Chapter 60 Management of Postoperative Cerebrospinal Fluid Leaks
 Videos corresponding to this chapter are available online at www.expertconsult.com.
Videos corresponding to this chapter are available online at www.expertconsult.com.
PRESSURE DRESSING
Technique
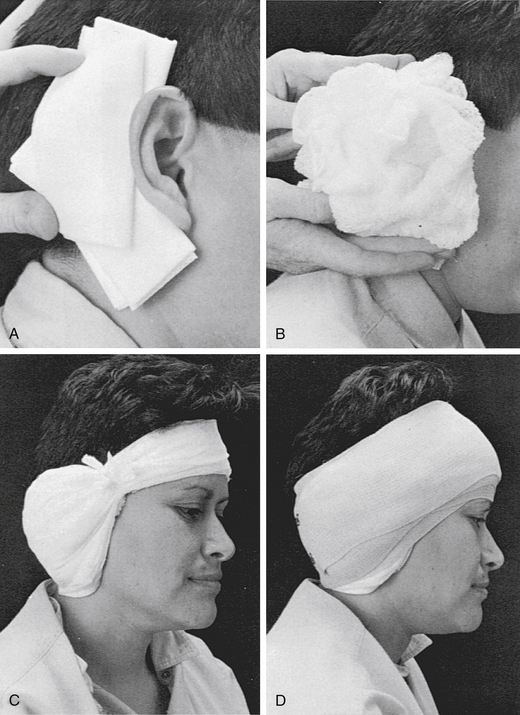
The dressing is applied in similar manner to the initial postoperative dressing. First, a vertical gathering tie is placed in the temporal fossa. Dressing sponges (4 × 4) are folded in half and placed directly over the fat graft and in the postauricular sulcus to support the auricle (Fig. 60-1A). Next, fluffed Kerlix is placed over the 4 × 4 sponges and the auricle (Fig. 60-1B). A tight wrap of roller gauze is applied (Fig. 60-1C). The direction of the wrap should be from the ear toward the occiput, which ensures that the auricle is not damaged by anterior folding. Also, the vertical tie must be as lateral as possible in the temporal fossa to avoid a pressure point on the forehead. Pressure necrosis of forehead skin can develop easily if attention is not given to this point.
The last layer of this dressing is a 3-inch elastic bandage, which provides the final compression (Fig. 60-1D). The elastic bandage should be wrapped firmly, but patient comfort must be accommodated. Usually, the last several turns can be altered to adjust the exact amount of compression. Ideally, a pressure dressing should be left in place for 4 or 5 days, which allows time for healing of the CSF leak site to occur. A minimum of 48 hours without leakage must pass before the dressing is removed.
LUMBAR DRAIN
Technique
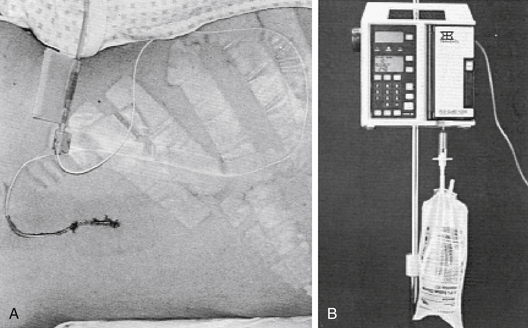
To place the catheter, a lumbar puncture is performed at the L4-L5 level. The patient is placed in either a sitting or a lateral decubitus position. The spinous processes are palpated, and L4-L5 is identified at the level of the iliac crest. The patient is asked to flex the back and bring the knees and chin to the chest. A prepared catheter kit contains all the necessary supplies for preparing, draping, and anesthetizing the site. A 14 gauge Tuohy needle is introduced in the midline with a slightly superior angle between the spinous processes (Fig. 60-2). The obturator is removed at intervals so that the surgeon can look for a flow of CSF. When a good flow of CSF is established, the epidural catheter is introduced through the needle and threaded into the epidural space. The opened side of the bevel of the needle faces the patient’s left or right side on penetrating the spinous ligaments and arachnoid. Before the catheter is threaded, the bevel is turned to open superiorly, facilitating directing the catheter cephalad. With a flow of CSF established, the needle is withdrawn, and the connector is placed on the end of the catheter so that a connection to intravenous tubing can be made. An empty intravenous fluid bag is attached to the tubing, and all connections are secured with tape (Fig 60-3A).
Several methods of regulating CSF output exist. This regulation is important because, if CSF is removed too rapidly, tension pneumocephalus and brain herniation can occur.1–3 Alternatively, if an inadequate amount of CSF is removed, the purpose of the drain is defeated. Humans produce approximately 18 mL of CSF per hour. The natural mechanisms of CSF absorption reduce some CSF; the lumbar drain should remove less than 18 mL of CSF per hour. Common neurosurgical practice is to order 50 mL of CSF removed every 8 hours.
To avoid some of these problems, we place the intravenous tubing in reverse direction through an intravenous infusion pump. The pump is set at a rate of 6 to 10 mL/hr, and a slow, controlled, continuous flow of CSF is withdrawn from the patient (Fig. 60-3B). Because this system is not gravity dependent, patients may move about without fear of a rapid discharge of CSF. Better patency of the catheter system is maintained because the flow is never stopped. If any occlusion of the system or air in the line is sensed, an alarm is sounded as the pump stops. Other physicians have employed flow-regulated systems,4,5 but the system described here is a definite improvement over those described. We have employed this system in a large series of patients and have found it vastly superior to gravity drainage.
WOUND EXPLORATION AND RECLOSURE
When leaks do not resolve within 48 hours with a pressure dressing or lumbar drain, wound exploration with repacking of the abdominal fat and reclosure is indicated. In wounds in which a dural defect was packed with fat, dislodging of the fat from the defect may cause a CSF leak. When the wound is explored, the fat is removed and repacked (with additional fat as required) into the defect, as described in Chapter 49.6 Some surgeons employ autologous fibrin glue as an adjunct to their closures.7,8 This material adds an additional seal to supplement the fat graft or, in other cases, a muscle or pericranial flap. Collagen matrix (DuraGen, Integra city) may also be used to augment the dural closure. A search should be made for opened mastoid air cells that can serve as pathways for CSF. These air cell tracts can be occluded with bone wax. In cases of severe hearing loss, the eustachian tube should be packed off with absorbable knitted fabric (Surgicel) and temporalis muscle after removal of the incus and cutting of the tensor tympani tendon.
Although a return to the operating room seems aggressive and is accompanied by the small risks brought by further surgery and anesthesia, this course of action has provided the most expedient control of CSF leaks that fail conservative therapy. The expeditious closure of a leak plays an important part in the prevention of infection. The longer a leak remains open, the greater the chance that meningitis has to develop. In a review of CSF leaks after translabyrinthine acoustic tumor removal at the House Ear Clinic, no statistically significant association between postoperative CSF leaks and the development of meningitis was found.9 One explanation for this is that leaks are aggressively treated and stopped quickly, limiting the time available for contamination to occur.
REFRACTORY CEREBROSPINAL FLUID LEAKS
Technique
Ear Canal Closure with Eustachian Tube and Middle Ear Obliteration
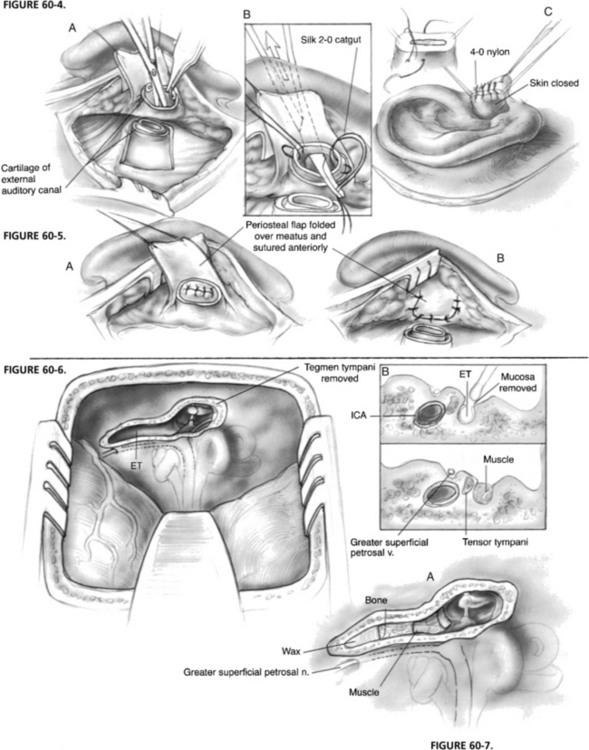
In cases with no residual hearing, blind sac closure is the technique of choice. Blind sac closure of the ear canal is accomplished through a postauricular incision with transection of the ear canal. The canal skin is separated from the cartilage of the lateral canal and everted through the external auditory meatus. This everted canal skin is oversewn with a nonabsorbable suture that is left in place for 10 days postoperatively (Fig. 60-4). A flap of mastoid periosteum is developed on a pedicle just posterior to the external auditory canal. This flap is rotated anteriorly and secured as a second layer of closure for the meatus (Fig. 60-5). Next, all canal skin, the tympanic membrane, the malleus, and the incus are removed. To ensure complete removal of squamous epithelium and to enlarge the canal, a canalplasty is performed with a cutting burr. The eustachian tube is curetted and packed with Surgicel, temporalis muscle, and bone wax. Before wound closure, the middle ear and remaining canal are packed with additional muscle. A pressure dressing is placed for 4 postoperative days; a lumbar spinal fluid drain can also be used in the initial postoperative period.
Middle Fossa Obliteration of the Eustachian Tube
In cases in which the patient has good hearing, and the tympanic membrane is intact, closure of the eustachian tube can be accomplished via the middle fossa.10 This procedure is approached as a middle fossa craniotomy, as outlined in Chapter 48. The bone flap is removed, and the dura is elevated from posterior to anterior. The arcuate eminence, greater superficial petrosal nerve, and middle meningeal artery are identified, and the middle fossa retractor is placed. A diamond burr is used to remove bone from the tegmen tympani and to expose the head of the malleus and the body of the incus (Fig. 60-6). Identification of the tensor tympani tendon and the cochleariform process allows identification of the eustachian tube, which is directly anterior. Also, the eustachian tube is just lateral to the greater superficial petrosal nerve. The bony eustachian tube is unroofed, and the mucosa is carefully curetted from the tube.
The surgeon must be aware that the internal carotid artery can be dehiscent in the medial or inferior eustachian tube. The tube is packed first with bone wax, then bone pâté, and finally temporalis muscle (Fig. 60-7). A split-thickness piece of bone from the bone flap is placed over the tegmen defect to avoid fixation of the ossicles against the dura. The wound is closed in the usual manner. In this setting, a pressure dressing is not likely to be helpful, but a lumbar drain could be placed for several postoperative days.
New Strategies
More recent strategies to reduce the rate of postoperative CSF leak after skull base surgery include the use of various materials for cranioplasty. At the House Ear Clinic, titanium mesh cranioplasty is used to secure the fat graft into the mastoid. In a series of 324 patients undergoing titanium mesh cranioplasty after translabyrinthine acoustic neuroma removal, the CSF leak rate was 3.3%.11
Hydroxyapatite compounds such as Bone-Source (Leibinger) have also been employed. A consecutive series of 108 translabyrinthine acoustic tumors at Pittsburgh Ear Associates showed a CSF leak in 3.7% of the 54 defects closed with hydroxyapatite cement versus 12% of the 54 cases closed with abdominal fat alone.12 In that series, a small quantity of abdominal fat was placed over the dural defect, and the mastoid cavity was filled with the hydroxyapatite cement. The ultimate role of these materials will be influenced by success rates, long-term costs, and ease of use.
1. Snow R.B., Kuhel W., Martin S.B. Prolonged spinal drainage after the resection of tumors of the skull base: A cautionary note. Neurosurgery. 1991;28:880-883.
2. Effron M.Z., Black F.O., Burns D. Tension pneumocephalus complicating the treatment of postoperative CSF otorrhea. Arch Otolaryngol Head Neck Surg. 1981;107:579-580.
3. Graf C.J., Gross C.E., Beck D.W. Complication of spinal drainage in the management of cerebrospinal fluid fistula: Report of three cases. J Neurosurg. 1981;54:392-395.
4. Swanson S.E., Kocan M.J., Chandler W.F. Flow-regulated continuous spinal drainage: Technical note with case report. Neurosurgery. 1981;9:163-165.
5. Swanson S.E., Chandler W.F., Kocan M.J. Flow-regulated continuous spinal drainage in the management of cerebrospinal fluid fistulas. Laryngoscope. 1985;95:104-106.
6. House J.L., Hitselberger W.E., House W.F. Wound closure and cerebrospinal fluid leak after translabyrinthine surgery. Am J Otol. 1982;4:126-128.
7. Epstein G.H., Weisman R.A., Zwillenberg S., Schreiber A. A new autologous fibrinogen-based adhesive for otologic surgery. Ann Otol Rhinol Laryngol. 1986;95:40-45.
8. Sierra D.H., Nissen A.J., Welch J. The use of fibrin glue in intracranial procedures: Preliminary results. Laryngoscope. 1990;100:360-363.
9. Rodgers G.K., Luxford W.M. Factors affecting the development of cerebrospinal fluid leak after translabyrinthine acoustic tumor surgery. Laryngoscope. 1993;103:959-962.
10. Friedman R.A., Cullen R.D., Ulis J., Brackmann D.E. Management of cerebrospinal fluid leaks after acoustic tumor removal. Neurosurgery. 2007;61:35-39.
11. Fayad J.N., Schwartz M.S., Slattery W.H., Brackmann D.E. Prevention and treatment of cerebrospinal fluid leak after translabyrinthine acoustic tumor removal. Otol Neurotol. 2007;28:387-390.
12. Arriaga M.A., Chen D.A. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2002;126:512-517.