Management of Laryngotracheal Obstruction in Children
Pediatric surgeons are often involved in the management of acute and chronic airway obstruction. Moreover, iatrogenic injury to the pediatric airway occasionally occurs. The large number of operative techniques for the treatment of laryngotracheal stenosis shows that no single procedure or technique is universally applicable and successful. Prevention of, or prompt therapy for, injury is all important.1,2
Practical Embryology and Anatomy
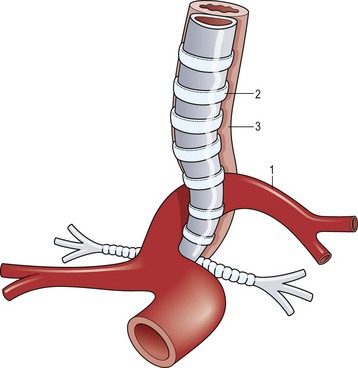
A working knowledge of the embryonic development of the mediastinal structures aids in understanding the etiology and associated anomalies of tracheal obstruction. Malformations of the great vessels (vascular rings) should be suspected and investigated when evaluating a child with complete tracheal rings. The most common vascular malformation associated with complete tracheal rings is a pulmonary vascular sling. This anomaly occurs when the left pulmonary artery arises to the right of the trachea, around which it curves and compresses just above the carina, and then passes between the trachea and esophagus before reaching the left lung (Fig. 21-1).3 Other vascular ring malformations may produce varying degrees of tracheal, bronchial, and esophageal compression.
Subglottic and Tracheal Malformations
Congenital Subglottic and Tracheal Stenosis
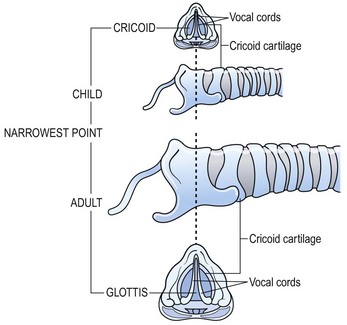
The anatomy of the pediatric airway has been compared to an inverted cone, with the trachea fitting telescopically into the cricoid above it, the cricoid into the thyroid cartilage, and then the thyroid into the hyoid space (Fig. 21-2).4 Congenital subglottic stenosis is the most common morphologic abnormality and presents as a narrowing of the airway at the distal end of the larynx, just at the beginning of the trachea. The subglottic region lies at the level of the cricoid cartilage, which is normally the only complete cartilaginous ring in the airway. Congenital subglottic abnormalities result in elliptical narrowing of the cricoid cartilage, the etiology of which is not known. Subglottic stenosis is exceeded only by laryngomalacia and vocal cord paralysis in the frequency of congenital airway anomalies.
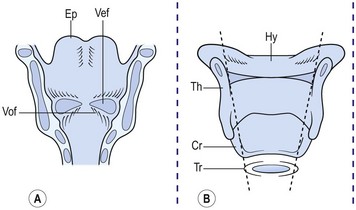
FIGURE 21-2 (A) Ventral area of the larynx in the neonate viewed from behind. The ventricle, or ‘third cavity,’ is bounded above by the ventricular folds (Vef) and below by the vocal folds (Vof). Ep, epiglottis. (B) Laryngeal cartilages (without arytenoids). Th, thyroid; Cr, cricoid; Tr, trachea; and Hy, hyoid viewed from behind. Inner dashed lines show telescopic configuration in the neonate as opposed to the rectangular shape in the adult (outer dashed lines). (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
When compared with an adult, the anatomy of the trachea and larynx of a child differs in several ways (Fig. 21-3). The child’s epiglottis is short and small, and the valleculae are shallow. Also, the larynx points posteriorly, and the arytenoid apparatus is large in relation to the lumen of the larynx. Finally, the narrowest point of the normal pediatric airway is the subglottis. In the adult, it is the glottis.
Acquired Subglottic and Tracheal Stenosis
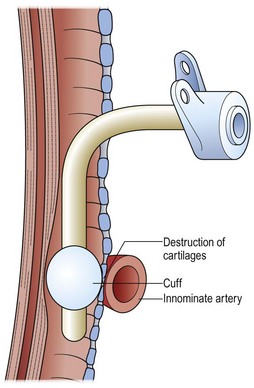
Acquired airway malformations usually result from intrinsic injury with subsequent inflammation, ulceration, and scarring, leading to subglottic or tracheal scarring and narrowing. Occasionally, trauma is the initiating event but an iatrogenic event can exacerbate an unstable situation.2 For example, a child with a congenitally small airway might be asymptomatic until an endotracheal tube is inserted. The tube may be appropriate in size but, because of the congenital stenosis, it will fit tightly and can lead to ulceration and stricture. Particularly difficult to treat are those injuries that occur well below the subglottic region, usually produced by an endotracheal balloon that caused compression and ulceration in the trachea. Frequently, these areas of injury are below the usual site for a tracheostomy. The cuff may even erode into overlying vessels (Fig. 21-4).
Vascular Compression
Compression and partial obstruction of the trachea may be caused by abnormalities of the aortic arch that impinge on, or encircle, the trachea or esophagus, or both.5,6 When both the trachea and esophagus are compressed, swallowing frequently produces airway compression and respiratory distress. Vascular rings are often asymptomatic in neonates and infants, yet can lead to significant airway obstruction in a child.7
The physiologic impingement on the trachea by a vascular ring is similar to that seen in patients after repair of esophageal atresia. The persistently distended upper esophageal pouch can displace the trachea anteriorly, producing tracheomalacia (Fig. 21-5). Particularly with swallowing, the distended esophageal pouch may compress the trachea against the innominate artery (Figs 21-6 and 21-7). Correction of this problem centers on anterior mobilization and suspension of the innominate artery (Fig. 21-8).8–12 The treatment of a pulmonary vascular sling may require not only relocation and reimplantation of the pulmonary artery, but also repair of the stenotic distal trachea.7,12,13
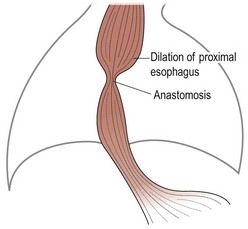
FIGURE 21-5 After repair of esophageal atresia, the proximal esophagus, which is already enlarged, is further dilated by an anastomotic stricture. (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
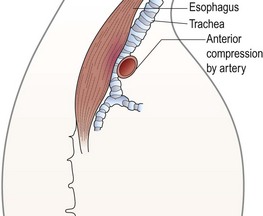
FIGURE 21-6 A lateral view shows how the dilated proximal esophagus displaces the trachea and compresses it against the overlying innominate artery. (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
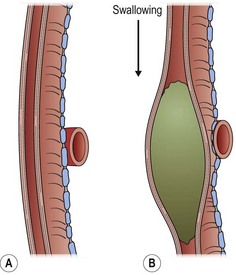
FIGURE 21-7 An enlarged diagram of Figure 21-6 illustrates how the compression is increased by ingestion of food. (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
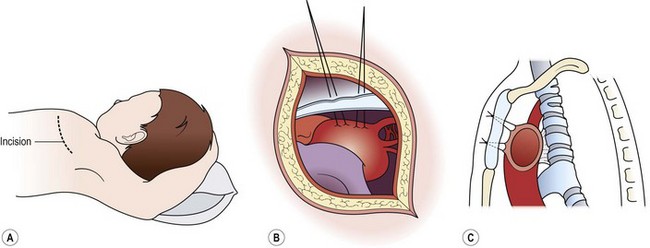
FIGURE 21-8 The operative technique for aortopexy. (A) Anterior left thoracotomy in the third interspace. (B) Sutures placed into the wall of the innominate artery and the aortic arch. (C) Sutures passed through the sternum and tied to elevate the compressing vessels. Tracheal attachments pull the anterior wall of the trachea forward. (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
Stridor and dyspnea are symptoms that can be produced by vascular impingement on the trachea. Patients with severe compromise from a double aortic arch are usually symptomatic, but their manifestations are variable (Fig. 21-9). Some patients are seen with frequent coughing episodes and stridor accompanied by dyspnea and cyanosis, whereas small infants may have apneic episodes. The symptoms of vascular impingement on the trachea are usually more dramatic than those from compression of the esophagus.
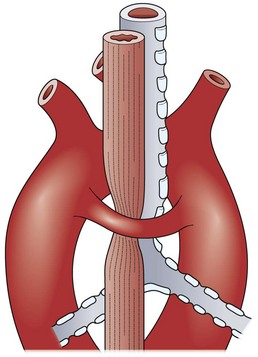
FIGURE 21-9 Both trachea and esophagus are compressed by a double aortic arch. (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
Classically, vascular ring anomalies are diagnosed on a barium esophagogram with indentations on the esophageal column of barium and a decrease in the tracheal air column. Offset of the axis of the barium column above and below the indentation is diagnostic of a double aortic arch (Fig. 21-10A). More recently, rapid computed tomographic (CT) scans allow a graphic reconstruction of the trachea and adjacent vessels (Fig. 21-10B). Magnetic resonance imaging (MRI) enhanced with intravenous administration of a contrast agent allows excellent visualization of the trachea and vessels as well.
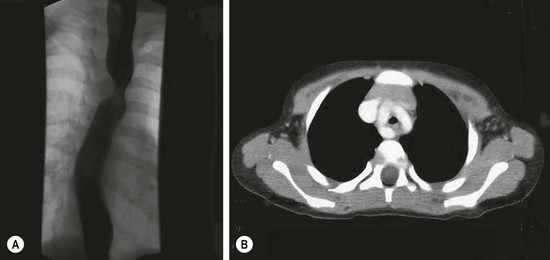
FIGURE 21-10 This infant presented with stridor. There was a suggestion of tracheal indentation on the chest radiograph. Therefore, a barium esophagogram was performed (A) and shows the double indentations diagnostic of a double aortic arch. (B) A CT scan shows contrast in the double arch that is encircling the trachea and esophagus (collapsed).
Occasionally, a child will appear with acute airway obstruction or other medical problems requiring intensive care, during which endotracheal intubation and a concomitant nasogastric tube are inserted. The presence of tubes in both airway and esophagus makes detection of a vascular ring difficult and can generate complications. In a child who is already intubated, performance of contrast radiographic procedures may not be possible. Ultrasonography (US), or CT, or contrast-enhanced MRI may delineate the vascular abnormality. When both tracheal and esophageal intubations are necessary in a patient with a double aortic arch, the encircling vessels may sustain pressure necrosis. Erosion into the aortic arch can produce an aortoesophageal fistula that may not be manifest until either the endotracheal or the esophageal tube is removed. A sentinel hemorrhage may occur before a massive, and often fatal, hemorrhage occurs into the esophagus. The passage of a Sengstaken–Blakemore tube with inflation of the esophageal balloon can be life-saving by tamponading the fistula.14 Because no reliable diagnostic study is available to demonstrate an aortoesophageal fistula, the observation of a sentinel hemorrhage in such a patient with ultrasound confirmation of a double aortic arch is a clear indication for urgent cardiopulmonary bypass and repair.14
Vascular rings cause airway constriction and not vascular problems. Thus, simple division of the vascular ring is often not enough to relieve tracheal compression. Following division of a vascular ring, if part of the ring continues to compress the airway, it should not be dissected away from the trachea but suspended anteriorly, often to the back of the sternum. The vascular-tracheal attachments will lift the anterior tracheal wall and enlarge the lumen (see Fig. 21-8). Traditionally, an open operation has been used for vascular ring repair. Significant numbers of patients are now being treated by the thoracoscopic approach.15 Regardless of the approach, whether from the right or from the left,16 or other technical variations,17 the recurrent laryngeal and phrenic nerves need to be identified and protected. Flexible endoscopic observation of the trachea during these maneuvers can corroborate relief of the compression.6
Tracheomalacia
Often, tracheomalacia is produced by constant pressure from a cardiovascular structure. Thus it is almost always necessary to suspend the offending vessel and utilize its attachments to the trachea to expand the tracheal lumen. Tracheomalacia can be primary in nature without evidence of compression. In these cases, suspension of the large mediastinal vessels may enlarge the tracheal lumen, or the peritracheal fascia can be suspended to the sternum to overcome collapse of the airway.18–21 In the UK (Scotland), guidelines have been promulgated for the use of thoracoscopic aortopexy to treat severe primary tracheomalacia. Interestingly, the National Health Service believed that these guidelines were necessary because individual surgeons would operate infrequently on infants and children who are good candidates for operative correction.
Inflammatory Obstructions
Viral laryngotracheitis (croup), bacterial or membranous tracheitis, and epiglottitis are inflammatory conditions that occasionally require operative intervention. In cases of inflammatory obstruction, endotracheal intubation is preferred instead of tracheostomy if possible. It is important to distinguish croup and bacterial tracheitis from epiglottitis because the treatments are quite different (Table 21-1).
TABLE 21-1
Characteristics of Laryngotracheobronchitis and Epiglottitis
| Characteristic | Laryngotracheobronchitis | Epiglottitis |
| Incidence | Common | Uncommon |
| Etiology | Viral | Haemophilus influenzae type b |
| Age | 6 months to 3 years | 2–6 years |
| Clinical picture | Gradual onset, preceding upper respiratory tract infection, barking cough | Rapid onset, fever, drooling, dysphagia |
| Physical examination | Respiratory distress, inspiratory stridor, low-grade temperature | Anxious, muffled voice, chin forward, drooling, high temperature |
| Laboratory studies | WBC usually <10,000/mm3 with lymphocytosis; radiograph shows narrowing of subglottic region | WBC often >10,000/mm3 with band cells increased; radiograph shows swollen epiglottis |
Adapted from McLain LG: Croup syndrome. Am Fam Physican 1987;36:213.
Children with epiglottitis characteristically tolerate endotracheal intubation without airway injury because the inflammation and edema are supraglottic and not circumferential. Conversely, with viral or bacterial laryngotracheitis, the inflammatory process involves the entire circumference of the airway and prolonged intubation may lead to permanent scarring.1,22 In the past, many hospitals had strict protocols requiring diagnostic laryngoscopy in the operating room with anesthesia standby for suspected cases of epiglottitis because an emergency tracheostomy was occasionally necessary. Fortunately, the widespread introduction of Haemophilus influenza type B vaccination has nearly eliminated pediatric epiglottitis in the U.S.22
Croup characteristically occurs during viral seasons in children age 3 months to 3 years. Children in whom the classic ‘croupy’ cough develops frequently have a history of an antecedent respiratory infection, usually with a high fever.23 Bacterial tracheitis, a nonviral infectious disease, is seen with fever and rapid development of upper airway obstruction, characterized by copious mucopurulent secretions.
Injuries
Intrinsic Injuries
Most intrinsic laryngotracheal injuries are iatrogenic and produced by inappropriate introduction of an endotracheal tube from instrumentation of the airway. Another intrinsic injury is a thermal burn. The inhalation of hot gases, steam, and toxic smoke produces acute injury that can lead to inflammation and edema in addition to burn necrosis.24,25 When an endotracheal tube is passed through an inflamed glottis and upper trachea, early tracheostomy should be considered. With more extensive involvement, prolonged stenting with a T-shaped tracheostomy or T-tube with open proximal and distal limbs may be required.24 Also, the overaggressive use of lasers or cautery may produce direct tissue thermal injury or may lead to an airway fire. Reconstruction after an airway burn injury should be delayed until the stenosis has matured.26
Extrinsic Injuries
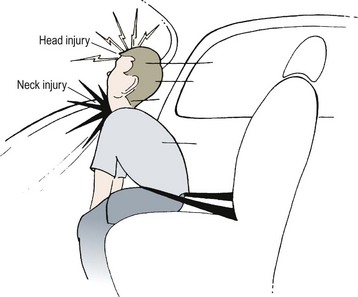
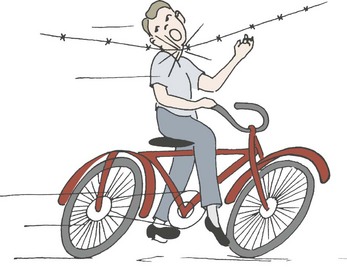
Extrinsic injury to the larynx and trachea may occur when an unrestrained child in an automobile strikes his or her neck on the dashboard or the back of the front seat (Fig. 21-11). A blow directly to the neck from a wire when falling or when riding a bicycle (‘clothesline injury’) may damage the larynx or trachea (Fig. 21-12). Transection of both the trachea and esophagus can occur without visible external neck injuries beyond slight erythema. Crepitus may be present. A good history is essential in determining the mechanism of injury.27 In these instances, and particularly in conjunction with severe craniofacial injuries, a tracheostomy performed under general anesthesia but without endotracheal intubation is usually advisable, because attempts at intubation may further compromise the tenuous airway. Penetrating injuries in children are infrequent, but the same general principles used for management in adults should be followed.28
Endotracheal Intubation
Usually a cuffed endotracheal tube is not necessary in children because compensation for air leaks can be accomplished by increasing the volume of air delivered by the ventilator. However, with massive craniofacial injuries and bleeding, or with significant gastroesophageal reflux, a cuff may be necessary to prevent aspiration of blood or gastric contents. Otherwise, it is best not to use a cuff to prevent damage to the subglottic trachea.29,30 Indications for tracheostomy are summarized in Table 21-2.
TABLE 21-2
Indications for Endotracheal Intubation and Tracheostomy
| Clinical Situation | Endotracheal Intubation | Tracheostomy |
| Emergencies | Always, except → | Severe craniofacial or head and neck injuries |
| Neonates and infants <6 months | Oral intubation unless no hope of extubation → | When long-term intubation is required or when there is difficulty in maintaining intubation because of activity |
| Infants >6 months and children | Maintain for 7–14 days and then → | When long-term intubation or ventilatory support is required for conditions such as severe head injuries |
| Epiglottitis | Until infection has cleared | Usually not necessary |
| Croup or other severe glottic inflammatory diseases | If does not respond to inhalations of racemic epinephrine or with airway obstruction as a temporary measure before → | When glottic edema and inflammation are severe |
Tracheostomy
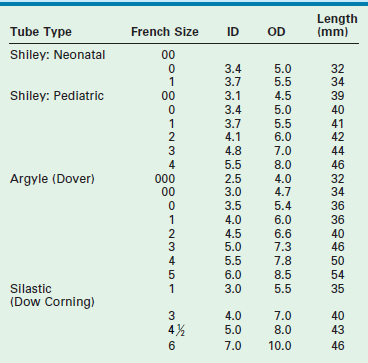
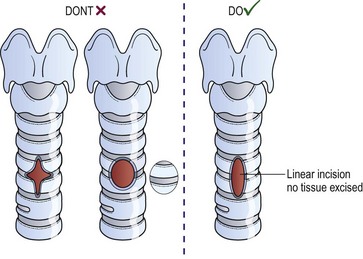
Tracheostomy is best performed with an endotracheal tube in place so that the airway is controlled. A transverse incision made in the lower neck approximately one finger-breadth above the sternal notch is deepened to allow lateral retraction of the strap muscles after the midline is opened. In infants, the subcutaneous fibrofatty tissue superficial to the strap muscles is generally removed to improve exposure. After the strap muscles are retracted, dissection is then carried down to the trachea. The thyroid isthmus is then elevated off the trachea and divided. In small children, palpation of the ridges of the tracheal rings with a small hemostat is frequently more valuable than visualization for determining the appropriate level of tracheotomy. Our experience is that a vertical midline linear tracheal incision through the third and fourth rings, without excising any of the anterior wall of the trachea, is the preferred technique (Fig. 21-13). Cruciate incisions should not be used because the flaps may become inverted and narrow the lumen. The tracheostomy tube specifications are listed in Table 21-3. Traction sutures of polypropylene, left long and labeled ‘left’ and ‘right,’ allow easier reintubation in the event of accidental dislodgement within the first week. Skin may be sewn to the trachea in four quadrants to allow better exposure of the tracheal incision. If the tracheostomy has been in place for longer than two weeks, bronchoscopy is recommended before decannulation. A suprastomal granuloma commonly develops at the superior rim of the tracheostomy stoma and may need excision.
Tracheal Repair
Congenital Stenosis
Many congenital stenotic lesions are asymptomatic until an acute event occurs, such as an injury, acute tracheal inflammation, or endotracheal intubation. Historically, congenital tracheal stenosis was managed with pericardial patch reconstruction or with an endoscopic procedure using either the KTP (potassium titanyl phosphate) or CO2 laser to divide each complete cartilaginous ring in the posterior midline followed by long-term stenting.31 With a short segment of complete rings, resection and anastomosis is effective, but short-segment congenital tracheal stenosis is very uncommon.32
More recently, these methods have been replaced by slide tracheoplasty.33 An improvement on standard resection techniques, slide tracheoplasty allows reconstruction without tension.34 The narrowed segment is transected in its midportion, and the remaining stenotic segments are incised. One end of the trachea is opened in the posterior midline, and the other is incised in the anterior midline. The diameter of the resulting anastomosis is broad enough to avoid airway narrowing. Long-term evaluation has shown excellent survival rates with a lower need for airway stenting.35
Acquired Stenosis
If a dense stenosis has already occurred, the previously described techniques may not be effective. Acquired tracheal obstruction can be classified as granulomatous, inflammatory, fibrous, or calcific. Congenital obstructions are usually cartilaginous. With dense fibrous and calcific strictures, open resection and reconstruction is usually necessary. However, endoscopic laser incision of the stricture with gradual and gentle balloon expansion of the lumen, combined with insertion of an endotracheal stent, may allow a functional airway to remodel over a period of time. Some authors advocate treatment of tracheal granulation tissue with mitomycin-C.36 If stenosis recurs when the stent is removed, the stent can be reinserted and balloon dilation performed with the stent in situ.
In the past, T-tubes have been used effectively as stents in both children and adults.24 Newer expandable metal stents are now frequently used in adults. Some of these nickel-titanium (nitinol)-coated stents have been used in children in selected cases.37 However, these stents may not be appropriate for children because the child will grow and the metal stent does not. Removal of the stent is then necessary and can be problematic. Moreover, the ingrowth of granulation tissue through the interstices of the metal stent may produce obstruction in itself and lead to severe hemorrhage when removal is attempted. Finally, the medical conditions for which the stents are placed are often different in children from those in adults. Stent use in adults is often due to neoplastic conditions that are associated with a short life expectancy. However, in children, a stent may be required for years.
Silicone rubber stents have been successfully used in adults, but fixation to the trachea using projections from the circumference of the tube is necessary to prevent migration.38,39 The small diameter of a child’s trachea makes these stents impractical. A T-shaped tube inserted through a tracheotomy with proximal and distal tracheal extensions can be readily inserted into a child’s airway and will not migrate.
Open Laryngotracheoplasty
There are variations in the operative techniques used for tracheal reconstruction in infants and children.40,41 An open procedure can be done with or without cardiopulmonary bypass.42 Recently, there has been a tendency to avoid bypass and use only endotracheal anesthesia. Second, the repair can be performed with or without an augmentation graft. Possible graft options include tracheal allografts or autografts, costal cartilage, cartilage from other sites such as thyroid or alar cartilage, autologous or allogeneic pericardium, or skin. Third, a stent can be used to maintain the lumen and can remain for hours, days, months, or years.
The anterior cricoid split procedure is useful in treating moderate subglottic stenosis in neonates and young infants.43,44 Infants selected for this procedure should weigh more than 1500 g and require assisted ventilation or inspired oxygen of more than 35%. They should also not be in cardiac failure. This technique is illustrated in Figure 21-14.45 Proper selection of patients for the anterior cricoid split is crucial. After undergoing the anterior cricoid split, infants who can be successfully extubated have excellent long-term outcomes, while those who continue to need intubation usually require tracheostomy.
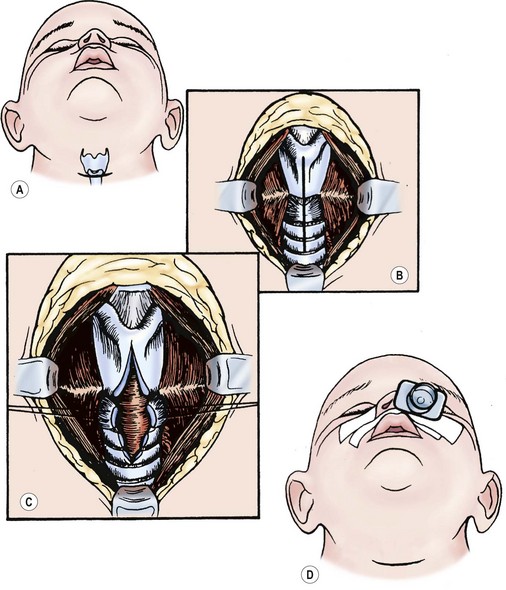
FIGURE 21-14 The anterior cricoid split procedure. (A) Make a horizontal incision over the cricoid cartilage. (B) Use a combination of sharp and blunt dissection to expose the larynx and upper trachea. (C) Split the lower portion of the thyroid cartilage, the cricoid cartilage, and upper tracheal rings. (D) Close the wound loosely over a drain with the airway stented by a nasotracheal tube. (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
The classic laryngotracheoplasty utilizes a cartilage graft (Fig. 21-15).46,47 An omental flap may help a long cartilaginous graft survive.48 The cartilage is inserted anteriorly after incising the stenotic segment.49,50 Cartilage inserts also can be placed posteriorly and laterally as well. Ciliated mucosa has been found on the surface of a mature costal cartilage graft if the perichondrium faces the airway lumen.51 Another option is resection of the stenotic tracheal segment and primary end-to-end anastomosis.52 A slide tracheoplasty technique can also be used.53,54
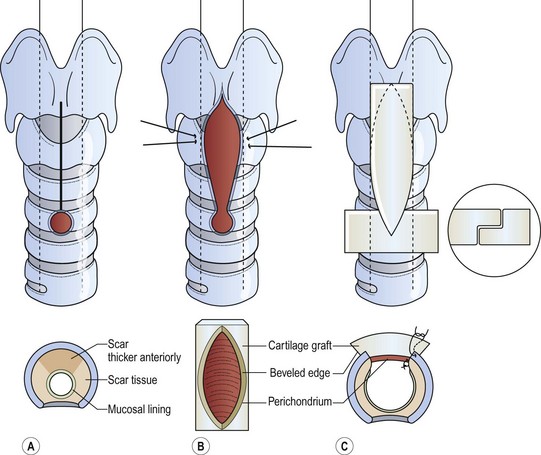
FIGURE 21-15 An autogenous costal cartilage graft reconstruction. (A) Expose the larynx and upper trachea. (B) Incise the aforementioned region, remaining superior to the tracheostomy stoma if the stenosis does not involve this site. (C) Sew the costal cartilage to the incised edges of the larynx and trachea, placing the perichondrium internally. (Adapted from Othersen HB Jr, editor. The Pediatric Airway. Philadelphia: WB Saunders; 1991.)
A fifth option for repair of tracheal stenosis utilizes an anterior tracheal incision with closure of the defect using pericardium. These pericardial patch operations are performed with cardiopulmonary bypass.55 However, experience at some centers has not been as favorable because of complications secondary to patch collapse.56 The original proponents of pericardial patching have now reported improved results with a free tracheal autograft in which the excised stenotic segment is flattened and used as a free anterior autograft to expand the tracheal lumen.57
Silicone T-tubes can be used as an internal stents to maintain the tracheal lumen and allow tracheal remodeling following tracheoplasty.58 These tubes can be placed temporarily to maintain the airway lumen while the airway heals following tracheoplasty. Alternatively, T-tubes can be effective as a permanent stent for the difficult airway stenosis or in cases of failed tracheoplasty. In very complicated and difficult cases, we have utilized custom-made T-tubes. One such custom T-tube is shown in Figure 21-16. This tube was necessary for the treatment of tracheomalacia at the carina. As a bifurcated Y-tube is difficult to insert, in this case, a bronchial arm extended into one bronchus and a hole allowed aeration of the other lung.
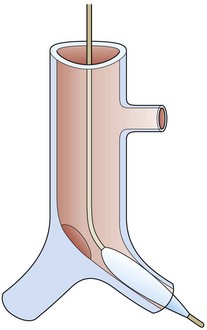
FIGURE 21-16 Diagram of a custom bifurcated T-tube with extension into the left main stem bronchus and a hole to allow ventilation for the right main-stem bronchus. A guide wire is placed with visualization by a flexible scope and a balloon catheter passed over it. With the balloon inflated, introduction of the stent is facilitated.
References
1. Othersen, HB, Jr. Intubation injuries of the trachea in children. Management and prevention. Ann Surg. 1979; 189:601–606.
2. Weber, TR, Connors, RH, Tracy, TF, Jr. Acquired tracheal stenosis in infants and children. J Thorac Cardiovasc Surg. 1991; 102:29–35.
3. Macpherson, RI. Radiologic aspects of airway obstruction. In: Othersen HB, Jr., eds. The Pediatric Airway. Philadephia: WB Saunders; 1991:30–65.
4. De Vries, PA, De Vries, CR. Embryology and Development. In: Othersen HB, Jr., eds. The Pediatric Airway. Philadelphia: WB Saunders; 1991:3–16.
5. Erwin, EA, Gerber, ME, Cotton, RT. Vascular compression of the airway: Indications for and results of surgical management. Int J Pediatr Otorhinolaryngol. 1997; 40:155–162.
6. Roberts, CS, Othersen, HB, Jr., Sade, RM, et al. Tracheoesophageal compression from aortic arch anomalies: Analysis of 30 operatively treated children. J Pediatr Surg. 1994; 29:334–337.
7. Braunstein, PW, Sade, RM. Vascular malformations with airway obstruction. In: Othersen HB, Jr., eds. The Pediatric Airway. Philadelphia: WB Saunders; 1991:81–96.
8. Adler, SC, Isaacson, G, Balsara, RK. Innominate artery compression of the trachea: Diagnosis and treatment by anterior suspension. A 25-year experience. Ann Otol Rhinol Laryngol. 1995; 104:924–927.
9. Clevenger, FW, Othersen, HB, Jr., Smith, CD. Relief of tracheal compression by aortopexy. Ann Thorac Surg. 1990; 50:524–529.
10. Corbally, MT, Spitz, L, Kiely, E, et al. Aortopexy for tracheomalacia in oesophageal anomalies. Eur J Pediatr Surg. 1993; 3:264–266.
11. Kamerkar, DR, Gladstone, DJ. Innominate artery compression of the trachea. A simplified technique for anterior suspension of the innominate artery. J Cardiovasc Surg (Torino). 1994; 35:549–552.
12. Pasic, M, von Segesser, L, Carrel, T, et al. Anomalous left pulmonary artery (pulmonary sling): Result of a surgical approach. Cardiovasc Surg. 1993; 1:608–612.
13. Ziemer, G, Heinemann, M, Kaulitz, R, et al. Pulmonary artery sling with tracheal stenosis: Primary one-stage repair in infancy. Ann Thorac Surg. 1992; 54:971–973.
14. Othersen, HB, Jr., Khalil, B, Zellner, J, et al. Aortoesophageal fistula and double aortic arch: Two important points in management. J Pediatr Surg. 1996; 31:594–595.
15. Koontz, CS, Bhatia, A, Forbess, J, et al. Video-assisted thoracoscopic division of vascular rings in pediatric patients. Am Surg. 2005; 71:289–291.
16. Kane, TD, Nadler, EP, Potoka, DA. Thoracoscopic aortopexy for vascular compression of the trachea: Approach from the right. J Laparoendosc Adv Surg Tech A. 2008; 18:313–316.
17. Jensen, AR, Le, D, Albanese, CT. Utilization of a transsternal spinal needle for retrograde sternal passage during thoracoscopic aortopexy. Pediatr Endosurg Innovative Tech. 2004; 8:333–338.
18. Decou, JM, Parsons, DS, Gauderer, MWL. Thoracoscopic aortopexy for severe tracheomalacia. Pediatr Endosurg Innovative Tech. 2001; 4:205–208.
19. Durkin, ET, Krawiec, ME, Shaaban, AF. Thoracoscopic aortopexy for primary tracheomalacia in a 12-year-old. J Pediatr Surg. 2007; 42:E15–E17.
20. Schaarschmidt, K, Kolberg-Schwerdt, A, Pietsch, L, et al. Thoracoscopic aortopericardiosternopexy for severe tracheomalacia in toddlers. J Pediatr Surg. 2002; 37:1476–1478.
21. van der Zee, DC, Bax, NM. Thoracoscopic tracheoaortopexia for the treatment of life-threatening events in tracheomalacia. Surg Endosc. 2007; 21:2024–2025.
22. Othersen, HB, Jr. Medical diseases of the airway: A surgeon’s role. In: Othersen HB, Jr., eds. The Pediatric Airway. Philadelphia: WB Saunders; 1991:64–70.
23. Mauro, RD, Poole, SR, Lockhart, CH. Differentiation of epiglottitis from laryngotracheitis in the child with stridor. Am J Dis Child. 1988; 142:679–682.
24. Gaissert, HA, Grillo, HC, Mathisen, DJ, et al. Temporary and permanent restoration of airway continuity with the tracheal T-tube. J Thorac Cardiovasc Surg. 1994; 107:600–606.
25. Gaissert, HA, Lofgren, RH, Grillo, HC. Upper airway compromise after inhalation injury. Complex strictures of the larynx and trachea and their management. Ann Surg. 1993; 218:672–678.
26. White, DR, Preciado, DA, Stamper, B, et al. Airway reconstruction in pediatric burn patients. Otolaryngol Head Neck Surg. 2005; 133:362–365.
27. Slimane, MA, Becmeur, F, Aubert, D, et al. Tracheobronchial ruptures from blunt thoracic trauma in children. J Pediatr Surg. 1999; 34:1847–1850.
28. Huh, J, Milliken, JC, Chen, JC. Management of tracheobronchial injuries following blunt and penetrating trauma. Am Surg. 1997; 63:896–899.
29. Cooper, JD, Grillo, HC. The evolution of tracheal injury due to ventilatory assistance through cuffed tubes: A pathologic study. Ann Surg. 1969; 169:334–348.
30. Othersen, HB, Jr. Subglottic tracheal stenosis. Semin Thorac Cardiovasc Surg. 1994; 6:200–205.
31. Othersen, HB, Jr., Hebra, A, Tagge, EP. A new method of treatment for complete tracheal rings in an infant: Endoscopic laser division and balloon dilation. J Pediatr Surg. 2000; 35:262–264.
32. Brown, JW, Bando, K, Sun, K, et al. Surgical management of congenital tracheal stenosis. Chest Surg Clin N Am. 1996; 6:837–852.
33. Acosta, AC, Albanese, CT, Farmer, DL, et al. Tracheal stenosis: The long and the short of it. J Pediatr Surg. 2000; 35:1612–1616.
34. Grillo, HC. Slide tracheoplasty for long-segment congenital tracheal stenosis. Ann Thorac Surg. 1994; 58:613–621.
35. Grillo, HC, Wright, CD, Vlahakes, GJ, et al. Management of congenital tracheal stenosis by means of slide tracheoplasty or resection and reconstruction, with long-term follow-up of growth after slide tracheoplasty. J Thorac Cardiovasc Surg. 2002; 123:145–152.
36. Ward, RF, April, MM. Mitomycin-C in the treatment of tracheal cicatrix after tracheal reconstruction. Int J Pediatr Otorhinolaryngol. 1998; 44:221–226.
37. Prasad, M, Bent, JP, Ward, RF, et al. Endoscopically placed nitinol stents for pediatric tracheal obstruction. Int J Pediatr Otorhinolaryngol. 2002; 66:155–160.
38. Puma, F, Ragusa, M, Avenia, N, et al. The role of silicone stents in the treatment of cicatricial tracheal stenoses. J Thorac Cardiovasc Surg. 2000; 120:1064–1069.
39. Vergnon, JM, Costes, F, Polio, JC. Efficacy and tolerance of a new silicone stent for the treatment of benign tracheal stenosis: Preliminary results. Chest. 2000; 118:422–426.
40. Ein, SH, Friedberg, J, Williams, WG, et al. Tracheoplasty: A new operation for complete congenital tracheal stenosis. J Pediatr Surg. 1982; 17:872–878.
41. Matute, JA, Villafruela, MA, Delgado, MD, et al. Surgery of subglottic stenosis in neonates and children. Eur J Pediatr Surg. 2000; 10:286–290.
42. Loukanov, T, Sebening, C, Springer, W, et al. Simultaneous management of congenital tracheal stenosis and cardiac anomalies in infants. J Thorac Cardiovasc Surg. 2005; 130:1537–1541.
43. Cotton, RT, Seid, AB. Management of the extubation problem in the premature child. Anterior cricoid split as an alternative to tracheotomy. Ann Otol Rhinol Laryngol. 1980; 89:508–511.
44. Silver, FM, Myer, CM, 3rd., Cotton, RT. Anterior cricoid split. Update 1991. Am J Otolaryngol. 1991; 12:343–346.
45. Myer, CM, 3rd., Cotton, RT. Cricoid split and cartilage tracheoplasty. In: Othersen HB, Jr., eds. The Pediatric Airway. Philadelphia: WB Saunders; 1991:117–124.
46. Kimura, K, Mukohara, N, Tsugawa, C, et al. Tracheoplasty for congenital stenosis of the entire trachea. J Pediatr Surg. 1982; 17:869–871.
47. Tsugawa, C, Kimura, K, Muraji, T, et al. Congenital stenosis involving a long segment of the trachea: Further experience in reconstructive surgery. J Pediatr Surg. 1988; 23:471–475.
48. Tsugawa, C, Nishijima, E, Muraji, T, et al. The use of omental pedicle flap for tracheobronchial reconstruction in infants and children. J Pediatr Surg. 1991; 26:762–765.
49. Forsen, JW, Jr., Lusk, RP, Huddleston, CB. Costal cartilage tracheoplasty for congenital long-segment tracheal stenosis. Arch Otolaryngol Head Neck Surg. 2002; 128:1165–1171.
50. Gustafson, LM, Hartley, BE, Liu, JH, et al. Single-stage laryngotracheal reconstruction in children: A review of 200 cases. Otolaryngol Head Neck Surg. 2000; 123:430–434.
51. Oue, T, Kamata, S, Usui, N, et al. Histopathologic changes after tracheobronchial reconstruction with costal cartilage graft for congenital tracheal stenosis. J Pediatr Surg. 2001; 36:329–333.
52. Har-El, G, Shaha, A, Chaudry, R, et al. Resection of tracheal stenosis with end-to-end anastomosis. Ann Otol Rhinol Laryngol. 1993; 102:670–674.
53. Lipshutz, GS, Jennings, RW, Lopoo, JB, et al. Slide tracheoplasty for congenital tracheal stenosis: A case report. J Pediatr Surg. 2000; 35:259–261.
54. Lang, FJ, Hurni, M, Monnier, P. Long-segment congenital tracheal stenosis: Treatment by slide-tracheoplasty. J Pediatr Surg. 1999; 34:1216–1222.
55. Backer, CL, Mavroudis, C, Gerber, ME, et al. Tracheal surgery in children: An 18-year review of four techniques. Eur J Cardiothorac Surg. 2001; 19:777–784.
56. Houel, R, Serraf, A, Macchiarini, P, et al. Tracheoplasty in congenital tracheal stenosis. Int J Pediatr Otorhinolaryngol. 1998; 44:31–38.
57. Backer, CL, Mavroudis, C, Dunham, ME, et al. Repair of congenital tracheal stenosis with a free tracheal autograft. J Thorac Cardiovasc Surg. 1998; 115:869–874.
58. Huang, CJ. Use of the silicone T-tube to treat tracheal stenosis or tracheal injury. Ann Thorac Cardiovasc Surg. 2001; 7:192–196.