23 Management of Gastrointestinal Bleeding
The incidence of upper GI bleeding is estimated to be 37 to 172 per 10,000 population per year. Upper GI bleeding is nearly twice as common in males as in females, and its incidence increases with age, a pattern that has been attributed to increased incidence of predisposing comorbid conditions.1,2 The mortality rate for patients with upper GI bleeding has remained relatively stable over the past 40 years, ranging from 3% to 14%.1 The risk of death depends on the patient’s age, presence of shock, comorbid medical conditions, presence of recent hemorrhage, location of the onset of bleeding (inpatient versus outpatient), and underlying cause of the hemorrhage (Table 23-1). Scoring systems to predict mortality and risk of rebleeding are based on host factors, the patient’s clinical course, and endoscopic findings.1,2 Variceal hemorrhage is associated with a mortality rate of 15% to 20%, and the risk of recurrent bleeding is about 30%.3,4
TABLE 23-1 Risk Factors for Death After Hospital Admission for Acute Upper Gastrointestinal Hemorrhage
 Causes of Lower Gastrointestinal Bleeding
Causes of Lower Gastrointestinal Bleeding
The most common cause of bleeding in patients younger than 50 years of age is hemorrhoids.5
 Major Causes of Gastrointestinal Bleeding
Major Causes of Gastrointestinal Bleeding
Peptic Ulcer Disease
Peptic ulcer disease accounts for as many as half of the cases of upper GI bleeding. It is also the most common cause of bleeding in patients with portal hypertension and varices.1 Bleeding from mucosal ulceration adjacent to a vessel can result from a Helicobacter pylori infection, use of nonsteroidal antiinflammatory drugs (NSAIDs), and/or critical illness. Concurrent aspirin and oral anticoagulation use further increases the risk of bleeding.6–9 Acid suppression therapy (H2-antagonists, proton pump inhibitors), however, has not affected the predominance of peptic ulcer bleeding as the cause of acute hemorrhage.10
Esophageal Varices
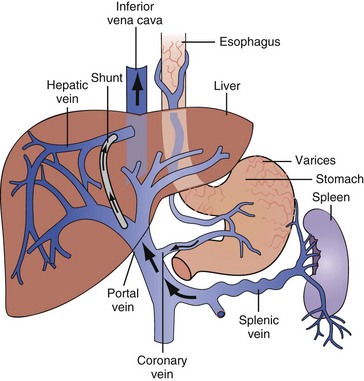
Gastroesophageal variceal hemorrhage is a major complication of portal hypertension from cirrhosis and accounts for 5% to 15% of all cases of bleeding from the upper GI tract.11–14 The most common site of varices is the distal 2 to 5 cm of the esophagus. Superficial veins in this anatomic region lack support from surrounding tissues (Figure 23-1).15 The dilation of distal esophageal varices depends on a threshold pressure gradient, most commonly measured by the hepatic venous pressure gradient, defined as the difference between the wedged, or occluded, hepatic venous pressure and the free hepatic venous pressure (normal gradient < 5 mm Hg). If the hepatic venous pressure gradient is below 12 mm Hg, varices do not form.16,17 Varices do not invariably develop in patients with gradients ≥12 mm Hg, so this pressure gradient is necessary but may not be sufficient in and of itself for varix formation.16,17 Gastroesophageal varices are present in 40% to 60% of patients with cirrhosis; their presence and size are related to the underlying cause, duration, and severity of cirrhosis.18
Esophagitis
Significant bleeding from esophagitis and erosive disease is the second most common cause of upper GI hemorrhage, often causing occult blood loss rather than acute bleeding.6–9 Clinically obvious bleeding is most likely in patients with extensive ulcerative disease or with an underlying coagulopathy.
Mallory-Weiss Tear
Mallory-Weiss tears usually occur in gastric mucosa, although 10% to 20% occur in esophageal mucosa. They account for approximately 5% to 7% of cases of upper GI hemorrhage.6–9 A history of retching is obtained in less than one third of patients.19 Bleeding from Mallory-Weiss tears remits spontaneously in most patients; 5% experience rebleeding. Patients who experience rebleeding from a Mallory-Weiss tear usually have an underlying bleeding diathesis.20,21
Diverticulosis
The prevalence of diverticular disease is age dependent, increasing from less than 5% at age 40 to 30% by age 60, to 65% by age 85. The high prevalence of the disease explains why diverticulosis is the most common cause of lower GI bleeding even though fewer than 15% of patients with diverticulosis develop significant diverticular bleeding. Diverticular bleeding typically occurs in the absence of diverticulitis, and the risk of bleeding is not further increased if diverticulitis is present.22 Risk factors for diverticular bleeding include23:
Neoplasms
Colon cancer is a relatively less common but serious cause of hematochezia. Neoplasms are responsible for approximately 10% of cases of rectal bleeding in patients older than 50 years, but neoplasms are rarely implicated as the etiology for GI bleeding in younger individuals.24 Bleeding occurs as the result of erosion or ulceration of the overlying mucosa. The bleeding tends to be low grade and recurrent. Bright red blood suggests left-sided lesions; right-sided lesions can manifest with maroon blood or melena.
Hemorrhoids
Hemorrhoidal bleeding typically is painless, often presenting as bright red blood on stools, in the toilet, or on toilet paper. Hemorrhoids are dilated submucosal veins in the anus, located above (internal) or below (external) the dentate line.25 They usually are asymptomatic but can manifest with hematochezia, thrombosis, strangulation, or pruritus. Hematochezia results from rupture of internal hemorrhoids that are supplied by the superior and middle hemorrhoidal arteries.
 Initial Management of Gastrointestinal Bleeding
Initial Management of Gastrointestinal Bleeding
Bleeding stops spontaneously in most patients, but aggressive management is required when bleeding does not quickly resolve or when patients are at high risk for rebleeding. Priorities include achieving hemodynamic stability and preventing complications such as pulmonary aspiration.26,27 The rate of bleeding dictates the urgency of management:
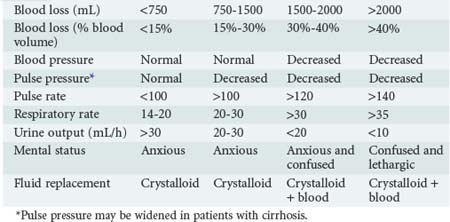
The degree of blood loss also can be estimated clinically by an evaluation of the heart rate, blood pressure, respiratory rate, urine output, and mental status (Table 23-2). The clinical estimation of blood loss is somewhat more difficult in patients with cirrhosis who have hyperdynamic circulation at baseline and a lower-than-normal systolic blood pressure and widened pulse pressure.
History and Examination
The clinical features of the GI bleeding provide clues to the probable source of bleeding within the GI tract (Table 23-3). When small amounts of bright red blood are passed per rectum, the lower GI tract can be assumed to be the source. In patients with large-volume maroon stools, aspiration via a nasogastric tube should be performed to assess the possibility of upper GI bleeding. Examination of nasogastric aspirate has diagnostic value, although in approximately 15% of patients with upper GI bleeding, the nasogastric aspirate fails to reveals blood or “coffee ground” material.26,27
TABLE 23-3 Clinical Indicators of Gastrointestinal Bleeding and the Probable Source Location Within the Gastrointestinal Tract
| Clinical Indicator | Probability of Upper Gastrointestinal Source | Probability of Lower Gastrointestinal Source |
|---|---|---|
| Hematemesis | Almost certain | Rare |
| Melena | Probable | Rare |
| Hematochezia | Possible | Probable |
| Blood-streaked stool | Rare | Almost certain |
| Occult blood in stool | Possible | Possible |
All patients with upper GI bleeding should have a nasogastric tube placed. Iced-saline lavage does not prevent or decrease upper GI bleeding.28 Gastric lavage with lukewarm tap water offers an equally safe and cost-effective alternative.29 Coffee-ground material or a frankly bloody gastric aspirate confirms an upper GI source of bleeding, whereas a nonbloody yellow-green nasogastric aspirate that contains duodenal secretions suggests the absence of bleeding proximal to the ligament of Treitz.30 However, in up to 50% of patients with a bleeding duodenal ulcer, a nonbloody gastric aspirate is obtained,29 possibly because of insufficient reflux of blood from the duodenum through the pylorus. Similarly, an intermittently bleeding upper GI lesion may result in a nonbloody gastric aspirate. The color of the gastric aspirate is of prognostic significance. Patients with coffee-ground or black gastric aspirates and whose stool is melanotic have a reported mortality rate of 9%.30 However, patients who have bright red blood per gastric aspirate and red blood per rectum have a 30% mortality rate.30 Red blood per rectum from an upper GI source usually signifies rapid bleeding.31
After the gastric contents have been aspirated, the nasogastric tube should be left in place to monitor ongoing bleeding and prevent pulmonary aspiration until there is no longer any evidence of bleeding. Maintaining this tube for a prolonged period, especially when the tube is attached to suction, may injure gastric mucosa and exacerbate GI hemorrhage.32
Initial Resuscitation
Volume resuscitation with crystalloids is the first priority in the management of any patient with GI bleeding. Two large-bore peripheral intravenous (IV) catheters should be inserted and/or a large-bore central line venous catheter should be established. Resuscitation should be initiated with crystalloid solutions, either normal saline (2 L) or lactated Ringer’s solution. Large-volume resuscitation with normal saline alone may cause a hyperchloremic metabolic acidosis and is possibly associated with coagulation abnormalities. Colloidal solutions have no role in the management of patients with acute GI bleeding. A complete blood count including platelet count should be obtained. Other key laboratory studies should include blood typing and cross-matching, prothrombin time (or international normalized ratio), activated partial thromboplastin time, blood chemistry panel, liver function panel. Transfusion of packed red blood cells should be initiated for patients with an estimated blood loss greater than 15%. Transfusion of fresh frozen plasma should be initiated for patients with preexisting coagulopathy (from liver disease or anticoagulation; see Table 23-2). Platelet transfusion is indicated if the platelet count is less than 50,000/µL.
The endpoints of resuscitation include normalization of heart rate, blood pressure, and indices of end-organ perfusion. Vasopressor agents initially should be avoided because pressor-mediated vasoconstriction in a hypovolemic patient can cause severe end-organ ischemia.33 Patients with a history of congestive heart failure, renal failure, or cirrhosis may require monitoring to assess cardiac parameters such as central venous pressure, cardiac output, stroke volume, and/or preload responsiveness. Although bedside pulmonary artery catheterization was widely used in the past for cardiac monitoring in the ICU, the recent trend in critical care medicine has been to use less invasive approaches such as bedside echocardiography or monitoring of pulse pressure variation.
Triage: Who to Admit to the Intensive Care Unit
The rate of rebleeding is approximately 3% in the low-risk group and 25% in the high-risk group. Patients in the low-risk group can be managed safely on a general medical floor. The decision regarding ICU admission should be individualized based on the patient’s risk stratification, age, comorbid diseases, clinical presentation, and endoscopic findings. Patients with active bleeding and two or more comorbidities have a mortality rate above 10% and should be observed in an ICU.34 Patients with coronary artery disease are best managed in an ICU because of the risk of myocardial ischemia secondary to hypovolemia and hypoperfusion.45 Admission to an ICU should be considered when endoscopic stigmata of recent hemorrhage, particularly visible vessels, are noted.
 Further Management of Upper Gastrointestinal Bleeding
Further Management of Upper Gastrointestinal Bleeding
Nonvariceal Bleeds
A meta-analysis of a large number of studies of nonvariceal bleeds demonstrated that endoscopic intervention decreased the mortality rate.35 Multiple endoscopic therapies, including injection of epinephrine, injection of alcohol, injection of thrombin, injection of fibrin glue, thermal contact, or application of hemostatic clips, have been evaluated. Monotherapy with epinephrine provides suboptimal hemostasis. However, epinephrine plus a second method significantly reduces the risk of rebleeding, surgery, or mortality.
Variceal Bleeding
Variceal bleeding stops spontaneously in more than half of patients; however, in those who continue to bleed, the mortality rate approaches 80%. Without treatment to obliterate the varices, there is a 60% to 70% risk of rebleeding. The risk for acute recurrent bleeding is highest within the first 72 hours of the initial bleed and decreases with time, similar to the case for peptic ulcer hemorrhage.36,37 Another option is variceal band ligation37,38; advantages over injection sclerotherapy include fewer local and systemic complications, lower rebleeding rates, fewer endoscopic treatment sessions to obliterate varices, and lower mortality rate.38–42
The diagnostic and therapeutic value of endoscopy in patients with upper GI bleeding is often limited by the presence of residual blood or clots.43 To avoid this problem, gastric lavage is usually performed with a large-diameter nasogastric tube just before endoscopy.44 Erythromycin induces rapid gastric emptying in healthy subjects and in patients with diabetic gastroparesis.44–46 Infusion of erythromycin (250 mg) just prior to endoscopy improves esophagogastroduodenal cleansing and enhances the quality of endoscopic findings.45
 Further Management of Bleeding Peptic Ulcers
Further Management of Bleeding Peptic Ulcers
Pharmacologic Therapy
Although gastric acid–suppressing agents such as histamine receptor 2 blockers (H2 blockers) have long been available as treatment options for patients with peptic ulcer disease, in acutely bleeding patients, their use has not reduced the number of transfusions, episodes of further bleeding or rebleeding, or the need for surgery.46
Proton pump inhibitors (PPIs) are now widely used to suppress gastric acid secretion in patients with a variety of acid-related disorders.47 Data from a number of studies48–54 suggest that IV administration of a PPI reduces the risk of recurrent upper GI bleeding, but this therapy may not affect other outcome variables. Somatostatin is effective for controlling hemorrhage from esophageal varices,55–57 but its efficacy in the setting of nonvariceal upper GI hemorrhage has not been demonstrated.58
Role of Surgery
Although surgical intervention for peptic ulcer bleeding is less common than in the past, the indications for operation remain unchanged, including severe hemorrhage unresponsive to initial resuscitative measures; unavailability or failure of endoscopic or other nonsurgical therapies to control persistent or recurrent bleeding; and a coexisting second indication for operation, such as perforation, obstruction, or suspicion of malignancy.59,60
In a clinical trial that enrolled patients with recurrent upper GI hemorrhage, patients who were randomized to receive endoscopic retreatment had significantly fewer complications and tended to have decreased transfusion requirements, 30-day mortality rate, and use of the ICU than patients who were randomized to surgery.61 Nevertheless, 10% to 12% of patients with acute ulcer hemorrhage still require operative intervention for adequate hemostasis.62
 Further Management of Esophageal Varices
Further Management of Esophageal Varices
Pharmacologic Interventions
Vasopressin causes direct splanchnic and systemic vasoconstriction mediated via the V1 receptor on vascular smooth muscle and thereby decreases portal venous flow and portal pressure.63 Vasopressin can be administered either IV or directly into the superior mesenteric artery. As with other potent vasoconstrictors, vasopressin must be administered via a central venous line. Higher doses are associated with increased toxicity without further benefit. Vasopressin achieves hemostasis in about 55% of patients.64 Systemic side effects, which occur in 20% to 30% of patients, can include myocardial ischemia, cerebral ischemia, acrocyanosis, congestive heart failure, cardiac arrhythmias, hyponatremia, hypertension, and phlebitis at the venous infusion site. Concomitant administration of nitroglycerin, either IV or sublingually, improves the safety and efficacy of vasopressin.65 The combination of vasopressin and nitroglycerin more effectively controls bleeding and reduces toxicity but does not reduce mortality compared to vasopressin alone.66 Terlipressin, a synthetic vasopressin analog, has been used instead of vasopressin to attempt to reduce the toxicity.67 Terlipressin can be administered as intermittent boluses and has a better side-effect profile than vasopressin. A recent meta-analysis showed reduction in all-cause mortality with terlipressin compared to placebo. No statistical difference in outcome was noted among terlipressin and octreotide, vasopressin, or balloon tamponade. Terlipressin is not currently available for use in the United States.
Somatostatin causes splanchnic vasoconstriction, reduces azygos blood flow, reduces portal collateral circulation, and decreases portal pressure.68 Somatostatin has been used successfully as an alternative to vasopressin to control variceal bleeding owing to its safer side-effect profile.69 Octreotide, a synthetic somatostatin analog, is more commonly used than somatostatin and is the drug of choice in the United States. Somatostatin or octreotide therapy in addition to sclerotherapy is superior to either therapy alone in controlling bleeding and preventing rebleeding but has not been shown to improve long-term mortality. Likewise, the combination of somatostatin and endoscopic variceal ligation does not improve long-term mortality. Although both agents control acute bleeding and prevent rebleeding, neither somatostatin nor octreotide have a clearly demonstrated role in improving mortality.70–74
Balloon Tamponade
Variceal hemorrhage that is unresponsive to combination therapy with octreotide and endoscopic therapy should be temporarily controlled by balloon tamponade, which initially can control hemorrhage in up to 90% of cases.75,76 Rebleeding occurs in approximately 50% of cases after balloon deflation if balloon tamponade is used alone.77 Endotracheal intubation and adequate sedation is essential before placement of the balloon.78,79 Relative contraindications to balloon tamponade include esophageal stricture, recent caustic ingestion, recent esophageal surgery, large hiatal hernia, recent sclerotherapy, an unproven variceal source of bleeding, and an improperly trained support staff.80,81 Esophageal rupture occurs in about 3% of cases. Other complications include pulmonary aspiration, alar necrosis, nasopharyngeal bleeding, and balloon impaction.77,80,81
Transjugular Intrahepatic Portosystemic Shunt
Transjugular intrahepatic portosystemic shunt (TIPS) is an intrahepatic low-resistance shunt between the hepatic and portal veins created by angiographic methods (see Figure 23-1). The shunt is kept patent by a fenestrated metal stent and decompresses the portal vein, similar to a surgical side-to-side portacaval shunt, but avoids the need for laparotomy.
Approximately 10% to 20% of patients fail to stop bleeding with standard medical therapy. Others rebleed in the first few days after cessation of the index bleed. A second attempt at endoscopic hemostasis is sometimes effective and is generally recommended.82 TIPS has been shown to achieve hemostasis in patients with refractory hemorrhage from varices. Among high-risk patients, placement of TIPS should be considered sooner rather than later, as significant improvement in mortality has been demonstrated in recent studies. TIPS also has been shown to improve long-term outcomes in patients who are poor candidates for surgery, such as those with sepsis, multiorgan failure, or cardiopulmonary compromise.83–86 Principal complications of TIPS are listed in Table 23-4.
TABLE 23-4 Complications of Transjugular Intrahepatic Portosystemic Shunt (TIPS)
| Technique-Related Complications | Complications Related to Portosystemic Shunting | Stent-Related Complications |
|---|---|---|
| Neck hematoma Cardiac arrhythmias Perihepatic hematoma Extrahepatic puncture of portal vein |
Hepatic encephalopathy Increased risk of bacteremia Liver failure |
TIPS-associated hemolysis |
| Infection of stent | ||
| Stent stenosis or ruptured liver capsule malfunction |
Nonselective Beta-Blockers
Nonselective beta-blockers such as propranolol and nadolol have been used to prevent recurrent bleeding. Treatment with these agents can reduce the risk of recurrent bleeding and death from bleeding by about 40%. Sympathetic adrenergic activity regulates splanchnic arteriolar resistance.87 Blockade of β-adrenergic receptors allows unrestricted α-adrenergic activity, producing splanchnic arteriolar vasoconstriction and decreasing portal venous inflow.
After an oral or IV dose of propranolol, portal pressure decreases by 9% to 31%.88–95 It has been suggested that a decrease in heart rate and cardiac output also contributes to the decrease in portal venous inflow.87–90 Findings suggest that the portal decompressive effect of propranolol is a specific splanchnic effect rather than a consequence of its systemic effects.96 Nitrates such as isosorbide mononitrate have been shown to act synergistically with beta-blockers in reducing hepatic venous pressure gradient. The cumulative risk of hemorrhage was decreased from 29% among those who received nadolol alone to 12% among those who received the combination of nadolol and isosorbide mononitrate.97 Nitrates, however, may worsen systemic arteriolar vasodilation due to cirrhosis and impair tissue oxygenation, presumably by dilation of arteriovenous channels in the peripheral circulation.
Nadolol has a longer half-life of biological activity98,99 and can be administered once a day. It is more hydrophilic than propranolol; hydrophilicity limits intestinal absorption after oral administration as well as passage across the blood-brain barrier.100,101 Propranolol is administered orally twice a day. The dose should be increased slowly until the heart rate decreases by 25% from baseline but remains above 55 beats per minute. Once a stable dose is achieved, propranolol can be changed to a once-a-day, sustained-release form102 that is equally effective.103–109
Surgical Management
Surgery for bleeding esophagogastric varices continues to be the most reliable method to control acute hemorrhage and prevent its recurrence. Operative approaches generally consist of either (1) decompression of the high-pressure portal venous system into the low-pressure systemic venous system by creation of a shunt or (2) devascularization of the distal esophagus and proximal stomach with or without disconnection of the portal and azygous venous systems. In most instances, surgical procedures are used for prevention of recurrent hemorrhage rather than treatment of the initial bleeding episode. Because of the effectiveness of endoscopic therapies, emergency surgery for variceal hemorrhage in most centers is reserved for patients who have failed initial nonsurgical treatment and have reasonable hepatic function.110
Antibiotics in Variceal Bleeding
Bacterial infections are very common in patients with cirrhosis. Most common causes are urinary tract infections and spontaneous bacterial peritonitis (SBP). Mortality has been shown to be higher in patients with infections than in noninfected patients.111,112 Infections also predispose patients to recurrent variceal hemorrhage.113 A meta-analysis of five trials of short-term antibiotic prophylaxis in patients with variceal bleeding showed both a decrease in the number of infections in treated patients and improved survival.114 Any patient with cirrhosis and GI bleeding should receive a short course of antibiotic therapy (oral norfloxacin, 400 mg twice a day; or IV ciprofloxacin, 1 g once a day).115 The latter therapy may be appropriate in areas with high prevalence of fluoroquinolone-resistant organisms.
 Further Management of Lower Gastrointestinal Bleeding
Further Management of Lower Gastrointestinal Bleeding
A careful digital rectal examination and sigmoidoscopy should be done to exclude anorectal pathology and confirm the patient’s description of the symptoms. Of rectal carcinomas diagnosed by proctoscopy, 40% are palpable on digital rectal examination.116
Colonoscopy
Colonoscopy is the mainstay of early and rapid diagnosis and treatment of lower GI bleeding. Colonoscopy has a very high diagnostic yield for patients presenting with lower GI bleeding.117 In addition, endoscopic therapy is applied to lower GI bleeding for many cases. Modes of endoscopic therapy for acute lower GI bleeding, in particular for angiodysplasia and diverticular disease, include thermal contact probes, laser, monopolar electrocautery (hot biopsy forceps), injection sclerotherapy, and band ligation.
Scintigraphy and Angiography
If the source of bleeding is not detected on colonoscopy, a bleeding scan followed by angiography should be considered if bleeding is severe. Although not as precise in identifying the site of bleeding as angiography, scintigraphy is safe and more sensitive, detecting active bleeding reliably at rates less than 0.1 mL/min.118,119 Angiographic demonstration of a tumor, neovascularization, or vascular lesions may identify a presumed source of bleeding in the absence of extravasation. The specificity of this procedure is 100%, but sensitivity varies from 47% with acute bleeding to 30% with recurrent bleeding.
Angiography permits transcatheter administration of vasoconstrictors (vasopressin or terlipressin) for lower GI bleeding.120 Although hemostasis is frequently achieved, rebleeding can occur in up to 50% of patients after cessation of therapy. Complications include abdominal pain, fluid retention, hyponatremia, transient hypertension, sinus bradycardia, premature ventricular contractions, and atrial fibrillation. Major complications have been reported and include pulmonary edema, serious arrhythmias, myocardial ischemia, and hypertension.121
Surgery
Age, probably by association with increased comorbidity, is an important risk factor for postoperative mortality. The postoperative mortality rate in patients undergoing emergent colon surgery for colorectal cancer is 3.7% in patients aged 70 to 79 years, 9.8% in those aged 80 to 89 years, and 12.9% in those older than 90 years.122 Surgery should be considered when a definite source of bleeding has been identified, but conservative measures have failed to achieve hemostasis. Accurate preoperative localization of the bleeding site is essential for successful segmental colonic resection. Blind segmental resection of the colon or segmental resection is associated with substantial risk of rebleeding and morbidity.123
van Leerdam ME, Vreeburg EM, Rauws EA, et al. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98(7):1494-1499.
Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98(3):653-659.
D’Amico G, Pietrosi G, Tarantino I, Pagliaro L, et al. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: a Cochrane meta-analysis. Gastroenterology. 2003;124(5):1277-1291.
Garcia-Pagán JC, Caca K, Bureau C, et al. An early decision for PTFE-TIPS improves survival in high risk cirrhotic patients admitted with an acute variceal bleeding: a multicenter RCT. Hepatology. 2008;48(Suppl):373A-374A.
Bernard B, Grange JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with GI bleeding: a meta-analysis. Hepatology. 1999;29(6):1655-1661.
1 van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22(2):209-224.
2 van Leerdam ME, Vreeburg EM, Rauws EA, et al. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494-1499.
3 Carbonell N, Pauwels A, Serfaty L, et al. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659.
4 Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653-659.
5 Korkis AM, McDougall CJ. Rectal bleeding in patients less than 50 years of age. Dig Dis Sci. 1995;40:1520-1523.
6 Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14-22.
7 van Leerdam ME, Tytgat GN. Review article: Helicobacter pylori infection in peptic ulcer haemorrhage. Aliment Pharmacol Ther. 2002;16:66-78.
8 Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717-727.
9 Chan FK, To KF, Wu JC, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: a randomised trial 2002. Lancet. 2002;359:9-13.
10 Van Dam J, Brugge WR. Endoscopy of the upper gastrointestinal tract. N Engl J Med. 1999;341:1738-1748.
11 Vreeburg EM, Snel P, de Bruijne JW, et al. Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92:236-243.
12 Longstreth, Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206-210.
13 Czernichow P, Hochain P, Nousbaum JB, et al. Epidemiology and course of acute upper gastro-intestinal haemorrhage in four French geographical areas. Eur J Gastroenterol Hepatol. 2000;12:175-181.
14 Paspatis GA, Matrella E, Kapsoritakis A, et al. An epidemiological study of acute upper gastrointestinal bleeding in Crete, Greece. Eur J Gastroenterol Hepatol. 2000;12:1215-1220.
15 Polio J, Groszmann RJ. Hemodynamic factors involved in the development and rupture of esophageal varices: A pathophysiologic approach to treatment. Semin Liver Dis. 1986;6:318-331.
16 Garcia-Tsao G, Groszmann RJ, Fisher RL, et al. Portal pressure, presence of gastro esophageal varices and variceal bleeding. Hepatology. 1985;5:419-424.
17 Lebrec D, De Fleury P, Rueff B, et al. Portal hypertension, size of esophageal varices, and risk of gastrointestinal bleeding in alcoholic cirrhosis. Gastroenterology. 1980;79:1139-1144.
18 Cales P, Zabotto B, Meskens C, et al. Gastroesophageal endoscopic features in cirrhosis: Observer variability, interassociations, and relationship to hepatic dysfunction. Gastroenterology. 1990;98:156-162.
19 Graham DY, Schwartz JT. The spectrum of the Mallory-Weiss tear. Medicine (Baltimore). 1978;57:307-318.
20 Hixson SD, Burns RP, Britt LG. Mallory-Weiss syndrome: Retrospective review of eight years’ experience. South Med J. 1979;72:1249-1251.
21 Bharucha AE, Gostout CJ, Balm RK. Clinical and endoscopic risk factors in the Mallory-Weiss syndrome. Am J Gastroenterol. 1997;92:805-808.
22 Meyers MA, Alonso DR, Gray GF, Baer JW. Pathogenesis of bleeding colonic diverticulosis. Gastroenterology. 1976;71:577-583.
23 Consensus Development Conference. Therapeutic endoscopy and bleeding ulcers. JAMA. 1989;262:1369-1372.
24 Macrae FAM, St John DJ. Relationship between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891-898.
25 Haas PA, Fox TA, Haas G. The pathogenesis of hemorrhoids. Dis Colon Rectum. 1984;27:442-450.
26 Barkun AN, Bardou M, Marshall JK, Nonvariceal upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843-857.
27 Barkun AN, Bardou Mm Kuipers E, Sung J, Hunt R, Martel M, Sinclair P. and for the International Consensus Upper Gastrointestinal Bleeding Conference Group: International Consensus Recommendations on the Management of Patients With Nonvariceal Upper Gastrointestinal Bleeding. Ann Intern Med. January 19, 2010;152:101-113.
28 Kankaria AG, Fleischer DE. The critical care management of nonvariceal upper gastrointestinal bleeding. Crit Care Clin. 1995;11:347-368.
29 Hawkey CJ. Nonsteroidal anti-inflammatory drugs and peptic ulcers. BMJ. 1990;300:278-284.
30 McGuirk TD, Coyle WJ. Upper gastrointestinal tract bleeding. Emerg Med Clin North Am. 1996;14:523-545.
31 Greene JF, Sawicki JE, Doyle WF. Gastric ulceration: A complication of double-lumen nasogastric tubes. JAMA. 1973;224:338-339.
32 Cappell MS. Intestinal (mesenteric) vasculopathy: I. Acute superior mesenteric arteriopathy and venopathy. Gastroenterol Clin North Am. 1998;27:783-825.
33 Hawkey CJ. Nonsteroidal anti-inflammatory drugs and peptic ulcers. BMJ. 1990;300:278-284.
34 Cappell MS. A study of the syndrome of simultaneous acute upper gastrointestinal bleeding and myocardial infarction in 36 patients. Am J Gastroenterol. 1995;90:1444-1449.
35 Savides TJ, Jensen DM. Therapeutic endoscopy for non-variceal bleeding. Gastroenterol Clin North Am. 2000;29:465-487.
36 Stiegmann GV, Goff JS, Sun JH, et al. Endoscopic elastic band ligation for active variceal hemorrhage. Am Surg. 1989;55:124-128.
37 Laine L, Cook D. Endoscopic ligation compared with sclerotherapy for treatment of esophageal variceal bleeding: A meta-analysis. Ann Intern Med. 1995;123:280-287.
38 Hou MC, Lin HC, Kuo BI, et al. Comparison of endoscopic variceal injection sclerotherapy and ligation for the treatment of esophageal variceal hemorrhage: A prospective randomized trial. Hepatology. 1995;21:1517-1522.
39 Lo GH, Lai KH, Shen MT, et al. A comparison of the incidence of transient bacteremia and infectious sequelae after sclerotherapy and rubber band ligation of bleeding esophageal varices. Gastrointest Endosc. 1994;40:675-679.
40 Stiegmann GV, Goff JS, Michaletz-Onody PA, et al. Endoscopic sclerotherapy as compared with endoscopic ligation for bleeding esophageal varices. N Engl J Med. 1992;326:1527-1532.
41 Young MF, Sanowski RA, Rasche R. Comparison and characterization of ulcerations induced by endoscopic ligation of esophageal varices versus endoscopic sclerotherapy. Gastrointest Endosc. 1993;39:119-122.
42 Stollman NH, Putcha RV, Neustater BR, et al. The uncleared fundal pool in acute upper gastrointestinal bleeding: Implications and outcomes. Gastrointest Endosc. 1997;46:324-327.
43 Peeters TL. Erythromycin and other macrolides as prokinetic agents. Gastroenterology. 1993;105:1886-1899.
44 Mantides A, Xynos E, Chrysos F, et al. The effect of erythromycin in gastric emptying of solids and hypertonic liquids in healthy subjects. Am J Gastroenterol. 1993;88:198-202.
45 Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin: Preliminary studies. N Engl J Med. 1990;322:1028-1031.
46 Nion I, Andant C, Jouet P, et al. Role of intravenous erythromycin in the preparation for endoscopy in case of upper digestive hemorrhage. Gastroenterol Clin Biol. 1998;22:554-555.
47 Younes Z. Medical therapies for bleeding peptic ulcer. Resident Reporter. 1999;4:52-56.
48 Daneshmend TK, Hawkey CJ, Langman MJS, et al. Omeprazole versus placebo for acute upper gastrointestinal bleeding: Randomised double-blind controlled trial. Br Med J. 1992;304:143-147.
49 Khuroo MS, Yattoo GN, Javid G, et al. A comparison of omeprazole and placebo for bleeding peptic ulcer. N Engl J Med. 1997;336:1054-1058.
50 Schaffalitzky de Muckadell OB, Havelund T, Harling H, et al. Effect of omeprazole on the outcome of endoscopically treated bleeding peptic ulcers. Scand J Gastroenterol. 1997;32:320-327.
51 Lau JY, Sung JJ, Lee KKC, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343:310-316.
52 Nietsch HH. Management of nonvariceal upper gastrointestinal bleeding. Resident Reporter. 2000;5:38-42.
53 Van Dam J, Brugge WR. Endoscopy of the upper gastrointestinal tract. N Engl J Med. 1999;34:1738-1748.
54 Cook DJ, Guyatt GH, Salena BJ, et al. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Gastroenterology. 1992;102:139-148.
55 Jenkins SA, Baxter SN, Corbett W, et al. A prospective, randomized controlled clinical trial comparing somatostatin and vasopressin in controlling acute variceal hemorrhage. Br Med J (Clin Res Ed). 1985;290:275-278.
56 Saari A, Klvilaasko E, Inberg M, et al. Comparison of somatostatin and vasopressin in bleeding esophageal varices. Am J Gastroenterol. 1990;85:804-807.
57 Imperiale TF, Birgisson S. Somatostatin or octreotide compared with H2 antagonists and placebo in the management of acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Ann Intern Med. 1997;127:1062-1071.
58 Stabile BE. Current surgical management of duodenal ulcers. Surg Clin North Am. 1992;72:335-356.
59 Stabile BE, Passaro EJr. Duodenal ulcer: A disease in evolution. Curr Prob Surg. 1984;21:1-79.
60 Lau JYW, Sung JJY, Lam Y, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
61 Rockall TA. Management and outcome of patients undergoing surgery after acute upper gastrointestinal haemorrhage. Steering Group for the National Audit of Acute Upper Gastrointestinal Haemorrhage. J R Soc Med. 1998;91:518-523.
62 Rodriguez-Perez F, Groszmann RJ. Pharmacologic treatment of portal hypertension. Gastroenterol Clin North Am. 1992;21:15-40.
63 Aronsen KF, Nylander G. The mechanism of vasopressin hemostasis in bleeding esophageal varices: An angiographic study in the dog. Acta Chir Scand. 1966;131:443-453.
64 Gimson AES, Westaby D, Hegarty J, et al. A randomized trial of vasopressin and vasopressin plus nitroglycerin in the control of acute variceal hemorrhage. Hepatology. 1986;6:410-413.
65 Bosch J, D’Amico G, Luca A, et al. Drug therapy for variceal hemorrhage. In: Bosch J, Groszmann RJ, editors. Portal Hypertension: Pathophysiology and Treatment. Oxford: Blackwell Scientific; 1994:108-123.
66 Wolf DC. The management of variceal bleeding: Past, present and future. Mt Sinai J Med. 1999;66:1-13.
67 Soderlund C, Magnusson I, Torngren S, et al. Terlipressin (triglycyl-lysine vasopressin) controls acute bleeding of esophageal varices: A double blind, randomized placebo-controlled trial. Scand J Gastroenterol. 1990;25:622-630.
68 Planas R, Quer JC, Boix J, et al. A prospective randomized trial comparing somatostatin and sclerotherapy in the treatment of acute variceal bleeding. Hepatology. 1994;20:370-375.
69 Besson I, Ingrand P, Person B, et al. Sclerotherapy with or without octreotide for acute variceal bleeding. N Engl J Med. 1995;333:555-560.
70 Ioannou G, Doust J, Rockey D. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev 2003;CD002147.
71 Imperiale T, Teran J, McCullough A. A meta-analysis of somatostatin versus vasopressin in the management of acute esophageal variceal hemorrhage. Gastroenterology. 1995;109:1289-1294.
72 D’Amico G, Pietrosi G, Tarantino I, Pagliaro L, et al. Emergency sclerotherapy versus vasoactive drugs for variceal bleeding in cirrhosis: a Cochrane meta-analysis. Gastroenterology. 2003;124:1277-1291.
73 Besson I, Ingrand P, Person B, et al. Sclerotherapy with or without octreotide for acute variceal bleeding. N Engl J Med. 1995;333:555-560.
74 Garcia-Tsao G, Bosh J. Management of Varices and Variceal Hemorrhage in Cirrhosis. N Engl J Med. 2010;362:823-832.
75 Chojkier M, Conn HO. Esophageal tamponade in the treatment of bleeding varices: A decadal progress report. Dig Dis Sci. 1980;25:267-272.
76 Haddock G, Garden OJ, McKee RF, et al. Esophageal tamponade in the management of acute variceal hemorrhage. Dig Dis Sci. 1989;34:913-918.
77 Mandelstam P, Zeppa R. Endotracheal intubation should precede esophagogastric balloon tamponade for control of variceal bleeding. J Clin Gastroenterol. 1983;5:493-494.
78 Pasquale MD, Cerra FB. Sengstaken-Blakemore tube placement: Use of balloon tamponade to control bleeding varices. Crit Care Clin. 1992;8:743-753.
79 McCormick PA, Burroughs AK, Mclntyre N. How to insert a Sengstaken-Blakemore tube. Br J Hosp Med. 1990;43:274-277.
80 McGuirk TD, Coyle WJ. Upper gastrointestinal tract bleeding. Emerg Med Clin North Am. 1996;14:523-545.
81 Hunt PS, Korman MG, Hansky T, et al. An 8-year prospective experience with balloon tamponade in emergency control of bleeding esophageal varices. Dig Dis Sci. 1982;27:413-416.
82 Rikkers L, Gongliang J, Burnett DA, et al. Shunt surgery versus endoscopic sclerotherapy for variceal hemorrhage: Late results of a randomized trial. Am J Surg. 1993;165:27-32.
83 Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801.
84 Garcia-Pagán JC, Caca K, Bureau C, et al. An early decision for PTFE-TIPS improves survival in high risk cirrhotic patients admitted with an acute variceal bleeding: a multicenter RCT. Hepatology. 2008;48(Suppl):373A-374A.
85 Rossle M, Haag K, Ochs A, et al. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994 Jan 20;330(3):165-171.
86 Freedman A, Sanyal A, Tisnado J, et al. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13:1185-1210.
87 Shand DG. Propranolol. N Engl J Med. 1975;293:280-285.
88 Bosch J, Masti R, Kravetz D, et al. Effects of propranolol on azygous venous blood flow and hepatic and systemic hemodynamics in cirrhosis. Hepatology. 1984;4:1200-1205.
89 Cales P, Braillon A, Jiron MI, et al. Superior portosystemic collateral circulation estimated by azygos blood flow in patients with cirrhosis: Lack of correlation with oesophageal varices and gastrointestinal bleeding—Effect of propranolol. J Hepatol. 1984;1:37-46.
90 Garcia-Tsao G, Grace ND, Groszmann RJ, et al. Short-term effects of propranolol on portal hypertension. Hepatology. 1986;6:101-106.
91 Kanazawa H, Matsusaka S, Tada N, et al. Measurement of azygos blood flow by a continuous thermodilution method in liver disease. Acta Hepatol Jpn. 1986;27:1132-1136.
92 Lebrec D, Hillon P, Munoz C, et al. The effect of propranolol on portal hypertension in patients with cirrhosis: A hemodynamic study. Hepatology. 1982;2:523-527.
93 Mastai R, Bosch J, Navasa M, et al. Effects of alpha-adrenergic stimulation and beta-adrenergic blockade on azygos blood flow in patients with cirrhosis and portal hypertension. J Hepatol. 1987;4:71-79.
94 Westaby D, Bihari DJ, Gimson AES, et al. Selective and non-selective beta receptor blockade in the reduction of portal pressure in patients with cirrhosis and portal hypertension. Gut. 1984;25:121-124.
95 Bendtsen F, Henriksen JH, Becker U, et al. Long-term effects of oral propanolol on splanchnic and systemic hemodynamics in patients with cirrhosis and oesophageal varices. Scand J Gastroenterol. 1991;26:933-939.
96 Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology. 1990;99:1401-1407.
97 Merkel C, Marin R, Sacerdoti D, et al. Long-term results of a clinical trial of nadolol with or without isosorbide mononitrate for primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;31:324-329.
98 Gatta A, Merkel C, Sacerdoti D, et al. Nadolol for prevention of variceal rebleeding in cirrhosis: A controlled clinical trial. Digestion. 1987;37:22-28.
99 Drayer DE. Lipophilicity, hydrophilicity, and the central nervous system side effects of beta blockers. Pharmacotherapy. 1987;7:87-91.
100 Grace ND. A hepatologist’s view of variceal bleeding. Am J Surg. 1990;160:26-31.
101 Nace GS, Wood AJ. Pharmacokinetics of long-acting propranolol: Implications for therapeutic use. Clin Pharmacokinet. 1987;13:51-64.
102 Colman J, Jones P, Finch C, et al. Propranolol in the prevention of variceal hemorrhage in alcoholic cirrhotic patients. Hepatology. 1990;12:851-856.
103 Conn HO, Grace ND, Bosch J, et al. Propranolol in the prevention of the first hemorrhage from esophagogastric varices: A multicenter, randomized clinical trial. Hepatology. 1991;13:902-912.
104 Pascal JP, Cales P, and the Multicenter Study Group. Propranolol in the prevention of first upper gastrointestinal hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1998;317:856-861.
105 The PROVA Study Group. Prophylaxis of first hemorrhage from esophageal varices by sclerotherapy, propranolol or both in cirrhotic patients: A randomized multicenter trial. Hepatology. 1991;14:1016-1024.
106 Hoffman BB, Lefkowitz RJ. Adrenergic receptor antagonists. In: Goodman-Gilman A, Rall TW, Nies AS, et al, editors. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990:221-243.
107 Frishman WH. Nadolol: A new beta-adrenoceptor antagonist. N Engl J Med. 1982;305:678-682.
108 Epstein M, Oster JR. Beta-blockers and the kidney. Miner Electrolyte Metab. 1982;8:237-254.
109 Hasan F, Levine BA. The role of endoscopic sclerotherapy in the management of esophageal varices. Dig Dis Sci. 1992;10(suppl I):38-45.
110 Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353-358.
111 Bleichner G, Boulanger R, Squara P, et al. Frequency of infections in cirrhotic patients presenting with acute gastrointestinal haemorrhage. Br J Surg. 1986;73:724-726.
112 Bernard B, Cadranel JF, Valla D, et al. Prognostic significance of bacterial infection in bleeding cirrhotic patients: A prospective study. Gastroenterology. 1995;108:1828-1834.
113 Bernard B, Grange JD, Khac EN, et al. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: A meta-analysis. Hepatology. 1999;29:1655-1661.
114 Steer ML, Silen W. Diagnostic procedures in gastrointestinal hemorrhage. N Engl J Med. 1983;309:646-650.
115 Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922.
116 Collachio TA, Forde KA, Patos TJ, et al. Impact of modern diagnostic methods on the management of active rectal bleeding—ten year experience. Am J Surg. 1982;143:607-610.
117 Jensen DM, Machicado GA. Diagnosis and treatment of severe hematochezia: The role of urgent colonoscopy after purge. Gastroenterology. 1988;95:1569-1574.
118 Thorne DA, Datz FL, Remley K, et al. Bleeding rates necessary for detecting acute gastrointestinal bleeding with technetium 99m labeled red cells in an experimental model. J Nucl Med. 1987;28:514-520.
119 Zuckerman GR, Prakash C. Acute lower intestinal bleeding: Part 1. Clinical presentation and diagnosis. Gastrointest Endosc. 1998;48:606-616.
120 Athanasoulis CA, Baum S, Rosch J, et al. Mesenteric arterial infusions of vasopressin for hemorrhage from colonic diverticulosis. Am J Surg. 1975;129:212-216.
121 Sherman LM, Shenoy SS, Cerra FB. Selective intra-arterial vasopressin: Clinical efficacy and complications. Ann Surg. 1979;189:298-302.
122 Eaton AC. Emergency surgery for acute colonic hemorrhage: A retrospective study. Br J Surg. 1981;68:109-112.
123 Drapanas T, Pennington G, Kappelman M, et al. Emergency subtotal colectomy: Preferred approach to management of massively bleeding diverticular disease. Ann Surg. 1973;177:519-526.