Chapter 28 Management of Bladder Dysfunction
Neuroanatomy and Physiology
Receptors and Neurotransmitters
The receptors active during bladder contraction are cholinergic muscarinic (M2 and M3) receptors widely distributed in the body of the bladder, trigone, bladder neck, and urethra. The M2 receptors predominate structurally in normal bladders, but the M3 receptors might be more important functionally. The cholinergic nicotinic receptors are primarily located in the striated sphincter. Adrenergic receptors are concentrated in the trigone, bladder neck, and urethra and are predominantly α1. These have recently been subdivided into α1a, α1b, and α1d. Identification of these α1 subgroups should allow increased specificity with regard to future therapeutic agents. Norepinephrine-containing nerve cells are also found in the paravesical and intramural ganglia. Some authors describe norepinephrine terminals in the striated muscle of the distal sphincter, although most would dispute this. When these cells are active, they have excitatory effects and maintain continence by contraction of the bladder neck and urethral smooth muscle. β2– and β3-adrenergic receptors are found in the bladder neck and also in the body of the bladder. These receptors are inhibitory when activated and can produce relaxation at the bladder neck on initiation of voiding and relax the bladder body to enhance storage (Figure 28-1). In humans, however, the storage role seems to be a minor one.
The main effector transmitter for contraction of the urethra is norepinephrine, via the α1 receptors. Smooth muscle relaxation is mediated by the effects of acetylcholine in the pelvic ganglia. This releases nitric oxide in the urethral wall, resulting in relaxation of urethral smooth muscle. Prostaglandins, in contrast to their effects on the detrusor, cause a relaxation of the urethral muscle. Prostaglandins have been tried in various clinical states of urinary retention but without consistent results. Serotonin appears to be an antagonist that causes urethral muscle contraction. It might be important in the production of irritable urethral symptoms. The role of estrogens on the lower urinary tract in women is confined to the modification of tissues and receptors. Apparently estrogens have no direct transmitter effects. In the brainstem and spinal cord the various transmitters described above can have a variety of inhibitory and facilitative actions, depending on their site of action. Serotonin might have inhibitory detrusor effects at the midbrain level, and uptake of serotonin might be blocked by tricyclics (used in treating nocturnal enuresis). Activation of opiate receptors in the brainstem and sacral spinal cord inhibits voiding. This might partly explain the retention of urine seen with the use of these agents.35 The pudendal motor neuron bodies are situated in the lateral border of the ventral horn of the sacral cord (Onuf’s nucleus). Serotonin and norepinephrine reuptake inhibitors prolong the effect of these agents in the synaptic cleft of Onuf’s nucleus and increase the activity of the external sphincter. (Such a dual agent [duloxetine] is currently under trial for stress incontinence in women and might be useful for some neurologic bladder conditions.15)
A complete discussion of the pharmacology of the lower urinary tract can be found in Steers.35
Peripheral Innervation
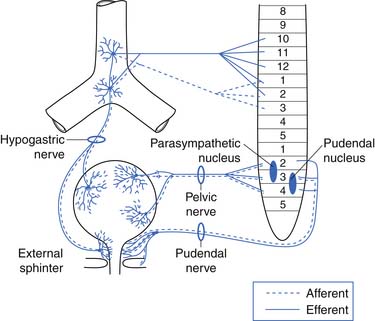
The afferent and efferent peripheral pathways include the autonomic fibers traversing the pelvic (parasympathetic) and hypogastric (sympathetic) nerves, and the somatic fibers traversing the pudendal nerves (Figure 28-2 and Table 28-1). In healthy individuals the volume of the bladder and the normal voiding reflex is routed via the afferent Aδ fibers. In pathologic states, stimulation of capsaicin or vanilloid receptor subtype 1 (VR1) (receptors expressed by unmyelinated afferents in the bladder) lead to excitation of C-afferent fibers, possibly mediating bladder dysfunction as a result of inflammatory reactions. These receptors are cation channels, expressed almost exclusively by the primary sensory neurons involved in nociception and neurogenic inflammation, and can also be activated by noxious heat and changes in pH. In suprasacral neurogenic bladder disease these capsaicin-sensitive vanilloid receptors and C-afferent fibers have a major role in the pathogenesis of hyperactivity. Intravesical capsaicin and resiniferatoxin, which block transmission through C-afferent fibers for several months, have been used experimentally to treat hyperactivity when it does not respond to the usual pharmacologic agents.20,23
Central Connections and Control
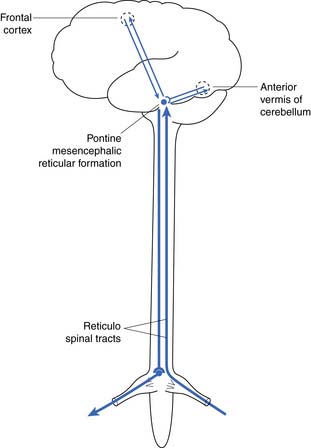
The reflex center for the bladder lies in the pons along with the other autonomic centers (Figure 28-3). Not shown in Figure 28-3 is a reflex with afferent axons originating from the bladder and synapsing on the pudendal nerve nucleus at S2, S3, and S4 (Onuf’s nucleus). This allows inhibition of pelvic floor activity during voiding. Another important reflex uses the local segmental innervation of the external sphincter with afferents from the urethra, sphincter, and pelvic floor and efferents in the pudendal nerve. Higher (voluntary) control over the pelvic floor is achieved through afferents that ascend to the sensory cortex. Descending fibers from the motor cortex synapse with the pudendal motor nucleus.6
Bladder Function
Urodynamic studies in both healthy people and those with neurologic disease have yielded clinical insights into the normal and abnormal function of the lower urinary tract over the course of life(Figure 28-4).
Elderly
Frequency, urgency, and incontinence with incomplete emptying are common in elderly persons. Urodynamic studies show that many elderly persons have bladder contractions during filling, producing frequency, urgency, and incontinence. These contractions are poorly sustained during voiding and result in incomplete evacuation. Elderly men can have prostatic obstruction, and women can have incontinence related to impaired sphincter activity or stress incontinence. In the absence of these mechanical factors, changes in bladder function in elderly persons have been ascribed to loss of cerebral control as a result of minor strokes and changes in the bladder wall caused by collagen deposition. Changes in bladder function can also result from polyuria secondary to reduced renal concentrating ability, diuretic use, lack of normal increase in antidiuretic hormone secretion at night, and mobilization of lower extremity edema during sleep.
Neurogenic Bladder Dysfunction and Classification
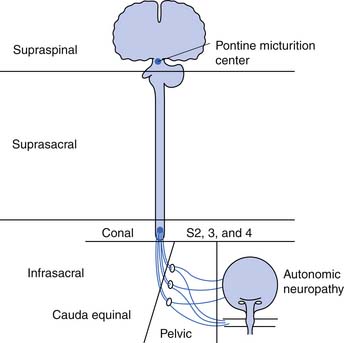
The neurogenic bladder has been classified in a variety of ways, beginning with the anatomic classification of Bors and Comarr.5 The first functional classification was based on cystometric findings, and five basic groups were described: (1) reflex, (2) uninhibited, (3) autonomous, (4) motor paralytic, and (5) sensory neurogenic bladders.31 Later a more anatomic classification system was proposed in which the neurogenic bladder was subdivided into types including supraspinal, suprasacral spinal, infrasacral, peripheral autonomic, and muscular lesions (Figure 28-5). At the same time others developed functional classifications, all of which were based on conventional urodynamic evaluations. This was an attempt to categorize the lower urinary tract according to the passive storage ability of the bladder and the activities and coordination of the detrusor and sphincter mechanisms (Table 28-2). In practice it is common to use a combination of both anatomic and functional classifications. Clinical management is based on functional changes demonstrated by urodynamic testing.
Table 28-2 Functional Classification of the Neurogenic Bladder
| Type of Failure | Bladder Factors | Outlet Factors |
|---|---|---|
| Failure to store | Hyperactivity | |
| Decreased compliance | Denervated pelvic floor | |
| Bladder neck descent | ||
| Intrinsic bladder neck sphincter failure | ||
| Failure to empty | Areflexia | |
| Hypocontractility | Detrusor–sphincter dyssynergia (striated sphincter and bladder neck) | |
| Nonrelaxing voluntary sphincter | ||
| Mechanical obstruction (benign prostatic hypertrophy or stricture) |
Evaluation
History and Physical Examination
The physical examination should assess mental status and confirm the level of the neurologic deficit (if present). Perineal sensation and pelvic floor muscle tone are particularly important. Reflexes are also important, but the bulbocavernous, cremasteric, and anal reflexes are sometimes difficult to elicit, even in neurologically intact patients. The skin of the perineum and the state of bladder support should be assessed. In women, a standard pelvic examination is warranted to assess for adequate estrogenization and any evidence of vaginal wall support defects. Good estrogenization is evidenced by a pink and moist vaginal mucosa with good rugae, whereas lack of estrogenization reveals a pale, dry, and smooth vaginal mucosa. Evidence of cystocele or pelvic organ prolapse can be seen in vaginal wall support defects. The prostate in males should be evaluated, but prostate size or consistency alone is not a good indicator of obstruction. It is also important to assess the patient’s motivation, lifestyle, body habitus, and other physical impairments including an assessment of upper limb function, lower limb function, and spine range of motion.
Diagnostic Tests
Indications
The bladder findings on urodynamic studies cannot be used alone to determine the level of neurologic lesion. For example, a suprasacral neurogenic bladder from a complete spinal cord injury can remain areflexic, and a conal or cauda equinal bladder can exhibit high pressures from poor compliance (Table 28-3). Although the anatomic level of the neurologic lesion can suggest to the clinician the most likely pattern of bladder dysfunction, urodynamic testing should be performed to confirm this. Functional bladder studies in traumatic spinal cord injury are best deferred until spinal shock has cleared.
| Term | Definition |
|---|---|
| Bladder | |
| Hyperactivity | Contractions of the detrusor during filling |
| Hypocontractility | Unsustained contractions causing failure to empty |
| Areflexia | Absent contractions with attempt to void |
| Compliance | Change in volume divided by change in baseline pressure with filling (<10 mL/cm H2O abnormal; 10 to 20 mL/cm H2O borderline if capacity reduced) |
| Outlet | |
| Detrusor–sphincter dyssynergia | |
| 1. At bladder neck | Usually in high tetraplegic patients with autonomic hyperactivity |
| 2. At striated sphincter | Uncoordinated pelvic floor and striated sphincter contraction with detrusor contraction during attempts to void |
| Nonrelaxing sphincter | Poor voluntary relaxation of voluntary sphincter in patients with areflexia attempting to void by the Valsalva maneuver |
| Decreased outlet resistance | Incontinence caused by damage to the bladder neck or striated sphincter, pelvic floor descent, or denervation |
Upper Tract Tests
Lower Tract Tests
Urinalysis, Culture, and Sensitivity Testing
These tests are done routinely for all patients with neurogenic bladder disease and should be repeated as often as necessary or at the very least at routine follow-up annually. These would also be recommended before invasive procedures in cases of suspected UTI (i.e., with cloudy, foul smelling, or bloody urine) or with new lower urinary tract symptoms such as incontinence, frequency, etc. Bacteriuria should be treated before any invasive urologic procedure or test is performed.
Postvoid Residual
By itself, a low postvoid residual (PVR) of less than 20% of capacity is not indicative of a “balanced” bladder as it was once defined because high intravesical pressures can be present despite low PVR values. The PVR is simple to determine and clinically useful, especially when compared with previous recordings and considered in conjunction with the bladder pressure, clinical symptoms, and the appearance of the bladder wall. PVRs can vary throughout the day. A catheter insertion has been used for PVR in the past, but there are now simple US machines that noninvasively obtain the PVR.9
Cystometrography
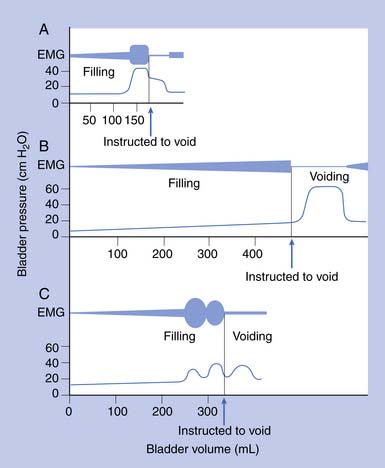
Water CMGs are best obtained with a two-channel catheter, with one channel used for filling and the other for pressure recording. A rectal pressure trace is also helpful in many patients to help distinguish intravesical pressure variations (resulting from intraabdominal transmission) from contractions of the detrusor itself. Reported filling rates vary but usually range from 25 to 60 mL/min. During filling, patients are asked to suppress voiding. Normal values include a capacity of 300 to 600 mL, with an initial sensation of filling at approximately 50% of capacity. The sensation of normal fullness is said to be appreciated in the lower abdomen with a sense of urgency in the perineum. The change in volume divided by the increase in baseline pressure during filling (i.e., in the absence of a detrusor contraction) describes the bladder’s compliance. This value should be greater than 10 mL/cm H2O, and 10 to 20 mL/cm H2O is considered borderline if the bladder capacity is reduced. The patient is asked to suppress detrusor contractions during this test. Any detrusor contraction during the test, usually defined as a phasic pressure change of more than 15 cm H2O, is abnormal. If the patient is neurologically intact, these contractions are referred to as uninhibited. If the patient has a suprasacral or supraspinal lesion, these contractions are called hyperreflexic.2 Overactive or hyperactive are terms now used to describe both types of contractions.
Sphincter Electromyography
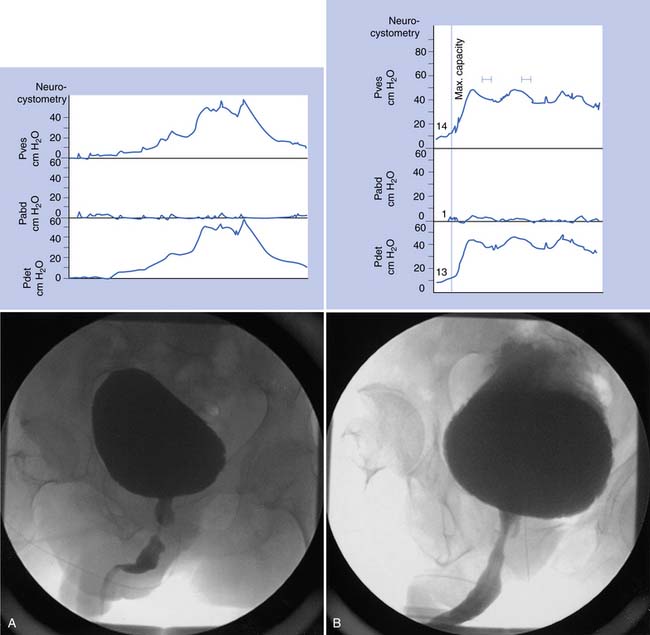
Sphincter electromyography (EMG) can be combined with the CMG or preferably with a full multichannel videourodynamic study.28 Recordings have been made with a variety of electrodes (monopolar, coaxial needle, and surface electrodes) from the levator, perianal, or periurethral muscles. Because some authors claim there is a functional dissociation between these muscle groups, periurethral recordings are preferred. The integrated EMG is displayed on the same trace as the bladder pressure. EMG activity gradually increases as bladder capacity is reached during bladder filling, and then becomes silent just before voiding. Low levels of EMG activity with no recruitment during filling are a common pattern in complete spinal cord injury. When a reflex detrusor contraction occurs in these patients, the EMG activity in the sphincter can increase rather than decrease. With this detrusor–sphincter dyssynergia, voiding often occurs toward the end of the detrusor contraction because the striated sphincter relaxes more quickly than the smooth muscle of the bladder. This type of sphincter EMG does not display individual motor units and cannot be used for the evaluation of infrasacral denervation of the pelvic floor musculature (for which standard needle EMG is needed). Diagnostic integrated EMG recordings from the external urethral sphincter are difficult to obtain and are invasive and painful in patients who are sensate. The fluoroscopic appearance of the urethra is an alternative method of determining sphincter dysfunction and is preferred after distal sphincterotomy if recurrent distal sphincter dyssynergia is suspected (Figure 28-6).
Videourodynamics
This study is designed to give the maximum information about the filling and voiding phases of lower urinary tract function, and every effort is made to make it as physiologic as possible.4 A videourodynamic study is indicated in the following patients: those with incomplete spinal cord lesions with incontinence who have some ability to void and inhibit voiding voluntarily but empty incompletely; persons with mechanical obstruction (e.g., benign prostatic hyperplasia) with neuropathy; candidates for sphincterotomy, to assess detrusor contraction and the presence or absence of bladder neck obstruction in addition to striated sphincter dyssynergia; those who fail to respond to pharmacotherapy; those who may be candidates for surgical procedures such as augmentation, continent diversion, or placement of an artificial sphincter or a suprapubic catheter; patients who have deterioration of the upper tracts; and patients who relapse frequently with symptomatic bacteriuria. The procedure requires placement of a 7-French two-channel catheter in the bladder and an 8-French balloon catheter in the rectum. EMG of the sphincter can be recorded along with bladder, rectal, and detrusor (bladder minus rectal) pressures. A contrast solution delivered at 50 mL/min is used to fill the bladder, with the patient sitting or lying as appropriate. The blood pressure is recorded in patients with spinal cord lesions above T6 to determine whether there is any autonomic dysreflexia. The bladder image is monitored intermittently with fluoroscopy, and the combined radiographic and urodynamic image is mixed on the same screen and can be recorded on videotape (see Figure 28-6). If the patient can sit and void during the study, a flow rate can also be recorded. A videourodynamic study in children with myelodysplasia or spinal cord injury might have to be modified, and adequate clinical information can often be obtained by recording bladder pressure combined with fluoroscopy. Table 28-3 lists urodynamic terms used to categorize bladder and outlet abnormalities.
Cystoscopy
The only routine indication for cystoscopy is the presence of a long-term indwelling suprapubic or urethral catheter because the presence of the catheter increases the risk for bladder tumor development.35 Cystoscopy is recommended after 5 years in high-risk patients, such as smokers, or after 10 years in those with no risk factors. Cystoscopy should also be performed after CT– intravenous pyelography (CT-IVP) in patients who have gross or microscopic hematuria that cannot be clearly associated with UTI, stones, or trauma. Often CT-KUB is the only study that will pick up small bladder stones, especially if the bladder is collapsed around an indwelling catheter. Repeated lower tract infections can be an indication for cystoscopy and can reveal nonopaque foreign bodies, such as hairs, that have been introduced by catheterization.
Management
General Principles
Bladder management should be undertaken in the context of the whole person. Patient goals are to empty the bladder not more than every 3 to 4 hours, remain continent, sleep without interference from incontinence or a urinary drainage system, and avoid recurrent UTI or other complications. Less than optimal bladder management decreases the person’s social, vocational, and avocational potential. The following discussion describes specific management approaches(Table 28-4).
| Problem | Options |
|---|---|
| Failure to store | |
| Bladder factors | |
| Behavioral | Timed voids |
| Collecting devices | Diaper, condom catheter, indwelling catheter |
| CIC | With medications to lower bladder pressure |
| Medications | Anticholinergics, intrathecal baclofen,∗ calcium channel blockers,∗ intravesical vanilloids (resiniferatoxin),∗ botulinum A injection∗ |
| Surgery | Augmentation, continent diversion, denervation procedures∗ |
| Outlet factors | |
| Behavioral | Timed voids, pelvic floor exercises |
| Collecting devices | Diaper, condom catheter, indwelling catheter |
| Medications | α-Agonists, imipramine, estrogens |
| Surgery | Collagen injection, fascial sling, artificial sphincter, Teflon injection∗ |
| Failure to empty | |
| Bladder factors | |
| Behavioral | Timed voids, bladder stimulation, Valsalva and Credé maneuvers |
| Collecting devices | Indwelling catheter |
| CIC | |
| Medications | Bethanechol |
| Surgery | Neurostimulation∗ |
| Outlet factors | |
| Behavioral | Anal stretch void |
| Collecting devices | Indwelling catheters |
| CIC | |
| Medications | α-Blockers, oral striated muscle relaxant, intrathecal baclofen∗ |
| Surgery | Sphincterotomy incision, stent sphincterotomy, bladder neck incision, prostate resection, pudendal neurectomy∗ |
| Failure of storage and emptying with nonusable urethra | |
| Surgery | Suprapubic catheter with or without bladder neck closure, ileal conduit, continent diversion |
CIC, Clean intermittent catheterization.
Behavioral Management
Bladder Stimulation
Various maneuvers have been tried to stimulate the bladder. Stroking or pinching the perineal skin to cause reflex stimulation is only rarely effective. Suprapubic tapping or jabbing over the bladder causes a mechanical stretch of the bladder wall and subsequent contraction. Controlled studies have shown that deeper indentation of the bladder with a jabbing technique is the most effective maneuver.10 This can be used by spinal cord–injured patients with condom catheters. It is most effective in patients with paraplegia who have good upper limb function.
Anal Stretch Voiding
In patients with paraplegia who have a spastic pelvic floor, effective voiding has been achieved by an anal stretch technique. This technique involves relaxing the pelvic floor by first stretching the anal sphincter and then emptying the bladder by the Valsalva maneuver.24 It requires transfer onto a toilet for bladder emptying, absence of anal sensation, and the ability to generate adequate intraabdominal pressure. For these reasons it is not widely used, even though the technique was well described more than 30 years ago.
Urine Collection Devices
Indwelling Catheters
Indwelling catheters can be either urethral or suprapubic and are typically used either because other programs have failed or for patient convenience. The combination of sphincterotomy and condom drainage, although ideal for men with tetraplegia, often fails because of inadequate detrusor contractions or penile skin problems. In the past, indwelling catheters had a justifiably negative reputation, but there are recent reports that some patients with indwelling catheters do no worse than those using other methods of management.14,17 This change is due to a number of factors, including improved catheter materials. Good catheter care is still very important. Some of the important aspects of care include monthly catheter changes, copious fluid intake, control of hyperactivity with medication, sterilization of the collecting bags with bleach, and avoidance of traction on the catheter. The prevalence of squamous cell carcinoma of the bladder associated with an indwelling catheter might be lower than reported.37
Clean Intermittent Catheterization
Intermittent catheterization using a sterile technique was introduced by Guttmann and Frankel in the 1950s for the management of patients with acute spinal cord injury. Lapides et al.25 in 1972 proposed a nonsterile but “clean” technique for the management of chronic retention and infection. The technique has since been used extensively for neurogenic bladder disease. An intermittent catheter program requires a low-pressure bladder of adequate capacity (>300 mL) and enough outflow resistance to maintain continence with normal daily activities. If the bladder is not sufficiently areflexic and compliant, anticholinergics can be used. If these fail, some form of surgery, such as augmentation, can be done to achieve a low-pressure reservoir. Men with lesions at C6 and below and women with lesions at C7 and below can usually manage self-catheterization. Although attendants and family members can perform intermittent catheterization in persons who cannot manage self-catheterization, the program often breaks down if the patient is at school or work. Patients should restrict fluid intake to maintain an output of not more than 600 mL in the period chosen. Some patients have enough sensation to be able to catheterize on demand, but most have to do so on a timed schedule. A minimum of three catheterizations per 24 hours is recommended because longer intervals between catheterizations theoretically increase the risk of symptomatic bacteriuria. Most patients wash their catheters with soap and tap water and reuse the catheters. In those patients with recurrent UTIs, other types of catheters (touchless, enclosed systems, or hydrophilic catheters) or sterilization of catheters can be helpful in reducing infections, although evidence for the efficacy of hydrophilic catheters is scant. A completely sterile technique can also be used but is rarely done in clinical practice.
Medications
Anticholinergic Agents
Anticholinergic agents have been used for many years for the suppression of detrusor activity. Propantheline bromide (15 to 30 mg three times a day) is the prototype, and hyoscyamine (0.125 to 0.25 mg three or four times a day) has also been used. Oxybutynin is currently the most commonly used agent. Oxybutynin hydrochloride can be taken at up to 5 mg four times a day or sustained release 15 mg once or twice daily in patients with no renal impairment concerns. Lower doses are needed in patients with severe hepatic impairment. Oxybutynin in solution can be administered as an intravesical instillation in patients requiring intermittent catheterization. It appears to be effective, although somewhat delayed serum levels result that are almost as high as when given orally. The side effects include mostly dry mouth and constipation, and appear to be less severe than when the drug is given orally.27 A problem with this technique is that, at present, there is no sterile liquid form, and the 5-mg tablet has to be dissolved in sterile water. An oxybutynin transdermal preparation is available, which avoids much of the drug being broken down in the liver (which occurs after oral administration). This is important because the side effects are caused by the breakdown product. Although this is a promising treatment, skin irritation might limit its use. Tolterodine is a muscarinic receptor antagonist used at a dose of 2 mg twice daily or 1 mg twice daily for patients with severe renal or hepatic impairment or with concomitant ketoconazole administration. A long-acting formulation is also available.
Cholinergic Agents
The detrusor is innervated by cholinergic muscarinic (M2 and M3) receptors. Bethanechol, a cholinergic agonist, can be helpful in detrusor areflexia by increasing detrusor activity. Although a pharmacologic effect can be seen with a parenteral dose when the bladder is partially innervated, oral doses are not effective at levels that can be tolerated by patients. Double-blind, controlled clinical trials have not been performed, and the use of this drug has declined.18
Adrenergic Antagonists
The α-adrenergic receptor antagonist phenoxybenzamine (10 to 30 mg daily) has α1– and α2-blocking actions and has been used for inhibiting smooth muscle activity at the bladder neck and in the prostate. Newer agents with more specific α1-blocking actions are available, such as prazosin, terazosin, and doxazosin.38 These are typically given in doses of 1 to 20 mg daily as tolerated. These agents have a number of effects. They appear to reduce the irritative symptoms in men with obstruction resulting from benign prostatic hyperplasia, and to increase emptying in patients with neurogenic voiding dysfunction. A more specific agent, tamsulosin (0.4 to 0.8 mg daily), has been used for the treatment of benign prostatic hyperplasia. This agent has fewer vascular effects and rarely causes hypotension. After suprasacral spinal cord injury it improved bladder storage and emptying and decreased the symptoms of autonomic dysreflexia.1 All of these agents are effective in control of the vascular manifestations of autonomic dysreflexia. Phenoxybenzamine, with its α1– and α2-blocking action, might be a better choice in this regard than the pure α1-blocking agents.
Adrenergic Agonists
Adrenergic agonists have been used to increase urethral resistance in patients with mild stress incontinence. Anecdotally, ephedrine (25 to 75 mg/day) has been effective in controlling mild stress incontinence in children with myelodysplasia, but controlled studies are lacking. This is also a consideration when treating congestion from upper respiratory infections in patients with spinal cord injury, depending on their bladder emptying system. Adrenergic agonists are rarely used in adults with bladder neuropathy. Prolongation of the α-adrenergic effects on the external urethral sphincter could be possible in the future using duloxetine, a serotonin and norepinephrine reuptake inhibitor, which acts on the pudendal (Onuf’s) nucleus in the sacral cord.
Muscle Relaxants
Baclofen, tizanidine, and dantrolene sodium are frequently used for skeletal muscle spasticity (see Chapter 30) but have never been shown to be effective in controlled studies in patients with detrusor-striated sphincter dyssynergia. Baclofen given intrathecally by infusion pump for severe lower extremity spasticity depresses pelvic floor reflexes but also depresses the detrusor reflex.36 This net result is a lower-pressure bladder that might empty less effectively. Insofar as intrathecal baclofen is indicated in some patients with tetraplegia, this overall decrease in bladder emptying might not be desirable.
Intravesical Therapy
Botulinum A toxin, given by injection at 30 to 40 sites in the bladder wall up to a total dose of 300 units, reduces or abolishes hyperactivity for up to 6 months. Repeated injections have been given with success. Although botulinum A toxin is widely available, it is an off-label use and might not be covered by insurance.26 Resiniferatoxin instillations are also under trial. These are not as effective as botulinum A toxin but are simpler to administer.20,23
Surgery
To Increase Bladder Capacity
Bowel Procedures
Augmentation
Bladder augmentation is often recommended for patients who have detrusor hyperactivity or reduced compliance that fails to respond to anticholinergic drugs.34 The patient must be motivated to continue indefinitely with clean intermittent catheterization and must have adequate outflow resistance. The patient must be fully informed of the immediate surgical risks and the possible long-term sequelae of this procedure. Immediate surgical risks include prolonged intestinal ileus or obstruction, anastomotic leak with peritonitis, wound infection, and pulmonary complications such as pneumonia and deep venous thrombosis with pulmonary emboli. Bladder augmentation has only been performed since the 1950s. The long-term complications reported so far have included chronic bacteriuria, a theoretical risk of neoplastic change, possible diarrhea or malabsorption from a shortened gut or decreased intestinal transit time, and hyperchloremic acidosis caused by absorption of urine with secondary mobilization of skeletal calcium (acting as a buffer).
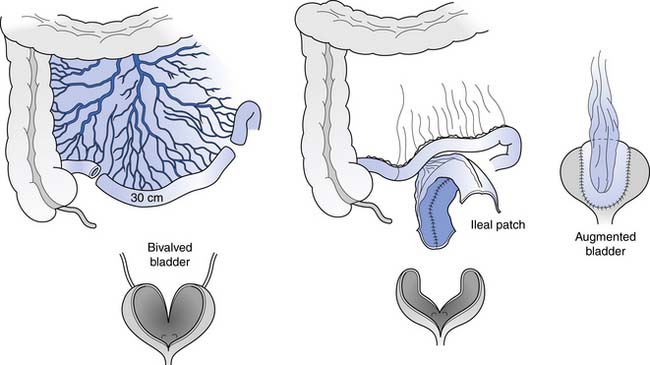
The bladder is opened widely in this procedure, and a detubularized and reconfigured segment of bowel is sewn in. A 20- to 30-cm segment of distal ileum is usually used, but an ileocecal segment, sigmoid, or even a wedge of stomach also can be used (Figure 28-7). The procedure is intended to result in a 600-mL, low-pressure reservoir without the use of any drugs. Mucus production is the main day-to-day problem initially, especially in those with active urinary infection. Mucus production is rarely a problem after the first 3 months if good intermittent catheterization technique and bladder irrigations are used as needed. Because the risk of neoplastic change is unknown with this procedure, yearly cystoscopy should probably begin 10 to 15 years after the augmentation.
Continent Diversion
In this procedure, a section of bowel is used not just to increase effective bladder capacity but also to form a continent catheterizable channel that opens onto the abdominal wall. It is particularly useful in women for whom intermittent self-catheterization via the urethra is difficult or impossible because of leg spasticity, body habitus, severe urethral incontinence, or the need to transfer from a wheelchair. Men who are unable to perform intermittent catheterization because of strictures, false passages, or fistulas are also potential candidates. Severe urethral disease in men is frequently the result of poor personal care, and it is inappropriate to perform these procedures on patients who cannot or will not follow through with appropriate techniques. If the patient fails to catheterize after augmentation and continent diversion, the bowel segment can rupture internally before overflow incontinence occurs through the urethra or catheterizable channel.
Denervation Procedures
The denervation technique for bladder hyperactivity is theoretically attractive but is not widely used. Operative approaches include sectioning of the sacral nerve roots or interrupting the peripheral nerve supply near the bladder. Selective sacral rhizotomies have been attempted. The technique involves identifying the nerve root (usually S3 on one side) that carries the detrusor reflex by doing differential sacral local anesthetic blocks while monitoring the detrusor reflex with CMG. Surgical or chemical destruction of S3 usually results in only temporary areflexia. Over time the detrusor reflex typically reroutes through the intact sacral nerves. Bilateral S2, S3, and S4 rhizotomies permanently abolish the reflex but at the expense of loss of reflex erections and worsening of the bowel evacuation problem.19
Peripheral denervation of the detrusor has been attempted by transecting the detrusor above the trigone and resuturing it, by removing the paravesical ganglia via a vaginal approach, or by overdistending the bladder with the intent of damaging the intramural nerves and muscle fibers. In one clinical study of patients with complete paraplegia, the bladder was intentionally distended in the spinal shock phase with the intent of preventing the return of the bladder reflex. The prevalence of areflexia in the short term following distention was 63% compared with an expected 15%.22 None of these peripheral denervation procedures have become commonly accepted, and long-term results of intentional overdistention of the bladder in spinal shock have not yet been reported. Chemical denervation procedures have been discussed under intravesical therapy.
To Increase Bladder Contraction
Electrical Stimulation
Attempts have been made to stimulate detrusor contraction using electrodes implanted on the bladder wall, pelvic nerves, sacral roots, and conus. At present, the only site being used clinically is the sacral roots, and most of the reported series come from Europe.7 The electrodes are placed on the anterior roots either intradurally or extradurally. To prevent spontaneous hyperactive contractions and antidromic reflex contractions, bilateral S2, S3, and S4 dorsal rhizotomies are usually performed. Pelvic floor contraction with anterior root stimulation will still obstruct voiding, and European centers have elected to stimulate intermittently. This leads to intermittent voiding, as the striated pelvic floor muscle relaxes more quickly than the smooth muscle detrusor. In a center developing this device in the United States, pudendal neurectomies were performed to decrease outflow resistance.39 Adequate bladder emptying at acceptable intravesical pressures with preservation of the bladder wall and upper tract morphology and function has been reported.7 One important disadvantage of bilateral S2, S3, and S4 rhizotomies is that reflex erections are abolished, and usable erections occurring as a result of sacral root stimulation occur in less than 30% of patients. Bowel evacuation, however, is improved in many patients. Further refinements such as supraselective rhizotomies, with modification of stimulus parameters and electrode design, are future possibilities. This technique is approved for routine clinical use in the USA, but there is no company marketing the device at this time.
To Increase Outlet Resistance
External Compressive Procedures
In the fascial sling procedure, a 2-cm strip of fascia is taken from the anterior rectus abdominis fascia or tensor fascia lata. It is wrapped around the bladder neck and fixed anteriorly to the abdominal fascia or pubic tubercle. Patients who are candidates for this procedure must have compliant low-pressure bladders. They will be unable to void by the Valsalva maneuver after a successful sling procedure and must be willing to perform self-catheterization indefinitely in exchange for being continent.
To Decrease Outlet Resistance
Sphincterotomy
In male spinal cord–injured patients unable or unwilling to do self-catheterization, the use of a condom catheter is a practical alternative. Because it is unusual to find a lower urinary tract that has adequate detrusor contraction and a coordinated pelvic floor in these patients, some procedure to decrease outflow resistance is usually indicated. The results are poor in patients without adequate detrusor contractions. Preoperative parameters suggested for a good outcome are presented in Box 28-1. Ablation of the striated sphincter, usually by incision, is the standard procedure. It is now performed anteriorly to avoid the cavernous artery and nerve, which lie lateral to the membranous urethra. Damage of the cavernous artery and nerve can lead to impotence. Some patients also have bladder neck obstruction. This can be due to primary hyperactivity (e.g., high tetraplegic patients) or total bladder wall hypertrophy (which follows striated sphincter dyssynergia). These patients need bladder neck ablation either by resection or by incision. In older men, prostatic obstruction from benign disease can also contribute to increased outflow resistance and might require prostatic resection. The immediate morbidity from sphincterotomy—bleeding, clot retention, and infection—is relatively high. The long-term results can be compromised because of recurrent obstruction from stricture or recurrent dyssynergia. An implantable stainless steel stent is now available and should reduce morbidity and improve long-term results. This stent material is inert, and the epithelium grows through the spaces between the wires of the stent, completely covering it.30 Unfortunately, the stent is not well tolerated in men with sensory incomplete spinal cord injury.
Other Methods of Decreasing Outflow Resistance
Intrathecal baclofen given for severe spasticity decreases the pudendal reflexes, but the detrusor reflex and contractions are reduced as well. Consequently, it cannot be used as a chemical sphincterotomy. Botulinum A toxin injected into the striated sphincter has also been used experimentally, but (as in the bladder) its effects last only a few months.16
Management of Specific Diseases
Diseases of the Brain
Stroke
After an initial period of areflexia, stroke patients typically have hyperactivity with frequency and urge incontinence but coordinated voiding and complete emptying (see Chapter 50). Anticholinergics frequently help ameliorate symptoms without adversely affecting emptying. Persistent areflexia and retention can occur with bilateral lesions. Prostatic obstruction can cause retention in elderly men after a stroke. Videourodynamic studies are helpful in differentiating these conditions.
Parkinson’s Disease
The prevalence of bladder symptoms in patients with Parkinson’s disease is high (70%) (see Chapter 51). Most have frequency, urgency, and urge incontinence, and 50% complain of difficulty voiding. Evaluation typically shows bladder hyperactivity, but the contractions are poorly sustained and result in incomplete emptying. Failure to empty can also be caused by bradykinesia, secondary to failure of pelvic floor relaxation, adrenergic effects of levodopa, or even the anticholinergic effects of other antiparkinsonian drugs.3 Treatment is difficult because there is frequently a combination of incontinence and retention. Detrusor inhibition with drugs makes emptying worse, and α-adrenergic blockers have a marginal effect in decreasing outflow resistance.38 Intermittent catheterization and detrusor inhibition are often the best choice, but many patients do not have sufficient upper extremity dexterity to catheterize independently.
Dementia, Brain Tumors, and Trauma
Dementia, brain tumors, and trauma can all cause hyperactivity with reflex or urge incontinence with complete emptying. If cognitive impairment is severe, incontinence often persists despite detrusor inhibition. Some type of collecting device is appropriate for many of these patients (see Chapter 49).
Diseases of the Brain and Spinal Cord
Multiple sclerosis is the most common disease in this category, with 90% of patients developing urinary manifestations in the course of the disease (see Chapter 52). The bladder symptoms usually present because of an incomplete spinal cord lesion with hyperactivity and hypocontractility. Detrusor inhibition with drugs worsens emptying in this case. In the rare, predominantly encephalopathic variety of multiple sclerosis, these agents might be useful. Patients with multiple sclerosis and a predominantly conal lesion have bladder areflexia. Intermittent catheterization is eventually indicated in most patients with multiple sclerosis, but few are able to undertake it because of poor upper extremity strength and coordination. High-pressure bladders resulting from hyperactivity and detrusor–sphincter dyssynergia are rare, but sphincterotomy in men is sometimes indicated.29
Diseases of the Spinal Cord
Injury, tumors, and vascular lesions of the spinal cord cause the majority of suprasacral neurogenic bladder problems (see Chapter 55). The detrusor reflex typically returns after a varying period of spinal shock. The center for the detrusor reflex develops in the sacral cord in patients with complete injury. Inhibitory control by the higher center is impaired, and because the long-routed detrusor reflex is interrupted, the detrusor contraction might not be completely sustained. Coordination and control of the pelvic floor are also impaired, leading to lack of voluntary contraction and relaxation. In complete lesions there is often dyscoordinated activity during voiding. This dyscoordination or detrusor–sphincter dyssynergia affects the striated voluntary sphincter, but in high complete tetraplegic patients excessive sympathetic activity can also lead to detrusor–bladder neck dyssynergia. Incomplete spinal cord lesions can produce the supraspinal pattern with urgency and adequate emptying, whereas patients with complete lesions have reflex incontinence and incomplete emptying because of detrusor–sphincter dyssynergia (in most cases). Some patients have hypocontractility or areflexia and retention. A truly balanced bladder with sustained detrusor contraction and coordinated pelvic floor is uncommon.
In male patients unable or unwilling to do self-catheterization, as well as for those who refuse augmentation, sphincterotomy followed by use of an external catheter is probably the best alternative. Other options include intermittent catheterization by an attendant, although this has a greater risk of febrile UTIs.11 Wearing an external collector alone can be considered, but only 15% of men with spinal cord injury have a suitable, truly “balanced” bladder with coordinated voiding at low pressure. Some men with tetraplegia end up with an indwelling catheter because of sphincterotomy failure, inadequate detrusor contractions, or skin breakdown on the penile shaft. Women using intermittent catheterization but unable to control urinary incontinence with medications might also choose to use an indwelling catheter. A regular long-term urinary tract surveillance program (Box 28-2) should be set up for spinal cord–injured patients who might, with good care, have a near-normal life expectancy.
Diseases of the Spinal Cord and Conus
Myelodysplasia is the most common disease producing a mixed pattern of lower urinary tract dysfunction. Any combination of detrusor and sphincter activity can be found, but it is most common to have a hyperactive or noncompliant bladder with dyssynergia or a nonrelaxing sphincter. Intermittent catheterization is used initially along with medication in infancy and childhood. In many cases, reconstructive surgery is necessary early if more conservative measures fail.
Management of Complications
Bacteriuria
About half of all hospital-acquired infections originate in the urinary tract in association with urinary catheters and other drainage devices. UTIs are a common source of morbidity in patients with neurogenic bladders. Frequent exposure to antibiotics increases the risk of infection with antibiotic-resistant organisms, further complicating the treatment of UTI. The diagnosis of UTI can be delayed or missed in patients with neurologic disorders affecting bladder sensation. In patients with spinal cord disorders, signs and symptoms suggestive of UTI include fever, onset of urinary incontinence, increased spasticity, autonomic dysreflexia, increased sweating, cloudy and odorous urine, malaise, lethargy, and sense of unease.32 Unexplained signs and symptoms suggestive of UTI in the presence of pyuria warrant empirical therapy for UTI. The absence of pyuria makes the diagnosis of UTI unlikely but does not exclude it.
Asymptomatic bacteriuria is very common in patients with neurogenic bladder, especially those using intermittent or indwelling catheterization. Most authorities recommend against routine treatment of asymptomatic bacteriuria, although the presence of significant bacteriuria with urease-producing organisms that are associated with stone formation might warrant treatment.33
The spectrum of uropathogens that cause catheter-associated UTI is much broader than that causing uncomplicated UTI. Escherichia coli bacteria cause the majority of uncomplicated UTIs. E. coli and organisms such as species of Proteus, Klebsiella, Pseudomonas, Serratia, Providencia, enterococci, and staphylococci are relatively more common in patients with catheter-associated UTI.32 Polymicrobic bacteriuria is the rule in patients with indwelling catheters.
In more seriously ill, hospitalized patients, piperacillin plus tazobactam, ampicillin plus gentamicin, or imipenem plus cilastatin provide coverage against most expected pathogens, including P. aeruginosa and most enterococci.8 A number of other parenteral antimicrobial agents can also be used. Patients can be switched to oral treatment after clinical improvement. At least 7 to 14 days of therapy is generally recommended, depending on the severity of the infection.33 No convincing evidence shows that regimens longer than this are beneficial. Patients undergoing effective treatment for UTI with an antibiotic to which the infecting pathogen is susceptible should have definite improvement within 24 to 48 hours. If not, a repeat urine culture and imaging studies (US or CT) are indicated.
Autonomic Dysreflexia
Paroxysmal hypertension, sweating, piloerection, headache, and reflex bradycardia are brought on by increased stimulation into and sympathetic output from the isolated spinal cord below a complete lesion. Spinal cord injuries below T6 are rarely associated with this problem. Stimulation from the bladder from high pressures or overdistention is the most common cause of autonomic dysreflexia. The best treatment is prevention of such stimuli. If symptoms persist when the bladder has been emptied or if the blood pressure is at a dangerously high level, 10 mg of sublingual nifedipine, a calcium channel blocking agent, can be given. It is often safer to apply 1⁄2 to 1 inch of nitroglycerin ointment on the skin because this can be wiped off if the blood pressure response is too exaggerated. If a patient has recently ingested sildenafil, then prazosin or captopril can be used instead.12 Long-term management with phenoxybenzamine (10 to 30 mg daily) has been used to prevent autonomic dysreflexia when all findable causes have been eliminated (see Chapter 55 for further explanation of the treatment of autonomic dysreflexia).
Hypercalciuria and Stones
Loss of calcium from the bones occurs in all spinal cord–injured patients and is worse in young males. Increased urinary calcium (>200 mg/24 hours) begins about 4 weeks after injury, reaches a maximum at 16 weeks, and can persist for 12 to 18 months. Renal stone incidence in the first 9 months is approximately 1.0% to 1.5% and is due mainly to hypercalciuria. Over the next 10 years upper tract stones are found in 8%, with many of these secondary to infection. The incidence of bladder stones in the first 9 months in patients on intermittent catheterization is 2.3%. In the presence of an indwelling catheter, and despite greater urine output, the prevalence is much higher (8.8%).13
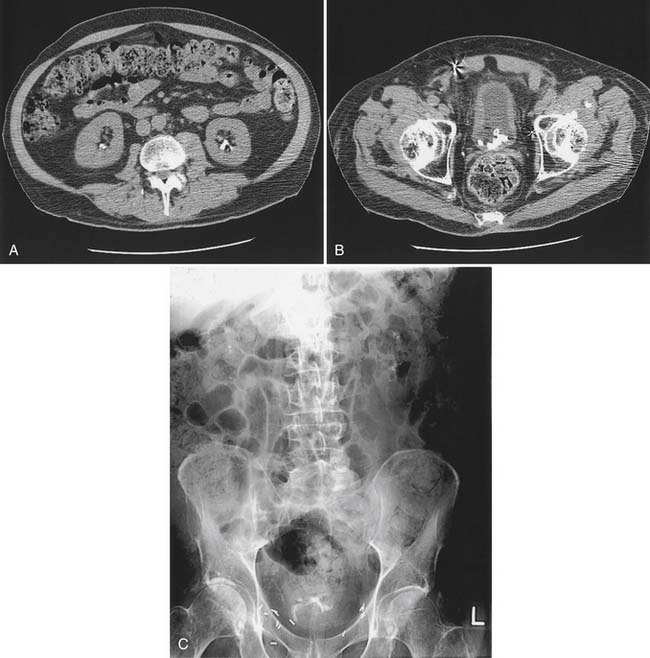
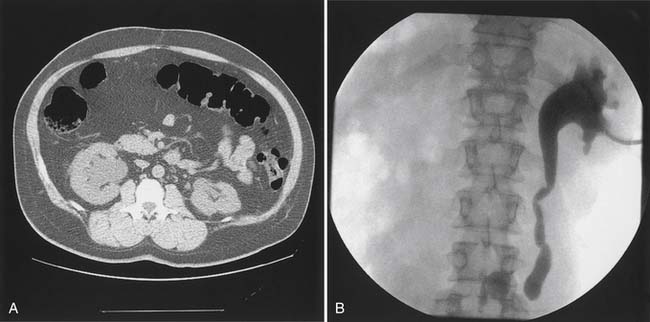
Bladder stones are effectively treated with cystoscopy and holmium:yttrium–aluminum–garnet laser lithotripsy. Small stones and particles can be dissolved by daily bladder irrigations with 30 mL of 10% hemiacidrin (Renacidin) solution, which is left in the bladder for 30 minutes. Some patients with recurrent stones use this once or twice a week for prophylaxis. In patients who have ureteral reflux, it should be used with caution because of potential nephrotoxicity and absorption of magnesium. Calyceal calculi that are small (<1 cm) and asymptomatic can be followed expectantly, but 50% of these patients become symptomatic over 5 years, and half of these will need some sort of invasive procedure.21 Calculi that are growing or that are located in the renal pelvis should probably be treated before they pass into the ureter and cause obstruction (Figure 28-8). Extracorporeal shock wave lithotripsy (ESWL) is the standard treatment. For large stones (>3 cm diameter), a percutaneous approach is preferred because clearance of fragments is poor if patients are inactive.
Ureteral Reflux and Upper Tract Dilation
Ureteral reflux or high bladder pressure in the absence of reflux can cause upper tract dilation (Figure 28-9). Dilation without reflux is said to be caused by decreased compliance, but data from long-term monitoring suggest that baseline pressure elevations are minimal with natural rates of filling, and that increased phasic activity might be more important.40 With reflux, or ureteral dilation without reflux, the bladder pressure should be lowered with intermittent catheterization and anticholinergics. If bladder pressure responds but reflux fails to improve, a surgical procedure to repair the reflux can be considered. If bladder pressures do not improve, the options are to augment the bladder or, in men, to perform a sphincterotomy and rely on free drainage.
1. Abrams P., Amarenco G., Bakke A., et al. Tamsulosin: efficacy and safety in patients with neurogenic lower urinary tract dysfunction due to suprasacral spinal cord injury. J Urol. 2003;170(4):1242-1251.
2. Abrams P., Blaivas J.G., Stanton S.L., et al. Standardization of terminology of lower urinary tract function. In Krane R.J., Siroky M.B., editors: Clinical neurology, ed 2, Boston: Little, Brown, 1991.
3. Berger Y., Blaivas J.G., DeLaRocha E.R., et al. Urodynamic findings in Parkinson’s disease. J Urol. 1987;138(4):836-838.
4. Blaivas J.G. Videourodynamics. In Krane R.J., Siroky M.B., editors: Clinical neurology, ed 2, Boston: Little, Brown, 1991.
5. Bors E., Comarr A.E. Neurological urology. Baltimore, MD: University Park Press; 1971.
6. Bradley W.E. Physiology of the urinary bladder. In: Walsh P.C., Gittes R.F., Perlmutter A.D., et al, editors. Campbell’s neurology. Philadelphia: WB Saunders, 1986.
7. Brindley G.S., Rushton D.N. Long-term follow-up of patients with sacral anterior root stimulator implants. Paraplegia. 1990;28(8):469-475.
8. Cardenas D.D., Hooton T.M. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;75(3):272-280.
9. Cardenas D.D., Kelly E., Krieger J.N., et al. Residual urine volumes in patients with spinal cord injury: measurement with a portable ultrasound instrument. Arch Phys Med Rehabil. 1988;69(7):514-516.
10. Cardenas D.D., Kelly E., Mayo M.E. Manual stimulation of reflex voiding after spinal cord injury. Arch Phys Med Rehabil. 1985;66(7):459-462.
11. Cardenas D.D., Mayo M.E. Bacteriuria with fever after spinal cord injury. Arch Phys Med Rehabil. 1987;68:291-293.
12. Consortium for Spinal Cord Medicine, Linsenmeyer T.A., Baker E.R., et al. Clinical practice guidelines for health-care professionals: acute management of autonomic dysreflexias: individuals with spinal cord injury presenting to health-care facilities. J Spinal Cord Med. 2002;25(S1):S67-S88.
13. DeVivo M.J., Fine P.R., Cutter G.R., et al. The risk of renal calculi in spinal cord injury patients. J Urol. 1984;131(5):857-860.
14. Dewire D.M., Owens R.S., Anderson G.A., et al. A comparison of the urological complications associated with long-term management of quadriplegics with and without chronic indwelling urinary catheters. J Urol. 1992;147(4):1069-1071.
15. Dmochowski R.R., Miklos J.R., Norton P.A., et al. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170(4):1259-1263.
16. Dykstra D.D., Sidi A.A. Treatment of detrusor-sphincter dyssynergia with botulinum A toxin: a double-blind study. Arch Phys Med Rehabil. 1990;71(1):24-26.
17. Feifer A., Corcos J. Contemporary role of suprapubic cystostomy in treatment of neuropathic bladder dysfunction in spinal cord injured patients. Neurourol Urodyn. 2008;27(6):475-479.
18. Finkbeiner A.E. Is bethanechol chloride clinically effective in promoting bladder emptying? A literature review. J Urol. 1985;134(3):443-449.
19. Gasparini M.E., Schmidt R.A., Tanagho E.A. Selective sacral rhizotomy in the management of the reflex neuropathic bladder: a report on 17 patients with long-term followup. J Urol. 1992;148(4):1207-1210.
20. Giannantoni A., Di Stasi S.M., Stephen R.L., et al. Intravesical resiniferatoxin versus botulinum-A toxin injections for neurogenic detrusor overactivity: a prospective randomized study. J Urol. 2004;172(1):240-243.
21. Glowacki L.S., Beecroft M.L., Cook R.J., et al. The natural history of asymptomatic urolithiasis. J Urol. 1992;147(2):319-321.
22. Iwatsubo E., Komine S., Yamashita H., et al. Over-distension therapy of the bladder in paraplegic patients using self-catheterization: a preliminary study. Paraplegia. 1984;22(4):210-215.
23. Kim J.H., Rivas D.A., Shenot P.J., et al. Intravesical resiniferatoxin for refractory detrusor hyperreflexia: a multicenter, blinded, randomized, placebo-controlled trial. J Spinal Cord Med. 2003;26(4):358-363.
24. Kiviat M.D., Zimmermann T.A., Donovan W.H. Sphincter stretch: a new technique resulting in continence and complete voiding in paraplegics. J Urol. 1975;114(6):895-897.
25. Lapides J., Diokno A.C., Silber S.J., et al. Clean, intermittent self-catheterization in the treatment of urinary tract disease. J Urol. 1972;107(3):458-461.
26. MacDonald R., Monga M., Fink H., et al. Neurotoxin treatments for urinary incontinence in subjects with spinal cord injury or multiple sclerosis: a systematic review of effectiveness and adverse effects. J Spinal Cord Med. 2008;31:157-165.
27. Madersbacher H., Jilg G. Control of detrusor hyperreflexia by the intravesical instillation of oxybutynin hydrochloride. Paraplegia. 1991;29(2):84-90.
28. Mayo M.E. The value of sphincter electromyography in urodynamics. J Urol. 1979;122(3):357-360.
29. Mayo M.E., Chetner M.P. Lower urinary tract dysfunction in multiple sclerosis. Urology. 1992;39(1):67-70.
30. McInerney P.D., Vanner T.F., Harris S.A., et al. Permanent urethral stents for detrusor sphincter dyssynergia. Br J Urol. 1991;67(3):291-294.
31. McLellan F.C. The neurogenic bladder. Springfield, IL: Charles C Thomas; 1939.
32. Montgomerie J.Z., Chan E., Gilmore D.S., et al. Low mortality among patients with spinal cord injury and bacteremia. Rev Infect Dis. 1991;13(5):867-871.
33. National Institute on Disability and Rehabilitation Research Consensus Statement. The prevention and management of urinary tract infection among people with spinal cord injuries. J Am Paraplegia Soc. 1992;15:194-204.
34. Sidi A.A., Becher E.F., Reddy P.K., et al. Augmentation enterocystoplasty for the management of voiding dysfunction in spinal cord injury patients. J Urol. 1990;143(1):83-85.
35. Steers W.D. Physiology and pharmacology of the bladder and urethra. In Walsh P.C., Retch A.B., et al, editors: Campbell’s urology, ed 7, Philadelphia: WB Saunders, 1998.
36. Steers W.D., Meythaler J.M., Haworth C., et al. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J Urol. 1992;148(6):1849-1855.
37. Subramonian K., Cartwright R.A., Harnden P., et al. Bladder cancer in patients with spinal cord injuries. BJU Int. 2004;93(6):739-743.
38. Swierzewski S.J.I., Gormley E.A., Belville W.D., et al. The effect of terazosin on bladder function in the spinal cord injured patient. J Urol. 1994;151(4):951-954.
39. Tanagho E.A., Schmidt R.A., Orvis B.R. Neural stimulation for control of voiding dysfunction: a preliminary report in 22 patients with serious neuropathic voiding disorders. J Urol. 1989;142(2):340-345.
40. Webb R.J., Styles R.A., Griffiths C.J., et al. Ambulatory monitoring of bladder pressure in low compliance neurogenic bladder dysfunction. J Urol. 1992;48:1477-1488.