152 Management of Neutropenic Cancer Patients
Among patients with chemotherapy-induced neutropenia, 1% to 5% experience toxic side effects or infections and benefit from intensive care unit (ICU) management.1 The outlook for cancer patients requiring ICU admission has long been considered dismal. Several recent studies have shown improved ICU outcomes in the overall population of patients with hematologic malignancies,2–6 highlighting that it is no longer relevant to deny ICU admission to patients with neutropenia or after autologous bone marrow transplantation.7,8–13
The prognosis of these neutropenic patients is determined by the number of organ failures at ICU admission. The proliferative potential and other characteristics of the underlying malignancy seem to have a far smaller impact on survival.25–27 The general severity scores (Simplified Acute Physiology Score II and Acute Physiology and Chronic Health Evaluation II)28 are of limited assistance for several reasons:
Finally, although bone marrow transplantation has been associated with a poor prognosis in many studies,10,30,31 these studies failed to separate autologous from allogeneic bone marrow transplant recipients or bone marrow transplant recipients from patients given “peripheral” hematopoietic stem cells (i.e., cells collected after mobilization out of the marrow). Allogeneic bone marrow transplant recipients who require ICU management have extremely high mortality rates,32,33 and mortality is highest when the need for life-supporting treatment arises late after the transplantation procedure.31 Allogeneic bone marrow transplantation differs from autologous bone marrow transplantation in important ways, including the risk of graft-versus-host disease and the intensity of the immunosuppressive treatment required for this complication.
 Management of Neutropenic Cancer Patients in the Intensive Care Unit
Management of Neutropenic Cancer Patients in the Intensive Care Unit
Immunodeficiency
Vulnerability to infections occurs in cancer patients for several reasons. Neutropenia diminishes the ability to fight against infectious agents. Neutrophil counts less than 1000/mm3 are associated with a significant risk of infection, and the lower the count, the greater the risk.34 Infections are far more likely to occur when counts fall below 500/mm3, and risk is even greater at neutrophil counts less than 100/mm3. The duration of neutropenia also influences the rate and the severity of infections.35
Fever
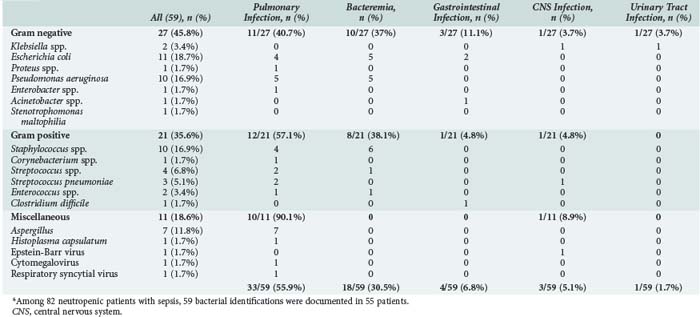
Probabilistic antibiotic therapy should be given routinely if a fever develops. The antibiotics should be active against gram-positive cocci (e.g., streptococci infecting mucositis lesions or staphylococci in intravascular catheters) and gram-negative rods (enterobacteria or Pseudomonas aeruginosa) (Table 152-1). The Infectious Diseases Society of America (IDSA) has updated its recommendations.36 A good first-line regimen in an ICU patient with prolonged neutropenia (as often occurs in hematologic malignancies) is a penicillin that is active against P. aeruginosa and gram-positive cocci, given either alone or in combination with an aminoglycoside or a fluoroquinolone active against P. aeruginosa. Although not given routinely, vancomycin is usually added. Indeed, many neutropenic ICU patients meet IDSA criteria for introducing a glycopeptide, including suspected catheter-associated infection, methicillin-resistant Staphylococcus aureus colonization, gram-positive cocci in blood cultures before identification of the organism, shock, and two situations associated with infection by gram-positive cocci—grade III or IV mucositis and abrupt body temperature elevation to greater than 40°C.36 Fluconazole, 400 mg/d, as prophylactic treatment of fungal infections has been found to be beneficial only in allogeneic bone marrow transplant recipients.18 After 5 to 7 days with febrile neutropenia, the risk of fungal infection (not only with Candida but also with Aspergillus) is sufficiently high to warrant routine antifungal therapy in combination with antibacterial agents. In our ICU, we use amphotericin B as the first-line drug. Finally, the need for antiviral agents or trimethoprim-sulfamethoxazole should be evaluated on a case-by-case basis according to patient-related factors and the clinical picture.36 Initiation of treatment for herpesvirus infection should be considered in all patients with grade III or IV mucositis.
When the organism is recovered and identified, antimicrobial therapy should be adjusted accordingly. ICU patients whose body temperature returns to normal on the third treatment day but who have negative tests for causative organisms should continue to receive antibiotics until their blood cell counts return to normal.36
 Hematopoietic Growth Factors
Hematopoietic Growth Factors
Among available hematopoietic growth factors, granulocyte colony-stimulating factor (G-CSF) is the most widely used in patients with hematologic or solid malignancies. G-CSF increases neutrophil counts and enhances neutrophil functions. In non-ICU patients, G-CSF has been shown to decrease the duration of neutropenia, reducing the rate of serious infections.37,38 G-CSF also decreased mortality related to bone marrow transplantation complications39 or dose-intensive chemotherapy.40
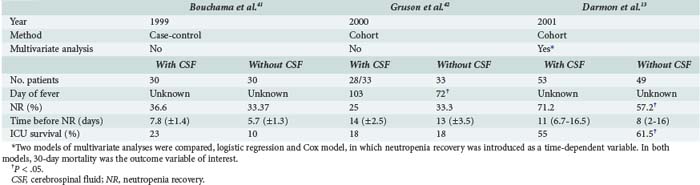
Intensivists and hematologists place considerable emphasis on correcting neutropenia. However, neutropenia recovery during the ICU stay was not associated with better survival in a study conducted at our institution.13 For instance, G-CSF therapy that was associated with more rapid recovery from neutropenia did not contribute to increased survival. Nevertheless, using a statistical model appropriate for the time dependency of neutropenia recovery contradicted two earlier studies in which G-CSF provided no benefit in ICU patients (Table 152-2).41,42 G-CSF should be given to all neutropenic ICU patients in whom neutropenia recovery can be expected within 7 days.43 Examination of a bone marrow smear may be more accurate for predicting the time to neutropenia recovery but is not performed routinely in patients given standard chemotherapy regimens. A bone marrow smear may be useful, however, after dose-intensive chemotherapy with bone marrow transplantation or after the first induction course for leukemia. G-CSF can stimulate the leukemic clone in patients receiving induction chemotherapy for acute leukemia and is contraindicated in this setting.
TABLE 152-2 Comparison of Studies Evaluating Impact of Colony-Stimulating Factors on Outcome of Neutropenic Patients in the ICU
In contrast, G-CSF is given to nearly every patient with Hodgkin’s or non-Hodgkin’s lymphoma. Close monitoring is needed in patients with respiratory symptoms or lung infiltrates, as respiratory failure may get worse at time of recovery from leukopenia. It is imperative that G-CSF be discontinued as soon as bone marrow function improves (neutrophils > 500/mm3).44 G-CSF can be given intravenously or subcutaneously; in the ICU, the intravenous route is simplest. Dosages recommended for adults are 10 µg/kg/d for filgrastim and 150 µg/m2/d for lenograstim; however, the optimal dosages in ICU patients have not been determined. The drug is given as a single injection daily. No dosage adjustment is required in patients with kidney dysfunction. Blood cell counts should be obtained daily, and the G-CSF should be stopped as soon as the leukocyte count increases to greater than 1000/mm3 or the neutrophil count increases to greater than 500/mm3.
Isolation Modalities
Protective isolation involves reducing the patient’s exposure to potentially infective microorganisms via geographic and technical measures (routine use of nonsterile gloves, gown, head covering, mask, and in some cases, overshoes). Because the gut lumen is a reservoir for bacteria that can cause bacteremia, selective digestive decontamination (SDD) is often added to isolation measures. In our ICU, we use oral colimycin capsules and oral amphotericin B. Efficacy data on these regimens come from old and methodologically flawed studies that often produced conflicting results. No data are available on neutropenic ICU patients. Finally, there is a paucity of studies comparing isolation measures. A combination of geographic isolation with air filtering (laminar flow or high-efficiency particulate-arresting filters), technical isolation (usually involving use of a mask, head covering, and gown, although variations exist across studies), and SDD have been found to decrease the mortality rate or the infection rate in many prospective and retrospective studies.45 Although the optimal modalities for protective isolation and their usefulness in the ICU have not been determined, a reasonable approach to the management of neutropenic ICU patients is maximal protective isolation, including geographic isolation with air filtration, technical isolation with at least a mask and gown, and SDD.
 Specific Organ Failures
Specific Organ Failures
Acute Respiratory Failure
Together with circulatory shock, acute respiratory failure is the most common organ failure leading to ICU admission of neutropenic patients.20 In these patients, acute respiratory failure often stems from a combination of factors that may be closely intertwined, such as infection and cardiogenic edema or alveolar hemorrhage. The causes of acute respiratory failure in cancer patients can be divided into infectious and noninfectious categories. At least three distinctive features characterize them:
Septic Shock
Survival rates in cancer patients with septic shock have increased over the years.7 Earlier treatment is one component. There is a need for studies evaluating the impact of new management strategies in these patients, who were excluded from large multicenter randomized studies.46–48
Macrophage Activation Syndrome
Lymphohistiocytic activation syndrome is another name for macrophage activation syndrome, which may develop in a neutropenic patient or cause neutropenia. Multiple organ failure with vasoplegic shock may occur.49 Fever, thrombocytopenia, and hepatosplenomegaly are almost universally present. Other manifestations include low counts of other cell lines, cholestasis with jaundice, high serum levels of ferritin and triglycerides, and low serum albumin and fibrinogen. Bone marrow smear findings are typical, with activated macrophages phagocytizing platelets, erythrocytes, and leukocytes, although false-positive results are encountered occasionally. Corticosteroids and etoposide are the mainstays of treatment and should be considered on an emergency basis.50
Typhlitis or Neutropenia-Associated Enterocolitis
Typhlitis occurs chiefly after intensive chemotherapy and manifests as any combination of abdominal pain, fever, and diarrhea.34,51 The protean nature of the manifestations raises diagnostic challenges. Typhlitis is probably a multifactorial condition related to chemotherapy-induced colonic mucosal damage, thrombopenia-related bleeding within the colonic wall, and bowel colonization by pathogenic microorganisms.51 Complications include bacteremia (28%-82% of cases), gastrointestinal bleeding (65% of cases), and gastrointestinal perforation (5%-10%).52 Ultrasonography or computed tomography (CT) of the gastrointestinal tract confirms the diagnosis and evaluates the severity of the disease. CT may show pneumoperitoneum or colonic pneumatosis, indicating severe parietal damage with imminent perforation. Bowel-wall thickening on ultrasound scan confirms the diagnosis.53 In a retrospective study, bowel-wall thickening was significantly associated with a high mortality rate (29% versus 0%), especially when the bowel wall was thicker than 10 mm.54 Conservative treatment should be used if possible, but surgery is required in patients with life-threatening gastrointestinal bleeding, perforation, or uncontrolled sepsis.52 A diagnosis of typhlitis requires prior elimination of other abdominal conditions, most notably classic surgical conditions and pseudomembranous colitis.52
Acute Tumor Lysis Syndrome
Although the onset usually precedes the development of neutropenia by several days, the two problems of acute tumor lysis syndrome and neutropenia are frequently interrelated. The risk of tumor lysis syndrome varies with the tumor burden and with the nature and intensity of induction chemotherapy. Neutropenia develops soon afterward. Although a detailed description of tumor lysis syndrome is beyond the scope of this chapter, five key words come to mind: Hyperuricemia stems from the metabolism and lysis of tumor cells and can cause precipitates to form within the renal tubules if the urine is acidic. The administration of recombinant urate oxidases (rasburicase) completely prevents this problem, obviating the need for alkalinization.55 Hyperphosphatemia is an absolute contraindication to alkalinization (the risk being nephrocalcinosis related to precipitation) but can be controlled by hyperhydration and renal support therapy. Dehydration is almost always present and requires volume repletion with nonalkaline isotonic solutions.
Key Points
Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360:1131-1135.
Darmon M, Azoulay E, Alberti C, et al. Impact of neutropenia duration on short-term mortality in neutropenic critically ill cancer patients. Intensive Care Med. 2002;28:1775-1780.
Hughes WT, Armstrong D, Bodey GP, et al. 2002 Guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730-751.
Larche J, Azoulay E, Fieux F, et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688-1695.
Massion PB, Dive AM, Doyen C, et al. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit Care Med. 2002;30:2260-2270.
1 Blot F, Cordonnier C, Buzin A, et al. Severity of illness scores: Are they useful in febrile neutropenic adult patients in hematology wards? A prospective multicenter study. Crit Care Med. 2001;29:2125-2131.
2 Azoulay E, Recher C, Alberti C, et al. Changing use of intensive care for hematological patients: The example of multiple myeloma. Intensive Care Med. 1999;25:1395-1401.
3 Azoulay E, Alberti C, Bornstain C, et al. Improved survival in cancer patients requiring mechanical ventilatory support: Impact of noninvasive mechanical ventilatory support. Crit Care Med. 2001;29:519-525.
4 Kress JP, Christenson J, Pohlman AS, et al. Outcomes of critically ill cancer patients in a university hospital setting. Am J Respir Crit Care Med. 1999;160:1957-1961.
5 Staudinger T, Stoiser B, Mullner M, et al. Outcome and prognostic factors in critically ill cancer patients admitted to the intensive care unit. Crit Care Med. 2000;28:1322-1328.
6 Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: A period analysis. Lancet. 2002;360:1131-1135.
7 Khassawneh BY, White PJr, Anaissie EJ, et al. Outcome from mechanical ventilation after autologous peripheral blood stem cell transplantation. Chest. 2002;121:185-188.
8 Schuster DP, Marion JM. Precedents for meaningful recovery during treatment in a medical intensive care unit: Outcome in patients with hematologic malignancy. Am J Med. 1983;75:402-408.
9 Rubenfeld GD, Crawford SW. Withdrawing life support from mechanically ventilated recipients of bone marrow transplants: A case for evidence-based guidelines. Ann Intern Med. 1996;125:625-633.
10 Carlon GC. Admitting cancer patients to the intensive care unit. Crit Care Clin. 1988;4:183-191.
11 Brunet F, Lanore JJ, Dhainaut JF, et al. Is intensive care justified for patients with haematological malignancies? Intensive Care Med. 1990;16:291-297.
12 Darmon M, Azoulay E, Alberti C, et al. Impact of neutropenia duration on short-term mortality in neutropenic critically ill cancer patients. Intensive Care Med. 2002;28:1775-1780.
13 Blot F, Guiguet M, Nitenberg G, et al. Prognostic factors for neutropenic patients in an intensive care unit: Respective roles of underlying malignancies and acute organ failures. Eur J Cancer. 1997;33:1031-1037.
14 Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-242.
15 Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201-1214.
16 O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
17 Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845-851.
18 Shanholtz C. Acute life-threatening toxicity of cancer treatment. Crit Care Clin. 2001;17:483-502.
19 Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481-487.
20 Larche J, Azoulay E, Fieux F, et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Med. 2003;29:1688-1695.
21 Mayaud C, Cadranel J. A persistent challenge: The diagnosis of respiratory disease in the non-AIDS immunocompromised host. Thorax. 2000;55:511-517.
22 Afessa B, Tefferi A, Hoagland HC, et al. Outcome of recipients of bone marrow transplants who require intensive-care unit support. Mayo Clin Proc. 1992;67:117-122.
23 Gruson D, Hilbert G, Valentino R, et al. Utility of fiberoptic bronchoscopy in neutropenic patients admitted to the intensive care unit with pulmonary infiltrates. Crit Care Med. 2000;28:2224-2230.
24 Hilbert G, Gruson D, Vargas F, et al. Bronchoscopy with broncho-alveolar lavage via the laryngeal mask airway in high-risk hypoxemic immunosuppressed patients. Crit Care Med. 2001;29:249-255.
25 Azoulay E, Moreau D, Alberti C, et al. Predictors of short-term mortality in critically ill patients with solid malignancies. Intensive Care Med. 2000;26:1817-1823.
26 Massion PB, Dive AM, Doyen C, et al. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit Care Med. 2002;30:2260-2270.
27 Boussat S, El’rini T, Dubiez A, et al. Predictive factors of death in primary lung cancer patients on admission to the intensive care unit. Intensive Care Med. 2000;26:1811-1816.
28 Sculier JP, Paesmans M, Markiewicz E, et al. Scoring systems in cancer patients admitted for an acute complication in a medical intensive care unit. Crit Care Med. 2000;28:2786-2792.
29 Guiguet M, Blot F, Escudier B, et al. Severity-of-illness scores for neutropenic cancer patients in an intensive care unit: Which is the best predictor? Do multiple assessment times improve the predictive value? Crit Care Med. 1998;26:488-493.
30 Price KJ, Thall PF, Kish SK, et al. Prognostic indicators for blood and marrow transplant patients admitted to an intensive care unit. Am J Respir Crit Care Med. 1998;158:876-884.
31 Huaringa AJ, Leyva FJ, Giralt SA, et al. Outcome of bone marrow transplantation patients requiring mechanical ventilation. Crit Care Med. 2000;28:1014-1017.
32 Leleu G, Azoulay E, Chen Y, et al. Prognostic factors of allogenic bone marrow recipients admitted to ICU [abstract]. Am J Respir Crit Care Med. 1998;157:A299.
33 Afessa B, Tefferi A, Dunn WF, et al. Intensive care unit support and Acute Physiology and Chronic Health Evaluation III performance in hematopoietic stem cell transplant recipients. Crit Care Med. 2003;31:1715-1721.
34 Bodey GP. Unusual presentations of infection in neutropenic patients. Int J Antimicrob Agents. 2000;16:93-95.
35 Seropian S, Nadkarni R, Jillella AP, et al. Neutropenic infections in 100 patients with non-Hodgkin’s lymphoma or Hodgkin’s disease treated with high-dose BEAM chemotherapy and peripheral blood progenitor cell transplant: Out-patient treatment is a viable option. Bone Marrow Transplant. 1999;23:599-605.
36 Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002;34:730-751.
37 Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992;327:28-35.
38 Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2). N Engl J Med. 1992;327:99-106.
39 Guardiola P, Runde V, Bacigalupo A, et al. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99:4370-4378.
40 Bergh J, Wiklund T, Erikstein B, et al. Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: A randomised trial. Scandinavian Breast Group 9401 study. Lancet. 2000;356:1384-1391.
41 Bouchama A, Khan B, Djazmati W, et al. Hematopoietic colony-stimulating factors for neutropenic patients in the ICU. Intensive Care Med. 1999;25:1003-1005.
42 Gruson D, Hilbert G, Vargas F, et al. Impact of colony-stimulating factor therapy on clinical outcome and frequency rate of nosocomial infections in intensive care unit neutropenic patients. Crit Care Med. 2000;28:3155-3160.
43 Hughes WT, Armstrong D, Bodey GP, et al. 1997 guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Infectious Diseases Society of America. Clin Infect Dis. 1997;25:551-573.
44 Azoulay E, Darmon M, Delclaux C, et al. Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med. 2002;30:781-786.
45 Darmon M, Azoulay E. Isolement du patient immunodéprimé en réanimation. Réanimation. 2002;11:1-4.
46 Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
47 Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862-871.
48 Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
49 Gauvin F, Toledano B, Champagne J, et al. Reactive hemophagocytic syndrome presenting as a component of multiple organ dysfunction syndrome. Crit Care Med. 2000;28:3341-3345.
50 Ravelli A. Macrophage activation syndrome. Curr Opin Rheumatol. 2002;14:548-552.
51 Blijlevens NM, Donnelly JP, De Pauw BE. Mucosal barrier injury: Biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: An overview. Bone Marrow Transplant. 2000;25:1269-1278.
52 Gomez L, Martino R, Rolston KV. Neutropenic enterocolitis: Spectrum of the disease and comparison of definite and possible cases. Clin Infect Dis. 1998;27:695-699.
53 Gorschluter M, Marklein G, Hofling K, et al. Abdominal infections in patients with acute leukaemia: A prospective study applying ultrasonography and microbiology. Br J Haematol. 2002;117:351-358.
54 Cartoni C, Dragoni F, Micozzi A, et al. Neutropenic enterocolitis in patients with acute leukemia: Prognostic significance of bowel wall thickening detected by ultrasonography. J Clin Oncol. 2001;19:756-761.
55 Wossmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160-165.