deformity with a loss of teeth, an autogenous corticocancellous iliac bone graft fixed in place with microplates and screws is generally a reliable option (see Figs. 35-4, 35-9, 35-12, 35-13, 35-16, and 35-17). This is followed by a soft-tissue vestibuloplasty and a keratinized mucosa grafting procedure, when required. Approximately 4 months after successful bone grafting and soft tissue re-arrangment, dental implants are placed. This is followed several months later with crown restorations. Comprehensive skeletal reconstruction and dental rehabilitation of segmental dentoalveolar defects generally involves a surgeon, a periodontist, and a restorative dentist and is best coordinated from the onset.87
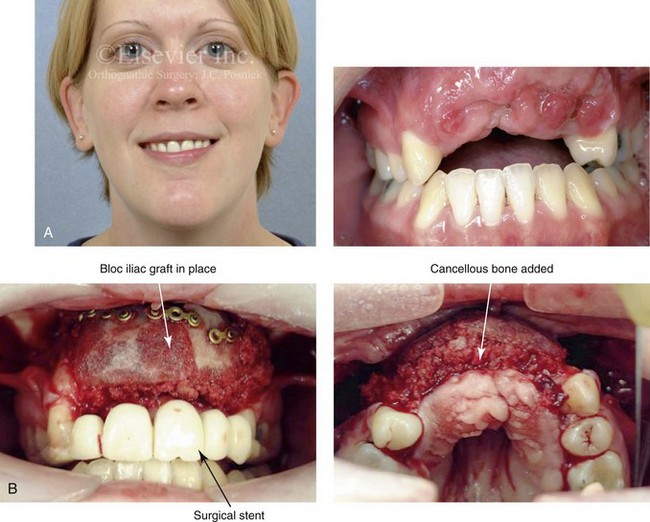
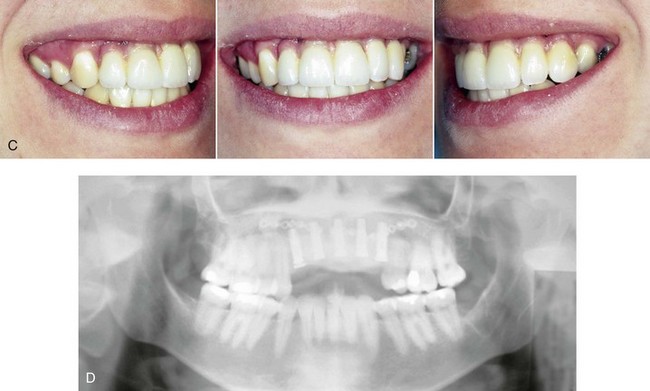
Figure 35-16 A 28-year-old woman was in a motor vehicle accident and sustained maxillary dentoalveolar trauma with a loss of five anterior teeth and associated alveolar bone. She had previously undergone orthodontic treatment including 4 bicuspid extractions to close an anterior open bite. A, Frontal view with temporary partial denture in place. Occlusal view with the removal of the partial denture indicates the extent of dentoalveolar injury and bone loss. B, Intraoperative view with a crafted corticocancellous iliac graft fixed in place. A prefabricated stent was used to assist with graft positioning and contouring. C, Occlusal views after successful grafting, placement of 5 implants and then dental restorative work. D, Panorex radiograph after successful grafting and the placement of five dental implants.
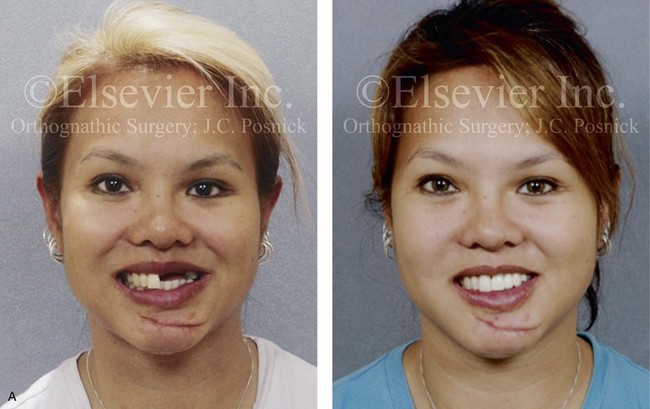
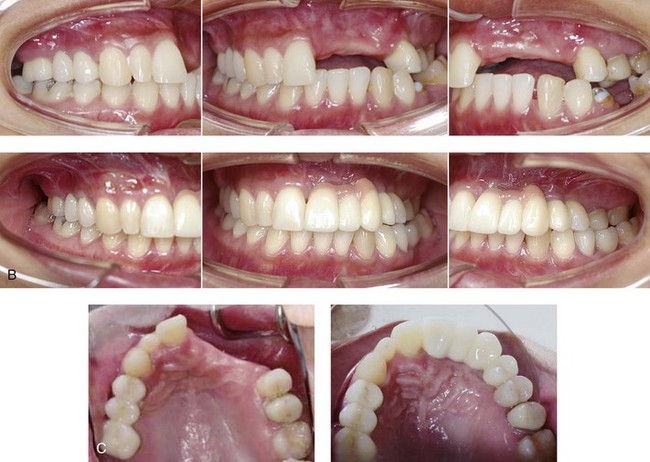
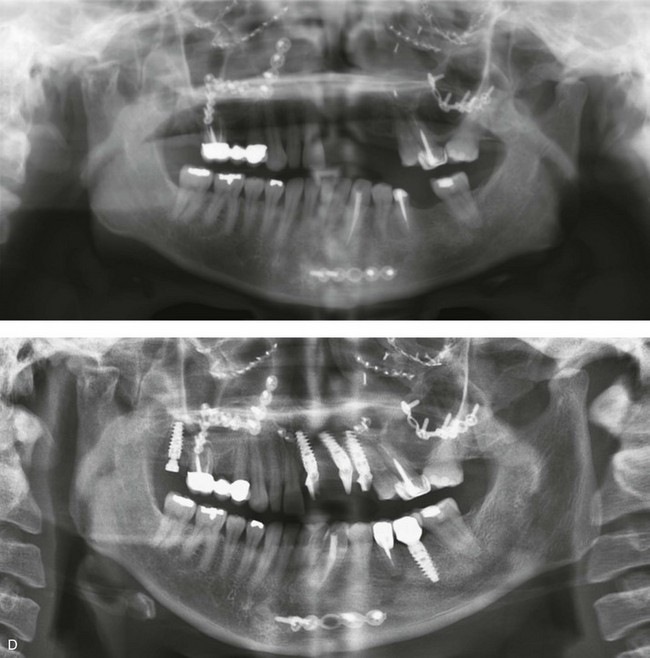
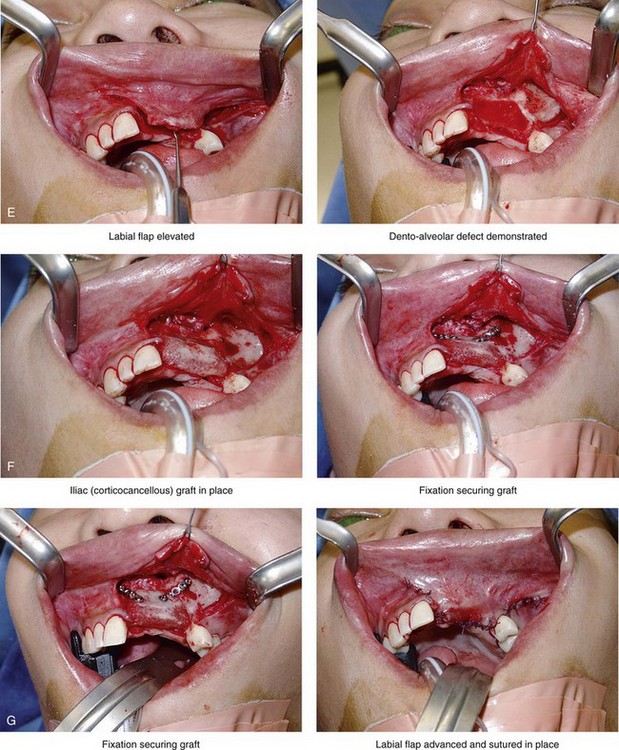
Figure 35-17 A 21-year-old woman was in a motor vehicle accident and sustained naso–orbital–ethmoid fractures, left anterior maxillary dentoalveolar trauma with a loss of three teeth, and associated alveolar bone and left posterior mandibular dental injuries. She was initially treated at another institution. She was referred to this surgeon 6 months later, with left and right enophthalmus, saddle-nose deformity, and right and left medial canthal drift. She underwent left and right orbital reconstruction, left and right medial canthopexies, and the correction of the saddle-nose deformity with a full-thickness cranial graft (radix to tip) through a coronal scalp incision. She was treated by her local dentist with a removable maxillary partial denture. She returned to this surgeon for further maxillofacial evaluation 14 years later in the hopes of achieving improved dental rehabilitation. Examination confirmed left anterior maxillary dentoalveolar defect, left posterior mandibular absent second bicuspid and first molar, and several teeth with root canals. She agreed to a comprehensive dental rehabilitation approach. Additional evaluation by a periodontist, and a prosthodontist, was carried out. The patient also complained of chronic obstructed nasal breathing. Intranasal examination confirmed septal deviation and enlarged inferior turbinates. She underwent periodontal treatment followed by temporization of the dentition. Surgical procedures included septoplasty; inferior turbinate reduction; and reconstruction of the maxillary dentoalveolar defect with an autogenous corticocancellous iliac bloc graft. Four months later, three dental implants were placed in the anterior maxillary region. She also underwent implant placement in the left posterior mandible and the right posterior maxilla. Six months after implant placement, definitive crown placement and bridgework were initiated. A, Frontal views with smile before and after comprehensive secondary jaw and dental rehabilitation. B and C, Occlusal and palatal views before grafting and then after grafting, implant placement, and dental restorative work. D, Panorex radiographs before and then after successful secondary jaw and dental rehabilitation including grafting and the placement of dental implants. E, F, and G, Intraoperative views of autogenous iliac corticocancellous graft reconstruction of the left maxillary dentoalveolar defect.
References
1. Adekeye, EO. Ankylosis of the mandible: Analysis of 76 cases. J Oral Maxillofac Surg. 1983; 41:442.
2. Allori, AC, Chang, CC, Fariña, R, et al. Current concepts in pediatric temporomandibular joint disorders: Part 1. Etiology, epidemiology, and classification. Plast Reconstr Surg. 2010; 126:1263–1275.
3. Becking, AG, Zijderveld, SA, Tuinzing, DB. Management of posttraumatic malocclusion caused by condylar process fractures. J Oral Maxillofac Surg. 1998; 56:1370–1374.
4. Becking, AG, Zijderveld, SA, Tuinzing, DB. Management of posttraumatic malocclusion caused by condylar process fractures. J Oral Maxillofac Surg. 1998; 56:1370–1377.
5. Blair, VP. Operative treatment of ankylosis of the mandible. Surg Gynecol Obstet. 1914; 19:436–456.
6. Blair, VP. The consideration of contour as well as function in operations for chronic ankylosis. Surg Gynecol Obstet. 1928; 46:167–179.
7. Bloomquist, DS. Mandibular body sagittal osteotomy in the correction of malunited mandibular fractures. J Maxillofac Surg. 1982; 10:18–23.
8. Bounds, GA, Hopkins, R, Sugar, A. Septic arthritis of the temporomandibular joint: A problematic diagnosis. Br J Oral Maxillofac Surg. 1987; 25:61–67.
9. Carlson, DS. (Discussion of) Growth of a costochondral graft in the rat temporomandibular joint. J Oral Maxillofac Surg. 1992; 50:857.
10. Coccaro, PJ. Restitution of mandibular form after condylar injury in infancy. Am J Orthod Dentofacial Orthop. 1969; 55:32.
11. Converse, JM, Smith, B, Wood-Smith, D. Deformities of the midface resulting from malunited orbital and naso-orbital fractures. Clin Plast Surg. 1975; 2:107.
12. Dahlstrom, L, Kahnberg, K-E, Lindahl, L. 15 years follow-up on condylar fractures. Int J Oral Maxillofac Surg. 1989; 18:18–23.
13. Daramola, JO, Ajagbe, HA, Oluwasanmi, JO. Ankylosis of the temporomandibular joint following mandibular injury: Case reports. Niger Med J. 1979; 9:395.
14. De Amaratunga, NA. Mouth opening after release of maxillomandibular fixation in fracture patients. J Oral Maxillofac Surg. 1987; 45:383–385.
15. Dodson, TB, Bays, RA, Pfeffle, RC, et al. Cranial bone graft to reconstruct the mandibular condyle in Macaca mulatta. J Oral Maxillofac Surg. 1997; 55:260.
16. He, D, Yang, C, Chen, M, et al. Traumatic temporomandibular joint ankylosis: Our classification and treatment experience. J Oral Maxillofac Surg. 2011; 69:1600–1607.
17. El-Mofty, S. Cephalometric studies of patients with ankylosis of the temporomandibular joint following surgical treatment. Oral Surg. 1979; 48:92.
18. El-Sheikh, MM, Medra, AM. Management of unilateral temporomandibular ankylosis associated with facial asymmetry. J Craniomaxillofac Surg. 1997; 25:109.
19. El-Sheikh, MM, Medra, AM, Warda, MH. Bird face deformity secondary to bilateral temporomandibular joint ankylosis. J Craniomaxillofac Surg. 1996; 24:96.
20. Ellis, E, III. Mobility of the mandible following advancement using maxillomandibular fixation and rigid internal fixation: an experimental investigation in Macaca mulatta. J Oral Maxillofac Surg. 1988; 46:118–123.
21. Ellis, E, III., Throckmorton, GS. Treatment of mandibular condylar process fractures: Biological considerations. J Oral Maxillofac Surg. 2005; 63:115–134.
22. Ellis, E, III., Throckmorton, GS, Palmieri, C. Open treatment of condylar process fractures: assessment of adequacy of repositioning and maintenance of stability. J Oral Maxillofac Surg. 2000; 58:27–34.
23. Ellis, E, III., Walker, R. Treatment of malocclusion and TMJ dysfunction secondary to condylar fractures. Craniomaxillofac Trauma Reconstr. 2009; 2:1–18.
24. Engel, MB, Brodie, AG. Condylar growth and mandibular deformities. Surgery. 1947; 22:976.
25. Evans, HJ, Burwell, RG, Merville, LC, et al, Residual deformities. Maxillofacial injuries. Rowe, NL, Williams, JL, eds. Maxillofacial injuries; vol 2. Churchill Livingstone, Edinburgh, UK, 1985:1765.
26. Funke, FW. Quantitative evaluation of mandibular condylar growth using tritiated thymidine in autoradiographic analysis. J Dent Res. 1971; 50:1500.
27. Furlan, S. Correction of saddle nose deformities by costal cartilage grafts: A technique. Ann Plast Surg. 1982; 9:32.
28. Gibson, T, Davis, WB. The distortion of autogenous cartilage grafts: Its cause and prevention. Br J Plast Surg. 1957–1958; 10:257.
29. Gilhus-Moe, O. Fractures of the mandibular condyle in the growth period. Stockholm, Sweden: Scandinavian University Books, Universitatsforlaget; 1969.
30. Gillies, HD. Plastic surgery of the face. London, UK: Oxford University Press; 1920.
31. Greenfield, FM, Hirsch, AC. Delayed treatment of fractured condyles. J Oral Surg. 1965; 19:295.
32. Gruber, RP. Lengthening the short nose. Plast Reconstr Surg. 1993; 91:1252.
33. Hekkenberg, RJ, Piedade, L, Mock, D, et al. Septic arthritis of the temporomandibular joint. Otolaryngol Head Neck Surg. 1999; 120:780–782.
34. Hennig, TB, Ellis, E, III., Carlson, DS. Growth of the mandible following replacement of the mandibular condyle with the sternal end of the clavicle: An experimental investigation in Macaca mulatta. J Oral Maxillofac Surg. 1992; 50:1196.
35. Heurlin, RJ, Gans, BJ, Stuteville, OH. Skeletal changes following fracture dislocation of the mandibular condyle in the adult rhesus monkey. Oral Surg Oral Med Oral Pathol. 1961; 14:1490–1500.
36. Hincapie, JW, Tobon, D, Diaz-Reyes, GA. Septic arthritis of the temporomandibular joint. Otolaryngol Head Neck Surg.. 1999; 121:836–837.
37. Hinds, EC, Parnes, EL. Late management of condylar fractures by means of subcondylar osteotomy: Report of cases. J Oral Surg. 1966; 24:54–59.
38. Jackson, IT, Smith, J, Mixter, RC. Nasal bone grafting using split skull grafts. Ann Plast Surg. 1983; 11:533.
39. Järvinen, S. Traumatic injuries to upper permanent incisors related to age and incisal overjet. Acta Odontol Scand. 1979; 37:335–338.
40. Joondeph, DR, Bloomquist, D. Open-bite closure with mandibular osteotomy. Am J Orthod Dentofacial Orthop. 2004; 126:296–298.
41. Kaban, LB. Acquired abnormalities of the temporomandibular joint. In: Pediatric oral and maxillofacial surgery. Philadelphia: Saunders; 1990:307–342.
42. Kaban, LB, Perrott, DH, Fisher, K. A protocol for management of temporomandibular joint ankylosis. J Oral Maxillofac Surg. 1990; 48:1145.
43. Kawamoto, HK, Jr. Correction of established traumatic deformities of the facial skeleton using craniofacial principles in facial injuries. St Louis: Mosby-Year Book; 1988.
44. Kazanjian, VH. Ankylosis of the temporomandibular joint. Surg Gynecol Obstet. 1938; 67:333–348.
45. Kennett, S. Temporomandibular joint ankylosis: The rationale for grafting in the young patient. J Oral Surg. 1973; 31:744.
46. Kiehn, CI, DesPrez, JD, Converse, CF. Total prosthetic replacement of the temporomandibular joint. Ann Plast Surg. 1979; 2:5.
47. Ohno, K, Michi, K, Ueno, T. Mandibular growth following ankylosis operation in childhood. Int J Oral Surg. 1981; 10(Suppl 1):324–328.
48. Leighty, SM, Spach, DH, Myall, RW, Burns, JL. Septic arthritis of the temporomandibular joint: Review of the literature and report of two cases in children. Int J Oral Maxillofac Surg. 1993; 22:292–297.
49. Lindahl, L, Hollender, L. Condylar fractures of the mandible: II. Radiographic study of remodeling processes in the temporomandibular joint. Int J Oral Surg. 1977; 6:153–165.
50. Lindquist, C, Pihakari, A, Tasanen, A, Hampf, G. Autogenous costochondral graft in temporomandibular joint arthroplasty. A survey of 66 arthroplasties in 60 patients. J Maxillofac Surg. 1986; 14:143–149.
51. Link, JO, Hoffman, DC, Laskin, DM. Hyperplasia of a costochondral graft in an adult. J Oral Maxillofac Surg. 1993; 51:1392–1394.
52. Lund, K. Mandibular growth and remodeling processes after mandibular fractures. Acta Odontol Scand Suppl. 1974; 32:64.
53. MacIntosh, RB, Henry, FA. A spectrum of application of autogenous costochondral grafts. J Maxillofac Surg. 1977; 5:257–267.
54. Manchester, WM. Immediate reconstruction of the mandible and temporomandibular joint. Br J Plast Surg. 1965; 18:291.
55. Manchester, WM. Some technical improvements in the reconstruction of the mandible and temporomandibular joint. Plast Reconstr Surg. 1972; 50:249.
56. Martinez-Garcia, WR. Surgical correction of recurrent bony ankylosis of the temporomandibular joint. Br J Oral Surg. 1971; 9:110.
57. Matukas, VJ, Szymela, VF, Schmidt, JF. Surgical treatment of bony ankylosis in a child using a composite cartilage-bone iliac crest graft. J Oral Surg. 1980; 38:903.
58. McNamara JA, ed. Determinants of mandibular form and growth. Ann Arbor, Mich: Center for Human Growth and Development, University of Michigan, 1995.
59. Melsen, B, Bjerregaard, J, Bundgaard, M. The effect of treatment with functional appliance on a pathologic growth pattern of the condyle. Am J Orthod Dentofacial Orthop. 1986; 90:503.
60. Millard, R. (Discussion of) Nasal reconstruction with full-thickness cranial bone grafts and rigid internal skeletal fixation through a coronal incision. Plast Reconstr Surg. 1990; 86:903.
61. Moorthy, AP, Finch, LD. Interpositional arthroplasty for ankylosis of the temporomandibular joint. Oral Surg. 1983; 55:545.
62. Mosby, EL, Hiatt, WR. A technique of fixation of costochondral grafts for reconstruction of the temporomandibular joint. J Oral Maxillofac Surg. 1989; 47:209.
63. Moss, ML. The primacy of functional matrices in orofacial growth. Dent Pract Dent Rec. 1968; 19:65–73.
64. Moss, ML. The role of muscular functional matrices in development and maintenance of occlusion. Bull Pac Coast Soc Orthod. 1970; 45:29–30.
65. Moss, ML, Salentijn, L. The primary role of functional matrices in facial growth. Am J Orthod. 1969; 55:566–577.
66. Moss, ML, Salentijn, L. The compensatory role of the condylar cartilage in mandibular growth: Theoretical and clinical implications. Dtsch Zahn Mund Kieferheilkd Zentralbl Gesamte. 1971; 56:5–16.
67. Munro, IR, Chen, YR, Park, BY. Simultaneous total correction of temporomandibular ankylosis and facial asymmetry. Plast Reconstr Surg. 1986; 77:517.
68. Nelson, CL, Buttrum, JD. Costochondral grafting for posttraumatic temporomandibular joint reconstruction: A review of six cases. J Oral Maxillofac Surg. 1989; 47:1030.
69. Nørholt, SE, Krishnan, V, Sindet-Pedersen, S, Jensen, I. Pediatric condylar fractures: a long-term follow-up study of 55 patients. J Oral Maxillofac Surg. 1993; 51:1302.
70. Nowak, AJ, Casamassimo, PS. Oral opening and other selected facial dimensions of children 6 weeks to 36 months of age. J Oral Maxillofac Surg. 1994; 52:845.
71. Nwoku, AL. Rehabilitating children with temporomandibular joint ankylosis. Int J Oral Surg. 1979; 8:271.
72. Obeid, G, Guttenberg, SA, Connole, PW. Costochondral grafting in condylar replacement and mandibular reconstruction. J Oral Maxillofac Surg. 1988; 46:177.
73. Ohno, K, Michi, K, Ueno, T. Mandibular growth following ankylosis operation in childhood. Int J Oral Surg. 1981; 10(Suppl 1):324.
74. Peer, L. Transplantation of tissues, vol 1. Baltimore, Md: Williams & Wilkins; 1955.
75. Peltomaki, T. Growth of a costochondral graft in the rat temporomandibular joint. J Oral Maxillofac Surg. 1992; 50:851.
76. Pensler, JM, Christopher, RD, Bewyer, DC. Correction of micrognathia with ankylosis of the temporomandibular joint in childhood. Plast Reconstr Surg. 1993; 91:799.
77. Perren, SM. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin Orthop. 1979; 138:175.
78. Perrott, DH. (Discussion of) Clinical and computed tomographic findings in costochondral grafts replacing the mandibular condyle. J Oral Maxillofac Surg. 1996; 54:1400.
79. Perrot, DH, Umeda, H, Kaban, LB. Costochondral graft construction/reconstruction of the ramus/condyle unit: Long-term follow-up. Int J Oral Maxillofac Surg. 1994; 23:321–328.
80. Pickerill, HP. Ankylosis of the jaw: Cartilage graft restoration of the joint: A new operation. Aust N Z J Surg. 1942; 11:197.
81. Politis, C, Fossion, E, Bossuyt, M. The use of costochondral grafts in arthroplasty of the temporomandibular joint. J Craniomaxillofac Surg. 1987; 15:345–354.
82. Posnick, JC. Craniomaxillofacial fractures in children. Oral Maxillofac Surg Clin North Am. 1994; 6:169–185.
83. Posnick, JC. (Discussion of) Mandibular fractures in infants: Review of the literature and report of seven cases. J Oral Maxillofac Surg. 1994; 52:245–246.
84. Posnick, JC. Management of facial fractures in children and adolescents. Ann Plast Surg. 1994; 33(4):442–457.
85. Posnick, JC. (Discussion of) Pediatric facial fractures: A demographic analysis outside an urban environment. Ann Plast Surg. 1997; 38:584–585.
86. Posnick, JC, Primary craniomaxillofacial fracture management. Craniofacial and maxillofacial surgery in children and young adults. Posnick, JC, eds. Craniofacial and maxillofacial surgery in children and young adults; vol 30. W. B. Saunders, Philadelphia, 2000:697–745.
87. Posnick, JC, Secondary craniomaxillofacial traumatic deformities: Evaluation and treatment: TMJ ankylosis, skull defects, orthognathic deformities, saddle nose deformities, enophthalmus. Craniofacial and maxillofacial surgery in children and young adults. Posnick, JC, eds. Craniofacial and maxillofacial surgery in children and young adults; vol 31. W. B. Saunders, Philadelphia, 2000:746–781.
88. Posnick, JC, Goldstein, JA. Surgical management of temporomandibular joint ankylosis in the pediatric population. Plast Reconstr Surg. 1993; 91:791.
89. Posnick, JC, Ruiz, R. (Discussion of) Age-related changes in the pattern of midface fractures in children: A chronographic analysis. J Craniomaxillofac Trauma. 2001; 12–13.
90. Posnick, JC, Ruiz, R. (Discussion of) Management of condylar fractures in children. J Craniomaxillofac Trauma. 2001; 6:16–17.
91. Posnick, JC, Seagle, MB, Armstrong, D. Nasal reconstruction with full-thickness cranial bone grafts and rigid internal skeletal fixation through a coronal incision. Plast Reconstr Surg. 1990; 86:894.
92. Posnick, JC, Wells, M, Pron, GE. Pediatric facial fractures: Evolving patterns of treatment. J Oral Maxillofac Surg. 1993; 51(8):836–844.
93. Poswillo, D. Experimental reconstruction of the mandibular joint. Int J Oral Surg. 1974; 3:400.
94. Poswillo, DE. The late effects of mandibular condylectomy. Oral Surg. 1972; 33:500.
95. Proffit, WR, Vig, KWL, Turvey, TW. Early fracture of the mandibular condyles: Frequently an unsuspected cause of growth disturbances. Am J Orthod Dentofacial Orthop. 1980; 78:1.
96. Rajgopal, A, Banerji, PK, Batura, V, et al. Temporomandibular ankylosis. J Maxillofac Surg. 1983; 11:37.
97. Raustia, A, Pernu, H, Pyhtinen, J, Oikarinen, K. Clinical and computed tomographic findings in costochondral grafts replacing the mandibular condyle. J Oral Maxillofac Surg. 1996; 54:1393–1400.
98. Raveh, J, Vuillemin, T, Ladrach, K, et al. Temporomandibular joint ankylosis: Surgical treatment and long-term results. J Oral Maxillofac Surg. 1989; 47:900.
99. Regev, E, Koplewitz, BZ, Nitzan, DW, Bar-Ziv, J. Ankylosis of the temporomandibular joint as a sequela of septic arthritis and neonatal sepsis. Pediatr Infect Dis J. 2003; 22:99–101.
100. Risdon, F. Ankylosis of the temporomandibular joint. J Am Dent Assoc. 21, 1933.
101. Rowe, NL. Surgery of the temporomandibular joint. Proc R Soc Med. 1972; 65:383.
102. Rowe, NL. Ankylosis of the temporomandibular joint: Part 1. J R Coll Surg. 1982; 27:67.
103. Rowe, NL. Ankylosis of the temporomandibular joint: Part 2. J R Coll Surg. 1982; 27:167.
104. Rowe, NL. Ankylosis of the temporomandibular joint: Part 3. J R Coll Surg. 1982; 27:209.
105. Rubens, BC, Stoelinga, PJW, Weaver, TJ, Blijdorp, PA. Management of malunited mandibular condylar fractures. Int J Oral Maxillofac Surg. 1990; 19:22–25.
106. Rubenstein, LK. (Discussion of) Oral opening and other selected facial dimensions of children 6 weeks to 36 months of age. J Oral Maxillofac Surg. 1994; 52:848.
107. Samman, N, Cheung, LK, Tiderman, H. Overgrowth of a costochondral graft in an adult male. Int J Oral Maxillofac Surg. 1995; 24:333–335.
108. Sarnat, BG. Developmental facial abnormalities and the temporomandibular joint. J Am Dent Assoc. 1969; 79:108–117.
109. Sarnat, BG, Muchnic, H. Facial skeletal changes after mandibular condylectomy in growing and adult monkeys. Am J Orthod. 1971; 60:33–45.
110. Sarnat, BG, Muchnic, H. Facial skeletal changes after mandibular condylectomy in the adult monkey. J Anat. 1971; 108:323–338.
111. Sarnat, BG, Muchnic, H. Facial skeletal changes after mandibular condylectomy in growing and adult monkeys. Am J Orthod. 1972; 62:428.
112. Silvennoinen, U, Iizuka, T, Oikarinen, K, Lindqvist, C. Analysis of possible factors leading to problems after nonsurgical treatment of condylar fractures. J Oral Maxillofac Surg. 1994; 52:793–799.
113. Skolnik, J, Iranpour, B, Westesson, PL, Adair, S. Pubertal trauma and mandibular asymmetry in orthognathic surgery and orthodontic patients. Am J Orthod Dentofacial Orthop. 1994; 105:73–77.
114. Snyder, CC, Levine, GA, Dingman, DL. Trial of a sternoclavicular whole joint graft as a substitute for the temporomandibular joint. Plast Reconstr Surg. 1971; 48:447.
115. Spitzer, WJ, Vanderborght, G, Dumbach, J. Surgical management of mandibular malposition after malunited condylar fractures in adults. J Craniomaxillofac Surg. 1997; 25:91.
116. Steinhauser, EW. The treatment of ankylosis in children. Int J Oral Surg. 1973; 2:129.
117. Tessier, P. Aesthetic aspects of bone grafting to the face. Clin Plast Surg. 1981; 8:279.
118. Tessier, P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg. 1982; 9:531.
119. Topazian, RG. Etiology of ankylosis of temporomandibular joint: Analysis of 44 cases. J Oral Surg. 1964; 22:227.
120. Topazian, RG. (Discussion of) A protocol for management of temporomandibular joint ankylosis. J Oral Maxillofac Surg. 1990; 48:1152.
121. Trimble, LD, Schoenaers, JA, Stoelinga, PJ. Acute suppurative arthritis of the temporomandibular joint in a patient with rheumatoid arthritis. J Maxillofac Surg. 1983; 11:92–95.
122. Troulis, MJ, Williams, WB, Kaban, LB. Endoscopic condylectomy and costochondral graft reconstruction of the ramus condyle unit. J Oral Maxillofac Surg. 2003; 61:63.
123. Troulis, MJ, Williams, WB, Kaban, LB. Endoscopic mandibular condylectomy and reconstruction: Early clinical result. J Oral Maxillofac Surg. 2004; 62:460.
124. Vargervik, K. (Discussion of) Cranial bone graft to reconstruct the mandibular condyle in Macaca mulatta. J Oral Maxillofac Surg. 1997; 55:267.
125. Walker, RV. Traumatic mandibular condyle fracture dislocations: Effect on growth in the Macaca rhesus monkey. Am J Surg. 1960; 100:850.
126. Walker RV: Condylar abnormalities. Transactions of the 2nd International Conference on Oral Surgery, Copenhagen, 1965, Munksgaard Copenhagen, pp 81–96, 1967.
127. Walker, RV. Condylar fractures: Nonsurgical management. J Oral Maxillofac Surg. 1994; 52:1185.
128. Ware, WH, Brown, SL. Growth centre transplantation to replace mandibular condyles. J Maxillofac Surg. 1981; 9:50–58.
129. Ware, WH, Taylor, RC. Replantation of growing mandibular condyles in rhesus monkeys. Oral Surg Oral Med Oral Pathol. 1965; 19:669–677.
130. Weiss, P. Regeneration of the mandibular and temporomandibular joints following subperiosteal exarticulation of the mandible in the young dog [German]. Dtsch Zahnarztl Z. 1969; 24:355–360.
131. Wheeler, ES, Kawamoto, HK, Jr., Zarem, HA. Bone grafts for nasal reconstruction. Plast Reconstr Surg. 1982; 69:9.
132. Zachariades, N, Mezitis, M, Michelis, A. Posttraumatic osteotomies of the jaws. Int J Oral Maxillofac Surg. 1993; 22:328–331.
133. Zide, MF. (Discussion of) An accurate method for open reduction and internal fixation of high and low condylar process fractures. J Oral Maxillofac Surg. 1994; 52:812.
134. Zou, Z-J, Wu, W-T, Sun, G-X, et al. Remodeling of the temporomandibular joint after conservative treatment of condylar fractures. Dentomaxillofac Radiol. 1987; 16:91–98.