195 Management of Patients After Heart, Heart-Lung, or Lung Transplantation
 Lung Transplantation
Lung Transplantation
Lung transplantation offers hope for improved survival and quality of life for selected patients with end-stage lung disease. The availability of suitable donor organs and preservation injury remain the initial limiting factors to successful transplantation. Like other transplants, rejection and infection as well as organ system dysfunction associated with the perioperative course remain challenges. However, experience over 40 years has led to substantial improvements in early outcome. This experience has been reflected in changes in various aspects of the field, including a different allocation system where priority is given based on medical urgency and expected outcome,1,2 donor and recipient assessments,3 innovative surgical techniques, better understanding of early complications, and the development of newer immunosuppressive medications. Nevertheless, obliterative bronchiolitis (OB) resulting from chronic rejection and non-cytomegalovirus (CMV) infections limit the long-term quality of life and is largely responsible for the 47% 5-year mortality rate for lung transplantation.4
Diagnoses for which adults receive lung transplantation include chronic obstructive pulmonary disease (COPD)/emphysema (35.8%), idiopathic pulmonary fibrosis (20.8%), cystic fibrosis (15.9%), α1-antitrypsin deficiency (7.1%), idiopathic pulmonary arterial hypertension (3.3%), and others including sarcoidosis, congenital heart diseases, and connective tissue disease complicated by advanced lung disease. These diagnoses have remained relatively unchanged, with the exception of procedures offered to patients with idiopathic pulmonary fibrosis going from 15% of all procedures in 2000 to 27% in 2007, and procedures offered to patients with idiopathic pulmonary arterial hypertension (previously called primary pulmonary hypertension) going from 13% in 1990 to 2% in 2007. Transplantation options include single lung transplant (SLT), bilateral lung transplant (i.e., sequential bilateral single lung transplantation [BLT]), heart-lung transplant (HLT), or living-donor lobar lung transplant (LDLLT). Over the last 15 years, the number of SLT procedures has remained stable, with a steady increase in the number of BLT procedures accounting for 69% of transplant procedures in 2007.4 The trend toward bilateral transplantation has been most noticeable in patients with chronic obstructive lung disease, either from emphysema or α1-antitrypsin deficiency, which is the most frequent diagnosis leading to transplantation.5
Donor selection, procurement, and lung preservation protocols tend to be individualized on an institutional basis. The limited availability of donor lungs, however, has increased the scrutiny with which organs are judged in order to avoid rejecting them inappropriately.3 Significant lung contusion, smoking-related lung damage, pneumonia, pulmonary edema, and significant aspiration are prime concerns in evaluating the suitability of donor organs. Although already described as an independent association for primary graft dysfunction (PGD, also known as primary graft failure [PGF] or pulmonary reimplantation response [PRR]),6 donor’s older age is being challenged at some centers as a risk factor for worsened outcomes.7 Procurement and lung preservation protocols often include administration of antiinflammatory agents, pulmonary vasodilators, and antioxidants.
Hyperinflation
In SLT with emphysema, the transplanted lung can be relatively noncompliant. As a result, the native lung may be hyperinflated. This is one reason that chronic obstructive lung disease recipients are preferably offered bilateral lung transplantation.4 This problem becomes even more apparent when higher levels of PEEP are needed because of allograft dysfunction. Hyperinflation of the native, more compliant lung leads to mediastinal shift, deterioration in gas exchange, and hemodynamic instability. Although inserting an expiratory pause into the ventilator cycle can be used to assess the level of intrinsic PEEP (“autopeep”), excessive air trapping is easily diagnosed by disconnecting the patient’s ETT from the ventilator tubing for 5 to 10 seconds. In patients with significant air trapping and hyperinflation, “popping the patient off” leads to a significant improvement in blood pressure and oxygenation. Management strategies using only a single ventilator to provide ventilation include reducing PEEP, reducing tidal volume, and accepting a modest level of respiratory acidosis. Alternatively, conversion to independent lung ventilation may be appropriate, particularly in the setting of significant allograft dysfunction and high PEEP requirements. In order to switch to independent lung ventilation, an EBT is advanced into the left mainstem bronchus, and the bronchial balloon is inflated. Positioning can be verified by measuring tidal volumes delivered to and returned from each lumen of the EBT. Bronchoscopy with a small-caliber bronchoscope is appropriate to verify that (1) the left bronchial balloon is distal to the carina and (2) that the end of the left endobronchial tube does not protrude too far distally into the left lung, compromising flow to either the upper or lower division bronchi. Ventilator settings are adjusted for each machine individually. Initially, PEEP for the allograft is set at 10 cm H2O, tidal volume is set at 3 mL/kg and rate is set at 20 to 25 breaths/min. Initial settings for the emphysematous native lung typically use a larger tidal volume and a slower rate with 0 to 2.5 cm PEEP. There is no need to synchronize the ventilators. Lung hyperinflation is associated with a longer ICU stay, longer duration of mechanical ventilation, and a trend toward worsened mortality.8
Early Postoperative Respiratory Complications
Airways are affected variably by the ischemic/implantation insult. Anastomotic dehiscences are rare, although a recent report suggests incidence of this complication is increased when sirolimus was used as an immunosuppressive agent.9 Anatomically, the transplanted bronchus derives its blood supply from the lung and pulmonary blood flow, since the bronchial arteries are not typically anastomosed. The longer left mainstem bronchus, particularly adjacent to the anastomosis, is at higher risk for ischemic injury compared with the right bronchus, which generally is anastomosed adjacent to the right upper lobe take-off. Early bronchoscopy often demonstrates relatively normal epithelium. However, more severe airway injury patterns can become apparent over the next several days. The earliest findings are patchy areas of subepithelial hemorrhage that can become confluent. In more severe cases, white plaques can form, and frank areas of desiccated sloughed epithelium become evident. In the most severe cases, eschar is evident, and bronchial cartilage may be exposed. Severe airway injury poses the risk of infection and bronchomalacia. The infections are typically due to Candida and Aspergillus species.10 In many centers, lung transplant recipients are treated prophylactically with antifungal agents such as inhaled amphotericin or an azole such as voriconazole. This strategy seems to reduce the rate of airway infection.10 If suspicious plaques are evident bronchoscopically, we perform bronchial biopsy to exclude invasive disease. Inhaled amphotericin B (50 mg twice daily) is generally administered to patients with severe airway injury and those with cultures demonstrating growth of fungus. Bronchomalacia is generally a long-term complication, although in some cases, this complication can become evident within the first 6 weeks after transplantation. Dynamic airway collapse or fixed stenoses are diagnosed by bronchoscopy. In addition to endobronchial infections, malacia, and stenosis, other airway complications include dehiscence, granulation tissue formation, and fistulas.11 These are not necessarily early complications, but increased awareness of their potential occurrence is warranted.
Primary graft dysfunction (PGD) is a severe form of ischemia-reperfusion injury (IRI) and is the leading cause of respiratory failure and morbidity early after transplantation.12 A recent consensus statement by the International Society of Heart and Lung Transplantation (ISHLT) Working Group on PGD standardized the grading of PGD on the basis of gas exchange (PaO2/FIO2 ratio) and plain chest radiologic findings.13 With this grading system, a grade 3 PGD (PaO2/FIO2 < 200 plus the presence of diffuse radiographic infiltrates) resembles the definition of acute respiratory distress syndrome (ARDS), and with this in mind, it has already been validated by demonstrating a worsened mortality and prolonged hospital stay.14 The reported incidence of grade 3 PGD ranges from 10% to 25%, with 30-day mortality close to 50%.15,16 Over 95% of patients have infiltrates in the allograft by chest roentgenogram during the first 72 hours.17 Although edema and atelectasis contribute to these early changes, worsening or persistent infiltrates most likely reflect diffuse alveolar damage (DAD) secondary to PGD. Although many cases of PGD are evident on chest films obtained on the first postoperative day, in some cases, it does not become apparent radiographically or physiologically for up to 72 hours post transplant. The timing of appearance of clinical and radiologic respiratory failure is extremely helpful, then, to elaborate a judicious differential diagnosis, understanding that while PGD occurs within hours and up to 3 days after transplantation, infection and rejection are more common past the first 24 to 36 hours. In some cases, patients will be successfully extubated only to deteriorate 24 hours later. When uncertain about the etiology, aggressive diagnostic efforts should be made, employing bronchoscopy, bronchoalveolar lavage (BAL), and biopsy to exclude superimposed infection or rejection. If pulmonary edema is unilateral, a diagnosis of pulmonary venous obstruction must be entertained. Although the incidence of this problem is extremely low, a transesophageal echocardiogram (TEE) should be performed to exclude unilateral venous obstruction.
The level of respiratory dysfunction secondary to IRI depends on the extent of the injury and the residual lung reserve. The latter factor is particularly important in SLT for emphysema, because the remaining native lung may have substantial residual function. The functional capacity of the native lung often allows the transplant recipient to tolerate a significant degree of allograft dysfunction.18 Such is typically not the case for IPF patients or recipients with significant pulmonary hypertension, since perfusion to the native lung is minimal once a donor lung with low pulmonary vascular resistance is implanted.
The management of PGD is largely supportive, including judicious diuresis and a protective ventilatory approach.19 Inhaled nitric oxide may be utilized to help address early postoperative hypoxemia.20,21 Extracorporeal membrane oxygenation (ECMO) remains a salvage therapy for severe PGD.22–24
Muscular weakness or mechanical issues can embarrass postoperative respiratory function. Patients requiring delayed closure of the clamshell incision, which is required in some patients with excessive bleeding, are at higher risk for respiratory dysfunction on this basis. Preoperative muscle wasting is a major contributing factor in most cases. Clinically significant phrenic nerve injury is rare. However, when dissection of the native lungs was difficult due to dense pleural adhesions, phrenic nerve injury can be present and significantly prolong the weaning process. Postoperative neuromuscular blockade is rarely used because of synergistic adverse effects on long-term neuromuscular function that have been associated with simultaneous administration of corticosteroids (a component of most immunosuppressive regimens) and neuromuscular blocking agents.25 If the patient has difficulty clearing secretions, tracheostomy should be performed early.
Patients are treated with broad-spectrum antibiotics perioperatively. In patients without suppurative lung disease, antibiotics are stopped within 72 hours if samples from the donor trachea and from the explanted lung are without pathogens. If cultures are positive for potential pulmonary pathogens, a directed course of antibiotics is continued for 7 to 10 days. In patients with septic lung disease, an antibiotic regimen based on preoperative cultures is continued for a minimum of 2 to 3 weeks. Patients also may be treated with prophylactic regimens to prevent CMV infection with valganciclovir if either the patient or the donor is CMV immunoglobulin G(IgG) positive before surgery (dose will vary with kidney function). Aggressive prophylaxis against deep vein thrombosis (DVT) is mandatory.26 At our center, critically ill transplant patients are screened liberally for DVT with lower extremity Doppler ultrasound examinations, with a low threshold for placement of an inferior vena caval filter when DVT is diagnosed.
New Pulmonary Infiltrates
Because the transplanted lung is in close contact with the external environment, the risk for infection is higher than is the case for other forms of organ transplantation. Accordingly, aggressive diagnostic efforts are the key to management of graft dysfunction after lung transplantation. Bacterial infections with traditional nosocomial pathogens are most common in the first 30 days. Subsequently, bacterial pneumonia is still responsible for the bulk of new infiltrates, although other etiologies must be considered as well. CMV infection, causing pneumonia among other problems, occurs in a large fraction of cases. Prophylactic and surveillance strategies to deal with CMV vary from center to center. Some institutions carry out weekly assays, seeking to detect CMV antigen in the bloodstream, and only institute early preemptive therapy when the antigen is present. Other centers provide prophylaxis using ganciclovir or valganciclovir for 3 to 6 months. Dosing of these antiviral medications will vary with the kidney function. Our center monitors CMV status via frequent plasma PCR assays.27 Other viruses such as adenovirus, parainfluenza virus, influenza virus, and respiratory syncytial virus are community-acquired pathogens that can cause significant morbidity and mortality.28
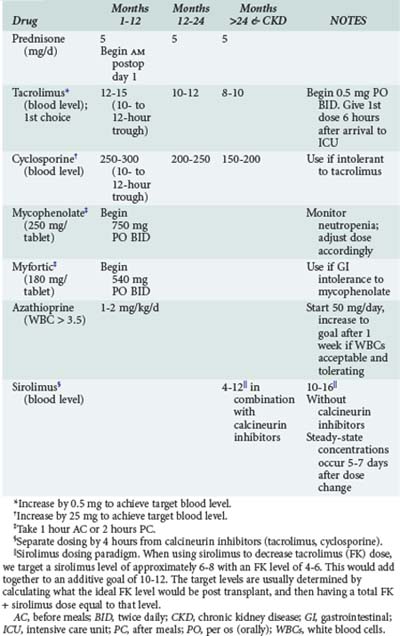
Acute cellular rejection (ACR) occurs in 36% of patients at some time in the first year after transplant.4 Patients typically present with allograft infiltrates, worsening hypoxemia and dyspnea. Fever and pleural effusion may occur, and pulmonary secretions are uncommon. Clinical findings have been shown to be inadequate for diagnosing ACR; establishing the diagnosis requires transbronchial biopsy.29 Maintenance immunosuppression in most centers is based on a three-drug regimen including either cyclosporine or tacrolimus, prednisone, and either mycophenolate or azathioprine. Sirolimus is also often included in the maintenance regimen in selected instances (Table 195-1). Episodes of significant acute rejection are treated with methylprednisolone (10-15 mg/kg intravenously [IV] daily × 3 days). Response usually occurs within 24-72 hours. Failure to respond should prompt re-biopsy to exclude refractory rejection.
Chronic rejection presents more insidiously. Findings are increased dyspnea, worsening pulmonary function test results, and sometimes cough. Pathologically, patients with chronic rejection manifest findings of OB with a lymphocytic infiltrate in the submucosa and epithelium plus submucosal fibrosis. These findings, however, can be missed on transbronchial biopsy. OB also can develop in the wake of other insults such as acute rejection, airway ischemia, lymphocytic bronchiolitis, and certain infections such as CMV. Patients with OB, in addition to developing progressive deterioration of lung function, are also at high risk for bacterial pneumonia and acute-on-chronic bouts of respiratory failure.30 Pneumonia is commonly caused by Pseudomonas aeruginosa followed by Staphylococcus and Acinetobacter baumannii.10
Nonpulmonary Organ Support and Complications
Hemodynamic management following lung transplantation is similar to that of other ICU patients, with the exception of volume administration. Given the lack of lymphatics in the allograft and potential ischemic injury of pulmonary endothelium and epithelium, pulmonary edema occurs at lower filling pressures. For that reason, lung transplant recipients with postoperative hypoxemia should be maintained “on the dry side” using vasopressors or inotropes as needed to support blood pressure and cardiac output. Patients have been screened preoperatively for coronary artery disease and ventricular dysfunction, and ischemia or congestive heart failure (CHF) should rarely be complicating factors in lung transplant recipients. Atrial arrhythmias are common and can effectively be managed with β-adrenergic blockade or sotalol in most cases. Amiodarone should be avoided because of its potential pulmonary toxicity.31 Diltiazem can unpredictably and markedly affect calcineurin inhibitor levels (tacrolimus and cyclosporine) and should be used cautiously.
Gastrointestinal problems include gastritis/ulcers, ileus, Clostridium difficile colitis, and CMV enteritis. In some centers, patients receive an H2 blocker during their initial hospitalization as well as enteral metronidazole (500 mg orally [PO] 3 times daily) as prophylaxis against C. difficile infection. CMV enteritis can be difficult to diagnose, since tests for circulating CMV antigen can be negative in patients with disease localized to the GI tract. Endoscopy with biopsy is appropriate, particularly if thickened bowel is identified radiographically. Patients with cystic fibrosis should be placed on a bowel regimen with lactulose (10 mg PO twice daily) or polyethylene glycol (17 g PO 4 times daily) to prevent mucous impaction. Gastroesophageal reflux disease is common after lung transplantation, and there is evidence that effective treatment, including surgical interventions,32,33 can improve lung allograft function as well as reduce the incidence of chronic rejection.34 Pancreatitis, bowel perforation, and cholecystitis are uncommon GI problems. These issues should be addressed in the standard manner.
Neurologic sequelae after lung transplant include tremors, seizures, encephalopathy, myopathy, and neuropathy. Drugs such as tacrolimus and cyclosporine, antibiotics, corticosteroids, and perioperative neuromuscular blocking agents can be contributing factors. Hyperammonemia after lung transplantation is a devastating complication presenting as lethargy and unexplained hyperammonemia. Its mechanism is poorly understood and carries a grave prognosis despite aggressive measures including gut decontamination, high levels of dialysis, and pharmacologic treatments targeted at urea-cycle enzyme deficiencies.35,36
 Heart Transplantation
Heart Transplantation
Heart transplantation has been performed for over 35 years for end-stage heart disease. In 2007, 3355 heart transplants were reported to the International Society of Heart and Lung Transplantation (ISHLT) database.37 The primary indication for heart transplantation has interestingly shifted over the last 10 years from an equal split of ischemic and nonischemic heart disease to a greater proportion of patients with nonischemic cardiomyopathy (39.5% versus 49.5%); other indications include congenital disease, valvular disease, amyloidosis, sarcoidosis, and re-transplantation. Donor availability still remains a primary deterrent to more widespread use of cardiac transplantation.
Hemodynamic Support
Patients with preexisting pulmonary hypertension require more prolonged inotropic support of the unconditioned donor RV, typically with a milrinone taper. Overt RV failure generally becomes apparent in the OR when the chest is still open, but in some cases, increasing CVP and decreasing stroke volume will necessitate evaluation by TEE. RV failure is confirmed by visualizing the absence of tamponade, a hypokinetic and distended RV, and underfilling of the LV. The addition of inhaled nitric oxide (20-40 ppm) or inhaled prostaglandin E1 or I238 may be appropriate adjuncts immediately postoperatively in this setting. Care must be taken to avoid overdistension of the RV chamber in the setting of postoperative RV dysfunction, since continued volume loading with CVPs above 20 cm H2O are unlikely to significantly improve flow and may lead to an acute hepatic congestion picture. Diuretics and ultrafiltration are employed early postoperatively if hemodynamically tolerated to keep CVP below 20 cm H2O. Mechanical support with an RVAD may be indicated and should be entertained before a picture of shock emerges. RV dysfunction in the setting of relatively normal pulmonary vascular resistance suggests that the primary problem is myocardial dysfunction rather than excessive afterload. Humoral (hyperacute) rejection should be ruled out in such cases, particularly if issues of preservation were not a concern.
Rejection
Cardiac allograft rejection can occur early post transplant. Three drug regimens similar to lung transplants are the mainstays of maintenance therapy. Induction therapy with antilymphocyte antibodies (either interleukin [IL]-2 receptor antibodies or polyclonal antilymphocyte globulin/antithymocyte globulin) is used in about half of patients to minimize renal toxicity caused by calcineurin inhibitors—more so in those with preexisting renal disease.37 Acute cellular rejection may present with arrhythmias, CHF, fatigue, abdominal pain, low cardiac output, or hypotension. Surveillance endomyocardial biopsies and right heart catheterizations are performed weekly in the early postoperative period. Methylprednisolone (1000 mg IV daily for 3 days) is the standard treatment for acute cellular rejection. A particularly aggressive form of rejection is called hyperacute rejection, and is mediated primarily by humoral factors. Myocardial biopsy reveals vascular deposition of immunoglobulin and complement, with evidence of vascular injury in the absence of a mononuclear cell infiltrate. Treatment includes therapy with corticosteroids, urgent plasmapheresis, and immunoglobulin infusion. Patients undergoing re-transplantation, multiparous women, and patients having received multiple blood transfusions are at particular risk for hyperacute rejection. For potential recipients with screening studies suggesting undesirable preformed antibodies, a negative prospective crossmatch or an induction regimen including preoperative plasmapheresis is undertaken before proceeding with transplantation in most programs.
Infections
Infectious complications include routine nosocomial infections such as pneumonia, catheter-related sepsis, and mediastinitis. Patients having been bridged to transplant with a VAD complicated by infection of the VAD pocket or driveline are more prone to wound infections following removal of the device and transplantation. CMV occurs in 10% to 25% of transplants.37 CMV-negative recipients who receive a CMV-positive organ are at the highest risk. Surveillance with serum CMV antigen assays is the mainstay of management. Some centers employ prophylactic regimens with ganciclovir (5 mg/kg IV twice daily or daily) or valganciclovir (900 mg PO daily) for 3 to 6 months. Toxoplasma and P. carinii prophylaxis are also standard with trimethoprim/sulfamethoxazole (160/800 PO, 3 times a week).
Long-Term Complications
The bulk of early mortality (within 1 month) is caused by graft failure (primary nonspecific) (41%), multiple organ dysfunction syndrome (MODS) (13%) and non-CMV infection (13%). Between 31 days and 1 year, non-CMV infections account for almost 30% of deaths, followed by graft failure (18%) and acute rejection (12%). After 5 years, coronary artery vasculopathy, malignancies, and non-CMV infections are the main causes of death.37 Coronary vasculopathy is the “OB” of heart transplantation and leads to deterioration in cardiac allograft function following transplantation.39 Endothelial cell injury can be triggered by graft ischemia, rejection, viral infections, and hyperlipidemia. Endothelial injury or activation leads to concentric, distal coronary intimal proliferation, ultimately occluding coronary flow.40 The absence of cardiac re-innervation in most heart transplant recipients precludes warning symptoms of angina. New onset of CHF, myocardial infarction, angina, ECG changes, or syncope, particularly in patients several years post transplant, are indications for cardiac catheterization. Stents may be of value in selected cases. Retransplantation is an option, although re-transplantation as an indication for transplant carries with it a significantly inferior outcome.37
Although coronary vasculopathy impairs long-term results, heart transplantation nevertheless provides a durable treatment for patients with end-stage heart disease. Patients enjoy 50% survival at 10 years.37 Donor availability remains the major limitation to more widespread treatment and success.
Key Points
Hachem RR, Trulock EP. The new lung allocation system and its impact on waitlist characteristics and post-transplant outcomes. Semin Thorac Cardiovasc Surg. 2008;20:139-142.
Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult lung and heart-lung transplantation report—2009. J Heart Lung Transplant. 2009;28:1031-1049.
Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161-165.
1 Hachem RR, Trulock EP. The new lung allocation system and its impact on waitlist characteristics and post-transplant outcomes. Semin Thorac Cardiovasc Surg. 2008;20(2):139-142.
2 Davis SQ, Garrity ERJr. Organ allocation in lung transplant. Chest. 2007 Nov;132(5):1646-1651.
3 Yeung JC, Cypel M, Waddell TK, van Raemdonck D, Keshavjee S. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques—non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009 May;19(2):261-274.
4 Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The registry of the international society for heart and lung transplantation: Twenty-sixth official adult lung and heart-lung transplantation report-2009. J Heart Lung Transplant. 2009 Oct;28(10):1031-1049.
5 Thabut G, Christie JD, Ravaud P, Castier Y, Brugiere O, Fournier M, et al. Survival after bilateral versus single lung transplantation for patients with chronic obstructive pulmonary disease: A retrospective analysis of registry data. Lancet. 2008 Mar 1;371(9614):744-751.
6 Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003 Oct;124(4):1232-1241.
7 Fischer S, Gohrbandt B, Struckmeier P, Niedermeyer J, Simon A, Hagl C, et al. Lung transplantation with lungs from donors fifty years of age and older. J Thorac Cardiovasc Surg. 2005 Apr;129(4):919-925.
8 Angles R, Tenorio L, Roman A, Soler J, Rochera M, de Latorre FJ. Lung transplantation for emphysema. lung hyperinflation: Incidence and outcome. Transplant Int. 2005 May;17(12):810-814.
9 King-Biggs MB, Dunitz JM, Park SJ, Kay Savik S, Hertz MI. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation. 2003 May 15;75(9):1437-1443.
10 Remund KF, Best M, Egan JJ. Infections relevant to lung transplantation. Proc Am Thorac Soc. 2009 January 15;6(1):94-100.
11 Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: Ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. 2009 January 15;6(1):79-93.
12 Lee JC, Christie JD. Primary graft dysfunction. Proc Am Thorac Soc. 2009 Jan 15;6(1):39-46.
13 Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D, et al. Report of the ISHLT working group on primary lung graft dysfunction part II: Definition. A consensus statement of the international society for heart and lung transplantation. J Heart Lung Transplant. 2005 Oct;24(10):1454-1459.
14 Prekker ME, Nath DS, Walker AR, Johnson AC, Hertz MI, Herrington CS, et al. Validation of the proposed international society for heart and lung transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2006 Apr;25(4):371-378.
15 Christie JD, Sager JS, Kimmel SE, Ahya VN, Gaughan C, Blumenthal NP, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005 Jan;127(1):161-165.
16 Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB, ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT working group on primary lung graft dysfunction part V: Predictors and outcomes. J Heart Lung Transplant. 2005 Oct;24(10):1483-1488.
17 Anderson DC, Glazer HS, Semenkovich JW, Pilgram TK, Trulock EP, Cooper JD, et al. Lung transplant edema: Chest radiography after lung transplantation—the first 10 days. Radiology. 1995 Apr;195(1):275-281.
18 Boujoukos AJ, Martich GD, Vega JD, Keenan RJ, Griffith BP. Reperfusion injury in single-lung transplant recipients with pulmonary hypertension and emphysema. J Heart Lung Transplant. 1997 Apr;16(4):439-448.
19 Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S, et al. Report of the ISHLT working group on primary lung graft dysfunction part VI: Treatment. J Heart Lung Transplant. 2005 Oct;24(10):1489-1500.
20 Rea RS, Ansani NT, Seybert AL. Role of inhaled nitric oxide in adult heart or lung transplant recipients. Ann Pharmacother. 2005 May;39(5):913-917.
21 Ardehali A, Laks H, Levine M, Shpiner R, Ross D, Watson LD, et al. A prospective trial of inhaled nitric oxide in clinical lung transplantation. Transplantation. 2001 Jul 15;72(1):112-115.
22 Bermudez CA, Adusumilli PS, McCurry KR, Zaldonis D, Crespo MM, Pilewski JM, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: Long-term survival. Ann Thorac Surg. 2009 Mar;87(3):854-860.
23 Fischer S, Bohn D, Rycus P, Pierre AF, de Perrot M, Waddell TK, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: Analysis of the extracorporeal life support organization (ELSO) registry. J Heart Lung Transplant. 2007 May;26(5):472-477.
24 Dahlberg PS, Prekker ME, Herrington CS, Hertz MI, Park SJ. Medium-term results of extracorporeal membrane oxygenation for severe acute lung injury after lung transplantation. J Heart Lung Transplant. 2004 Aug;23(8):979-984.
25 Lacomis D, Giuliani MJ, Van Cott A, Kramer DJ. Acute myopathy of intensive care: Clinical, electromyographic, and pathological aspects. Ann Neurol. 1996 Oct;40(4):645-654.
26 Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation. 2003 Sep 27;76(6):964-968.
27 Hadaya K, Wunderli W, Deffernez C, Martin PY, Mentha G, Binet I, et al. Monitoring of cytomegalovirus infection in solid-organ transplant recipients by an ultrasensitive plasma PCR assay. J Clin Microbiol. 2003 Aug;41(8):3757-3764.
28 Kumar D, Erdman D, Keshavjee S, Peret T, Tellier R, Hadjiliadis D, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005 Aug;5(8):2031-2036.
29 Martinu T, Chen DF, Palmer SM. Acute rejection and humoral sensitization in lung transplant recipients. Proc Am Thorac Soc. 2009 Jan 15;6(1):54-65.
30 Belperio JA, Weigt SS, Fishbein MC, Lynch JP3rd. Chronic lung allograft rejection: Mechanisms and therapy. Proc Am Thorac Soc. 2009 Jan 15;6(1):108-121.
31 Diaz-Guzman E, Mireles-Cabodevila E, Arrossi A, Kanne JP, Budev M. Amiodarone pulmonary toxicity after lung transplantation. J Heart Lung Transplant. 2008 Sep;27(9):1059-1063.
32 Gasper WJ, Sweet MP, Hoopes C, Leard LE, Kleinhenz ME, Hays SR, et al. Antireflux surgery for patients with end-stage lung disease before and after lung transplantation. Surg Endosc. 2008 Feb;22(2):495-500.
33 Robertson AG, Ward C, Pearson JP, Corris PA, Dark JH, Griffin SM. Lung transplantation, gastroesophageal reflux, and fundoplication. Ann Thorac Surg. 2010 Feb;89(2):653-660.
34 King BJ, Iyer H, Leidi AA, Carby MR. Gastroesophageal reflux in bronchiolitis obliterans syndrome: A new perspective. J Heart Lung Transplant. 2009 Sep;28(9):870-875.
35 Moffatt-Bruce SD, Pesavento T, Von Viger J, Nunley D, Pope-Harman A, Martin S, et al. Successful management of immunosuppression in a patient with severe hyperammonemia after lung transplantation. J Heart Lung Transplant. 2008 Jul;27(7):801-803.
36 Lichtenstein GR, Yang YX, Nunes FA, Lewis JD, Tuchman M, Tino G, et al. Fatal hyperammonemia after orthotopic lung transplantation. Ann Intern Med. 2000 Feb 15;132(4):283-287.
37 Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, et al. Registry of the international society for heart and lung transplantation: Twenty-sixth official adult heart transplant report-2009. J Heart Lung Transplant. 2009 Oct;28(10):1007-1022.
38 Khan TA, Schnickel G, Ross D, Bastani S, Laks H, Esmailian F, et al. A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg. 2009 Dec;138(6):1417-1424.
39 Uretsky BF, Murali S, Reddy PS, Rabin B, Lee A, Griffith BP, et al. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827-834.
40 Valantine HA. Cardiac allograft vasculopathy: Central role of endothelial injury leading to transplant “atheroma”. Transplantation. 2003 Sep 27;76(6):891-899.