CHAPTER 108 Management of Early Glottic Cancer
Background
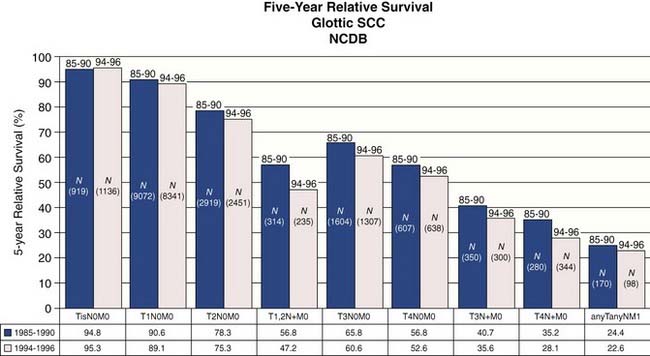
Statistics from the National Cancer Database (NCDB) provide insight into contemporary demographics and management of laryngeal cancer in the United States.2 The most common site for laryngeal squamous cell carcinoma is the glottic larynx (Fig. 108-1). The role for surgery as single-modality therapy for advanced laryngeal cancer is diminishing as combined chemoradiation has become more common in the initial management (Fig. 108-2). Five-year relative survival was best for early-stage (stages 0, I, and II) glottic cancer (Fig. 108-3).

Figure 108-1. Cancers isolated to the glottic larynx account for approximately one half of all laryngeal cancer cases in the United States.
(From Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116[9 Pt 2 Suppl 111]:1-13.)

Figure 108-2. General treatment type by year for laryngeal squamous cell carcinoma.
(From Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116[Suppl 111]:1-13.)
Analysis of this dataset demonstrated that squamous cell carcinoma is the dominant histologic type of laryngeal cancer in the United States, representing approximately 95% of cases (Fig. 108-4). Unless indicated otherwise, the remainder of the discussion in this chapter focuses on squamous cell carcinoma.3,4
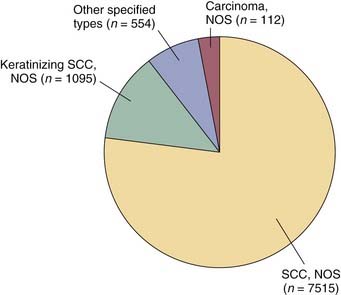
Figure 108-4. Histology of laryngeal cancer cases diagnosed in 2000 national cancer database.
(From National Cancer Database Benchmark Report: Histology of Laryngeal Cancer in the U.S. in 2000. © Commission on Cancer, American College of Surgeons. NCDB Benchmark Reports, v1.1. Chicago, 2002. [The content reproduced from the applications remains the full and exclusive copyrighted property of the American College of Surgeons. The American College of Surgeons is not responsible for any ancillary or derivative works based on the original text, tables, or figures.])
Etiology
Squamous cell carcinoma of the larynx is strongly associated with the use of tobacco and alcohol. An increase in the prevalence of cigarette use among females in the past has been linked to a proportionate increase in the number of female patients with laryngeal cancer. Other factors implicated in the development of laryngeal cancer include passive smoking, chronic laryngeal irritation from gastroesophageal reflux, and viral infection.5–9
Since 1979 gastric acid reflux has been identified as a risk factor for laryngeal and pharyngeal cancer.10,11 Alkaline reflux has more recently been identified as a laryngeal carcinogen by Galli and colleagues.12 These investigators identified a higher than expected number of patients with pharyngolaryngeal cancer associated with achlorhydria and presumed alkaline reflux after gastric resection. They concluded that periodic endoscopy of the upper aerodigestive tract should be performed on gastrectomized patients because of their higher risk for pharyngolaryngeal disorders in general and cancer in particular. These investigators also identified that, among patients with laryngeal squamous cell carcinoma with an intact gastric acid secreting mechanism, 81% showed abnormal acid reflux on 24-hour pH testing.
Human papillomavirus (most notably the oncogenic type 16) has been associated with head and neck cancer.13–15 The role for human papillomavirus (HPV) infection in laryngeal oncogenesis has been supported by previous study but does not have as strong an association as has been identified with cancer of the oropharynx and oral cavity.16,17 Initial study suggests that a difference exists in behavior between HPV and non–HPV-related squamous cell carcinoma of the upper aerodigestive tract, with a better prognosis afforded the HPV-positive cases.18
Differences in exposure to carcinogens or genetic susceptibility may explain the differences in the predominant types of laryngeal cancer throughout the world. Glottic cancers predominate over supraglottic cancers in the United States at a ratio of 2 : 1. In France the opposite occurs, where the number of supraglottic cancers exceeds that of glottic cancers at a ratio of 2 : 1.19 In Finland the incidence of supraglottic cancer decreased and the incidence of glottic cancers increased between the decades 1976 to 1985 and 1986 to 1995.20 Supraglottic cancers were more common than glottic cancers in the earlier decade (ratio of 1.4 : 1). Glottic cancers predominated in the later decade at a ratio of 2 : 1. The reason for this change in distribution of laryngeal cancers in Finland is not clear. A low exposure to alcohol and a high availability of medical services were factors Brenner and colleagues identified as important determinants to the characteristics of laryngeal cancer in Israel. These investigators linked these factors to the high proportion of laryngeal cancers treated at an early stage with an overall better outcome when compared with other countries.21
Staging
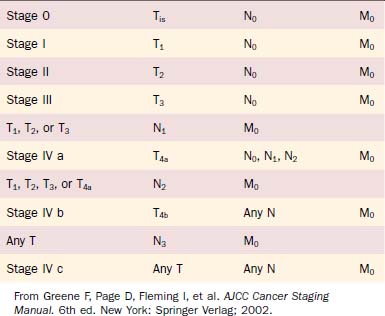
The most widely accepted system for classifying cancer in the United States, as is currently promoted by the American Joint Committee on Cancer (AJCC), had its beginning in 1959 with the organization of the American Joint Committee for Cancer Staging and End-Results Reporting (AJC).22 This staging system was first published in 1977 in the Manual for Staging of Cancer.23 The staging system has evolved to its current form as it is codified in the AJCC Staging Manual, Sixth Edition published in May of 2002 and implemented as of January 1, 2003.24 Major revisions made to the fifth edition in the head and neck section were directed primarily to the advanced-stage cases. Although the definitions for stages 0, I, and II laryngeal cancers remained unchanged, alterations to the T3 definitions in the sixth edition have impacted on the interpretation of T1 and T2 classification.
The anatomic definitions and boundaries between sites, as published in the AJCC Staging Manual, have remained constant across the multiple editions. The glottis has been defined as the true vocal cords including the anterior and posterior commissures. Unfortunately, the definition of the posterior commissure remains vague. It is difficult to discriminate among the glottis, the adjacent supraglottis (arytenoids), and the hypopharynx (postcricoid region) in the region of the posterior commissure.25 The superior border of the glottis is more clearly defined by a horizontal plane passing through the apex of the ventricle. The inferior limit of the glottis is defined by a line parallel to and 1 cm inferior to this plane.
Glottic cancers are staged primarily by clinical assessment of degree of vocal cord mobility (Table 108-1).24 Cancers confined to the glottis with normal mobility are staged T1. Subdivision of T1 vocal cord cancers is based on extension to involve the opposite cord. A tumor limited to one vocal cord is T1a. A horseshoe lesion that extends around the anterior commissure to involve both vocal cords without impairing mobility is T1b. Despite consistency across all previous editions of AJCC staging manuals indicating that T4 status is based on “tumor invasion through the thyroid cartilage …, ” it has been common clinical practice to consider any extent of thyroid cartilage invasion from a glottic cancer to be cause for T4 classification. Clinically apparent stage I glottic cancers (T1N0M0) were reassigned stage IV status (T4N0M0) when “microscopic invasion of adjacent cartilage” was identified.26 Revision to the AJCC Staging Manual, Sixth Edition identifies that minor cartilage erosion (e.g., inner cortex) should be classified as T3 rather than T4.
Table 108-1 Primary Tumor (T) Classification of Glottic Cancer
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor limited to the vocal cord(s) (may involve anterior or posterior commissure) with normal mobility |
| T1a | Tumor limited to one vocal cord |
| T1b | Tumor involves both vocal cords |
| T2 | Tumor extends to supraglottis or subglottis, or with impaired vocal cord mobility |
| T3 | Tumor limited to the larynx with vocal cord fixation or invades paraglottic space, or minor thyroid cartilage erosion (e.g., inner cortex) |
| T4a | Tumor invades through the thyroid cartilage or invades tissues beyond the larynx (e.g., trachea, soft tissues of neck including deep extrinsic muscle of the tongue, strap muscles, thyroid, or esophagus) |
| T4b | Tumor invades prevertebral space, encases carotid artery, or invades mediastinal structures |
From Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer Verlag; 2002.
Still further clarification is necessary to address inconsistencies that persist among publications describing the paraglottic space.12,27–29 Berman defined the borders of the paraglottic space as they have been commonly reported by others:
This definition fails to address the controversy as to whether all, part, or none of the thyroarytenoid muscle is part of the paraglottic space.30 An assessment by Weinstein and colleagues31 defines the medial border of the paraglottic space as the tissue lateral to the conus elasticus at the glottic level and lateral to the quadrangular membrane at the supraglottic level. By this convention, the thyroarytenoid muscle is considered part of the paraglottic space. Others define the glottic portion of the paraglottic space as the fat deep to the intrinsic laryngeal musculature. For the purposes of T classification according to the revised AJCC criteria, it is reasonable to consider that paraglottic space invasion (T3) occurs when the fat compartment deep to the intrinsic laryngeal musculature is involved with tumor. Impaired vocal cord mobility (T2) (without paraglottic space involvement) may be considered when the thyroarytenoid or lateral cricoarytenoid muscles are infiltrated but not fully transgressed.
Carcinoma in situ is classified as Tis. Accuracy in classifying premalignant changes affecting the laryngeal epithelium is compromised by the subjective nature of the assessment and by a lack of uniformity in terminology. Some pathologists discriminate between severe dysplasia and carcinoma in situ, whereas others group them together as type III intraepithelial neoplasia.32–34 The grading of laryngeal dysplasia as mild, moderate, and severe or as carcinoma in situ originated from a similar classification of preneoplastic diseases of the uterine cervix. Keratinizing dysplastic lesions of the upper airway are more common than in the cervix and are also more difficult to categorize. The differences that exist in the behavior of carcinoma in situ originating at these two distinctly different anatomic sites has also undermined the transfer of concepts governing uterine cervical carcinoma in situ to laryngeal carcinoma in situ.35 Despite the clearly stated pathologic principle that high-grade dysplasia and carcinoma in situ are equivalent terms in describing abnormal laryngeal squamous epithelium, clinical management is generally less aggressive for high-grade dysplasia than for carcinoma in situ.
The stage grouping of primary tumor, regional lymph nodes, distant metastatic (TNM) classifications for laryngeal cancer is the same as for other head and neck mucosal sites (Table 108-2). Although the stage groupings have been revised—with the sixth edition introducing the division of stage IV into IV a, IV b, and IV c—the staging of early glottic cancer has remained constant (stages 0, I, and II).
Definitions: Early Glottic Cancer
The appropriate definition of the term early as it applies to laryngeal cancer has been debated. Some investigators have loosely used the term to describe laryngeal cancer in the context of management options. By this convention, a laryngeal cancer is considered early if it can be treated by partial laryngeal (conservative) surgery without a neck dissection, by endoscopic excision, or by radiotherapy alone.36 Others apply the term early to stage 0, I, or II tumors and late to stage III or IV tumors. This definition of early may be inappropriate. Because the extent of disease—not the rate of growth—determines staging, the term localized is more accurate than the term early.37 Similarly, the term advanced may be more appropriate than the term late.38 The most accurate grouping uses the terms localized for any-TN0M0 disease, regionally spread or vicinally spread for any-TN+M0 disease, and widely disseminated for any-T-any-NM1 disease.39 Convention and tradition dictate that this chapter continue to use the terms early to refer to lower stage (0, I, and II) and late to refer to higher stage (III and IV).
Diagnosis
Endoscopic Imaging
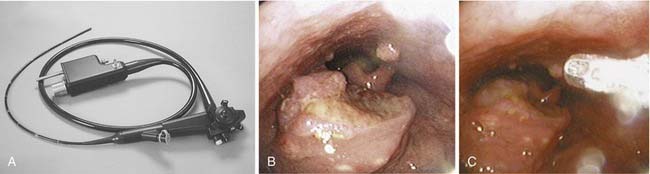
The development of flexible fiberoptic endoscopes designed for transnasal endoscopy has also permitted use of these instruments to evaluate the larynx and trachea.40–43 The improved optics of these instruments—coupled with the presence of a side port that permits the use of suction, placement of biopsy instruments, and instillation of topical anesthetics—has expanded the capacity to perform in-office laryngeal evaluations (Fig. 108-5A). Although direct laryngoscopy remains the most appropriate approach to evaluate and biopsy the majority of lesions suspected of being malignant, in selected cases biopsy can be effected in the clinic (see Fig. 108-5B and C).
When videostroboscopy was introduced as a common clinical tool, enthusiasm was high for its potential to help discriminate invasive cancer from epithelial atypia on the basis of the stroboscopic appearance of the vocal fold epithelial pliability.44,45 Subsequent experience of Colden and colleagues46 has identified that laryngeal stroboscopy is neither reliable in distinguishing vocal fold atypia from invasive cancer nor useful in determining the depth of invasion of vocal fold cancer. These investigators did suggest that the stroboscopic appearance of a relatively normal mucosal wave indicates that a lesion is superficial and does not extensively involve the underlying vocal ligament.
Advances in technology, coupling physical and biologic analysis to endoscopy, have identified several adjuncts to complement standard imaging of the larynx at the time of direct laryngoscopy. Lugol’s solution, as well as toluidine blue, have been used to stain laryngeal tissue in an effort to discriminate normal tissue from tumor.47,48 These approaches have not met with uniform success and therefore have not been widely adopted. Fluorescent tumor markers such as tetracyclines and hematoporphyrin derivatives are preferentially absorbed by tumor cells and can be used to discriminate tumor from adjacent normal tissue by the red fluorescence they emit when exposed to ultraviolet light.49 Additional cost and operating time, as well as problems with skin photosensitivity, have limited the usefulness of this technique. A more promising approach employing autofluorescence laryngoscopy has been reported. Zargi and colleagues50 have employed the lung imaging fluorescence endoscope (LIFE) system to evaluate laryngeal lesions. Their preliminary work identified differences in the intensity of autofluorescence between normal and tumor tissue when exposed to blue or violet light. They also identified shortcomings in the use of the LIFE system for this purpose, noting that further technical advances are necessary to make this endeavor a useful clinical tool.
Stone and colleagues51 reported that the fluorescence of biologic tissue actually may impede the use of Raman spectroscopic analysis to discriminate normal from malignant laryngeal lesions. These investigators have developed strategies that negate the impact of the natural biologic fluorescence to permit evaluation of the distinctive spectral signatures of tissues elicited when exposed to monochromatic laser excitation. This strategy may develop into a noninvasive tool in the early detection of cancerous and precancerous lesions.
Radiology
Chest X-Ray
A chest radiograph is necessary in the evaluation of patients with glottic cancers to assess for concurrent pulmonary disease and to screen for second primary lung cancers. The incidence of distant metastatic spread of an early glottic cancer to the lung is sufficiently rare that a preoperative chest radiograph is not generally intended to survey for that specific purpose. Annual chest x-rays should be obtained in the course of clinic follow-up examinations primarily because of the high incidence of second primary lung cancers in patients managed for laryngeal cancer.52
Magnetic Resonance Imaging and Computed Tomography
In our institution most early laryngeal cancers will be assessed clinically and with a fine cut computed tomography (CT) to determine invasion into or through the thyroid cartilage and to assess for spread into the paraglottic space. The appropriate role for magnetic resonance imaging (MRI) and CT in early glottic cancers is still being defined.53 These advanced imaging studies are of questionable value in the assessment of most superficial T1 lesions of the vocal cords. Selected T1 lesions with anterior commissure involvement may benefit from MRI or CT imaging if extension of the tumor superiorly into the preepiglottic space or anteriorly into the thyroid cartilage is detected. The more routine use of MRI or CT is advocated in the evaluation of T2 lesions not only to help determine tumor extent (and hence T classification) but also tumor volume. Some authors have suggested that CT or MRI of T2 lesions may help determine radiocurability by assessing tumor volume. Other investigators have not found the size of the tumor to be useful for predicting radiocurability.54
Wenig and colleagues55 contend that surgical treatment of laryngeal cancer with less than a total laryngectomy mandates preoperative MRI in almost all cases. These investigators identify that tumor involvement of the entire vocal cord or the anterior commissure, as well as the presence of vocal fold paresis, is considered an indication for the use of MRI. They relate that only the most superficial T1 lesions of the mobile portion of the vocal cord need not be imaged because MRI will not detect the extent of these lesions. This philosophy is distinctly opposite from that of Steiner and Ambrosch,56 who relate that advanced imaging of laryngeal cancer is fraught with artifact. Steiner and Ambrosch find little value in preoperative imaging of laryngeal cancers and relate that the most accurate assessment of tumor extent comes at the time of endoscopic laser microsurgical resection.
Debate continues as to whether CT or MRI offers the most useful information when imaging the larynx.57 Advances in the speed with which CTs can now be done offer an advantage to this technique. In 2002 Stadler and colleagues58 reported that use of rapid imaging spiral CTs of the larynx permits complete evaluation during the course of a single maneuver. This rapid imaging of the larynx may be accomplished as the patient performs either a modified Valsalva maneuver or phonation of the utterance “E.” This functional imaging (done during the maneuver) was compared with nonfunctional imaging (done with quiet breathing) and correlated with postsurgical pathology assessments and findings at microlaryngoscopy. Although the numbers in their study were small, Stadler and colleagues conclude that functional CT imaging appears to be more accurate in determining tumor extent than nonfunctional imaging.
Positron Emission Tomography
The role for positron emission tomography (PET) is continuing to expand. Although distant metastases from early laryngeal cancers are rare, the most common cause of death in patients with early laryngeal cancer is second primary tumor.59,60 The most common second primary tumors are lung followed by other head and neck primaries, which are more easily detected clinically and with endoscopy. It has become standard practice in our institution to order a PET scan in all but the earliest T1 laryngeal cancers.
F-18-fluoro-deoxy-glucose (FDG) PET scanning has been reported as useful in detecting subclinical recurrent or persistent cancer at a stage when it is highly curable.61 Terhaard and colleagues62 propose an algorithm for the follow-up of patients treated for laryngeal or hypopharyngeal cancers that emphasizes the use of PET imaging. Through a prospective study of 109 PET scans done on 75 patients with either laryngeal or hypopharyngeal cancer, these investigators concluded that an FDG PET scan should be the first diagnostic step when a recurrence is suspected after treatment with irradiation. As a result of this study, in which the majority of cases were glottic and classified as either T1 or T2, these investigators suggested that no biopsy is necessary if the scan is negative. In the face of a positive scan and a negative biopsy, a follow-up scan showing decreased FDG uptake would indicate that a recurrence is unlikely. Caution should be exercised in following these guidelines if other clinical indicators support the need for a biopsy. At this point in our experience, endoscopy with a biopsy to sample tissue suspicious in appearance for cancer remains the most critical means by which recurrence is evaluated.
Shortcomings of PET imaging have been noted. Most investigators suggest waiting at least 3 months after treatment to perform PET imaging because false-positive results are common if the imaging is done soon after radiotherapy is completed.63 False positives may also result from infection, radionecrosis, or accumulation of saliva in the vallecula.64,65 Some of these shortcomings of PET imaging are being addressed by use of pharmaceuticals other than FDG. The presence of viable tumor has been identified through PET imaging with 1-[1-11C]-tyrosine (TYR). TYR PET imaging analyzes protein synthesis activity and has been reported to be successful in detecting recurrent squamous cell carcinoma of the larynx.66 Further progress with PET imaging is anticipated with the introduction of other pharmaceuticals.
Treatment
Surgery
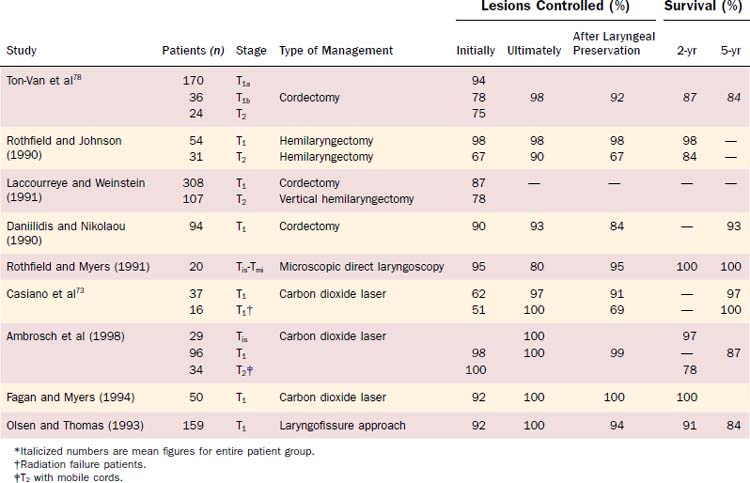
Physicians’ views vary on the indications for use of a specific procedure or radiotherapy technique. General guidelines for selection of an approach based on the extent of a lesion are listed in Table 108-3 and depicted in Fig. 108-6. The choice of therapeutic approach for a patient requires consideration of many other variables in addition to the anatomic extent of the cancer. Overall cure rates and laryngeal preservation figures are high for the surgical management of early glottic squamous cell carcinoma (Table 108-4).
| Procedure | Indications | Study |
|---|---|---|
| Microlaryngoscopy partial cordectomy with or without carbon dioxide laser (excisional biopsy) | T1 midcord leukoplakia, Tis/microinvasion | Strong and Jako (1972) |
| Kleinsasser (1990) | ||
| Shapshay, Hybels, and Bohigian (1990) | ||
| Cordectomy complete with or without carbon dioxide laser (endoscopy) | T1 midcord | Strong (1975) |
| Annyas et al (1990) | ||
| Shapsay, Hybels, and Bohigian (1990) | ||
| Eckel and Thumfart (1992) | ||
| Cordectomy (laryngofissure) | T1 midcord | DeSanto et al (1977) |
| Bailey (1985) | ||
| Frontolateral partial laryngectomy | T1 with extension to anterior commissure | Leroux-Robert (1975) |
| Hemilaryngectomy and extended hemilaryngectomy | T1/T2 with extension to arytenoid, without anterior commissure fixation (minimal) | Norris (1958) |
| Som (1975) | ||
| Mohr et al (1983) | ||
| Anterior partial laryngectomy with epiglottoplasty or with keel | T1/T2 with extension to anterior commissure (without arytenoid) | Tucker et al (1979) |
| Som and Silver (1968) | ||
| Subtotal laryngectomy with cricohyoidoepiglottopexy | T1b/T2 bilateral anterior involvement; may include removal of one arytenoid | Laccourreye et al (1990) |
| Charlin et al (1988) |
Endoscopic Excision
The histopathologic correlate of a superficial T1 primary cancer may reveal a lesion without deep extension beyond the superficial layer of the lamina propria.67 Endoscopic surgical excision of such a lesion may allow for resection with a clear margin without damaging the underlying vocal ligament or vocalis muscle (Fig. 108-7).68 However, not all T1 glottic cancers are amenable to superficial conservative resection. Up to 20% of T1 cancers will show normal mobility despite invasion of the vocal ligament.69 Carcinoma in situ, which by definition does not invade past the basement membrane, is most amenable to complete excision without disruption of the underlying vocal ligament. The importance of achieving clear surgical margins at the first attempt at resection is highlighted by a review of 1467 patients treated with laser microsurgery. Patients with residual tumor at re-resection had significantly worse locoregional control regardless of clear margins at the second surgery.70
Open Partial Laryngectomy
The topic of open partial laryngectomy is covered well in Chapter 110 and is not addressed here.
Voice Considerations
There are two central issues when discussing voice outcomes of various treatment options for early laryngeal cancer: absolute quality of voice, which generally favors radiotherapy; and final laryngeal preservation, which generally favors surgery.71
Cragle and Brandenburg72 reported that surgical treatment of early glottic cancers may produce a vocal result that is equivalent to that following irradiation. These investigators evaluated voice profiles in 11 patients treated with laser cordectomy and 20 patients treated with irradiation. Both groups had similar voice profiles, characterized by decreased maximum phonation times and increased jitter, shimmer, and signal-to-noise ratios.
Most investigators contend that, in cases of early glottic cancers, a better voice more reliably follows treatment with radiotherapy rather than with surgery.73–77 Unlike surgery, radiotherapy does not require removal of adjacent healthy tissue to provide a clear margin around a cancer. However, vocal deterioration may result from radiotherapy and may be significant if loss of vocal cord bulk results from tumor necrosis or if fibrosis develops.
Ton-Van and colleagues78 evaluated 356 patients with early glottic cancer and determined that the quality of voice after treatment with radiotherapy is “indisputably superior to that after conservation surgery.” However, these investigators pointed out that a functional larynx was preserved in 92% of patients treated surgically compared with 81% initially treated with radiotherapy. The investigators acknowledged that these results could have been biased by management, which generally used total laryngectomy for surgical salvage. As a result of these findings, Ton-Van and colleagues78 advocate the use of surgery as a primary mode of therapy in patients capable of safely undergoing an anesthetic. Exceptions include patients who are willing to accept a greater risk of total loss of the larynx in the effort to preserve the highest-quality voice.
For most patients with laryngeal cancer, the absolute quality of their voice may not be as important a consideration in selecting treatment as is their general ability to communicate. For these patients, speech intelligibility may be more meaningful as a criterion to assess results rather than acoustic and aerodynamic measurements. Schuller and colleagues80 used interviews and questionnaires to evaluate 75 patients treated surgically for early laryngeal cancers. They found that 88% of the respondents were content with the postoperative voice.
The vocal behaviors of most patients after partial laryngectomy are similar to those of patients with glottic incompetence caused by vocal cord paralysis. Leeper and colleagues81 evaluated six patients who had undergone vertical hemilaryngectomy operations. They found that, whereas large variability was noted, the general tendencies for patients after hemilaryngectomy were (1) incomplete glottic closure; (2) vibratory action of the supraglottic structures (ventricular folds, arytenoids); and (3) high average transglottal airflow with reduced maximum phonation time.
The ability of the vocal tract to regain normal appearance and function is determined by the extent of tumor damage and by the extent of surgical resection. Benninger and colleagues82 evaluated factors associated with recurrence and voice quality after radiotherapy for early glottic carcinomas. They identified that large excisional biopsies before irradiation increased the risk of poor voice quality.
Radiotherapy
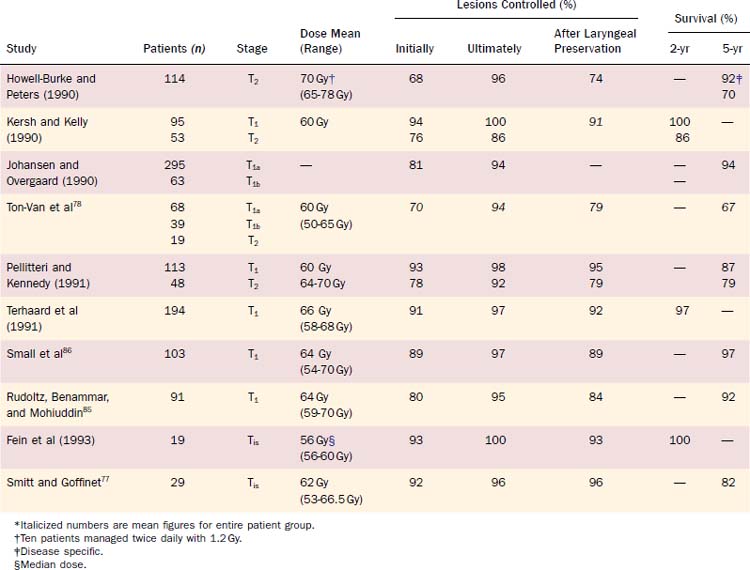
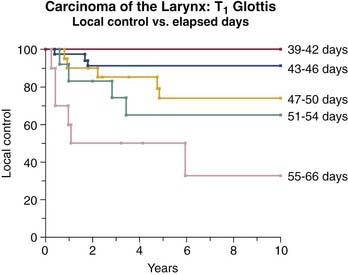
Radiotherapy is considered effective treatment for early glottic cancer (Table 108-5). Broad comparisons between outcomes after initial treatment with either irradiation or surgery are often made without addressing the differences in the various approaches to the administration of radiotherapy or different techniques in performing the surgery.83 Variables in the application of radiotherapy that affect tumor control include fraction size, total dose of radiation delivered, treatment duration, field size, energy of the x-rays, and method of delivery.84–87 Rudoltz and colleagues85 identified duration of treatment as a significant variable in predicting local control and survival in the management of early glottic cancer. Patients completing treatment within 46 days had better local control than those treated over a longer period (Fig. 108-8). Treatment applied through fractions of at least 200 cGy for the standard once-a-day, 5-day-a-week dosing also improved control rates relative to treatment with less than 200 cGy per day.84,88 Ricciardelli and colleagues89 also supported the use of higher per-dose fractions through a clinical study and in an in vivo tumor model. In 1993 Rudoltz and colleagues85 reported that accelerated regimens employing higher-dose fractions were of value primarily because they allowed for shorter treatment duration.
The benefit derived from accelerated fractionation needs to be balanced against the additional morbidity induced. In a phase III study Hliniak and colleagues90 compared an accelerated regimen (AF) of 66 Gy given in 33 fractions during 38 days (2 fractions every Thursday) with conventional regimen (CF) of 66 Gy given in 33 fractions during 45 days. A total of 395 patients with T1-3N0 glottic and supraglottic cancer treated between 1995 and 1998 were evaluated. More acute morbidity (acute mucositis with pain and dysphagia) was seen in the AF arm at 1 and 2 months. At 4 months after treatment all types of toxicity except skin telangiectasia were similar in both arms. Although there was no difference in the disease-free survival between the two arms, these investigators calculated that shortening of the overall treatment time by 1 week resulted in a 3% to 5% therapeutic gain (P = 0.37). They suggest that further shortening of the treatment time could be done within the limits of acceptable morbidity, especially if the volume of the irradiated tissue was small (as for T1 and T2 tumors).
Despite this simple and common sense approach to treatment setup, dosimetric misses occurring because of field misplacement, as well as dose calculation considerations in the small air-filled and irregular cavity of the larynx, were problematic. Reports using higher (6 meV) energy beams (which today are more commonly available than lower energy beams) were initially associated with higher failure rates because of a lack of adequate dosimetric considerations.91,92 With the advent of virtual simulation it has become possible to better account for the 3D anatomic considerations within the glottis. More sophisticated corrections for the air interfaces can also now be made. These advances permit better estimation of the optimal wedge used to shape the beam profile. Multiple field techniques can now be employed to assure homogeneous and accurate doses. These new techniques improve success but still depend on verification for accurate daily reproducibility and dosimetry.93,94
Most radiation failures of early glottic cancer represent local recurrences rather than regional or distant metastasis.95 The ability to salvage radiation failures with partial laryngectomy or endoscopic laser resection contributes to the overall success of radiotherapy for initial treatment modality (Table 108-6).96–101 The extent to which conservation laryngeal surgery (partial laryngectomy) rather than total laryngectomy is used to salvage irradiation failures of T1 and T2 glottic squamous cell carcinoma depends on multiple factors: the intensity of follow-up (designed to identify persistent tumor at an early stage), the desires of the patient, the presence of comorbidity, and the management philosophy of the treating physicians. Differing philosophies likely contribute to the variable success reported with salvage partial laryngectomy.
Table 108-6 Recurrence Rates of Early Stage Laryngeal Cancer Following Initial Treatment with Irradiation*
| Study | T1 Recurrence (%) | T2 Recurrence (%) |
|---|---|---|
| Jose et al, J Surg Oncol. 1984 | T1, 18 | T2, 18 |
| (summarized 8 reports before 1984) | T1, 15 | T2, 31 |
| Fisher et al, Arch Otolaryngol Head Neck Surg. 1986 | T1, 8 | T2, 36 |
| Strauss, Laryngoscope. 1988 | T1, 11 | T2, 23 |
| Maipang et al, J Surg Oncol. 1989 | T1, 20 | T2, 36 |
| Foote et al, Mayo Clin Proc. 1992 | T1, 16 | T2, 16 |
| Suzuki et al, Acta Otolaryngol. 1994 | T1, 7 | T2, 82 |
| Parsons et al, Int J Radiat Oncol Biol Phys. 1995 | T1, 4 | T2, 15 |
| McLaughlin et al, Head Neck. 1996 | T1, 6 | T2, 16 |
| Nibu et al, Head Neck. 1997 | — | T2, 11-73 |
| Mendenhall, J Clin Oncol. 2001 | T1, 7 | T2, 28 |
| Warde et al, Int J Radiat Oncol Biol Phys. 1998 | T1, 9-18 | T2, 31 |
| Wang et al, 1997 | T1, 7 | T2, 29 |
| Garden, Int J Radiat Oncol Biol Phys. 2003 | — | T2, 28 |
* T1 approximately 10%; T2 approximately 30%.
Modified from Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
Toma and colleagues102 expanded on their initial criteria to permit partial laryngectomy salvage of early glottic irradiation failures unless the tumor invaded:
Additionally, Toma and colleagues did not address the role that supracricoid laryngectomy may play in salvaging irradiation failures. Laccourreye and colleagues103 reported successful use of supracricoid laryngectomy as a salvage procedure with a laryngeal preservation rate among 12 patients who had failed radiotherapy. The major contraindications they pointed to in use of supracricoid laryngectomy were fixation of the ipsilateral arytenoid cartilage, cricoid cartilage invasion, extralaryngeal spread of tumor, and infraglottic spread to the cricoid cartilage.
Chemotherapy
Because early glottic cancers are highly curable with conventional surgery or radiotherapy, the addition of chemotherapy to most treatment algorithms has not been strongly supported. In addition, past dogma has dictated that invasive squamous cell carcinoma of the upper aerodigestive tract is not curable with chemotherapy alone.104 Laccourreye and colleagues reported that some of the patients with early glottic squamous carcinoma that they treated with an induction chemotherapy regimen (platinum based) had a complete response clinically and chose to forgo the further treatment that was suggested.105 Although local recurrence was noted in almost one third of these patients, none of the patients who had local recurrence ultimately died from their disease or lost their larynx. This finding has prompted further interest in the use of chemotherapy for early laryngeal cancer to the point that prospective trials evaluating this modality are now under way.
Choosing a Treatment Modality
Carcinoma in Situ
Isenberg and colleagues106 reviewed 2188 biopsies for leukoplakia revealing no dysplasia (53.6%), mild-moderate dysplasia (33.5%), and severe dysplasia or carcinoma in situ (15.2%). Followed for 3 years, 3.7% of patients with no dysplasia, 10.1% with mild/moderate dysplasia, and 18.1% with severe dysplasia or carcinoma in situ developed invasive squamous cell carcinoma.
Close interaction between the clinician and pathologist is necessary to manage optimally carcinoma in situ (TisN0M0). Depending on the bias among several pathologists evaluating a biopsy, the same lesion may be reported as grade III intraepithelial neoplasia, high-grade dysplasia, or TisN0M0.107 Despite the often stated pathologic principle that high-grade dysplasia and TisN0M0 are equivalent terms in describing abnormal squamous epithelium, the clinical management is generally less aggressive for high-grade dysplasia than for TisN0M0. Whereas radiotherapy has commonly been supported as a reasonable treatment alternative for TisN0M0, it is not recommended in the management of high-grade dysplasia.
Difficulty in discriminating between reactive processes and carcinoma in situ is underscored by a study evaluating imprecision in histopathologic diagnoses. Westra and colleagues108 reported three cases of laryngeal TisN0M0 submitted from outside hospitals that were, on review with a second opinion at their hospital, considered to be reactive atypia.
Controversy persists regarding the role for radiotherapy in the management of TisN0M0. A common management approach has been to address laryngeal TisN0M0 surgically when conservative endoscopic removal is possible.109 Persistence after multiple resections or the presence of extensive involvement of multiple sites has been used as an indication for irradiating TisN0M0.110 Factors such as the anticipated need for multiple endoscopic resections, cost of treatment, voice considerations, reliability of the patient for follow-up, patient’s age, and presence of comorbidities are all factors that may influence initial definitive treatment with irradiation.111
Issues regarding the appropriate management of TisN0M0 are unresolved. Local excision and radiotherapy are effective management options.112 Either approach requires close follow-up.
Invasive Squamous Cell Carcinoma
Selection of an appropriate treatment modality for early glottic cancers (T1N0M0, T2N0M0) is made difficult by the many opinions that support conflicting approaches. The simplest conceptual approach is offered by Hinerman and colleagues,113 who identified that, in the past at the University of Florida, “Radiation therapy has been the treatment of choice for all previously untreated T1 and T2 vocal cord cancers.” These investigators later amended this practice to prefer transoral laser excision over radiotherapy for the small subset of patients with limited T1 midthird vocal cord lesions for whom surgical excision is expected to preserve voice quality.
Although the NCDB survey of laryngeal cancer indicates that in the United States radiotherapy is used most commonly in the management of T1 and T2 cancers, there are well-considered arguments for a more liberal use of surgery as the initial management.114 Morris and colleagues,115 through an intensive literature review, identified an overall 8.6% failure rate at the primary site for T1 glottic cancers managed surgically (cordectomy) compared with a 16.7% failure rate among similarly staged cancers managed with radiotherapy.116 Stronger support comes for use of surgery as the primary treatment for T2 glottic cancers. In 1990 Howell-Burke and colleagues reported from MD Anderson that, among patients treated with primary irradiation for T2 glottic cancers, only 74% maintained a functional larynx.117 Some investigators contend that T2 glottic cancers initially treated with irradiation have a higher risk that laryngeal function will be lost than when conservation surgery is the initial treatment.118
Further support for this concept comes from review of published recurrence rates following initial treatment of early-stage laryngeal cancer (see Tables 108-6 and 108-7). A review of these same rates identifies that the great majority of early laryngeal cancers that recur are ultimately treated with total laryngectomy. A problem leading to this need for total laryngectomy may be identified as difficulty in surveillance permitting early detection. Suzuki and colleagues119 reported in 1994 that 60% of patients initially identified with an early laryngeal cancer are identified with an increased stage at the time of recurrence.
Table 108-7 Percentage of T1/T2 Laryngeal Cancers with Recurrence Requiring Total Laryngectomy
| Study | Recurrence (%) |
|---|---|
| Jose et al, J Surg Oncol. 1984 | 95 |
| Fisher et al, Arch Otolaryngol Head Neck Surg. 1986 | 100 |
| Maipang et al, J Surg Oncol. 1989 | 100 |
| Nichols and Mickelson, Ann Otol Rhinol Laryngol. 1991 | >30 |
| Suzuki et al, Acta Otolaryngol. 1994 | 91 |
| Parsons et al, Int J Radiat Oncol Biol Phys. 1995 | >40 |
| McLaughlin et al, Head Neck. 1996 | 83 |
| Stoeckli et al, Arch Otolaryngol Head Neck Surg. 2000 | 92 |
Barthel and Esclamado120 reviewed management of 45 patients with early glottic cancer who were treated with primary irradiation at the Cleveland Clinic between 1986 and 1994. These investigators reported that their local control rates of 87.5% for T1 and 75% for T2 lesions were consistent with prior published series. Among the nine recurrences that developed, six had tumors that were (retrospectively) considered to be amenable to treatment with a partial laryngectomy. Among these six cases (all treated with irradiation), only one was successfully salvaged with conservation laryngeal surgery. These investigators concluded that patients with T2 glottic tumors may be better treated primarily with surgery because T2 glottic cancers have a high recurrence rate after irradiation and surgical salvage with less than a total laryngectomy was unlikely to be successful. Valuable insight is provided by a report by Jorgenson and colleagues that addresses 1005 Danish patients treated at a single referral center between 1965 and 1998.121 The philosophy of treating all but those who had previous treatment with irradiation resulted in remarkably little selection bias regarding treatment choice, with 99% of patients receiving initial management with radiotherapy. Jorgenson and colleagues121 identify that the presence of a stable patient base within a cachement area of 1.33 million people, coupled with excellent follow-up (only three patients were lost to follow-up), lends further credibility to this study. Among 312 T1 glottic cancers treated with irradiation, 5-year locoregional control was 88%, which, coupled with surgical salvage, resulted in a 99% 5-year disease-specific survival. Among 233 T2 glottic cancers treated with irradiation, 5-year locoregional control was 67.4%, which, when coupled with surgical salvage, resulted in an 88.4% 5-year disease-specific survival. These investigators identify that this high recurrence rate (one out of three) for T2 glottic cancers resulted in an overall laryngeal preservation of 80%, which is substantially lower than the 95% organ preservation reported by Chevalier and colleagues when supracricoid laryngectomy was used as the initial treatment.122 Based on these data, Jorgensen and colleagues raised the logical question: Why not introduce supracricoid partial laryngectomy as primary standard treatment of T2 glottic cancer? Jorgenson and colleagues argued that part of the excellent results reported by Chevalier and colleagues reflected a selection bias that favoring surgical results by reserving supracricoid laryngectomy for the more favorable cases. Jorgensen and colleagues additionally pointed out that the voice quality is better after irradiation than after supracricoid laryngectomy. For these reasons, they have not altered their standard approach to managing T2 glottic cancers with irradiation. They also observed that improved radiotherapy techniques, as well as the capacity to salvage irradiation failures with supracricoid laryngectomy, will likely decrease the ultimate need for total laryngectomy further.103
Motamed and colleagues123 reviewed all series of laryngeal preserving surgical salvage following localized radiotherapy failure for early laryngeal cancer. They estimated that one third of recurrences following radiotherapy are amenable to localized treatment. In this highly select group of patients the average laryngeal conservation rate was 77% following open conservation surgery and 65% following the endolaryngeal laser microsurgery. The trend favoring surgery over radiotherapy with regards to final laryngeal preservation may therefore be due to differing salvage philosophies, as well as submucosal or diffuse recurrence patterns and the difficulties in early detection of recurrence due to edema and tissue changes following radiotherapy.
The preceding studies have focused on AJCC (or the International Union Against Cancer) TNM classification as the factor determining treatment. Others have identified specific features of early glottic cancers separate from this classification system that may be helpful in assessing the likelihood of cure with radiotherapy.124 It is useful to individually evaluate these characteristics that are either not included in the TNM staging (anterior commissure extension and large tumor bulk) or are used to discriminate T2 from T1 lesions (impaired vocal cord mobility and subglottic extension). Many investigators support surgery as the preferred initial treatment for patients who have such characteristics.125
In general, the survival outcomes for early laryngeal cancer are very good regardless of treatment modality. In a series of 410 patients treated with primary radiotherapy, Franchin and colleagues59 showed that second primary tumors were the major cause of death. Patients who developed a second primary (most commonly lung followed by head and neck) had a 32% 10 year survival compared with 77% 10-year survival for those without a second primary tumor. In a series of 240 early-stage laryngeal cancers treated with radiotherapy followed for a median 6 years, Holland and colleagues60 showed that 28% developed second primary tumors (6% in the head and neck) with median survival 14 months.
The anterior commissure has been, in a contradictory fashion, either considered as a barrier to tumor spread or as an early pathway for cancer extension into the laryngeal framework.126 Shvero and colleagues127 related that surgical treatment is preferred for cancers arising in this region because of a higher local recurrence rate after radiotherapy with an increased risk for distant metastasis. They contend that the behavior of small cancers in this location is much different from that of other early glottic cancers.79 Other investigators have attributed the higher rates of failure after radiotherapy for anterior commissure lesions to problems with adequate dosing.128,129 The distance from the anterior commissure to the skin varies greatly among patients. This variability and the thick overlying thyroid cartilage have been cited as impediments to consistent dosing of radiation to this region.
Insight into poor outcomes after irradiation of cancer affecting the anterior commissure is offered through a retrospective review by Maheshwar and Gaffney of 53 patients with T1 glottic cancer treated between 1989 and 1996 with a minimum dose of 63 Gy in 30 fractions during 6 weeks with parallel opposed fields.130 These investigators reported an overall 20.8% locoregional recurrence rate. The recurrence rate (57.1%) with anterior commissure involvement was much higher than the recurrence rate (15.8%) when the anterior vocal cord was involved but did not include the anterior commissure. They hypothesized that a lack of pretreatment CT imaging may have contributed to their poor results. Without this advanced imaging, they may have understaged the group of anterior commissure cancers, which have a high chance of cartilage involvement and hidden tumor bulk. Although some investigators relate that modern radiotherapy techniques with improved dosimetry have adequately addressed these concerns, Maheshwar and Gaffney130 report in their study that they had addressed the potential to underdose the anterior commissure by adding wax bolus to the region during treatment.
T2b cancers and those with subglottic extension generally have a larger tumor volume that may contribute to the lower cure rates with radiotherapy.131,132 Many contend that surgery as a single modality is more effective than radiotherapy for managing these larger “early” glottic lesions. Questions persist about the capacity of a management plan that uses definitive radiotherapy coupled with salvage by partial laryngectomy to provide equivalent rates of laryngeal preservation.69
One final factor weighing against radiotherapy is the absence of definitive histologic specimen. From the early days of laryngeal conservation surgery it has been known that up to 20% of biopsy proven cases will have no residual cancer found on definitive resection.133 Such cases improve results in both radiotherapy and surgical outcome studies and would not require radiotherapy to the unaffected residual tissues.
A systematic review of the world’s literature (including the Cochrane Controlled Trials Register, as well as MEDLINE, EMBASE, CINAHL, and Cancer Lit, up to October 2000) identified three randomized clinical trials (RCTs) comparing treatment of patients with stages I and II glottic cancer with either irradiation or surgery.134 One trial was excluded because of insufficient numbers of patients135 and another because of the combination of suboptimal radiotherapy dosimetry, small numbers of patients with glottic cancer, and improper randomization.136
The most relevant RCT was an Eastern European multicenter trial to which 234 patients with early laryngeal cancer were randomly assigned, starting in 1979. They were treated with open surgery, radiotherapy, or a combination of radiotherapy and chemotherapy (prospodine) after stratification by anatomical site (glottis or supraglottis) and by tumor stage (T1 or T2).137 The published results (which did not report the number of events or patients at risk in each treatment arm) identified 5-year survival rates of 100% after surgery and 91.7% after radiotherapy for T1 tumors. For T2 tumors the 5-year survival rates were 97.4% after surgery and 88.8% after radiotherapy. Although these higher survival rates for patients receiving surgery were not statistically significant, the 5-year disease-free survival rates of 78.7% for surgery and 60.1% for radiotherapy were significant (P = 0.036). In subsequent review, Dey and colleagues134 questioned the validity of these results because they believed the patients in the study were inadequately staged before being randomly assigned, that the radiotherapy may have been suboptimal, and that the follow-up was poor. Dey and colleagues concluded from their systematic review that uncertainty remains as to the comparative benefits and societal costs of these different treatment modalities.
A large survey employing Canadian and American data sets to assess outcome as a function of differing management philosophies acknowledged that “current practice is based on reports from individual institutions recommending differing treatment policies.”138 These investigators opine that a clear advantage to one approach will be difficult to identify because randomized trials comparing surgical and nonsurgical treatment of glottic cancer will never be conducted because of entrenched beliefs. Although it is clear from the review by Dey and colleagues that RCTs have been done addressing early laryngeal cancer, none has yet compared the effectiveness of endolaryngeal resection with either radiotherapy or open surgery.
Ambrosch P, Kron M, Steiner W. Carbon dioxide laser microsurgery for early supraglottic carcinoma. Ann Otol Rhinol LARYNGOL. 1998;107(8):680-689.
American Cancer Society. Facts and figures. http:www.cancer.org/docroot/STT/stt_0.asp, 2003.
Commission on Cancer, American College of Surgeons. NCDB benchmark reports. Vol 1. Chicago, 2002.
Franchin G, Minatel E, Gobitti C, et al. Radiotherapy for patients with early stage glottic carcinoma. Cancer. 2003;98:765-772.
Hoffman HT, Buatti J. Update on the Endoscopic Management of Laryngeal Cancer. Curr Opin Otolaryngol Head Neck Surg. 2004;12(Dec (6)):525-531.
Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(Sep (9 Pt 2 Suppl 111)):1-13.
Jackel MC, Ambrosch P, Alexios M, et al. Impact of re-resection for inadequate margins on the prognosis of upper aerodigestive tract cancer treated by laser microsurgery. Laryngoscope. 2007;117(2):350-356.
Kennedy JT, Paddle PM, Cook BJ, et al. Voice outcomes following transoral laser microsurgery for early glottic squamous cell carcinoma. J Laryngol Otol. 2007;20:1-5.
Laccourreye O, Laccourreye H, El-Sawy M, et al. Supracricoid partial laryngectomy with cricohyoidoepiglottopexy. In: Weinstein G, Laccourreye O, Brasnu D, et al, editors. Organ Preservation Surgery for Laryngeal Cancer. San Diego: Singular Thomson Learning, 2000.
Lydiatt WM, Shah JP, Hoffman HT. AJCC stage groupings for head and neck cancer: should we look at alternatives? A report of the Head and Neck Sites Task Force. Head Neck. 2001;23:607-612.
McWorter AJ, Hoffman HT. Transoral laser microsurgery for laryngeal malignancies. Curr Probl Cancer. 2005;29(July-August (4)):180-189.
Mork J, Lie KA, Glattre E, et al. Human papilloma virus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125-1131.
Motamed M, Laccourreye O, Bradley P. Salvage conservation laryngeal surgery after irradiation failure for early laryngeal cancer. Larygoscope. 2006;116(3):451-455.
Remacle M, Eckel HE, Anteonelli A, et al. Endoscopic cordectomy: a proposal for a classification by the working committee: European Laryngological Society. Eur Arch Otohinolaryngol. 2000;257:227-231.
Steiner W, Ambrosch P. The role of the phoniatrician in laser surgery of the larynx. In: Steiner W, Ambrosch P, editors. Endoscopic Laser Surgery of the Upper Aerodigestive Tract. New York: Thieme; 2000:124-129.
von Leden H. The history of phonosurgery. In: Ford CN, Bless DM, editors. Phonosurgery: Assessment and Surgical Management of Voice Disorders. New York: Raven Press, Ltd.; 1991:3-24.
Ward PH, Hanson DG. Reflux as an etiological factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98:1195.
Weinstein GS, Laccourreye O, Brasnu D, et al. Laryngeal anatomy: surgical and clinical implications. In: Weinstein GS, Brasnu D, Laccourreye H, editors. Organ Preservation Surgery for Laryngeal Cancer. San Diego: Singular; 2000:18-21.
1. Ferlito A, Silver C, Howard D, et al. The role of partial laryngeal resection in current management of laryngeal cancer: a collective review. Acta Otolaryngol. 2000;120:456-465.
2. Commission on Cancer, American College of Surgeons: NCDB benchmark reports. Vol 1. Chicago, 2002.
3. Ferlito A. Histological classification of larynx and hypopharynx cancers and their clinical implications: pathologic aspects of 2052 malignant neoplasms diagnosed at the ORL department of Padua University from 1966 to 1976. Acta Otolaryngol (Stockh). 342(1 Suppl), 1976.
4. Shah JP, Loree TR, Kowalski L. Conservation surgery for radiation-failure carcinoma of the glottic larynx. Head Neck. 1990;12:326-331.
5. Bradford CR, Hoffman HT, Wolf GT, et al. Squamous carcinoma of the head and neck in organ transplant recipients: possible role of oncogenic viruses. Laryngoscope. 1990;100:190.
6. Harrison DFN. Laryngeal cancer: a preventable disease. In: Ferlito A, editor. Laryngeal Cancer: A Preventable Disease, Neoplasms of the Larynx. Edinburgh: Churchill Livingstone, 1993.
7. Hoshikawa T, Nakajima T, Uhara H, et al. Detection of human papillomavirus DNA in laryngeal squamous cell carcinomas by polymerase chain reaction. Laryngoscope. 1990;100:647-650.
8. Ritchie JM, Smith EM, Summergill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336-344.
9. Ward PH, Hanson DG. Reflux as an etiological factor of carcinoma of the laryngopharynx. Laryngoscope. 1988;98:1195.
10. El-Serag HB, Hepworth EJ, Lee P, et al. Gastroesophageal reflux disease is a risk factor for laryngeal and pharyngeal cancer. Am J Gastroenterology. 2001;96:2013-2018.
11. Olson N. Effects of stomach acid on the larynx. Proc Am Laryngol Assoc. 1983;104:108-112.
12. Galli J, Cammarota G, Calo L, et al. The role of acid and alkaline reflux in laryngeal squamous cell carcinoma. Laryngoscope. 2002;112:1861-1865.
13. Gillison ML, Koch WM, Capone RB, et al. Evidence for casual association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709-720.
14. Mork J, Lie KA, Glattre E, et al. Human papilloma virus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125-1131.
15. Smith EM, Hoffman HT, Summersgill KS, et al. Human papillomavirus and risk of oral cancer. Laryngoscope. 1998;108:1098-1103.
16. Almadoria G, Galli J, Cadoni G, et al. Human papilloma virus infection and cyclin D1 gene amplification in laryngeal squamous cell carcinoma: biologic function and clinical significance. Head Neck. 2002;24:597-604.
17. Smith EM, Summersgill KF, Allen J, et al. Human papillomavirus and risk of laryngeal cancer. Ann Otol Rhinol Laryngol. 2000;109:1069-1076.
18. Bailey BJ, Biller JF. Surgical anatomy of the larynx. In: Surgery of the Larynx. Philadelphia: WB Saunders; 1985:23.
19. Waterhouse JAH. Epidemiology. In: Ferlito A, editor. Neoplasms of the larynx. Edinburgh: Churchill Livingstone, 1993.
20. Teppo H, Koivunen P, Sipila S, et al. Decreasing incidence and improved survival of laryngeal cancer in Finland. Acta Oncological. 2001;40:791-795.
21. Brenner B, Marshak G, Rakowsky E, et al. Laryngeal carcinoma—Epidemiological and clinical features: experience of the Rabin Medical Center in Israel. Oncol Rep. 2001;8:141-144.
22. Lydiatt WM, Shah JP, Hoffman HT. AJCC stage groupings for head and neck cancer: should we look at alternatives? A report of the Head and Neck Sites Task Force. Head Neck. 2001;23:607-612.
23. American Joint Committee on Cancer Staging. Manual for Staging of Cancer. Philadelphia: J.B. Lippincott; 1977.
24. Greene F, Page D, Fleming I, et al. AJCC Cancer Staging Manual: Part II Head and Neck Sites, 6th ed. New York: Springer Verlag; 2002.
25. Hoffman HT, Overholt EM, Karnell MP, et al. The larynx. In: Steele GD, Jessup JM, Winchester DP, et al, editors. Granuloma Contact Ulcers and Other Posterior Lesions. Philadelphia: Lippincott Williams & Wilkins; 2003:203-224.
26. Ferlito A, Carbone A, DeSanto L, et al. Early cancer of the larynx: the concept as defined by clinicians, pathologists, and biologists. Ann Otol Rhinol Laryngol. 1996;105:245-250.
27. Kim M, Sun D, Park K, et al. Paraglottic space in supracricoid laryngectomy. Arch Otolaryngol Head Neck Surg. 2002;128:304-307.
28. Maguire A, Dayal VS. Supraglottic anatomy: the pre- or the periepiglottic space? Can J Otolaryngol. 1974;3:432-445.
29. Tucker GF, Smith HR. A histological demonstration of the development of laryngeal connective tissue compartments. Trans Am Acad Ophthalmol Otolaryngol. 1962;66:308-318.
30. Silver C, Silver CE, Ferlito A, editors. Chapter 2 Surgical Anatomy in Surgery for Cancer of the Larynx and Related Structures, 2nd ed. Philadelphia: WB Saunders Co. 25. 1996.
31. Weinstein GS, Laccourreye O, Brasnu D, et al. Laryngeal anatomy: surgical and clinical implications. In: Weinstein GS, Brasnu D, Laccourreye H, editors. Organ Preservation Surgery for Laryngeal Cancer. San Diego, CA: Singular; 2000:18-21.
32. Crissman JD, Zarbo RJ, Drozdowicz S, et al. Carcinoma in situ and microinvasive squamous cell carcinoma of the laryngeal glottis. Arch Otolaryngol Head Neck Surg. 1988;114:299.
33. Flint PW. Minimally invasive techniques for management of early glottic cancer. Otolaryngol Clin N Am. 2002;35:1055-1066.
34. Miller AH. Premalignant laryngeal lesions, carcinoma in situ, superficial carcinoma: Definition and management. Can J Otolaryngol. 1974;3:573.
35. Bouquot JE, Gnepp DR. Pathology of the head and neck. New York. Epidemiology. 1988:263-314.
36. Ferlito A, Rinaldo A. A comment on misuse of the term “early” laryngeal cancer. Eur Arch Otorhinolaryngol. 2002;257:347-348.
37. Feinstein AR. A new staging system for cancer and reappraisal of “early” treatment and “cure” by radical surgery. N Engl J Med. 1968;279:747.
38. Diaz E, Laccourreye L, Veivers D, et al. Laryngeal stenosis after supracricoid partial laryngectomy. Ann Otol Rhinol Laryngol. 2000;109:1077-1081.
39. Feinstein AR, Wells CK. A clinical-severity staging system for patients with lung cancer. Medicine (Baltimore). 1990;69:1.
40. Aviv JE, Takoudes TG, Ma G, et al. Office-based esophagoscopy: a preliminary report. Otolaryngol Head Neck Surg. 2001;125:170-175.
41. Belafsky PC, Postma GN, Daniel E, et al. Transnasal esophagoscopy. Otolaryngol Head Neck Surg. 2001;125:588-589.
42. Belafsky PC, Postma GN, Koufman JA. Normal transnasal esophagoscopy (comment). Ear Nose Throat J. 2001;80:438.
43. Postma GN, Bch KK, Belafsky PC, et al. The role of transnasal esophagoscopy in head and neck oncology. Laryngoscope. 2002;112:2242-2243.
44. Sessions RB, Miller SD, Martin GF, et al. Videolaryngostroboscopic analysis of minimal glottic cancer. Trans Am Laryngol Assoc. 1989;110:56-59.
45. Zhao R, Hirano M, Tanaka S, et al. Vocal fold epithelial hyperplasia: vibratory behavior versus extent of lesion. Arch Otolaryngol Head Neck Surg. 1991;117:1015-1018.
46. Colden D, Zeitels SM, Hillman RE, et al. Stroboscopic assessment of vocal fold keratosis and glottic cancer. Ann Otol Rhinol Laryngol. 2001;110:293-298.
47. Kambic V, Gale N. Epithelial Hyperplastic Lesions of the Larynx. Amsterdam: Elsevier; 1995.
48. Kleinsasser O. Tumors of the Larynx and Hypopharynx. Stuttgart: Thieme International; 1988.
49. Leornard JR, Beck WL. Hematoporphyrin fluorescence: an aid in diagnosis of malignant neoplasm. Laryngoscope. 1971;81:365-372.
50. Zargi M, Smid L, Fajdiga I, et al. Detection and localization of early laryngeal cancer with laser-induced fluorescence: preliminary report. Eur Arch Otorhinolarynngol. 1997;254:S113-S116.
51. Stone N, Stavroulaki P, Kendall C, et al. Raman: spectroscopy for early detection of laryngeal malignancy: preliminary results. Laryngoscope. 2000;110(10 Pt 1):1756-1763.
52. Rachmat L, Vreeburg GC, de Vries N, et al. The value of twice yearly bronchoscopy in the workup and follow-up of patients with laryngeal cancer. Eur J Cancer. 1993;29A:1096-1099.
53. Weber RS, et al. CT vs MRI for staging carcinoma of the larynx (L) and hypopharynx (HP), American Laryngological Association, Orlando, Florida, May 4, 1996 (abstract).
54. Mukherji SK, Mancuso AA, Mendenhall W, et al. Can pretreatment CT predict local control of T2 glottic carcinomas treated with radiation therapy alone? Am J Neuroradiol. 1995;16:655-662.
55. Wenig BL, Ziffra KL, Mafee MF, et al. MR imaging of squamous cell carcinoma of the larynx and hypopharynx. Otolaryngol Clin North Am. 1995;28:609.
56. Steiner W, Ambrosch P. The role of the phoniatrician in laser surgery of the larynx. In: Steiner W, Ambrosch P, editors. Endoscopic Laser Surgery of the Upper Aerodigestive Tract. New York: Thieme; 2000:124-129.
57. Weinstein GS, El-Sawy MM, Ruiz C, et al. Laryngeal preservation with supracricoid partial laryngectomy results in improved quality of life when compared with total laryngectomy. Laryngoscope. 2001;111:191-199.
58. Stadler A, Dontrus M, Kornfehl J, et al. Tumor staging of laryngeal and hypopharyngeal carcinomas with functional spiral CT: comparison with nonfunctional CT, histopathology, and microlaryngoscopy. J Comput Assist Tomogr. 2002;26:279-284.
59. Franchin G, Minatel E, Gobitti C, et al. Radiotherapy for patients with early stage glottic carcinoma. Cancer. 2003;98:765-772.
60. Holland JM, Arsanjani A, Liem BJ, et al. Second malignancies in early stage laryngeal carcinoma patients treated with radiotherapy. J Laryngol Otol. 2002;116:190-193.
61. Austin JR, Wong FC, Kim EE. Positron emission tomograph in the detection of residual laryngeal carcinoma. Otolaryngol Head Neck Surg. 1995;113:404.
62. Terhaard C, Bongers V, Van Rijk P, et al. F-18-fluoro-deozy-glucose positron-emission tomography scanning in detection of local recurrence after radiotherapy for laryngeal/pharyngeal cancer. Head Neck. 2001;23(11):933-941.
63. McGuirt WF, Keyes JW, Geising KR, et al. Positron emission tomography in the evaluation of laryngeal carcinoma. Ann Otol Rhinol Laryngol. 1995;104:274-278.
64. Jabour BA, Choi Y, Hoh CK, et al. Extracranial head and neck: PET imaging with 2-[f-18]-fluoro-2-deoxy-D-glucose and MR imaging correlation. Radiology. 1993;186:27-35.
65. Uematsu H, Sadato N, Yonekura Y, et al. Coregistration of FDB, PET and MRI of the head and neck using normal distribution of FDG. J Nucl Med. 1998;39:2121-2127.
66. de Boer JR, Pruim J, Burlage F, et al. Therapy evaluation of laryngeal carcinomas by tyrosine-pet. Head Neck. 2003;25:634-644.
67. Kirchner JA. One hundred laryngeal cancers studied by serial section. Ann Otol Rhinol Laryngol. 1969;78:689.
68. Vaughon CW, Gates GA, editors. Glottic Cancer: Current Therapy in Otolaryngology Head and Neck Surgery, 5th ed, St Louis: Mosby, 1993.
69. DeSanto LW, Olsen KD. Early glottic cancer. Am J Otolaryngol. 1994;15:242.
70. Jackel MC, Ambrosch P, Alexios M, et al. Impact of re-resection for inadequate margins on the prognosis of upper aerodigestive tract cancer treated by laser microsurgery. Laryngoscope. 2007;117(2):350-356.
71. Kennedy JT, Paddle PM, Cook BJ, et al. Voice outcomes following transoral laser microsurgery for early glottic squamous cell carcinoma. J Laryngol Otol. 2007;20:1-5.
72. Cragle S, Brandenburg J. Laser cordectomy or radiotherapy: cure rates, communication and cost. Otolaryngol Head Neck Surg. 1993;108:648.
73. Casiano RR, Cooper JD, Lundy DS, et al. Laser cordectomy for T1 glottic cancer: a 10-year experience and videostroboscopic findings. Otolaryngol Head Neck Surg. 1991;104:831.
74. Epstein BE, Lee DJ, Kashima H, et al. Stage T1 glottic carcinoma: results of radiation therapy or laser excision. Radiology. 1992;101:49.
75. Hoyt DJ, Lettinga JW, Leopold KA, et al. The effect of head and neck radiation therapy on voice quality. Laryngoscope. 1992;102:477-480.
76. Rydell R, Schalén L, Fex S, et al. Voice evaluation before and after laser excision vs. radiotherapy of T1A glottic carcinoma. Acta Otolaryngol (Stockh). 1995;115:560-565.
77. Smitt MC, Goffinet DR. Radiotherapy for carcinoma-in situ of the glottic larynx. Int J Radiat Oncol Biol Phys. 1994;28:251.
78. Ton-Van J, LaFebvre JL, Stern JC, et al. Comparison of surgery and radiotherapy in T1 and T2 glottic carcinomas. Am J Surg. 1991;162:337-340.
79. Shvero J, Hadar R, Segal K, et al. T1 Glottic carcinoma involving the anterior commissure. Eur J Surg Oncol. 1994;20:557-560.
80. Schuller DE, Trudeau M, Bistline J, et al. Evaluation of voice by patients and close relatives following different laryngeal cancer treatments. J Surg Oncol. 1990;44:10-14.
81. Leeper HA, Heeneman H, Reynolds C. Vocal function following vertical hemilaryngectomy: a preliminary investigation. J Otolaryngol. 1990;19:62.
82. Benninger MS, Bellomo A, Ferrero FE, et al. Factors associated with recurrence and voice quality following radiation therapy for T1 and T2 glottic carcinomas. Laryngoscope. 1994;104:294.
83. Biacabe B, Crevier-Buchman L, Laccourreye O, et al. Phonatory mechanisms after vertical partial laryngectomy with glottic reconstruction by false vocal fold flap. Ann Otol Rhinol Laryngol. 2001;110:935-940.
84. Kim RY, Marks ME, Salter MM. Early-stage glottic cancer: importance of dose fractionation in radiation therapy. Radiology. 1992;182:273.
85. Rudoltz MS, Benammar A, Mohiuddin M. Prognostic factors for local control and survival in T1 squamous cell carcinoma of the glottis. Int J Radiat Oncol Biol Phys. 1993;26:767.
86. Small WJr, Mittal BB, Brand WN, et al. Results of radiation therapy in early glottic carcinoma: multivariate analysis of prognostic and radiation therapy variables. Radiology. 1992;183:789-794.
87. Stevenson JM, Juillard GJ, Selch MT. Stages 1 and 2 epidermoid carcinoma of the glottic larynx: involvement of the anterior commissure. Radiology. 1992;182:797.
88. Mendenhall WM, Parsons JT, Million RR, et al. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiation therapy: relationship of dose-fractionation factors to local control and complications. Int J Radiat Oncol Biol Phys. 1988;15:1267-1273.
89. Ricciardelli EJ, Weymuller EAJr, Koh WJ, et al. Effect of radiation fraction size on local control rates for early glottic carcinoma. Arch Otolaryngol Head Neck Surg. 1994;120:737-742.
90. Hliniak A, Gwiazdowska B, Szutkowski Z, et al. A multicenter randomized/controlled trial of conventional versus modestly accelerated radiotherapy in the laryngeal cancer: influence of a 1 week shortening overall time. Radiother Oncol. 2002;62:1-10.
91. Devineni VR, King K, Perez C, et al. Early glottic carcinoma treated with radiotherapy: impact of treatment energy on success rate. Int J Radiat Oncol Biol Phys. 1992;24:106.
92. Warde PR, O’Sullivan B, Panzarella T, et al. T1/T2 glottic cancer managed by external beam radiotherapy-the influence of beam energy on local control. Int J Radiat Oncol Biol Phys. 1998;42:2202.
93. Foote RL, Grado GL, Buskirk SJ, et al. Radiation therapy for glottic cancer using 6 MV photons. Cancer. 1996;77:381-386.
94. Parson JT, Greene BD, Speer TW, et al. Treatment of early and moderately advanced vocal cord carcinoma with 6 MV x-rays. J Radiat Oncol Biol Phys. 2001;50:953-959.
95. Cellai E, Chiavacci A, Olmi P. Causes of failure of curative radiation therapy in 205 early glottic cancers. Int J Radiat Oncol Biol Phys. 1990;19:1139.
96. Kerrebijn JD, deBoer MF, Knegt PP. CO2-laser treatment of recurrent glottic carcinoma. Clin Otolaryngol. 1992;17:430.
97. Lavey RS, Calcaterra TC. Partial laryngectomy for glottic cancer after high-dose radiotherapy. Am J Surg. 1991;162:341.
98. Rothfield RE, Johnson JT, Myers EN, et al. Hemilaryngectomy for salvage of radiation therapy failures. Otolaryngol Head Neck Surg. 1990;103:792-794.
99. Shah JP, Loree TR, Kowalski L, et al. Conservation surgery for radiation-failure carcinoma of the glottic larynx. Head Neck. 1990;12:326.
100. Shapshay SM. Laser technology in the diagnosis and treatment of head and neck carcinoma of the glottic larynx. Head Neck. 1990;12:326.
101. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
102. Toma M, Kenichi N, Nakao K, et al. Partial laryngectomy to treat early glottic cancer after failure of radiation therapy. Arch Otolaryngol Head Neck Surg. 2002;128:909-912.
103. Laccourreye O, Weinstein G, Nauda P, et al. Supracricoid partial laryngectomy after failed laryngeal radiation therapy. Laryngoscope. 1996;106:495-498.
104. Laccourreye O, Veivers D, Hans S, et al. Chemotherapy alone with curative intent in patients with invasive squamous cell carcinoma of the pharyngolarynx classified as T1-T4N0M0 complete clinical responders. Cancer. 2001;92:1504-1511.
105. Laccourreye O, Veivers D, Bassot V, et al. Analysis of local recurrence in patients with selected T1-3N0M0 squamous cell carcinoma of the true vocal cord managed with a platinum-based chemotherapy-alone regimen for cure (comment). Ann Otol Rhinol Laryngol. 2002;111:315-322.
106. Isenberg JS, Crozier DL, Dailey SH. Institutional and comprehensive review of laryngeal leukoplakia. Ann Otol Rhinol Laryngol. 2008;117:74-79.
107. Gallo A, de Vincentiis M, Della Rocca C, et al. Evolution of precancerous laryngeal lesions: a clinicopathologic study with long-term follow-up on 259 patients. Head Neck. 2001;23:42-47.
108. Westra WH, Kronz JD, Eisele DW. The impact of second opinion surgical pathology on the practice of head and neck surgery: a decade experience at a large referral hospital. Head Neck. 2002;24:684-693.
109. Schweinfurth JM, Powitzky E, Ossoff RH. Regression of laryngeal dysplasia after serial microflap excision. Ann Otol Rhinol Laryngol. 2001;110:811-814.
110. Spayne JA, Warde P, O’Sullivan, et al. Carcinoma-in situ of the glottic larynx: results of treatment with radiation therapy. Int J Radiation Oncology Biol Phys. 2001;49:1235-1238.
111. Garcia-Serra A, Hinerman RW, Amdur RJ, et al. Radiotherapy for carcinoma in situ of the true vocal cords. Head Neck. 2002;24:390-394.
112. Nguyen C, Naghibzadeh B, Black MJ, et al. Carcinoma in situ of the glottic larynx: excision or irradiation. Head Neck. 1996;8(3):225-228.
113. Hinerman R, Mendenhall W, Amdur R, et al. Early laryngeal cancer. Curr Treat Options Oncol. 2002;3:3-9.
114. Hoffman HT, Karnell LK. Laryngeal cancer. In: Steele GD, et al, editors. National Cancer Data Base Annual Review of Patient Care 1995. Atlanta: American Cancer Society; 1995:84-99.
115. Morris MR, Canonico D, Blank C. A critical review of radiotherapy in the management of T1 glottic carcinoma. Am J Otolaryngol. 1994;15:276.
116. DeSanto LW. Early supraglottic cancer. Ann Otol Rhinol Laryngol. 1990;99:593.
117. Howell-Burke D, Peters LJ, Goepfert H, et al. T2 glottic cancer: recurrence, salvage, and survival after definitive radiotherapy. Arch Otolaryngol Head Neck Surg. 1990;116:830-835.
118. Weinstein GS. Surgical approach to organ preservation in the treatment of cancer of the larynx. Oncology, Huntington.
119. Yamada K, Kaga K, Suzuki J. Temporal bone pathology in patients without caloric response. Acta Otolaryngol. 1994;114:586-594.
120. Barthel S, Esclamado R. Primary radiation therapy for early glottic cancer. Otolaryngol Head Neck Surg. 2001;124:35-39.
121. Jorgensen K, Godballe C, Hansen O, et al. Cancer of the larynx treatment results after primary radiotherapy with salvage surgery in a series of 1005 patients. Acta Oncologica. 2002;41:69-76.
122. Chevalier D, Laccourreye O, Brasnu D, et al. Cricohyoidoepiglottopexy for glottic carcinoma with fixation or impaired motion of the true vocal cord: 5-year oncology results with 112 patients. Ann Otol Rhinol Laryngol. 1997;106:364-369.
123. Motamed M, Laccourreye O, Bradley P. Salvage conservation laryngeal surgery after irradiation failure for early laryngeal cancer. Larygoscope. 2006;116(3):451-455.
124. Franchin S, Boiocchi M. Brief report: prognostic importance of cellular DNA content in T1-2N0 laryngeal squamous cell carcinomas treated with radiotherapy. Laryngoscope. 1995;105:649.
125. Coman W, Grigg R, Tomkinson A, et al. Supracricoid laryngectomy: a significant advance in the management of laryngeal cancer. Aust N Z Surg. 1998;68:630-634.
126. Kirchner JA. What have whole organ sections contributed to the treatment of laryngeal cancer? Ann Otol Rhinol Laryngol. 1989;98:661.
127. Shvero J, Hadar T, Segal K, et al. Early glottic carcinoma involving the anterior commissure. Clin Otolaryngol. 1994;19:105-108.
128. Krespi YP, Meltzer CJ. Laser surgery for vocal cord carcinoma involving the anterior commissure. Ann Otol Rhinol Laryngol. 1989;98:105.
129. Zohar Y, Rahima M, Shvili Y, et al. The controversial treatment of anterior commissure carcinoma of the larynx. Laryngoscope. 1992;102:69-72.
130. Maheshwar A, Gaffney C. Radiotherapy for T1 glottic carcinoma: impact of anterior commissure involvement. J Laryngol Otol. 2001;115:298-301.
131. van den Bogaert W. What is the treatment of choice in early glottic cancer. In: Johnson JT, Didolkar MS, editors. Head and Neck Cancer: Proceedings of the Third International Conference on Head and Neck Cancer, San Francisco. July 26-30, 1992, Vol 3. Amsterdam: Elsevier Science Publishing; 1993.
132. Yamamoto M, Hada Y, Shirane M, et al. The results of radiation therapy for glottic carcinoma: prognostic significance of tumor size in laryngoscopic findings. Oncol Rep. 2000;7:1275-1277.
133. Strong MS, Jako GJ. Laser surgery in the larynx: early clinical experience with continuous CO2 laser. Ann Otol Rhinol Laryngol. 1972;1:791-793.
134. Dey P, Arnold D, Wight R, et al. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev. 2, 2003.
135. Li GZ. A comparison of the therapeutic effect between preoperative radiotherapy plus operation and operation alone for laryngocarcinoma: a prospective study of 260 cases. Chung Hua Erh Pi Yen Hou Ko Tsa Chih-Chinese J Otorhinol. 1993;28:170-173.
136. Hintz B, Charyula K, Chandler JR, et al. Randomized study of local control and survival following radical surgery or radiation therapy in oral and laryngeal carcinoma. J Surg Oncology. 1979;12:61-74.
137. Ogoltsova ES, Paches AI, Matyakin EG, et al. Comparative evaluation of the efficacy of radiotherapy, surgery and combine treatment of stage I-II laryngeal cancer (T1-2N0M0) on the basis of co-operative studies. J Otorhinolaryngol (Moscow). 1990;3:3-7.
138. Groome P, O’Sullivan G, Irish J, et al. Glottic cancer in Ontario, Canada and the SEER areas of the United States: do different management philosophies produce different outcome profiles? A detailed analysis of glottic cancer management. J Clin Epidemiology. 2001;54:301-315.