196 Management of Patients after Kidney, Kidney-Pancreas, or Pancreas Transplantation
 Background
Background
Kidney Transplants
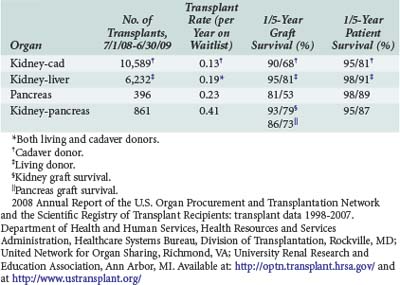
Kidneys are the most frequently transplanted organ; more than 285,000 transplants have been performed through 2007, with over 16,000 transplants performed per year in the United States1 (Table 196-1). Numerous causes of chronic renal failure result in the need for transplantation, the most common being diabetes mellitus and glomerular disorders (Box 196-1). The source of donors for renal transplantation are both cadavers and living donors. In 2009, there were 10,442 cadaveric donor transplants and 6387 living donor transplants performed.1,2 The living donor pool consists of both living related donors, who have a higher likelihood for a favorable crossmatch, and living unrelated donors. Recent surgical innovations such as using laparoscopy to obtain the donor kidney have decreased morbidity for donors and decreased costs.3 The living donor pool may be expanded through the use of programs to utilize nonrelated donors (e.g., kidney paired donations, non-directed donation).
Box 196-1
Common Indications for Kidney Transplant*
2008 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: transplant data 1998-2007. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association, Ann Arbor, MI. Available at: http://optn.transplant.hrsa.gov/
Pancreas Transplants
Pancreas transplantation for control of diabetes was first successfully reported by Lillehei and colleagues in 1970.4 The major indication for transplantation of this organ is diabetes mellitus. Because of the significant morbidity associated with immunosuppression, transplantation of this organ in isolation is uncommon; most pancreatic transplants are carried out in conjunction with a simultaneous or previous kidney transplant. In 2009, there were 854 simultaneous kidney-pancreas transplants and 379 pancreas-after-kidney transplants or solitary pancreas transplants.2 Isolated pancreatic islet cell transplantation (autotransplantation) has been utilized as an adjunct to total pancreatectomy for patients with intractable pain due to chronic pancreatitis and is an active area of research using human and genetically modified animal islet cells to produce insulin while minimizing the risks of immunosuppression.5 However, these techniques are difficult to apply outside of specialized centers.
Compared with other solid-organ transplant operations, rejection is more difficult to diagnose in pancreas transplantation for a number of reasons. Hyperglycemia is not manifested until a significant portion of the graft is lost. For grafts drained into the bladder, decreases in urinary amylase concentrations sometimes suggest that rejection is occurring, although this test is not very sensitive. Needle core biopsies under ultrasound guidance using 18- or 20-gauge needles have reduced complications associated with biopsies to 2% to 3%.6 The problem of detecting rejection of pancreatic grafts has prompted efforts to carry out simultaneous pancreatic and kidney transplants, using the kidney as a “canary” to detect rejection of both organs. This indicator of rejection is not as effective in pancreas-after-kidney transplants, because the two organs are immunologically distinct, as evidenced by higher pancreas graft loss rates after pancreas-after-kidney procedures as compared with simultaneous transplants (22% versus 15%).7 The advantage of early identification of rejection must be balanced against the increased risk of perioperative complications as a consequence of the more challenging simultaneous operation.
 Ethical Issues
Ethical Issues
A number of ethical issues are related to transplantation. Unstated (and/or unintended) coercion to donate can be overwhelming for the family members or loved ones of a patient with renal failure. The physician must act as an advisor, not only for the recipient but also for potential donors. The risk of mortality for donors is low (0%-0.03%), but there is a complication rate of 18%.8 There is some evidence that renal donors are at slightly increased risk for late renal failure after donation.9,10 These issues mandate a frank and open discussion prior to donation.
 Current Immunosuppressive Agents/Regimens
Current Immunosuppressive Agents/Regimens
The field of immunosuppression has undergone many changes over the past decade, driven by a much better understanding of the immune system, allowing the development of targeted therapies. Most patients will receive a combination of agents to prevent rejection. These agents include calcineurin antagonists (cyclosporine, tacrolimus), proliferation signal inhibitors (sirolimus, rapamycin, or everolimus), proliferation inhibitors (azathioprine, mycophenolate mofetil), and corticosteroids. Other agents frequently used to combat rejection include antilymphocyte antibodies and interleukin (IL)-2 receptor antagonists. Induction therapy with anti-T-cell antibodies or IL-2 receptor antibodies are commonly utilized in pancreas transplantation. A summary of these agents and mechanisms of action is provided in Table 196-2.
TABLE 196-2 Immunosuppressive Agents and Mechanism of Action
| Class of Agent | Uses | Mechanism of Action |
|---|---|---|
| Corticosteroids (methylprednisolone, prednisone) | Induction, maintenance, rejection | Redistribution of lymphocytes Block T-cell proliferation, IL-2 synthesis |
| Antilymphocyte antibodies (antithymocyte globulin, OKT-3) | Induction, rejection | Lymphocyte depletion |
| Humanized antibodies (basiliximab, daclizumab) | Induction, rejection | Specific targets: IL-2 receptor |
| Calcineurin inhibitors (cyclosporine, tacrolimus) | Maintenance, rejection | Inhibit IL-2 production Inhibit expansion and differentiation of T cells |
| Proliferation signal inhibitors (sirolimus [rapamycin], everolimus) | Maintenance | Block cytokine-driven cell cycle progression |
| Antimetabolites/antiproliferative agents (azathioprine, mycophenolate mofetil) | Maintenance | Inhibit RNA/DNA synthesis |
IL-2, interleukin-2.
Most of the immunosuppressants have significant side effects and toxicities and significant drug interactions. For a complete discussion of this issue, please see Chapter 176. Common side effects of immunosuppressive agents are summarized in Table 196-3.
TABLE 196-3 Side Effects of Common Immunosuppressive Agents
| Antithymocyte globulin | Fever, leukopenia, thrombocytopenia, serum sickness |
| Azathioprine, mycophenolate mofetil | Leukopenia, thrombocytopenia, anemia, diarrhea, abdominal pain, hepatotoxicity, pancreatitis |
| Basiliximab, daclizumab | Hypersensitivity (anaphylaxis), fever |
| Corticosteroids | Hyperglycemia, osteoporosis, impaired wound healing, hypertension, Cushingoid facies, Addisonian crisis (from rapid withdrawal) |
| Cyclosporine | Nephrotoxicity, neurotoxicity, drug interactions, hypertension, hyperkalemia, hirsutism, gingival hyperplasia |
| Sirolimus, everolimus | Hyperlipidemia, myelosuppression, impaired wound healing, diarrhea, arthralgia, pneumonitis |
| Tacrolimus | Nephrotoxicity, neurotoxicity, drug interactions, hypertension, hyperkalemia, diarrhea, diabetes, tremor |
| OKT-3 | Pulmonary edema, fever, rigors, diarrhea, headache, bronchospasm, increased cytomegalovirus infection, risk of posttransplant lymphoproliferative disorder |
 Common Related Diseases and Conditions
Common Related Diseases and Conditions
The vast majority of patients receiving kidney and/or pancreas transplants do not require admission to the intensive care unit (ICU). For those patients who do require admission, most are admitted because of perioperative difficulties, which are frequently related to an underlying medical disorder (Box 196-2). A number of medical illnesses are more common in patients with chronic renal failure, including atherosclerotic heart disease, hypertension, congestive heart failure, diabetes mellitus, chronic obstructive pulmonary disease, peripheral vascular disease, and cerebrovascular disease. Discussions with the patient or family often will reveal a history of one or more of these illnesses, allowing evaluation and treatment to be tailored appropriately for the patient.
 Routine Perioperative Care: Kidney or Kidney/Pancreas Transplant
Routine Perioperative Care: Kidney or Kidney/Pancreas Transplant
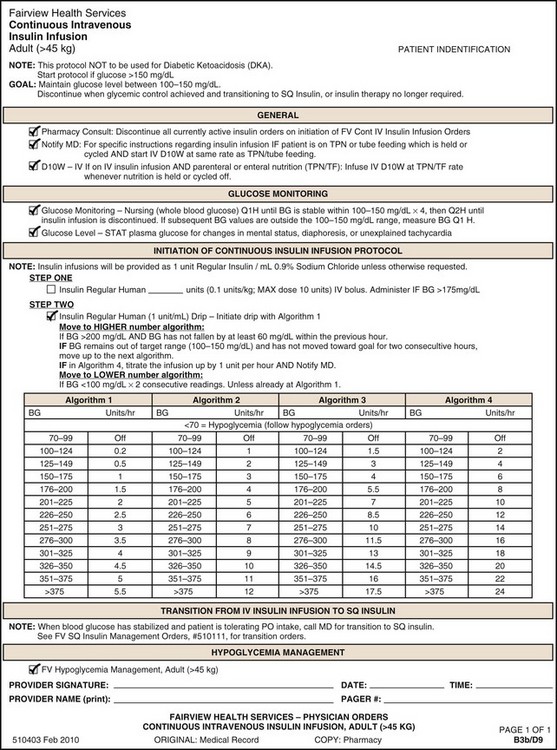
For typical kidney transplant recipients without acute tubular necrosis, a brisk diuresis begins within minutes of revascularization of the kidney graft. This diuresis is due to a number of factors including intraoperative administration of diuretics, proximal tubular damage related to allograft ischemia, fluid and electrolyte disturbances as a result of chronic renal failure, and osmotic factors related to uremia. In patients after kidney-pancreas transplantation, the diuresis also can be related to hyperglycemia. Tight control of blood glucose concentration should be achieved using an insulin infusion. Many patients who were euglycemic before transplantation become hyperglycemic after transplantation, owing to the effects of corticosteroids (occasionally administered to prevent rejection) and the stress of surgery. The appropriate target for blood glucose control remains controversial, with recent evidence showing no benefit to tight glucose control.11 Nonetheless, in pancreas transplant patients in particular, insulin infusions around the time of transplant have been associated with improved islet function.12 Our current practice is to maintain blood glucose concentration at 80 to 140 mg/dL. An example of an insulin drip protocol is noted in Figure 196-1. Urinary losses should be corrected with a hypotonic solution; a common prescription is 2.5% dextrose in 0.2% saline infused at a rate of 1 mL per milliliter of urinary output for the first 12 to 24 hours after transplantation. Sodium bicarbonate and potassium chloride should be added as needed, based on frequent measurements of serum electrolyte concentrations. Urine volumes of less than 100 to 200 mL/h within the first 12 hours after renal transplant may represent a problem with the graft, and this finding should be immediately communicated to the transplant service (Box 196-3).
Immunosuppression is typically initiated in the operating room and continued postoperatively. At most transplant centers, the dosing of the immunosuppressive agents is protocol driven and determined by the transplant service. Examples of standard protocols for kidney transplant and simultaneous kidney-pancreas transplant patients are illustrated in Table 196-4.
TABLE 196-4 Examples of Immunosuppression Protocols for Kidney Transplant and Simultaneous Pancreas-Kidney Transplant in the Immediate Postoperative Period*
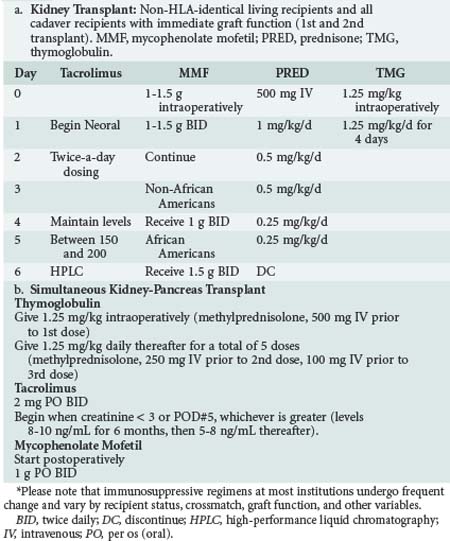
Several specific issues should be considered in pancreas transplantation aside from the usual management of kidney transplantation. The first of these is related to the high rate of graft loss in pancreas transplants owing to portal venous thrombosis. Many centers use a low-dose anticoagulation regimen of unfractionated heparin (100-500 units IV hourly as a continuous drip) in an effort to reduce graft loss from this complication. Systemic anticoagulation increases the risk of postoperative hemorrhage. Second, there is a high incidence of wound and intraabdominal infections after pancreas transplantation, being as great as 47% in some centers.13,14 Some centers advocate longer courses of broad-spectrum antibiotics because of concerns about infection, although data to support this practice are lacking.
 Posttransplant Complications
Posttransplant Complications
Posttransplant issues requiring ICU admission can be divided into those occurring immediately post transplant and those occurring at some time remote to the perioperative period. Kidney transplant patients are admitted to the ICU at a frequency of 16 per 1000 patient-years and have a mortality rate associated with admission of 40%, significantly higher than the general population.15 Common postoperative complications after kidney and/or pancreas transplantation are listed in Table 196-5.
TABLE 196-5 Common Postoperative Complications: Kidney, Kidney-Pancreas, Pancreas Transplant
| Early | Late |
|---|---|
| Myocardial infarction | Myocardial infarction |
| Renal failure | Renal failure |
| Hyperglycemia | Transplant artery stenosis |
| Graft thrombosis | Respiratory failure |
| Hemorrhage | Posttransplant infection (immune-compromised host) |
| Wound infection | |
| Respiratory failure | Posttransplant lymphoproliferative disorder |
| Posttransplant infection (hospital acquired) | Graft pancreatitis |
| Deep venous thrombosis | Acute and chronic rejection |
| Metabolic acidosis | |
| Graft pancreatitis | |
| Hyperacute and acute rejection | |
| Bladder leak | |
| Pseudomembranous colitis |
Respiratory Failure Distant To Transplant
Initial treatment for posttransplant respiratory failure distant to surgery requires broad-spectrum antibacterial, fungal, and viral therapy until a definitive diagnosis is reached. It is not unusual in such circumstances to have patients on agents that will cover common bacterial organisms, Candida and Aspergillus, and CMV (see also Chapter 195). Common regimens include broad-spectrum antibiotic agents with antipseudomonal and antianaerobic activity, an agent with gram-positive activity, a broad-spectrum antifungal agent, and ganciclovir to provide antiviral coverage for cytomegalovirus and other members of the herpesvirus family. Similarly to other work in the ICU care, delay to appropriate antibiotics in transplant patients has been associated with worsened outcomes.16 A number of appropriate agents for this purpose are listed in Table 196-6. In situations where Pseudomonas is strongly suspected, an additional agent should be added to provide double coverage of this organism. In situations where the patient has high risk for or has known vancomycin-resistant Enterococcus faecium, one of the new gram-positive agents should be chosen. Another key component of treatment in this setting is strong consideration for short-term discontinuation of most immunosuppressive medications. The practice at our institution is to hold all but maintenance doses of corticosteroids when infection is strongly suspected. It is frequently possible to tailor antimicrobial therapy as results return. For instance, in the setting of a patient with a low white blood cell count, diffuse pneumonitis, and positive screen for CMV, it is not unreasonable to discontinue antifungal therapy. It is important for the intensivist to be willing to revisit the diagnosis on at least a daily basis, especially if the clinical course is not consistent with the working diagnosis.
TABLE 196-6 Empirical Agents for Early Treatment of Infection in Kidney/Pancreas Transplant Patients
| Class of Agent | Agent | Dose* |
|---|---|---|
| Broad-spectrum antibiotic agents† | Piperacillin/tazobactam | 3.375 g IV q 6 h |
| Meropenem | 0.5-1 g IV q 8 h | |
| Imipenem/cilastatin | 0.5-1 g IV q 6-8 h | |
| Gram-positive agents‡ | Vancomycin | 1-1.5 g IV q 12-24 h |
| Daptomycin§ | 4-6 mg/kg IV daily | |
| Quinupristin/dalfopristin | 7.5 mg/kg IV q 8 h | |
| Linezolid | 600 mg IV q 12 h | |
| Tigecycline|| | 100 mg IV load, 50 mg IV q 12 h | |
| Antifungal agents | Voriconazole | 6 mg/kg IV q 12 h × 2, then 4 mg/kg IV q 12 h |
| Posaconazole | Oral only: 200 mg 3-4 times daily | |
| Caspofungin | 70 mg load, 50 mg IV daily | |
| Anidulafungin | 200 mg load, 100 mg IV daily | |
| Liposomal amphotericin B | 3-10 mg/kg IV daily | |
| Antiviral agents¶ | Ganciclovir | 2.5-5 mg/kg IV q 12 h |
| Foscarnet | 90 mg/kg IV q 12 h |
* Please note that doses given do not account for renal or hepatic insufficiency common in critically ill patients. Prior to choosing an empirical antibiotic regimen, the clinician should carefully consider the patient scenario and medication side effects related to the specific patient.
† Rather than a single agent, combination agents covering both gram-negative organisms and anaerobes may be chosen (e.g., fluoroquinolone plus clindamycin or metronidazole). For cases with a strong suspicion for Pseudomonas aeruginosa infection, additional Pseudomonas coverage should be added (e.g., fluoroquinolone or aminoglycoside).
‡ When vancomycin-resistant Enterococcus faecium infection is suspected, one of the latter 4 choices should be employed.
§ Daptomycin is not indicated for treatment of pneumonia (package insert).
|| Tigecycline is not indicated for treatment of hospital-acquired pneumonia (package insert).
¶ Antiviral agents directed toward herpesvirus family (most commonly CMV). Adjust for other viruses.
Postoperative Oliguria
Postoperative oliguria (see Box 196-3) is a frequent problem in the renal transplant patient. Common causes include blood clots in the bladder causing outflow obstruction, acute tubular necrosis, arterial or venous thrombosis, and acute rejection. Many patients present in the immediate postoperative period with oliguria and suspected acute tubular necrosis (also called delayed graft function in this context). In these patients, it is important to monitor fluid balance closely; many will require urgent dialysis for fluid overload or hyperkalemia. Most patients with acute tubular necrosis in the early postoperative period recover adequate renal function and become able to function without dialysis, albeit with less renal reserve than those patients with immediate graft function.17 Recovery can be delayed for as long as 3 months.
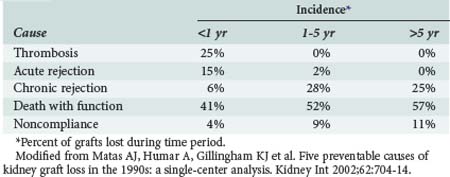
Among patients who underwent transplantation in the more distant past, the likely causes of oliguria are quite different and include acute or chronic rejection, renal artery stenosis, toxic effects of medications, especially calcineurin inhibitors, and BK virus nephropathy. Major causes of graft loss after kidney and/or pancreas transplant are listed in Table 196-7.18 Important studies in addition to baseline laboratory assays should include drug levels of calcineurin inhibitors, Doppler ultrasound of the transplant, and radioisotope scan. Renal biopsy and angiography also may be indicated. BK viremia and viruria can be detected by qualitative and quantitative polymerase chain reaction (PCR) techniques.19 Ultrasound is an excellent noninvasive way to screen for vascular complications, including renal artery stenosis, arteriovenous fistulas, and pseudoaneurysms. Radioisotope scans are a very useful noninvasive modality for assessment of renal function.20 Management depends on diagnosis but requires careful titration of IV fluids based upon clinical assessment of intravascular volume status and control of hypertension. Renal artery stenosis is typically treated successfully with angiographic stent placement.
Hypertension
Hypertension is common both immediately post transplant and long term. There is evidence that early postoperative hypertension is associated with delayed graft function,21,22 making perioperative control of hypertension an important feature of postoperative care. Acute management of hypertension in the ICU consists of appropriate parenteral antihypertensives, including β-adrenergic blockers or hydralazine.23 There are no specific guidelines for appropriate agents in transplant patients. We use an intermediate-acting beta-blocker such as labetalol (10-20 mg IV every 4 to 6 hours) until heart rate is less than 90 beats per minute, then IV hydralazine (10-20 mg IV every 4-6 hours) as needed. A continuous infusion of esmolol offers the benefits of rapid titration. Sodium nitroprusside is reserved for hypertension not controlled with other measures, because of concerns about cyanide toxicity. It is important in this population to titrate blood pressure so that perfusion pressure to the transplanted organ is maintained.
Tacrolimus and cyclosporine are associated with development of new hypertension in patients after renal transplant (25% and 35% of cases, respectively).22 Long-term control of hypertension after renal transplantation can be managed with a number of classes of agents, including calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin-II type-1 receptor blockers, diuretics, and β-adrenergic blockers. Many authors suggest use of a calcium channel blocker as first-line therapy for chronic use, owing to evidence that these agents can reduce cyclosporine-induced renal damage.23
Myocardial Infarction
Patients receiving chronic dialysis and those with diabetes mellitus are at increased risk of MI. In a single-center study of approximately 2700 kidney transplant recipients, the incidence of perioperative cardiac complications was 6.1%.24 Risk factors for posttransplant cardiac events include age, pretransplant cardiac disease, diabetes, arrhythmia, and low ejection fraction (<40%).25 Preoperative cardiac evaluation and percutaneous coronary intervention in this population may help reduce the perioperative risk of death but does not necessarily reduce the risk of perioperative MI.26 It is prudent in a patient with risk factors to consider perioperative β-adrenergic blockade and aspirin.
The clinical diagnosis of MI is difficult in many cases because of perioperative pain. It is prudent to evaluate at-risk patients with perioperative measurements of circulating troponin levels. Cardiac screening should be considered in diabetics, in patients with a history of cardiac disease, and in patients with intraoperative hypotension. Elevated circulating troponin levels should be followed by transthoracic echocardiography to evaluate for new wall-motion abnormalities in addition to ECG testing. Treatment of MI in early perioperative patients typically does not include thrombolytic therapy, owing to concerns for hemorrhage. This factor and the different pathophysiology of perioperative MI contributes to increased mortality in the transplant population (20%)25 as well as others (25%).26 For hemodynamically stable patients with only slight increases in circulating troponin levels and new wall-motion abnormalities on echocardiography, the most prudent course may be medical therapy consisting of aspirin and β-adrenergic blockade with or without systemic heparinization. Invasive intervention may be indicated for patients with hemodynamic instability or other signs of progression of MI.
Gastrointestinal Problems
The transplant population is at risk for development of upper and lower gastrointestinal (GI) tract involvement with CMV, leading to abdominal pain, bleeding, and (rarely) perforation. CMV will most commonly occur the first time within about 6 months of transplantation, correlating with the highest immunosuppressive load. CMV infections are more common in patients when the recipient was serologically negative, but the donor was CMV positive. CMV-related problems are also more common among those patients with known CMV infection and those treated with relatively high doses of immunosuppression.27 Diagnostic endoscopy should include tissue biopsies of the stomach or colon to determine whether CMV is present. Initial treatment consists of IV ganciclovir or foscarnet, with a switch to maintenance therapy by oral agents as tolerated for a period of weeks to months (see Table 196-6 for initial IV dosing).
Colon perforation and lower GI hemorrhage are the most common lower tract complications in kidney transplant recipients. Immunosuppressive therapy can mask the signs and symptoms of peritonitis, delaying diagnosis of perforation. Colonic perforation can be due to pseudomembranous enterocolitis, acute colonic pseudo-obstruction (Ogilvie’s syndrome), diverticulitis, ischemic colitis, stercoral perforation, fecal impaction, or other forms of colitis. Diverticulitis may be more common in patients with polycystic kidney disease (20% versus 3% in one small retrospective analysis),28 and this group of patients also had a higher incidence of GI surgical complications.29 Colonic perforation is an infrequent complication post transplant (21 of 1611 transplants at one center) with a high risk of death (24%). Perforation in this setting is associated with high-dose immunosuppression.30 Surgical therapy for perforated diverticulitis typically includes colostomy, because a fresh anastomosis in this setting is more likely to leak. Perioperative therapy should include broad-spectrum antibiotics directed at gram-negative and gram-positive aerobes, anaerobes, and fungi. Stress-dose corticosteroids should be administered as clinically indicated.
Pseudomembranous Colitis
Pseudomembranous colitis due to Clostridium difficile should be suspected in any transplant patient presenting with diarrhea. Risk factors for development of pseudomembranous colitis include previous antibiotic therapy and immunosuppression. Pseudomembranous colitis is diagnosed by detecting C. difficile toxin in stool. Controversy exists regarding the need for treatment in patients who are C. difficile culture positive but C. difficile toxin negative, because C. difficile may be present but not pathogenic. The development of serious complications due to C. difficile, such as toxic megacolon, are directly related to the time from onset of symptoms to the time of initiation of therapy. There is a new virulent strain of C. difficile (NAP1/B1/027) associated with increased morbidity and mortality, making the need for early diagnosis a high priority.31 Empirical therapy should be started when the diagnosis is considered and then discontinued if stool samples are negative for C. difficile toxin. Therapy for C. difficile enterocolitis consists of either metronidazole (250 mg PO every 6 hours for 10 days) or vancomycin (125 mg PO every 6 hours for 10 days). The cure rate for C. difficile enterocolitis seems to be higher for vancomycin than metronidazole, especially in more severe disease (97% versus 76%).32 Given this concern, it seems prudent to consider oral vancomycin for transplant patients with a clinical scenario consistent with C. difficile infection.
Rejection
Rejection is classified according to its temporal relation to the transplant and includes hyperacute, acute, and chronic rejection. Each of these types is mediated via different immunologic mechanisms. Hyperacute rejection occurs within minutes to hours of the transplant and is caused by preformed antibody directed against the transplanted organ. This type of rejection is very uncommon owing to appropriate pretransplant tissue typing. Acute rejection is the most common type of rejection in current clinical transplantation (occurring in 15%-60% of renal transplant patients).33 This type of rejection is most frequent within the first 6 weeks to 6 months after transplantation and is the result of activation of host T lymphocytes by antigens in the transplanted organ. Chronic rejection is common in transplanted organs, developing typically over years to decades. Its etiology is less well understood, but it appears to be related to accumulation of microvascular injury over time.
Diagnosis of Rejection
Acute rejection of a renal allograft is typically suspected when the serum creatinine and BUN concentrations increase. Other causes should be considered as well, including hypovolemia, drug toxicity, ureteral obstruction, lymphocele, or vascular anastomotic complications. Diagnosis of acute rejection is confirmed by percutaneous biopsy and histopathologic examination, which show edema and focal infiltration of the interstitium and peritubular capillaries by lymphocytes. Another characteristic finding of acute rejection is invasion of tubular epithelial cells by lymphocytes. Diagnosis of pancreas allograft rejection is more problematic. Increased rates of rejection have been reported after simultaneous kidney-pancreas transplant compared with kidney transplant alone.34 Hyperglycemia is a late finding and occurs after loss of significant islet cell mass. For simultaneous kidney-pancreas grafts, increases in the serum creatinine concentration may prompt suspicion of pancreatic rejection as well. If the pancreatic duct has been anastomosed to the bladder, a decrease in urinary amylase concentration may be helpful as a marker of graft rejection.35 New techniques using biomarkers have much promise but have not yet been widely evaluated.36 Biopsy of the pancreas graft may be performed either percutaneously or via cystoscopy to confirm the presence of rejection. This procedure has a complication rate of 2.8%.6 Because the incidence of venous thrombosis is high in pancreas transplantation, Doppler ultrasound should be performed to evaluate this possibility.
Treatment of acute rejection varies between transplant centers. A common initial approach is bolus therapy with high-dose methylprednisolone at a dose of 500 mg to 1 g IV daily. Severe rejection is more commonly treated with antibody therapy consisting of OKT3 or one of the newer antibodies. These treatments are beyond the scope of the present discussion and have been recently reviewed.37–39 Treatment of acute rejection is usually successful and is typically followed by adjustment of immunosuppression with a switch to different agents.
Graft Thrombosis
Arterial or venous thrombosis of the kidney allograft should be considered promptly if an established diuresis abruptly ceases in the immediate postoperative period. The transplant service should be immediately notified because prompt reoperation provides the only opportunity for salvage. The diagnosis can be rapidly established either by Doppler ultrasound or by inspection at the time of reoperation. Pancreatic allograft thrombosis may be related to either technical problems or high vascular resistance in the graft from preservation-related or immunologic injury. The incidence of graft thrombosis for pancreas allografts is 6%.2 Many centers routinely administer low-dose heparin, dextran, or antiplatelet agents to prevent this complication (e.g., unfractionated heparin, 100-300 units IV/h; aspirin, 325 mg PO/d). The use of anticoagulant and/or antiplatelet therapy in this population may be associated with an increased risk of bleeding complications. Signs of pancreatic graft thrombosis include hematuria, tenderness, and swelling of the graft. Treatment for this condition is removal of the graft.
Deep Venous Thrombosis
Deep venous thrombosis is a common complication of most major surgical procedures, including kidney or pancreas transplantation.40 After these procedures patients should receive standard prophylaxis consisting of low-dose fractionated or unfractionated heparin (unfractionated heparin, 5000 units subcutaneously [SQ] twice daily; Lovenox, 0.5 mg SQ twice daily), and application of sequential compression devices.
Iliofemoral thrombosis occasionally follows renal or pancreas transplant, presumably owing to injury of the vein at the time of transplantation. Typically these thromboses respond to standard-dose anticoagulation. Thrombolytics may be considered, especially in the patient who is more than 2 to 3 weeks out from surgery. The use of vena cava filters for patients with proximal deep venous thrombosis, persistent pulmonary embolus, or bleeding complications of anticoagulation is potentially an issue because of the theoretical risk of occlusion of the transplanted renal or portal vein. However, compromised transplant function is rare after placement of a vena cava filter,41 and it is our practice to place a vena cava filter in this situation.
Transplant-Associated Infectious Disease
The price of success in transplantation is increased susceptibility to infections due to the need for suppression of the host’s immune response (see also Chapter 176). As many as 63% of solid-organ transplant recipients experience an infectious complication within the first year of transplant.42 The risk of infection is highest during the period of most intensive immunosuppression (typically the first 6-12 months) and increases with treatment of rejection. The most frequent infections seen early and late after transplantation are presented in Table 196-8.43 Infectious complications in the first month after transplantation are frequently caused by those organisms likely to cause disease in immunocompetent hosts. The time of greatest immunosuppression (1-6 months post transplant) is the time when the majority of opportunistic infections occur. These infections include a number of viral infections (most commonly CMV) and opportunistic fungal infections (most frequently Candida and Aspergillus).43 A high index of suspicion for the presence of infection should be maintained when evaluating transplant patients in the ICU. A key component to treatment of infection in transplant patients is decreasing immunosuppression, because many infections will not be successfully treated without this step.
TABLE 196-8 Risk of Infection After Transplant with Respect to Time After Transplant
| Within 6 Weeks | 6 Weeks to 6 Months | Greater Than 6 Months |
|---|---|---|
| Viral | ||
| Herpes simplex | Cytomegalovirus (pneumonia) | Cytomegalovirus (retinitis, colitis) |
| Hepatitis B, C | Hepatitis B, C | Hepatitis B, C |
| Epstein-Barr virus | Papillomavirus | |
| Varicella-zoster | Posttransplant lymphoproliferative disorder | |
| Influenza | ||
| Respiratory syncytial virus | ||
| Adenovirus | ||
| Polyoma (BK) virus | ||
| Bacterial | ||
| Nosocomial infection (e.g., line, pneumonia, wound, urinary tract infection) | Nocardiosis | Listeriosis |
| Listeriosis from Listeria monocytogenes | Tuberculosis | |
| Tuberculosis | ||
| Fungal | ||
| Candidosis | Candidosis | Cryptococcosis |
| Aspergillosis | Coccidioidomycosis | |
| Cryptococcosis | Histoplasmosis | |
| Coccidioidomycosis | P. jiroveci infection | |
| Histoplasmosis | ||
| Pneumocystis jiroveci infection | ||
| Parasitic | ||
| Strongyloidosis | Strongyloidosis | |
| Toxoplasmosis | ||
| Leishmaniasis | ||
| Trypanosoma cruzi infection | ||
Modified from Snydman DR. Epidemiology of infections after solid-organ transplantation. Clin Infect Dis 2001;33:S5-S8.
Bacterial infections are common in the first 30 days after transplant and are related both to the site of surgery and the presence of indwelling lines and catheters. Infection of the surgical site is uncommon in the renal transplant recipient (1%-2%) and is comparable to the incidence seen in surgery of immunocompetent patients. Pancreas transplantation, on the other hand, is associated with a 10% to 40% incidence of wound infection.13,14 Infections from these wounds reflect skin flora, flora of the duodenum and bladder, and flora associated with previous exposure to antibiotics.
Fungal Infections
The immunosuppression associated with solid-organ transplantation increases the risk of fungal infection. The incidence of these infections also may be increased because of the use of broad-spectrum antibacterial agents. Useful agents for treating fungal pathogens include amphotericin B, azoles, and echinocandins. Amphotericin B acts to prevent fungal growth and kills fungi by binding to fungal cell wall sterols and causing cell death via lysis. Azoles inhibit the cytochrome P450 enzyme responsible for ergosterol synthesis. Echinocandins inhibit glucan synthesis, disrupting cell wall structure. The different mechanisms of action of the echinocandins and azoles make consideration of dual therapy attractive. A recent report of transplant recipients with invasive aspergillosis receiving combination therapy of voriconazole and caspofungin showed improved survival in patients with either renal failure or Aspergillus fumigatus infection compared to those receiving a lipid formulation of amphotericin B.44
The most common fungal pathogens seen are Candida species. The widespread use of fluconazole has likely contributed to the increased isolation of Candida species resistant to fluconazole. Treatment of suspected fungal infection in the transplant patient in the ICU should therefore consist of an agent with more broad-spectrum antifungal activity, such as amphotericin B (most commonly one of the liposomal forms), caspofungin or anidulafungin (an echinocandin), or voriconazole (an azole with broader antifungal activity). Aspergillus infection occurs in approximately 1% of transplant patients and should be considered in patients failing to respond to appropriate initial antimicrobial therapy. The diagnosis of aspergillosis is frequently difficult, and the intensivist may need to empirically initiate therapy well before a final diagnosis is established. Newer diagnostic methods such as galactomannan assay or real-time polymerase chain reaction for Aspergillus in the serum or bronchoalveolar lavage (BAL) fluid may allow an earlier diagnosis.45,46 Sensitivity and specificity of the galactomannan assay is significantly higher in BAL than serum.46 The high mortality associated with invasive aspergillosis in this population (60%)47 mandates early empirical therapy.
Viral infections are important causes of morbidity and mortality in renal and pancreas transplant recipients. Endemic viruses of little concern to the immunocompetent population may produce life-threatening infection in the immunosuppressed host. Common viral pathogens in the kidney and pancreas transplant patient include members of the human herpesvirus family, most notably CMV. Infection with this agent affects nearly 50% of kidney and transplant patients; infection occurs during the period from 2 weeks to 3 months after transplantation.48 The major risk factors for CMV infection include CMV seronegativity when the donor is seropositive, need for higher doses of immunosuppression, or repeated treatment for rejection.27 The range and severity of infection with CMV is broad. The most commonly affected organs are the lungs, GI tract, liver, retina, and pancreas. The diagnosis of CMV has been recently enhanced by assays identifying CMV antigen in blood or body fluid.49,50 The primary treatment of CMV infection is prevention, and many transplant centers include ganciclovir or other antiviral therapy in their protocols (valganciclovir, 900 mg once daily; or oral ganciclovir, 1000 mg three times daily within 10 days of transplant and continued through 100 days).51 Treatment of suspected or identified CMV infection typically consists of IV ganciclovir followed by oral valganciclovir (see Table 196-6).
Polyoma (BK) Virus Infection
Polyomavirus has a prevalence worldwide of about 98% in the general population, with nearly all exposed as a child. After infection, the virus resides in the kidney in a latent form. Reactivation of the virus in kidney transplant recipients can result in an inflammatory interstitial nephritis progressing to renal failure known as BK- or polyoma-associated nephropathy. Interestingly, this reactivation has so far been associated with complications only in kidney transplant patients. Reactivation has been most closely associated with tacrolimus use and recent treatment for rejection. A prospective study of renal transplant patients has identified the time course of this reactivation process.52 BK viruria proceeds BK viremia and in renal transplant patients occurred an average of 54 days after transplant. Blood BKV PCR was positive a median of 32 weeks prior to diagnosis of BK nephropathy. In patients with unexplained renal failure after renal transplant, the presence of BK virus should be evaluated using PCR in urine and blood. Patients with a viremia should undergo reduction of immunosuppression. Cidofovir has been evaluated as further treatment but has not demonstrated additional benefit in outcome.53
Pneumocystis jiroveci (Previously carinii)
Pneumocystis jiroveci is a common cause of pneumonia in immunosuppressed patients and should be considered in any patient presenting with respiratory illness who has had prophylactic therapy (trimethoprim/sulfamethoxazole or dapsone) interrupted. Recent work has led to the reclassification of pneumocystis as an unusual fungus,54 although some authors have disputed this reclassification.55 Empirical therapy with IV trimethoprim/sulfamethoxazole (15 mg/kg of the trimethoprim component per day given in 3 divided doses) or pentamidine (4 mg/kg/d) should be initiated before established diagnosis in this patient population because of the high mortality rate of the untreated disease.
Posttransplant Lymphoproliferative Disorder
Posttransplant lymphoproliferative disorder (PTLD) includes a broad range of conditions ranging from simple lymphoid hyperplasia to lymphoma. The etiology of this disorder in the transplant patient is closely related to infection with Epstein-Barr virus (EBV). PTLD typically occurs during times of most intensive immunosuppression. The incidence of PTLD is low in renal and pancreas transplantation compared with other solid-organ transplants (2.6% at 10 years).1 The clinical presentation of this disorder varies widely, and many patients present with nonspecific symptoms such as malaise, fever, and weight loss. Occasionally, patients present to the ICU acutely ill with a markedly elevated blood lactate level that is unresponsive to aggressive fluid resuscitation. Evaluation for suspected PTLD should include imaging of the brain, chest, and abdomen, with targeted biopsies to provide a tissue diagnosis. Treatment of patients with PTLD has not been well codified but may include reduction of immunosuppression, administration of interferon alfa (IFN-α), antiviral therapy, chemotherapy, and treatment with an anti-B-cell antibody (rituximab).56–57 A new approach in monitoring transplant patients is to follow serial Epstein-Barr viral load and use increases as a tool to direct reduction of immunosuppression or other therapy.58
 Pancreas Transplant
Pancreas Transplant
Bladder leak is most commonly from the duodenal segment of the donor pancreas; this is due to devascularization during the graft preparation process or during placement of the graft. A frequent complication (10% of cases), bladder leak is most common during the first several weeks after transplantation.59 Diagnosis is aided by having a high index of clinical suspicion and may be confirmed with a high degree of accuracy by CT of the area using contrast agent instilled into the bladder.60 Treatment for smaller leaks consists of prolonged Foley catheter drainage, whereas larger or chronic leaks require operative intervention.
Graft pancreatitis occurs in 16% of pancreas transplant patients and is a significant cause of graft loss.61,62 Graft pancreatitis early after transplantation is due to preservation-related or ischemic injury to the pancreas and typically is self-limited. However, the development of peripancreatic fluid collections necessitates evaluation for infection and may require operative intervention ranging from opening a deep abscess, to débridement of the involved portions of the pancreas, to removal of the entire pancreatic allograft. Removal, if necessary, is best performed early before development of established organ dysfunction.62 Late graft pancreatitis is related to reflux from the bladder or CMV infection and can be treated by conversion to enteric drainage or specific antiviral therapy, respectively.
Key Points
Klouche K, Amigues L, Massanet P, et al. Outcome of renal transplant recipients admitted to an intensive care unit: a 10-year cohort study. Transplantation. 2009;87:889-895.
Jeloka TK, Ross H, Smith R, et al. Renal transplant outcome in high-cardiovascular risk recipients. Clin Transplant. 2007;21:609-614.
Thomas MC, Mathew TH, Russ GR, et al. Perioperative blood pressure control, delayed graft function, and acute rejection after renal transplantation. Transplantation. 2003;75:189-195.
Matas AJ, Humar A, Gillingham KJ, et al. Five preventable causes of kidney graft loss in the 1990s: a single-center analysis. Kidney Int. 2002;62:704-714.
Catena F, Ansaloni L, Gazzotti F, Bertelli R, et al. Gastrointestinal perforations following kidney transplantation. Transplant Proc. 2008;40:1895-1896.
Evens AM, David KA, Helenowski I, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010;28:1038-1046.
1 http://optn.transplant.hrsa.gov/latestData/rptdata.asp, 4 May, 2010. accessed
2 2008 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1998-2007. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association, Ann Arbor, MI.
3 Nanidis TG, Antcliffe D, Kokkinos C, Borysiewicz CA, Darzi AW, Tekkis PP, et al. Laparoscopic versus open live donor nephrectomy in renal transplantation: a meta-analysis. Ann Surg. 2008 Jan;247(1):58-70.
4 Lillehei RC, Simmons RL, Najarian JS, et al. Pancreaticoduodenal allotransplantation: Experimental and clinical experience. Ann Surg. 1970;172:405.
5 Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008 Dec 27;86(12):1799-1802.
6 Drachenberg CB, Odorico J, Demetris AJ, et al. Banff schema for grading pancreas allograft rejection: working proposal by a multi-disciplinary international consensus panel. Am J Transplant. 2008 Jun;8(6):1237-1249.
7 Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 2005 Aug;19(4):433-455.
8 Friedman AL, Cheung K, Roman SA, Sosa JA. Early clinical and economic outcomes of patients undergoing living donor nephrectomy in the United States. Arch Surg. 2010 Apr;145(4):356-362.
9 Ellison MD, McBride MA, Taranto SE, et al. Living kidney donors in need of kidney transplants: A report from the organ procurement and transplantation network. Transplantation. 2002;74:1349-1351.
10 Fehrman-Ekholm I, Nordén G, Lennerling A, et al. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82:1646.
11 NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009 Mar 26;360(13):1283-1297.
12 Koh A, Senior P, Salam A, Kin T, et al. Insulin-heparin infusions peritransplant substantially improve single-donor clinical islet transplant success. Transplantation. 2010 Feb 27;89(4):465-471.
13 Perdiz LB, Furtado GH, Linhares MM, et al. Incidence and risk factors for surgical site infection after simultaneous pancreas-kidney transplantation. J Hosp Infect. 2009 Aug;72(4):326-331.
14 Michalak G, Kwiatkowski A, Bieniasz M, et al. Infectious complications after simultaneous pancreas-kidney transplantation. Transplant Proc. 2005 Oct;37(8):3560-3563.
15 Klouche K, Amigues L, Massanet P, et al. Outcome of renal transplant recipients admitted to an intensive care unit: A 10-year cohort study. Transplantation. 2009;87(6):889-895.
16 Lupei MI, Mann HJ, Beilman GJ, et al. Inadequate antibiotic therapy in solid organ transplant recipients is associated with a higher mortality rate. Surg Infect (Larchmt). 2010 Feb;11(1):33-39.
17 Gonwa TA, Mai ML, Smith LB, et al. Immunosuppression for delayed or slow graft function in primary cadaveric renal transplantation. Clin Transplant. 2002;16:144-149.
18 Matas AJ, Humar A, Gillingham KJ, et al. Five preventable causes of kidney graft loss in the 1990s: A single-center analysis. Kidney Int. 2002;62:704-714.
19 Babel N, Fendt J, Karaivanov S, et al. Sustained BK viruria as an early marker for the development of BKV-associated nephropathy. Transplantation. 2009;88(1):89-95.
20 Brown ED, Chen MY, Wolfman NT, et al. Complications of renal transplantation: Evaluation with US and radionuclide imaging. Radiographics. 2000;20:607-622.
21 Thomas MC, Mathew TH, Russ GR, et al. Perioperative blood pressure control, delayed graft function, and acute rejection after renal transplantation. Transplantation. 2003;75:189-195.
22 Nichelle L, Canet S, Garrigue V, et al. Arterial hypertension in renal transplant recipients treated with tacrolimus or cyclosporin-Neoral. Transplant Proc. 2002;34:2824-2825.
23 Zhang R, Leslie B, Boudreaux JP, et al. Hypertension after kidney transplantation: Impact, pathogenesis, and therapy. Am J Med Sci. 2003;325:202-208.
24 Humar A, Kerr SR, Ramcharan T, et al. Peri-operative cardiac morbidity in kidney transplant recipients: Incidence and risk factors. Clin Transplant. 2001;15:154-158.
25 Jeloka TK, Ross H, Smith R, et al. Renal transplant outcome in high-cardiovascular risk recipients. Clin Transplant. 2007;21:609-614.
26 Mangano DT, Browner WS, Hollenberg M, et al. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781-1788.
27 Browne BJ, Young JA, Dunn TB, Matas AJ. The impact of cytomegalovirus infection ≥1 year after primary renal transplantation. Clin Transplant. 2010 Jan 24. [Epub ahead of print]
28 Lederman ED, McCoy G, Conti DJ, Lee EC. Diverticulitis and polycystic kidney disease. Am Surg. 2000;66(2):200-203.
29 Benjamin ER, Jim J, Kim TJ, et al. Acute care surgery after renal transplantation. Am Surg. 2009 Oct;75(10):882-886.
30 Catena F, Ansaloni L, Gazzotti F, Bertelli R, et al. Gastrointestinal perforations following kidney transplantation. Transplant Proc. 2008 Jul-Aug;40(6):1895-1896.
31 Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009 Apr 7;15(13):1554-1580.
32 Al-Nassir WN, Sethi AK, Nerandzic MM, et al. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis. 2008;47:56-62.
33 Djamali A, Premasathian N, Pirsch JD. Outcomes in kidney transplantation. Semin Nephrol. 2003;23(3):306-316.
34 Rerolle JP, Thervet E, Anglicheau D, et al. Long-term renal allograft outcome after simultaneous kidney and pancreas transplantation. Nephrol Dial Transplant. 2002;17:905-909.
35 Prieto M, Sutherland DER, Fernandez-Cruz L, et al. Experimental and clinical experience with urine amylase monitoring for early diagnosis of rejection in pancreas transplantation. Transplantation. 1987;43:73-79.
36 Cashion AK, Sabek O, Driscoll C. Serial analysis of biomarkers of acute pancreas allograft rejection. Clin Transplant. 2010 May 17. [Epub ahead of print]
37 Gruessner AC, Sutherland DE, Gruessner RW. Pancreas transplantation in the United States: a review. Curr Opin Organ Transplant. 2010 Feb;15(1):93-101.
38 Webster AC, Ruster LP, McGee R, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010 Jan 20;(1):CD003897.
39 Durrbach A, Francois H, Beaudreuil S, et al. Advances in immunosuppression for renal transplantation. Nat Rev Nephrol. 2010 Mar;6(3):160-167.
40 Poli D, Zanazzi M, Antonucci E, et al. Renal transplant recipients are at high risk for both symptomatic and asymptomatic deep vein thrombosis. J Thromb Haemost. 2006 May;4(5):988-992.
41 Pasquale MD, Abrams JH, Najarian JS, Cerra FB. Use of Greenfield filters in renal transplant patients—are they safe? Transplantation. 1993;55:439-442.
42 Rostambeigi N, Kudva YC, John S, et al. Epidemiology of infections requiring hospitalization during long-term follow-up of pancreas transplantation. Transplantation. 2010 May 15;89(9):1126-1133.
43 Snydman DR. Epidemiology of infections after solid-organ transplantation. Clin Infect Dis. 2001;33(Suppl 1):S5-S8.
44 Singh N, Limaye AP, Forrest G, et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: a prospective, multicenter, observational study. Transplantation. 2006 Feb 15;81(3):320-326.
45 Bennett JE, Kauffman C, Walsh T, et al. Forum report: Issues in the evaluation of diagnostic tests, use of historical controls, and merits of the current multicenter collaborative groups. Clin Infect Dis. 2003;36(Suppl 3):S123-S127.
46 Meersseman W, Lagrou K, Maertens J, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med. 2008;177:27-34.
47 Singh N, Avery RK, Munoz P, et al. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46-52.
48 Geddes CC, Church CC, Collidge T, et al. Management of cytomegalovirus infection by weekly surveillance after renal transplant: Analysis of cost, rejection and renal function. Nephrol Dial Transplant. 2003;18:1891-1898.
49 Degre M, Kristiansen KI, Rollag H, et al. Detection of human cytomegalovirus (HCMV) pp67-mRNA and pp65 antigenemia in relation to development of clinical HCMV disease in renal transplant recipients. Clin Microbiol Infect. 2001;7:254-260.
50 Hadaya K, Wunderli W, Deffernez C, et al. Monitoring of cytomegalovirus infection in solid-organ transplant recipients by an ultrasensitive plasma PCR assay. J Clin Microbiol. 2003;41:3757-3764.
51 Paya C, Humar A, Dominguez E, et al. Valganciclovir Solid Organ Transplant Study Group. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4(4):611-620.
52 Agha I, Brennan DC. BK Virus and immunosuppressive agents. In: Ahsan N, editor. Polyomaviruses and human diseases. New York: Springer Science+Business Media, 2006.
53 Johnston O, Jaswal D, Gill JS, et al. Treatment of polyoma virus infection in kidney transplant recipients: A systematic review. Transplantation. 2010;89(9):1057-1070.
54 Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis proved) for Pneumocystis from humans. Emerg Infect Dis. 2002;8(9):891-896.
55 Hughes WT. Pneumocystis carinii vs. Pneumocystis jiroveci: Another misnomer. Emerg Infect Dis. 2003;9(2):276-277.
56 Ganne V, Siddiqi N, Kamaplath B, et al. Humanized anti-CD20 monoclonal antibody (rituximab) treatment for post-transplant lymphoproliferative disorder. Clin Transplant. 2003;17(5):417-422.
57 Evens AM, David KA, Helenowski I, et al. Multicenter analysis of 80 solid organ transplantation recipients with post-transplantation lymphoproliferative disease: outcomes and prognostic factors in the modern era. J Clin Oncol. 2010 Feb 20;28(6):1038-1046.
58 Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 2010 Apr;23(2):350-366.
59 Hakim NS, Gruessner AC, Papalois BE, et al. Duodenal complications in bladder-drained pancreas transplantation. Surgery. 1997;121:618-624.
60 Bischof TP, Thoeni RF, Melzer JS. Diagnosis of duodenal leaks from kidney-pancreas transplants in patients with duodenovesical anastomoses: Value of CT cystography. AJR Am J Roentgenol. 1995;165:349-354.
61 Fernandez-Cruz L, Sabater L, Gilabert, et al. Native and graft pancreatitis following combined pancreas-renal transplantation. Br J Surg. 1993;80:1429-1432.
62 Troppmann C, Gruessner AC, Dunn DL, et al. Surgical complications requiring early re-laparotomy after pancreas transplantation: A multi-variate risk factor and economic impact analysis of the cyclosporine era. Ann Surg. 1998;227:255-268.